Abstract
During development, the environment exerts a profound influence on the wiring of brain circuits. Due to the limited resolution of studies in fixed tissue, this experience-dependent structural plasticity was once thought to be restricted to a specific developmental time window. The recent introduction of two-photon microscopy for in vivo imaging has opened the door to repeated monitoring of individual neurons and the study of structural plasticity mechanisms at a very fine scale. In this review, we focus on recent work showing that synaptic structural rearrangements are a key mechanism mediating neural circuit adaptation and behavioral plasticity in the adult brain. We examine this work in the context of classic studies in the visual systems of model organisms, which have laid much of the groundwork for our understanding of activity-dependent synaptic remodeling and its role in brain plasticity.
Keywords: visual cortex, retinogeniculate afferents, retinotectal system, circuit remodeling, structural plasticity, synapse dynamics
1. INTRODUCTION
In vertebrates, brain connectivity and function are plastic in the sense that they can be strongly influenced by an animal’s experience within its environment. This plasticity is common across brain regions and is manifest over a wide range of scales, from entire circuits to their individual neuronal components. Each neuron receives and integrates inputs from multiple sources and transforms these into relevant outputs. Altering the input activity onto even a small subset of neurons within a circuit can modify the responses of the circuit as a whole. At the simplest level, changes in input activity can occur by modulating the strength of individual synaptic connections. For example, long-term potentiation and long-term depression are mechanisms for synaptic strengthening or weakening, respectively, which can have pronounced circuit level effects (Malenka & Bear 2004). Although long-term potentiation and long-term depression are mostly thought of as modulators of synaptic efficacy, one might imagine that in the extreme case they could lead to synaptic gain/loss. Indeed, new technologies enabling synaptic visualization in vivo are providing evidence that even the adult brain can add or remove synaptic connections (Chen & Nedivi 2010, 2013).
The impact of synapse formation and elimination as part of broader neuronal structural plasticity has long been appreciated during late brain development, when the connectivity of neuronal circuits is strongly influenced by experience. In studies now considered classic, David Hubel (1982) and Torsten Wiesel (1982) showed in felines and primates that normal vision is required for the appropriate development of cortical wiring supporting binocular vision. Thalamocortical afferents carrying visual drive from each eye are not hardwired. Rather, their final connectivity patterns are sculpted by activity-dependent competition during a limited developmental time window that Hubel & Wiesel (1970) termed the critical period. Implicit in the concept of a developmental critical period is that rewiring of neuronal circuits does not occur past development. Yet, the capacity to form or remove individual synaptic connections could greatly expand the storage capacity of the brain by allowing neurons to assume different partner configurations dependent on circuit requirements (Chklovskii et al. 2004, Stepanyants et al. 2002). In this review, we focus on recent work showing that synaptic structural rearrangements are a key mechanism mediating neural circuit adaptation and behavioral plasticity in the adult brain. We pay particular attention to studies in the visual systems of model organisms, ranging from cats and primates to rodents and amphibians, which have laid much of the groundwork for our understanding of activity-dependent synaptic remodeling and its role in brain plasticity.
2. EXPERIENCE IS INSTRUCTIVE TO CIRCUIT DEVELOPMENT
The capacity for experience-dependent plasticity, the ability to alter the structural connectivity and the functional efficacy of neuronal circuits, is not constant throughout life. Hubel and Wiesel were the first to establish this experimentally when they demonstrated in monkeys and cats that binocular vision is required during a postnatal critical period for normal development of cortical responses to both eyes. They showed that closing one eye, monocular deprivation (MD), during the critical period results in a permanent shift of cortical responses toward the open eye, an ocular dominance (OD) shift (Hubel & Wiesel 1970; Hubel et al. 1977; Wiesel & Hubel 1963, 1965). After the close of the critical period, no amount of visual deprivation could induce an OD shift. Critical periods, when the sensory environment can profoundly impact the wiring of neuronal circuits, have since been observed in a variety of model systems. The principles are now recognized as general to the development of nonvisual sensory regions, including the somatosensory and auditory cortices, and are also relevant to the development of higher-level social and cognitive functions (Hensch 2004).
Following the demonstration that activity during development serves as a critical modifier of neuronal functional properties, Hubel and Wiesel were also the first to show the influence of activity on neuronal structure and connectivity. They injected a radiolabeled amino acid into the monkey eye to label neurons within the visual pathway and later processed the brains for autoradiography. Tangential sections through the binocular visual cortex from control animals showed a clear segregation of thalamocortical afferents into distinct columns dominated by one eye or the other, with cortical area near equally allocated to labeled and unlabeled inputs (Wiesel et al. 1974). MD during the developmental critical period resulted in an expansion of cortical space allocated to the nondeprived eye (Hubel et al. 1977), accompanied by a functional OD shift toward nondeprived eye responses. These observations suggested a competitive mechanism where more active afferents gain cortical territory and form more connections at the expense of less active inputs. The same mechanism was thought to bring about the natural segregation of initially intermixed inputs from the two eyes (Constantine-Paton et al. 1990, Shatz 1990), prenatally in monkeys (Hubel et al. 1977, Rakic 1976) and just after birth in cats (LeVay et al. 1978).
Experiments in the optic tectum of the highly visual frog, Rana pipiens, further established that activity-driven competition between afferents from the two eyes is sufficient to induce the developmental segregation of their central terminals and that disrupting the balance between them could alter their allocation of tectal space. In amphibians, visual inputs are fully crossed so that right eye afferents innervate only the left optic tectum and left eye afferents the right. However, competition for the same target can be induced by transplanting a supernumerary third eye primordium into the diencephalon of developing embryos. This causes the optic tectum on one side to be innervated by both the normal and the supernumerary eye. The functional additional eye competes with the normal eye for space in the optic tectum, and inputs from the two eyes form stripes similar to the OD columns in primary visual cortex of monkeys and cats (Constantine-Paton & Law 1978). Action potential blockade with tetrodotoxin results in desegregation of the eye-specific stripes. In subsequent experiments (Cline et al. 1987), a slow-release plastic infused with the N-methyl-D-aspartate (NMDA) receptor antagonist aminophosphonovaleric acid (APV) was placed over the tecta of tadpoles innervated by a normal and a supernumerary retina. In this experiment, the retinal inputs desegregated and overlapped, but removal of the drug resulted in their resegregation once NMDA receptor activity recovered. Because the optic tectum is not normally dually innervated, the ability of the third eye to form tectal connections that are spatially segregated from the normal eye is, to this day, one of the strongest demonstrations that activity-dependent competition is the major determinant of OD column segregation and maintenance, rather than molecular cues (Reh & Constantine-Paton 1985). These experiments in the amphibian retinotectal system were also the first to show the requirement for the NMDA receptor in activity-dependent developmental competition, suggesting that this molecular coincidence detector required for synaptic strengthening links activity patterns with connectivity outcomes (Constantine-Paton et al. 1990).
Although in the cat and monkey, anatomical changes went hand in hand with the alterations in circuit function, whether the structural rearrangements were the underlying basis of the functional shift in OD was less clear. Several days are required for detecting an MD-induced functional OD shift. Yet several weeks of deprivation are required to detect the gross circuit level alterations visible by autoradiography. Because autoradiography is not sensitive enough to resolve fine changes in individual afferents, in order to directly label single thalamocortical afferents and monitor the role of activity in their development, Phaseolus lectin was injected into lamina A of both the right and left cat lateral geniculate nucleus (LGN). The axons carrying this protein could be identified by post hoc immunohistochemistry in fixed preparations, representing the deprived eye in one hemisphere and the nondeprived eye in the other. This approach allowed comparison of the two populations by examining the two hemispheres of visual cortex in the same animal. Brief MD induced significant shrinkage of the deprived eye’s axonal arbors, demonstrating rapid structural alterations shortly after MD (Antonini & Stryker 1993b). With longer MD, the initial shrinkage was followed by expansion of nondeprived eye arbors (Antonini & Stryker 1996). These findings demonstrated that the anatomical rearrangement of individual thalamic afferents in response to MD occurs on a sufficiently fast timescale to account for a functional OD shift.
Given earlier studies in the neuromuscular junction (Sanes & Lichtman 1999) and of thalamic axon arborization in the cortex (LeVay et al. 1978, Rakic 1976), the thought was that overproduction of neural connections with later activity-dependent pruning was a general developmental theme. However, studies of LGN innervation in kittens revealed that activity was also critical for the initial development of retinogeniculate afferents. Similar to thalamocortical afferents in the primary visual cortex, retinogeniculate afferents from both eyes initially overlap in the LGN before segregating into eye-specific regions (Shatz 1983). Despite this similarity, retinogeniculate afferent segregation does not seem to occur by refinement of initially overlapping axonal arbors. Early in development, retinogeniculate axons appeared simpler than arbors later in development. Instead of an initially widespread arbor that is eventually refined to its ultimate structure, axonal arbors showed a gradual increase in elaboration with only minimal retractions of some side branches (Sretavan & Shatz 1984, 1986b). Enucleation prior to this developmental stage revealed that inputs from both eyes are required for segregated retinogeniculate arbor ingrowth into eye-specific layers, suggesting that, as in the three-eyed frogs, competition (rather than molecular markers) leads to segregation of afferents from the two eyes (Sretavan & Shatz 1986a). Later, implantation of minipumps chronically delivering tetrodotoxin to the optic nerves of fetal cats further showed that activity was required for the segregation of eye-specific inputs but not for the elaboration of retinogeniculate arbors (Shatz & Stryker 1988, Sretavan et al. 1988).
If visual experience is a requirement for circuit refinement, how do areas that develop prior to eye opening undergo circuit optimization? A closed eye does not necessarily mean no retinal activity. Prior to eye opening, random calcium waves sweeping across the retina coactivate adjacent retinal ganglion cells (Wong et al. 1995). Disrupting these waves disrupts eye-specific segregation of retinogeniculate afferents (Penn et al. 1998). Activity resulting from retinal calcium waves propagates throughout the entire visual system and is the dominant source of visual activity prior to eye opening (Ackman et al. 2012). Because this retinal activity is random it results in asynchronous activity between the two eyes, a feature critical to normal development of eye-specific inputs (Stryker & Strickland 1984, Zhang et al. 2012), and is instructive for the early development of circuit function (Burbridge et al. 2014, Feller 2009). Thus, developmental experience-dependent plasticity essentially follows the Hebbian learning rule “neurons that fire together, wire together” (Constantine-Paton et al. 1990, Hebb 1949).
These studies were foundational in terms of our thinking about the role of activity in the development of brain circuitry; however, the methods applied were not able to reveal the existence of similar processes in the adult brain.
3. CLASSIC ANATOMY VERSUS MODERN IMAGING METHODS
In the feline and primate cortex, where visual inputs from the two eyes are spatially segregated, methods for visualizing afferents typically involved injection of one eye with an anterograde transneuronal tracer, such as tritiated proline, tritiated fucose, or wheat germ agglutinin conjugated to horseradish peroxidase, at specific developmental time points or following visual manipulations. Animals were later perfused with a fixative, and their brains sliced into thin sections for autoradiography and staining with the enzyme horseradish peroxidase, or other postmortem histological staining techniques (Hubel et al. 1977; Law et al. 1988; LeVay et al. 1978, 1980; Ruthazer et al. 1999; Shatz & Stryker 1978). Such methods can detect populations of terminals that are separated into discrete eye-specific zones and change as a group, but they would not resolve changes in single terminal arbors. When circuitry is poorly segregated, anatomical studies have relied on serial reconstruction and tracing techniques, whereby individual neurons are filled with biocytin (Callaway 1998, Yabuta & Callaway 1998), Lucifer yellow (Callaway & Katz 1992), or the enzyme horseradish peroxidase (Gilbert & Wiesel 1979; McGuire et al. 1984, 1991). Alternatively, afferent fibers are filled by anterograde transport of Phaseolus lectin (Antonini et al. 1998, 1999; Antonini & Stryker 1993a,b, 1996) injected into the LGN, by biocytin injected into the cortex (Darian-Smith & Gilbert 1994, Malach et al. 1993), or by retrograde uptake of fluorescent latex microspheres injected into the cortex (Callaway & Katz 1990, 1991). Labeling is always followed by serial reconstruction of the fixed tissue and/or tracing of individual arbors.
All of these anatomical techniques provide only static pictures of a dynamic system. Moreover, because they require tissue fixation, there is no option of comparing before and after treatment. Differences between experimental populations have to be large enough to detect when averaged across an entire sample population, and thus significantly larger than the general variance in the size and shape of individual neurons within the population. Given the scale of change that occurs during development and the robust effect of visual manipulations across the population, these anatomical methods were clearly sufficient for resolving structural plasticity. However, if changes in the adult happen on a smaller scale, are unequal across the sample population, and have net-zero growth, it is perhaps unrealistic to expect that they would be detected using conventional anatomical techniques. To detect and monitor structural changes that are within the variance of the neuronal population studied, one would need to follow the same cells over time.
The first experiments allowing the chronic tracking of individual terminals in the same animal came from studies in the developing Xenopus retinotectal system. The transparency of the Xenopus tadpole allowed relatively easy in vivo visualization of individual tectal neurons or retinotectal axons labeled with the lipophilic dye DiI over several days using fast-scanning confocal microscopy. The ability to image the same neurons in vivo at short time intervals revealed that structural changes in dendritic and axonal arbors can occur very rapidly (Witte et al. 1996, Wu et al. 1999) and are strongly influenced by patterned activity (Rajan & Cline 1998, Rajan et al. 1999, Sin et al. 2002). Using the vaccinia virus, foreign proteins were introduced into retinal or tectal neurons revealing the role of important plasticity molecules, such as CaMKII, CPG15, and Homer, on neuronal structure in vivo. This proved to be a powerful tool for elucidating the cellular role of various molecular signals on the development and growth of dendritic and axonal arbors, long before such experiments were possible in a mammalian system (Cantallops et al. 2000, Javaherian & Cline 2005, Li et al. 2000, Nedivi et al. 1998, Wu & Cline 1998, Zou & Cline 1999).
Only after the introduction and development of two-photon microscopy for biological imaging (Denk et al. 1990; Denk & Svoboda 1997; Helmchen et al. 1999; Maletic-Savatic et al. 1999; Shi et al. 1999; Svoboda et al. 1996, 1997, 1999), combined with the advent of fluorescent protein use for labeling individual cells (Chalfie et al. 1994, Chen et al. 2000, Moriyoshi et al. 1996), did similar experiments monitoring individual neuronal structures in vivo over time become possible in the mammalian brain (Figure 1a–e) (Lendvai et al. 2000). The first of these studies was performed in the two-week-old rat barrel cortex, where highly motile dendritic spines and filopodia were observed on dendrites imaged at 10-min intervals. Trimming whiskers on the contralateral side led to a decrease in this motility only in the barrel cortex and no other somatosensory region, suggesting that the dynamics of these fine structural changes were correlated with experience-dependent plasticity (Lendvai et al. 2000). Dendritic spine dynamics were also modified by sensory experience in the developing mouse visual cortex, where MD led to an increase in spine dynamics (Majewska & Sur 2003, Mataga et al. 2004, Oray et al. 2004). During postnatal development of both primary somatosensory and visual cortices in mice, spine eliminations dominated over additions, suggesting a refinement process that continued into adulthood (Grutzendler et al. 2002, Holtmaat et al. 2005, Zuo et al. 2005). In mice, by the second postnatal week, the majority of spines contained excitatory synapses (Blue & Parnavelas 1983a,b), so these dendritic protrusions can be considered morphological surrogates for excitatory synaptic presence, especially if they have been present for at least 4 days (Knott et al. 2006). Thus, their elimination and addition likely represented the removal and addition, respectively, of excitatory synapses.
Figure 1.
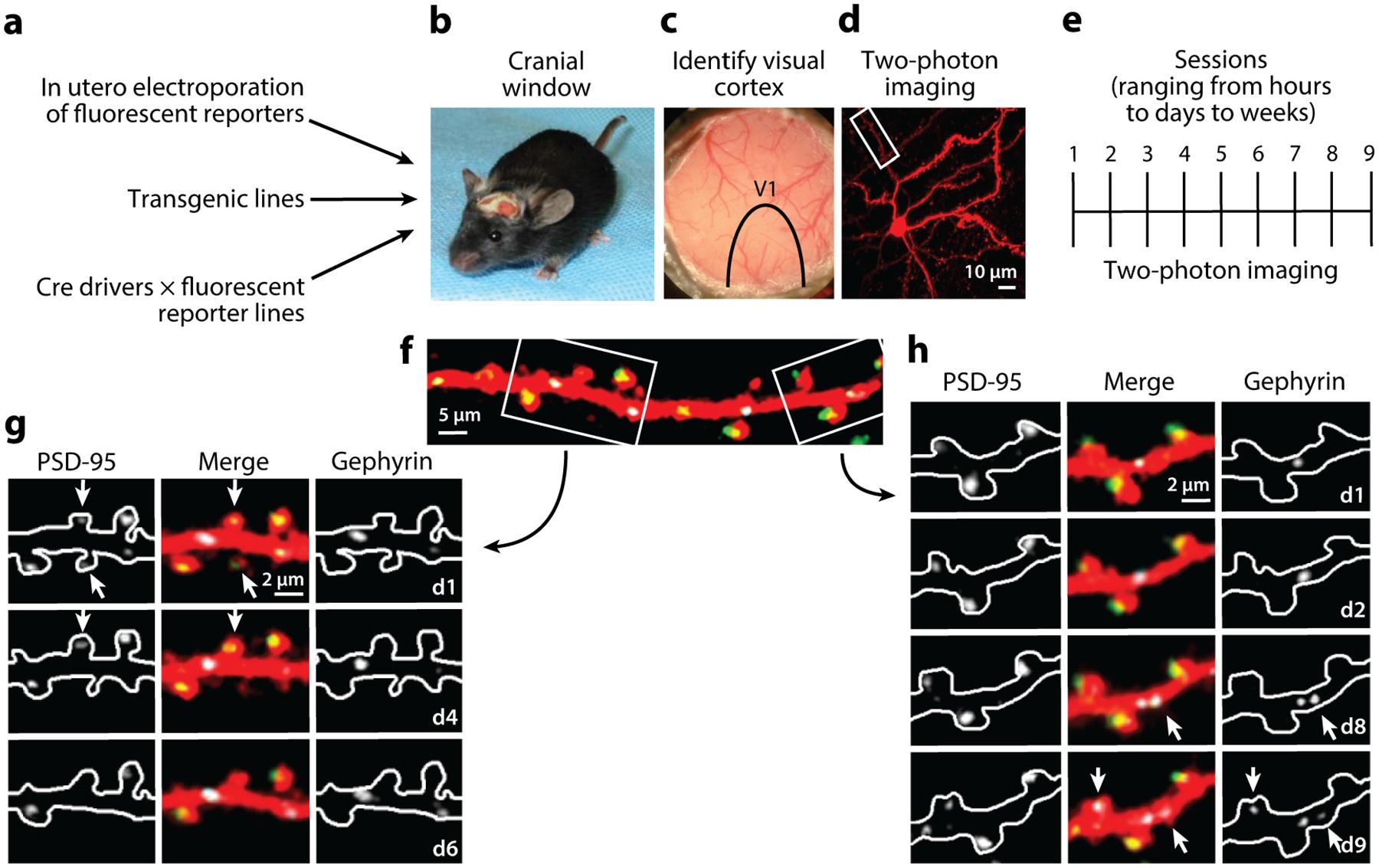
In vivo two-photon imaging experimental pipeline. (a) Multiple methods for sparsely labeling individual neurons in vivo. (b) Cranial windows are implanted at various times after the mouse is born. (c) Intrinsic signal imaging is used to locate the visual cortex within the cranial window. Blood vessels are used as landmarks to relocate the same cell for multiple imaging sessions. (d) Maximum Z-projection of a cell in the visual cortex. (e) The same cell can be imaged over a range of time periods ranging from hours to days to weeks.(f) Zoomed-in view of boxed region in panel d. Cell fill pseudocolored red, with labeled inhibitory synapses (white), and excitatory synapses (pseudocolored green). (g,h) Examples of dynamic synapses from boxed regions in panel f on the indicated days. Left, middle, and right subpanels show a postsynaptic density protein 95 (PSD-95)-mCherry alone, three-channel merge, and Teal-gephyrin alone, respectively. Arrows denote dynamic synapses. Inhibitory synapses in panel h appear on days 8 and 9 (d8 and d9). The excitatory synapse in panel g disappears on day 4 (d4), and its spine is removed on day 6 (d6).
The high spine dynamics coinciding with developmental critical periods in multiple sensory areas, as well as their responsiveness in paradigms of experience-dependent plasticity such as whisker trimming or MD, suggest that, in addition to arbor refinement, synapse refinement through activity-driven spine dynamics can facilitate partner selection. Once critical periods end, there is a clear quantitative decline in spine dynamics with age (Grutzendler et al. 2002, Holtmaat et al. 2005, Zuo et al. 2005), consistent with prior views of a hardwired adult brain after critical period closure.
4. FUNCTIONAL PLASTICITY IN THE MATURE BRAIN
The lack of extensive structural rewiring in the adult brain in response to manipulations of the sensory periphery led to a prevailing view that, once past the critical period, the brain was essentially hardwired. This was despite the fact that studies using single-electrode recordings found evidence for functional remapping in sensory cortices in mature animals. Studies in the somatosensory system showed that, after finger amputation in adult monkeys, the region innervated by this finger in the somatosensory cortex eventually began responding to the two adjacent fingers (Merzenich et al. 1984). Similarly, surgically attaching two fingers together led to a merging of their adjacent cortical projection regions (Clark et al. 1988). In the auditory cortex, ablations of small regions in the cochlea, selectively eliminating perception of the corresponding frequencies, resulted in cortical remapping of nearby frequencies to the deafferented cortical regions (Robertson & Irvine 1989). Focal retinal lesions also revealed post-critical period plasticity in the visual cortex. Neurons in the primary visual cortex innervated by the lesioned part of the retina were initially silenced, but this was followed by a filling-in process, whereby the neurons in the silenced lesion projection zone (LPZ) began responding to the same retinal stimuli as the surrounding cortex (Gilbert & Wiesel 1992, Kaas et al. 1990).
In these initial studies, the observed remapping occurred within a 1- or 2-mm range, similar in size to thalamocortical axon projection zones, and thus could be explained without invoking structural remodeling. But deafferentation studies in monkeys showed that adult functional plasticity was possible on a much larger scale. In monkeys, a decade after nerves from a forelimb were severed, remapping was shown to occur over a distance of as much as 10 mm. The cortical region originally responsive to the deafferented forelimb became responsive to sensory stimuli normally mapping to adjacent facial representation areas (Pons et al. 1991). Similar results were seen from human amputees and were found to occur within as little as 4 weeks after amputation (Ramachandran et al. 1992). This was thought to be too short an interval for structural remapping in the cortex so the functional changes were attributed to a reweighing of existing synapses. In this hypothesis, preexisting but functionally silenced thalamocortical connections from cortical areas neighboring the LPZ are unmasked by the loss of the previously dominant afferents from the amputated area (Ramachandran et al. 1992). Post hoc analysis of deafferented regions soon began to suggest otherwise.
In cats that were given retinal lesions and injections of biocytin just outside of the LPZ, axons projecting to the LPZ showed denser labeling than ones projecting to the unaffected surrounding cortex. This suggested that outgrowth from normal surrounding regions into deafferented territory may play a role in the reorganization process, leading to the functional fill-in of the LPZ (Darian-Smith & Gilbert 1994). The role, if any, of the thalamocortical afferents remained unclear. Later, electrode recordings from cats and monkeys showed that, even after remapping had occurred in the cortex, there was still a silent region in the LGN corresponding to the lesioned part of the retina (Darian-Smith & Gilbert 1995). The ability of intracortical, but not thalamocortical, afferents to structurally remodel past the critical period was consistent with functional studies recording from the cat visual cortex suggesting that a degree of OD plasticity was maintained in superficial layers of the cortex after the thalamocortical circuit was stabilized (Daw et al. 1992). Intracortical and thalamic afferents were also examined in the deafferented regions of the somatosensory cortex of monkeys after accidental forearm injuries. Tracers injected into the area representing the hand in normal monkeys, and the equivalent region in injured monkeys, revealed an increase in intracortical afferents but no difference in the density of labeled thalamocortical afferents (Florence et al. 1998). Similarly, trimming all but two whiskers in adult rats led to an increase in their paired responses within layer 2/3 (L2/3) but not L4 of barrel cortex (Diamond et al. 1993). Although one surprising study in the rat barrel cortex found that whisker trimming could still induce thalamocortical axon restructuring, specifically a 25% reduction in arbor sizes (Oberlaender et al. 2012), the general trend suggests that in the adult brain thalamocortical circuitry is more structurally stable than the intracortical circuitry.
Altogether, these experiments, on top of a large body of literature suggesting an increase in synapse number in response to learning (Greenough et al. 1979, 1985; Greenough & Volkmar 1973; Sirevaag & Greenough 1987; Volkmar & Greenough 1972), were suggestive of at least some capacity for new synapse formation and potentially limited afferent growth in mediating plasticity in the adult cortex. Because these structural changes are on a much smaller scale than ones occurring during development, the classic methodologies so successfully used in developmental studies were not sensitive enough to draw strong conclusions regarding the extent of adult circuit remodeling. It was not until the advent of new cell labeling and imaging technologies that allowed repeated monitoring of individual dendrites and axons in vivo that, as discussed below, the evidence for changes in circuit structure in the adult brain became indisputable.
5. DENDRITIC AND AXONAL ARBOR STRUCTURAL DYNAMICS IN ADULT VISUAL CORTEX
Although the new imaging methods were initially used to study developmental remodeling, perhaps their biggest impact has been in demonstrating remodeling in the adult brain, where the scale and type of structural changes are virtually invisible by classic anatomical methods. The contribution of in vivo imaging to our understanding of experience-dependent plasticity in the adult brain comes mostly from sensory cortices owing to their relative accessibility through a cranial window (Figure 2) (Holtmaat et al. 2009). The first in vivo imaging studies of cells in the adult brain labeled with a fluorescent protein fill revealed that the dendritic arbors of pyramidal neurons in visual, somatosensory, and olfactory cortices are extremely stable over weeks to months (Lee et al. 2006, Mizrahi & Katz 2003, Trachtenberg et al. 2002). Consistent with the classical anatomical studies, they remained unchanged after manipulations to the sensory periphery, such as whisker trimming (Trachtenberg et al. 2002), environmental enrichment and olfactory learning (Mizrahi & Katz 2003), MD (Chen et al. 2012), or retinal scotomas (Keck et al. 2008).
Figure 2.
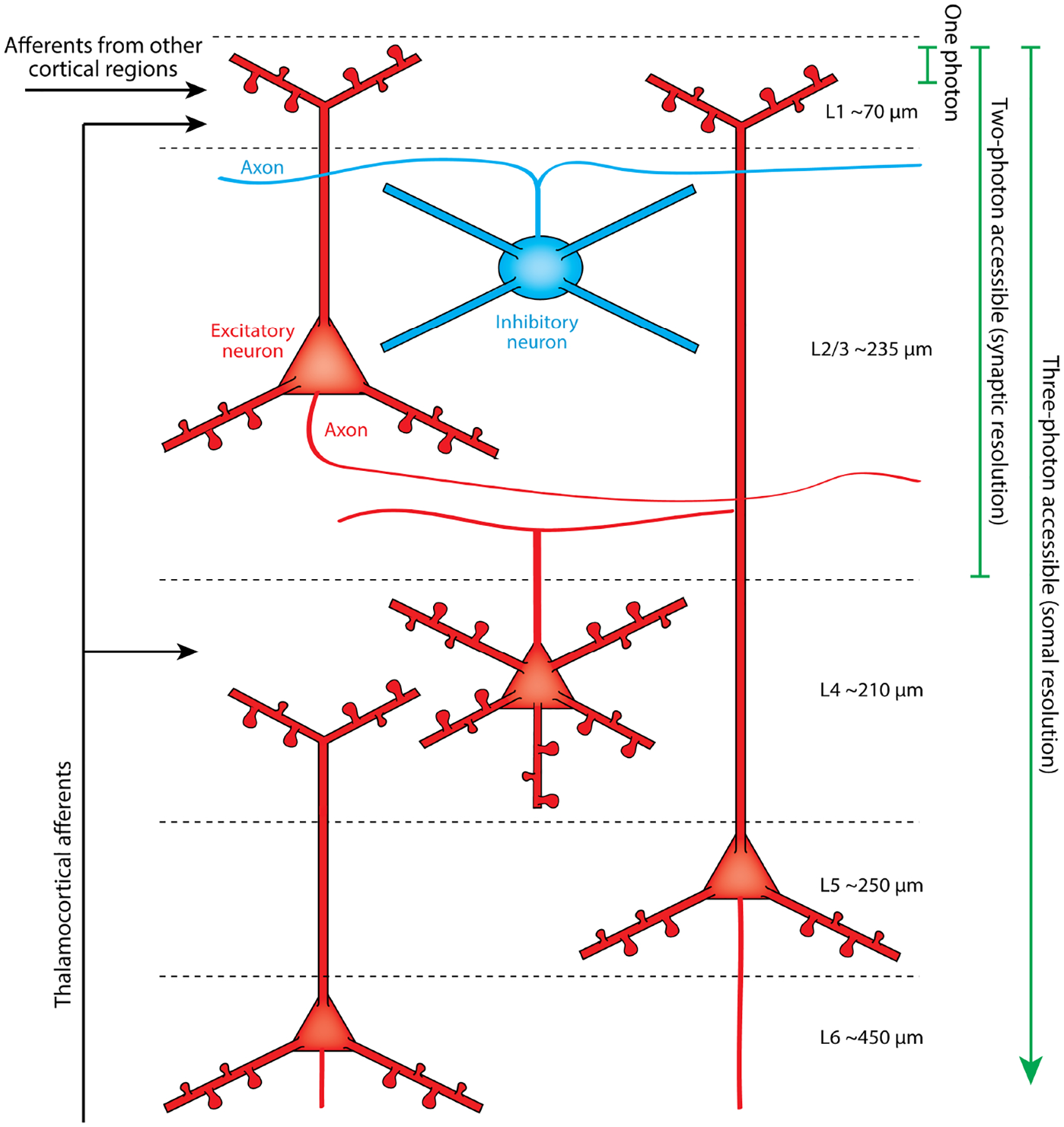
Cortical circuits accessible through a cranial window. This schematic shows the neuron types accessible in vivo with one-photon, two-photon, or three-photon microscopy. Axons are illustrated as thin lines. One-photon techniques, such as confocal microscopy, cannot image more than a few tens of microns into scattering tissue. Two-photon microscopy can provide high synaptic resolution images throughout 300–400 μm of tissue. Three-photon microscopy can image as deep as a millimeter, and the resolution currently allows cellular but not synaptic imaging. Cortical layer thicknesses according to DeFelipe et al. (2002).
In the case of inhibitory interneurons, in vivo imaging in the adult yielded some unexpected findings. Although inhibitory interneuron dendritic arbors proved largely stable, unlike those of pyramidal neurons, the branch tips of inhibitory interneuron dendrites showed significant extensions and retractions over the course of several weeks (Lee et al. 2006). A large fraction of the dynamic events involved addition and elimination of new branches on tertiary dendrites at the arbor periphery (Lee et al. 2008) and represented the gain and loss of multiple synapses per dendritic segment (Chen et al. 2011b). The capacity to remodel dendritic segments at the arbor periphery was common to all interneuron subtypes as long as they were located within a dynamic zone corresponding to the superficial 100 or so microns of L2/3. Interneurons of the same subtypes in L1 or deeper than the dynamic zone did not remodel. Branch tips of remodeling interneurons did not extend past the lower dynamic zone border (Lee et al. 2008). Thus, dendritic arbor remodeling in the adult not only has a cell-type specificity, restricted to inhibitory interneurons, but also appears to be circuit specific in its restricted laminar location. Of note, the dynamic zone for interneuron remodeling in the neocortex corresponds to the laminar location shown in physiological studies to be susceptible to functional plasticity in the adult (Daw et al. 1992, Diamond et al. 1994). The general features of interneuron remodeling are not unique to the primary visual cortex; these features are also evident in somatosensory and higher-order visual cortices and are likely integral to the neocortical microcircuit (Chen et al. 2011a). Similar to remodeling events during development, interneuron branch tip dynamics are responsive to sensory experience. MD as well as binocular deprivation (BD) increases the rate of branch tip retractions, exclusively in binocular V1 (Chen et al. 2011b). In the case of MD, initial retractions are followed by an increase in additions, whereas after BD, retractions continue. The lack of additions in the BD animal suggests that these additions are not homeostatic compensations in response to the decrease in visual drive but rather are constructive events meditating restructuring of nondeprived eye connections. The fact that monocular V1 responds with a transient decrease in dynamics, instead of an increase, further suggests that events in binocular V1 are driven by competitive interactions between the two eyes. Perhaps the capacity of inhibitory neuron dendrites to remodel in response to sensory deprivation accounts for the one reported example of dendritic arbor rearrangements in response to whisker trimming in the adult (Hickmott & Steen 2005), because in this case they could not discriminate the neuron subtype.
The remodeling of inhibitory interneuron dendritic arbors is, not surprisingly, accompanied by changes to their axons (Chen et al. 2011b). When interneuron dendrites retract in response to deprivation and lose excitatory inputs, their output is also dialed down by removal of axonal boutons. The architecture of axonal arbors remains largely stable in the adult rodent and macaque neocortex, but individual axons of excitatory and inhibitory neurons in visual and somatosensory cortices can be dynamic, with branch tips extending or retracting on the order of tens of microns over several days (De Paola et al. 2006; Marik et al. 2010, 2014; Stettler et al. 2006; Yamahachi et al. 2009). Under normal conditions, these changes represent only a small percentage of the total axonal arbor. However, major loss of sensory input can lead to profound axonal restructuring. Retinal lesions in monkeys induce large-scale sprouting and pruning of excitatory and inhibitory axonal arbors (Marik et al. 2014, Yamahachi et al. 2009). A similar result can be induced by whisker trimming in the mouse barrel cortex (Marik et al. 2010). Because the segments being added or removed contain boutons, axonal remodeling events obviously represent the addition or removal of synapses. Boutons on stable axons are also dynamic (more on this below in Section 6).
In vivo imaging confirmed the overall stability of neuronal arbors in the adult, as expected from classical anatomy, and revealed that interneuron dendritic arbors are capable of small-scale branch tip growth and remodeling. It also validated earlier findings showing that axons are capable of peripheral remodeling, especially when challenged with large-scale perturbations to the sensory periphery. Perhaps the biggest contribution of this modern anatomical method has been the discovery that across the stable excitatory dendritic scaffold there is significant capacity for synaptic remodeling.
6. SYNAPSE DYNAMICS IN ADULT VISUAL CORTEX AND THE ROLE OF EXPERIENCE
The presence of dendritic spines has long been considered a hallmark of excitatory neuronal morphology (Beaulieu & Colonnier 1985). Electron microscopy (EM) showed that dendritic spine heads were the sites of excitatory synaptic innervation onto pyramidal neurons and that most dendritic spines harbor an excitatory synapse (Harris et al. 1989). Thus, spine number and density were considered representative of excitatory innervation and indicators of normal circuit health and development (Rochefort & Konnerth 2012). One of the first surprises of in vivo imaging in the adult brain was how dynamic dendritic spines were, given that their removal and addition essentially represent the removal and addition of synaptic sites on the stable arbor. Although in the adult mouse brain the majority of dendritic spines on pyramidal neurons are stable, there is a significant amount of spine turnover under baseline conditions. The baseline rates of spine turnover vary between cortical areas; spines on L2/3 pyramidal neurons in the somatosensory cortex are more dynamic than those in the visual cortex (Holtmaat et al. 2005, Majewska et al. 2006). Spine dynamics can also vary between neurons from different layers in the same region. Spines on L2/3 pyramidal neurons are more stable than those on the L5 pyramidal neuron apical tufts that course through L2/3 (Holtmaat et al. 2005). Sensory manipulations can strongly influence spine dynamics, and here too there is cell-type specificity. In mice, adult MD increases spine dynamics on L5 apical dendrites (Hofer et al. 2009) but not on L2/3 pyramidal neurons (Chen et al. 2012, Hofer et al. 2009). Retinal lesions can result in an almost complete turnover of preexisting dendritic spines on L5 apical dendrites (Keck et al. 2008). A subpopulation of inhibitory neurons also have dendritic spines that carry excitatory synapses (Kawaguchi et al. 2006, Keck et al. 2011), and these spines as well respond to retinal lesions (Keck et al. 2011).
Axonal boutons representing presynaptic terminals in the adult are also dynamic in a cell-type-specific manner, even under normal conditions (De Paola et al. 2006, Stettler et al. 2006). Neocortical neurons that are accessible to imaging, L2/3 neurons as well as pyramidal neurons from deeper layers with apical tufts projecting into L2/3 and L1, receive excitatory inputs from multiple sources (Figure 2). These include thalamocortical afferents as well as intracortical connections from different lamina. Tracking the bouton dynamics on axons of targeted populations found that the thalamocortical afferents are largely stable (De Paola et al. 2006). L6 axons had the most dynamic bouton population, followed by L2/3 axons. Boutons on inhibitory axons are also dynamic under baseline conditions, and their dynamics are influenced by experience-dependent plasticity (Chen et al. 2011b; Marik et al. 2010, 2014).
Overall, the capacity for spine dynamics to rewire the local microcircuit in the adult through excitatory synapse addition and elimination seems excessive in relation to the rates of bouton turnover (De Paola et al. 2006, Holtmaat et al. 2005, Majewska et al. 2006). One potential reason for this discrepancy is that not all spine dynamics may actually represent excitatory synaptic changes. Newly formed spines fall into two dynamic classes. The first includes transient spines, the most dynamic category of spines, which form de novo and are removed within a few days. Transient spines may not appose a bouton (Knott et al. 2006). In fact, recent in vivo imaging studies, where the postsynaptic density protein 95 (PSD-95) is tagged with a second fluorescent tag that is spectrally complementary to the spine fill (Figure 1f–h), show that transient spines usually lack PSD-95 (Cane et al. 2014, Villa et al. 2016). The second type of dynamic spines are ones that persist for at least 4 days, gain PSD-95, and then remain for weeks to months (Cane et al. 2014, Holtmaat et al. 2005, Knott et al. 2006, Villa et al. 2016). Only the persistent category may in fact represent stable changes to circuit connectivity, and these may be the only ones with a concomitant change to the matching presynaptic bouton. However, even dynamic spines that persist may not require a change to the presynaptic bouton. One study found that the majority of newly formed spines formed onto multisynaptic boutons with preexisting synapses (Knott et al. 2006). This suggests a competitive mechanism where new spines form and compete with preexisting spines for stable presynaptic boutons.
Because there is no structural surrogate for inhibitory synapses comparable to spines for excitatory synapses, their in vivo characterization has lagged behind that of their excitatory counterparts. The recent introduction of two-color two-photon imaging has enabled, for the first time, the direct monitoring of inhibitory synapses by expressing the fluorescently tagged inhibitory postsynaptic scaffolding protein gephyrin in addition to a cell fill (Figure 1f–h) (Chen et al. 2012, van Versendaal et al. 2012). One of the most surprising findings from these studies was that a large fraction of inhibitory synapses on pyramidal neurons are located on dendritic spines rather than on the shaft. Although EM studies had previously reported the existence of inhibitory synapses on spines (Jones & Powell 1969, Knott et al. 2002, Kubota et al. 2007), their prevalence had not previously been appreciated due to the low sampling capacity of EM. One-third of all inhibitory synapses were found located on spines, always side by side with an excitatory synapse (Chen et al. 2012). A second important finding was that inhibitory synapses on these dually innervated spines are significantly more dynamic than inhibitory shaft synapses and the dendritic spines themselves (Chen et al. 2012, van Versendaal et al. 2012, Villa et al. 2016). They are also more responsive to MD than spines (Chen et al. 2012, van Versendaal et al. 2012, Villa et al. 2016). In terms of synaptic changes on neocortical pyramidal arbors, there is no question that inhibitory synapses are by far the most dynamic category.
Short-term in vivo imaging of synapse dynamics at 24-h intervals shows that one reason for the high dynamics of inhibitory spine synapses is that many are eliminated and reoccur at the same location on a relatively short timescale. These findings suggest a potentially different logic behind inhibitory synapse dynamics relative to excitatory synapses (Figure 3). Rather than exchanging or sampling new partners, these inhibitory synapse dynamics allow high-precision local modulation of specific circuit elements. Because dually innervated spines are extremely stable, as are the excitatory synapses on these spines (Villa et al. 2016), recurrent inhibitory dynamics could serve to effectively add or eliminate these essentially hardwired synapses. An immuno-EM study reports that dually innervated spines in L2/3 are preferentially innervated by axons positive for VGLUT2, a marker for subcortical, presumably thalamic, inputs (Kubota et al. 2007). It is interesting to think that stable feed-forward inputs onto dually innervated L2/3 spines can still be taken on/off line through sensory modulation of inhibitory synaptic presence.
Figure 3.
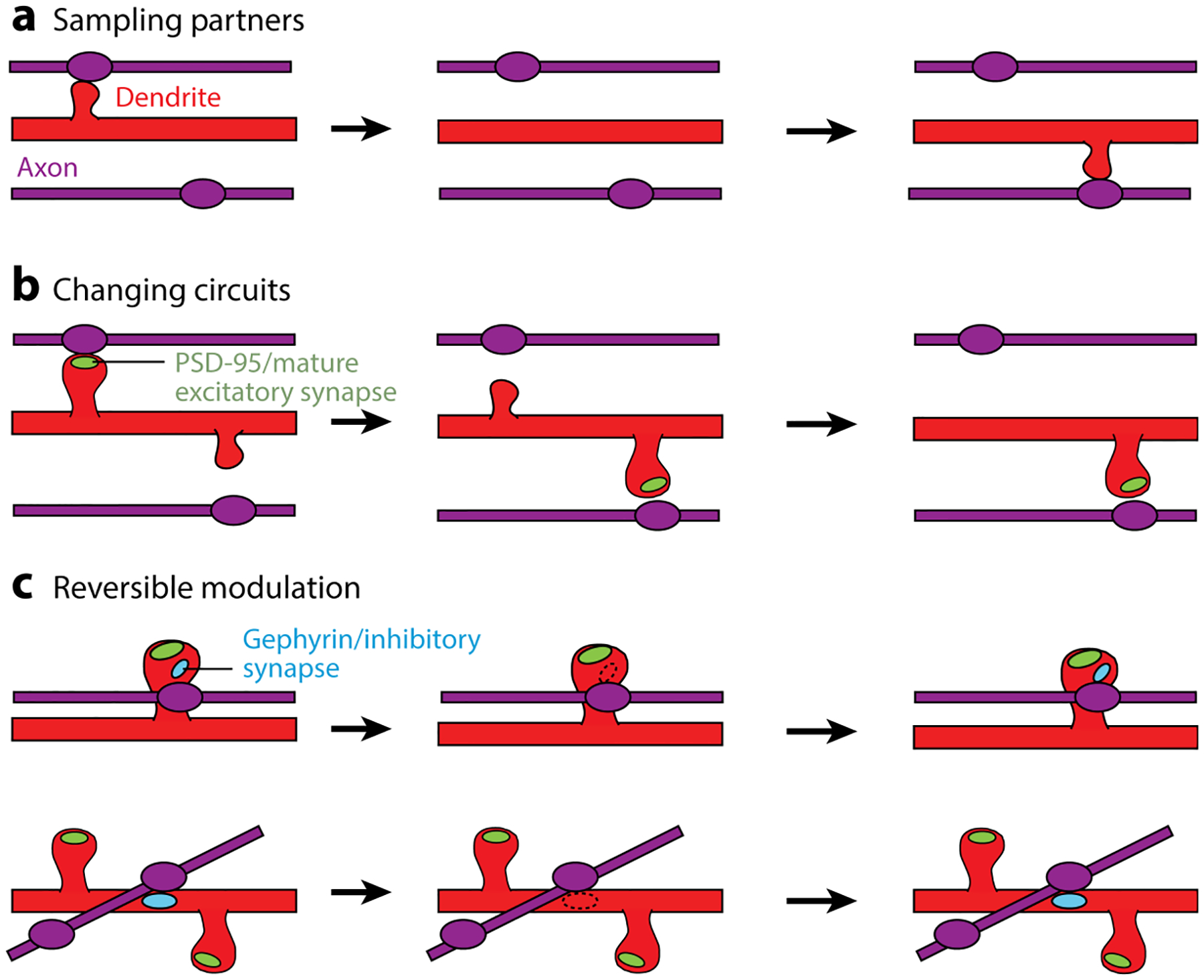
Different logic for excitatory versus inhibitory synaptic changes. (a–c) Schematics illustrating the most prevalent categories of dynamic events for spines without postsynaptic density protein 95 (PSD-95), spines with PSD-95, and inhibitory synapses on dually innervated spine (DiS) and on the shaft. (a) The dynamics of spines without PSD-95 are rapid and sample different locations, potentially testing for different partners.(b) Spines that lose an excitatory synapse are destabilized, whereas those that gain one are stabilized and persist. In both cases, they represent a local rewiring of excitatory circuits by exchanging partners.(c) Inhibitory synapses on the shaft or on DiS are removed and reassembled at stable locations, providing a mechanism for reversible inhibitory modulation of excitatory circuits. Figure adapted from Villa et al. (2016).
7. CONCLUSIONS
In summary, structural remodeling of neuronal circuits is a common underlying feature of brain plasticity across developmental ages and cortical regions. The key difference between the developing and adult brain is one of scale. During development, large-scale alterations in axonal and dendritic arbors mediate an immense capacity for experience-dependent plasticity. Later in adults, structural plasticity occurs on a finer scale and permits exchanging partners within local circuits. The capacity for adult structural plasticity appears to be more pronounced in some neuronal types and in some circuits more than others. Inhibitory neurons and synapses maintain a significant dynamic capacity in the adult and are particularly responsive to changes in experience, suggesting that modifications to inhibitory circuits are a dominant force in adult experience-dependent plasticity. Intracortical excitatory circuits, especially in the superficial lamina, maintain some degree of functional, and to a lesser extent structural, plasticity more so than the thalamocortical inputs that dominate developmental experience-dependent plasticity.
Aside from the lower magnitude of excitatory synaptic changes in the adult, as compared to inhibitory ones, excitatory synapse dynamics appear to follow a different logic than inhibitory dynamics. Excitatory synapses are generally very stable once established in the naive animal, but many spines are added and removed all along the dendritic branch on a relatively rapid timescale. These short-lived transient spines potentially represent a sampling strategy to search for and create connections with new presynaptic partners, and most of these attempts fail. In contrast, many inhibitory synapses are added and removed at the same location on a rapid timescale and likely represent input-specific regulation at particular dendritic locales (Figure 3, based on Villa et al. 2016).
SUMMARY POINTS.
During development, the ultimate wiring of neuronal circuits is determined by sensory experience. Disrupting normal vision during a restricted developmental window, the critical period, induces large-scale changes in visual circuit connectivity.
Although there are many examples of functional plasticity in adult primary sensory areas, studies in fixed tissue are not sufficiently sensitive to reveal the extent of experience-dependent structural plasticity.
The recent introduction of two-photon microscopy for in vivo imaging has opened the door to chronic monitoring of individual neurons and the study of structural plasticity mechanisms at a very fine scale.
The capacity for adult structural plasticity appears to be more pronounced in some neuronal cell types and circuits.
Intracortical excitatory circuits, especially in the superficial lamina, maintain some degree of functional, and to a lesser extent structural, plasticity in the adult brain, in contrast to the thalamocortical inputs that dominate developmental experience-dependent plasticity.
Inhibitory neurons and synapses in the adult are particularly dynamic and are responsive to changes in experience, suggesting that modifications to inhibitory circuits are a dominant force in adult experience-dependent plasticity.
Dendritic spine dynamics reflect sampling of potential partners and selection of new contacts, leading to circuit rewiring.
Inhibitory synapses can be added and removed at stable sites and may not always represent changes of synaptic partners but rather reversible modulation of excitatory circuits.
FUTURE ISSUES.
What are the presynaptic sources of dynamic synapses in vivo? Are some sources more stable than others?
What are the spatial and temporal relationships between dynamic synapses on the same neuron? Are excitatory and inhibitory dynamics locally coordinated?
Does inhibitory circuit refinement during development follow rules similar to those of excitatory circuits? How do inhibitory synapse and interneuron branch tip dynamics differ between the adult and developing brain?
ACKNOWLEDGMENTS
We thank Dr. Martha Constantine-Paton, Dr. Hollis Cline, and members of the Nedivi lab for comments on the manuscript. The authors are supported by National Eye Institute grants RO1 EY017656, RO1 EY025437, and RO1 EY011894 to E.N. Partial support for K.B. was provided by National Institutes of Health Predoctoral Training Grant T32GM007287.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Ackman JB, Burbridge TJ, Crair MC. 2012. Retinal waves coordinate patterned activity throughout the developing visual system. Nature 490:219–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A, Fagiolini M, Stryker MP. 1999. Anatomical correlates of functional plasticity in mouse visual cortex. J. Neurosci 19:4388–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A, Gillespie DC, Crair MC, Stryker MP. 1998. Morphology of single geniculocortical afferents and functional recovery after reverse monocular deprivation in kitten. J. Neurosci 18:9896–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A, Stryker MP. 1993a. Development of individual geniculocortical arbors in cat striate cortex and effects of binocular impulse blockade. J. Neurosci 13:3549–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A, Stryker MP. 1993b. Rapid remodeling of axonal arbors in the visual cortex. Science 260:1819–21 [DOI] [PubMed] [Google Scholar]
- Antonini A, Stryker MP. 1996. Plasticity of geniculocortical afferents following brief or prolonged monocular occlusion in the cat. J. Comp. Neurol 369:64–82 [DOI] [PubMed] [Google Scholar]
- Baker FH, Grigg P, Von Noorden GK. 1974. Effects of visual deprivation and strabismus on the response of neurons in visual cortex of monkey, including studies on the striate and prestriate cortex in normal animal. Brain Res. 66:185–208 [Google Scholar]
- Beaulieu C, Colonnier M. 1985. A laminar analysis of the number of round-asymmetrical and flat-symmetrical synapses on spines, dendritic trunks, and cell bodies in area 17 of the cat. J. Comp. Neurol 231:180–89 [DOI] [PubMed] [Google Scholar]
- Blue ME, Parnavelas JG. 1983a. The formation and maturation of synapses in the visual cortex of the rat. I.Qualitative analysis. J. Neurocytol 12:599–616 [DOI] [PubMed] [Google Scholar]
- Blue ME, Parnavelas JG. 1983b. The formation and maturation of synapses in the visual cortex of the rat. II.Quantitative analysis. J. Neurocytol 12:697–712 [DOI] [PubMed] [Google Scholar]
- Burbridge TJ, Xu HP, Ackman JB, Ge X, Zhang Y, et al. 2014. Visual circuit development requires patterned activity mediated by retinal acetylcholine receptors. Neuron 84:1049–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM. 1998. Prenatal development of layer-specific local circuits in primary visual cortex of the macaque monkey. J. Neurosci 18:1505–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM, Katz LC. 1990. Emergence and refinement of clustered horizontal connections in cat striate cortex. J. Neurosci 10:1134–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM, Katz LC. 1991. Effects of binocular deprivation on the development of clustered horizontal connections in cat striate cortex. PNAS 88:745–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM, Katz LC. 1992. Development of axonal arbors of layer 4 spiny neurons in cat striate cortex. J. Neurosci 12:570–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cane M, Maco B, Knott G, Holtmaat A. 2014. The relationship between PSD-95 clustering and spine stability in vivo. J. Neurosci 34:2075–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantallops I, Haas K, Cline HT. 2000. Postsynaptic CPG15 promotes synaptic maturation and presynaptic axon arbor elaboration in vivo. Nat. Neurosci 3:1004–11 [DOI] [PubMed] [Google Scholar]
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. 1994. Green fluorescent protein as a marker for gene expression. Science 263:802–5 [DOI] [PubMed] [Google Scholar]
- Chen BE, Lendvai B, Nimchinsky EA, Burbach B, Fox K, Svoboda K. 2000. Imaging high-resolution structure of GFP-expressing neurons in neocortex in vivo. Learn. Mem 7:433–41 [DOI] [PubMed] [Google Scholar]
- Chen JL, Flanders GH, Lee WC, Lin WC, Nedivi E. 2011a. Inhibitory dendrite dynamics as a general feature of the adult cortical microcircuit. J. Neurosci 31:12437–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Lin WC, Cha JW, So PT, Kubota Y, Nedivi E. 2011b. Structural basis for the role of inhibition in facilitating adult brain plasticity. Nat. Neurosci 14:587–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Nedivi E. 2010. Neuronal structural remodeling: Is it all about access? Curr. Opin. Neurobiol 20:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Nedivi E. 2013. Highly specific structural plasticity of inhibitory circuits in the adult neocortex. Neuroscientist 19:384–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Villa KL, Cha JW, So PT, Kubota Y, Nedivi E. 2012. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron 74:361–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chklovskii DB, Mel BW, Svoboda K. 2004. Cortical rewiring and information storage. Nature 431:782–88 [DOI] [PubMed] [Google Scholar]
- Clark SA, Allard T, Jenkins WM, Merzenich MM. 1988. Receptive fields in the body-surface map in adult cortex defined by temporally correlated inputs. Nature 332:444–45 [DOI] [PubMed] [Google Scholar]
- Cline HT, Debski EA, Constantine-Paton M. 1987. N-methyl-d-aspartate receptor antagonist desegregates eye-specific stripes. PNAS 84:4342–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine-Paton M, Cline HT, Debski E. 1990. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu. Rev. Neurosci 13:129–54 [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M, Law MI. 1978. Eye-specific termination bands in tecta of three-eyed frogs. Science 202:639–41 [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Gilbert CD. 1994. Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature 368:737–40 [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Gilbert CD. 1995. Topographic reorganization in the striate cortex of the adult cat and monkey is cortically mediated. J. Neurosci 15:1631–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw NW, Fox K, Sato H, Czepita D. 1992. Critical period for monocular deprivation in the cat visual cortex. J. Neurophysiol 67:197–202 [DOI] [PubMed] [Google Scholar]
- De Paola V, Holtmaat A, Knott G, Song S, Wilbrecht L, et al. 2006. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron 49:861–75 [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Alonso-Nanclares L, Arellano J. 2002. Microstructure of the neocortex: comparative aspects. J. Neurocytol 31(3):299–316 [DOI] [PubMed] [Google Scholar]
- Denk W, Strickler JH, Webb WW. 1990. Two-photon laser scanning fluorescence microscopy. Science 248:73–76 [DOI] [PubMed] [Google Scholar]
- Denk W, Svoboda K. 1997. Photo upmanship: Why multiphoton imaging is more than a gimmick. Neuron 18:351–57 [DOI] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. 1993. Experience-dependent plasticity in adult rat barrel cortex. PNAS 90:2082–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ME, Huang W, Ebner FF. 1994. Laminar comparison of somatosensory cortical plasticity. Science 265:1885–88 [DOI] [PubMed] [Google Scholar]
- Feller MB. 2009. Retinal waves are likely to instruct the formation of eye-specific retinogeniculate projections. Neural Dev. 4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence SL, Taub HB, Kaas JH. 1998. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science 282:1117–20 [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. 1979. Morphology and intracortical projections of functionally characterized neurons in the cat visual cortex. Nature 280:120–25 [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. 1992. Receptive field dynamics in adult primary visual cortex. Nature 356:150–52 [DOI] [PubMed] [Google Scholar]
- Greenough WT, Hwang H- MF, Gorman C. 1985. Evidence for active synapse formation or altered postsynaptic metabolism in visual cortex of rats reared in complex environments. PNAS 82:4549–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Juraska JM, Volkmar FR. 1979. Maze training effects on dendritic branching in occipital cortex of adult rats. Behav. Neural Biol 26:287–97 [DOI] [PubMed] [Google Scholar]
- Greenough WT, Volkmar FR. 1973. Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exp. Neurol 40:491–504 [DOI] [PubMed] [Google Scholar]
- Grutzendler J, Kasthuri N, Gan W- B. 2002. Long-term dendritic spine stability in the adult cortex. Nature 420:812–16 [DOI] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao BH. 1989. Ultrastructure, development and plasticity of dendritic spine synapses in area CA1 of the rat hippocampus: extending our vision with serial electron microscopy and three-dimensional analyses The Hippocampus: New Vistas, ed. Chan-Palay V, Kohler C, pp. 33–52. New York: Liss [Google Scholar]
- Hebb DO. 1949. The Organization of Behavior: A Neuropsychological Theory. New York: Wiley [Google Scholar]
- Helmchen F, Svoboda K, Denk W, Tank DW. 1999. In vivo dendritic calcium dynamics in deep-layer cortical pyramidal neurons. Nat. Neurosci 2:989–95 [DOI] [PubMed] [Google Scholar]
- Hensch TK. 2004. Critical period regulation. Annu. Rev. Neurosci 27:549–79 [DOI] [PubMed] [Google Scholar]
- Hickmott PW, Steen PA. 2005. Large-scale changes in dendritic structure during reorganization of adult somatosensory cortex. Nat. Neurosci 2:140–42 [DOI] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M. 2009. Experience leaves a lasting structural trace in cortical circuits. Nature 457:313–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Bonhoeffer T, Chow DK, Chuckowree J, De Paola V, et al. 2009. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc 4:1128–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, et al. 2005. Transient and persistent dendritic spines in the neocortex in vivo. Neuron 45:279–91 [DOI] [PubMed] [Google Scholar]
- Hubel DH. 1982. Exploration of the primary visual cortex, 1955–78. Nature 299:515–24 [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. 1970. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J. Physiol 206:419–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S. 1977. Plasticity of ocular dominance columns in monkey striate cortex. Philos. Trans. R. Soc. B 278:377–409 [DOI] [PubMed] [Google Scholar]
- Javaherian A, Cline HT. 2005. Coordinated motor neuron axon growth and neuromuscular synaptogenesis are promoted by CPG15 in vivo. Neuron 45:505–12 [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TPS. 1969. Morphological variations in the dendritic spines of the neocortex. J. Cell Sci 5:509–29 [DOI] [PubMed] [Google Scholar]
- Kaas JH, Krubitzer LA, Chino YM, Langston AL, Polley EH, Blair N. 1990. Reorganization of retinotopic cortical maps in adult mammals after lesions of the retina. Science 248:229–31 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Karube F, Kubota Y. 2006. Dendritic branch typing and spine expression patterns in cortical nonpyramidal cells. Cereb. Cortex 16:696–711 [DOI] [PubMed] [Google Scholar]
- Keck T, Mrsic-Flogel TD, Vaz Afonso M, Eysel UT, Bonhoeffer T, Hübener M. 2008. Massive restructuring of neuronal circuits during functional reorganization of adult visual cortex. Nat. Neurosci 11:1162–67 [DOI] [PubMed] [Google Scholar]
- Keck T, Scheuss V, Jacobsen RI, Wierenga CJ, Eysel UT, et al. 2011. Loss of sensory input causes rapid structural changes of inhibitory neurons in adult mouse visual cortex. Neuron 71:869–82 [DOI] [PubMed] [Google Scholar]
- Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. 2006. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat. Neurosci 9:1117–24 [DOI] [PubMed] [Google Scholar]
- Knott GW, Quairiaux C, Genoud C, Welker E. 2002. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron 34:265–73 [DOI] [PubMed] [Google Scholar]
- Kubota Y, Hatada S, Kondo S, Karube F, Kawaguchi Y. 2007. Neocortical inhibitory terminals innervate dendritic spines targeted by thalamocortical afferents. J. Neurosci 27:1139–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law MI, Zahs KR, Stryker MP. 1988. Organization of primary visual cortex (area 17) in the ferret. J. Comp.Neurol 278:157–80 [DOI] [PubMed] [Google Scholar]
- Lee WC, Chen JL, Huang H, Leslie JH, Amitai Y, et al. 2008. A dynamic zone defines interneuron remodeling in the adult neocortex. PNAS 105:19968–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Huang H, Feng G, Sanes JR, Brown EN, et al. 2006. Dynamic remodeling of dendritic arbors in GABAergic interneurons of adult visual cortex. PLOS Biol. 4:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendvai B, Stern EA, Chen B, Svoboda K. 2000. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature 404:876–81 [DOI] [PubMed] [Google Scholar]
- LeVay S, Stryker MP, Shatz CJ. 1978. Ocular dominance columns and their development in layer IV of the cat’s visual cortex. J. Comp. Neurol 179:223–44 [DOI] [PubMed] [Google Scholar]
- LeVay S, Wiesel TN, Hubel DH. 1980. The development of ocular dominance columns in normal and visually deprived monkeys. J. Comp. Neurol 191:1–51 [DOI] [PubMed] [Google Scholar]
- Li Z, Van Aelst L, Cline HT. 2000. Rho GTPases regulate distinct aspects of dendritic arbor growth in Xenopus central neurons in vivo. Nat. Neurosci 3:217–25 [DOI] [PubMed] [Google Scholar]
- Majewska A, Sur M. 2003. Motility of dendritic spines in visual cortex in vivo: changes during the critical period and effects of visual deprivation. PNAS 100:16024–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska AK, Newton JR, Sur M. 2006. Remodeling of synaptic structure in sensory cortical areas in vivo. J. Neurosci 26:3021–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malach R, Amir Y, Harel M, Grinvald A. 1993. Relationship between intrinsic connections and functional architecture revealed by optical imaging and in vivo targeted biocytin injections in primate striate cortex. PNAS 90:10469–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. 2004. LTP and LTD: an embarrassment of riches. Neuron 44:5–21 [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K. 1999. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 283:1923–27 [DOI] [PubMed] [Google Scholar]
- Marik SA, Yamahachi H, McManus JNJ, Szabo G, Gilbert CD. 2010. Axonal dynamics of excitatory and inhibitory neurons in somatosensory cortex. PLOS Biol. 8:e1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marik SA, Yamahachi H, Meyer zum Alten Borgloh S, Gilbert CD. 2014. Large-scale axonal reorganization of inhibitory neurons following retinal lesions. J. Neurosci 34:1625–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataga N, Mizuguchi Y, Hensch TK. 2004. Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron 44:1031–41 [DOI] [PubMed] [Google Scholar]
- McGuire BA, Gilbert CD, Rivlin PK, Wiesel TN. 1991. Targets of horizontal connections in macaque primary visual cortex. J. Comp. Neurol 305:370–92 [DOI] [PubMed] [Google Scholar]
- McGuire BA, Hornung J- P, Gilbert CD, Weisel TN. 1984. Patterns of synaptic input to layer 4 of cat striate cortex. J. Neurosci 4:3021–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. 1984. Somatosensory cortical map changes following digit amputation in adult monkeys. J. Comp. Neurol 224:591–605 [DOI] [PubMed] [Google Scholar]
- Mizrahi A, Katz LC. 2003. Dendritic stability in the adult olfactory bulb. Nat. Neurosci 6:1201–7 [DOI] [PubMed] [Google Scholar]
- Moriyoshi K, Richards LJ, Akazawa C, O’Leary DD, Nakanishi S. 1996. Labeling neural cells using adenoviral gene transfer of membrane-targeted GFP. Neuron 16:255–60 [DOI] [PubMed] [Google Scholar]
- Nedivi E, Wu GY, Cline HT. 1998. Promotion of dendritic growth by CPG15, an activity-induced signaling molecule. Science 281:1863–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlaender M, Ramirez A, Bruno RM. 2012. Sensory experience restructures thalamocortical axons during adulthood. Neuron 74:648–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oray S, Majewska A, Sur M. 2004. Dendritic spine dynamics are regulated by monocular deprivation and extracellular matrix degradation. Neuron 44:1021–30 [DOI] [PubMed] [Google Scholar]
- Penn AA, Riquelme PA, Feller MB, Shatz CJ. 1998. Competition in retinogeniculate patterning driven by spontaneous activity. Science 279:2108–12 [DOI] [PubMed] [Google Scholar]
- Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M. 1991. Massive cortical reorganization after sensory deafferentation in adult macaques. Science 252:1857–60 [DOI] [PubMed] [Google Scholar]
- Rajan I, Cline HT. 1998. Glutamate receptor activity is required for normal development of tectal cell dendrites in vivo. J. Neurosci 18:7836–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan I, Witte S, Cline HT. 1999. NMDA receptor activity stabilizes presynaptic retinotectal axons and postsynaptic optic tectal cell dendrites in vivo. J. Neurobiol 38:357–68 [DOI] [PubMed] [Google Scholar]
- Rakic P 1976. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature 261:467–71 [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Rogers-Ramachandran D, Stewart M. 1992. Perceptual correlates of massive cortical reorganization. Science 258:1159–60 [DOI] [PubMed] [Google Scholar]
- Reh T, Constantine-Paton M. 1985. Eye-specific segregation requires neural activity in three-eyed Rana pipiens. J. Neurosci 5:1132–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Irvine DR. 1989. Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. J. Comp. Neurol 282:456–71 [DOI] [PubMed] [Google Scholar]
- Rochefort NL, Konnerth A. 2012. Dendritic spines: from structure to in vivo function. EMBO Rep. 13:699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthazer ES, Baker GE, Stryker MP. 1999. Development and organization of ocular dominance bands in primary visual cortex of the sable ferret. J. Comp. Neurol 407:151–65 [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. 1999. Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci 22:389–442 [DOI] [PubMed] [Google Scholar]
- Shatz CJ. 1983. The prenatal development of the cat’s retinogeniculate pathway. J. Neurosci 3:482–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ. 1990. Impulse activity and the patterning of connections during CNS development. Neuron 5:745–56 [DOI] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. 1978. Ocular dominance in layer IV of the cat’s visual cortex and the effects of monocular deprivation. J. Physiol 281:267–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. 1988. Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents. Science 242:87–89 [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, et al. 1999. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 284:1811–16 [DOI] [PubMed] [Google Scholar]
- Sin WC, Haas K, Ruthazer ES, Cline HT. 2002. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature 419:475–80 [DOI] [PubMed] [Google Scholar]
- Sirevaag AM, Greenough WT. 1987. Differential rearing effects on rat visual cortex synapses. III. Neuronal and glial nuclei, boutons, dendrites, and capillaries. Brain Res. 424:320–32 [DOI] [PubMed] [Google Scholar]
- Sretavan D, Shatz CJ. 1984. Prenatal development of individual retinogeniculate axons during the period of segregation. Nature 308:845–48 [DOI] [PubMed] [Google Scholar]
- Sretavan DW, Shatz CJ. 1986a. Prenatal development of cat retinogeniculate axon arbors in the absence of binocular interactions. J. Neurosci 6:990–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sretavan DW, Shatz CJ. 1986b. Prenatal development of retinal ganglion cell axons: segregation into eye-specific layers. J. Neurosci 6:234–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sretavan DW, Shatz CJ, Stryker MP. 1988. Modification of retinal ganglion cell axon morphology by prenatal infusion of tetrodotoxin. Nature 336:468–71 [DOI] [PubMed] [Google Scholar]
- Stepanyants A, Hof PR, Chklovskii DB. 2002. Geometry and structural plasticity of synaptic connectivity. Neuron 34:275–88 [DOI] [PubMed] [Google Scholar]
- Stettler DD, Yamahachi H, Li W, Denk W, Gilbert CD. 2006. Axons and synaptic boutons are highly dynamic in the adult visual cortex. Neuron 49:877–87 [DOI] [PubMed] [Google Scholar]
- Stryker MP, Strickland SL. 1984. Physiological segregation of ocular dominance columns depends on the pattern of afferent electrical activity. Investig. Ophthalmol. Vis. Sci 25:2786321388 [Google Scholar]
- Svoboda K, Denk W, Kleinfeld D, Tank DW. 1997. In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature 385:161–65 [DOI] [PubMed] [Google Scholar]
- Svoboda K, Helmchen F, Denk W, Tank DW. 1999. Spread of dendritic excitation in layer 2/3 pyramidal neurons in rat barrel cortex in vivo. Nat. Neurosci 2:65–73 [DOI] [PubMed] [Google Scholar]
- Svoboda K, Tank DW, Denk W. 1996. Direct measurement of coupling between dendritic spines and shafts. Science 272:716–19 [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, et al. 2002. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420:788–94 [DOI] [PubMed] [Google Scholar]
- van Versendaal D, Rajendran R, Saiepour MH, Klooster J, Smit-Rigter L, et al. 2012. Elimination of inhibitory synapses is a major component of adult ocular dominance plasticity. Neuron 74:374–83 [DOI] [PubMed] [Google Scholar]
- Villa KL, Berry KP, Subramanian J, Cha JW, Oh WC, et al. 2016. Inhibitory synapses are repeatedly assembled and removed at persistent sites in vivo. Neuron 89:756–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR, Greenough WT. 1972. Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science 176:1445–47 [DOI] [PubMed] [Google Scholar]
- Wiesel TN. 1982. Postnatal development of the visual cortex and the influence of environment. Nature 299:583–91 [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. 1963. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol 26:1003–17 [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. 1965. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J. Neurophysiol 28:1029–40 [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH, Lam DMK. 1974. Autoradiographic demonstration of ocular-dominance columns in monkey striate cortex by means of transneuronal transport. Brain Res. 79:273–79 [DOI] [PubMed] [Google Scholar]
- Witte S, Stier H, Cline HT. 1996. In vivo observations of timecourse and distribution of morphological dynamics in Xenopus retinotectal axon arbors. J. Neurobiol 31:219–34 [DOI] [PubMed] [Google Scholar]
- Wong ROL, Chernjavsky A, Smith SJ, Shatz CJ. 1995. Early functional neural networks in the developing retina. Nature 374:716–18 [DOI] [PubMed] [Google Scholar]
- Wu GY, Cline HT. 1998. Stabilization of dendritic arbor structure in vivo by CaMKII. Science 279:222–26 [DOI] [PubMed] [Google Scholar]
- Wu GY, Zou DJ, Rajan I, Cline H. 1999. Dendritic dynamics in vivo change during neuronal maturation. J. Neurosci 19:4472–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuta NH, Callaway EM. 1998. Cytochrome-oxidase blobs and intrinsic horizontal connections of layer 2/3 pyramidal neurons in primate V1. Vis. Neurosci 15:1007–27 [DOI] [PubMed] [Google Scholar]
- Yamahachi H, Marik SA, McManus JNJ, Denk W, Gilbert CD. 2009. Rapid axonal sprouting and pruning accompany functional reorganization in primary visual cortex. Neuron 64:719–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ackman JB, Xu HP, Crair MC. 2012. Visual map development depends on the temporal pattern of binocular activity in mice. Nat. Neurosci 15:298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou DJ, Cline HT. 1999. Postsynaptic calcium/calmodulin-dependent protein kinase II is required to limit elaboration of presynaptic and postsynaptic neuronal arbors. J. Neurosci 19:8909–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Yang G, Kwon E, Gan WB. 2005. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature 436:261–65 [DOI] [PubMed] [Google Scholar]


