Abstract
Objective
Kawasaki Disease (KD) is the leading cause of acute vasculitis and acquired heart disease in children in developed countries. Notably, KD is more prevalent in males than females. We previously established a key role for IL-1 signaling in KD pathogenesis, but whether this pathway underlies the sex-based difference in susceptibility is unknown.
Approach and Results
The role of IL-1 signaling was investigated in the Lactobacillus casei cell wall extract (LCWE)-induced experimental mouse model of KD vasculitis. 5-week-old male and female mice were injected intraperitoneally with PBS, LCWE, or a combination of LCWE and the IL-1 receptor antagonist Anakinra. Aortitis, coronary arteritis inflammation score and abdominal aorta dilatation and aneurysm development were assessed. mRNA-seq analysis was performed on abdominal aorta tissue. Publicly available human transcriptomics data from KD patients was analyzed to identify sex differences and disease-associated genes.
Male mice displayed enhanced aortitis and coronary arteritis as well as increased incidence and severity of abdominal aorta dilatation and aneurysm, recapitulating the increased incidence in males that is observed in human KD. Gene expression data from KD patients and abdominal aorta tissue of LCWE-injected mice showed enhanced Il1b expression and IL-1 signaling genes in males. While the more severe IL-1β-mediated disease phenotype observed in male mice was ameliorated by Anakinra treatment, the milder disease phenotype in female mice failed to respond.
Conclusions
IL-1β may play a central role in mediating sex-based differences in KD, with important implications for the use of anti-IL-1β therapies to treat male and female KD patients.
Introduction
Kawasaki disease (KD) is an acute febrile disease of unknown etiology characterized by systemic vasculitis and myocarditis. KD is the leading cause of acquired heart disease in children in developed countries 1, and if left untreated, KD leads to coronary artery abnormalities in up to 30% of patients 2. Treatment with high dose intravenous immunoglobulin (IVIG) reduces the risk of an aneurysm from 25–30% down to 5–7% 3–6. However, nearly 20% of patients with KD are resistant to the initial IVIG dose, which leads to a 3-fold increase in risk for the development of coronary artery aneurysms (CAA) 6, 7.
In the Lactobacillus casei cell wall extract (LCWE)-induced mouse model of KD, a single intraperitoneal injection of LCWE results in systemic inflammation, coronary artery vasculitis, aortitis, myocarditis and abdominal aorta aneurysms (AAA) 8, 9, closely resembling human KD pathology 9, 10. In the LCWE mouse model, genetic or antibody-mediated blockade of the interleukin-1 (IL-1) pathway results in protection from vasculitis and AAA formation, indicating a crucial role for IL-1 signaling in pathogenesis 9, 11. Treatment with an IL-1 receptor antagonist (Anakinra, IL-1Ra) also blocks LCWE-induced AAA formation 9, 11. Further, pre-treatment with Anakinra significantly reduces LCWE-induced myocardial inflammation and N-terminal pro B-type Natriuretic peptide (NT-proBNP) release, and improves ejection fraction and left ventricular function 12. Recent studies also indicate that IL-1β and the NLRP3 inflammasome are required for the Candida albicans water-soluble fraction (CAWS) mouse model of KD vasculitis 13–15. In humans, serum levels of IL-1β 16 and IL-1 related genes are upregulated in KD peripheral blood during the acute phase of illness 17. Taken together, these studies provide strong rationale for therapeutically targeting IL-1 signaling in KD, although whether this approach will be universally effective remains in question.
The incidence of KD is consistently higher in males, with a reported male-to-female KD incidence ratio of 1.5 in the US, 1.31 in Japan, and 1.62 in Taiwan 18, and similar trends reported in Finland, Norway, and Sweden 19. However, the sex-based differences in KD development have remained largely understudied, limiting optimization of health management and treatment strategies. Here, we find that the LCWE-induced KD mouse model displays sex-based differences that resemble those observed in human patients, specifically increased incidence and severity in males. Patient and mouse transcriptomics revealed IL-1β signaling as a predominant factor in mediating this disparity. Furthermore, while the more severe disease phenotype observed in male mice was treated successfully by Anakinra, the milder phenotype in female mice failed to respond. Given the on-going clinical trials using Anakinra treatment for KD patients 20, these findings are highly pertinent and have wide reaching implications for the management of KD.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Mice
Wild-type (WT) C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All animals were housed under specific pathogen-free conditions at the animal center of Cedars-Sinai Medical Center. Experiments were conducted under approved Institutional Animal Care and Use Committee protocols.
Preparation of LCWE
LCWE (ATCC 11578) was prepared as previously described 21. In brief, L. casei were grown in Lactobacillus de Man, Rogosa, and Sharpe broth (Difco) for 48 hours, harvested, and washed with PBS. The harvested bacteria were disrupted by 2 packed volumes of 4% SDS/PBS overnight. Cell wall fragments were washed 8 times with PBS to remove any residual SDS. Cell wall fragments were sonicated for 2 hours with a 3/4-in horn and a garnet tip at maximum power. During sonication, the cell wall fragments were maintained by cooling in a dry ice/ethanol bath. After sonication, the cell wall fragments were spun for 20 minutes at 11,000 g and 4°C. The supernatant was centrifuged for 1 hour at 180,000 g and 4°C, and the pellet was discarded. The total rhamnose content of the cell wall extract was determined by a colorimetric phenol-sulfuric assay as described previously 8
LCWE-induced Kawasaki Disease model
5-week-old male or female mice (as indicated) were injected intraperitoneally with 400 μg LCWE or PBS (Day 0). For Anakinra treatment; mice were injected intraperitoneally with Anakinra (500μg) every 2 days starting the day before LCWE injection (Days −1, 1, 3, 5, 7, 9, 11 and 13) for a total of 8 injections. At 7- or 14-days post LCWE injection (as indicated), mice were euthanized and perfused with PBS. After dissection of aorta, the diameters of abdominal aorta were measured at 5 different parts (below the left renal artery) and maximal abdominal aorta diameters were calculated. Aortas were either placed in RNA later for RNA extraction as described below or embedded in optimal cutting temperature (OCT) compound for histological analysis. Hearts were removed and embedded in OCT compound for histological analysis. Serial cryosections (7 μm) were prepared from the tissues and stained with hematoxylin and eosin (H&E). Histopathological examination and inflammation severity scoring of the coronary arteries and aortic root vasculitis were performed by a senior investigator blinded to the experimental groups. KD lesions were assessed with the scoring system as described previously22
RNA Isolation
Aortas were stored in RNA later (Qiagen) prior to RNA extraction. RNA extraction was performed using the miRNEasy micro kit (Qiagen) according to the manufacturer’s instructions.
qRT-PCR
qRT-PCR was performed with the Power SYBR Green RNA-to-Ct 1 step kit according to the manufacturer’s instructions (Thermo Fisher Scientific). Primer sequences: Il1b: F’ CAGGCAGGCAGTATCACTCA, R’ TGTCCTCATCCTGGAAGGTC; Nlrp3: F’ TCCACAATTCTGACCCACAA, R’ ACCTCACAGAGGGTCACCAC; Il1r1: F’ GTGCTACTGGGGCTCATTTGT, R’ GGAGTAAGAGGACACTTGCGAAT; Stat1: F’ TCACAGTGGTTCGAGCTTCAG, R’ GCAAACGAGACATCATAGGCA; Ccr5: F’ ATGGATTTTCAAGGGTCAGTTCC, R’ CTGAGCCGCAATTTGTTTCAC; Selp: F’ AACCACTGCCAACCTGTGAA, R’ GGTTCCTGTAGATGGGACGC; Hprt: F’ CTCATGGACTGATTATGGACAGGAC; R’ GCAGGTCAGCAAAGAACTTATAGCC.
Murine mRNA-seq
Library construction was performed using the Lexogen QuantSeq 3’ mRNA-Seq Library Prep Kit FWD for Illumina (Lexogen, Vienna, Austria). Briefly, total RNA samples were assessed for concentration using a Qubit fluorometer (ThermoFisher Scientific, Waltham, MA), and for quality using the 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). Up to 100 ng of total RNA per sample was used for reverse transcription using oligo(dT) priming. After removal of the RNA template, random priming was used to perform second stand synthesis. Next, PCR amplification with indexing adapters for Illumina sequencing was followed by library purification. The concentration of the amplified library was measured with a Qubit fluorometer and an aliquot of the library was resolved on a Bioanalyzer. Sample libraries were multiplexed and sequenced on the NextSeq 500 platform (Illumina) using 75bp single-end sequencing. On average, approximately 10 million reads were generated from each sample. Raw sequencing data were demultiplexed and converted to FASTQ format by using bcl2fastq v2.20 (Illumina). Then, the raw reads were uploaded to Bluebee® Genomics Platform for quality control, alignment, and expression quantification using QuantSeq FWD-UMI Data Analysis Pipeline (Lexogen). Briefly, the umi2index (Lexogen) process added the 6 nucleotide UMI sequence to the identifier of each read and trimmed the UMI from the start of each read. Reads were then processed by BBDuk v35.92 to trim the low-quality tails, poly(A) tails, and adapter contamination. Trimmed reads were aligned to reference genome GRCm38 using STAR (v2.5.2a)23. To remove PCR duplicates, the mapped reads were collapsed if they had the same mapping coordinate and identical UMI sequences. HTSeq-count (v0.6.0) was employed for gene expression quantification24. Normalization and analysis of gene expression data was performed in R using edgeR and Limma-voom. Genes were considered differentially expressed with an adjusted p value of < 0.05 and a fold change of > 2. DE genes were analyzed by Ingenuity Pathway Analysis (IPA; QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis) and DAVID Bioinformatics Resources25, 26. Cell composition was analyzed with CIBERSORT27 using the cell signature file as described28.
The murine abdominal aorta mRNA-seq data have been deposited in NCBI’s Gene Expression Omnibus29, and are accessible through GEO Series accession number GSE141072 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE141072).
Human Transcriptome Data Analysis
Published gene expression data (GSE68004) was analyzed with GEO2R to identify gene expression differences in whole blood between 44 male and 32 female complete KD patients, or between healthy controls and KD patients 30. Differentially expressed genes (p value < 0.05) were analyzed by IPA. For plotting of individual probe counts, significant outliers were removed based on Grubb’s test. Published gene expression data (GSE7084) was analyzed with GEO2R to identify gene expression differences in abdominal aorta tissue from controls or patients with abdominal aortic aneurysm 31. Published gene expression data (GSE64486) was analyzed with GEO2R to identify gene expression differences in coronary arteries from controls or KD patients 32
Western Blotting
Abdominal aorta tissue (pooled) from male and female LCWE-injected mice were homogenized in RIPA lysis buffer. Lysates were centrifuged at 13,000 x g for 10 minutes at 4°C. 25ug of cell lysate was assessed by SDS-PAGE and western blot using the enhanced chemiluminescent system using the following antibodies: anti-mouse IL-1b (RRID: AB_2233636), anti-rat IgG HRP (RRID: AB_2338128), anti-beta-actin (RRID: AB_476743) and anti-mouse IgG HRP (RRID: AB_10015289).
Statistical Analysis
For data involving single comparisons, a 2-tailed unpaired Student’s t-test was used for normally distributed data. For non-parametric data, the Mann-Whitney test was used. For multiple comparison testing, significance was evaluated by one- or two-way ANOVA with Tukey’s post-hoc test where appropriate. Data were tested for normality using the D’Agostino & Pearson normality test, where indicated.
Results
Male KD patients display an enhanced IL-1β signature compared to females
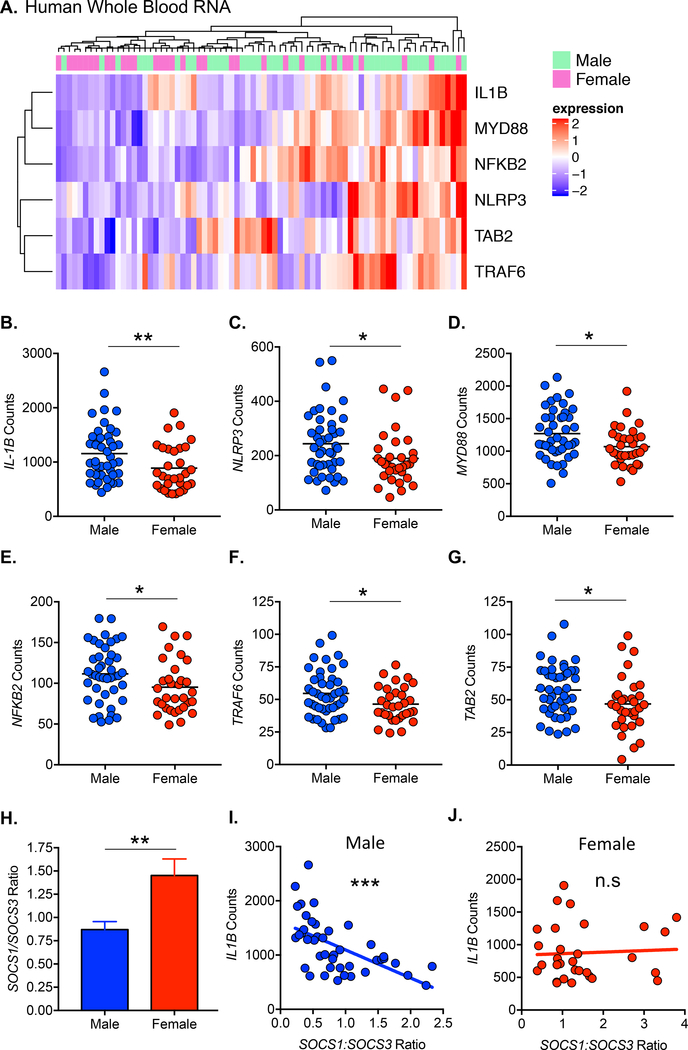
Since the incidence of KD development is higher in males, we analyzed published and publicly available transcriptomic data to assess gene expression difference between male and female KD patients (GSE68004) 30. We performed pathway analysis of differentially expressed genes (p < 0.05) and identified significant enrichment of the IL-1 signaling pathway (p = 0.00019), which was enhanced in male patients (z score = 1.387). Male patients displayed significantly enhanced expression of IL1B, NLRP3, MYD88, NFKB2, TRAF6 and TAB2 (Figure 1A–1G), suggesting higher IL-1β production and signaling compared to females. IL1A expression was not significantly different between male and female patients (data not shown). We therefore hypothesized that enhanced IL-1β signaling in males versus females may contribute to the observed sex bias in KD development and pathogenesis. To determine the effect of sex on macrophage polarization, we analyzed the ratio of SOCS1:SOCS3 expression33, 34. Males had a significantly lower SOCS1:SOCS3 ratio than females (Figure 1H), indicative of greater M1 polarization. IL1B expression was negatively correlated with the SOCS1:SOCS3 expression ratio in males but not females (Figure 1I,J). We therefore hypothesized that increased IL1B expression may be due to enhanced M1 macrophage polarization in males.
Figure 1: Sex differences in the expression of IL-1 signaling components in KD patients.
Transcriptomic data from whole blood of KD patients was analyzed for differential gene expression (p < 0.05) between males and females. A. Hierarchical clustering of KD patients and differentially expressed (p < 0.05) IL-1 associated/signaling genes. B-G. Expression counts of IL-1 associated/signaling genes in male (n=44) and female (n=32) KD patients. H. SOCS1:SOCS3 expression ratio in male (n=44) and female (n=32) KD patients. I-J. Correlation of SOCS1:SOCS3 expression ratio with IL1B expression in male (I) and female (J) KD patients. Statistical Analysis: Mann Whitney test (B-E, I) or student’s t-test (F-G) were performed. Outliers were removed based on Grubbs’ test (B-C,E and H). Data was tested for normality using the D’Agostino & Pearson normality test. Data is represented as mean. A probability value of p<0.05 was considered statistically significant; *p<0.05, **p<0.01, ***p<0.001.
Male have higher incidence and a more severe disease phenotype than females in a mouse model of KD
We next investigated disease development in male and female mice using the LCWE-induced KD mouse model. LCWE-injected male mice showed significantly more inflammation of the aorta and coronary artery than females (Figure 2A and 2B), and had increased incidence of disease (Figure 2C). LCWE-injected male mice also displayed greater dilatation of the abdominal aorta and increased incidence of abdominal aorta aneurysms (AAA) than females (Figure 2D through 2F). We performed qPCR analysis of Il1b and Il1r1 expression in male and female LCWE treated mice. We observed a significant correlation between Il1b and maximum abdominal aorta (AA) diameter in both males and females (Figure 2G), yet to a greater degree in males. While we observed a significant correlation between Il1r1 and maximum AA diameter in males, there was no correlation in females (Figure 2H). Furthermore, Western Blot assessment of IL-1β protein indicates higher expression in males than females (Figure II in the online-only Data Supplement).
Figure 2: Sex differences in the development of coronary arteritis, aortitis and aortic aneurysm in the LCWE-induced KD mouse model.
Male or female mice were injected i.p. with PBS or LCWE and analyzed 7 days post injection. A. Representative H&E stained heart vessel sections. B. Heart vessel inflammation scores (Histopathological examination and inflammation severity scoring of the coronary arteries and aortic root vasculitis, see methods) (male: n=14; female: n=25). C. Incidence of coronaritis and aortitis development (max AA > 0.6 mm). D. Representative gross photographs and H&E stained sections of abdominal aorta. E. Quantification of maximal AA diameter (PBS: n=5–8; LCWE: n=10–11). F. Incidence of AAA development (Heart Inflammation > 4). G. Correlation of max AA diameter and Il1b expression in AA tissue as measured by RT-PCR. AA: abdominal aorta. H. Correlation of max AA diameter and Il1r1 expression in AA tissue as measured by RT-PCR. AA: abdominal aorta. LCWE: Lactobacillus casei cell wall extract. Statistical Analysis: student’s t-test (B), one-way anova with tukey post hoc test (E) or spearman’s correlation test (G-H). Data is represented as mean. A probability value of p<0.05 was considered statistically significant; **p<0.01, ***p<0.001.
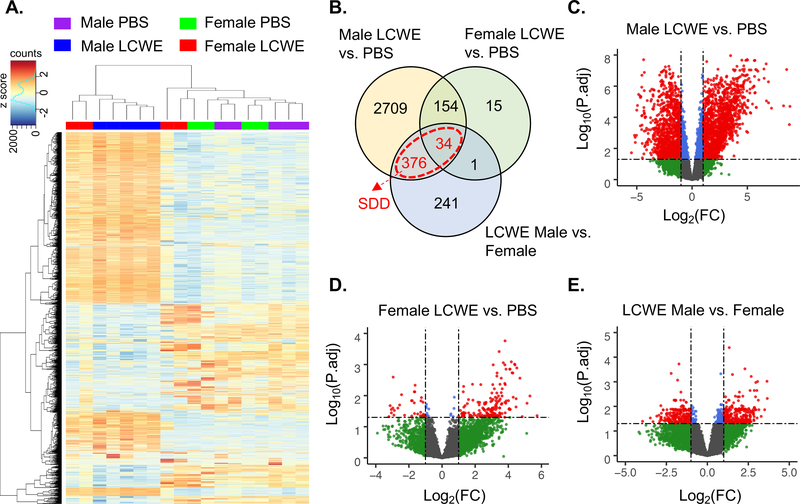
We performed mRNA-seq analysis of AA tissue harvested from PBS or LCWE-injected male and female mice and visualized gene expression differences between the groups using principal component analysis (Figure IA in the online-only Data Supplement) and hierarchal clustering (Figure 3A). Gene expression of AA tissue from male and female PBS treated mice appeared almost identical, with only 9 genes differentially expressed (> 2-fold) between the sexes (Figure IB in the online-only Data Supplement). AA tissue from male LCWE-treated mice formed a distinct cluster from male PBS controls, with 3,273 genes differentially expressed (> 2-fold) (Figure 3B and 3C). Female LCWE-injected mice showed high variability in gene expression. Two female LCWE-treated mice showed similarity to male LCWE-treated mice, while two showed greater similarity to the PBS controls (Figure 3A). This is in consensus with the disease incidence and decreased severity we observed in the female mice (Figure 2D through 2F). Despite broad differences in gene expression amongst female LCWE samples (as shown by clustering methods), male and female groups still formed distinct clusters based on maximum AA diameter and Il1b expression (Figure IC in the online-only Data Supplement). We therefore analyzed the female LCWE mice as a single group to help identify core differences in male and female gene expression. Between female LCWE-injected mice and PBS controls, 204 genes were differentially expressed (> 2-fold) (Figure 3B and 3D) while 652 genes were differentially expressed (> 2-fold) between female and male LCWE-injected mice (Figure 3B and 3E) indicating that on average, female LCWE-treated mice show more similarity to PBS controls than male LCWE-treated mice. Overall, these data indicate that LCWE-induced KD vasculitis results in greater gene expression changes in the AA of male mice than in female mice, reflective of disease incidence and severity.
Figure 3: Sex differences in abdominal aorta gene expression changes in the mouse model of KD.
Male or female mice were injected i.p. with PBS or LCWE (n=4–5 per group). AA was harvested 14 days post injection for mRNA-seq analysis. A. Heatmap and clustering of gene expression data from mRNA-seq analysis of AA tissue from PBS and LCWE-treated male and female mice (top 2000 variable genes). B. Venn diagram of differentially expressed genes (P.adj<0.05, FC>2) between indicated groups. Genes highlighted in red are the sex difference in disease (SDD) gene set. C-E. Volcano plots of differentially expressed genes between PBS and LCWE treated male mice (C), PBS and LCWE treated female mice (D) and LCWE treated male and female mice (E), blue = P.adj < 0.05 and FC < 2, red = P.adj < 0.05 and FC > 2. FC: fold change.
Abdominal aorta gene expression profiles indicate enhanced IL-1β mediated inflammation and vascular tissue disruption in males
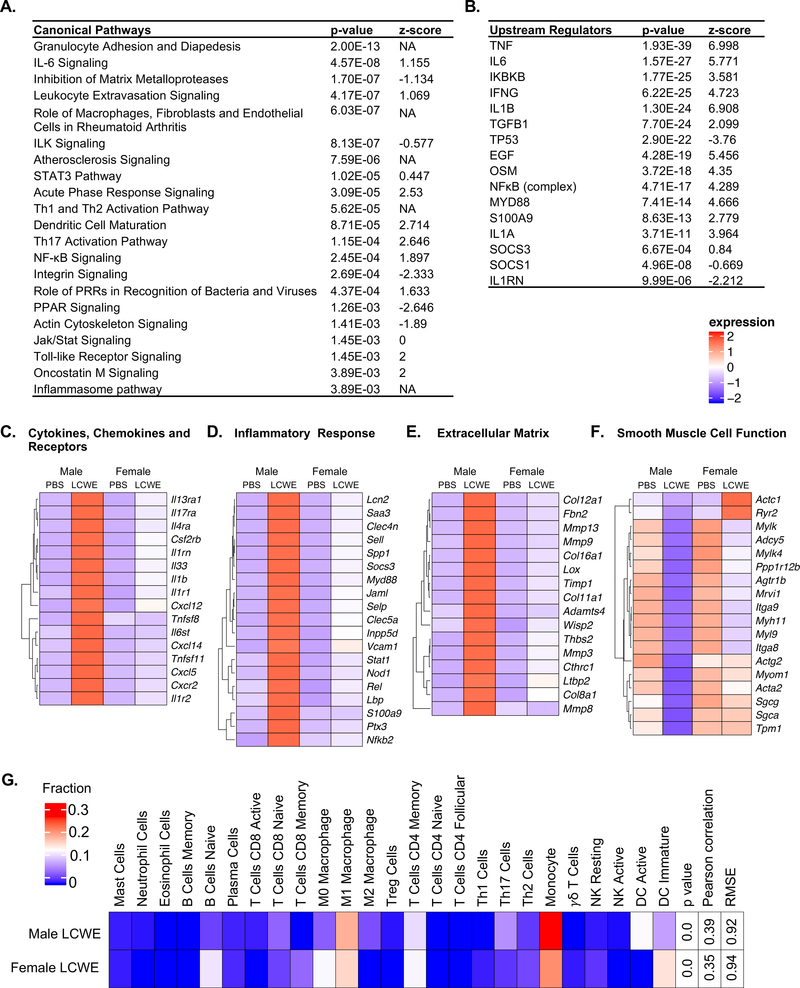
We identified a set of genes associated with disease development based on differential expression between male PBS and LCWE treated mice (Figure 3C), and a subset that were also differentially expressed between male and female LCWE-treated mice (highlighted red in Figure 3B), which we refer to as the sex difference in disease (SDD) gene set. We performed pathway and upstream regulator analysis of the SDD gene set (Figure 4A and 4B, Tables I and II in the online-only Data Supplement) and plotted heatmaps of selected genes separated into functional groups (Figure 4C through 4F). These functional analyses revealed key sex differences that may impact differential disease development and response to treatment.
Figure 4: Differentially regulated canonical pathways and upstream regulators between males and females in the mouse model.
Male or female mice were injected i.p. with PBS or LCWE (n=4–5 per group). AA was harvested 14 days post injection for mRNA-seq analysis. Functional analysis was performed on the SDD gene set (differentially expressed genes between male LCWE and PBS overlapping with those between male LCWE and female LCWE). A. Canonical pathway analysis of the SDD gene set. B. Upstream regulator analysis of the SDD gene set. C-F. Heatmaps of selected DE genes between male and female LCWE treated mice. G. CIBERSORT analysis of AA gene expression data from male and female LCWE treated mice.
Similar to our observations in peripheral blood mononuclear cells (PBMCs) from male versus female KD patients (Figure 1), we found that compared with female mice, LCWE-injected male mice had enhanced upregulation and expression of Il1b as well as components of the IL1-signaling pathway including Il1r1, Il1r2, Myd88, Nfkb2 and the NFκB subunit Rel (Figure 4 C and 4D). Validation of selected genes by qPCR on AA tissues from LCWE-injected male and female mice confirmed this observation (Figure ID in the online-only Data Supplement). While Nlrp3 did not reach significance as a differentially expressed gene between LCWE-injected male and female mice (P.adj = 0.105, FC = 4.04), we observed significant upregulation of Nlrp3 in LCWE-injected males, but not females, compared to PBS controls (Figure IE in the online-only Data Supplement). Furthermore, we found enhanced expression of Socs3 in males compared to females (Figure 4D). Functional analysis indicated that IL-1β, IL-1α, MYD88 and NFκB act as upstream regulators of genes within the SDD gene set, and their activation is enhanced in males compared to females (Figure 4B). Furthermore, we observed enrichment of the inflammasome pathway and NFκB signaling in males (Figure 4A). IL-1α and IL-1β signal through a common receptor, however we did not observe a significant difference in Il1a gene expression between males and females (Figure IF in the online-only Data Supplement). Overall these results point to an enhanced IL-1β signature within the SDD gene set in males compared to females.
Functional analysis of the SDD gene set indicated a heightened inflammatory state in the AA from LCWE-injected male mice compared with the AA of LCWE-injected female mice (Figure 4, Tables I and II in the online-only Data Supplement). LCWE injection enhanced expression of pathways involved in the innate immune response to a greater degree in males than females, including the acute phase response, TLR signaling and the role of pattern recognition receptors (PRRs) in detection of bacteria and viruses (Figure 4A and Table I in the online-only Data Supplement). In addition, pathway analysis identified enrichment of genes involved in granulocyte adhesion and diapedesis as well as leukocyte extravasation signaling, indicating key differences in the recruitment and infiltration of immune cells to the AA (Figure 4A and Table I in the online-only Data Supplement). Numerous genes involved in promoting these pathways, including Cxcl5, Cxcl14, Stat1, Vcam1, Selp, Sell, s100a9 and Mmp9, were enhanced in the AA of LCWE-injected male compared with female mice (Figure 4C through 4F and Figure ID in the online-only Data Supplement). Furthermore, functional analysis indicated heightened activation of immune cells promoting adaptive immunity, including enrichment of genes involved in dendritic cell (DC) maturation as well as Th1, Th2 and Th17 activation (Figure 4A and Table I in the online-only Data Supplement). In addition to IL1β, other cytokines were also predicted regulators of the SDD gene set including TNFα, IL6, IFNγ and oncostatin-M (OSM) (Figure 4B). Furthermore, we found that IL6, Jak/Stat, OSM, STAT3 and NFκB signaling pathways were enhanced in LCWE-injected males compared to females (Figure 4B and Table II in the online-only Data Supplement). While we did not detect significantly increased expression of Tnf, Il6, Ifng or Osm in LCWE-injected males compared with LCWE-injected females, we did find significant upregulation of Tnf, Il6, Osm and receptors Il6st and Ifngr1 in LCWE-injected males compared to PBS controls, but this upregulation did not occur in females (Figure ID in the online-only Data Supplement).
Functional analysis also pointed to a gene signature associated with disruption of vascular structure in males. We found enrichment of genes involved in actin cytoskeleton signaling, integrin signaling, integrin-like kinase (ILK) signaling and inhibition of matrix-metalloproteinase (MMP) signaling in the SDD gene set (Figure 4E and 4F). These pathways were reduced in LCWE-injected males compared with LCWE-injected females, indicative of reduced integrity of the abdominal aorta vessel structure. Consistent with this, we found enhanced expression of Mmp-3, −8, −9 and −13 in LCWE-injected males (Figure 4E). Furthermore, compared to LCWE-injected female mice, LCWE-injected male mice had a more pronounced downregulation of genes involved in smooth muscle cell function including Acta2, Mhy11 and Mylk (Figure 4F), which play important roles in vascular smooth muscle cell contraction, and are associated with thoracic abdominal aorta aneurysm development 35
We next performed CIBERSORT analysis using our mRNA-seq data to infer the immune cell composition of the abdominal aorta tissue in LCWE-treated male and female mice (Figure 4G). Males exhibited a greater degree of M1 macrophage polarization than females, as predicted, which may account for the enhanced IL-1β expression in males.
Males and female mice show differential responses to Anakinra treatment in the LCWE-induced KD mouse model
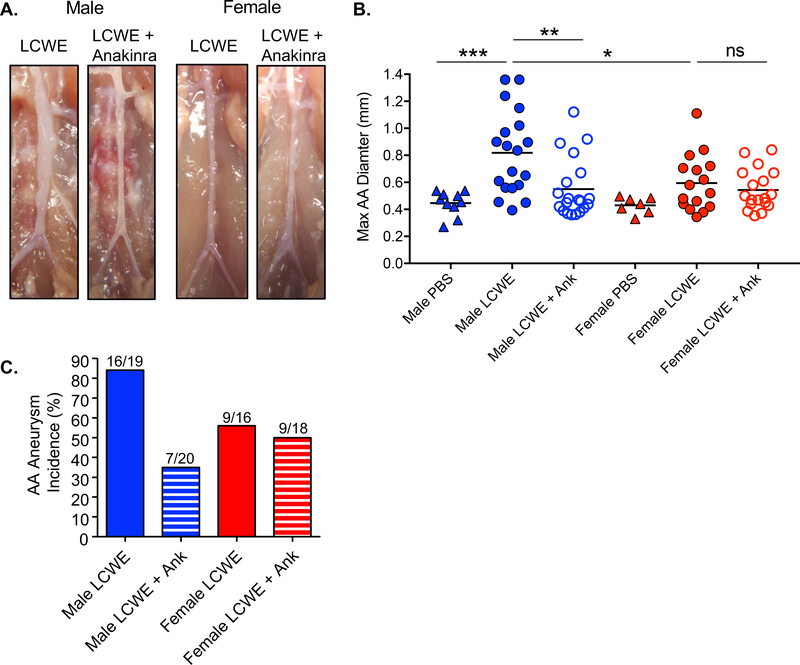
Anakinra treatment prevents LCWE-induced coronary lesions, myocarditis and AAA formation in male mice 9, 12, 22. Given the differences in Il1b expression and IL-1 signaling observed between males and females in human KD and our murine model, we investigated whether sex influences the response to Anakinra. As expected, Anakinra treatment reduced disease severity (Figure 5A and 5B) and incidence of AAA formation (Figure 5C) in LCWE-injected male mice. In contrast, Anakinra treatment did not significantly affect disease severity (Figure 5A and 5B) or incidence of AAA formation (Figure 5C) in LCWE-injected female mice. These results indicate that Anakinra treatment, while effective in reducing LCWE-induced KD vasculitis in male mice, is not sufficient to inhibit disease in LCWE-injected female mice, which display milder disease and low Il1b expression.
Figure 5: Sex differences in the response to Anakinra treatment in mouse model.
Male or female mice were injected i.p. with LCWE or LCWE and Anakinra and analyzed 14 days post injection or as indicated. A. Representative gross photographs of AA. B. Quantification of maximal AA diameter (mm) (PBS; n=7–10, LCWE; n=16–19, LCWE and Anakinra; n=18–20). C. Incidence of AAA formation (max AA > 0.6 mm). Statistical Analysis: one-way Anova with Tukey post hoc test (C). A probability value of p<0.05 was considered statistically significant; *p<0.05, **p<0.01, ns: not significant.
Anakinra treatment effectively inhibits LCWE-induced gene expression changes in male but not female mice
We further performed mRNA-seq analysis of AA tissue from PBS controls (no LCWE or Anakinra) and LCWE-injected male and female mice treated with or without Anakinra. PCA, hierarchical clustering and differential expression analysis demonstrated large changes in gene expression associated with Anakinra treatment in male but not female mice (Figure 6A–6C and Figure III in the online-only Data Supplement). Clustering and principal component analysis (PCA) show that the AA expression profile in male LCWE-injected mice treated with Anakinra is highly similar to that of PBS controls, and that LCWE-injected male mice treated with Anakinra form a separate cluster from untreated LCWE-injected male mice (Figure 6A, 6C and Figure IIIA in the online-only Data Supplement). 1592 genes were differentially expressed (> 2-fold) between male LCWE-injected mice treated with Anakinra or not, while only 7 genes were differentially expressed between PBS only controls and LCWE-injected male mice treated with Anakinra (Figure 6D). Taken together, these results indicate than in male mice, Anakinra treatment inhibits LCWE-induced gene expression changes in AA tissue. In contrast, PCA and clustering analyses show that in female mice, Anakinra treatment did not significantly alter gene expression in the AA after LCWE-injection (Figure 6B, 6C and Figure IIIB in the online-only Data Supplement). Only 1 gene was found to be differentially expressed between LCWE mice treated with Anakinra and those without, while 54 genes were found differentially expressed between Anakinra-treated LCWE mice and PBS controls (Figure 6E). Thus, Anakinra treatment has limited effect on AA gene expression in females.
Figure 6: Sex differences in gene responses following Anakinra treatment KD mice.
Mice were injected i.p. with PBS, LCWE or LCWE and Anakinra (n=4–5 per group), and AA was harvested 14 days post injection for mRNA-seq analysis. A-B. Heatmap and clustering (top 500 variable genes) of gene expression data from mRNA-seq analysis of male (A) and female (B) mice. C. Volcano plots of differentially expressed genes between indicated groups, blue = P.adj < 0.05 and FC < 2, red = P.adj < 0.05 and FC > 2. D-E. Venn diagrams of differentially expressed genes (P.adj < 0.05, FC > 2) between indicated groups.
Anakinra is more efficient in suppressing inflammation and vascular tissue disruption in male mice than in females
We next performed comparative pathway and upstream regulator analysis of genes differentially expressed in the AA across treatment groups and sex (Figure 7A and 7B). LCWE injection resulted in the enrichment of multiple pathways associated with inflammation, immune cell activation, cytokine signaling and tissue disruption (Figure 7A). As expected, given that male mice show increased severity of LCWE-induced KD vasculitis, these pathways had stronger enrichment (lower p value) and increased activation (higher z-score) in males than in females. A similar pattern was evident with upstream regulator analysis, which identified enrichment of multiple inflammatory mediators such as cytokines, their downstream signaling intermediates and transcription factors (Figure 7B). The majority of these pathways and upstream regulators were also enriched and activated in LCWE-injected mice compared to LCWE-injected male mice treated with Anakinra, indicating that Anakinra treatment is effective at reducing inflammation in male mice (Figure 7A and 7B). In contrast, these pathways were unchanged between LCWE-injected female mice with and without Anakinra (Figure 7A and 7B). Those pathways remained enriched, although to a lesser degree, in the comparison between LCWE-injected female mice treated with Anakinra with female PBS controls (Figure 7A and 7B). The differential change in the enrichment of these pathways further highlights the decreased disease severity and the resulting reduced effectiveness of Anakinra treatment in females.
Figure 7: Functional analysis of genes differentially expressed in response to Anakinra treatment in KD mice.
Mice were injected i.p. with PBS, LCWE or LCWE and Anakinra (n=4–5 per group). AA was harvested 14 days post injection for mRNA-seq analysis. A. Comparative canonical pathway analysis of differentially expressed genes between indicated groups. B. Comparative upstream regulator analysis of differentially expressed genes between indicated groups. Ank indicates Anakinra treatment.
We also generated gene expression plots of selected genes regulated by both LCWE and Anakinra in males, categorized into functional groups (Figure 8A through 8E). Selected genes were further validated by qPCR (Figure IV in the online-only Data Supplement). In males, LCWE-induced gene expression changes in cytokines, chemokines and their receptors, as well as genes involved in the inflammatory response and the extracellular matrix were regulated by Anakinra. As expected, since LCWE treated females have mild disease and low Il1b expression, female mice failed to show a significant change in gene expression when treated with Anakinra. To understand the relevance of genes within our dataset to human pathology, we compared our gene set to one associated with aneurysms in human abdominal aorta tissue (healthy vs. AAA tissue), a gene set associated with human KD whole blood (healthy vs. KD patient whole blood), and a gene set associated with human KD coronary artery (controls vs. KD coronary artery) (Figure 8). Numerous genes overlapped with our dataset (genes shown with a green mark), indicating that Anakinra can suppress the expression of LCWE-induced genes relevant to human KD pathology and/or aortic aneurysm development. Genes involved in inflammation, as well as many associated with vascular smooth muscle cell function, were rescued by treatment with Anakinra in males but not females. These findings could have wide reaching implications for the use of anti-IL1β therapies in human KD patients.
Figure 8: Gene expression changes in male and female mice in response to Anakinra treatment in KD mice.
Mice were injected i.p. with PBS, LCWE or LCWE and Anakinra (n=4–5 per group). AA was harvested 14 days post injection for mRNA-seq analysis. Heatmaps were generated of selected genes in the following functional categories: A. Cytokines and chemokines. B. Cytokine and chemokine receptors. C. Inflammatory response. D. Extracellular matrix. E. Vascular smooth muscle cell function. Green represents genes found differentially expressed (p.adj < 0.05, FC > 2) between human control abdominal aorta and abdominal aortic aneurysm tissue (AAA), genes found differentially expressed (p.adj < 0.05, FC > 2) between healthy control blood and KD patient blood (PBMC) and genes found differentially expressed (p.adj < 0.05, FC > 2) between control and KD coronary artery tissue (Coronary). Ank indicates Anakinra treatment.
Discussion
KD is the leading cause of acquired heart disease in children in the developed world, affecting males at 1.5 times the rate of females 1, 18. Evidence also suggests disease severity is enhanced in males, as sex based stratification of data published by Hoang et al. shows that in acute KD patients, males have a higher percentage of dilatation (23.5% compared to 17.4% in females) and aneurysms (14.7% compared to 7.2% in females) 17. However, the cause of this sex bias is unknown and largely understudied. The importance of separating clinical trial analysis by sex to identify both appropriate dosage and potential differential responses to therapy was highlighted by the finding that females experience more adverse drug responses than males 36. Here, we demonstrate that the LCWE-induced KD mouse model, which closely resembles human KD pathology 10, also recapitulates sex differences observed in the human disease. Male mice displayed enhanced aortitis and coronary arteritis as well as increased incidence and severity of abdominal aorta aneurysm. Taking a transcriptomics approach, we used this model to study underlying causes of sex bias in KD. Our results point to a central role of IL-1β in mediating sex-based differences in disease, with important implications for the use of anti-IL-1β therapies to treat male and female KD patients.
The vascular wall consists of a highly structured network of endothelial and smooth muscle cells, and involves complex interactions between actin, integrins and extracellular matrix. Central to the development of KD is an uncontrolled chronic inflammation involving immune cell infiltration into the arterial wall and progressive remodeling and destruction of vascular tissue 1. IL-1β is a potent inflammatory cytokine involved in autoimmune and chronic inflammatory conditions that has been implicated in regulating many aspects of cardiovascular disease relevant to KD pathogenesis 37–39. Enhanced circulating levels of IL-1β and the expression of IL1-regulated genes are found in KD patients 16, 17. Genetic studies have identified SNPs in IL1B that are associated with increased risk of coronary artery lesions (CAL) as well as IVIG treatment failure 39, 40. Furthermore, a SNP in the inositol 1,4,5-triphosphare 3 kinase (ITPKC) gene, which leads to increased NLRP3-inflammasome activation and IL-1β, was associated with an increased risk of KD and development of CAA 41. In line with this, we have previously shown that IL-1 pathways are important for the development or coronary arteritis, myocarditis and abdominal aorta aneurysm in the LCWE-induced KD mouse model 9, 11, 22. Additionally, IL-1β is also required for pathogenesis of the CAWS mouse model of KD vasculitis 13–15
In this study, we demonstrate a central role for IL-1 signaling pathways in mediating sex differences in the development and severity of KD. Analysis of human transcriptomic data from whole blood identified enhanced expression of IL1B, NLRP3 and IL-1 signaling molecules, including MYD88, in male KD patients. Similarly, in the LCWE-induced KD mouse model, males had enhanced expression of genes encoding IL-1β and components of IL-1 signaling pathways in the abdominal aorta, including IL-1 receptor subunits and MYD88. Our analysis identified IL-1β, IL-1α, MYD88 and NFκB as significant regulators of LCWE-induced genes that were differentially expressed between the sexes. Interestingly, the incidence and severity of adult abdominal aortic aneurysms are also greater in males than females 42, 43 and transcriptional analysis of AAA patient PBMCs found enhanced expression of inflammasome components including AIM2, NLRP3, ASC (PYCARD), CASP1, CASP5, and IL1B in males compared to females44. Thus, a role for IL-1β in mediating sex differences in abdominal aortic aneurysms may be universally relevant.
In addition to IL-1β and inflammasome signaling, we identified many key signaling pathways within the murine expression data that have previously been implicated in human KD and AAA. Furthermore, the top upstream regulators predicted to mediate gene expression changes in the LCWE model—TNF, IFNγ, IL6 and TGFB1—have all been implicated in human KD. These similarities strongly support the translational value of our murine data.
Interestingly, males have shown a higher production of IL-1β in response to lipopolysaccharides (LPS) in both mouse 45 and human 46, indicating immune cells from males may be primed to produce a more robust IL-1β response than females. Our data indicate that enhanced IL-1β expression may be a result of a greater degree of inflammatory M1 macrophage polarization in males compared to females. SOCS3 promotes M1 polarization, while SOCS1 promotes M2 polarization34. Recently, Barrett et al. showed that in atherosclerosis, the ratio of Socs1:Socs3 expression negatively correlates with Il1b expression33. Similarly, we found that the SOCS1:SOCS3 ratio negatively correlated with IL1B expression in PBMCs from male KD patients, and the SOCS1:SOCS3 ratio was lower in male patients. Furthermore, analysis of the murine abdominal aorta revealed more M1 macrophages and monocytes in male mice treated with LCWE than in females, and LCWE-treated male mice had enhanced Socs3 expression compared with females. In support of a more reactive M1 response in males, Li et al. demonstrated enhanced M1 macrophage polarization in the myocardium of male mice in response to Coxsackievirus infection47.
Sex-differences in the immune response and disease development may be driven by genetic and epigenetic factors. Sex chromosomes themselves may also play a role in driving differential expression between males and females. For example, incomplete X-chromosome inactivation may result in females receiving a double dose of X-linked genes that modulate immune responses 48. Whether this phenomenon drives changes in gene expression linked to sex-differences in KD remains to be determined. A recent study also demonstrates conserved sex-bias in the expression of numerous genes, across multiple species, that are not restricted to just X and Y chromosomes 49. These may account for differences in immune function between the sexes. Indeed, within this study, Il1b showed a trend for male bias expression in the spleen 49, which may translate into enhanced Il1b production by immune cells in males. Sex-bias in gene polymorphisms associated with KD have been identified. A recent GWAS in a Korean population found that FCGR2A, SEMA6A, and IL17REL loci were significantly associated with KD in males, but not females. A functional polymorphism of FCGR2A (p.His167Arg), which encodes a low-affinity receptor for IgG, was specifically associated with KD susceptibility in males in a Japanese population 50. Interestingly, epigenetic modifications of this gene are associated with increased IVIG-resistance in KD 51, 52, although sex differences in such modifications were not investigated in those studies. In the LCWE model we identified the related gene FCGR2B as differentially regulated between males and females and differentially modulated by anakinra treatment.
Recently, much attention has been focused on hormonal differences impacting cardiovascular disease, in relation to both immune and vascular cell function 53. However, KD occurs mainly in prepubescent children under the age of five1, and displays a consistent sex bias across infancy and childhood54. Similarly, our studies were performed on 5-week-old sexually immature mice. Given that sex-hormone levels are similar in male and female pre-pubescent children55, 56, sex hormone signaling is unlikely to play a direct role in mediating the sex-differences observed in KD. However, given the important role of sex hormones during fetal development, we cannot rule out the possibility that those developmental differences have long lasting effects on the immune response, perhaps due to epigenetic modifications. Indeed, sex differences in epigenetic modifications of genes associated with cardiovascular disease pathogenesis have been identified 57, and the expression of DNA methylation modifiers DNMT1 and TET2 is altered in KD patients 58. Future studies investigating epigenetic modifications and their relationship to KD, particularly in regard to IL-1β mediated pathways, should be stratified by sex to help identify such potential mechanisms.
The microbiome plays a central role in regulation of the immune system and has been implicated in patients with KD 59 and the LCWE-induced KD mouse model 60. Sex differences in the composition of gut microbiota have been described in both mouse and human 61, although whether they contribute to IL-1 signaling or KD pathogenesis is unknown.
Given our data pointing to altered IL-1β signaling in male and female KD mice, we hypothesized that there may be differential responses of male and female mice to treatment with the IL-R antagonist Anakinra. Indeed, we found that Anakinra was only effective at reducing AAA development and severity and associated gene expression changes in male mice. A number of case reports have highlighted the successful use of Anakinra in IVIG-non-responder KD patients, leading to two Phase II clinical trials for Anakinra in IVIG-resistant patients 20. Case reports to date have shown effective Anakinra treatment in both male and female IVIG resistant patients 62–65. Overall, our results highlight the importance of incorporating independent analyses of male and female KD patients in trials testing the therapeutic efficacy of Anakinra as well as other emerging therapeutics. Additional biomarkers, i.e. serum IL-β or presence of polymorphisms, may also be beneficial in determining which patients will respond to Anakinra treatment. Ongoing research in KD, both in human and mouse, should incorporate sex-based stratification of results to further tease out the mechanisms driving differences in KD development and response to treatment between the sexes.
Supplementary Material
Highlights.
The incidence of Kawasaki Disease (KD) is consistently higher in males, however sex-based differences in KD development are largely under investigated.
Compared with females, males with KD have enhanced Il1b expression and IL-1 signaling genes.
In experimental LCWE-induced murine model of KD vasculitis, anti-IL-1β therapy with Anakinra treatment is more efficient in males than females.
Differential expression of IL-1β may play a role in mediating sex-based differences during KD.
Acknowledgements
We thank Malcolm Lane, Debbie Moreira and Daisy Martinon for their technical support and the lab members for their helpful discussions. We would like to thank the Cedars-Sinai Genomic Core.
Sources of Funding
Supported by R01 HL139766 to MNR and R01 1AI07272-07 to MA
Nonstandard abbreviations and acronyms
- AA
abdominal aorta
- AAA
abdominal aorta aneurysms
- IL-1
interleukin-1
- IL-1Ra
IL-1 receptor antagonist
- SOCS
Suppressor of cytokine signaling
- IPA
Ingenuity Pathway Analysis
- IVIG
Intravenous immunoglobulin
- KD
Kawasaki disease
- LCWE
Lactobacillus casei cell wall extract
- MMP
matrix-metalloproteinase (MMP)
- NT-proBNP
N-terminal pro B-type Natriuretic peptide
- PBMCs
peripheral blood mononuclear cells
- PCA
principal component analysis
- PRRs
pattern recognition receptors
- SDD
sex difference in disease
- WT
Wild-type
Footnotes
Disclosures
The authors have nothing to disclose.
References
- 1.McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of kawasaki disease: A scientific statement for health professionals from the american heart association. Circulation. 2017;135:e927–e999 [DOI] [PubMed] [Google Scholar]
- 2.Burns JC. Kawasaki disease update. Indian J Pediatr. 2009;76:71–76 [DOI] [PubMed] [Google Scholar]
- 3.Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, Glode MP, Mason WH, Reddy V, Sanders SP, et al. The treatment of kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341–347 [DOI] [PubMed] [Google Scholar]
- 4.Burns JC, Capparelli EV, Brown JA, Newburger JW, Glode MP. Intravenous gamma-globulin treatment and retreatment in kawasaki disease. Us/canadian kawasaki syndrome study group. The Pediatric Infectious Disease Journal. 1998;17:1144–1148 [DOI] [PubMed] [Google Scholar]
- 5.Sundel RP, Burns JC, Baker A, Beiser AS, Newburger JW. Gamma globulin re-treatment in kawasaki disease. The Journal of pediatrics. 1993;123:657–659 [DOI] [PubMed] [Google Scholar]
- 6.Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, Eichenfield JR, Martin DD, Newburger JW, Burns JC. Resistance to intravenous immunoglobulin in children with kawasaki disease. J Pediatr. 2008;153:117–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong YM, Jin H-S, Park IS, Hong S-J. Association of the matrix metalloproteinase-3 (−439c/g) promoter polymorphism with kawasaki disease in korean children. Heart Vessels. 2008;23:341–347 [DOI] [PubMed] [Google Scholar]
- 8.Lehman TJ, Walker SM, Mahnovski V, McCurdy D. Coronary arteritis in mice following the systemic injection of group b lactobacillus casei cell walls in aqueous suspension. Arthritis Rheum. 1985;28:652–659 [DOI] [PubMed] [Google Scholar]
- 9.Wakita D, Kurashima Y, Crother TR, et al. Role of interleukin-1 signaling in a mouse model of kawasaki disease-associated abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2016;36:886–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noval Rivas M, Lee Y, Wakita D, Chiba N, Dagvadorj J, Shimada K, Chen S, Fishbein MC, Lehman TJ, Crother TR, Arditi M. Cd8+ t cells contribute to the development of coronary arteritis in the lactobacillus casei cell wall extract-induced murine model of kawasaki disease. Arthritis Rheumatol. 2017;69:410–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee Y, Wakita D, Dagvadorj J, Shimada K, Chen S, Huang G, Lehman TJ, Fishbein MC, Hoffman HM, Crother TR, Arditi M. Il-1 signaling is critically required in stromal cells in kawasaki disease vasculitis mouse model: Role of both il-1alpha and il-1beta. Arterioscler Thromb Vasc Biol. 2015;35:2605–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorelik M, Lee Y, Abe M, Andrews T, Davis L, Patterson J, Chen S, Crother TR, Aune GJ, Noval Rivas M, Arditi M. Il-1 receptor antagonist, anakinra, prevents myocardial dysfunction in a mouse model of kawasaki disease vasculitis and myocarditis. Clin Exp Immunol. 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anzai F, Watanabe S, Kimura H, Kamata R, Karasawa T, Komada T, Nakamura J, Nagi-Miura N, Ohno N, Takeishi Y, Takahashi M. Crucial role of nlrp3 inflammasome in a murine model of kawasaki disease. J Mol Cell Cardiol. 2019;138:185–196 [DOI] [PubMed] [Google Scholar]
- 14.Miyabe C, Miyabe Y, Bricio-Moreno L, Lian J, Rahimi RA, Miura NN, Ohno N, Iwakura Y, Kawakami T, Luster AD. Dectin-2-induced ccl2 production in tissue-resident macrophages ignites cardiac arteritis. J Clin Invest. 2019;130:3610–3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stock AT, Jama HA, Hansen JA, Wicks IP. Tnf and il-1 play essential but temporally distinct roles in driving cardiac inflammation in a murine model of kawasaki disease. J Immunol. 2019;202:3151–3160 [DOI] [PubMed] [Google Scholar]
- 16.Maury CP, Salo E, Pelkonen P. Circulating interleukin-1 beta in patients with kawasaki disease. N Engl J Med. 1988;319:1670–1671 [DOI] [PubMed] [Google Scholar]
- 17.Hoang LT, Shimizu C, Ling L, Naim AN, Khor CC, Tremoulet AH, Wright V, Levin M, Hibberd ML, Burns JC. Global gene expression profiling identifies new therapeutic targets in acute kawasaki disease. Genome Med. 2014;6:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uehara R, Belay ED. Epidemiology of kawasaki disease in asia, europe, and the united states. J Epidemiol. 2012;22:79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salo E, Griffiths EP, Farstad T, Schiller B, Nakamura Y, Yashiro M, Uehara R, Best BM, Burns JC. Incidence of kawasaki disease in northern european countries. Pediatr Int. 2012;54:770–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tremoulet AH, Jain S, Kim S, Newburger J, Arditi M, Franco A, Best B, Burns JC. Rationale and study design for a phase i/iia trial of anakinra in children with kawasaki disease and early coronary artery abnormalities (the anakid trial). Contemp Clin Trials. 2016;48:70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenkranz ME, Schulte DJ, Agle LM, Wong MH, Zhang W, Ivashkiv L, Doherty TM, Fishbein MC, Lehman TJ, Michelsen KS, Arditi M. Tlr2 and myd88 contribute to lactobacillus casei extract-induced focal coronary arteritis in a mouse model of kawasaki disease. Circulation. 2005;112:2966–2973 [DOI] [PubMed] [Google Scholar]
- 22.Lee Y, Schulte DJ, Shimada K, Chen S, Crother TR, Chiba N, Fishbein MC, Lehman TJ, Arditi M. Interleukin-1beta is crucial for the induction of coronary artery inflammation in a mouse model of kawasaki disease. Circulation. 2012;125:1542–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. Star: Ultrafast universal rna-seq aligner. Bioinformatics. 2013;29:15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anders S, Pyl PT, Huber W. Htseq--a python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using david bioinformatics resources. Nat Protoc. 2009;4:44–57 [DOI] [PubMed] [Google Scholar]
- 26.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z, Huang A, Sun J, Jiang T, Qin FX, Wu A. Inference of immune cell composition on the expression profiles of mouse tissue. Sci Rep. 2017;7:40508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edgar R, Domrachev M, Lash AE. Gene expression omnibus: Ncbi gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaggi P, Mejias A, Xu Z, Yin H, Moore-Clingenpeel M, Smith B, Burns JC, Tremoulet AH, Jordan-Villegas A, Chaussabel D, Texter K, Pascual V, Ramilo O. Whole blood transcriptional profiles as a prognostic tool in complete and incomplete kawasaki disease. PLoS One. 2018;13:e0197858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenk GM, Tromp G, Weinsheimer S, Gatalica Z, Berguer R, Kuivaniemi H. Whole genome expression profiling reveals a significant role for immune function in human abdominal aortic aneurysms. BMC Genomics. 2007;8:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rowley AH, Wylie KM, Kim KY, Pink AJ, Yang A, Reindel R, Baker SC, Shulman ST, Orenstein JM, Lingen MW, Weinstock GM, Wylie TN. The transcriptional profile of coronary arteritis in kawasaki disease. BMC Genomics. 2015;16:1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrett TJ, Schlegel M, Zhou F, Gorenchtein M, Bolstorff J, Moore KJ, Fisher EA, Berger JS. Platelet regulation of myeloid suppressor of cytokine signaling 3 accelerates atherosclerosis. Sci Transl Med. 2019;11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Stewart KN, Bishop E, Marek CJ, Kluth DC, Rees AJ, Wilson HM. Unique expression of suppressor of cytokine signaling 3 is essential for classical macrophage activation in rodents in vitro and in vivo. J Immunol. 2008;180:6270–6278 [DOI] [PubMed] [Google Scholar]
- 35.Michel JB, Jondeau G, Milewicz DM. From genetics to response to injury: Vascular smooth muscle cells in aneurysms and dissections of the ascending aorta. Cardiovasc Res. 2018;114:578–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franconi F, Brunelleschi S, Steardo L, Cuomo V. Gender differences in drug responses. Pharmacol Res. 2007;55:81–95 [DOI] [PubMed] [Google Scholar]
- 37.Mertens M, Singh JA. Anakinra for rheumatoid arthritis: A systematic review. J Rheumatol. 2009;36:1118–1125 [DOI] [PubMed] [Google Scholar]
- 38.Mitroulis I, Skendros P, Ritis K. Targeting il-1beta in disease; the expanding role of nlrp3 inflammasome. Eur J Intern Med. 2010;21:157–163 [DOI] [PubMed] [Google Scholar]
- 39.Weng KP, Hsieh KS, Ho TY, Huang SH, Lai CR, Chiu YT, Huang SC, Lin CC, Hwang YT, Ger LP. Il-1b polymorphism in association with initial intravenous immunoglobulin treatment failure in taiwanese children with kawasaki disease. Circ J. 2010;74:544–551 [DOI] [PubMed] [Google Scholar]
- 40.Fu LY, Qiu X, Deng QL, Huang P, Pi L, Xu Y, Che D, Zhou H, Lu Z, Tan Y, Jiang Z, Zhang L, Liu T, Gu X. The il-1b gene polymorphisms rs16944 and rs1143627 contribute to an increased risk of coronary artery lesions in southern chinese children with kawasaki disease. J Immunol Res. 2019;2019:4730507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Onouchi Y, Ozaki K, Burns JC, et al. A genome-wide association study identifies three new risk loci for kawasaki disease. Nat Genet. 2012;44:517–521 [DOI] [PubMed] [Google Scholar]
- 42.Singh K, Bønaa KH, Jacobsen BK, Bjørk L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study : The tromsø study. Am J Epidemiol. 2001;154:236–244 [DOI] [PubMed] [Google Scholar]
- 43.Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111:816–828 [DOI] [PubMed] [Google Scholar]
- 44.Wu X, Cakmak S, Wortmann M, Hakimi M, Zhang J, Bockler D, Dihlmann S. Sex- and disease-specific inflammasome signatures in circulating blood leukocytes of patients with abdominal aortic aneurysm. Mol Med. 2016;22:505–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marriott I, Bost KL, Huet-Hudson YM. Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: A possible mechanism for gender-based differences in endotoxic shock susceptibility. J Reprod Immunol. 2006;71:12–27 [DOI] [PubMed] [Google Scholar]
- 46.Imahara SD, Jelacic S, Junker CE, O’Keefe GE. The influence of gender on human innate immunity. Surgery. 2005;138:275–282 [DOI] [PubMed] [Google Scholar]
- 47.Li K, Xu W, Guo Q, Jiang Z, Wang P, Yue Y, Xiong S. Differential macrophage polarization in male and female balb/c mice infected with coxsackievirus b3 defines susceptibility to viral myocarditis. Circ Res. 2009;105:353–364 [DOI] [PubMed] [Google Scholar]
- 48.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in x-linked gene expression in females. Nature. 2005;434:400–404 [DOI] [PubMed] [Google Scholar]
- 49.Naqvi S, Godfrey AK, Hughes JF, Goodheart ML, Mitchel RN, Page DC. Conservation, acquisition, and functional impact of sex-biased gene expression in mammals. Science. 2019;365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon YC, Kim JJ, Yun SW, et al. Male-specific association of the fcgr2a his167arg polymorphism with kawasaki disease. PLoS One. 2017;12:e0184248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuo HC, Chang JC, Kuo HC, Yu HR, Wang CL, Lee CP, Huang LT, Yang KD. Identification of an association between genomic hypomethylation of fcgr2a and susceptibility to kawasaki disease and intravenous immunoglobulin resistance by DNA methylation array. Arthritis Rheumatol. 2015;67:828–836 [DOI] [PubMed] [Google Scholar]
- 52.Kuo HC, Hsu YW, Wu MS, Woon PY, Wong HS, Tsai LJ, Lin RK, Klahan S, Hsieh KS, Chang WC. Fcgr2a promoter methylation and risks for intravenous immunoglobulin treatment responses in kawasaki disease. Mediators Inflamm. 2015;2015:564625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vitale C, Mendelsohn ME, Rosano GM. Gender differences in the cardiovascular effect of sex hormones. Nat Rev Cardiol. 2009;6:532–542 [DOI] [PubMed] [Google Scholar]
- 54.Nakamura Y, Yashiro M, Uehara R, Sadakane A, Chihara I, Aoyama Y, Kotani K, Yanagawa H. Epidemiologic features of kawasaki disease in japan: Results of the 2007–2008 nationwide survey. J Epidemiol. 2010;20:302–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9:911–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soldin OP, Hoffman EG, Waring MA, Soldin SJ. Pediatric reference intervals for fsh, lh, estradiol, t3, free t3, cortisol, and growth hormone on the dpc immulite 1000. Clin Chim Acta. 2005;355:205–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hartman RJG, Huisman SE, den Ruijter HM. Sex differences in cardiovascular epigenetics-a systematic review. Biol Sex Differ. 2018;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang YH, Chen KD, Lo MH, Cai XY, Chang LS, Kuo YH, Huang WD, Kuo HC. Decreased DNA methyltransferases expression is associated with coronary artery lesion formation in kawasaki disease. Int J Med Sci. 2019;16:576–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kinumaki A, Sekizuka T, Hamada H, Kato K, Yamashita A, Kuroda M. Characterization of the gut microbiota of kawasaki disease patients by metagenomic analysis. Front Microbiol. 2015;6:824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wakita D, Kurashima Y, Takasato Y, Lee Y, Shimada K, Chen S, Crother TR, Fishbein MC, Lehman T, Kiyono H, Arditi M. Abstract o.24: Gut microflora influences pathology in the kawasaki disease vascultis mouse model. Circulation. 2015;131 [Google Scholar]
- 61.Kim YS, Unno T, Kim BY, Park MS. Sex differences in gut microbiota. World J Mens Health. 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shafferman A, Birmingham JD, Cron RQ. High dose anakinra for treatment of severe neonatal kawasaki disease: A case report. Pediatr Rheumatol Online J. 2014;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cohen S, Tacke CE, Straver B, Meijer N, Kuipers IM, Kuijpers TW. A child with severe relapsing kawasaki disease rescued by il-1 receptor blockade and extracorporeal membrane oxygenation. Ann Rheum Dis. 2012;71:2059–2061 [DOI] [PubMed] [Google Scholar]
- 64.Kone-Paut I, Cimaz R, Herberg J, Bates O, Carbasse A, Saulnier JP, Maggio MC, Anton J, Piram M. The use of interleukin 1 receptor antagonist (anakinra) in kawasaki disease: A retrospective cases series. Autoimmun Rev. 2018;17:768–774 [DOI] [PubMed] [Google Scholar]
- 65.Sánchez-Manubens J, Gelman A, Franch N, Teodoro S, Palacios JR, Rudi N, Rivera J, Antón J. A child with resistant kawasaki disease successfully treated with anakinra: A case report. BMC Pediatr. 2017;17:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.