Abstract
Widespread use of pesticides has resulted in the accumulation of pesticide residues in the environment due to their persistence and stability. To reduce potential exposures, we have developed broad-acting clay-based sorbents that can be included in the diet as enterosorbents to reduce the bioavailability and toxicity of chemicals. In this study, parent and acid-processed calcium montmorillonite clays (CM and APM, respectively) were used to determine their potential as sorbents of the organochlorine insecticide dieldrin. We used adsorption isotherms, thermodynamics and dosimetry studies to determine the capacities and affinities of the clays, enthalpies of the binding reactions and potential doses of sorbent that could protect against high exposures. Adsorption isotherms for APM fit a Langmuir model with high enthalpy (suggesting chemisorption) and high capacity (Qmax value equal to 0.45 mol kg−1), indicating tight binding of dieldrin. Cultures of Hydra vulgaris were used to determine the ability of sorbents to protect a living organism from dieldrin toxicity. The inclusion of acid-processed clays resulted in the highest reduction of dieldrin toxicity (70%) in the hydra. Further work indicated that both CM and APM can significantly reduce the bioavailability of dieldrin from soil (p≤0.01). These results suggest that APM (and similar clays) can be effective sorbents of dieldrin and may be included in the diet and/or soil to protect against environmental exposures.
Keywords: dieldrin, adsorption, organochlorines, isotherm, montmorillonite
INTRODUCTION
Widespread use of pesticides has resulted in the accumulation of pesticide residues in the environment due to their persistence and stability. Dieldrin, an organochlorine insecticide, was used extensively in agricultural and commercial settings until the 1970s when many countries began to prohibit its use due to toxicity concerns (ATSDR 2002). However, dieldrin and other persistent organic pollutants (POPs) can still be found throughout the environment, posing significant contamination threats. High levels of dieldrin have been detected in soil from vegetable farms and other agricultural fields years after discontinuation of use (Getenga et al. 2004; Harner et al. 1999; Hashimoto 2005; Zhang et al. 2012). The high affinity of dieldrin for organic matter such as animal fat and plant wax can result in its bioaccumulation (USEPA 2003). Reports of dieldrin uptake by plants such as root crops indicate potential for exposure to dieldrin through consumption of contaminated produce (Donnarumma et al. 2009; Doong et al. 1999; Otani et al. 2006; Saito et al. 2012). Dieldrin uptake by grasses and other herbaceous plants is of concern for livestock and domestic animals (Paton et al. 1997). Moreover, exposure to dieldrin has been associated with neurotoxic, reproductive and carcinogenic effects (Abramovich et al. 2007; Kanthasamy et al. 2005; Stern 2014).
Exposure to pesticides and other chemicals can be enhanced during natural and manmade disasters such as floods and chemical spills. Chemicals can be mobilized and redistributed throughout the environment following these disasters and further contaminate food and water sources, increasing the risk of harmful exposures. Humans and animals can be exposed to these chemicals through contaminated food and water or hand-to-mouth exposures resulting from contact with contaminated soil. Pesticides such as dieldrin are of particular concern due to their widespread use and persistence in the environment. Protecting humans and animals from harmful exposures to these environmental contaminants is an emerging issue in public health.
Natural clay materials can be used as sorbents to reduce the bioavailability of hazardous chemicals. Montmorillonite clays have a 2:1 aluminosilicate layered structure with high surface areas that contribute to their abilities to bind various chemicals in pores and on active interlayer surfaces. Our laboratory has shown that calcium montmorillonite (CM) clays are exceptional sorbents that are safe for human and animal consumption on a short-term basis and can tightly bind aflatoxin in the gastrointestinal tract, reducing its bioavailability (Phillips et al. 2008; Phillips et al. 2019). Previous studies indicate that acid treatment of clays increases their sorption capacities. Acid processing induces structural changes such as exchange of extra-lattice cations and dissociation of octahedral and tetrahedral sheets, resulting in the formation of amorphous silicon and increased surface area and pore volume (Kumar et al. 1995; Narayanan et al. 1998; Tomić et al 2010). For example, treatment of CM with 17% sulfuric acid can increase montmorillonite surface area by a factor of 2 (Novikova et al. 2006). Recent studies in our laboratory have shown that acid-processed calcium montmorillonite clays (APMs) can have increased capacities for mycotoxins and organophosphate pesticides compared to the parent CM clay (Wang et al. 2019). These novel, broad-acting sorbents can bind a variety of environmental chemicals and could be included in diets on a short-term basis to reduce human and animal exposures to chemicals in water or food.
Previous studies have evaluated the potential of various natural and amended clay minerals as sorbents for pesticides. A natural kaolinite/montmorillonite clay was able to reduce levels of methomyl in aqueous solutions by 27 to 33% (El-Geundi et al. 2012). Additionally, clays modified with cationic surfactants show increased binding potentials for pesticides compared to natural clays. Montmorillonite clay modified with OTDMA was 100-fold more effective than the natural montmorillonite in binding the hydrophobic pesticides penconazole and metalaxyl, and could be used to decrease the mobility of these pesticides in soil (Sanchez-Martin et al. 2006). Organosmectites reduced bentazone availability in soil almost instantaneously from 124 μg g−1 to 1 μg g−1 (Carrizosa et al. 2000).
There are currently no reports of acid-modified clay-based sorbents that can be used as enterosorbents to reduce bioavailability (bioaccessibility) of dieldrin. This study was designed to determine the effectiveness of CM and APMs as sorbents for dieldrin using in vitro isothermal analyses (including thermodynamics and binding mechanisms), dosimetry studies and the hydra assay. Additionally, CM and APMs were evaluated for their ability to bind dieldrin in soil and reduce potential exposures.
MATERIALS AND METHODS
Reagents
High pressure liquid chromatography (HPLC) grade acetonitrile and pH buffers (4.0, 7.0 and 10.0) were purchased from VWR (Atlanta, GA). HPLC grade hexane and dieldrin analytical standard were purchased from Sigma-Al (Saint Louis, MO). HPLC grade acetone was purchased from Fisher Scientific (Hampton, NH). Calcium montmorillonite (CM) was obtained from Engelhard Corp. (Cleveland, OH) with an average total surface area of 850 m2 g−1, an external surface area of approximately 70 m2 g−1, and a cation exchange capacity of 97 cmol kg−1 (Grant and Phillips 1998). The general formula for CM is (Ca)0.3(Al,Mg)2Si4O10(OH)2·nH2O and the compound contains some quartz, mica, calcite, orthoclase feldspars and sanidine as impurities (Marroquin-Cardona et al. 2011). Deionized water (18.2 MΩ) was generated in the laboratory using an Elga™ automated filtration system (Woodridge, IL) and was used in all experiments.
Synthesis of sorbents
Acid-processed montmorillonite clays (APMs) were synthesized following methods previously described (Wang et al. 2019). Briefly, CM was treated with sulfuric acid to produce sorbents with high surface areas and greater porosities than the parent clay. CM clay suspensions (6% w/w) were treated with 18N or 12N (APM1 or APM2) sulfuric acid. The solutions were stirred vigorously in an oven at 60°C overnight. The slurry was cooled, centrifuged at 2000 g for 20 min and washed thoroughly with distilled water. This centrifugation/washing process was repeated until the pH was constant. Samples were oven-dried overnight at 110°C, ground and sieved through a 125-μm screen. Grinding and sieving was repeated until uniform particle sizes were obtained.
To determine the importance of clay interlayers in binding dieldrin, clays were heat-collapsed to significantly reduce interlayer surfaces. Collapsed CM and APM1 clays were prepared by heating at 200°C for 30 min, then 800°C for 1 h (Grant and Phillips 1998).
In vitro analysis: isothermal adsorption
The dieldrin stock solution was prepared by dissolving pure powder into the analytical mobile phase (66:34 - acetonitrile:water). Sorbents were added at 0.002% w/vol to dieldrin solutions with an increasing dieldrin concentration gradient. The 0.002% inclusion level was achieved by adding 40 μL of 0.5 mg ml−1 clay suspension in mobile phase which was mixed vigorously during transfer to ensure equal distribution of clay to each sample. The concentration gradients of dieldrin solutions were achieved by adding a calculated amount of dieldrin stock solution to a complementary volume of mobile phase in 1.5 mL centrifuge tubes to a total volume of 1 mL. In addition to these samples, 3 controls were tested (mobile phase, dieldrin solution and 0.002% clay solution). All samples were agitated on an orbital shaker at 1000 rpm for 2 h at ambient temperature (25°C) and high temperature (37°C) for thermodynamics experiments. Samples were then centrifuged at 2000 g for 20 min to separate clay/dieldrin complexes from the solution.
HPLC was used to measure the amount of free dieldrin in the supernatant (Pantaleoni et al. 1989). Chromatography was conducted on a Waters HPLC equipped with a 717 plus autosampler, model 1525 binary pumps, model 2487 Duel Absorbance Detector and a Symmetry® C18 5μm (4.6 × 150 mm) column. Chemical separation was achieved by a mobile phase of 66% acetonitrile and 34% water at a flow rate of 1.5 mL min−1 and injection volume of 20 μL. The UV detector was set at 215 nm and 254 nm. Breeze® software was used to control the HPLC system and collect the data. The standard curve for deldrin was linear with r2 > 0.99.
Data calculations and curve fitting
Dieldrin was detected by HPLC and quantified using standard calibration curves. Dieldrin concentrations in solution were calculated from peak area at the dieldrin retention time (5.1 min). The amount adsorbed for each data point was calculated from the concentration difference between test and control groups. The resulting data was input into a Microsoft Excel program and plotted using Table-Curve 2D to derive values for each parameter. The best fit for the data was the Langmuir model, which was then used to plot equilibrium isotherms for each analysis. The isotherm equation was entered as a user-defined function,
where q = toxin adsorbed (mol kg−1), Qmax = maximum capacity (mol kg−1), Kd = distribution constant and Cw = toxin equilibrium concentration. Estimates for the Qmax and Kd were derived from a double logarithmic plot of the data. The plot will normally display a break in the curve, where the value of the x-axis is an estimate of Kd, and the value of the y-axis is an estimate of Qmax. The Kd value is derived from the Langmuir equation by solving for Kd:
The enthalpy (ΔH) is a parameter of the thermodynamics of the binding reaction, indicating total heat released or absorbed. It is calculated by the Van’t Hoff equation, comparing individual Kd values at two temperatures (25°C and 37°C):
where R (ideal gas constant) = 8.314 J/mol K, and T (absolute temperature) = 273 + t (°C).
Dosimetry extrapolations
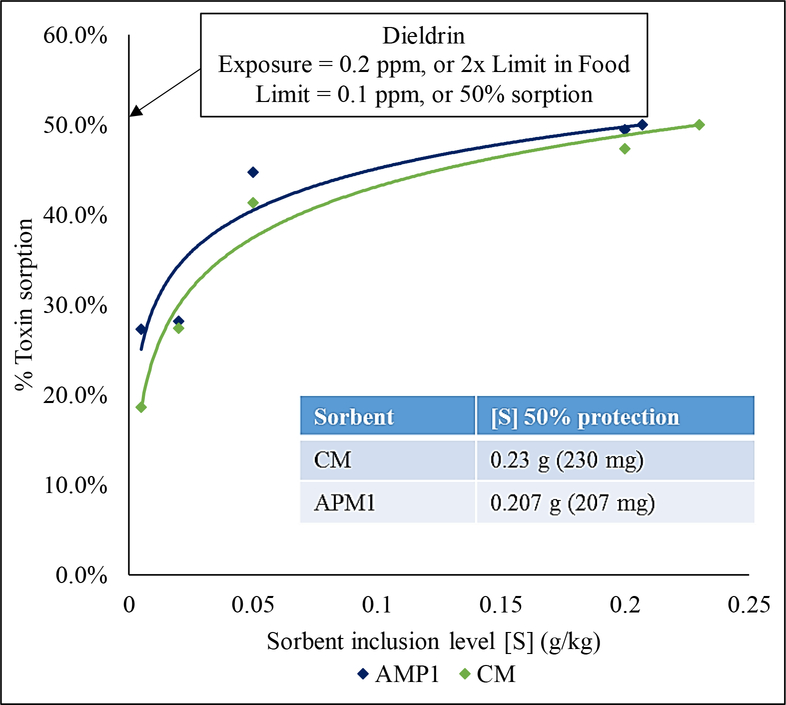
Dosimetry studies were conducted to estimate the doses of sorbents necessary to maintain threshold limits of dieldrin. The dieldrin stock solution was diluted with mobile phase to produce 0.2 ppm dieldrin, which is twice its threshold limit. The regulatory limit of dieldrin in food is equal to 0.1 ppm (ATSDR 2002). An increasing gradient of sorbent (0.0005, 0.002, 0.005, and 0.2% w/vol) was added to dieldrin solution to make 1 mL. Control groups included mobile phase, dieldrin solution, and clay solution. Control and test groups in 1.5 mL centrifuge tubes were agitated at 1000 rpm for 2 h and then centrifuged at 2000 g for 20 min. Aliquots of dieldrin were measured by HPLC and concentrations were calculated from the signal peak area. The toxicant sorption percentage for each group was calculated from the difference between control and test groups. The sorbent inclusion levels were plotted against % dieldrin sorption, and the values were fit to a natural log trend to extrapolate the sorbent inclusion levels that would be required to reduce high exposures of dieldrin from food to below the threshold limit.
In vivo analysis: hydra assay
Hydra vulgaris were obtained from Environment Canada (Montreal, QC) and maintained at 18°C. The original hydra classification method (Wilby et al. 1990) was used with modification to rate morphology of the adult hydra as an indicator of solution toxicity. Following our modifications (Brown et al., 2014), the morphological scoring of hydra in this assay was objective and repeatable. A score of 0 to 10 was assigned individually to three hydra in each experimental group. Scores of 7 to 10 indicated minor toxicity, 4 to 6 indicated moderate toxicity, and 0 to 3 indicated severe toxicity. The modified assay included monitoring at shorter time intervals during the first two days (0, 4, 20, and 28 h) and 24 h intervals during the last three days (44, 68, 92 h). Solutions were not changed during the testing period. Control groups included hydra media (18.2 MΩ water, 4 mg L−1 EDTA, 115 mg L−1 N-tris[hydroxymethyl]methyl-2- aminoethanesulfonic acid (TES), and 147 mg L−1 CaCl2 adjusted to pH 6.9 – 7.0). Toxin treatment groups included 30 ppm dieldrin in hydra media based on the minimum effective dose (MED) that caused 100% mortality of the hydra in 92 h. Sorbent inclusion levels were equal to 0.5% w/vol. All test groups in disposable culture tubes were capped and agitated at 1000 rpm for 2 h followed by centrifugation at 2000 g for 20 min. Groups of hydra were then exposed to each solution and the hydra morphological responses for each group were scored and recorded at each time point. The score or average score for each group was used to determine the average toxicity rating at each time point. Each group contained three adult hydra in 4 mL of test solution at 18°C.
Soil studies
Garden soil (with a composition including compost, processed forest products, sphagnum peat moss, a wetting agent and fertilizer containing 0.09% total nitrogen, 0.05% available phosphate and 0.07% soluble potash) was obtained from The Scotts Miracle-Gro Company (Marysville, OH). Soil was air-dried and sieved through a 1 mm screen before use. 1 g aliquots of soil were added to disposable culture tubes. Each soil sample was spiked with 2 mL of dieldrin/acetone solution and mixed to ensure even distribution of dieldrin. Samples were left uncapped in a fume hood until the acetone had completely evaporated (48 h). Sorbents were added at 1% w/w to a gradient of dieldrin concentrations and mixed. The samples were hydrated by adding 4 mL of distilled water and then slowly agitated at 200 rpm for 24 h. Soil extraction methods (Correia-Sá et al. 2012; Fish & Revesz 1996) with modification were used to extract dieldrin from soil samples. Briefly, 4 mL of 40:60 - hexane:acetone was added to each sample before agitating at 1000 rpm for 1 h. Samples were centrifuged at 2000 g for 20 min and the supernatants were transferred to new culture tubes. The samples were extracted twice. The supernatants were evaporated with nitrogen gas and then made up with 500 μL of the mobile phase. All samples were analyzed using HPLC. Calibration curves were conducted for each group of dieldrin extracts to ensure linearity of peak concentrations and consistency of the extraction method. Water and soil blanks and controls (spiked with different levels of dieldrin standard) were included in each experiment to validate the accuracy of our method and to determine the % recovery of dieldrin from the soil matrix. Peak areas of dieldrin from samples that included sorbents were compared to the dieldrin control samples to determine the percent reduction in dieldrin bioavailability from soil. Results were reproducible and based on at least three independent replicates.
Statistical analysis
A two-way t-test was used to determine statistical significance. Each experiment was conducted in triplicate to derive means and standard deviations. Qmax and Kd values from isothermal analyses, toxicity scores from the hydra assay and peak areas of dieldrin from soil studies were used to calculate t-values. T-values and degrees of freedom were used to determine the p-value. Results were considered significant at p≤0.05.
RESULTS
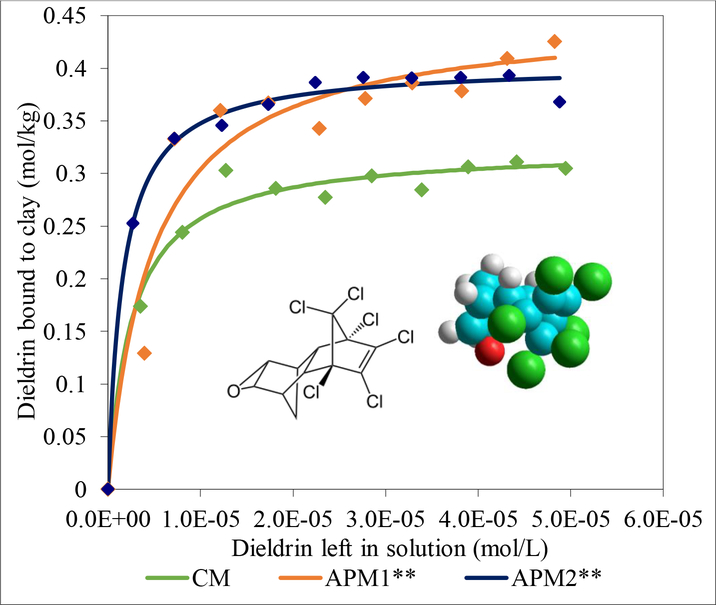
Adsorption isotherms for CM, APM1 and APM2 were generated using a Microsoft Excel program developed in our laboratory and plotted with Table-Curve 2D to derive sorbent-dieldrin binding parameters including Qmax (capacity), Kd (affinity) and ΔH (enthalpy). Fig. 1 shows the isothermal plots for dieldrin on the parent CM clay and the modified APM1 and APM2 clays. All three plots fit a Langmuir model with r2 values above 0.93, indicating tight binding of dieldrin. The curved shape of the Langmuir trend shows that binding of dieldrin onto active surfaces of the three sorbents was saturable. Both APM1 and APM2 had significantly higher binding capacities compared to the parent CM clay with Qmax values of 0.45 and 0.4 mol/kg, respectively, compared to 0.32 mol/kg for CM (p≤0.01).
Fig. 1.
Langmuir plots of dieldrin on CM, APM1, and AMP2 indicating tight binding of dieldrin by all three sorbents (CM: Qmax = 0.324 mol kg-1, Kd = 3.82E+05; APM2: Qmax = 0.403 mol kg-1, Kd = 6.21E+05; APM1: Qmax = 0.450 mol kg-1, Kd = 2.07E+05). APM1 and APM2 showed significantly higher Qmax values than the parent clay (**p≤0.01).
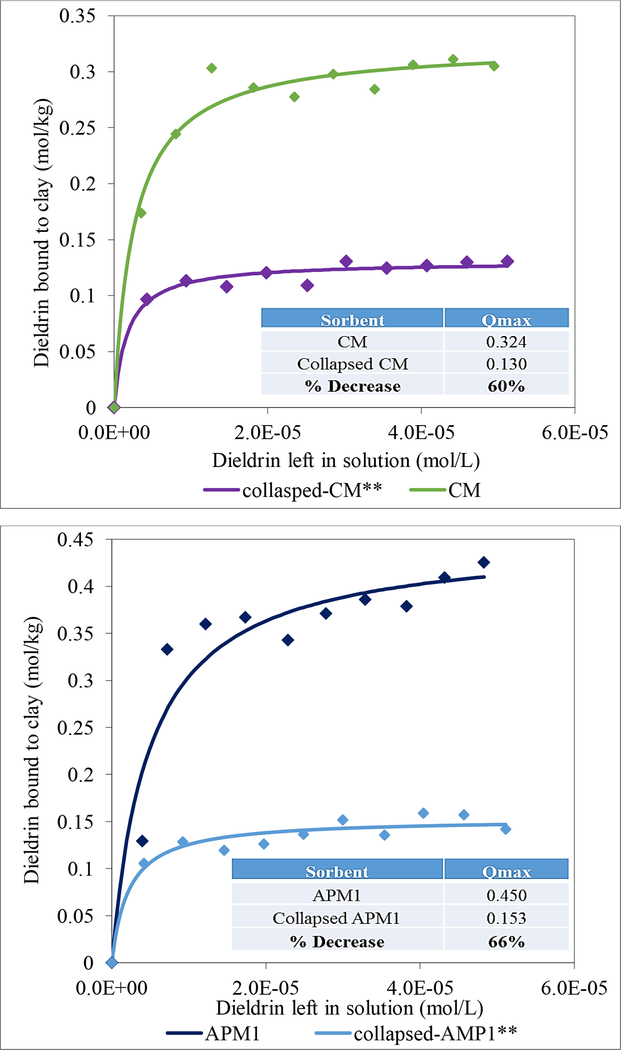
CM and APM1 were heat treated to determine the importance of interlayer surfaces in binding of dieldrin. Heating clays at 200°C and 800°C has been shown to considerably dehydroxylate and collapse interlayer surfaces, thus reducing interlayer binding sites available for dieldrin. The isothermal plots in Fig. 2 show Langmuir trends for heat-collapsed CM and APM1 clays. The Qmax values were significantly decreased by 60 and 66% for the collapsed CM and APM1 clays compared to the intact parent CM and APM1 clays (p≤0.01). Based on the extent of collapse in these experiments, the interlayer surfaces of both clays are clearly important for binding dieldrin.
Fig. 2.
Langmuir plots of dieldrin on CM and APM1 compared to heat-collapsed CM and APM1. The Qmax values indicated significantly reduced binding capacities of dieldrin with the collapsed clays (collapsed CM: Qmax = 0.130, collapsed APM1: Qmax = 0.153) (*p≤0.01).
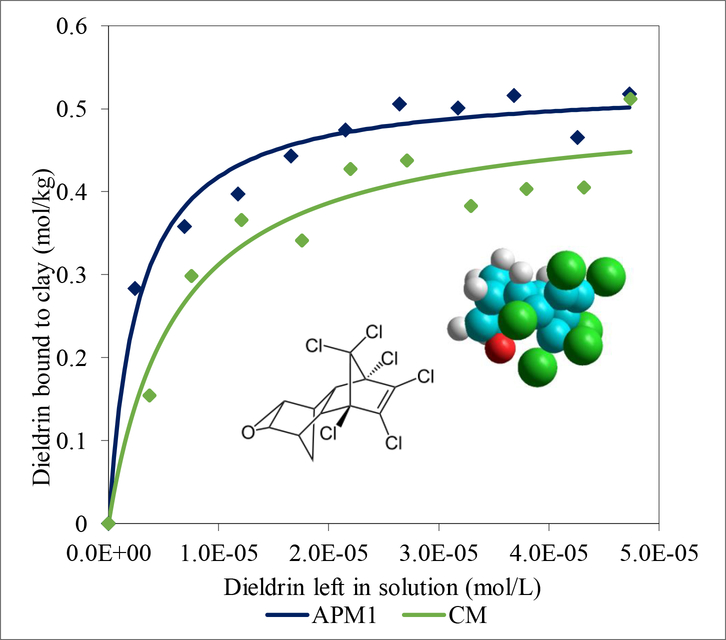
To determine the enthalpy values for dieldrin-sorbent binding reactions, equilibrium isotherms were conducted at 25°C and 37°C. Isothermal plots for CM and APM1 at 37°C are shown in Fig. 3. The Kd values for these plots were compared to the Kd values for the plots conducted at ambient temperature (25°C) to calculate the enthalpies (ΔH) of the binding reactions using the Van’t Hoff equation. Enthalpy values for CM and APM1 were −55.7 kJ mol−1 and −61.4 kJ mol−1, respectively.
Fig. 3.
Langmuir plots of dieldrin on CM and APM1 at 37°C (CM: Kd = 1.60E+05; APM1: Kd = 4.33E+05). Kd values for these plots were compared to those conducted at 25°C to determine the enthalpy of the binding reactions.
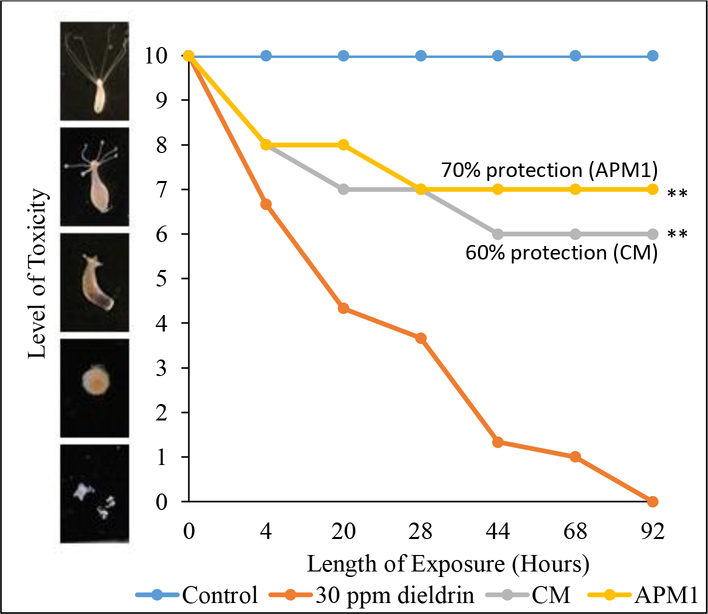
Fig. 4 shows results of the hydra assay for CM and APM1 and the efficacy of these sorbents in protecting a living organism from dieldrin toxicity. Hydra groups were exposed to 30 ppm dieldrin solution, based on the minimum effective dose (MED) that caused 100% mortality of the hydra in 92 h. The control group received no sorbent and the treatment groups were dosed with sorbent (0.5% w/vol). Inclusion of 0.5% sorbent provided significant reduction in mortality of the hydra, with 60% and 70% protection for CM and APM1, respectively (p≤0.01).
Fig. 4.
Hydra assay showing dieldrin toxicity at 30 ppm and protection of hydra with inclusion of 0.5% w/vol CM and APM1. Control groups with no dieldrin (hydra media and sorbent controls) are included for comparison and show scores of 10 throughout the test period. Sorbent inclusion at 0.5% was able to significantly protect the hydra from dieldrin toxicity (**p≤0.01).
Dosimetry studies were conducted to determine the amounts of CM and APM1 that would be necessary to reduce exposures to high levels of dieldrin. The exposure level was set to 0.2 ppm, which is twice the regulatory limit in food (0.1 ppm) (USEPA 2003). The sorbent inclusion levels (g kg−1) were plotted against % toxicant sorption and the data were fit to a log function, with CM being described by the equation y = 0.0822 ln(x) + 0.6206 and APM1 by the equation y = 0.067 ln(x) 0.6055 (Fig. 5). The sorbent inclusion levels needed to reduce dieldrin exposure of 0.2 ppm to below threshold limits were calculated to be 0.230 g kg−1 for CM and 0.207 g kg−1 for APM1.
Fig. 5.
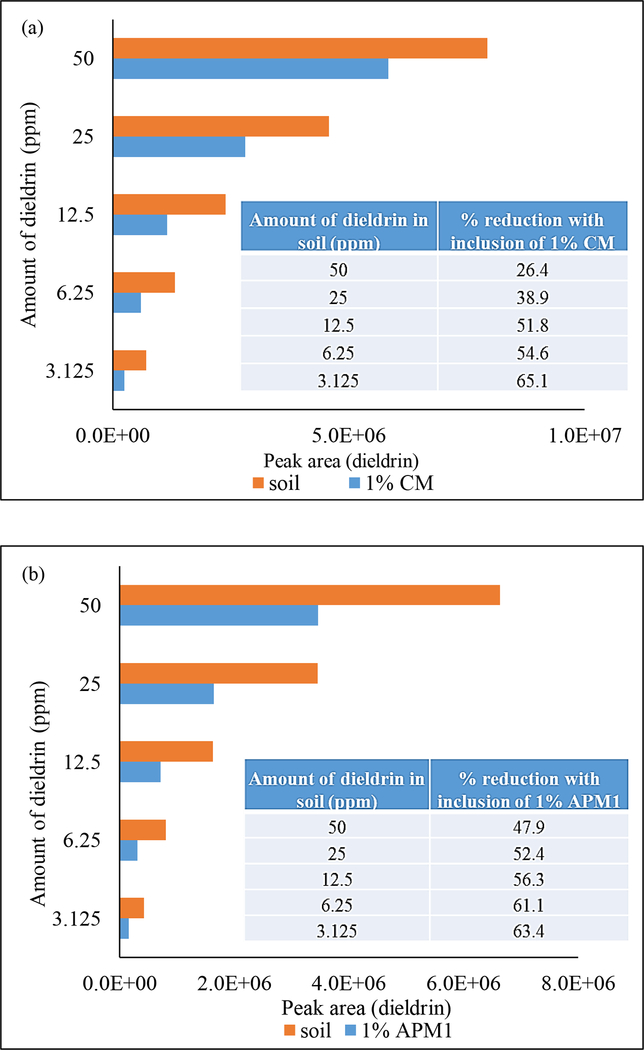
Soil studies showing significant reduction of dieldrin bioavailability with inclusion of (a) 1% CM and (b) 1% APM1 (*p≤0.01). 1% CM and 1% APM1 inclusion provided 26.4 to 65.1% and 47.9 to 63.4% reduction in dieldrin bioavailability, respectively.
Soil studies were conducted to determine the potential of CM and APM1 to reduce bioavailability of dieldrin from soil. Soil samples (1.0 g) were spiked with a gradient of dieldrin ranging from approximately 3 to 50 ppm. Recovery of dieldrin from soil samples ranged from 74 to 82%. Fig. 6 shows reduced recovery of dieldrin from spiked soil with CM and APM1 treatment at 1% w/w compared to recovery without treatment. Inclusion of 1% CM reduced the amount of dieldrin extracted from soil in a dose-dependent manner ranging from 26.4% to 65.1%. Inclusion of 1% APM1 also reduced the amount of dieldrin extracted from soil in a dose-dependent manner ranging from 47.9% to 63.4%. These results indicate that CM and APM1 at low inclusion levels (1%) can significantly reduce the bioavailability of dieldrin from soil (p≤0.01).
Fig. 6.
Soil studies showing significant reduction of dieldrin bioavailability with inclusion of (a) 1% CM and (b) 1% APM1 (*p≤0.01). 1% CM and 1% APM1 inclusion provided 26.4 to 65.1% and 47.9 to 63.4% reduction in dieldrin bioavailability, respectively.
DISCUSSION
Dieldrin is considered a persistent organic pollutant (POP) and is shown to be toxic to humans and animals. Exposure to dieldrin has been associated with neurological, developmental and carcinogenic effects in animal models (Richardson et al. 2006, Nebeker et al. 1992, Bachowski et al. 1998). Dieldrin residues have been detected in adipose tissue of sheep, ducks, cows and swine (Paton et al. 1997, Falandysz and Kannan 1991, Corrigan and Seneviratna 1990). Additionally, dieldrin is the active product of aldrin, another organochlorine insecticide that was widely used to treat seed and soil. Because of the extensive use of these two chemicals and their abilities to bioaccumulate, high levels can be found throughout the environment (Jorgenson 2001). Dieldrin and aldrin are ranked #18 and #25, respectively, on the ATSDR Substance Priority List of chemicals that are commonly found in contaminated facilities and pose a significant risk to human health due to their potential toxicities (ATSDR 2017). Subsequently, strategies are needed to reduce the risk of exposures to dieldrin and protect humans and animals from adverse health effects associated with dieldrin exposure. Previous studies have investigated removal of dieldrin from environmental media with strategies such as magnetic nanoparticles, activated carbon sorbents and bioremediation strategies (Shrivas et al. 2017; Hilber et al. 2009; Matsumoto et al. 2009). However, no studies have looked at the safety and efficacy of clays to mitigate unintended exposures to dieldrin in the environment. To develop an effective enterosorbent for dieldrin, we have characterized the sorption of the toxicant onto active interlayer surfaces of CM and APMs.
Various sorbents have been evaluated for binding potential with dieldrin in aqueous environments. Activated carbons from bamboo and coconut shell reduced 100 μg L−1 of dieldrin in drinking water sources by 68 and 71%, respectively (Thuy et al. 2011). A triolen-embedded activated carbon composite sorbent decreased dieldrin concentrations in an aqueous solution from 10 μg L−1 to 1.94 μg L−1 (Ru et al. 2007). Adsorption curves for silica aerogel and dieldrin showed 92% removal of dieldrin in 3 h (Liu et al. 2009). Bromopropyl functionalized silica nanofibers had a removal rate of 91% for dieldrin residues in water (Yue et al. 2012). All of these sorbents for dieldrin resulted in Freundlich trends, suggesting partitioning of dieldrin on and off sorbent surfaces and potentially weak bonding.
Equilibrium isothermal analyses were conducted for CM, APM1, and APM2. All three isotherms fit Langmuir models with high Qmax and Kd values, indicating efficient binding of dieldrin with saturable binding sites and high capacities and affinities for dieldrin. The APM1 and APM2 modified clays had significantly increased binding capacities for dieldrin compared to the CM clay. These findings are consistent with previous studies indicating that acid processed clays have increased surface areas and toxin binding capacities compared to parent clays. APM1 had a higher capacity for dieldrin than APM2, so it was chosen for use in subsequent studies along with the parent CM clay.
The plateau surface density of CM was also calculated to determine the clay surface area available for dieldrin binding at saturation. The surface area of CM interlamellar binding sites is equal to 850 – 70 = 780 m2 g−1 = 7.8 × 1022 Å2 g−1. Using the Qmax value (maximum binding capacity) for dieldrin binding to CM, the number of dieldrin molecules bound (plateau surface density) is equal to 0.32 mol kg−1 x 6.02 × 1023 molecules mol−1 = 1.9 × 1020 molecules g−1. The topological polar surface area of one dieldrin molecule is 12.5 Å2, so the total binding area for dieldrin is equal to 1.9 × 1020 molecules g−1 x 12.5 Å2 = 2.4 × 1021 Å2 g−1 (smaller than the available CM surface area of 7.8 × 1022 Å2 g−1). The high binding capacity of CM for dieldrin along with the plateau surface density indicate that CM contains more than enough binding sites for monolayer sorption, which is consistent with the curved shape and homogeneous binding site requirements of the Langmuir plots shown in Fig 2.
To investigate binding sites of dieldrin on both CM and APM1, the sorbents were heat-collapsed to reduce interlayer surfaces. Heating CM and APM1 at 800°C significantly collapsed the interlayers. Results in Fig. 3 show a 60% decrease in Qmax of collapsed CM compared to the intact CM (40% binding capacity remaining), and a 66% decrease in Qmax of collapsed APM1 compared to the intact APM1 (34% binding capacity remaining). This suggests that the interlayer surfaces of the clays are important in dieldrin binding to CM and APM1. Upon further heating, we would expect to see a greater degree of collapsed interlayer surfaces and a greater decrease in Qmax for both CM and APM1.
Enthalpy is another important parameter to consider in terms of sorbent-chemical interactions. Equilibrium isotherms were conducted at room temperature (25°C) and high temperature (37°C) and the Kd values were compared using the Van’t Hoff equation. The negative enthalpy values of CM and APM1 (−55.7 and −61.4 kJ mol−1, respectively) indicate a high heat of sorption for dieldrin binding to clay surfaces. The high absolute values of ΔH for both sorbents (more than −20 kJ mol−1) indicate chemisorption (instead of physisorption) of dieldrin. The results suggest that the dieldrin-sorbent complex is stable and dieldrin would not be readily dissociated from either sorbent.
The hydra assay predicted safety and efficacy of the sorbents in protecting an aquatic species against dieldrin toxicity. Both sorbents were not toxic to the hydra with inclusion levels as high as 0.5% w/vol. The results indicate that CM and APM1 were able to significantly protect the hydra from dieldrin toxicity (up to 30 ppm). Inclusion of 0.5% of CM and APM1 reduced the toxicity of dieldrin from severe toxicity (score of 0) to moderate toxicity (score of 6) and minor toxicity (score of 7), respectively. These results agree with the isothermal analyses, further indicating that CM and APM1 are both effective sorbents for dieldrin, with APM1 having a higher capacity. The in vivo results of this study were limited to an aquatic testing species (i.e., Hydra vulgaris) and toxin binding in an aqueous environment. Further work to investigate any potential differences in non-aqueous environments is warranted.
Dosimetry studies were conducted to determine the doses of CM and APM1 that would be necessary to protect humans and animals from high levels of dieldrin exposure. Based on the assumption that humans consume 1 kg of food at each meal, the low inclusion levels of sorbent (0.230 g kg−1 for CM and 0.207 g kg−1 for APM1) would equate to a small amount of sorbent taken 3 times a day (at least 2 h before or after medications to prevent any potential interactions).
Based on these in vitro and aquatic toxicity studies, CM and APM1 were effective sorbents of dieldrin. Upon further investigation, CM and APM1 could be delivered in tablets, capsules, condiments, snacks and flavored water at low levels on a short-term basis to reduce harmful exposures to dieldrin. Including these sorbents could be important for vulnerable populations such as first responders and people living near contaminated sites or working in settings susceptible to spills or disasters. Further studies are necessary to determine the efficacy of these materials as sorbents of dieldrin in humans and animals, as various processes may alter their effectiveness in more complex biological systems.
Extensive research has been conducted on the use of activated charcoals as soil amendments to sequester environmental contaminants in soil (Sarmah et al. 2010; Yang et al. 2010). Reports of charcoals (i.e. biochars) used as soil amendments to reduce bioavailability and plant uptake of dieldrin support their effective sorption capacities for dieldrin and their potential to protect against harmful exposures (Donnarumma et al. 2009; Hilber et al. 2009). However, potential safety and adverse environmental impacts (e.g. changes in soil chemistry, nutrient availability and metal contamination) associated with activated charcoals warrant the development of alternative materials with similar sorption abilities (Kookana et al. 2011). Based on isothermal and dosimetry results from this study, CMs and APMs are highly effective sorbents of dieldrin. APM1 has a very high capacity for dieldrin (Qmax = 0.45 kJ mol−1), so it could be an effective sorbent for high concentrations of dieldrin that might occur as a result of a disaster or spill. APM1 was approximately twice as effective at reducing high levels (50 ppm) of dieldrin versus CM (47.9 vs. 26.4% reduction). CM had a slightly lower capacity for dieldrin than APM1 (Qmax = 0.324 kJ mol−1). At 1% inclusion, it reduced dieldrin’s bioavailability by 65% at a concentration of 3 ppm. Importantly, CM has been shown to be safe for use in animals and humans in earlier animal studies and clinical trials (Phillips et al. 2019). Because environmental levels of dieldrin are relatively low (< 3 ppm), inclusion of CM as a soil amendment at inclusion rates as low as 1% could be used routinely to reduce dieldrin exposures from soil (Hashimoto 2005, Otani et al. 2006). In the case of a disaster or chemical spill, the more active APM1 could be added to protect against higher level exposures. Thermodynamic studies suggested that the clay-dieldrin complex is not easily dissociated, indicating that soil-based clay-dieldrin complexes would be stable in a soil environment. Montmorillonites and other clay materials are natural components of many soil types, so minimal adverse environmental impacts are expected (Ito et al. 2017).
CONCLUSION
These results collectively indicate that calcium montmorillonites and acid-processed calcium montmorillonites are effective sorbents of dieldrin. Acid-processed CM clay has significantly increased binding capacity and affinity for dieldrin compared to the parent clay. These results agree with previous studies which indicate that acid processed clays have increased surface areas and capacities. Future studies should be conducted to evaluate the safety and efficacy of APMs in reducing bioavailability of environmental chemicals in animals and humans to determine if APMs could be used as enterosorbents on a short-term basis to protect against harmful exposures.
The use of clay materials such as montmorillonites as toxicant enterosorbents may be effective in reducing the bioavailability of chemicals that have been ingested by humans and animals and from contaminated soil. Further studies will investigate APMs as broad-acting sorbents that can mitigate exposures to environmentally-relevant mixtures of chemicals.
Acknowledgment
This work was supported by funding through NIEHS SRP (Superfund Hazardous Substance Research and Training Program), P42 ES0277704 and USDA Hatch 6215.
Footnotes
Data availability
Data, associated metadata, and calculation tools are available from the corresponding author (tphillips@cvm.tamu.edu).
REFERENCES
- Abramovich DR, Fowler PA, Haites NE, Cash P, Al-Qahtani A, Groome NP, Murray TJ & Lea RG 2007. Human fetal testis Leydig cell disruption by exposure to the pesticide dieldrin at low concentrations. Human Reproduction 22(11): 2919–2927. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). 2002. Toxicological profile for aldrin and dieldrin Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). 2017. 2017 Substance Priority List. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. [Google Scholar]
- Bachowski S, Xu Y, Stevenson DE, Walborg EF, Klaunig JE 1998. Role of Oxidative Stress in the Selective Toxicity of Dieldrin in the Mouse Liver. Toxicology and Applied Pharmacology 150(2): 301–309. [DOI] [PubMed] [Google Scholar]
- Brown KA, Mays T, Romoser A, Marroquin-Cardona A, Mitchell NJ, Elmore SE & Phillips TD 2014. Modified hydra bioassay to evaluate the toxicity of multiple mycotoxins and predict the detoxification efficacy of a clay-based sorbent. Journal of Applied Toxicology 34(1): 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrizosa MJ, Calderón MJ, Hermosín MC, Cornejo J 2000. Organosmectites as sorbent and carrier of the herbicide bentazone. Science of The Total Environment 247(2): 285–293. [DOI] [PubMed] [Google Scholar]
- Correia-Sá L, Fernandes VC, Carvalho M, Calhau C, Domingues VF & Delerue-Matos C 2012. Optimization of QuEChERS method for the analysis of organochlorine pesticides in soils with diverse organic matter. Journal of Separation Science 35(12): 1521–1530. [DOI] [PubMed] [Google Scholar]
- Corrigan P and Seneviratna P 1990. Occurrence of organochlorine residues in Australian meat. Australian Veterinary Journal 67(2): 56–58. [DOI] [PubMed] [Google Scholar]
- Donnarumma L, Pompi V, Faraci A & Conte E 2009. Dieldrin uptake by vegetable crops grown in contaminated soils. Journal of Environmental Science and Health, Part B 44(5): 449–454. [DOI] [PubMed] [Google Scholar]
- Doong RA, Lee CY & Sun YC 1999. Dietary intake and residues of organochlorine pesticides in foods from Hsinchu, Taiwan. J AOAC Int 82(3): 677–682. [PubMed] [Google Scholar]
- El-Geundi MS, Nassar MM, Farrag TE, Ahmed MH 2012. Removal of an insecticide (methomyl) from aqueous solutions using natural clay. Alexandria Engineering Journal 51(1): 11–18. [Google Scholar]
- Falandysz J and Kannan K 1992. Organochlorine pesticide and polychlorinated biphenyl residues in slaughtered and game animal fats from the northern part of Poland. Zeitschrift für Lebensmittel-Untersuchung und Forschung 195(1): 17–21. [DOI] [PubMed] [Google Scholar]
- Fish JR & Revesz R 1996. Microwave solvent extraction of chlorinated pesticides from soil. LC GC: Liquid Chromatography, Gas Chromatography 14(3): 230–234. [Google Scholar]
- Getenga ZM,Keng’ara FO &Wandiga SO 2004. Determination of Organochlorine Pesticide Residues in Soil and Water from River Nyando Drainage System Within Lake Victoria Basin, Kenya. Bulletin of Environmental Contamination and Toxicology 72(2): 335–343. [DOI] [PubMed] [Google Scholar]
- Grant PG & Phillips TD 1998. Isothermal Adsorption of Aflatoxin B1 on HSCAS Clay. Journal of Agricultural and Food Chemistry 46(2): 599–605. [DOI] [PubMed] [Google Scholar]
- Harner T,Wideman JL,Jantunen LMM,Bidleman TF & Parkhurst WJ 1999. Residues of organochlorine pesticides in Alabama soils. Environmental Pollution 106(3): 323–332. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y 2005. Dieldrin Residue in the Soil and Cucumber from Agricultural Field in Tokyo. Journal of Pesticide Science 30(4): 397–402. [Google Scholar]
- Hilber I, Wyss GS,Mäder P,Bucheli TD, Meier I, Vogt L &R. Schulin 2009. Influence of activated charcoal amendment to contaminated soil on dieldrin and nutrient uptake by cucumbers. Environmental Pollution 157(8): 2224–2230. [DOI] [PubMed] [Google Scholar]
- Ito A &Wagai R 2017. Global distribution of clay-size minerals on land surface for biogeochemical and climatological studies. Scientific data 4: 170103–170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgenson JL 2001. Aldrin and dieldrin: a review of research on their production, environmental deposition and fate, bioaccumulation, toxicology, and epidemiology in the United States. Environmental health perspectives 109 Suppl 1(Suppl 1): 113–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M,Kanthasamy A &Anantharam V 2005. Dieldrin-Induced Neurotoxicity: Relevance to Parkinson’s Disease Pathogenesis. NeuroToxicology 26(4): 701–719. [DOI] [PubMed] [Google Scholar]
- Kookana RS,Sarmah AK, Van Zwieten L,Krull E & Singh B 2011Chapter three - Biochar Application to Soil: Agronomic and Environmental Benefits and Unintended Consequences In Advances in Agronomy, Vol. 112, 103–143 (Ed Sparks DL). Academic Press. [Google Scholar]
- Kumar P,Jasra RV & Bhat TSG 1995. Evolution of Porosity and Surface Acidity in Montmorillonite Clay on Acid Activation. Industrial & Engineering Chemistry Research 34(4): 1440–1448. [Google Scholar]
- Liu H, Sha W, Cooper AT, Fan M 2009. Preparation and characterization of a novel silica aerogel as adsorbent for toxic organic compounds. Colloids and Surfaces A: Physicochemical and Engineering Aspects 347(1): 38–44. [Google Scholar]
- Marroquín-Cardona A, Deng Y, Garcia-Mazcorro J, Johnson NM, Mitchell N, Tang L, Robinson A, 2nd, Taylor J, Wang JS & Phillips TD 2011. Characterization and Safety of Uniform Particle Size NovaSil Clay as a Potential Aflatoxin Enterosorbent. Applied clay science 54(3–4): 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto E,Kawanaka Y, Yun S-J & Oyaizu H 2009. Bioremediation of the organochlorine pesticides, dieldrin and endrin, and their occurrence in the environment. Applied Microbiology and Biotechnology 84(2): 205–216. [DOI] [PubMed] [Google Scholar]
- Narayanan S & Deshpande K 1998Acid activation of montmorillonite : Effect on structural and catalytic properties In Studies in Surface Science and Catalysis, Vol. 113, 773–778 (EdsT. S. R. P Rao and G. M. Dhar). Elsevier. [Google Scholar]
- Nebeker AV, Griffis WL, Stutzman TW, Schuytema GS, Carey LA, Scherer SM 1992. Effects of aqueous and dietary exposure of dieldrin on survival, growth and bioconcentration in mallard ducklings. Environmental Toxicology and Chemistry 11(5): 687–699. [Google Scholar]
- Novikova LA,Bel’chinskaya LI &Roessner F 2006. Effect of treatment with acids on the state of the surface of natural clay minerals. Russian Journal of Physical Chemistry 80(1): S185–S188. [Google Scholar]
- Otani T,Seike N & Sakata Y 2007. Differential uptake of dieldrin and endrin from soil by several plant families and Cucurbita genera. Soil Science and Plant Nutrition 53(1): 86–94. [Google Scholar]
- Pantaleoni GC, Palumbo G,Fanini D, Giorgi R, Carlucci G &Sponta AM 1989. A high-performance liquid chromatographic method for the evaluation of aldrin epoxidation by cytochrome P-450 dependent monooxygenase in small liver samples. Journal of Pharmaceutical and Biomedical Analysis 7(6): 783–788. [DOI] [PubMed] [Google Scholar]
- Paton M &Petterson D 1997. Absorption by sheep of dieldrin from contaminated soil. Australian Veterinary Journal 75(6): 441–445. [DOI] [PubMed] [Google Scholar]
- Phillips TD,Afriyie-Gyawu E, Williams J, Huebner H,Ankrah NA,Ofori-Adjei D, Jolly P, Johnson N, Taylor J, Marroquin-Cardona A, Xu L, Tang L & Wang JS 2008. Reducing human exposure to aflatoxin through the use of clay: A review. Food Additives & Contaminants: Part A 25(2): 134–145. [DOI] [PubMed] [Google Scholar]
- Phillips TD, Wang M, Elmore SE,Hearon S & Wang J-S 2019. NovaSil Clay for the Protection of Humans and Animals from Aflatoxins and Other Contaminants. Clays and Clay Minerals 67(1): 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Wang M, Dean ED, Pennell KD, Miller GW 2006. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson’s disease. The FASEB Journal 20(10): 1695–1697. [DOI] [PubMed] [Google Scholar]
- Ru J, Liu H, Qu J, Wang A, Dai R 2007. Removal of dieldrin from aqueous solution by a novel triolein-embedded composite adsorbent. Journal of Hazardous Materials 141(1): 61–69. [DOI] [PubMed] [Google Scholar]
- Saito T, Otani T, Seike N & Okazaki M 2012. A comparison of dieldrin residues in various vegetable crops cultivated in a contaminated field. Soil Science and Plant Nutrition 58(3): 373–383. [Google Scholar]
- Sanchez-Martin MJ, Rodriguez-Cruz MS, Andrades MS, Sanchez-Camazano M 2006. Efficiency of different clay minerals modified with a cationic surfactant in the adsorption of pesticides: Influence of clay type and pesticide hydrophobicity. Applied Clay Science 31(3): 216–228. [Google Scholar]
- Sarmah AK, Srinivasan P,Smernik RJ, Manley-Harris M,Antal MJ, Downie A & van Zwieten L 2010. Retention capacity of biochar-amended New Zealand dairy farm soil for an estrogenic steroid hormone and its primary metabolite. Soil Research 48(7): 648–658. [Google Scholar]
- Shrivas K,Ghosale A,Nirmalkar N, Srivastava A, Singh SK & Shinde SS 2017. Removal of endrin and dieldrin isomeric pesticides through stereoselective adsorption behavior on the graphene oxide-magnetic nanoparticles. Environmental Science and Pollution Research 24(32): 24980–24988. [DOI] [PubMed] [Google Scholar]
- Stern AH 2014. Hazard identification of the potential for dieldrin carcinogenicity to humans. Environmental Research 131: 188–214. [DOI] [PubMed] [Google Scholar]
- Thuy PT, Anh NV, van der Bruggen B 2012. Evaluation of Two Low-Cost–High-Performance Adsorbent Materials in the Waste-to-Product Approach for the Removal of Pesticides from Drinking Water. CLEAN – Soil, Air, Water 40(3): 246–253. [Google Scholar]
- Tomić ZP, Mladenović SBA, Babić BM, Logar VAP, Ðorđević AR & Cupać SB 2011. Modification of smectite structure by sulfuric acid and characteristics of the modified smectite. Journal of Agricultural Sciences, Belgrade 56(1): 25–35. [Google Scholar]
- United States Environmental Protection Agency (USEPA). 2003. Health Effects Support Document for Aldrin/Dieldrin. Health and Ecological Criteria Division, Office of Science and Technology, Office of Water. [Google Scholar]
- Wang M, Hearon SE & Phillips TD 2019. Development of enterosorbents that can be added to food and water to reduce toxin exposures during disasters. Journal of Environmental Science and Health, Part B 54(6): 514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilby OK, Tesh JM & Shore PR 1990. Application of the Hydra regeneration assay: Assessment of the potential teratogenic activity of engine exhaust emissions. Toxicology in Vitro 4(4): 612–613. [DOI] [PubMed] [Google Scholar]
- Yang X-B, Ying G-G, Peng P-A, Wang L, Zhao J-L, Zhang L-J, Yuan P & He H-P 2010. Influence of Biochars on Plant Uptake and Dissipation of Two Pesticides in an Agricultural Soil. Journal of Agricultural and Food Chemistry 58(13): 7915–7921. [DOI] [PubMed] [Google Scholar]
- Yue X, Feng S, Li S, Jing Y, Shao C 2012. Bromopropyl functionalized silica nanofibers for effective removal of trace level dieldrin from water. Colloids and Surfaces A: Physicochemical and Engineering Aspects 406: 44–51. [Google Scholar]
- Zhang A, Fang L, Wang J, Liu W, Yuan H, Jantunen L & Li Y-F 2012. Residues of Currently and Never Used Organochlorine Pesticides in Agricultural Soils from Zhejiang Province, China. Journal of Agricultural and Food Chemistry 60(12): 2982–2988. [DOI] [PubMed] [Google Scholar]