Abstract
Objective
Tramadol has been widely used among patients with osteoarthritis (OA); however, there is paucity of information on its cardiovascular risk. We aimed to examine the association of tramadol with risk of myocardial infarction (MI) among patients with OA.
Design
Among OA patients aged 50 to 90 years without history of MI, cancer, or opioid use disorder in The Health Improvement Network database in the United Kingdom (2000-2016), three sequential propensity-score matched cohort studies were assembled, i.e., (1) patients who initiated tramadol or naproxen (negative comparator); (2) patients who initiated tramadol or diclofenac (positive comparator); and (3) patients who initiated tramadol or codeine (a commonly used weak opioid). The outcome was incident MI over six-months.
Results
Among tramadol and naproxen initiators (n=33,024 in each cohort), 77 (4.8/1000 person-years) and 46 (2.8/1000 person-years) incident MI occurred, respectively. The rate difference (RD) and hazard ratios (HR) for incident MI with tramadol initiation were 1.9 (95% confidence interval [CI] 0.6 to 2.3)/1000 person-years and 1.68 (95% CI 1.16 to 2.41) relative to naproxen initiation, respectively. Among tramadol and diclofenac initiators (n=18,662 in each cohort), 58 (6.4/1000 person-years) and 47 (5.1/1000 person-years) incident MIs occurred, respectively. The corresponding RD and HR for incident MI were 1.2 (95%CI −2.1 to 14.1)/1000 person-years and 1.24 (95%CI 0.84 to 1.82), respectively. Among tramadol and codeine initiators (n=42,722 in each cohort), 127 (6.1/1000 person-years) and 103 (5.0/1000 person-years) incident MI occurred, respectively, and the corresponding RD and HR were 1.1 (95%CI:−0.3 to 2.5)/1000 person-years and 1.23 (95%CI:0.95 to 1.60), respectively.
Conclusions
In this population-based cohort of patients with OA, the six-month risk of MI among initiators of tramadol was higher than that of naproxen, but comparable to, if not lower than, those of diclofenac or codeine.
Keywords: Osteoarthritis, Tramadol, Myocardial Infarction, Cohort
INTRODUCTION
Osteoarthritis (OA) is a leading cause of pain, disability, and socioeconomic cost worldwide1. To date, there is no effective treatment available that can halt OA progression, and the main goal of clinical management remains pain control with treatments such as oral non-steroidal anti-inflammatory drugs (NSAIDs)2. However, the safety of NSAIDs, particularly cardiovascular risk, has raised a great concern. Of the commonly used NSAIDs, diclofenac had the highest, whereas naproxen had the lowest risk of cardiovascular risk (mainly myocardial infarction [MI])3–9.
Tramadol, a weak opioid agonist, is a commonly used pain relief medication and is currently available in more than 100 countries10. Owing to its perceived lower risk of serious cardiovascular adverse effects than NSAIDs11–13, as well as a lower risk of addiction and respiratory depression compared with traditional opioids14, 15, tramadol has been considered a reasonable option for treatment of many pain conditions, e.g., OA. The use of tramadol among patients with OA has been increasing rapidly around the world16, 17. For example, the use of tramadol for management of knee OA doubled from 5% in 2003 to 10% in 2009 in the United States16, and the prevalence of patients with OA with prescriptions for tramadol increased from 3.4% to 9.8% between 2000 and 2015 in the United Kingdom (UK)17.
Tramadol inhibits the reuptake of serotonin18, 19, a crucial mediator of platelet aggregation in vascular homeostasis and thrombosis20. Tramadol has been frequently associated with the serotonin syndrome but codeine has not 21. In addition, tramadol has been showed to increase the free plasma concentration of serotonin22, and an elevated plasma serotonin is a common feature of cardiovascular disease often associated with enhanced platelet activation and thrombosis23. To date, there is paucity of information on the risk of cardiovascular diseases with tramadol use24–27. Results from two randomized controlled trials (tramadol versus nonuse or placebo) were inconclusive owing to the relatively short follow-up time (ranging from 1 to 42 days) and small number of participants (ranging from 31 to 64 in each arm)24, 25. Of two observational studies that compared tramadol with either non-users or users of other opioids, neither found that tramadol use increased risk of cardiovascular disease26, 27. Nevertheless, our recent population-based cohort study of patients with OA reported a higher mortality rate from cardiovascular diseases among initiators of tramadol than initiators of several commonly used NSAIDs (e.g., naproxen and diclofenac). However, because of relatively small number of deaths from each specific cause, most studies were lack of power to detect clinically meaningful association17.
To address this knowledge gap, we conducted three population-based cohort studies among patients with OA to compare the risk of incident MI, a major cardiovascular disease and a leading cause of morbidity and mortality worldwide, among initiators of tramadol with initiators of two commonly used NSAIDs, i.e., naproxen (negative comparator)3–8 and diclofenac (positive comparator)4–9, respectively, as well as with initiators of codeine, one of the most commonly used weak opioids. With this design, the potential selection bias and indication bias, if it occurred, could be minimized.
METHODS
Data Source
The Health Improvement Network (THIN) is an electronic medical record database derived from the records of general practitioners (GPs) in the UK. THIN contains health information on approximately 17 million patients from 770 general practices in the UK. Health care information is recorded on site at each practice and includes socio-demographics, anthropometrics, lifestyle factors, details from GP visits, diagnoses from specialists’ referrals and hospital admissions, as well as results of laboratory tests. The Read classification system is used to code specific diagnoses28, and a drug dictionary based on data from the Multilex classification system is used to code drugs29. THIN is a population-based cohort representative of the UK general population since individuals in the UK are required to be registered with a GP, regardless of health status. THIN data reflect a routine medical practice environment and have been shown to be valid for use in clinical and epidemiological research studies30.
Study Design and Cohort Definition
Eligible participants consisted of those who were aged 50 to 90 years old with history of OA based on Read codes between January 2000 and December 2016 who had not been prescribed tramadol or its active comparator (naproxen, diclofenac, or codeine) one year before entering the study cohort. Participants with history of MI, cancer, or opioid use disorder before study entry were ineligible for the current analysis (Codes lists for OA, MI, tramadol, naproxen, diclofenac and codeine were available in Supplement).
We conducted three sequential propensity-score matched cohort studies to compare the risk of incident MI among tramadol initiators with that among initiators of naproxen, diclofenac, or codeine, respectively. For example, to compare the risk of incident MI between tramadol initiators and naproxen initiators, eligible participants were required to be prescribed neither tramadol nor naproxen one year before entering the study. The date of initiation of tramadol and naproxen was considered as the index date for the corresponding participant. We divided calendar time into 17 one-year blocks from January 2000 to December 2016. Within each time block propensity-score for tramadol initiation was calculated for each participant using logistic regression. The variables included in the model were sociodemographic factors (i.e., age at index date, sex, Townsend Deprivation Index31), body mass index (BMI), lifestyle factors (i.e., alcohol use, smoking status), OA site, OA duration, comorbidities prior to the index date, medication use prior to the index date, and healthcare utilization during the past one year before the index date (see Table 1). Within each time block, each tramadol initiator was matched to one naproxen initiator using a greedy matching algorithm. We took the same approach to assemble another two cohort studies, i.e., initiators of tramadol vs. initiators of diclofenac, and initiators of tramadol vs. initiators of codeine.
Table 1.
Basic Characteristics of Propensity-score Matched Patients with Osteoarthritis
| Tramadol vs. Naproxen | Tramadol vs. Diclofenac | Tramadol vs. Codeine | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Tramadol (n=33,024) | Naproxen (n=33,024) | Standardized differences | Tramadol (n=18,662) | Diclofenac (n=18,662) | Standardized differences | Tramadol (n=42,722) | Codeine (n=42,722) | Standardized differences | |
| Demographics | |||||||||
| Age, mean (SD), y | 68.3 (9.6) | 68.4 (9.4) | 0.010 | 69.5 (9.7) | 69.7 (9.5) | 0.023 | 70.1 (9.6) | 70.1 (9.7) | 0.002 |
| Socioeconomic deprivation index, mean (SD)† | 2.7 (1.4) | 2.7 (1.4) | 0.007 | 2.8 (1.4) | 2.9 (1.4) | 0.008 | 2.7 (1.4) | 2.7 (1.3) | 0.001 |
| Female (%) | 61.8 | 62.4 | 0.013 | 64.5 | 65.5 | 0.021 | 64.0 | 64.2 | 0.004 |
| BMI, mean (SD), kg/m2 | 29.0 (5.8) | 29.0 (5.7) | 0.005 | 28.6 (5.7) | 28.7 (5.6) | 0.005 | 28.6 (5.6) | 28.6 (5.5) | <0.001 |
| OA site (%) | |||||||||
| Knee OA | 25.0 | 25.0 | 0.001 | 22.1 | 22.3 | 0.005 | 25.8 | 25.6 | 0.005 |
| Hip OA | 12.9 | 12.9 | 0.001 | 12.5 | 12.7 | 0.006 | 13.4 | 13.3 | 0.004 |
| Hand OA | 5.3 | 5.0 | 0.015 | 4.3 | 4.1 | 0.011 | 5.4 | 5.4 | 0.001 |
| OA duration, mean (SD), y | 7.8 (7.3) | 7.9 (7.3) | 0.002 | 7.6 (7.4) | 7.6 (7.6) | 0.001 | 8.3 (7.6) | 8.3 (7.4) | 0.002 |
| Lifestyle factors | |||||||||
| Alcohol (%) | 0.006 | 0.005 | 0.007 | ||||||
| None | 20.3 | 20.5 | 23.2 | 23.4 | 21.6 | 21.4 | |||
| Past | 2.7 | 2.8 | 2.5 | 2.5 | 2.9 | 2.9 | |||
| Current | 76.9 | 76.7 | 74.2 | 74.1 | 75.4 | 75.7 | |||
| Smoking (%) | 0.007 | 0.009 | 0.004 | ||||||
| None | 53.1 | 52.8 | 53.5 | 53.3 | 54.7 | 54.5 | |||
| Past | 32.6 | 32.8 | 31.3 | 31.2 | 32.7 | 32.9 | |||
| Current | 14.2 | 14.4 | 15.1 | 15.5 | 12.6 | 12.5 | |||
| Comorbidity (%) | |||||||||
| Peptic ulcer | 6.3 | 6.1 | 0.008 | 8.7 | 8.5 | 0.009 | 7.4 | 7.5 | 0.002 |
| Chronic kidney disease | 10.3 | 10.2 | 0.002 | 7.2 | 7.0 | 0.008 | 10.9 | 10.7 | 0.005 |
| Stroke | 3.3 | 3.2 | 0.006 | 3.7 | 3.8 | 0.003 | 4.3 | 4.5 | 0.008 |
| Diabetes | 14.5 | 14.5 | 0.001 | 13.4 | 13.7 | 0.010 | 14.9 | 14.9 | 0.002 |
| Hypertension | 50.0 | 50.3 | 0.006 | 50.9 | 51.7 | 0.017 | 52.9 | 52.8 | 0.002 |
| Liver disease | 2.4 | 2.4 | <0.001 | 2.4 | 2.4 | 0.004 | 2.6 | 2.6 | 0.001 |
| Transient ischemic attack | 3.1 | 3.0 | 0.005 | 3.8 | 3.6 | 0.010 | 4.0 | 4.1 | 0.003 |
| Ischemic heart disease | 9.9 | 9.7 | 0.004 | 12.9 | 12.8 | 0.002 | 12.9 | 12.6 | 0.006 |
| Congestive heart failure | 1.9 | 1.7 | 0.011 | 3.2 | 2.9 | 0.013 | 3.5 | 3.5 | <0.001 |
| Angina | 7.4 | 7.3 | 0.005 | 9.9 | 9.9 | <0.001 | 9.8 | 9.6 | 0.007 |
| Peripheral vascular disease | 1.5 | 1.4 | 0.011 | 2.1 | 1.9 | 0.012 | 1.8 | 1.9 | 0.004 |
| Venous thromboembolism | 3.4 | 3.5 | 0.003 | 3.9 | 3.7 | 0.010 | 4.4 | 4.4 | 0.001 |
| Pneumonia or infection | 7.2 | 7.2 | 0.002 | 7.0 | 6.8 | 0.007 | 8.0 | 7.9 | 0.004 |
| Hyperlipidaemia | 17.0 | 17.3 | 0.007 | 15.5 | 15.9 | 0.010 | 16.9 | 16.8 | 0.003 |
| Dementia | 0.9 | 0.9 | 0.004 | 0.8 | 0.8 | 0.001 | 1.2 | 1.3 | 0.006 |
| Varicose veins | 12.8 | 12.7 | 0.003 | 12.5 | 12.7 | 0.005 | 13.8 | 13.8 | 0.001 |
| Other circulatory disease | 30.3 | 30.1 | 0.004 | 29.1 | 29.2 | 0.001 | 34.0 | 33.8 | 0.003 |
| Depression | 14.7 | 14.9 | 0.006 | 14.1 | 14.0 | 0.004 | 14.0 | 14.0 | 0.001 |
| Chronic obstructive pulmonary disease | 5.4 | 5.3 | 0.002 | 6.2 | 6.0 | 0.007 | 6.2 | 6.2 | 0.003 |
| Atrial fibrillation | 4.1 | 3.7 | 0.020 | 4.7 | 4.3 | 0.019 | 7.0 | 6.9 | 0.002 |
| Anxiety | 16.2 | 16.2 | 0.001 | 15.3 | 15.5 | 0.007 | 16.2 | 16.2 | <0.001 |
| Seizure | 0.5 | 0.5 | 0.001 | 0.5 | 0.4 | 0.001 | 0.5 | 0.5 | 0.006 |
| Sleep disorder or sleep apnea | 1.9 | 1.9 | 0.004 | 1.5 | 1.5 | 0.007 | 1.8 | 1.8 | 0.001 |
| Rheumatoid arthritis | 2.1 | 2.0 | 0.002 | 2.2 | 2.1 | 0.006 | 2.5 | 2.5 | <0.001 |
| Medication (%) | |||||||||
| Other NSAIDs# | 85.0 | 85.1 | 0.003 | 70.3 | 71.7 | 0.031 | 86.4 | 86.2 | 0.006 |
| Other opioids# | 32.8 | 33.1 | 0.006 | 30.3 | 30.4 | 0.002 | 15.6 | 15.7 | 0.001 |
| Aspirin | 33.2 | 33.3 | 0.003 | 34.1 | 34.5 | 0.008 | 36.3 | 36.3 | <0.001 |
| ACE inhibitors | 34.0 | 34.1 | 0.001 | 32.2 | 32.3 | 0.002 | 36.7 | 36.6 | 0.002 |
| Calcium channel blockers | 32.4 | 33.0 | 0.013 | 32.9 | 33.2 | 0.005 | 35.9 | 35.6 | 0.006 |
| Angiotensin receptor blocker | 13.5 | 13.4 | 0.002 | 12.3 | 12.3 | <0.001 | 13.6 | 13.6 | <0.001 |
| Beta receptor inhibitor | 33.4 | 33.4 | <0.001 | 31.8 | 32.3 | 0.011 | 35.9 | 35.9 | 0.001 |
| Statins | 41.0 | 41.5 | 0.010 | 35.6 | 35.9 | 0.006 | 39.9 | 39.8 | 0.002 |
| Loop diuretics | 16.2 | 16.1 | 0.001 | 20.0 | 19.6 | 0.012 | 20.8 | 20.9 | 0.003 |
| Thiazide diuretics | 36.8 | 37.1 | 0.005 | 38.9 | 39.4 | 0.010 | 39.9 | 40.0 | 0.002 |
| Potassium-sparing diuretics | 6.8 | 6.9 | <0.001 | 9.4 | 9.3 | 0.001 | 9.0 | 8.9 | 0.002 |
| Glucocorticoids | 22.6 | 22.7 | 0.003 | 21.1 | 21.2 | 0.001 | 24.2 | 24.0 | 0.003 |
| Nitrates | 11.3 | 11.1 | 0.007 | 12.9 | 12.9 | 0.002 | 13.5 | 13.5 | 0.001 |
| Antidiabetic medicine | 10.4 | 10.4 | <0.001 | 9.8 | 10.0 | 0.005 | 10.7 | 10.6 | 0.002 |
| Anticoagulants | 5.4 | 5.2 | 0.012 | 5.9 | 5.4 | 0.025 | 8.8 | 8.7 | 0.004 |
| Benzodiazepines | 36.1 | 36.6 | 0.011 | 33.5 | 33.8 | 0.007 | 35.5 | 35.3 | 0.004 |
| SSRI | 24.0 | 24.0 | 0.001 | 20.7 | 20.6 | 0.002 | 22.1 | 22.1 | 0.002 |
| SNRI | 6.5 | 6.6 | 0.004 | 5.2 | 5.3 | 0.005 | 5.7 | 5.7 | <0.001 |
| Antiepileptic medicine | 9.1 | 9.3 | 0.005 | 6.9 | 6.9 | <0.001 | 8.5 | 8.5 | <0.001 |
| PPIs | 57.0 | 56.8 | 0.003 | 50.1 | 50.3 | 0.002 | 53.7 | 53.4 | 0.005 |
| H2 blockers | 23.1 | 23.3 | 0.005 | 24.8 | 24.7 | 0.002 | 25.6 | 25.5 | 0.003 |
| Healthcare utilization, mean (SD) | |||||||||
| Hospitalizations‡ | 0.4 (0.8) | 0.4 (0.9) | 0.024 | 0.3 (0.8) | 0.3 (0.9) | 0.030 | 0.5 (1.0) | 0.5 (1.1) | 0.002 |
| General practice visits‡ | 6.9 (5.6) | 6.9 (5.8) | 0.006 | 7.0 (6.0) | 7.0 (6.2) | 0.005 | 7.7 (6.8) | 7.7 (6.5) | 0.006 |
| Specialist referrals‡ | 0.7 (1.1) | 0.7 (1.1) | 0.005 | 0.5 (0.9) | 0.5 (0.9) | 0.003 | 0.7 (1.0) | 0.7 (1.1) | 0.003 |
| Propensity score (SD) | 0.5 (0.2) | 0.5 (0.2) | 0.002 | 0.4 (0.2) | 0.4 (0.2) | 0.002 | 0.6 (0.1) | 0.6 (0.1) | 0.009 |
OA, osteoarthritis; BMI, body mass index; n, number; y, years; SD, standard deviation; NSAID, non-steroidal anti-inflammatory drug; ACE, angiotensin converting enzyme; SSRI, Selective serotonin reuptake inhibitor; SNRI, Serotonin-norepinephrine reuptake inhibitor; PPIs, proton pump inhibitors; H2 blockers, histamine-2 blockers.
The Socio-Economic Deprivation Index (i.e., Townsend Deprivation Index) was grouped into quintiles from 1 (least deprived) to 5 (most deprived).
Other NSAIDs or opioids means other NSAIDs or opioids use prior to the index date.
Frequency during the past one year.
Assessment of Outcome
The outcome was incident MI (including fatal and non-fatal MI) within the first six months after initiation of tramadol or its comparative medication26. MI was identified using Read codes. Previous studies have used this approach to define MI8,32,33 and demonstrated a high confirmation rate (i.e., 95%)32.
Statistical Analysis
The baseline characteristics of the tramadol cohort were compared with the naproxen, diclofenac, and codeine cohorts, respectively. For each subject, person-years of follow-up were calculated as the amount of time from the index date to the first of the following events: incident MI, disenrollment from a GP practice participating in THIN, death, or the end of six month follow-up period. We calculated the risk of incident MI for each cohort and plotted cumulative incidence curves while accounting for competing risk of death34. The absolute rate difference (RD) in MI was estimated between the tramadol cohort with each of the comparison cohorts using the following formula: RD = rate (tramadol) - rate (comparison); where a and b refer to the number of events in each cohort, and PTa and PTb refer to the total person-time accumulated in each cohort, and 95% CI: RD ± 1.96*SERD. We applied cause-specific Cox proportional hazard models adjusting for propensity score to obtain the hazard ratio (HR) of incident MI for the tramadol cohort related to each of its comparator cohorts accounting for competing risk of death34. We used the “COVSANDWICH” statement in the PROC PHREG procedure in SAS to account for the correlation in the matched pair35. We tested the proportional hazards assumption by using the Kolmogorov supremum test36.
Four sensitivity analyses were performed to assess the robustness of our study findings. First, we conducted an “as-treated” analysis to account for non-adherence of medications under investigation. Specifically, we censored the follow-up at the time when participants either changed (e.g., switching from tramadol to naproxen or vice versa, when comparing tramadol with naproxen) or discontinued (i.e., no prescription refill for the respective class of medication with a period of over 60 days37) their initiated medication. Second, we performed an analysis among participants whose OA was diagnosed during the study period (i.e., incident OA) to minimize potential misclassification of the duration of OA. Third, since individuals with missing values (i.e., BMI, alcohol use, smoking status, and Townsend Deprivation Index) were not included in our primary analyses, we used a sequential regression method to impute missing values for these four variables based on a set of covariates as predictors. To minimize random error, we imputed five datasets, calculating effect estimates from each imputed dataset, and using PROC MIANALYZE in SAS to combine the results from the five datasets to generate average estimates and their confidence intervals (CIs)38. Fourth, since approximately half of the eligible participants were not included in the analysis after propensity-score matching, we also used the conventional covariate adjustment approach, i.e., classic multivariable Cox-proportional hazard model, to test the study hypothesis39, 40.
All P values were 2-sided and P < 0.05 was considered significant for all tests. All statistical analyses were conducted using SAS V9.4.
RESULTS
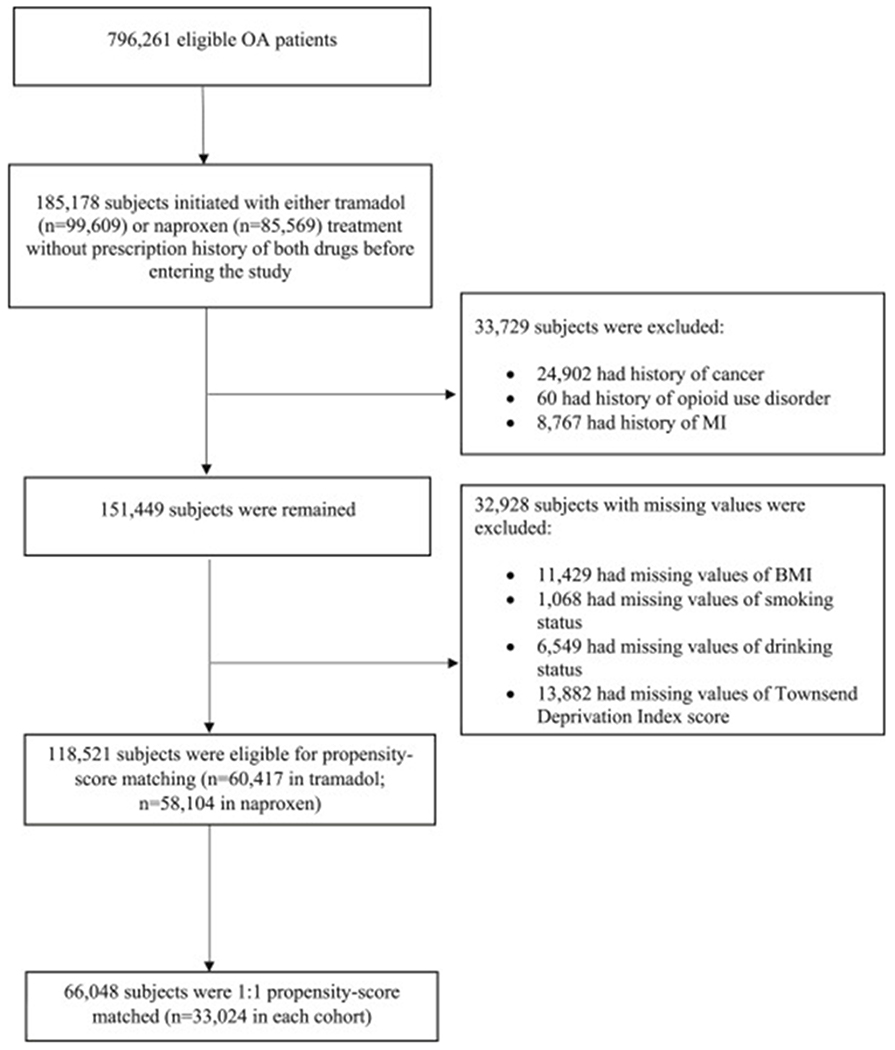
In total, 796,261 OA patients met our inclusion criteria for the comparison between tramadol and naproxen. Of them 99,609 initiated a tramadol and 85,569 initiated a naproxen, without prescription history of either drug before entering the study. We excluded 33,729 subjects who had history of MI, cancer, or opioid use disorder, and 32,928 subjects who had missing information on BMI, smoking status, alcohol drinking, or Townsend Deprivation Index Score. Of the remaining (n=118,521), 33,024 initiators of tramadol were successfully propensity-score matched to the same number of initiators of naproxen (Figure 1). Similarly, the selection process for the comparison between tramadol and diclofenac or tramadol and codeine are shown in the Supplement.
Figure 1. Selection Process of Included Subjects for the Comparison between Tramadol and Naproxen.
OA, osteoarthritis; MI, myocardial infarction; BMI, body mass index.
The baseline characteristics of each propensity-score matched cohort are shown in Table 1. The mean age of participants ranged from 68.3 to 70.1 years, and slightly more than 60% were women. Overall, the characteristics in the propensity-score matched cohorts were well-balanced, with all standardized differences < 0.1.41
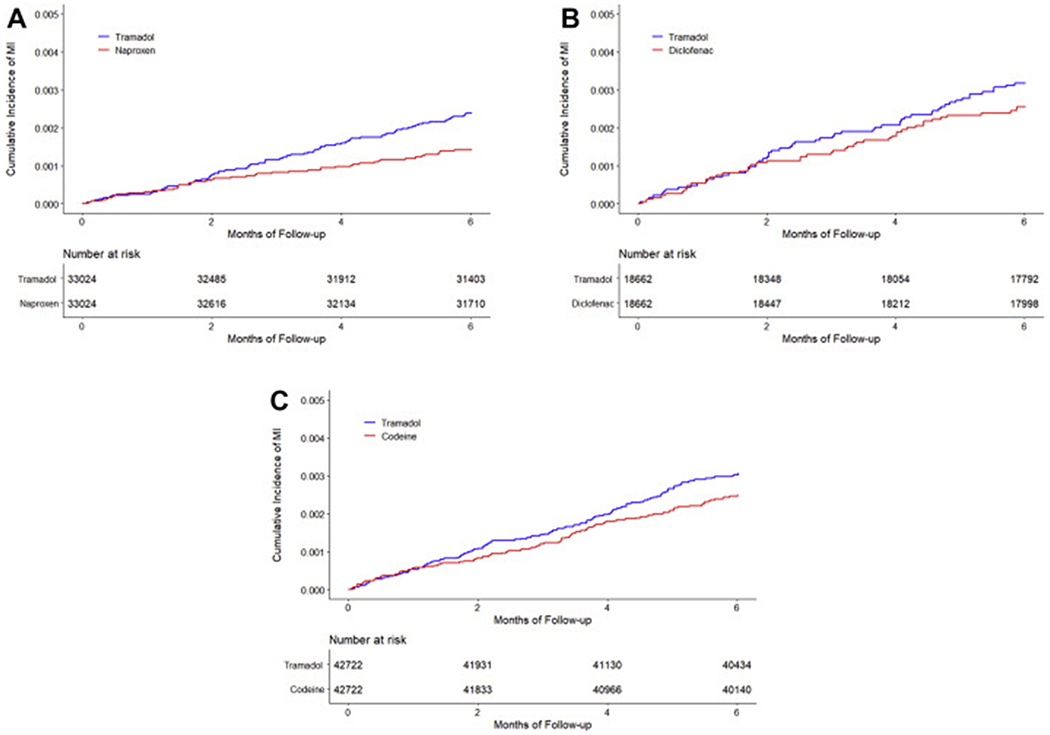
The risk of incident MI was higher in the tramadol cohort than that in the naproxen cohort (Figure 2A). As shown in Table 2, during the six months follow-up period 77 (4.8 per 1000 person-years) incident MI occurred in the tramadol cohort and 46 (2.8 per 1000 person-years) in the naproxen cohort. The RD for incident MI in the tramadol cohort was 1.9 (95% CI: 0.6 to 3.3) per 1000 person-years, compared with the naproxen cohort. The corresponding HR was 1.68 (95% CI: 1.16 to 2.41). The proportional hazard assumption was not violated (P = 0.25). Sensitivity analyses including the “as-treated” approach, restricting to participants with incident OA, missing data imputation, and the conventional covariate adjustment approach did not change the results materially (Table 2).
Figure 2. Time to Incident Myocardial Infarction for the Propensity-score Matched Cohorts of Patients with Osteoarthritis and Tramadol Initiation Comparing with Initiation of Naproxen (A), Diclofenac (B), or Codeine (C).
A, risk of MI for tramadol and naproxen were 2.4/1000 and 1.4/1000 over six-month follow-up, respectively, with corresponding risk difference of 1.0/1000 (95%CI: 0.3/1000 to 1.6/1000) over six months; B, risk of MI for tramadol and diclofenac were 3.1/1000 and 2.5/1000 over six-month follow-up, respectively, with corresponding risk difference of 0.6/1000 (95%CI: −0.2/1000 to 1.4/1000) over six months; C, risk of MI for tramadol and codeine were 3.0/1000 and 2.5/1000 over six-month follow-up , respectively, with corresponding risk difference of 0.6/1000 (95%CI: −0.2/1000 to 1.4/1000) over six months.
Table 2.
Association between Tramadol Initiation and Risk of Incident Myocardial Infarction within Six-month Follow-up Comparing with Initiation of Naproxen among Patients with Osteoarthritis
| Tramadol | Naproxen | |
|---|---|---|
| Primary analysis | ||
| Participants (n) | 33,024 | 33,024 |
| Incident myocardial infarction (n) | 77 | 46 |
| Mean follow-up (year) | 0.49 | 0.49 |
| Rate (1000 person-years)* | 4.8 | 2.8 |
| RD (1000 person-years, 95% CI) | 1.9 (0.6, 3.3) | 0.0 (reference) |
| HR (95% CI) | 1.68 (1.16, 2.41) | 1.00 (reference) |
| “As-treated” approach** | ||
| Participants (n) | 33,024 | 33,024 |
| Incident myocardial infarction (n) | 40 | 23 |
| Mean follow-up (year) | 0.25 | 0.24 |
| Rate (1000 person-years) | 4.9 | 2.9 |
| RD (1000 person-years, 95% CI) | 2.0 (0.1, 3.9) | 0.0 (reference) |
| HR (95% CI) | 1.66 (1.00, 2.76) | 1.00 (reference) |
| Incident OA patients | ||
| Participants (n) | 20,159 | 20,159 |
| Incident myocardial infarction (n) | 35 | 20 |
| Mean follow-up (year) | 0.49 | 0.49 |
| Rate (1000 person-years) | 3.6 | 2.0 |
| RD (1000 person-years, 95% CI) | 1.5 (0.1, 3.0) | 0.0 (reference) |
| HR (95% CI) | 1.75 (1.01, 3.03) | 1.00 (reference) |
| Missing data imputation | ||
| HR (95% CI) | 1.57 (1.13, 2.17) | 1.00 (reference) |
| Conventional covariate adjustment approach | ||
| HR (95% CI) | 1.59 (1.19, 2.12) | 1.00 (reference) |
RD, rate difference; HR, hazard ratio; n, number; 95% CI, 95% confidence interval; OA, osteoarthritis.
Number (rate) of competing event (i.e., death) in tramadol and naproxen cohort was 457 (28.3/1000 person-years) and 207 (12.8/1000 person-years), respectively.
82% and 85% participants discontinued or switched their initiated treatment in tramadol and naproxen cohort, respectively.
The risk of incident MI in the tramadol cohort (6.4 per 1000 person-years) was comparable to, if not lower than that in the diclofenac cohort (5.1 per 1000 person-years) (Figure 2B, Table 3). The RD of incident MI for the tramadol cohort was 1.2 (95% CI: −1.0 to 3.4) per 1000 person-years compared with the diclofenac cohort, and the corresponding HR was 1.24 (95% CI: 0.84 to 1.82). Sensitivity analyses (i.e., “as-treated” approach, restricting to participants with incident OA, missing data imputation, and the conventional covariate adjustment approach) did not change the results materially (Table 3).
Table 3.
Association between Tramadol Initiation and Risk of Incident Myocardial Infarction within Six-month Follow-up Comparing with Initiation of Diclofenac among Patients with Osteoarthritis
| Tramadol | Diclofenac | |
|---|---|---|
| Primary analysis | ||
| Participants (n) | 18,662 | 18,662 |
| Incident myocardial infarction (n) | 58 | 47 |
| Mean follow-up (year) | 0.49 | 0.49 |
| Rate (1000 person-years)* | 6.4 | 5.1 |
| RD (1000 person-years, 95% CI) | 1.2 (−1.0, 3.4) | 0.0 (reference) |
| HR (95% CI) | 1.24 (0.84, 1.82) | 1.00 (reference) |
| “As-treated” approach** | ||
| Participants (n) | 18,662 | 18,662 |
| Incident myocardial infarction (n) | 39 | 27 |
| Mean follow-up (year) | 0.25 | 0.23 |
| Rate (1000 person-years) | 8.4 | 6.2 |
| RD (1000 person-years, 95% CI) | 2.3 (−1.3, 5.8) | 0.0 (reference) |
| HR (95% CI) | 1.32 (0.81, 2.15) | 1.00 (reference) |
| Incident OA patients | ||
| Participants (n) | 9,902 | 9,902 |
| Incident myocardial infarction (n) | 26 | 20 |
| Mean follow-up (year) | 0.49 | 0.49 |
| Rate (1000 person-years) | 5.4 | 4.1 |
| RD (1000 person-years, 95% CI) | 1.3 (−1.5, 4.0) | 0.0 (reference) |
| HR (95% CI) | 1.30 (0.73, 2.33) | 1.00 (reference) |
| Missing data imputation | ||
| HR (95% CI) | 1.04 (0.73, 1.48) | 1.00 (reference) |
| Conventional covariate adjustment approach | ||
| HR (95% CI) | 1.23 (0.88, 1.73) | 1.00 (reference) |
RD, rate difference; HR, hazard ratio; n, number; 95% CI, 95% confidence interval; OA, osteoarthritis.
Number (rate) of competing event (i.e., death) in tramadol and diclofenac cohort was 370 (40.6/1000 person-years) and 205 (22.3/1000 person-years), respectively.
81% and 86% participants discontinued or switched their initiated treatment in tramadol and diclofenac cohort, respectively.
Similarly, the risk of incident MI in the tramadol cohort (6.1 per 1000 person-years) was comparable to, if not lower than that in the codeine cohort (5.0 per 1000 person-years) (Figure 2C, Table 4). The RD of incident MI for tramadol was 1.1 (95% CI: −0.3 to 2.5) per 1000 person-years, compared with codeine cohort. The corresponding HR was 1.23 (95% CI: 0.95 to 1.60). The results of sensitivity analyses remained consistent (Table 4).
Table 4.
Association between Tramadol Initiation and Risk of Incident Myocardial Infarction within Six-month Follow-up Comparing with Initiation of Codeine among Patients with Osteoarthritis
| Tramadol | Codeine | |
|---|---|---|
| Primary analysis | ||
| Participants (n) | 42,722 | 42,722 |
| Incident myocardial infarction (n) | 127 | 103 |
| Mean follow-up (year) | 0.49 | 0.49 |
| Rate (1000 person-years)* | 6.1 | 5.0 |
| RD (1000 person-years, 95% CI) | 1.1 (−0.3, 2.5) | 0.0 (reference) |
| HR (95% CI) | 1.23 (0.95, 1.60) | 1.00 (reference) |
| “As-treated” approach** | ||
| Participants (n) | 42,722 | 42,722 |
| Incident myocardial infarction (n) | 66 | 47 |
| Mean follow-up (year) | 0.25 | 0.21 |
| Rate (1000 person-years) | 6.2 | 5.1 |
| RD (1000 person-years, 95% CI) | 1.1 (−1.0, 3.2) | 0.0 (reference) |
| HR (95% CI) | 1.21 (0.83, 1.76) | 1.00 (reference) |
| Incident OA patients | ||
| Participants (n) | 25,104 | 25,104 |
| Incident myocardial infarction (n) | 60 | 59 |
| Mean follow-up (year) | 0.49 | 0.49 |
| Rate (1000 person-years) | 4.9 | 4.8 |
| RD (1000 person-years, 95% CI) | 0.1 (−1.7, 1.8) | 0.0 (reference) |
| HR (95% CI) | 1.01 (0.71, 1.45) | 1.00 (reference) |
| Missing data imputation | ||
| HR (95% CI) | 1.13 (0.88, 1.46) | 1.00 (reference) |
| Conventional covariate adjustment approach | ||
| HR (95% CI) | 1.16 (0.92, 1.47) | 1.00 (reference) |
RD, rate difference; HR, hazard ratio; n, number; 95% CI, 95% confidence interval; OA, osteoarthritis.
Number (rate) of competing event (i.e., death) in tramadol and codeine cohort was 791 (38.0/1000 person-years) and 822 (39.6 /1000 person-years), respectively.
82% and 89% participants discontinued or switched their initiated treatment in tramadol and codeine cohort, respectively.
DISCUSSION
Using data collected from THIN, the six-month risk of incident MI among initiators of tramadol was higher than that of naproxen initiators, but comparable to initiators of diclofenac and codeine, two analgesics that have been associated with an increased risk of cardiovascular adverse effects in the previous studies4–9, 26. Our findings were independent of the major confounders and remained consistent in various sensitivity analyses, suggesting that the observed associations were robust.
Comparison with Previous Studies
One large propensity-score matched cohort study using the US Medicare database reported that the incidence rates of composite cardiovascular events (including MI, stroke, heart failure, revascularization, and out-of-hospital cardiac death) over the 180 days follow-up period was slightly lower among tramadol initiators (11 per 100 person-years) than that among codeine initiators (17 per 100 person-years)26. The study, however, did not examine the relation of tramadol prescription to individual cardiovascular event (e.g., MI), and some potential confounders (e.g., BMI, smoking, and drinking) were not controlled for in the analyses26. Another case-control study conducted among patients with osteoarthritis in Spain did not show a statistically significant association between tramadol use and the risk of acute coronary events (i.e., acute MI or unstable angina) when nonuse served as the referent exposure (odds ratio [OR] = 1.10, 95% CI: 0.93 to 1.29)27. However, in the same study the association of naproxen use vs. nonuse with acute coronary events (OR = 1.25, 95% CI: 1.04 to 1.48) was stronger than that of diclofenac use vs. nonuse with acute coronary events (OR = 1.16, 95% CI: 1.06 to 1.27)27; this finding contradicts most previous studies3–9.
Possible Explanations
Biological mechanisms linking tramadol use to the risk of MI are not well understood. One proposed explanation is that tramadol inhibits the reuptake of serotonin19, a key factor in the process of platelet aggregation, mediating vascular homeostasis and thrombosis21. A previous in vivo study demonstrated that mice selectively deficient in serotonin exhibited reduced risk of thrombosis and thromboembolism42. Others have postulated that tramadol use may enhance coagulation of plasma proteins and suppress thrombocyte de-aggregation process43–46. Furthermore, tramadol use may induce oxidative stress which has a critical role in the process of atherosclerotic diseases47–51. Finally, tramadol use may decrease the expression of inducible nitric oxide synthases and worsen myocardial injury in patients undergoing cardiac surgery25.
Strengths and Limitations
Several characteristics of our study are noteworthy. We adopted a new-user design to include only initiators of tramadol, naproxen, diclofenac, and codeine. This method minimizes potential selection bias (i.e., immortal bias) introduced if prevalent medication users were included. In addition, the results from various sensitivity analyses were consistent, suggesting robustness of observed associations. Nevertheless, the present findings should be interpreted with caution. First, the hazard ratio generated from imputed data analysis (HR=1.57) was smaller than that from complete data analysis (HR=1.68); however, the difference in these effect estimates is small ([1.68-1.57)]/1.68=6.5%). Nevertheless, as in any observational study we can’t rule out the residual confounding, despite our use of propensity-score matching and several sensitivity analyses. Future studies are needed to verify our findings. Second, physician-ordered prescriptions may not reflect the actual medication use by patients. For instance, patients may not fill prescriptions, may not take the medication once filled, or may not use the medication according to physician’s instruction. As a result, misclassification of the medication use could occur and bias the study findings. However, such bias, if occurred, is likely to be non-differential and would bias the observed associations towards the null.
Clinical Implications
Our findings may have clinical implications. Although our study found tramadol use was associated with an increased risk of MI when compared with naproxen, the effect was relatively modest (RD=1.9/1000 person-years). This study suggests that tramadol may be not as safe as some clinicians have perceived with respect to cardiovascular adverse effects. Considering tramadol prescriptions have been increased rapidly worldwide, especially among patients with OA16, 17, 52–54 and MI is a leading cause of morbidity and mortality around the world, precautions should be taken when prescribing tramadol to the patients with OA.
CONCLUSION
In this population-based cohort of patients with OA, the six-month risk of MI among initiators of tramadol was higher than that of naproxen, but comparable to, if not lower than risk with diclofenac and codeine.
Supplementary Material
Acknowledgements
Everyone who contributed significantly to the work has been listed.
Funding
This work was supported by the National Natural Science Foundation of China (81772413, 81702207, 81702206), the National Institutes of Health (K23 AR069127, P60 AR047785, K23 DA042168), the Spondylitis Association of America (Bruckel Early Career Investigator Award), and the OptumLabs.
Role of the Funder/Sponsor
The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; praparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures
The authors have declared that no competing interests exist.
Ethical approval
The Institutional Review Board approved this study, with waiver of informed consent.
Scientific approval
The protocol of this study was approved by the THIN Scientific Review Committee (18THIN078).
Transparency
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
References
- 1.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet 2019; 393: 1745–59. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Niu J. Editorial: Shifting Gears in Osteoarthritis Research Toward Symptomatic Osteoarthritis. Arthritis Rheumatol 2016; 68: 1797–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jüni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger M. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. The Lancet 2004; 364: 2021–9. [DOI] [PubMed] [Google Scholar]
- 4.McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA 2006; 296: 1633–44. [DOI] [PubMed] [Google Scholar]
- 5.McGettigan P, Henry D. Cardiovascular risk with non-steroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS Med 2011; 8: e1001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coxib and traditional NSAID Trialists’ (CNT) Collaboration. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. The Lancet 2013; 382: 769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGettigan P, Henry D. Use of non-steroidal anti-inflammatory drugs that elevate cardiovascular risk: an examination of sales and essential medicines lists in low-, middle-, and high-income countries. PLoS Med 2013; 10: e1001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubreuil M, Louie-Gao Q, Peloquin CE, Choi HK, Zhang Y, Neogi T. Risk of myocardial infarction with use of selected non-steroidal anti-inflammatory drugs in patients with spondyloarthritis and osteoarthritis. Ann Rheum Dis 2018; 77: 1137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt M, Sorensen HT, Pedersen L. Diclofenac use and cardiovascular risks: series of nationwide cohort studies. BMJ 2018; 362: k3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet 2004; 43: 879–923. [DOI] [PubMed] [Google Scholar]
- 11.Katz WA. Pharmacology and clinical experience with tramadol in osteoarthritis. Drugs 1996; 52 Suppl 3: 39–47. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin JL, Kraemer JJ, Bajwa ZH. The use of opioids in the treatment of osteoarthritis: when, why, and how? Curr Rheumatol Rep 2009; 11: 5–14. [DOI] [PubMed] [Google Scholar]
- 13.Barkin RL. Extended-release Tramadol (ULTRAM ER): a pharmacotherapeutic, pharmacokinetic, and pharmacodynamic focus on effectiveness and safety in patients with chronic/persistent pain. Am J Ther 2008; 15: 157–66. [DOI] [PubMed] [Google Scholar]
- 14.Hassamal S, Miotto K, Dale W, Danovitch I. Tramadol: Understanding the Risk of Serotonin Syndrome and Seizures. Am J Med 2018. [DOI] [PubMed] [Google Scholar]
- 15.Miotto K, Cho AK, Khalil MA, Blanco K, Sasaki JD, Rawson R. Trends in Tramadol: Pharmacology, Metabolism, and Misuse. Anesth Analg 2017; 124: 44–51. [DOI] [PubMed] [Google Scholar]
- 16.Wright EA, Katz JN, Abrams S, Solomon DH, Losina E. Trends in prescription of opioids from 2003-2009 in persons with knee osteoarthritis. Arthritis Care Res (Hoboken) 2014; 66: 1489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng C, Dubreuil M, LaRochelle MR, Lu N, Wei J, Choi HK, et al. Association of Tramadol With All-Cause Mortality Among Patients With Osteoarthritis. JAMA 2019; 321: 969–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barann M, Stamer UM, Lyutenska M, Stuber F, Bonisch H, Urban B. Effects of opioids on human serotonin transporters. Naunyn Schmiedebergs Arch Pharmacol 2015; 388: 43–9. [DOI] [PubMed] [Google Scholar]
- 19.Gong L, Stamer UM, Tzvetkov MV, Altman RB, Klein TE. PharmGKB summary: tramadol pathway. Pharmacogenet Genomics 2014; 24: 374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassamal S, Miotto K, Dale W, Danovitch I. Tramadol: Understanding the Risk of Serotonin Syndrome and Seizures. Am J Med 2018; 131: 1382 e1–e6. [DOI] [PubMed] [Google Scholar]
- 21.Koupenova M, Kehrel BE, Corkrey HA, Freedman JE. Thrombosis and platelets: an update. Eur Heart J 2017; 38: 785–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rickli A, Liakoni E, Hoener MC, Liechti ME. Opioid-induced inhibition of the human 5-HT and noradrenaline transporters in vitro: link to clinical reports of serotonin syndrome. Br J Pharmacol 2018; 175: 532–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biere-Rafi S, Di Nisio M, Gerdes V, Porreca E, Souverein P, Boer A, et al. Non-steroidal anti-inflammatory drugs and risk of pulmonary embolism. Pharmacoepidemiol Drug Saf 2011; 20: 635–42. [DOI] [PubMed] [Google Scholar]
- 24.Boureau F, Legallicier P, Kabir-Ahmadi M. Tramadol in post-herpetic neuralgia: a randomized, double-blind, placebo-controlled trial. Pain 2003; 104: 323–31. [DOI] [PubMed] [Google Scholar]
- 25.Wagner R, Piler P, Bedanova H, Adamek P, Grodecka L, Freiberger T. Myocardial injury is decreased by late remote ischaemic preconditioning and aggravated by tramadol in patients undergoing cardiac surgery: a randomised controlled trial. Interact Cardiovasc Thorac Surg 2010; 11: 758–62. [DOI] [PubMed] [Google Scholar]
- 26.Solomon DH, Rassen JA, Glynn RJ, Garneau K, Levin R, Lee J, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med 2010; 170: 1979–86. [DOI] [PubMed] [Google Scholar]
- 27.Pontes C, Marsal JR, Elorza JM, Aragon M, Prieto-Alhambra D, Morros R. Analgesic Use and Risk for Acute Coronary Events in Patients With Osteoarthritis: A Population-based, Nested Case-control Study. Clin Ther 2018; 40: 270–83. [DOI] [PubMed] [Google Scholar]
- 28.Stuart-Buttle CD, Read JD, Sanderson HF, Sutton YM. A language of health in action: Read Codes, classifications and groupings. Proc AMIA Annu Fall Symp 1996: 75–9. [PMC free article] [PubMed] [Google Scholar]
- 29.First Databank. Multilex drug data file.
- 30.Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf 2007; 16: 393–401. [DOI] [PubMed] [Google Scholar]
- 31.Morris R, Carstairs V Which deprivation? A comparison of selected deprivation indexes. J Public Health Med 1991; 13: 318–26. [PubMed] [Google Scholar]
- 32.Garcia Rodriguez LA, Tacconelli S, Patrignani P. Role of dose potency in the prediction of risk of myocardial infarction associated with nonsteroidal anti-inflammatory drugs in the general population. J Am Coll Cardiol 2008; 52: 1628–36. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez LA, Cea-Soriano L, Martin-Merino E, Johansson S. Discontinuation of low dose aspirin and risk of myocardial infarction: case-control study in UK primary care. BMJ 2011; 343: d4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation 2016; 133: 601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei LJ, Lin DY, Weissfeld L. Regression Analysis of Multivariate Incomplete Failure Time Data by Modeling Marginal Distributions. Journal of the American Statistical Association 1989; 84: 1065–73. [Google Scholar]
- 36.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of Martingale based residuals. Biometrika 1993; 80: 557–72. [Google Scholar]
- 37.Graham DJ, Campen D, Hui R, Spence M, Cheetham C, Levy G, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet 2005; 365: 475–81. [DOI] [PubMed] [Google Scholar]
- 38.Rubin DB. Multiple imputation for nonresponse in surveys. New York, John Wiley & Sons; 1987. [Google Scholar]
- 39.Elze MC, Gregson J, Baber U, Williamson E, Sartori S, Mehran R, et al. Comparison of Propensity Score Methods and Covariate Adjustment: Evaluation in 4 Cardiovascular Studies. J Am Coll Cardiol 2017; 69: 345–57. [DOI] [PubMed] [Google Scholar]
- 40.Glynn RJ, Schneeweiss S, Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol 2006; 98: 253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franklin JM, Rassen JA, Ackermann D, Bartels DB, Schneeweiss S. Metrics for covariate balance in cohort studies of causal effects. Stat Med 2014; 33: 1685–99. [DOI] [PubMed] [Google Scholar]
- 42.Iwagami M, Tomlinson LA, Mansfield KE, Douglas IJ, Smeeth L, Nitsch D. Gastrointestinal bleeding risk of selective serotonin reuptake inhibitors by level of kidney function: A population-based cohort study. Br J Clin Pharmacol 2018; 84: 2142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bol’shakov VV, Sapozhkov AV, Denisova SV. Effect of droperidol and tramadol combination on the hemostasis in rabbits. Eksp Klin Farmakol 2002; 65: 50–2. [PubMed] [Google Scholar]
- 44.Casella S, Giannetto C, Giudice E, Marafioti S, Fazio F, Assenza A, et al. ADP-induced platelet aggregation after addition of tramadol in vitro in fed and fasted horses plasma. Res Vet Sci 2013; 94: 325–30. [DOI] [PubMed] [Google Scholar]
- 45.Koenig W Haemostatic risk factors for cardiovascular diseases. Eur Heart J 1998; 19: C39–43. [PubMed] [Google Scholar]
- 46.Fabre JE, Nguyen M, Latour A, Keifer JA, Audoly LP, Coffman TM, et al. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nat Med 1999; 5: 1199–202. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed MA, Kurkar A. Effects of opioid (tramadol) treatment on testicular functions in adult male rats: The role of nitric oxide and oxidative stress. Clin Exp Pharmacol Physiol 2014; 41: 317–23. [DOI] [PubMed] [Google Scholar]
- 48.Bameri B, Shaki F, Ahangar N, Ataee R, Samadi M, Mohammadi H. Evidence for the Involvement of the Dopaminergic System in Seizure and Oxidative Damage Induced by Tramadol. Int J Toxicol 2018; 37: 164–70. [DOI] [PubMed] [Google Scholar]
- 49.Kinugawa S, Tsutsui H, Hayashidani S, Ide T, Suematsu N, Satoh S, et al. Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: role of oxidative stress. Circ Res 2000; 87: 392–8. [DOI] [PubMed] [Google Scholar]
- 50.Sia YT, Parker TG, Liu P, Tsoporis JN, Adam A, Rouleau JL. Improved post-myocardial infarction survival with probucol in rats: effects on left ventricular function, morphology, cardiac oxidative stress and cytokine expression. J Am Coll Cardiol 2002; 39: 148–56. [DOI] [PubMed] [Google Scholar]
- 51.Katakami N, Sakamoto K, Kaneto H, Matsuhisa M, Ohno K, Shimizu I, et al. Cumulative effect of oxidative stress-related gene polymorphisms on myocardial infarction in type 2 diabetes. Diabetes Care 2009; 32: e55. [DOI] [PubMed] [Google Scholar]
- 52.Fournier JP, Azoulay L, Yin H, Montastruc JL, Suissa S. Tramadol use and the risk of hospitalization for hypoglycemia in patients with noncancer pain. JAMA Intern Med 2015; 175: 186–93. [DOI] [PubMed] [Google Scholar]
- 53.Patterson E. Tramadol History and Statistics. 2017. [Google Scholar]
- 54.Wilson N, Sanchez-Riera L, Morros R, Diez-Perez A, Javaid MK, Cooper C, et al. Drug utilization in patients with OA: a population-based study. Rheumatology (Oxford) 2015; 54: 860–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.