Abstract
BACKGROUND:
De-intensification of adjuvant therapy is being considered for older women with early-stage, biologically-favorable breast cancer. Although radiation therapy (RT) can be omitted in some cases, toxicity from endocrine therapy (ET) is not trivial, and adherence rates vary. We hypothesized that adjuvant RT alone would produce comparable survival outcomes as adjuvant ET alone among elderly patients treated with lumpectomy.
METHODS:
We searched the National Cancer Database (2010–2014) for healthy women (≥70 years, Charlson/Deyo (CD) score 0–1) with T1N0 hormone-receptor–positive, HER-2-negative breast cancer treated with lumpectomy and adjuvant ET or RT. Propensity scores were used to match patients for analysis.
RESULTS:
We identified 2995 patients (median age, 78 years), most (81%) with a CD score of 0, clinical stage IA (77%), of whom 65% received ET alone and 35% RT only after lumpectomy. On multivariate analysis of the matched cohort, older age (HR 1.10 (95% CI 1.07–1.13), P<0.001) CD score 1 (HR 1.92 (95% CI 1.37–2.70), P=0.0002), and living in a metropolitan (vs. urban) area (HR 3.09 (95% CI 1.43–6.67), P=0.004) were associated with inferior OS, whereas treatment with ET (vs. RT) was not (HR 1.13, (95% CI 0.85–1.49), P=0.406). At a median follow-up of 45 months, no difference was found in OS between ET versus RT cohorts (85% and 86%, respectively; P=0.44).
CONCLUSIONS:
For healthy, older women with biologically favorable breast cancer treated with lumpectomy, adjuvant RT or ET is associated with equivalent 5-year OS rates. A randomized controlled trial is warranted to explore these adjuvant monotherapy options in elderly patients with hormone receptor-positive breast cancer.
Keywords: elderly patients, early-stage breast cancer, endocrine therapy, hormone therapy, radiation therapy, overall survival
INTRODUCTION
Breast cancer is the most common malignancy in women and predominately affects those older than 70 years.1–3 As the U.S. population continues to age, the incidence of invasive breast cancer in this population will also increase; however, gains from advances in screening, detection, and treatment of this disease have been less evident for older women.1,4–5 Surgical resection remains the cornerstone of treatment for early breast cancer for patients of any age, and several studies have examined the efficacy, morbidity, and mortality of subsequent adjuvant therapies for older patients, including endocrine therapy (ET) alone, radiation therapy (RT) alone, and combination ET+RT.1,6–9 Adding adjuvant RT to ET has consistently shown a local control benefit; however, given the biologically favorable nature of breast cancers among women diagnosed at age 70 or older, interest has been expressed in de-intensifying adjuvant treatment for breast cancer in elderly women. De-intensification remains up for debate, however, as many women in this age group are healthy, with minimal comorbid conditions, and thus may live for many years after diagnosis. In fact, the average life expectancy for women with no comorbidities (Charlson/Deyo (CD) score 0) aged 70 is 19 years and for women with low/medium comorbidities (CD score 1–2) is 16 years.10
Adjuvant ET has been shown to decrease the risk of disease recurrence by 41% and the risk of death by 34% in women with estrogen receptor (ER)–positive early-stage breast cancer. 11 ET also has the added benefit of preventing cancer, not only in the contralateral breast, but also elsewhere;12–13 however, this therapy also has the potential for negative side effects such as arthralgia and hot flashes, leading to poor patient adherence or discontinuation of the drug. On average, adherence rates for hormonal therapy range from 41% to 72%.14–17 To our knowledge, no study has directly compared survival outcomes for healthy elderly women treated with lumpectomy followed by either ET or RT alone. A patient-level microsimulation using the relative effectiveness between treatments of the NSABP B-21 trial was recently published and revealed similar findings to modern trials: ET was superior in preventing contralateral cancers and RT was superior in preventing ipsilateral breast-tumor recurrence.18 The authors concluded that adjuvant RT alone is a safe option for healthy elderly women with early stage breast cancer, therefore, providing further rationale for performing a population level analysis to validate this model.19 We thus undertook this study of the National Cancer Database (NCDB) to evaluate survival outcomes for women aged ≥70 years with hormone receptor–positive early breast cancer treated with lumpectomy followed by either adjuvant ET or RT alone.
METHODS
Patient Population
The NCDB, a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society, is a hospital-based registry that captures approximately 70% of newly diagnosed cancer cases in the United States and Puerto Rico and draws data from >1500 commission-accredited cancer programs. Originating in 1989, the NCDB now contains approximately 34 million records. Data registries contain patient characteristics, cancer stage, tumor histologic characteristics, type of first treatment administered, and outcomes. Vital status data is provided by CoC accredited hospitals, which requires a 90% annual follow-up rate for analytic patients diagnosed within the prior 5 years. The American College of Surgeons and the CoC have not verified and are not responsible for the analytic or statistical methods used, or for the conclusions drawn from these data, by the investigators of this study. The analysis was exempt from the institutional review board at our institution given its retrospective nature and the lack of identifying patient information provided in the NCDB User File.
We queried the NCDB User File for female patients, 70 years of age or older, with T1N0 hormone receptor–positive, HER-2–negative breast cancer diagnosed in 2010–2014 and treated with lumpectomy followed by adjuvant ET or RT (Fig. 1). Patients were included if they had a CD score of 0 or 1, where 0 meant no comorbid conditions and 1 included any one of the following: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatologic disease, peptic ulcer disease, mild liver disease, or diabetes. Patients could have ER+ and/or progesterone receptor–positive (PR+) disease. Exclusion criteria were receipt of chemotherapy at any time, receipt of RT to any area other than the breast or breast regional lymphatics, or receipt of both ET and RT. The lumpectomy had to have been done before ET or RT was begun, assessed by treatment start day from date of diagnosis or documentation of any neoadjuvant ET or RT. All patients had AJCC 7th Ed. clinical stage IA or IB breast cancer, with either no regional nodes examined or no nodes documented as being positive. Patients diagnosed in 2015 were excluded, as no survival data for such patients were included in the database. The start date of 2010 was the first year that data on HER-2 status had been captured in the NCDB.
Figure 1.
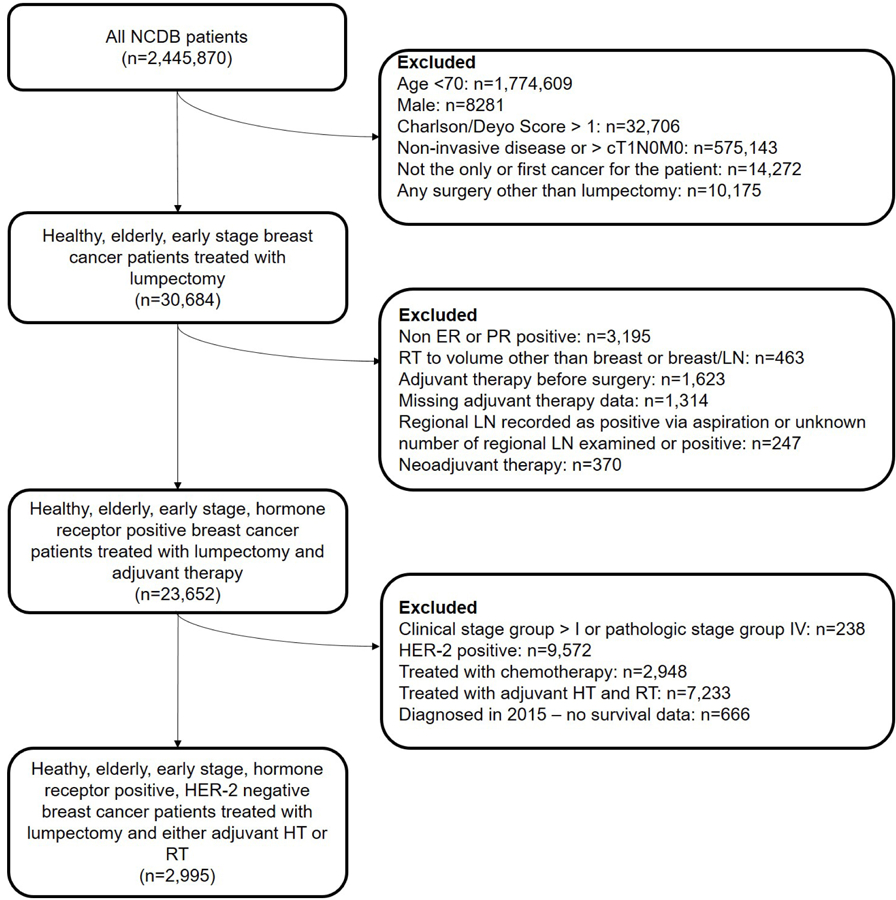
CONSORT diagram. NCDB, National Cancer Database; RT, radiation therapy; ET, endocrine therapy; LN, lymph node; ER, estrogen receptor; PR, progesterone receptor.
Statistical Analyses
We used propensity score matching to reduce the influence of selection bias on the estimates of treatment effect on overall survival (OS) from these observational data.19–21 The propensity score is the conditional probability of receiving a specific treatment (ET or RT) given a set of observed covariates. The following covariates were included in the multivariate logistic regression model (MVA) to create the propensity scores: age at diagnosis, CD score (0, 1), education level (proportion of patients within a zip code that did not graduate from high school [≥29%, 20%–28.9%, 14%–19.9%, <14%]), income (Median household income for each patient’s area of residence [<$30,000, $30,000-$34,999, $35,000-$45,999, $46,000+]) and geographic location of treatment (defined by matching the Federal Information Processing Standard county code for a patient against the U.S. Department of Agriculture Economic Research Service: metropolitan (urbanized county population of at least 50,000), urban (county population of more at least 2,500 outside of a metropolitan area), rural (county population of less than 2,500), or unknown). We identified 1:1 matched doublets, one case for each of the two treatments (RT vs. ET) by using a 5-to-1-digit greedy match algorithm.22 Absolute standardized differences were used to assess balance in the covariates between the two treatment groups, with an absolute standardized difference of <10% for the groups, suggesting a substantial and reasonable balance between the two groups.
OS time was measured from the date of diagnosis to the date of death, with OS for surviving patients right-censored at the time of last contact. The distribution of OS was estimated by the Kaplan-Meier method.23 For the non-matched cohort, log-rank tests24 were used to test differences in survival between treatment groups (RT vs. ET). Regression analyses of survival data based on the Cox proportional hazards model25 were conducted for OS in a multivariate setting. For the matched cohort, stratified log-rank tests with the matched pairs as strata were fitted to evaluate differences in OS between the treatment groups. We further adjusted for the matching factors by using double robust estimation under the Cox model.26 All tests were two-sided. P values of <0.05 were considered statistically significant. All analyses were done with SAS (version 9.4, Cary, NC) and S-plus (version 8.04, TIBCO Software Inc., Palo Alto, CA).
RESULTS
Population Characteristics
Of the 2,445,870 patients included in the obtained dataset, 2995 women were identified as meeting the inclusion criteria. The median age at diagnosis was 78 years (range 70–90 years). Most patients had a CD score of 0 (81%) and white ethnicity (91%). The majority of patients also had a median quartile income of >$46,000 (44%); lived in zip codes where <14% of the population did not graduate high school (45%); had public insurance (89%); lived in a metropolitan area (82%); and were treated at a Comprehensive Community Cancer Program (56%). Sixty-five percent of patients (n=1957) received adjuvant ET alone after lumpectomy and 35% (n=1038) received adjuvant RT alone after lumpectomy. Detailed population and treatment characteristics are summarized in Table 1.
TABLE 1.
Characteristics of healthy patients with stage I, hormone receptor positive, HER2 non-amplified breast cancer treated with adjuvant monotherapy captured in the NCDB 2010–2014.
| Value or No. of Patients (Column %) | ||||
|---|---|---|---|---|
| All Patients (n=2995) | ET only (n=1957) | RT only (n=1038) | P Valuea | |
| Age, median (range), years | 78 (70–90) | 78 (70–90) | 77 (70–90) | ≤0.0001 |
| Ethnicity | 0.2733 | |||
| White | 2729 (91) | 1774 (91) | 955 (93) | |
| Black | 164 (5) | 111 (6) | 53 (5) | |
| Other | 78 (3) | 57 (3) | 21 (2) | |
| Unknown | 24 (1) | 15 (0) | 9 (0) | |
| Charlson/Deyo Score* | 0.0005 | |||
| 0: no comorbid conditions | 2418 (81) | 1544 (79) | 874 (84) | |
| 1: single comorbid condition | 577 (19) | 413 (21) | 164 (16) | |
| Insurance Status | 0.1813 | |||
| Public | 2663 (89) | 1749 (89) | 914 (88) | |
| Private | 297 (10) | 181 (9) | 116 (11) | |
| Uninsured | 10 (0) | 8 (0) | 2 (0) | |
| Missing | 25 (1) | 19 (1) | 6 (1) | |
| Income level Quartiles | 0.0003 | |||
| <$30,000 | 253 (8) | 183 (9) | 70 (7) | |
| $30,000-$34,999 | 451 (15) | 322 (17) | 129 (12) | |
| $35,000-$45,999 | 900 (30) | 577 (30) | 323 (31) | |
| $46,000+ | 1307 (44) | 831 (43) | 467 (46) | |
| Missing | 84 (3) | 44 (2) | 40 (4) | |
| Education level† | 0.0032 | |||
| 29%+ | 312 (10) | 227 (12) | 85 (8) | |
| 20%−28.9% | 570 (19) | 382 (20) | 188 (18) | |
| 14%−19.9% | 684 (23) | 434 (22) | 250 (24) | |
| <14% | 1345 (45) | 870 (45) | 475 (46) | |
| Missing | 84 (3) | 44 (2) | 40 (4) | |
| Home location | 0.0060 | |||
| Metro | 2461 (82) | 1574 (80) | 887 (86) | |
| Urban | 411 (14) | 298 (15) | 113 (11) | |
| Rural | 53 (2) | 38 (3) | 15 (1) | |
| Unknown | 70 (2) | 47 (2) | 23 (2) | |
| Treatment Facility | 0.0352 | |||
| Academic/Research | 756 (25) | 523 (27) | 233 (22) | |
| Community Program | 572 (19) | 363 (19) | 209 (20) | |
| Comprehensive Community Program | 1667 (56) | 1071 (55) | 596 (57) | |
| Facility Location | ≤0.0001 | |||
| Midwest | 774 (26) | 529 (27) | 245 (24) | |
| Northeast | 615 (21) | 408 (21) | 207 (20) | |
| South | 1001 (33) | 677 (35) | 324 (31) | |
| West | 605 (20) | 343 (17) | 262 (25) | |
| Tumor size, median (range) mm | 14 (1–700) | 10 (1–700) | 9 (1–60) | <0.0001 |
P-value of comparison of patient’s characteristics between RT alone and ET alone
A Charlson/Deyo score of 1 represents having one of the following comorbid conditions: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatologic disease, peptic ulcer disease, mild liver disease, or diabetes.
Percentage of people in the patient’s zip code who did not graduate from high school.
Abbreviations: NCDB: National Cancer Database, ET: endocrine therapy, RT: radiation therapy.
Overall Survival Outcomes
The median follow-up time for all patients was 44.6 months. The 5-year OS for patients treated with ET alone was 82.8% and the 5-year OS for patients treated with RT alone was 86.1% (logrank P=0.09).
Propensity Matched Analysis and Outcomes
A total of 998 patients who received adjuvant ET were successfully propensity score matched with 998 patients who received adjuvant RT at a 1:1 ratio. The absolute standardized difference between groups for all variables was <10%, representing a well-matched cohort. In the matched cohort, the median age of patients was 77 years (range 70–90). Five-year OS rates were 84.6% for patients given adjuvant ET and 85.9% for those given adjuvant RT (logrank test P=0.44). Without adjusting for the other risk factors, the difference in OS between the propensity score matched RT-only and ET-only groups was not statistically significant (P=0.44, Fig. 2). After adjusting for the variables used for matching using the double robust estimation under the Cox model, the difference in OS between propensity score matched RT-only and ET-only subgroups remained insignificant (P= 0.41).
Figure 2.
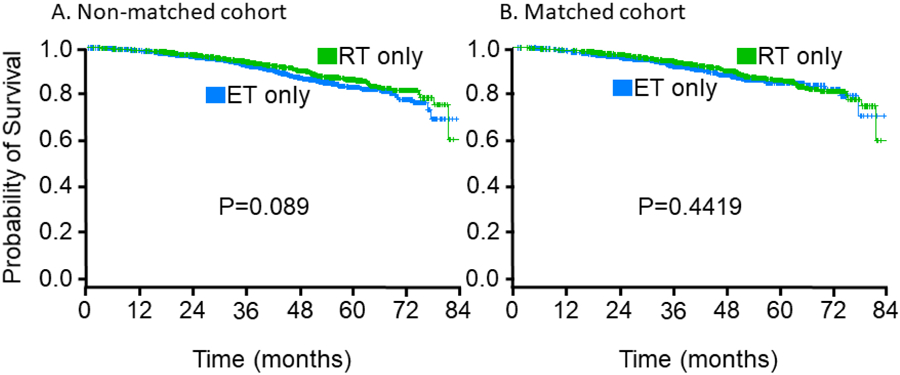
Kaplan-Meier estimates of overall survival by treatment group, in (A) all non-matched patients, and (B) patients matched for age at diagnosis, Charlson/Deyo comorbidity score, education, and geographic location. ET, endocrine therapy; RT, radiation therapy.
Multivariate Analyses
On MVA of the unmatched cohort, CD score of 1 (hazard ratio [HR] (95% confidence interval (CI)) 1.964 (1.53, 2.519), P<0.0001) and older age (HR (95% CI) 1.103 (1.081, 1.126), P<0.001) remained significantly associated with worse OS. After adjusting for age and CD score in the multivariate Cox proportional hazards model, the difference in OS between the RT-only and ET-only subgroups was not statistically significant (HR (95% CI) 0.96 (0.759, 1.213), P=0.73).
On MVA of the matched cohort, older age (HR 1.097, P<0.001) and a CD score of 1 (HR 1.922, P=0.0002) predicted worse survival, and living in an urban area (versus a metropolitan area) predicted improved survival (HR 0.323, P=0.004; Table 2). Neither income nor education predicted survival.
TABLE 2.
Multivariable analysis of factors associated with the risk of death in a propensity score matched cohort of elderly patients with early stage breast cancer treated with adjuvant ET or RT.
| Variable | Hazard Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Adjuvant Monotherapy | |||
| RT only | Reference | 0.4064 | |
| ET only | 1.125 | 0.852–1.485 | |
| Age | 1.097 | 1.069–1.125 | <0.0001 |
| Charlson/Deyo Score | 0.0002 | ||
| 0: no comorbid conditions | Reference | ||
| 1: single comorbid condition | 1.922 | 1.367–2.702 | |
| Income level | 0.3487 | ||
| <$30,000 | Reference | ||
| $30,000-$34,999 | 1.369 | 0.621–3.019 | |
| $35,000-$45,999 | 1.709 | 0.813–3.539 | |
| $46,000+ | 1.348 | 0.621–2.926 | |
| Education level | 0.9604 | ||
| <14% | Reference | ||
| 14%−19.9% | 1.014 | 0.692–1.487 | |
| 20%−28.9% | 1.012 | 0.655–1.565 | |
| 29%+ | 1.180 | 0.635–2.193 | |
| Facility rurality | 0.0139 | ||
| Urban | Reference | ||
| Metro | 3.091 | 1.434–6.665 | |
| Rural | 1.033 | 0.127–8.433 | |
| Unknown | 0.910 | 0.111–7.448 |
DISCUSSION
As the U.S. population continues to age, the optimal adjuvant treatment regimen for healthy, elderly women with early-stage breast cancer should be tailored in light of the biology of the disease, competing risks of morbidity and mortality, and a desire to optimize quality of life for this age group. Unfortunately, elderly patients are often excluded from clinical trials and are scarce in most prospective reviews.27 Strikingly, the disparity between the incident disease population and the age of cancer clinical trial participants appears to be increasing over time.
Three randomized phase III trials have particularly focused on outcomes in elderly women with early stage breast cancer, examining if RT can be omitted in the setting of ET. First, in the CALGB C9343 trial of women aged ≥70 with ER+ clinical stage I breast cancer treated by lumpectomy, patients were randomized to either adjuvant ET+RT or ET alone. This study found no difference in five year OS rates between those given ET+RT (87%) and those treated with ET alone (86%; p=0.94). Additionally, there were no differences in rates of mastectomy for local recurrence or distant metastasis (DM).28 Five-year overall survival in this study of 86–87% was comparable to the findings reported in our study, here, in the NCDB. Second, the PRIME II study evaluated women aged ≥65 years with early, low-risk breast cancer (hormone receptor–positive, N0, T1–2 up to 3 cm, and grade 3 or lymphovascular invasion [but not both]) treated with lumpectomy followed by randomization to adjuvant ET with or without RT.29 At a median follow-up of 5 years, no differences were found in regional recurrence, DM or OS. Rates of ipsilateral breast tumor recurrence (IBTR) were 1.3% in women who received RT and 4.1% in those who did not. Third, ABCSG 8A evaluated 869 postmenopausal women with favorable early breast cancer treated by lumpectomy followed by ET with or without RT and found a 5-year local relapse rate of 5.1% after ET and 0.4% after ET+RT (P=0.0001), with no difference in DM or OS.30 In sum, these studies demonstrated no survival advantage with the addition of RT to ET but consistently showed a local control benefit of adding RT to ET.
None of these studies, however, evaluated whether adjuvant RT alone was equivalent to adjuvant ET alone. In 2002, NSABP B-21 attempted to answer this question in all age groups and reported results after lumpectomy and adjuvant ET alone, RT alone, or ET+RT for women with ≤1 cm, node-negative breast cancer, the majority of which were HR positive. Only 16% of the study cohort included women 70 years or older. The addition of RT reduced the cumulative incidence of IBTR compared to that of ET alone (16.5% for ET alone, 9.3% for RT+placebo, and 2.8% for ET+RT). 6 Notably, there was no difference in the three arms in either DM DFS or OS with ET alone or RT alone, similar to the findings of our study.
Current clinical guidelines recommend at least 5 years of treatment with adjuvant ET to prevent recurrence and improve survival.31 However, the toxicity of ET is not trivial, particularly for older women,32–39 and reported adherence rates range from 41% to 88% for tamoxifen and 50% to 72% for aromatase inhibitors.14–17 Prior studies suggest that long-term adherence rates for ET are highest for women aged 51–69, but decline among women over 70.33–34
In contrast, RT for breast cancer is generally well tolerated by most patients and does not significantly impair their daily activities. Acute side effects, the most common being skin reactions (10%), are self-limiting and resolve within 4–6 weeks after treatment. Forms of late toxicity may include moderate to marked persistent breast edema (1–11%), change in breast skin appearance (6–23%) or fibrosis (7–38%).40–41 Moreover, adherence rates are high at 98%–99% 39–40 and treatment time ranges from 1 to 5 weeks.41
One of the goals of ET is to reduce not only the risk of local recurrence, but also the risk of contralateral breast cancer.6,43 However, with increasing age, the incidence of a contralateral breast cancer appears lower than that of patients with breast cancer diagnosed at younger ages.44–47 Thus, the potential benefit of ET monotherapy over RT may be reduced in older women. Theoretically, ET may also have a benefit of decreasing DM, but in biologically favorable early-stage breast cancer, the risk of DM is low at 1.5% to 3.3% and does not seem to be affected by adjuvant RT as opposed to adjuvant ET (P=0.28)6; indeed, among patients aged ≥70 in NSABP B-21, tumor recurrence rates were similar for those given RT versus ET, and no difference was found in OS (93%−94%, P=0.93) 6.
In our large retrospective analysis of healthy older women with hormone receptor–positive, HER-2 negative disease, adjuvant ET and adjuvant RT were both associated with outstanding outcomes, with median survival time not yet reached at a median follow-up time of 44.6 months and no differences in OS rates. These findings make the option of adjuvant RT alone worth investigating further, especially considering continued advances in hypofractionated and partial-breast irradiation enabling increasingly shortened treatment times for adjuvant RT. Therefore, RT alone may be a useful approach given the real-world risks of noncompliance with or discontinuation of ET for such patients.
The NCDB provides a valuable tool for studying large, real-world cohorts and for seeking answers to questions that are unlikely to be studied in prospective randomized clinical trials. Also, elderly patients are not well represented in clinical trials in oncology, and as such the NCDB is particularly valuable for understanding oncologic outcomes in this sizeable proportion of patients. For example, the NSABP B-14 trial of adjuvant ET excluded women aged ≥70,12 and only 16% of the 1000 women in NSABP B-21 were aged ≥70.6 The current study of 2995 healthy woman with breast cancer diagnosed at an advanced age is unlikely to be replicated in such large numbers prospectively. Nevertheless, the nature of the NCDB carries inherent limitations arising from potential miscoding of variables, undetectable selection bias, absence of recurrence data, and missing data for some variables, specifically adherence to either regimen (ET or RT). We restricted analysis to patients confirmed to have clinical node negative disease which did not require pathologic evaluation of the axilla, and our findings are not applicable to those patients with pathologic node positive disease. Of the patients included in our study, the vast majority (86%, 2563 patients) underwent a biopsy or aspiration of a regional lymph node, 14% underwent no lymph node surgery, and only 86 patients (2.9%) had pathologically confirmed lymph node involvement. Additionally, a median follow up time of less than 5 years in patients with early stage, hormone receptor positive breast cancer may be premature, as differences may not become apparent until longer follow-up.12 HER-2 status was included in the NCDB starting in 2010, which limited the numbers of patients for this study, as well as the duration of follow up. Finally, the NCDB provides information only on all-cause survival, but not progression-free survival, cancer-specific survival, or local recurrence.
In conclusion, this analysis of the NCDB suggests that healthy elderly women with hormone receptor–positive, HER-2–negative disease treated with lumpectomy followed by adjuvant ET or RT do very well with either type of adjuvant therapy. The availability of short radiation treatment times, with low toxicities, may be considered for such patients in light of the relatively higher risk of toxicity from ET, which could lead to non-adherence with or outright discontinuation of that therapy.
Acknowledgements:
Special thanks to Chistine Wogan for editorial assistance.
Funding: Cancer Center Support (Core) Grant P30 CA016672 from the National Cancer Institute, National Institutes of Health, provided biostatistics support.
Conflict of Interest: SFS has a grant from Varian for work unrelated to this research. Other authors report no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gandhi S, Verma S. Early breast cancer in the older woman. Oncologist 2011;16:479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016–2017. CA Cancer J Clin 2016;66:271–289. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer Facts and Figures 2019. Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf Accessed July 25, 2019.
- 5.Lodi M, Scheer L, Reix N, et al. Breast cancer in elderly women and altered clinico-pathological characteristics: a systematic review. Breast Cancer Res Treat 2017;166:657–668. [DOI] [PubMed] [Google Scholar]
- 6.Fisher B, Bryant J, Dignam JJ, et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol 2002; 20:4141–4149. [DOI] [PubMed] [Google Scholar]
- 7.Fyles AW, McCready DR, Manchul LA, et al. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med 2004; 351:963–970. [DOI] [PubMed] [Google Scholar]
- 8.Blamey RW, Bates T, Chetty U, et al. Radiotherapy or tamoxifen after conserving surgery for breast cancers of excellent prognosis: British Association of Surgical Oncology (BASO) II trial. Eur J Cancer 2013;49:2294–2302. [DOI] [PubMed] [Google Scholar]
- 9.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long term follow-up of CALGB 9343. J Clin Oncol 2013;31:2382–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho H, Klabunde CN, Yabroff KR, et al. Comorbidity-adjusted life expectancy: a new tool to inform recommendations for optimal screening strategies. Ann Intern Med 2013;159:667–676. [DOI] [PubMed] [Google Scholar]
- 11.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15 year survival: an overview of the randomized trials. Lancet 2005;365(2):1687–1717. [DOI] [PubMed] [Google Scholar]
- 12.Fisher B, Costantino J, Redmond C, et al. : A randomized clinical trial evaluating tamoxifen in the treatment of patients with node negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med 1989;320:479–484. [DOI] [PubMed] [Google Scholar]
- 13.Van’tVeer LJ, Yau C, Yu NY, et al. Tamoxifen therapy benefit for patients with 70-gene signature high and low risk. Breast Cancer Res Treat 2017;166(2):593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pourcelot C, Orillard E, Nallet G et al. Adjuvant hormonal therapy for early breast cancer: an epidemiologic study of medication adherence. Breast Cancer Res Treat 2018;169:153. [DOI] [PubMed] [Google Scholar]
- 15.Murphy CC, Bartholomew LK, Carpentier MY, et al. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat 2012;134:459–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verbrugghe M, Verhaeghe S, Lauwaert K et al. (2013) Determinants and associated factors influencing medication adherence and persistence to oral anticancer drugs: a systematic review. Cancer Treat Rev 2013;39:610–621. [DOI] [PubMed] [Google Scholar]
- 17.Puts MTE, Tu HA, Tourangeau A et al. (2014) Factors influencing adherence to cancer treatment in older adults with cancer: a systematic review. Ann Oncol 2014;25:564–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward MC, Vicini F, Chadha M, Pierce L, Recht A, Hayman J, Thaker NG, Khan A, Keisch M, Shah C, Radiation Therapy without Endocrine Therapy for Women Age 70 or Above with Low-Risk Early Breast Cancer: A Microsimulation, IJROBP (2019), doi: 10.1016/j.ijrobp.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med 2007;26(4):734–753. [DOI] [PubMed] [Google Scholar]
- 20.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med 2008;27(12):2037–2049. [DOI] [PubMed] [Google Scholar]
- 21.Dõagostino RB. Tutorial in biostatistics propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 22.Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques Proceedings of the 26th Annual SAS Users Group International Conference, Cary, NC: SAS Institute Inc., 2001. Available at ETtp://www2.sas.com/proceedings/sugi26/p214-26.pdf. [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimator from incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 24.Mantel N Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 60:163–170, 1966. [PubMed] [Google Scholar]
- 25.Cox DR. Regression models and life tables (with discussion). J Royal Stat Soc B 1972;34:187,220, 197. [Google Scholar]
- 26.Ho I, King S. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Political Analysis. 2007;15(3):199–236. [Google Scholar]
- 27.Ludmir EB, Mainwaring W, Lint TA, et al. Factors Associated with Age Disparities Among Cancer Clinical Trial Participants. JAMA Oncol, 2019. June 3 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 28.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus Tamoxifen with or without Irradiation in Women 70 Years of Age or Older with Early Breast Cancer. NEJM 2004;351(10):971–977. [DOI] [PubMed] [Google Scholar]
- 29.Kunkler IH, Williams L, Jack WJL, Cameron DA, Dixon JM. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomized controlled trial. Lancet 2015;16(3):266–273. [DOI] [PubMed] [Google Scholar]
- 30.Potter R, Gnant M, Kwasny W, et al. Lumpectomy plus tamoxifen or anastrozole with or without whole breast irradiation in women with favorable early breast cancer. Int J Radiat Oncol Biol Phys 2007;68(2):334–340. [DOI] [PubMed] [Google Scholar]
- 31.Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst 1996;88(21):1529–1542. [DOI] [PubMed] [Google Scholar]
- 32.Milata JL, Otte JL, Carpenter JS. Oral endocrine therapy nonadherence, adverse effects, decisional support, and decisional needs in women with breast cancer. Cancer Nurs 2018;41(1):E9–E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jinih M, Relihan N, Corrigan MA, O’Reilly S, Redmond HP. Extended adjuvant endocrine therapy in breast cancer: evidence and update—a review. Breast J 2017;23(6):694–705. [DOI] [PubMed] [Google Scholar]
- 34.Brett J, Fenlon D, Boulton M, et al. Factors associated with intentional and unintentional non-adherence to adjuvant endocrine therapy following breast cancer. Eur J Cancer Care 2018;27(1):e12601. [DOI] [PubMed] [Google Scholar]
- 35.Karmakar M, Pinto SL, Jordan TR, Mohamed I, Holiday-Goodman M. Predicting adherence to aromatase inhibitor therapy among breast cancer survivors: an application of the protection motivation theory. Breast Cancer (Auckl) 2017;11:1178223417694520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amir E, Seruga B, Niraula S, et al. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst 2011;103:1299–1309. [DOI] [PubMed] [Google Scholar]
- 37.Gallicchio L, Calhoun C, Helzlsouer K. A prospective study of aromatase inhibitor therapy initiation and self-reported side effects. Support Care Cancer. 2017;25(9):2697–2705. [DOI] [PubMed] [Google Scholar]
- 38.Tinari N, Fanizza C, Romero M, et al. Identification of subgroups of early breast cancer patients at high risk of nonadherence to adjuvant hormone therapy: results of an Italian survey. Clin Breast Cancer. 2015;15(2):e131–e137. [DOI] [PubMed] [Google Scholar]
- 39.Hopwood P, Haviland JS, Sumo G, Mills J, Bliss JM, Yarnold JR. Comparison of patient reported breast, arm, and shoulder symptoms and body image after radiotherapy for early breast cancer: 5-year follow up in the randomized Standardization of Breast Radiotherapy (START) trials. Lancet Oncol 2001;11:231–240. [DOI] [PubMed] [Google Scholar]
- 40.Bhattacharya IS, Haviland JS, Kirby AM, et al. Patient reported outcomes over 5 years after whole or partial breast radiotherapy: longitudinal analysis of the IMPORT LOW (CRUK/06/003) phase III randomized controlled trial. J Clin Oncol 2018(37):305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Badakhshi H, Gruen A, Sehouli J, Budach V, Boehmer D. The impact of patient compliance with adjuvant radiotherapy: a comprehensive cohort study. Cancer Med 2013;2(5):712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 1998;351(9114)1451–1467. [PubMed] [Google Scholar]
- 43.Akdeniz D, Schmidt MK, Seynaeve CM, et al. Risk factors for metachronous contralateral breast cancer: a systematic review and meta-analysis. Breast 2018;44:1–14. [DOI] [PubMed] [Google Scholar]
- 44.Adami HO, Bergström R, Hansen J. Age at first primary as a determinant of the incidence of bilateral breast cancer. Cumulative and relative risks in a population-based case-control study. Cancer. 1985;55(3):643–647. [DOI] [PubMed] [Google Scholar]
- 45.Harvey EB, Brinton LA. Second cancer following cancer of the breast in Connecticut, 1935–82. Natl Cancer Inst Monogr 1985;68:99–112. [PubMed] [Google Scholar]
- 46.Broët P, de la Rochefordière A, Scholl SM, et al. Contralateral breast cancer: annual incidence and risk parameters. J Clin Oncol 1995;13(7):1578–1583. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Thompson W, Semenciw R, Mao Y. Epidemiology of contralateral breast cancer. Cancer Epidemiol Biomarkers Prev 1999;8(10):855–861. [PubMed] [Google Scholar]


