Abstract
Beverages make important contributions to nutritional intake and their role in health has received much attention. This review focuses on the genetic determinants of common beverage consumption and how research in this field is contributing insight to what and how much we consume and why this genetic knowledge matters from a research and public health perspective. The earliest efforts in gene-beverage behavior mapping involved genetic linkage and candidate gene analysis but these approaches have been largely replaced by genome-wide association studies (GWAS). GWAS have identified biologically plausible loci underlying alcohol and coffee drinking behavior. No GWAS has identified variants specifically associated with consumption of tea, juice, soda, wine, beer, milk or any other common beverage. Thus far, GWAS highlight an important behavior-reward component (as opposed to taste) to beverage consumption which may serve as a potential barrier to dietary interventions. Loci identified have been used in Mendelian randomization and gene×beverage interaction analysis of disease but results have been mixed. This research is necessary as it informs the clinical relevance of SNP-beverage associations and thus genotype-based personalized nutrition, which is gaining interest in the commercial and public health sectors.
Keywords: genetics, beverage, coffee, alcohol, milk, Mendelian randomization, gene-diet interaction, beverage consumption, public health
1. INTRODUCTION
Water is an essential nutrient for life (Jéquier & Constant, 2009). Beverages contribute approximately 80% to total water intake with the remainder provided by solid foods (EFSA, 2010; Electrolytes & Water, 2005). After water, coffee, tea, beer, milk, 100% juice, sugar sweetened beverages (SSB) and wine are among the most widely consumed beverages in the world (Euromonitor, 2018; Neves, Trombin, Lopes, Kalaki, & Milan, 2012; Singh et al., 2015). Unlike plain water, beverages are also important sources of energy, other vitamins and minerals as well as 1000s of non-nutrients, many of which are bioactive. Coffee and tea, for example, are naturally energy-free but important sources of caffeine and polyphenols. Beer and wine both contain alcohol but also present with unique constituents including hops and resveratrol, respectively. Juice and SSB are both energy dense but the former is also a natural source of vitamins.
With their wide consumption and contributions to nutrient intake there is great interest in the role beverages play in health. Epidemiological studies support a beneficial role of moderate coffee intake in reducing risk of several chronic diseases but heavy intake is likely harmful on pregnancy outcomes (Poole et al., 2017). Tea might also reduce risk of type 2 diabetes (T2D), metabolic syndrome (MetS)(Marventano et al., 2016; Yang, J., Mao, Xu, Ma, & Zeng, 2014), osteoporosis (Sun et al., 2017) and cardiovascular diseases(CVD)(Pang et al., 2016). Wine drinking may have a dose-dependent association with health: low doses might protect against breast cancer and CVD while high doses offer no benefit or increased risk (Chen, J. Y. et al., 2016; de Gaetano, Di Castelnuovo, Rotondo, Iacoviello, & Donati, 2002). Health benefits or risks specific to beer and milk are unclear (Gijsbers et al., 2016; Guo, J. et al., 2017; Kaplan, Palmer, & Denke, 2000; Larsson, Crippa, Orsini, Wolk, & Michaelsson, 2015; Lee, J., Fu, Chung, Jang, & Lee, 2018; Li, F. et al., 2011; Liu et al., 2015; Mullie, Pizot, & Autier, 2016; Soedamah-Muthu et al., 2011). There are currently no health benefits to SSB consumption but rather convincing data supporting its role in obesity which is, in turn, a risk factor for several diseases (Malik et al., 2010; Malik, Schulze, & Hu, 2006). A caveat to our knowledge pertaining to beverage consumption and human health is that much of it is derived from epidemiological studies which have limitations (Rothman KJ, 2008; Taubes, 1995; Willett, 1998).
This review focuses on the genetic determinants of common beverage consumption and how research in this field is contributing insight to what and how much we consume and why this genetic knowledge matters from a research and public health perspective.
2. DETERMINANTS OF BEVERAGE CONSUMPTION
Understanding factors contributing to beverage consumption has important public health and research implications. Knowledge of both external and internal cues for beverage intake may inform the causal role each beverage has in health (research) and the potential population subgroups most susceptible to the health consequences of its regular consumption (public health). Thirst is an important determinant of beverage intake, but in today’s society the amount and choice of beverage consumed is governed by a multitude of individual and societal factors such as availability, mood, social context, health status, education, convenience, cost, cultural influences, and sensory attributes such as smell and taste (Block, Gillman, Linakis, & Goldman, 2013; Drewnowski, 1997; Drewnowski, Henderson, Levine, & Hann, 1999; Glanz, Basil, Maibach, Goldberg, & Snyder, 1998; Neumark-Sztainer, Story, Perry, & Casey, 1999; Wardle, Carnell, & Cooke, 2005). Consumption of coffee, for example, tends to positively correlate with age, smoking, and alcohol consumption and may also be impacted by perceived health consequences of the beverage (Cornelis, M. C., 2012). Degree of economic development, religious and cultural norms, the availability and the level and effectiveness of policies are societal factors contributing to alcohol consumption behaviors while age, gender, social economic status (SES) and prior alcohol exposure are important individual level factors (Babor et al., 2010). The acute positive or negative reinforcing properties of alcohol and caffeine are especially important in determining alcoholic beverage and coffee drinking patterns (Cornelis, M. C., 2012; Koob & Volkow, 2016; Kuntsche, Knibbe, Gmel, & Engels, 2005). Physiological effects of beverages are intrinsic and often vary between individuals. Genetics also play a role in beverage consumption behavior and more likely with regards to these physiological effects.
3. GENETIC DETERMINANTS OF BEVERAGE CONSUMPTION
Twin studies have largely been the standard approaches to estimating the genetic or heritable component of beverage consumption behaviors which can vary from 0 (not heritable) to 1 (completely inherited). Twin studies estimate heritability by comparing monozygotic twins, who share the common environment and have identical genetics, to dizygotic twins, who also share the common environment but only half their genetics (Neale & Cardon, 1994; Turkheimer, D’Onofrio, Maes, & Eaves, 2005).
In an earlier twin study by de Castro (de Castro, 1993), the heritability estimate for amount of drinking water consumed was 0.43, which was slightly higher than the 0.37 estimate for total water (food and beverages). Heritability estimates for alcohol, soda, milk, coffee and fruit juice were 0.45, 0.54, 0.04, 0.69 and 0.12, respectively. Hasselbalch et al (Hasselbalch, Ann Louise, 2010) reported lower estimates for soda: 0.26 and 0.30 for men and women, respectively, while Teucher et al (Teucher et al., 2007) reported a much higher estimate for fruit juice (0.70). Heritability estimates for self-reported total caffeine intake (derived from caffeine-containing coffee, tea and soda) ranged between 0.30 and 0.58, with higher estimates reported for heavy use (up to 0.77)(Yang, A., Palmer, & de Wit, 2010). Studies that separated heritability estimates by caffeine source report higher heritability for coffee relative to other sources (Luciano, Kirk, Heath, & Martin, 2005; Teucher et al., 2007; Vink, Staphorsius, & Boomsma, 2009). Twin studies as well as family and adoption studies of habitual alcohol consumption or alcoholism have largely focused on males and report heritability estimates between 0.30 and 0.60 (Carol A. Prescott & Kenneth S. Kendler, 1999; McGue, Matt, 1999; Reed et al., 1994; Teucher et al., 2007). Heath and Martin (Heath, A. & Martin, 1988) propose that the decision to abstain from drinking is not genetically determined, but the onset and amount consumed once that decision is made are influenced by genetic factors.
Twins studies are powerful epidemiological approaches to measuring the contribution of genetics to a given trait but are often underpowered and subject to bias which likely add to between study differences in estimates of heritability (Kendler, 1993; Zaitlen & Kraft, 2013; Zuk, Hechter, Sunyaev, & Lander, 2012). Furthermore, although results above provide compelling evidence for a genetic influence on beverage intake and choice, they do not indicate the specific genes that increase or decrease drinking behaviors.
3.1. Early Approaches: Linkage and Candidate Gene Analysis
The earliest efforts in gene-trait mapping involved genetic linkage and candidate gene analysis. Genetic linkage studies determine inheritance of a binary or quantitative trait among family members in extensive pedigrees by evaluating whether one or more genetic markers spaced across the 23 chromosomes segregate with the trait (Cantor, 2014).This approach roughly locates a broad chromosomal region, which may contain 10s of 100s of genes, that co-segregate with the trait. Other strategies such as fine mapping and targeted association analysis must be used to further refine the linked region and identify the gene of interest (Cantor, 2014).
Lactose intolerance (or lactase non-persistence) is a common autosomal recessive condition resulting from the physiological decline in activity of lactase-phlorizin hydrolase (LPH) in intestinal cells after weaning and has a significant impact on milk drinking behavior. The age of onset varies between populations and in some populations lactase activity persists at a high level throughout adult life (Sahi, Timo, 1994; Sahi, T, Isokoski, Jussila, & Launiala, 1972; Swallow, 2003; Wang, Y. et al., 1998). Although the sequence of the lactase gene (LCT, encoding LPH) had been known since 1991 (Boll, Wagner, & Mantei, 1991), the causative mechanism for lactase persistence remained elusive until 2002 when a linkage analysis of nine Finnish families with hypolactasia identified a variant upstream of the initiation codon of LCT (LCT-13910C>T) which demonstrated complete association with lactase persistence (Enattah et al., 2002).
The first linkage studies on alcohol dependence (AD) from the family-based Collaborative Study on the Genetics of Alcoholism (COGA) (Reich et al., 1998) and a sib-pair study from a Southwest American Indian tribe (Long et al., 1998) reported a broad risk locus on chromosome 4q that contains the genes that encode the isoforms of alcohol dehydrogenase (ADH). Hill et al (Hill et al., 2004) reported support for AD loci at chromosome 1q23.3-q25.1 in a genome-wide linkage analysis of double probands. This region was followed up using fine-mapping genotyping and confirmed single nucleotide polymorphism (SNP)-AD associations within ASTN1 (Hill, Weeks, Jones, Zezza, & Stiffler, 2012). Additional AD studies have implicated other chromosomal regions (Dick et al., 2010; Edenberg, Howard J & Foroud, 2014; Wang, Jen C et al., 2004). The genetic linkage approach, to the author’s knowledge, has not been applied to habitual beverage consumption.
A second approach to isolating genetic determinants of a trait involves genetic association in population or family base-studies and is essentially a form of linkage mapping but is allele-based rather than locus-based and is often hypothesis driven. Efforts focus on potentially functional SNPs in genes with biological plausibility or in regions identified by linkage. In the context of beverage consumption behavior a highly implicated pathway pertains to taste. Taste is one of the primary means of determining the acceptability of a food and might have been critical to the survival of early human subjects (Tepper, 2008). The perception of sweet, umami and bitter tastes are all mediated via G-coupled protein receptors, encoded by the TAS1R and TAS2R taste receptor gene families, while salty and sour tastes are transduced via ion channels (Wise, Hansen, Reed, & Breslin, 2007). There is little known regarding genetic variation in salty and sour tastes(Wise et al., 2007). In contrast, bitter taste quality is affected by variants in TAS2R16, TAS2R38, TAS2R43 and TAS2R44 while variants in TAS1R1 and TAS1R3 impact umami and sweet (Drayna, 2005; Feeney, O’Brien, Scannell, Markey, & Gibney, 2011; Kim, U. K. et al., 2003; Mainland & Matsunami, 2009; Shigemura, Shirosaki, Sanematsu, Yoshida, & Ninomiya, 2009). The most studied is TAS2R38, in particular its three SNPs, which result in two common haplotypes that are named for their amino acid substitutions: PAV (proline, alanine, and valine) and AVI (alanine, valine, and isoleucine) (Kim, U.-K., Breslin, Reed, & Drayna, 2004). TAS2R38 diplotype influences the ability to taste a family of bitter compounds including phenylthiocarbamide (PTC) and 6-n-propylthiouracil (PROP). Although these compounds are not found in food stuffs naturally, PTC/PROP-related compounds are present in several bitter tasting fruits and vegetables. PAV homozygotes and heterozygotes perceive greater bitterness than AVI homozygotes that perceive little or no bitterness (Kim, U.-K. et al., 2004). This locus has been subject to numerous association studies of food and beverage preferences. Variation in TAS2R38 has been linked to coffee, beer, spirits, green tea, sugar content of beverages and total alcohol or drinking status (Beckett et al., 2017; Choi, J. H., Lee, Yang, & Kim, 2017; Duffy et al., 2004; Hayes et al., 2011; Mennella, Pepino, & Reed, 2005; Ong et al., 2018; Ooi, Lee, Law, & Say, 2010; Perna et al., 2018; Ramos-Lopez, O. et al., 2015; Wang, J. C. et al., 2007). Variation in other TASR2 members have also been linked to bitter beverage consumption (Cornelis, M. C., 2012; Hayes et al., 2011; Ong et al., 2018; Pirastu et al., 2014; Wang, J. C. et al., 2007). Most of these findings, however, lack replication. Although bitterness is widely claimed to be an evolutionarily important indicator of toxicity (Behrens & Meyerhof, 2016; Drewnowski & Gomez-Carneros, 2000) not all bitter stimuli are toxic (Glendinning, 1994; Nissim, Dagan-Wiener, & Niv, 2017). Coffee and beer are prime examples whereby the innate eversion to bitter taste does not hold. Indeed, variants in TAS2R43 linked to increased perception of caffeine; a bitter compound, associates with increased coffee consumption and liking according to candidate-SNP analysis (Ong et al., 2018; Pirastu et al., 2014). Although coffee bitterness is easily offset by additives, some individuals may also learn to associate this sensory cue with social context or postingestive signals elicited by biologically active constituents of coffee. Variation in the TAS1R sweet and umami receptor family has also been linked to alcohol consumption behavior (Choi, J. H. et al., 2017), wine drinking (Choi, J. H. et al., 2017) and vodka liking (Pirastu et al., 2012).
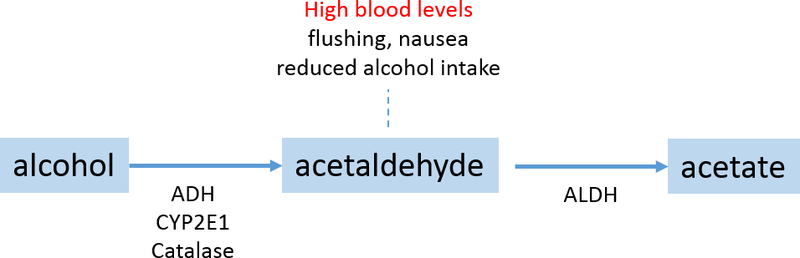
Besides taste-related genes, the candidate approach for alcohol-related traits has additionally focused on alcohol metabolism genes including ADH, CYP2E1 and ALDH (Figure 1), as well as gene members of several neurotransmitter systems: GIRK1, GABA-A, DRD2, SLC6A3, SLC6A4, TPH1, COMT, CHRM2 and OPRM1(Edenberg, H. J. & Foroud, 2006; Matsuo et al., 2006; Reilly, Noronha, Goldman, & Koob, 2017; Tawa, Hall, & Lohoff, 2016). For coffee and tea drinking, candidate genes involved in caffeine metabolism (CYP1A2) and caffeine’s target of action (ADORA2A, DRD2) have been examined (Cornelis, M. C., 2012; Cornelis, M. C., El-Sohemy, & Campos, 2007). Gene members of the brain reward system, particularly DRD2, have been tested for associations with SSB (Baik, J.-H., 2013; Eny, Corey, & El-Sohemy, 2009; Ramos-Lopez, Omar, Panduro, Rivera-Iñiguez, & Roman, 2018).
Figure 1.
Alcohol Metabolism
3.2. Recent Approaches: Genome-wide analysis
With the human genome sequenced in the early 2000’s and mapped frequencies and patterns of association among millions of common SNPs in diverse populations, the primary approach to identifying genetic variants for complex traits has quickly transitioned to genome-wide association studies (GWAS). GWAS is based on the premise that a causal variant is located on a haplotype, and therefore a marker allele in linkage disequilibrium (LD) with the causal variant should present with an association with a trait of interest (Hirschhorn & Daly, 2005). This approach is unbiased with respect to genomic structure and previous knowledge of the trait etiology, which contrasts with earlier approaches, and therefore has greater potential to reveal novel gene-trait associations.
Table 1 provides descriptions of GWAS listed in the GWAS catalogue (November 26, 2018) reporting significant SNP-drinking behavior associations. GWAS designs included single sample, 2-stage (i.e. discovery and replication) and meta-analysis. Table 2 presents those loci defined as significant according to authors’ a priori threshold. Results of GWAS of AD and beverage ‘liking’ were also included.
Table 1.
Genome-wide association studies of beverage consumption reporting significant loci
| Study Reference | Phenotype | Phenotype defined | Study design | N | Race |
|---|---|---|---|---|---|
| (Baik, I., Cho, Kim, Han, & Shin, 2011) | Alcohol drinking Alcohol Use Disorder (NULL) |
Self-reported alcohol consumption among ever drinkers. Questionnaire, alcohol g/day in the past month, derived Alcohol Use Disorder Identification Test (AUDIT) score:, <8 or ≥8 score |
Discovery Population-based males Replication Population-based males |
1721 1113 |
Korean |
| (Takeuchi et al., 2011) | Alcohol drinking | Self-reported ‘ever’ vs ‘non-drinkers’ alcohol consumption Questionnaire, alcohol gou/wk also derived |
Discovery Population-based Replication Health center-based |
733 ca 729 co 2794 ca 1351 co |
Japanese |
| (Schumann, G. et al., 2011) | Alcohol drinking | Self-reported alcohol consumption Self-reported alcohol consumption among ever drinkers (sex stratified) Questionnaire, alcohol g/day per kg (no detail) |
Discovery 12 Population-based Replication 7 Population-based |
26,316 21,185 |
EUR |
| (Yang, X. et al., 2013) | Alcohol drinking | Self-reported ‘drinkers’ (≥ 12 drinks in past year) vs ‘non-drinkers’ alcohol consumption In-person interviews. For drinkers, alcohol g/d also derived (NULL) |
Discovery 1 population-based 1 community-based Replication 1 population-based |
1420 ca 3590 co 4896 ca 13293 co |
Chinese |
| (Kapoor, Manav et al., 2013) | Alcohol drinking | Maximum number of drinks consumed among drinkers In-person interview: “What is the largest number of drinks you have ever had in a 24-hour period”? (standard serving sizes per beverage). |
Meta-analysis COGA: AD probands recruited through alcohol treatment programs; relatives of the probands and comparison families SAGE: non-overlapping COGA, Family Study of Cocaine Dependence (FSCD), and Collaborative Genetic Study of Nicotine Dependence (COGEND). |
4915 | EUR |
| (Quillen, E. E. et al., 2014) | Alcohol drinking | Maximum number of drinks consumed among drinkers in-person interview, AD: DSM-IV “What is the largest number of alcoholic drinks you have ever consumed in a day?” and “Does your face flush after drinking a little alcohol?” |
One Sample Male AD probands and pedigrees from isolated region |
272 | Chinese |
| (Xu et al., 2015) | Alcohol drinking | maximum number of alcoholic beverages consumed in any lifetime 24-hour period (“MAXDRINKS”) in-person interview |
Discovery Yale-UPenn (recruited for studies of genetics of drug or AD, families and unrelated) Replication SAGE |
2328 3215 2736 1276 |
EUR AFR EUR AFR |
| (Adkins et al., 2015) | Alcohol drinking | Mean alcohol intake over duration of follow-up (i.e. transition from adolescents to adulthood) trajectory estimate of alcohol consumption (drinks per week) across adolescence and early adulthood In-person interviews. |
Meta-analysis Great Smoky Mountain Study (US, children) Christchurch Health and Development Study (CHDS): birth cohort children (New Zealand, 1977) Virginia Twin Study on Adolescent Behavioral Development: twin cohort US 1974–1983 |
2126 | EUR |
| (Schumann, Gunter et al., 2016) | Alcohol drinking | Alcohol g/d among drinkers Heavy vs light/no drinking (NULL) Self-reported questionnaires/diaries In-person interviews |
Discovery Meta-analysis Population-based Replication Meta-analysis Population-based |
70,460 74,711 31,021 35,438 |
EUR |
| (Jorgenson et al., 2017) | Alcohol drinking | Individuals who reported drinking ≥1 day per week and ≥1 drink per day were defined as ‘drinkers’, whereas those who provided negative answers (‘no days’ and ‘none’) were considered as ‘non-drinkers’. For alcohol drinkers, the regular quantity of alcohol drinks consumed per week was calculated by multiplying the two answers. Self-reported questionnaire |
Meta-analysis Genetic Epidemiology Research in Adult Health and Aging (GERA) cohort : members of the Kaiser Permanente Medical Care Plan, Northern California Region (KPNC) |
23,104 co 47,967 ca 2,673 co 4,374 ca 3,288 co 2,746 ca 1,165 co 1,310 ca |
EUR Hisp/Latino East Asian AA |
| (Clarke et al., 2017) | Alcohol drinking | Alcohol units/week (excluded former drinkers) Sex-stratified GWAS Self-reported computer questionnaire |
One-sample UK Biobank | 112,117 (108,309 drinkers) | EUR |
| (Sanchez-Roige et al.) | AD | 10-item AUDIT questionnaire (scores 0 to 40) Included only subjects who answered yes to the question ‘Have you ever in your life used alcohol’. |
One-sample 23andMe Direct to consumer genotyping service |
20328 drinkers | EUR |
| (Gelernter, Joel et al., 2018) | Alcohol drinking | flushing, maximum number of alcoholic beverages consumed in any lifetime 24-hour period, and DSM-IV AD criterion count. Only alcohol-exposed subjects were included In-person interview |
Meta-analysis Two samples of methamphetamine users, dependent subjects and exposed but not dependent |
1045 drinkers | Thai |
| (Treutlein, J. et al., 2009) | AD | AD: based on DSM-IV criteria Interview |
Discovery Male in-patients and population-based controls Replication Male in-patients and matched controls |
476 ca 1358 co 1024 ca 996 co |
EUR |
| (Kendler et al., 2011) | AD | abstainers excluded, alcohol factor scores among drinkers based on alcohol-related symptoms online questionnaire: Composite International Diagnostic Interview—Short Form, modified to screen for lifetime diagnoses |
One sample, Race-strata survey-based recruitment |
2,357 812 |
EUR AA |
| (Wang, K. S. et al., 2011) | AD | Discovery: DSM-IV diagnosis (interview) Replication: telephone diagnostic interview |
Discovery Meta-analysis: COGA and SAGE Replication OZALC (family based) |
1283 ca 1416 co 1650 ca 1684 co |
EUR EUR |
| (Frank et al., 2012) | AD | DSM-IV criteria | Pooled sample analysis (males) extends Treutlien et al (2009) with more in-patients/controls | 1333 ca 2168 co |
EUR |
| (Zuo, L. et al., 2013) | AD | DSM-IV criteia for AD, controls: exposed to alchol but not addicted | Discovery: SAGE and COGA Replication OZ-ALC |
1409 ca 1518 co 6438 European-Australian family subjects with 1645 probands |
EUR |
| (Park et al., 2013) | AD | DSM-IV diagnosis interview Drinking habit questionnaire (controls) |
Discovery cases: in-patients controls: non-alcoholics (hospital based) Replication cases: in-patients controls: “normal” |
117 ca 279 co 504 ca 471 co |
Korean |
| (Quillen, E. E. et al., 2014) | AD | DSM-IV diagnosis interview Other traits: Maximum number of drinks consumed among drinkers “What is the largest number of alcoholic drinks you have ever consumed in a day?” and “Does your face flush after drinking a little alcohol?” |
One Sample Male AD probands and pedigrees from isolated region |
102 ca 212 co |
Chinese |
| (Gelernter, J. et al., 2013) | AD | Subjects were administered the semi-structured assessment for drug dependence and alcoholism (SSADDA) to derive DSM-IV diagnoses of lifetime AD | Discovery: 7 samples. Replication: 5 samples |
2415 ca 1798 co 2669 ca 2002 co 324 ca 327 co 2269 ca 2975 co |
AA AA EUR EUR AA AA EUR EUR |
| (Gelernter, J. et al., 2013) | AD score | AD symptom count (DSM-IV) | Discovery: 6 samples Replication: 5 samples |
4629 5131 801 1746 |
AA EUR AA EUR |
| (Zuo, Lingjun et al., 2015) | AD | DSM-IV criteria Controls: exposed to alcohol but not addicted |
Meta-analysis OZ-ALC SAGE + COGA SAGE + COGA Yale |
1645 ca 3922 co 1409 ca 1518 co 681 ca 508 co 1429 ca 498 co |
EUR EUR EUR EUR AFR AFR AFR AFR |
| (Chen, G. et al., 2017) | AD score | AD symptom count (DSM-IV) | One-sample SAGE |
2605 | EUR |
| (Treutlein, Jens et al., 2017) | AD | DSM-IV criteria Interview |
One-sample Patients and community controls |
1331 ca 1934 co |
EUR |
| (Gelernter, Joel et al., 2018) | AD score | AD symptom count (DSM-IV) All alcohol-exposed subjects In-person interview |
Meta-analysis Two samples of methamphetamine users, dependent subjects and exposed but not dependent |
1045 | Thai |
| (Cornelis, M. C. et al., 2011) | Caffeine | Total dietary caffeine, mg/d Self-reported, semi-quantitative FFQ. |
Meta-analysis Five population-based studies in US |
47,431 | EUR |
| (Sulem et al., 2011) | Coffee | Cups/d among drinkers Self-reported, questionnaire or in-person interviews |
Discovery 4 population-based, 1 genetic isolate Replication 2 population-based |
6611 4,050 |
EUR EUR |
| (Amin et al., 2012) | Coffee | 5 categories of intake Self-reported, semi-quantitative FFQ |
Discovery 8 population-based Replication 1 population-based |
18,176 7,929 |
EUR EUR |
| (Cornelis, M. C. et al., 2015) | Coffee | Cups/d among drinkers High vs low/non drinkers Self-reported, questionnaire or in-person interviews |
Discovery 28 population-based Replication 13 population based 7 population based |
91 462 30,062 7,964 |
EUR EUR AA |
| (Pirastu, Kooyman, Robino, et al., 2016) | Coffee | Cups/d Self-reported, questionnaire or in-person interviews |
Discovery 2 genetic isolate populations Replication 1 genetic isolate |
1213 1731 |
EUR EUR |
| (Nakagawa-Senda et al., 2018) | Coffee | Cups/d Self-reported, questionnaire |
Discovery Replication Both samples drawn from the same multi-site population-based study |
6312 4949 |
Japanese |
| (Pirastu, Kooyman, Traglia, et al., 2016) | Food/beverage liking | Liking/disliking scale (coffee, whole milk and beer amongst 20 items tested) Questionnaire administered by an operator |
Discovery 3 genetic isolate populations Replication 1 genetic isolate 1 community sample |
2240 1596 |
EUR EUR |
| (Pirastu et al., 2015) | Wine liking | Liking/disliking scale White and red wine tested Questionnaire administered by an operator |
Discovery 3 genetic isolate populations Replication 1 genetic isolate 1 community sample |
2240 1596 |
EUR EUR |
| (Zhong et al., 2018) | Total bitter beverages intake | Coffee (any type), tea (any type), grapefruit juice, beer/cider, red wine, spirit/liquor Servings/day, self-reported, questionnaire | Discovery UK Biobank Replication 3 US cohorts |
85,852 39,924 |
EUR EUR |
| Bitter alcoholic beverage intake | Beer/cider*, red wine, spirit/liquor Servings/day, self-reported, questionnaire |
Discovery UK Biobank Replication 3 US cohorts |
336,448 39,924 |
EUR EUR |
|
| Bitter non-alcoholic beverage intake | Coffee (any type), tea (any type), grapefruit juice Servings/day, self-reported, questionnaire |
Discovery UK Biobank Replication 3 US cohorts |
85,852 39,924 |
EUR EUR |
|
| Coffee intake | Any type (i.e., regular/decaf, instant/ground) Servings/day, self-reported, questionnaire |
Discovery UK Biobank Replication 3 US cohorts |
335,909 39,924 |
EUR EUR |
|
| Tea intake | Any type (i.e., regular/decaf, herbal/non-herbal) Servings/day, self-reported, questionnaire |
Discovery UK Biobank Replication 3 US cohorts |
85,852 39,924 |
EUR EUR |
|
| Grapefruit juice intake | Servings/day, self-reported, questionnaire | Discovery UK Biobank Replication 3 US cohorts |
85,852 39,924 |
EUR EUR |
|
| (Zhong et al., 2018) | Total sweet beverage intake | Sugary carbonated beverages, flavoured juice beverages with sugar, any low-calorie or diet non-alcoholic beverages, pure fruit juice (excluding grapefruit juice), flavored milk, hot chocolate Servings/day, self-reported, questionnaire |
Discovery UK Biobank Replication 3 US cohorts |
85,852 39,924 |
EUR EUR |
| Sugar sweetened beverages (SSBs) | Sugary carbonated beverages, flavoured juice beverages with sugar Servings/day, self-reported, questionnaire |
Discovery UK Biobank Replication 3 US cohorts |
85,852 39,924 |
EUR EUR |
|
| Artificially sweetened beverages (ASBs) | Any low-calorie or diet non-alcoholic beverages Servings/day, self-reported, questionnaire |
Discovery UK Biobank Replication 3 US cohorts |
85,852 39,924 |
EUR EUR |
|
| Pure non-grapefruit juices | Excluding grapefruit juice Servings/day, self-reported, questionnaire |
Discovery UK Biobank Replication 3 US cohorts |
85,852 39,924 |
EUR EUR |
Table 2.
Genome-wide significant loci for beverage consumption behavior
| Locus | Closest gene(s) | SNP, EA | EAF | Race Origin | Trait | Effect | Reference | Assoc. with other traits | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AFR | AMR | ASN | EU | ||||||||
| 1p35.2 | SERINC2 | rs4478858,G intronic | 0.55 | 0.5 | 0.79 | 0.45 | EU | AD | + | (Zuo, Lingjun et al., 2015; Zuo, L. et al., 2013) | |
| 1q21.3 | ANXA9 | rs12405726, A intronic | 0.09 | 0.5 | 0.43 | 0.36 | EU | bitter non-alcoholic beverage intake | + | (Zhong et al., 2018) | serum urate |
| 1q25.1 | KIAA0040, TNN | rs6701037, C intergenic | 0.33 | 0.32 | 0.09 | 0.46 | EU | AD | + | (Wang, K. S. et al., 2011) | |
| 1q25.1 | SERPINC1 | rs1799876, G intronic | 0.86 | 0.35 | 0.6 | 0.33 | EU | alcohol intake | − | (Xu et al., 2015) | |
| 1q25.2 | SEC16B | rs574367, T intergenic | 0.09 | 0.15 | 0.19 | 0.21 | EU | coffee intake | + | (Zhong et al., 2018) | BMI, obesity, menarche |
| 2p25.3 | TMEM18 | rs10865548, G intergenic | 0.93 | 0.85 | 0.9 | 0.83 | EU | coffee intake | + | (Zhong et al., 2018) | BMI, obesity, weight, menarche |
| 2p23.3 | GCKR | rs1260326, T missense | 0.12 | 0.41 | 0.56 | 0.41 | EU/AFR | coffee intake | − | (Cornelis, M. C. et al., 2015; Zhong et al., 2018) | waist circumference, lipid traits, serum glucose, chronic kidney disease, C-reactive protein, gamma-glutamyl transferase, serum albumin, serum urate, leptin |
| EU/AFR/ASN/HL | alcohol intake, bitter alcoholic beverage intake | − | (Clarke et al., 2017; Jorgenson et al., 2017; Zhong et al., 2018) borderline (Schumann, G. et al., 2011) | ||||||||
| EU | total bitter beverage intake | − | (Zhong et al., 2018) | ||||||||
| 2p16.1 | MTIF2 - CCDC88A | rs1437396,T intergenic | 0.11 | 0.17 | 0.5 | 0.2 | EU/AFR | AD | (Gelernter, J. et al., 2013) | ||
| 2p12 | CTNNA2 | rs140089781,A intronic | 0.0 | 0.0 | 0.02 | <0.01 | EU | alcohol intake (men) | − | (Clarke et al., 2017) | |
| 2q35 | PECR | rs7590720,G intronic | 0.25 | 0.26 | 0.31 | 0.29 | EU | AD | + | (Treutlein, J. et al., 2009) | |
| 3p12.1 | CADM2 | rs13078384,A intronic | 0.02 | 0.20 | 0.02 | 0.33 | EU | alcohol intake | + | (Clarke et al., 2017) | |
| 4p14 | KLB,U6 | rs7686419,A intergenic | 0.45 | 0.38 | 0.46 | 0.47 | EU/AFR/ASN/HL | alcohol intake | - | (Jorgenson et al., 2017) | |
| 4p14 | KLB | rs11940694,A intronic | 0.38 | 0.61 | 0.57 | 0.40 | EU | alcohol intake, bitter alcoholic beverage intake | − | (Clarke et al., 2017; Schumann, Gunter et al., 2016; Zhong et al., 2018) | |
| 4q22.1 | ABCG2 | rs1481012, A intronic | 0.98 | 0.85 | 0.71 | 0.89 | EU (AA) | coffee intake | + | (Cornelis, M. C. et al., 2015) | LDL, response to statin, serum urate, gout |
| total bitter beverage intake | + | (Zhong et al., 2018) | |||||||||
| 4q23 | ADH4-ADH7, ADH1A-C | rs1229984,T missense | 0.0 | 0.08 | 0.73 | <0.01 | EU/AFR/ASN/HL | AD, AD symptoms, alcohol intake, bitter alcoholic beverage intake | − | (Clarke et al., 2017; Frank et al., 2012; Gelernter, J. et al., 2013; Jorgenson et al., 2017; Kapoor, Manav et al., 2013; Park et al., 2013; Treutlein, Jens et al., 2017; Xu et al., 2015) | esophageal cancer, upper aerodigestive tract cancers, oral cavity cancer, oropharynx cancer, pulse pressure |
| 5q35.2 | CTB-33O18.3 | rs1363605,G intergenic | 0.6 | 0.73 | 0.43 | 0.81 | EU/AA | AD symptom count | + | (Chen, G. et al., 2017) | |
| 6p21.33 | NRM | rs2269705,A intronic | 0.71 | 0.88 | 0.95 | 0.94 | EU/AA | AD symptom count | − | (Chen, G. et al., 2017) | |
| 6p21.32 | HLA-DOA | rs9276975,T 3’ UTR | 0.06 | 0.15 | 0.17 | 0.15 | EU | white wine liking | + | (Pirastu et al., 2015) | |
| 6q21 | PDSS2 | rs2216084,T intronic | 0.96 | 0.64 | 0.99 | 0.67 | EU | coffee intake | + | (Pirastu, Kooyman, Robino, et al., 2016) | |
| 7p21.1 | AHR | rs4410790,T intergenic | 0.53 | 0.58 | 0.63 | 0.38 | EU/AA | coffee intake | − | (Cornelis, M. C. et al., 2015; Sulem et al., 2011; Zhong et al., 2018) borderline (Nakagawa-Senda et al., 2018) | albuminuria |
| EU | caffeine intake | (Cornelis, M. C. et al., 2011) | |||||||||
| EU | bitter non-alcoholic beverage intake | − | (Zhong et al., 2018) | ||||||||
| EU | total bitter beverage intake | − | (Zhong et al., 2018) | ||||||||
| 7q11.22 | AUTS2 | rs6943555,A intronic | 0.59 | 0.28 | 0.33 | 0.22 | EU | alcohol intake | + | (Schumann, G. et al., 2011) | |
| 7q11.23 | MLXIPL | rs7800944,T intronic | 0.61 | 0.82 | 0.89 | 0.72 | EU | coffee intake | − | (Cornelis, M. C. et al., 2015; Zhong et al., 2018) | lipid traits |
| bitter non-alcoholic beverage intake | − | (Zhong et al., 2018) | |||||||||
| 7q11.23 | POR | rs17685, A 3’UTR | 0.13 | 0.22 | 0.35 | 0.30 | EU/AA | coffee intake | + | (Cornelis, M. C. et al., 2015; Zhong et al., 2018) | |
| EU | bitter non-alcoholic beverage intake | + | (Zhong et al., 2018) | ||||||||
| EU | total bitter beverage intake | + | (Zhong et al., 2018) | ||||||||
| 7q31.1 | NRCAM | rs382140, A intergenic | 0.45 | 0.21 | 0.19 | 0.18 | EU | coffee intake | + | (Amin et al., 2012) | |
| 7q32.3 | PODXL | rs2909678, C Intergenic | 0.48 | 0.74 | 0.41 | 0.86 | EU/AA | AD symptom count | − | (Chen, G. et al., 2017) | |
| 11p14.2 | FIBIN | rs145671205,C intergenic | 0.04 | 0.07 | 0.06 | 0.07 | EU | coffee liking | − | (Pirastu, Kooyman, Traglia, et al., 2016) | |
| 11p11.2 | AGBL2 | rs7935528, A | 0.14 | 0.34 | 0.35 | 0.42 | EU | bitter alcoholic beverage intake | + | (Zhong et al., 2018) | |
| 11q12.1 | OR8U8 | rs597045, A intergenic | 0.97 | 0.82 | 0.86 | 0.69 | EU | coffee intake | + | (Zhong et al., 2018) | |
| 12q24.11-13 | ALDH2 | rs671, G missense | 1 | 1 | 0.88 | 1 | ASN | AD, AD symptoms, alcohol intake, flushing response | + | (Baik, I. et al., 2011; Frank et al., 2012; Gelernter, Joel et al., 2018; Jorgenson et al., 2017; Quillen, E. E. et al., 2014; Takeuchi et al., 2011) | lipid traits,, gamma-glutamyl transferase, serum urate, serum alpha-1-antitrypsin, esophageal cancer, hemoglobin, CHD, brain aneurysm, metabolic syndrome, BMI |
| ASN | coffee intake | − | (Nakagawa-Senda et al., 2018) | ||||||||
| 14q12 | AKAP6 | rs1956218, G intronic | 0.68 | 0.66 | 0.44 | 0.53 | EU | coffee intake | + | (Zhong et al., 2018) | |
| 14q23.1 | ARID4A | rs8012947, A intronic | 0.27 | 0.3 | 0.62 | 0.28 | EU | alcohol intake | + | (Clarke et al., 2017) | |
| 15q24.1 | CYP1A1-CYP1A2 | rs2472297, T intergenic | 0.02 | 0.09 | 0.0 | 0.24 | EU/AA | coffee intake | + | (Amin et al., 2012; Cornelis, M. C. et al., 2015; Sulem et al., 2011; Zhong et al., 2018) | albuminuria, urine albumin, |
| caffeine intake | + | (Cornelis, M. C. et al., 2011) | |||||||||
| bitter non-alcoholic beverage intake | + | (Zhong et al., 2018) | |||||||||
| total bitter beverage intake | + | (Zhong et al., 2018) | |||||||||
| 16q12.2 | FTO | rs55872725, c intronic | 0.95 | 0.74 | 0.84 | 0.59 | EU | SSB | + | (Zhong et al., 2018) | waist circumference, obesity, BMI, menarche, type 2 diabetes, adiposity, leptin |
| 17q11.2 | EFCAB5, NSRP1, SLC6A4 | rs9902453, A intronic | 0.92 | 0.48 | 0.29 | 0.55 | EU/AA | coffee intake | − | (Cornelis, M. C. et al., 2015) | |
| 18q21.32 | MC4R | rs66723169, A intergenic | 0.11 | 0.13 | 0.2 | 0.23 | EU | coffee intake | + | (Zhong et al., 2018) | BMI, obesity, height, |
| 22q11.23 | SPECC1L-ADORA2A | rs2330783, G intronic | 0.92 | 0.98 | 0 | 0.99 | EU | coffee intake | + | (Zhong et al., 2018) | |
| 22q13.2 | CSDC2 | rs9607819, G intronic | 0.74 | 0.52 | 0.91 | 0.81 | EU | bitter non-alcoholic beverage intake | + | (Zhong et al., 2018) | |
GWAS catalogue traits unrelated to traits listed in column “Trait”
Alcohol
Successful GWAS of alcohol-related traits have been undertaken in European, African American, Asian and Hispanic Latino populations. Most of these targeted AD and included study samples with a high proportion of individuals with comorbid psychiatric disorders and/or co-occurring drug dependence. Several efforts report null findings (Heath, A. C. et al., 2011; Kapoor, M. et al., 2014; Lydall et al., 2011; Mbarek et al., 2015; McGue, M. et al., 2013; Zuo, L. et al., 2012), but inability to replicate some loci may be a function of both case and control ascertainment (Frank et al., 2012; Mailman et al., 2007). Most robust are associations with potentially functional SNPs that alter alcohol metabolism (Fig.1). For example, the ADH1B rs1229984 T allele (48His) results in a 40 to 100-fold higher rate of alcohol to acetaldehyde metabolism (i.e ethanol oxidation)(Edenberg, Howard J, 2000; Hurley, Bosron, Stone, & Amzel, 1994). The ALDH2 rs671 A allele (504Lys) reduces ALDH2 activity and thus decreases acetaldehyde to acetate metabolism (i.e. acetaldehyde oxidation)(Bosron & Li, 1986; Enomoto, Takase, Yasuhara, & Takada, 1991; Harada, Misawa, Agarwal, & Goedde, 1980; Quillen, Ellen E. et al., 2014). Acetaldehyde is a toxic substance whose accumulation leads to a highly aversive reaction that includes facial flushing, nausea, and tachycardia. The ADH1B His and ALDH2 Lys variants influence alcohol drinking behavior by elevating blood acetaldehyde levels upon alcohol drinking which ultimately reduces susceptibility to developing alcohol drinking problems (Macgregor et al., 2009; Peng, Y. et al., 2010; Takeuchi et al., 2011; Yokoyama et al., 2008).
ALDH2 and ADH1B are the only susceptibility genes that were studied as candidate SNPs before they were highlighted via agnostic GWAS (Li, D., Zhao, & Gelernter, 2011; Takeuchi et al., 2011). Variants in SERINC2, KIAA0040, MTIF2-CCDC88A, and PECR have also been associated with AD in GWAS. Variation in SERINC2, encoding a transmembrane transporter of L-serine, may potentially alter glycine and glutamate neurotransmission contributing to hyperexcitability and negative affect during alcohol abstinence (Furuya & Watanabe, 2003; Hirabayashi & Furuya, 2008; Reilly et al., 2017; Smith, Q. R., 2000; Tabatabaie, Klomp, Berger, & De Koning, 2010). KIAA0040 has no obvious role in alcohol consumption behavior but is closely situated to i) ASTN1, which has been associated with AD and substance dependence (Gratacòs et al., 2009; Hill et al., 2012), ii) TNN encoding tenascin-N; involved in neurite outgrowth and cell migration in hippocampal explants and iii) TNR, encoding tenascin-R; an extracellular matrix protein expressed primarily in the central nervous system and has been related to multiple brain diseases (Reilly et al., 2017). CCDC88A is the most promising candidate at 2p16, a region also implicated in a previous linkage study of AD (Dick et al., 2010). CCDC88A is differentially expressed in AD (Gelernter, J. et al., 2013) and interacts with DISC1, a gene associated with both schizophrenia (Kim, J. Y. et al., 2012) and opioid dependence (Xie et al., 2014). PECR encodes a protein that participates in chain elongation of fatty acids and is an integral component of peroxisomes which play a key role in protection against oxidative stress particularly in glial cells (Di Cesare Mannelli, Zanardelli, Micheli, & Ghelardini, 2014; Varga, Czimmerer, & Nagy, 2011). Variants at 5q35, 6p21, and 7q32 have also been associated with AD symptoms but not clinically diagnosed AD.
Variants linked to AD may not necessarily equate with habitual alcohol consumption in general population. The latter has been addressed by independent GWAS often involving several 1000s of individuals. Indeed, aside from ALDH2 and ADH1B, none of the loci associated with habitual alcohol consumption associate with AD, although this may be a function of the study design rather than real biological differences between these traits. Variants in CADM2 associated with alcohol consumption have also been associated with cognitive ability, reproductive success, risk-taking propensity and cannabis use (Davies, G. et al., 2016; Day et al., 2016; Ibrahim-Verbaas et al., 2016; Stringer et al., 2017). Following-up on their well replicated KLB-alcohol association (Clarke et al., 2017; Jorgenson et al., 2017; Schumann, Gunter et al., 2016), Schuman et al (Schumann, Gunter et al., 2016) identified a liver-brain axis linking the liver hormone FGF21 with central regulation of alcohol intake involving β-Klotho receptor (encoded by KLB) in the brain. FGF21 is induced in liver and released into the blood in response to various metabolic stresses, including high-carbohydrate diets and alcohol (Dushay et al., 2015; Sanchez, Palou, & Pico, 2009; Zhao, C. et al., 2015). FGF21 was also shown to suppress sweet and alcohol preference in mice (Talukdar et al., 2016; von Holstein-Rathlou et al., 2016). The function of another GWAS AD candidate, AUTS2, is unknown, but significant differences in expression of AUTS2 in whole-brain extracts of mice selected for differences in voluntary alcohol consumption as well as reduced alcohol sensitivity in Drosophila with a downregulated AUTS2 homolog (Schumann, G. et al., 2011) support a potential role of AUTS2 in alcohol drinking behavior. The role of other loci associated with alcohol-related traits is unclear and/or require stronger support in independent studies.
Coffee
GWAS have identified multiple genetic variants associated with self-reported habitual coffee consumption (Amin et al., 2012; Coffee and Caffeine Genetics Consortium et al., 2015; Cornelis, M. C. et al., 2011; Sulem et al., 2011; Zhong et al., 2018), many of which point to caffeine-related pathways. All of these GWAS have been population-based but predominately of individuals of European Ancestry. The earlier GWAS confirmed loci near AHR, CYP1A2, POR, and ABCG2 which generally present with the largest effect sizes and likely impact drinking behavior indirectly by altering the metabolism of caffeine and thus the physiological levels of this compound available for its psychostimulant effects. CYP1A2, for example, is responsible for over 95% of caffeine metabolism (Thorn, Aklillu, Klein, & Altman, 2012). Indeed, a subsequent GWAS of circulating caffeine metabolite levels further informed the roles of these loci in caffeine metabolism (Cornelis, M. C. et al., 2016). Genetic variants leading to increased coffee/caffeine consumption associate with lower circulating caffeine levels and higher paraxanthine-to-caffeine ratio suggesting a fast caffeine metabolism phenotype (Cornelis, M. C. et al., 2016). Loci near ADORA2A, BDNF and SLC6A4 likely act directly on coffee drinking behavior by modulating the acute psychostimulant and rewarding properties of caffeine. GWAS and smaller follow-up studies have linked several of these loci to consumption of regular coffee, decaffeinated coffee, tea, total caffeine and water and further extended the findings to African American and Japanese populations (Coffee and Caffeine Genetics Consortium et al., 2015; McMahon, Taylor, Smith, & Munafo, 2014; Nakagawa-Senda et al., 2018; Taylor, Davey Smith, & Munafò, 2018). Only one locus is implicated in the sensory properties of coffee (OR8U8) and was discovered when GWAS sample sizes exceeded 300, 000 (Zhong et al., 2018). Variants near MLXIPL, GCKR, SEC16B, TMEM18, AKAP6 and MC4R have no obvious role in coffee or caffeine consumption but have previously been associated with other traits in GWAS notably obesity, glucose and lipids (Table 2)(MacArthur et al., 2017). A recent GWAS among Japanese reported an association between coffee consumption and the ALDH2 locus which persisted upon adjustment for alcohol intake, BMI and smoking and in stratified analysis based on alcohol drinking status (Nakagawa-Senda et al., 2018). Pirastu et al has performed GWAS of coffee intake (Pirastu, Kooyman, Robino, et al., 2016) and coffee liking (Pirastu, Kooyman, Traglia, et al., 2016) and identified associations with variants in PDSS2 and FIBIN, respectively. A role of proteins encoded by these genes in coffee intake or liking is unclear.
Bitter and Sweet Tasting Beverages
We recently conducted a GWAS of habitual bitter and sweet beverage consumption based on the premise that taste perceptions and preferences are heritable and determinants of beverage choice and intake (Zhong et al., 2018). Phenotypes consisted of groups of beverages characterized by similar taste (Table 1) as defined in earlier work (Cornelis, M. C., Tordoff, El-Sohemy, & van Dam, 2017). All loci associated with total bitter beverage consumption were previously associated with coffee intake in earlier GWAS (Table 2). Sub-phenotype analyses targeting the alcohol and caffeine components of beverages yielded additional loci and were discussed above with alcohol and coffee loci.
No locus was replicated for total sweet beverage consumption, but a GWAS of SSB yielded significant variants mapping to FTO, a well-established locus for BMI and obesity-related traits(MacArthur et al., 2017). We found that variants in FTO previously linked to higher BMI were associated with lower SSB consumption regardless of BMI adjustment and is consistent with a previous candidate gene study by Brunkwall et al (Brunkwall et al., 2013).
In summary, GWAS have discovered plausible as well as novel loci underlying alcohol and coffee drinking behavior. Many of these loci affect drinking behavior by modulating the physiological levels of bioactive constituents (i.e. acetaldehyde, caffeine). To our knowledge, no GWAS has identified variants specifically associated with consumption of tea, juice, milk or any other beverage intake. Differential success might be a function of heritability, SNP effect size, precision in drinking behavior measurement or other factors impeding power for detection.
Beverage-related loci mapping to GCKR, MLXIPL, FTO, MC4R, SEC16B, and TMEM18 are interesting since they are also GWAS loci for metabolic traits (Table 2). GCKR encodes the glucokinase regulatory protein that is produced by hepatocytes and is responsible for phosphorylation of glucose in the liver; a property that aligns more with metabolic traits than behavioral traits. FTO, MC4R, SEC16B, and TMEM18 are all obesity loci. The genetic variant in FTO that increases risk for obesity has also been associated with increased alcohol, coffee and sweet food consumption but lower consumption of soft drinks and SSB according to independent candidate SNP analysis (Brunkwall et al., 2013; Sobczyk-Kopciol et al., 2011; Zhong et al., 2018), adjusted for BMI. These findings might suggest that the effect of FTO on beverage choice might depend on the form (liquid versus solid) and energy density (high-caloric versus low-caloric), independent from FTO’s effect on BMI. Neverthless, increasing evidence suggests that a subset of BMI loci contribute to behavioral aspects of obesity by altering food and beverage intake (Brunkwall et al., 2013; Hasselbalch, Ann L et al., 2010; Loos & Yeo, 2014; Sobczyk-Kopciol et al., 2011; Willer et al., 2009).
GWAS of beverage drinking behavior highlight an important behavior-reward component to beverage choice and thus adds to understanding the link between genetics and beverage consumption and the potential barriers to dietary interventions. Interestingly, GWAS do not suggest a major role of variation in taste on drinking behaviors; a direction taken in prior candidate gene analysis.
4. IMPLICATIONS OF GENETIC KNOWLEDGE ON BEVERAGE CONSUMPTION
4.1. Research Tools
An immediate application of the loci identified via GWAS has been for optimizing epidemiological research on beverages and health. Nutritional epidemiology is often criticized for lack of reliable progress (Archer, Lavie, & Hill, 2018; Ioannidis, 2018; Trepanowski & Ioannidis, 2018). Difficulty detecting small effect sizes, accounting for confounding, measuring diet and suboptimal research reporting are amongst the problems with the field (Trepanowski & Ioannidis, 2018). Randomized control trials (RCTs) may address these problems but also bare limitations (Trepanowski & Ioannidis, 2018). Integrating genetic information by way of Mendelian randomization (MR) and gene- or SNP-diet interaction (G×D) analysis offers an efficient alternate solution to issues faced by traditional nutritional epidemiology. Because alleles segregate randomly from parents to offspring, offspring genotypes are unlikely to be associated with confounders in the population. Germ-line genotypes are fixed at conception, avoiding issues of reverse causation (Zheng et al., 2017). Moreover, the biological functions of the genes of interest might also provide insight to mechanisms linking a beverage to a disease. These genetic epidemiological approaches are not new but have garnered more attention in light of new and robust GWAS findings and the availability of large genetic data sets. While earlier applications have modeled single SNPs, more recent applications have, when possible, modeled several SNPs together as a “genetic score” (GS) to boost power while also addressing model assumption violations.
MR is a technique that uses genetic variants as instrumental variables (IVs) to assess whether an observational association between a risk factor and an outcome aligns with a causal effect. If a genetic variant alters the level of an exposure of interest, then this genetic variant should also be associated with disease risk and to the extent predicted by the effect of the genetic variant on the exposure (Davey Smith & Ebrahim, 2003; Katan, 1986). MR is a valid approach given the following assumptions are met: (1) the genetic variant is associated with the modifiable exposure of interest, (2) the genetic variant is not associated with confounders of the exposure to outcome association and (3) the genetic variant only influences the outcome through the exposure of interest (Davey Smith & Hemani, 2014). Study designs, statistical approaches and limitations of MR studies more generally have been reviewed in detail elsewhere (Davies, N. M., Holmes, & Smith, 2018; Glymour, Tchetgen, & Robins, 2012; Holmes, Michael V, Ala-Korpela, & Smith, 2017; Munafò, Tilling, Taylor, Evans, & Davey Smith, 2017; Paternoster, Tilling, & Smith, 2017; Zheng et al., 2017). Trait heterogeneity and pleiotropy are particular limitations concerning MRs of beverage consumption. A GS encompassing all beverage-trait SNPs will yield an IV reflecting multiple aspects of drinking behavior (Table 2). For coffee and alcohol, this may include caffeine or alcohol metabolism, reward-response and others. Such heterogeneity limits the ability to infer causality for particular dimensions of a beverage (e.g., alcohol vs non-alcohol) and makes interpretation of MR analyses more difficult (Holmes, Michael V et al., 2017; Zheng et al., 2017). Biological pleiotropy occurs when a genetic variant is associated with multiple exposures or traits and is therefore a violation of MR assumption 3 (Burgess & Thompson, 2015; Davey Smith & Hemani, 2014). Many loci associated with beverage drinking traits are more strongly associated with other traits based on GWAS (Table 2)(MacArthur et al., 2017). Whether this results from pleiotropy or a true causal relationship between a beverage and these other traits is unclear.
The term ‘interaction’ has various meanings but the focus of the current discussion is on G×D interaction, here defined as a joint effect of one or more genes with one or more dietary factors that cannot be readily explained by their separate marginal effects. By convention, a multiplicative model is taken as the null hypothesis: the relative risk of disease in individuals with both the genetic and dietary risk factors is the product of the relative risks of each separately. Therefore, any joint effect that differs from this prediction is considered to be a form of interaction (Rothman & Greenland, 1998; Thomas, 2010). The nature of the interaction can also vary. The main effect of both genotype and beverage intake may be greater in one stratum (i.e. intake level or genotype) than in the other strata, or it may have the opposite effect in one stratum compared with the others (Rothman & Greenland, 1998). Because some statistical techniques used in MR and G×D interactions are common it is sometimes difficult to distinguish between the approaches. While the primary goal of MR is to establish causality a unique feature of G×D interactions is they can potentially provide mechanistic insight into diet’s role in disease. Following are examples of how these methods have been applied to beverage and health research.
Alcohol
Most of the genetic epidemiological studies of alcohol have focused on the ALDH2 Glu504Lys (rs671) and ADH1B1 Arg48His (rs1229984) polymorphisms, wherein the ALDH2 Lys (A allele) and ADH1B1 His (T allele) variants associate with lower alcohol consumption due to adverse reaction to alcohol as a result of higher circulating acetaldehyde. These variants are most common in Asians (Table 2) and thus most genetic studies have included Asian populations. Several studies report that individuals with ALDH2 Lys/Lys genotype have no or a lower risk of esophageal cancer while those with Lys/Glu are at increased risk compared to Glu/Glu. This provides strong evidence that alcohol intake increases the risk of esophageal cancer and individuals whose genotype results in markedly lower intake (i.e. Lys/Lys) due to an adverse reaction to alcohol are thus protected (Fang et al., 2011; Lewis & Smith, 2005; Yang, S. J. et al., 2010; Zhang, G. H., Mai, R. Q., & Huang, B., 2010; Zhao, T. et al., 2015). Heterozygotes have a limited ability to metabolize acetaldehyde, but exhibit a less severe reaction than seen among Lys/Lys homozygotes, which enables them to drink considerable amounts of alcohol. An increased risk for this subgroup also suggests that alcohol increases risk through the carcinogenic action of acetaldehyde (Boccia et al., 2009). This is further supported by reports of ALDH2-alcohol interactions whereby both Lys/Lys and Lys/Glu are at increased risk of esophageal cancer when they also consume alcohol (Tanaka et al., 2010; Yang, S. J. et al., 2010; Zhang, G. H. et al., 2010). MRs and ALDH2×alcohol interaction analysis also support a causal role of alcohol intake (via acetaldehyde) in the development of gastric and head/neck cancers(Boccia et al., 2009; Hidaka et al., 2015; Matsuo et al., 2013; Shin et al., 2011; Wang, H. L., Zhou, Liu, & Zhang, 2014; Yang, S. et al., 2017). The genetic model of analysis (i.e. genotype-specific vs additive, dominant or recessive models) and the need to further account for drinking behavior appears important with regards to ALDH2 Glu504Lys and disregard for these may explain, in part, the inconsistent results in the literature pertaining to ALDH2 and other outcomes (Chen, B. et al., 2015; Choi, J. Y. et al., 2003; Guo, X.-F. et al., 2013; Kawase et al., 2009; Masaoka et al., 2016). ALDH2 Lys/Lys and Lys/Glu decreased risk of ovarian cancer vs Glu/Glu in a pooled analysis of Asians (Ugai et al., 2018) supporting a causal relationship between high alcohol intake and cancer risk. However, the association was independent of alcohol intake suggesting a potential violation of MR assumption 3 (or pleiotropy) or measurement error of alcohol.
Different findings arise for ALDH2, alcohol and cardiometabolic traits. The ALDH2 Lys/Lys or Lys/Glu genotypes increased risk of T2D compared to the Glu/Glu genotype (Li, G.-y. et al., 2017). ALDH2 Lys carriers have an increased risk of CHD, CAD and MI (Gu & Li, 2014; Han et al., 2013; Wang, Q. et al., 2013; Zhang, Wang, Fu, Zhao, & Kui, 2015) and present with an at-risk lipid profile (Cho et al., 2015; Sasakabe et al., 2018; Tabara et al., 2016). Although alcohol consumption was not assessed, by traditional MR interpretations this would suggest alcohol drinking is protective for these outcomes. ALDH2 also detoxifies reactive aldehydes, such as methylglyoxal and 4-hydroxynonenal, which derive from lipids and glucose and contribute to the formation of advanced glycation end products (Chen, C.-H. et al., 2008; Morita et al., 2013; Siraki & Shangari, 2005), implicated in T2D (Li, G.-y. et al., 2017). The formation of acetaldehyde adducts with apolipoprotein B may reduce the conversion of very low-density lipoprotein cholesterol to LDL cholesterol, which would decrease the serum LDL cholesterol level (Kesaniemi, Kervinen, & Miettinen, 1987; Savolainen, Baraona, & Lieber, 1987; Wehr, Rodo, Lieber, & Baraona, 1993). Inconsistencies in this area nevertheless arise whereby the Lys variant contributes to a lower risk of T2D (particularly in drinkers) (Peng, M. et al., 2018) and HTN(Chen, L., Smith, Harbord, & Lewis, 2008; Zhang, S. Y. et al., 2015).
For ADH1B1 Arg48His (rs1229984), each additional Arg variant (high alcohol consumer, slow acetaldehyde producer) increased risk of esophageal and other upper aerodigestive tract cancers (UADT) compared to His/His (Guo, H., Zhang, & Mai, 2012; Tanaka et al., 2010; Yang, S. J. et al., 2010; Zhang, G., Mai, R., & Huang, B., 2010; Zhang, G. H. et al., 2010). An interaction with alcohol drinking is also evident with risk being restricted to or even greater among drinkers with Arg/Arg or Arg/His genotypes (Guo, H. et al., 2012; Tanaka et al., 2010; Yang, S. J. et al., 2010; Zhang, G. et al., 2010; Zhang, G. H. et al., 2010). Taken together this suggests alcohol drinking and exposure to ethanol (not acetaldehyde) increases UADT cancer risk which differs slightly from results concerning ALDH2 and cancers. In a meta-analysis including individuals of European ethnicity, individuals with an ADH1B His variant presented with lower measures of adiposity, blood pressure and inflammation as well as a reduced risk of CHD than those with Arg/Arg; and most of these associations were null in non-drinkers. This suggests that reduction of alcohol consumption, even for light to moderate drinkers, is beneficial for cardiovascular health and is contrary to the J-shaped epidemiological associations between alcohol and CVD risk previously described (Holmes, M. V. et al., 2014; Toma, Pare, & Leong, 2017).
Three recent MRs of alcohol and lupus (Bae & Lee, 2018b), RA (Bae & Lee, 2018a) and BMD (Guo, R., Wu, & Fu, 2018) used 6 to 24 independent loci identified exclusively by Clark et al (Clarke et al., 2017) (Table 1) and provided no support for a causal role of alcohol intake in these outcomes. However, only variants near GCKR and KLB were among the selected loci that have been confirmed by others (Table 2) and several IV SNPs violate MR assumptions and are thus invalid.
Coffee
Cornelis and Munafo (Cornelis, Marilyn & Munafo, 2018) recently reviewed MR studies of coffee and caffeine consumption. To date, at least fifteen MR studies have investigated the causal role of coffee or caffeine use on risk of T2D, CVD, Alzheimer’s disease, Parkinson’s disease, gout, osteoarthritis, cancers, sleep disturbances and other substance use. The vast majority of study IVs included at least SNPs near CYP1A2 and AHR – the strongest and most robust variants linked to coffee drinking behavior (Table 2) and caffeine metabolite levels(Cornelis, M. C. et al., 2016). Single studies investigated and provided support for a causal role of coffee in reducing risk of gout (Poole et al., 2017) and increasing risk of osteoarthritis (Lee, Y. H., 2018). Four studies examined the co-occurrence of caffeine use and other substances with conflicting results (Bjørngaard et al., 2017; Treur et al., 2017; Verweij et al., 2013; Ware et al., 2017). For the remaining outcomes, studies did not provide clear support for a causal role of coffee or caffeine, but often acknowledged limitations (such as low statistical power, pleiotropy and collider bias), such that a causal role cannot yet be ruled out. In a 2014 review, over 30 gene–coffee interaction studies had been published(Cornelis, MC, 2014). Most have targeted the caffeine component of coffee but have examined a limited number of SNPs. Studies of cancers, CVD, Parkinson’s disease, and pregnancy outcomes were promising but rather preliminary. Studies suggest that the caffeine component of coffee may have adverse cardiovascular effects, but that these effects are limited to individuals with the genotype corresponding to impaired or slower caffeine metabolism (Cornelis, MC, 2014). Since 2014, additional studies have been published. For example, in a large UK study, coffee of any type was associated with lower risk of mortality regardless of genetic variation in caffeine metabolism (based on CYP1A2, AHR, POR and CYP2A6 (Table 2 variants)) (Loftfield et al., 2018). Casiglia et al (Casiglia et al., 2018) reported an inverse relationship between caffeine intake and incident atrial fibrillation and this was not modified by CYP1A2 variation. The same group reported better reasoning measures with higher caffeine intake, but this was apparent only among those with a CYP1A2 genotype corresponding to slow caffeine metabolism (Casiglia et al., 2017). Sasaki et al (Sasaki, Limpar, Sata, Kobayashi, & Kishi, 2017) reported an inverse association between maternal caffeine consumption and infant birth size only among mothers with the CYP1A2 genotype corresponding to rapid caffeine metabolism.
Milk
All MR studies of milk used the LCT-13910 C/T SNP as an IV for milk/dairy intake. Studies examined the causal role of milk/dairy in bone health, mortality, CVD and related traits, T2D, obesity and mortality (Bergholdt, H. K., Nordestgaard, & Ellervik, 2015; Bergholdt, H. K., Nordestgaard, Varbo, & Ellervik, 2015; Bergholdt, H. K. M., Larsen, Varbo, Nordestgaard, & Ellervik, 2018; Bergholdt, H. K. M., Nordestgaard, Varbo, & Ellervik, 2018; Hartwig, Horta, Smith, de Mola, & Victora, 2016; Lamri et al., 2013; Manco, Dias, Muc, & Padez, 2016; Smith, C. E. et al., 2016; Tognon et al., 2017; Yang, Q. et al., 2017). Results were null or inconsistent with the exception that the lactate persistant T allele (proxy for increased milk intake) may promote obesity (Manco et al., 2016; Yang, Q. et al., 2017).
4.2. Public Health
Personalized nutrition (PN) involves tailored dietary advice that can be delivered to individuals based on their diet and lifestyle factors. PN contrasts with the public health model which provides non-specific healthy eating advice. Since the completion of the human genome sequence, many direct-to-consumer (DTC) genetic testing services have been established and several target individuals who seek genetic–based PN (Bloss, Madlensky, Schork, & Topol, 2011; Guasch-Ferré, Dashti, & Merino, 2018). Whether genotype-based PN is scientifically sound, motivates behavior change beyond that provided by general advice or, rather, promotes a fatalistic attitude and decreased self-efficacy are just a few of the many questions being asked concerning genotype-based PN (Bouwman & te Molder, 2008; Guasch-Ferré et al., 2018).
In their recent review, O’Donovan et al (O’Donovan, Walsh, Gibney, Brennan, & Gibney, 2017) reported little evidence for the benefit of genotype-based PN on motivating behavior change. Test price and perceived seriousness of the disease were factors potentially impacting DTC testing on behavior. Horne et al (Horne, Madill, O’Connor, Shelley, & Gilliland, 2018) also reviewed research pertaining to lifestyle behavior change (nutrition, physical activity, sleep, and smoking) resulting from genetic testing interventions. The provision of actionable recommendations informed by genetic testing was more likely to facilitate behavior change than the provision of genetic information without actionable lifestyle recommendations. Several studies of good quality demonstrated changes in lifestyle habits, nutrition especially, arising from the provision of genetic interventions. More recently, the Food4Me proof-of-principle study set out to investigate the effect of varying levels of PN advice on health outcomes in comparison with general healthy eating advice (O’Donovan et al., 2017). PN advice resulted in greater dietary changes compared with general healthy eating advice, but no additional benefit was observed for PN advice based dietary intake, phenotype and genotype information than PN advise based solely on dietary intake (O’Donovan et al., 2017). Highly relevant to the current review is the work by Nielsen et al (Nielsen & El-Sohemy, 2012, 2014) who compared the effects of providing genotype-based dietary advice with general recommendations on behavioral outcomes. Participants in the intervention group were e-mailed a personalized dietary report providing recommendations for daily intakes of caffeine, vitamin C, sugar, and sodium based on genotypes for CYP1A2, GSTM1 and GSTT1, TAS1R2, and ACE, respectively. Compared to the control group, participants in the intervention group more likely agreed that they understood the dietary advice they were given and the dietary recommendations they received would be useful when considering their diet. However, the intervention resulted in greater changes in only sodium intake compared to general population-based dietary advice.
Overall, the current evidence does not appear to provide strong support for genotype-based PN with respect to motivating behavior change. Moreover, there is some evidence to suggest that personal genetic knowledge might demotivate or increase anxiety (Hollands et al., 2016; Marteau et al., 2010; O’Donovan et al., 2017). Because DTC genotyping services to prescribe PN are already available, more research in this area is warranted. Study design, SNP selection, control group for comparison, outcome measures and clinical relevance need all be considered in research going forward(O’Donovan et al., 2017; Shyam & Smith). Fundamental to initiating this research is scientific evidence supporting genotype-based PN advice which is currently sparse(Guasch-Ferré et al., 2018).
5. CONCLUSIONS
Beverages are important sources of water, energy, vitamins and minerals and non-nutrients. With their widespread consumption, availability and contributions to diet and nutrition there is great interest in the role beverages play in health. Understanding factors contributing to drinking behaviors therefore has important public health and research implications. Genetics is amongst these factors and in the last decade progress has been made in this area. GWAS have confirmed known candidate loci but have also identified novel loci underlying alcoholic beverage and coffee drinking behavior. Many of these loci indirectly affect drinking behavior by modulating the physiological levels of bioactive constituents (i.e. acetaldehyde, caffeine). Several loci overlap with those associated with other metabolic traits such as obesity. Relatively less progress has been made in identifying loci underlying the habitual consumption of other beverages such as tea, juice, SSB and milk. Thus far, GWAS of beverage drinking behavior highlight an important behavior-reward component (as opposed to taste) to beverage choice and thus adds to understanding the link between genetics and beverage consumption and the potential barriers to dietary interventions. Loci identified via GWAS have been used in epidemiological research involving MR and G×D interaction analysis. An excerpt of findings were discussed in the current review and highlights the need for careful design and results interpretation and, importantly, replication. This research is necessary as it informs the clinical relevance of SNP-beverage associations and thus genotype-based PN. The latter has and will continue to attract much interest in the commercial and public health sectors.
ACK
Funding: This work was supported by the National Institute on Deafness and Other Communication Disorders [R03DC01337301A1.
References
- Adkins DE, Clark SL, Copeland WE, Kennedy M, Conway K, Angold A, et al. (2015). Genome-Wide Meta-Analysis of Longitudinal Alcohol Consumption Across Youth and Early Adulthood. Twin Res Hum Genet, 18(4), 335–347. doi: 10.1017/thg.2015.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin N, Byrne E, Johnson J, Chenevix-Trench G, Walter S, Nolte IM, et al. (2012). Genome-wide association analysis of coffee drinking suggests association with CYP1A1/CYP1A2 and NRCAM. Mol Psychiatry, 17(11), 1116–1129. doi: 10.1038/mp.2011.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer E, Lavie CJ, & Hill JO (2018). The Failure to Measure Dietary Intake Engendered a Fictional Discourse on Diet-Disease Relations. Frontiers in Nutrition, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Babor T, Caetano R, Casswell S, Edwards G, & Giesbrecht N (2010). Alcohol: no ordinary commodity: research and public policy: Oxford University Press. [Google Scholar]
- Bae SC, & Lee YH (2018a). Alcohol intake and risk of rheumatoid arthritis: a Mendelian randomization study. Z Rheumatol. doi: 10.1007/s00393-018-0537-z [DOI] [PubMed] [Google Scholar]
- Bae SC, & Lee YH (2018b). Alcohol intake and risk of systemic lupus erythematosus: a Mendelian randomization study. Lupus, 961203318817832. doi: 10.1177/0961203318817832 [DOI] [PubMed] [Google Scholar]
- Baik I, Cho NH, Kim SH, Han BG, & Shin C (2011). Genome-wide association studies identify genetic loci related to alcohol consumption in Korean men. Am J Clin Nutr, 93(4), 809–816. [DOI] [PubMed] [Google Scholar]
- Baik J-H (2013). Dopamine signaling in food addiction: role of dopamine D2 receptors. BMB Rep, 46(11), 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett EL, Duesing K, Boyd L, Yates Z, Veysey M, & Lucock M (2017). A potential sex dimorphism in the relationship between bitter taste and alcohol consumption. Food Funct, 8(3), 1116–1123. doi: 10.1039/c6fo01759b [DOI] [PubMed] [Google Scholar]
- Behrens M, & Meyerhof W (2016). The vertebrate gustatory system Flavour, from food to perception, Oxford, UK: Wiley-Blackwell. [Google Scholar]
- Bergholdt HK, Nordestgaard BG, & Ellervik C (2015). Milk intake is not associated with low risk of diabetes or overweight-obesity: a Mendelian randomization study in 97,811 Danish individuals. Am J Clin Nutr, 102(2), 487–496. doi: 10.3945/ajcn.114.105049 [DOI] [PubMed] [Google Scholar]
- Bergholdt HK, Nordestgaard BG, Varbo A, & Ellervik C (2015). Milk intake is not associated with ischaemic heart disease in observational or Mendelian randomization analyses in 98,529 Danish adults. Int J Epidemiol, 44(2), 587–603. doi: 10.1093/ije/dyv109 [DOI] [PubMed] [Google Scholar]
- Bergholdt HKM, Larsen MK, Varbo A, Nordestgaard BG, & Ellervik C (2018). Lactase persistence, milk intake, hip fracture and bone mineral density: a study of 97 811 Danish individuals and a meta-analysis. J Intern Med. doi: 10.1111/joim.12753 [DOI] [PubMed] [Google Scholar]
- Bergholdt HKM, Nordestgaard BG, Varbo A, & Ellervik C (2018). Lactase persistence, milk intake, and mortality in the Danish general population: a Mendelian randomization study. Eur J Epidemiol, 33(2), 171–181. doi: 10.1007/s10654-017-0328-x [DOI] [PubMed] [Google Scholar]
- Bjørngaard JH, Nordestgaard AT, Taylor AE, Treur JL, Gabrielsen ME, Munafò MR, et al. (2017). Heavier smoking increases coffee consumption: findings from a Mendelian randomization analysis. Int. J. Epidemiol, 46(6), 1958–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block JP, Gillman MW, Linakis SK, & Goldman RE (2013). “If it tastes good, I’m drinking it”: qualitative study of beverage consumption among college students. Journal of Adolescent Health, 52(6), 702–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss CS, Madlensky L, Schork NJ, & Topol EJ (2011). Genomic information as a behavioral health intervention: can it work? Personalized medicine, 8(6), 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia S, Hashibe M, Galli P, De Feo E, Asakage T, Hashimoto T, et al. (2009). Aldehyde dehydrogenase 2 and head and neck cancer: a meta-analysis implementing a Mendelian randomization approach. Cancer Epidemiol Biomarkers Prev, 18(1), 248–254. doi: 10.1158/1055-9965.EPI-08-0462 [DOI] [PubMed] [Google Scholar]
- Boll W, Wagner P, & Mantei N (1991). Structure of the chromosomal gene and cDNAs coding for lactase-phlorizin hydrolase in humans with adult-type hypolactasia or persistence of lactase. Am. J. Hum. Genet, 48(5), 889. [PMC free article] [PubMed] [Google Scholar]
- Bosron WF, & Li TK (1986). Genetic polymorphism of human liver alcohol and aldehyde dehydrogenases, and their relationship to alcohol metabolism and alcoholism. Hepatology, 6(3), 502–510. [DOI] [PubMed] [Google Scholar]
- Bouwman LI, & te Molder HF (2008). About evidence based and beyond: a discourse-analytic study of stakeholders’ talk on involvement in the early development of personalized nutrition. Health education research, 24(2), 253–269. [DOI] [PubMed] [Google Scholar]
- Brunkwall L, Ericson U, Hellstrand S, Gullberg B, Orho-Melander M, & Sonestedt E (2013). Genetic variation in the fat mass and obesity-associated gene (FTO) in association with food preferences in healthy adults. Food Nutr Res, 57(1), 20028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, & Thompson SG (2015). Multivariable mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol, 181(4), 251–260. doi: 10.1093/aje/kwu283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor RM (2014). Analysis of Genetic Linkage In Reference Module in Biomedical Sciences: Elsevier. [Google Scholar]
- Prescott Carol A., & Kendler Kenneth S.. (1999). Genetic and Environmental Contributions to Alcohol Abuse and Dependence in a Population-Based Sample of Male Twins. American Journal of Psychiatry, 156(1), 34–40. doi: 10.1176/ajp.156.1.34 [DOI] [PubMed] [Google Scholar]
- Casiglia E, Tikhonoff V, Albertini F, Favaro J, Montagnana M, Danese E, et al. (2017). Caffeine intake and abstract reasoning among 1374 unselected men and women from general population. Role of the −163C>A polymorphism of CYP1A2 gene. Clin Nutr ESPEN, 20, 52–59. doi: 10.1016/j.clnesp.2017.04.001 [DOI] [PubMed] [Google Scholar]
- Casiglia E, Tikhonoff V, Albertini F, Gasparotti F, Mazza A, Montagnana M, et al. (2018). Caffeine intake reduces incident atrial fibrillation at a population level. Eur J Prev Cardiol, 25(10), 1055–1062. doi: 10.1177/2047487318772945 [DOI] [PubMed] [Google Scholar]
- Chen B, Hu KW, Zhang JW, Wei ZJ, Meng XL, & Xiong MM (2015). A critical analysis of the relationship between aldehyde dehydrogenases-2 Glu487Lys polymorphism and colorectal cancer susceptibility. Pathol Oncol Res, 21(3), 727–733. doi: 10.1007/s12253-014-9881-8 [DOI] [PubMed] [Google Scholar]
- Chen C-H, Budas GR, Churchill EN, Disatnik M-H, Hurley TD, & Mochly-Rosen D (2008). Activation of Aldehyde Dehydrogenase-2 Reduces Ischemic Damage to the Heart. Science, 321(5895), 1493–1495. doi: 10.1126/science.1158554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Zhang F, Xue W, Wu R, Xu H, Wang K, et al. (2017). An association study revealed substantial effects of dominance, epistasis and substance dependence comorbidity on alcohol dependence symptom count. Addiction biology, 22(6), 1475–1485. doi:doi: 10.1111/adb.12402 [DOI] [PubMed] [Google Scholar]
- Chen JY, Zhu HC, Guo Q, Shu Z, Bao XH, Sun F, et al. (2016). Dose-Dependent Associations between Wine Drinking and Breast Cancer Risk - Meta-Analysis Findings. Asian Pac J Cancer Prev, 17(3), 1221–1233. [DOI] [PubMed] [Google Scholar]
- Chen L, Smith GD, Harbord RM, & Lewis SJ (2008). Alcohol intake and blood pressure: a systematic review implementing a Mendelian randomization approach. PLoS Med, 5(3), e52. doi: 10.1371/journal.pmed.0050052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Shin SY, Won S, Relton CL, Davey Smith G, & Shin MJ (2015). Alcohol intake and cardiovascular risk factors: A Mendelian randomisation study. Sci Rep, 5, 18422. doi: 10.1038/srep18422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Lee J, Yang S, & Kim J (2017). Genetic variations in taste perception modify alcohol drinking behavior in Koreans. Appetite, 113, 178–186. doi: 10.1016/j.appet.2017.02.022 [DOI] [PubMed] [Google Scholar]
- Choi JY, Abel J, Neuhaus T, Ko Y, Harth V, Hamajima N, et al. (2003). Role of alcohol and genetic polymorphisms of CYP2E1 and ALDH2 in breast cancer development. Pharmacogenetics, 13(2), 67–72. doi: 10.1097/01.fpc.0000054060.98065.fc [DOI] [PubMed] [Google Scholar]
- Clarke TK, Adams MJ, Davies G, Howard DM, Hall LS, Padmanabhan S, et al. (2017). Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank (N=112 117). Mol Psychiatry, 22, 1376. doi: 10.1038/mp.2017.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffee and Caffeine Genetics Consortium, Cornelis MC, Byrne EM, Esko T, Nalls MA, Ganna A, et al. (2015). Genome-wide meta-analysis identifies six novel loci associated with habitual coffee consumption. Mol. Psychiatry, 20(5), 647–656. doi: 10.1038/mp.2014.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis M (2014). Gene-coffee interactions and health. Curr Nutr Rep, 3, 178–195. [Google Scholar]
- Cornelis M, & Munafo M (2018). Mendelian Randomization Studies of Coffee and Caffeine Consumption. Nutrients, 10(10), 1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis MC (2012). Coffee intake. Prog Mol Biol Transl Sci, 108, 293–322. [DOI] [PubMed] [Google Scholar]
- Cornelis MC, Byrne EM, Esko T, Nalls MA, Ganna A, Paynter N, et al. (2015). Genome-wide meta-analysis identifies six novel loci associated with habitual coffee consumption. Mol Psychiatry, 20(5), 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis MC, El-Sohemy A, & Campos H (2007). Genetic polymorphism of the adenosine A2A receptor is associated with habitual caffeine consumption. Am J Clin Nutr, 86(1), 240–244. [DOI] [PubMed] [Google Scholar]
- Cornelis MC, Kacprowski T, Menni C, Gustafsson S, Pivin E, Adamski J, et al. (2016). Genome-wide association study of caffeine metabolites provides new insights to caffeine metabolism and dietary caffeine-consumption behavior. Hum Mol Genet. doi: 10.1093/hmg/ddw334 [DOI] [PubMed] [Google Scholar]
- Cornelis MC, Monda KL, Yu K, Paynter N, Azzato EM, Bennett SN, et al. (2011). Genome-wide meta-analysis identifies regions on 7p21 (AHR) and 15q24 (CYP1A2) as determinants of habitual caffeine consumption. PLoS Genet, 7(4), e1002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis MC, Tordoff MG, El-Sohemy A, & van Dam RM (2017). Recalled taste intensity, liking and habitual intake of commonly consumed foods. Appetite, 109, 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey Smith G, & Ebrahim S (2003). ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol, 32(1), 1–22. [DOI] [PubMed] [Google Scholar]
- Davey Smith G, & Hemani G (2014). Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet, 23(R1), R89–98. doi: 10.1093/hmg/ddu328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G, Marioni RE, Liewald DC, Hill WD, Hagenaars SP, Harris SE, et al. (2016). Genome-wide association study of cognitive functions and educational attainment in UK Biobank (N= 112 151). Mol Psychiatry, 21(6), 758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies NM, Holmes MV, & Smith GD (2018). Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. Bmj, 362, k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day FR, Helgason H, Chasman DI, Rose LM, Loh P-R, Scott RA, et al. (2016). Physical and neurobehavioral determinants of reproductive onset and success. Nat. Genet, 48(6), 617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro JM (1993). A twin study of genetic and environmental influences on the intake of fluids and beverages. Physiol Behav, 54(4), 677–687. [DOI] [PubMed] [Google Scholar]
- de Gaetano G, Di Castelnuovo A, Rotondo S, Iacoviello L, & Donati MB (2002). A meta-analysis of studies on wine and beer and cardiovascular disease. Pathophysiol Haemost Thromb, 32(5–6), 353–355. doi: 10.1159/000073598 [DOI] [PubMed] [Google Scholar]
- Di Cesare Mannelli L, Zanardelli M, Micheli L, & Ghelardini C (2014). PPAR-γ impairment alters peroxisome functionality in primary astrocyte cell cultures. Biomed Res Int, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Meyers J, Aliev F, Nurnberger J Jr, Kramer J, Kuperman S, et al. (2010). Evidence for genes on chromosome 2 contributing to alcohol dependence with conduct disorder and suicide attempts. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 153(6), 1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayna D (2005). Human taste genetics. Annu. Rev. Genomics Hum. Genet, 6, 217–235. [DOI] [PubMed] [Google Scholar]
- Drewnowski A (1997). Taste preferences and food intake. Annu Rev Nutr, 17, 237–253. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, & Gomez-Carneros C (2000). Bitter taste, phytonutrients, and the consumer: a review. Am J Clin Nutr, 72(6), 1424–1435. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Henderson SA, Levine A, & Hann C (1999). Taste and food preferences as predictors of dietary practices in young women. Public Health Nutr, 2(4), 513–519. [DOI] [PubMed] [Google Scholar]
- Duffy VB, Davidson AC, Kidd JR, Kidd KK, Speed WC, Pakstis AJ, et al. (2004). Bitter receptor gene (TAS2R38), 6-n-propylthiouracil (PROP) bitterness and alcohol intake. Alcohol Clin Exp Res, 28(11), 1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushay JR, Toschi E, Mitten EK, Fisher FM, Herman MA, & Maratos-Flier E (2015). Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Molecular metabolism, 4(1), 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ (2000). Regulation of the mammalian alcohol dehydrogenase genes. [DOI] [PubMed]
- Edenberg HJ, & Foroud T (2006). The genetics of alcoholism: identifying specific genes through family studies. Addict Biol, 11(3–4), 386–396. doi: 10.1111/j.1369-1600.2006.00035.x [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, & Foroud T (2014). Genetics of alcoholism In Handbook of clinical neurology (Vol. 125, pp. 561–571): Elsevier. [DOI] [PubMed] [Google Scholar]
- EFSA (2010). Scientific opinion of the Panel on Dietetic Products, N., & Allergies on dietary reference values for water. EFSA Journal, 8(3), 1459. [Google Scholar]
- Electrolytes, I. o. M. P. o. D. R. I. f., & Water. (2005). DRI, dietary reference intakes for water, potassium, sodium, chloride, and sulfate: National Academy Press. [Google Scholar]
- Enattah NS, Sahi T, Savilahti E, Terwilliger JD, Peltonen L, & Järvelä I (2002). Identification of a variant associated with adult-type hypolactasia. Nat. Genet, 30(2), 233. [DOI] [PubMed] [Google Scholar]
- Enomoto N, Takase S, Yasuhara M, & Takada A (1991). Acetaldehyde metabolism in different aldehyde dehydrogenase-2 genotypes. Alcohol Clin Exp Res, 15(1), 141–144. [DOI] [PubMed] [Google Scholar]
- Eny KM, Corey PN, & El-Sohemy A (2009). Dopamine D2 receptor genotype (C957T) and habitual consumption of sugars in a free-living population of men and women. Lifestyle Genomics, 2(4–5), 235–242. [DOI] [PubMed] [Google Scholar]
- Euromonitor. (2018). Euromonitor International. Retrieved from https://www.euromonitor.com/ (accessed November, 2018)
- Fang P, Jiao S, Zhang X, Liu Z, Wang H, Gao Y, et al. (2011). Meta-analysis of ALDH2 variants and esophageal cancer in Asians. Asian Pac J Cancer Prev, 12(10), 2623–2627. [PubMed] [Google Scholar]
- Feeney E, O’Brien S, Scannell A, Markey A, & Gibney ER (2011). Genetic variation in taste perception: does it have a role in healthy eating? Proc Nutr Soc, 70(1), 135–143. doi: 10.1017/S0029665110003976 [DOI] [PubMed] [Google Scholar]
- Frank J, Cichon S, Treutlein J, Ridinger M, Mattheisen M, Hoffmann P, et al. (2012). Genome-wide significant association between alcohol dependence and a variant in the ADH gene cluster. Addiction biology, 17(1), 171–180. doi:doi: 10.1111/j.1369-1600.2011.00395.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya S, & Watanabe M (2003). Novel neuroglial and glioglial relationships mediated by L-serine metabolism. Arch Histol Cytol, 66(2), 109–121. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR, Sherva R, Almasy L, Koesterer R, Smith AH, et al. (2013). Genome-wide association study of alcohol dependence:significant findings in African- and European-Americans including novel risk loci. Mol Psychiatry, 19, 41. doi: 10.1038/mp.2013.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Zhou H, Nuñez YZ, Mutirangura A, Malison RT, & Kalayasiri R (2018). Genomewide Association Study of Alcohol Dependence and Related Traits in a Thai Population. Alcoholism: clinical and experimental research, 42(5), 861–868. doi:doi: 10.1111/acer.13614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijsbers L, Ding EL, Malik VS, de Goede J, Geleijnse JM, & Soedamah-Muthu SS (2016). Consumption of dairy foods and diabetes incidence: a dose-response meta-analysis of observational studies. Am J Clin Nutr, 103(4), 1111–1124. doi: 10.3945/ajcn.115.123216 [DOI] [PubMed] [Google Scholar]
- Glanz K, Basil M, Maibach E, Goldberg J, & Snyder D (1998). Why Americans eat what they do: taste, nutrition, cost, convenience, and weight control concerns as influences on food consumption. J Am Diet Assoc, 98(10), 1118–1126. [DOI] [PubMed] [Google Scholar]
- Glendinning JI (1994). Is the bitter rejection response always adaptive? Physiology & behavior, 56(6), 1217–1227. [DOI] [PubMed] [Google Scholar]
- Glymour MM, Tchetgen EJ, & Robins JM (2012). Credible Mendelian randomization studies: approaches for evaluating the instrumental variable assumptions. Am J Epidemiol, 175(4), 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratacòs M, Costas J, de Cid R, Bayés M, González J, Baca-García E, et al. (2009). Psychiatric Genetics Network Group: Identification of new putative susceptibility genes for several psychiatric disorders by association analysis of regulatory and non-synonymous SNPs of 306 genes involved in neurotransmission and neurodevelopment. Am J Med Genet B Neuropsychiatr Genet B, 150, 808–816. [DOI] [PubMed] [Google Scholar]
- Gu J. y., & Li L. w. (2014). ALDH2 Glu504Lys polymorphism and susceptibility to coronary artery disease and myocardial infarction in East Asians: a meta-analysis. Arch Med Res, 45(1), 76–83. [DOI] [PubMed] [Google Scholar]
- Guasch-Ferré M, Dashti HS, & Merino J (2018). Nutritional genomics and direct-to-consumer genetic testing: an overview. Advances in Nutrition, 9(2), 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Zhang G, & Mai R (2012). Alcohol dehydrogenase-1B Arg47His polymorphism and upper aerodigestive tract cancer risk: a meta-analysis including 24,252 subjects. Alcohol Clin Exp Res, 36(2), 272–278. doi: 10.1111/j.1530-0277.2011.01621.x [DOI] [PubMed] [Google Scholar]
- Guo J, Astrup A, Lovegrove JA, Gijsbers L, Givens DI, & Soedamah-Muthu SS (2017). Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol, 32(4), 269–287. doi: 10.1007/s10654-017-0243-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R, Wu L, & Fu Q (2018). Is There Causal Relationship of Smoking and Alcohol Consumption with Bone Mineral Density? A Mendelian Randomization Study. Calcif Tissue Int. doi: 10.1007/s00223-018-0452-y [DOI] [PubMed] [Google Scholar]
- Guo X-F, Wang J, Yu S-J, Song J, Ji M-Y, Zhang J-X, et al. (2013). Meta-analysis of the ADH1B and ALDH2 Polymorphisms and the Risk of Colorectal Cancer in East Asians. Internal Medicine, 52(24), 2693–2699. doi: 10.2169/internalmedicine.52.1202 [DOI] [PubMed] [Google Scholar]
- Han H, Wang H, Yin Z, Jiang H, Fang M, & Han J (2013). Association of genetic polymorphisms in ADH and ALDH2 with risk of coronary artery disease and myocardial infarction: a meta-analysis. Gene, 526(2), 134–141. doi: 10.1016/j.gene.2013.05.002 [DOI] [PubMed] [Google Scholar]
- Harada S, Misawa S, Agarwal DP, & Goedde HW (1980). Liver alcohol dehydrogenase and aldehyde dehydrogenase in the Japanese: isozyme variation and its possible role in alcohol intoxication. Am. J. Hum. Genet, 32(1), 8. [PMC free article] [PubMed] [Google Scholar]
- Hartwig FP, Horta BL, Smith GD, de Mola CL, & Victora CG (2016). Association of lactase persistence genotype with milk consumption, obesity and blood pressure: a Mendelian randomization study in the 1982 Pelotas (Brazil) Birth Cohort, with a systematic review and meta-analysis. Int J Epidemiol, 45(5), 1573–1587. doi: 10.1093/ije/dyw074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselbalch AL (2010). Genetics of dietary habits and obesity-a twin study. Dan Med Bull, 57(9), B4182–B4182. [PubMed] [Google Scholar]
- Hasselbalch AL, Angquist L, Christiansen L, Heitmann BL, Kyvik KO, & Sørensen TI (2010). A Variant in the Fat Mass and Obesity-Associated Gene (FTO) and Variants near the Melanocortin-4 Receptor Gene (MC4R) Do Not Influence Dietary Intake–3. The Journal of nutrition, 140(4), 831–834. [DOI] [PubMed] [Google Scholar]
- Hayes JE, Wallace MR, Knopik VS, Herbstman DM, Bartoshuk LM, & Duffy VB (2011). Allelic variation in TAS2R bitter receptor genes associates with variation in sensations from and ingestive behaviors toward common bitter beverages in adults. Chem Senses, 36(3), 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath A, & Martin N (1988). Teenage alcohol use in the Australian twin register: Genetic and social determinants of starting to drink. Alcoholism: clinical and experimental research, 12(6), 735–741. [DOI] [PubMed] [Google Scholar]
- Heath AC, Whitfield JB, Martin NG, Pergadia ML, Goate AM, Lind PA, et al. (2011). A quantitative-trait genome-wide association study of alcoholism risk in the community: findings and implications. Biol Psychiatry, 70(6), 513–518. doi: 10.1016/j.biopsych.2011.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka A, Sasazuki S, Matsuo K, Ito H, Sawada N, Shimazu T, et al. (2015). Genetic polymorphisms of ADH1B, ADH1C and ALDH2, alcohol consumption, and the risk of gastric cancer: the Japan Public Health Center-based prospective study. Carcinogenesis, 36(2), 223–231. doi: 10.1093/carcin/bgu244 [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Zezza N, Hoffman EK, Perlin M, & Allan W (2004). A genome wide search for alcoholism susceptibility genes. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 128B(1), 102–113. doi:doi: 10.1002/ajmg.b.30013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Weeks DE, Jones BL, Zezza N, & Stiffler S (2012). ASTN1 and alcohol dependence: Family-based association analysis in multiplex alcohol dependence families. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 159B(4), 445–455. doi:doi: 10.1002/ajmg.b.32048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi Y, & Furuya S (2008). Roles of l-serine and sphingolipid synthesis in brain development and neuronal survival. Prog Lipid Res, 47(3), 188–203. [DOI] [PubMed] [Google Scholar]
- Hirschhorn JN, & Daly MJ (2005). Genome-wide association studies for common diseases and complex traits. Nat Rev Genet, 6(2), 95–108. [DOI] [PubMed] [Google Scholar]
- Hollands GJ, French DP, Griffin SJ, Prevost AT, Sutton S, King S, et al. (2016). The impact of communicating genetic risks of disease on risk-reducing health behaviour: systematic review with meta-analysis. Bmj, 352, i1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MV, Ala-Korpela M, & Smith GD (2017). Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nature Reviews Cardiology, 14(10), 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MV, Dale CE, Zuccolo L, Silverwood RJ, Guo Y, Ye Z, et al. (2014). Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. Bmj, 349, g4164. doi: 10.1136/bmj.g4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne J, Madill J, O’Connor C, Shelley J, & Gilliland J (2018). A Systematic Review of Genetic Testing and Lifestyle Behaviour Change: Are We Using High-Quality Genetic Interventions and Considering Behaviour Change Theory? Lifestyle Genomics, 11(1), 49–63. doi: 10.1159/000488086 [DOI] [PubMed] [Google Scholar]
- Hurley TD, Bosron WF, Stone CL, & Amzel LM (1994). Structures of three human β alcohol dehydrogenase variants: Correlations with their functional differences. Journal of molecular biology, 239(3), 415–429. [DOI] [PubMed] [Google Scholar]
- Ibrahim-Verbaas C, Bressler J, Debette S, Schuur M, Smith A, Bis J, et al. (2016). GWAS for executive function and processing speed suggests involvement of the CADM2 gene. Mol Psychiatry, 21(2), 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JA (2018). The challenge of reforming nutritional epidemiologic research. Jama, 320(10), 969–970. doi: 10.1001/jama.2018.11025 [DOI] [PubMed] [Google Scholar]
- Jéquier E, & Constant F (2009). Water as an essential nutrient: the physiological basis of hydration. European journal of clinical nutrition, 64, 115. doi: 10.1038/ejcn.2009.111 [DOI] [PubMed] [Google Scholar]
- Jorgenson E, Thai KK, Hoffmann TJ, Sakoda LC, Kvale MN, Banda Y, et al. (2017). Genetic contributors to variation in alcohol consumption vary by race/ethnicity in a large multi-ethnic genome-wide association study. Mol Psychiatry, 22(9), 1359–1367. doi: 10.1038/mp.2017.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan NM, Palmer BF, & Denke MA (2000). Nutritional and health benefits of beer. The American journal of the medical sciences, 320(5), 320–326. [DOI] [PubMed] [Google Scholar]
- Kapoor M, Wang J-C, Wetherill L, Le N, Bertelsen S, Hinrichs AL, et al. (2013). A meta-analysis of two genome-wide association studies to identify novel loci for maximum number of alcoholic drinks. Human Genetics, 132(10), 1141–1151. doi: 10.1007/s00439-013-1318-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M, Wang JC, Wetherill L, Le N, Bertelsen S, Hinrichs AL, et al. (2014). Genome-wide survival analysis of age at onset of alcohol dependence in extended high-risk COGA families. Drug Alcohol Depend, 142, 56–62. doi: 10.1016/j.drugalcdep.2014.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan MB (1986). Apolipoprotein E isoforms, serum cholesterol, and cancer. Lancet, 1(8479), 507–508. [DOI] [PubMed] [Google Scholar]
- Kawase T, Matsuo K, Hiraki A, Suzuki T, Watanabe M, Iwata H, et al. (2009). Interaction of the effects of alcohol drinking and polymorphisms in alcohol-metabolizing enzymes on the risk of female breast cancer in Japan. J Epidemiol, 19(5), 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS (1993). Twin studies of psychiatric illness. Current status and future directions. Arch Gen Psychiatry, 50(11), 905–915. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kalsi G, Holmans PA, Sanders AR, Aggen SH, Dick DM, et al. (2011). Genomewide association analysis of symptoms of alcohol dependence in the molecular genetics of schizophrenia (MGS2) control sample. Alcohol Clin Exp Res, 35(5), 963–975. doi: 10.1111/j.1530-0277.2010.01427.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesaniemi YA, Kervinen K, & Miettinen TA (1987). Acetaldehyde modification of low density lipoprotein accelerates its catabolism in man. Eur J Clin Invest, 17(1), 29–36. [DOI] [PubMed] [Google Scholar]
- Kim JY, Liu CY, Zhang F, Duan X, Wen Z, Song J, et al. (2012). Interplay between DISC1 and GABA signaling regulates neurogenesis in mice and risk for schizophrenia. Cell, 148(5), 1051–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U-K, Breslin P, Reed D, & Drayna D (2004). Genetics of human taste perception. J Dent Res, 83(6), 448–453. [DOI] [PubMed] [Google Scholar]
- Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, & Drayna D (2003). Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science, 299(5610), 1221–1225. [DOI] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2016). Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry, 3(8), 760–773. doi: 10.1016/S2215-0366(16)00104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsche E, Knibbe R, Gmel G, & Engels R (2005). Why do young people drink? A review of drinking motives. Clin Psychol Rev, 25(7), 841–861. doi: 10.1016/j.cpr.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Lamri A, Poli A, Emery N, Bellili N, Velho G, Lantieri O, et al. (2013). The lactase persistence genotype is associated with body mass index and dairy consumption in the D.E.S.I.R. study. Metabolism, 62(9), 1323–1329. doi: 10.1016/j.metabol.2013.04.006 [DOI] [PubMed] [Google Scholar]
- Larsson SC, Crippa A, Orsini N, Wolk A, & Michaelsson K (2015). Milk Consumption and Mortality from All Causes, Cardiovascular Disease, and Cancer: A Systematic Review and Meta-Analysis. Nutrients, 7(9), 7749–7763. doi: 10.3390/nu7095363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Fu Z, Chung M, Jang DJ, & Lee HJ (2018). Role of milk and dairy intake in cognitive function in older adults: a systematic review and meta-analysis. Nutr J, 17(1), 82. doi: 10.1186/s12937-018-0387-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH (2018). Investigating the possible causal association of coffee consumption with osteoarthritis risk using a Mendelian randomization analysis. Clin Rheumatol, 1–7. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, & Smith GD (2005). Alcohol, ALDH2, and esophageal cancer: a meta-analysis which illustrates the potentials and limitations of a Mendelian randomization approach. Cancer Epidemiol Biomarkers Prev, 14(8), 1967–1971. doi: 10.1158/1055-9965.EPI-05-0196 [DOI] [PubMed] [Google Scholar]
- Li D, Zhao H, & Gelernter J (2011). Strong Association of the Alcohol Dehydrogenase 1B Gene (ADH1B) with Alcohol Dependence and Alcohol-Induced Medical Diseases. Biol Psychiatry, 70(6), 504–512. doi: 10.1016/j.biopsych.2011.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, An S. l., Zhou Y, Liang Z. k., Jiao Z. j., Jing Y. m., et al. (2011). Milk and Dairy Consumption and Risk of Bladder Cancer: A Meta-analysis. Urology, 78(6), 1298–1305. doi: 10.1016/j.urology.2011.09.002 [DOI] [PubMed] [Google Scholar]
- Li G. y., Li Z. b., Li F, Dong L. p., Tang L, Xiang J, et al. (2017). Meta-Analysis on the Association of ALDH2 Polymorphisms and Type 2 Diabetic Mellitus, Diabetic Retinopathy. Int J Environ Res Public Health, 14(2), 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Tang W, Sang L, Dai X, Wei D, Luo Y, et al. (2015). Milk, yogurt, and lactose intake and ovarian cancer risk: a meta-analysis. Nutr Cancer, 67(1), 68–72. doi: 10.1080/01635581.2014.956247 [DOI] [PubMed] [Google Scholar]
- Loftfield E, Cornelis MC, Caporaso N, Yu K, Sinha R, & Freedman N (2018). Association of coffee drinking with mortality by genetic variation in caffeine metabolism: Findings from the uk biobank. JAMA Internal Medicine, 178(8), 1086–1097. doi: 10.1001/jamainternmed.2018.2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JC, Knowler WC, Hanson RL, Robin RW, Urbanek M, Moore E, et al. (1998). Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet C Semin Med Genet, 81(3), 216–221. [DOI] [PubMed] [Google Scholar]
- Loos RJ, & Yeo GS (2014). The bigger picture of FTO—the first GWAS-identified obesity gene. Nature Reviews Endocrinology, 10(1), 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M, Kirk KM, Heath AC, & Martin NG (2005). The genetics of tea and coffee drinking and preference for source of caffeine in a large community sample of Australian twins. Addiction, 100(10), 1510–1517. [DOI] [PubMed] [Google Scholar]
- Lydall GJ, Bass NJ, McQuillin A, Lawrence J, Anjorin A, Kandaswamy R, et al. (2011). Confirmation of prior evidence of genetic susceptibility to alcoholism in a genome-wide association study of comorbid alcoholism and bipolar disorder. Psychiatr Genet, 21(6), 294–306. doi: 10.1097/YPG.0b013e32834915c2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur J, Bowler E, Cerezo M, Gil L, Hall P, Hastings E, et al. (2017). The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog). Nucleic acids research, 45(D1), D896–D901. doi: 10.1093/nar/gkw1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macgregor S, Lind PA, Bucholz KK, Hansell NK, Madden PA, Richter MM, et al. (2009). Associations of ADH and ALDH2 gene variation with self report alcohol reactions, consumption and dependence: an integrated analysis. Hum Mol Genet, 18(3), 580–593. doi: 10.1093/hmg/ddn372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailman MD, Feolo M, Jin Y, Kimura M, Tryka K, Bagoutdinov R, et al. (2007). The NCBI dbGaP database of genotypes and phenotypes. Nat Genet, 39(10), 1181–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainland JD, & Matsunami H (2009). Taste perception: how sweet it is (to be transcribed by you). Current Biology, 19(15), R655–R656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, & Hu FB (2010). Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care, 33(11), 2477–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik VS, Schulze MB, & Hu FB (2006). Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr, 84(2), 274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manco L, Dias H, Muc M, & Padez C (2016). The lactase −13910C>T polymorphism (rs4988235) is associated with overweight/obesity and obesity-related variables in a population sample of Portuguese young adults. European journal of clinical nutrition, 71, 21. doi: 10.1038/ejcn.2016.164 [DOI] [PubMed] [Google Scholar]
- Marteau TM, French DP, Griffin SJ, Prevost AT, Sutton S, Watkinson C, et al. (2010). Effects of communicating DNA-based disease risk estimates on risk-reducing behaviours. Cochrane Database of Systematic Reviews(10). [DOI] [PubMed] [Google Scholar]
- Marventano S, Salomone F, Godos J, Pluchinotta F, Del Rio D, Mistretta A, et al. (2016). Coffee and tea consumption in relation with non-alcoholic fatty liver and metabolic syndrome: A systematic review and meta-analysis of observational studies. Clinical nutrition, 35(6), 1269–1281. [DOI] [PubMed] [Google Scholar]
- Masaoka H, Ito H, Soga N, Hosono S, Oze I, Watanabe M, et al. (2016). Aldehyde dehydrogenase 2 (ALDH2) and alcohol dehydrogenase 1B (ADH1B) polymorphisms exacerbate bladder cancer risk associated with alcohol drinking: gene-environment interaction. Carcinogenesis, 37(6), 583–588. doi: 10.1093/carcin/bgw033 [DOI] [PubMed] [Google Scholar]
- Matsuo K, Oze I, Hosono S, Ito H, Watanabe M, Ishioka K, et al. (2013). The aldehyde dehydrogenase 2 (ALDH2) Glu504Lys polymorphism interacts with alcohol drinking in the risk of stomach cancer. Carcinogenesis, 34(7), 1510–1515. doi: 10.1093/carcin/bgt080 [DOI] [PubMed] [Google Scholar]
- Matsuo K, Wakai K, Hirose K, Ito H, Saito T, & Tajima K (2006). Alcohol dehydrogenase 2 His47Arg polymorphism influences drinking habit independently of aldehyde dehydrogenase 2 Glu487Lys polymorphism: analysis of 2,299 Japanese subjects. Cancer Epidemiol Biomarkers Prev, 15(5), 1009–1013. doi: 10.1158/1055-9965.EPI-05-0911 [DOI] [PubMed] [Google Scholar]
- Mbarek H, Milaneschi Y, Fedko IO, Hottenga JJ, de Moor MH, Jansen R, et al. (2015). The genetics of alcohol dependence: Twin and SNP-based heritability, and genome-wide association study based on AUDIT scores. Am J Med Genet B Neuropsychiatr Genet, 168(8), 739–748. doi: 10.1002/ajmg.b.32379 [DOI] [PubMed] [Google Scholar]
- McGue M (1999). Phenotyping Alcoholism. Alcoholism: clinical and experimental research, 23(5), 757–758. doi:doi: 10.1111/j.1530-0277.1999.tb04180.x [DOI] [PubMed] [Google Scholar]
- McGue M, Zhang Y, Miller MB, Basu S, Vrieze S, Hicks B, et al. (2013). A genome-wide association study of behavioral disinhibition. Behav Genet, 43(5), 363–373. doi: 10.1007/s10519-013-9606-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon G, Taylor AE, Smith GD, & Munafo MR (2014). Phenotype refinement strengthens the association of AHR and CYP1A1 genotype with caffeine consumption. PLoS ONE, 9(7), e103448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Pepino MY, & Reed DR (2005). Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics, 115(2), e216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Saruwatari J, Miyagawa H, Uchiyashiki Y, Oniki K, Sakata M, et al. (2013). Association between aldehyde dehydrogenase 2 polymorphisms and the incidence of diabetic retinopathy among Japanese subjects with type 2 diabetes mellitus. Cardiovascular Diabetology, 12(1), 132. doi: 10.1186/1475-2840-12-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullie P, Pizot C, & Autier P (2016). Daily milk consumption and all-cause mortality, coronary heart disease and stroke: a systematic review and meta-analysis of observational cohort studies. BMC Public Health, 16(1), 1236. doi: 10.1186/s12889-016-3889-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Tilling K, Taylor AE, Evans DM, & Davey Smith G (2017). Collider scope: when selection bias can substantially influence observed associations. Int. J. Epidemiol, 47(1), 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa-Senda H, Hachiya T, Shimizu A, Hosono S, Oze I, Watanabe M, et al. (2018). A genome-wide association study in the Japanese population identifies the 12q24 locus for habitual coffee consumption: The J-MICC Study. Sci Rep, 8(1), 1493. doi: 10.1038/s41598-018-19914-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, & Cardon LR (1994). Methodology for genetic studies of twins and families. Statistics in medicine, 13, 199–199. [Google Scholar]
- Neumark-Sztainer D, Story M, Perry C, & Casey MA (1999). Factors influencing food choices of adolescents: findings from focus-group discussions with adolescents. Journal of the American dietetic association, 99(8), 929–937. [DOI] [PubMed] [Google Scholar]
- Neves MF, Trombin VG, Lopes FF, Kalaki R, & Milan P (2012). World consumption of beverages In The orange juice business: A Brazilian perspective (pp. 118–118). Wageningen: Wageningen Academic Publishers. [Google Scholar]
- Nielsen DE, & El-Sohemy A (2012). A randomized trial of genetic information for personalized nutrition. Genes Nutr, 7(4), 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DE, & El-Sohemy A (2014). Disclosure of genetic information and change in dietary intake: a randomized controlled trial. PLoS ONE, 9(11), e112665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissim I, Dagan-Wiener A, & Niv MY (2017). The taste of toxicity: A quantitative analysis of bitter and toxic molecules. IUBMB Life, 69(12), 938–946. [DOI] [PubMed] [Google Scholar]
- O’Donovan CB, Walsh MC, Gibney MJ, Brennan L, & Gibney ER (2017). Knowing your genes: does this impact behaviour change? Proceedings of the Nutrition Society, 76(3), 182–191. doi: 10.1017/S0029665116002949 [DOI] [PubMed] [Google Scholar]
- Ong JS, Hwang DL, Zhong VW, An J, Gharahkhani P, Breslin PAS, et al. (2018). Understanding the role of bitter taste perception in coffee, tea and alcohol consumption through Mendelian randomization. Sci Rep, 8(1), 16414. doi: 10.1038/s41598-018-34713-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi SX, Lee PL, Law HY, & Say YH (2010). Bitter receptor gene (TAS2R38) P49A genotypes and their associations with aversion to vegetables and sweet/fat foods in Malaysian subjects. Asia Pac J Clin Nutr, 19(4), 491–498. [PubMed] [Google Scholar]
- Pang J, Zhang Z, Zheng TZ, Bassig BA, Mao C, Liu X, et al. (2016). Green tea consumption and risk of cardiovascular and ischemic related diseases: A meta-analysis. Int J Cardiol, 202, 967–974. doi: 10.1016/j.ijcard.2014.12.176 [DOI] [PubMed] [Google Scholar]
- Park BL, Kim JW, Cheong HS, Kim LH, Lee BC, Seo CH, et al. (2013). Extended genetic effects of ADH cluster genes on the risk of alcohol dependence: from GWAS to replication. Hum Genet, 132(6), 657–668. doi: 10.1007/s00439-013-1281-8 [DOI] [PubMed] [Google Scholar]
- Paternoster L, Tilling K, & Smith GD (2017). Genetic epidemiology and Mendelian randomization for informing disease therapeutics: Conceptual and methodological challenges. PLoS Genet, 13(10), e1006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M, Zhang J, Zeng T, Hu X, Min J, Tian S, et al. (2018). Alcohol consumption and diabetes risk in a Chinese population: a Mendelian randomization analysis. Addiction. doi: 10.1111/add.14475 [DOI] [PubMed] [Google Scholar]
- Peng Y, Shi H, Qi X. b., Xiao C. j., Zhong H, Ma R.-l. Z., et al. (2010). The ADH1B Arg47His polymorphism in East Asian populations and expansion of rice domestication in history. BMC Evolutionary Biology, 10(1), 15. doi: 10.1186/1471-2148-10-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna S, Riva A, Nicosanti G, Carrai M, Barale R, Vigo B, et al. (2018). Association of the bitter taste receptor gene TAS2R38 (polymorphism RS713598) with sensory responsiveness, food preferences, biochemical parameters and body-composition markers. A cross-sectional study in Italy. Int J Food Sci Nutr, 69(2), 245–252. doi: 10.1080/09637486.2017.1353954 [DOI] [PubMed] [Google Scholar]
- Pirastu N, Kooyman M, Robino A, van der Spek A, Navarini L, Amin N, et al. (2016). Non-additive genome-wide association scan reveals a new gene associated with habitual coffee consumption. Sci Rep, 6, 31590. doi: 10.1038/srep31590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirastu N, Kooyman M, Traglia M, Robino A, Willems SM, Pistis G, et al. (2016). A Genome-Wide Association Study in isolated populations reveals new genes associated to common food likings. Reviews in Endocrine and Metabolic Disorders, 17(2), 209–219. [DOI] [PubMed] [Google Scholar]
- Pirastu N, Kooyman M, Traglia M, Robino A, Willems SM, Pistis G, et al. (2015). Genome-wide association analysis on five isolated populations identifies variants of the HLA-DOA gene associated with white wine liking. Eur J Hum Genet, 23(12), 1717–1722. doi: 10.1038/ejhg.2015.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirastu N, Kooyman M, Traglia M, Robino A, Willems SM, Pistis G, et al. (2014). Association Analysis of Bitter Receptor Genes in Five Isolated Populations Identifies a Significant Correlation between TAS2R43 Variants and Coffee Liking. PLoS ONE, 9(3), e92065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirastu N, Robino A, Lanzara C, Athanasakis E, Esposito L, Tepper BJ, et al. (2012). Genetics of Food Preferences: A First View from Silk Road Populations. J Food Sci. [DOI] [PubMed] [Google Scholar]
- Poole R, Kennedy OJ, Roderick P, Fallowfield JA, Hayes PC, & Parkes J (2017). Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. Bmj, 359, j5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillen EE, Chen X-D, Almasy L, Yang F, He H, Li X, et al. (2014). ALDH2 is associated to alcohol dependence and is the major genetic determinant of “daily maximum drinks” in a GWAS study of an isolated rural chinese sample. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 165(2), 103–110. doi:doi: 10.1002/ajmg.b.32213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillen EE, Chen XD, Almasy L, Yang F, He H, Li X, et al. (2014). ALDH2 is associated to alcohol dependence and is the major genetic determinant of “daily maximum drinks” in a GWAS study of an isolated rural Chinese sample. Am J Med Genet B Neuropsychiatr Genet, 165B(2), 103–110. doi: 10.1002/ajmg.b.32213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Lopez O, Panduro A, Rivera-Iñiguez I, & Roman S (2018). Dopamine D2 receptor polymorphism (C957T) is associated with sugar consumption and triglyceride levels in West Mexicans. Physiology & behavior, 194, 532–537. [DOI] [PubMed] [Google Scholar]
- Ramos-Lopez O, Roman S, Martinez-Lopez E, Gonzalez-Aldaco K, Ojeda-Granados C, Sepulveda-Villegas M, et al. (2015). Association of a novel TAS2R38 haplotype with alcohol intake among Mexican-Mestizo population. Ann Hepatol, 14(5), 729–734. [PubMed] [Google Scholar]
- Reed T, Slemenda CW, Viken RJ, Christian JC, Carmelli D, & Fabsitz RR (1994). Correlations of Alcohol Consumption with Related Covariates and Heritability Estimates in Older Adult Males Over a 14- to 18-Year Period: The NHLBI Twin Study. Alcoholism: clinical and experimental research, 18(3), 702–710. doi:doi: 10.1111/j.1530-0277.1994.tb00934.x [DOI] [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, et al. (1998). Genome-wide search for genes affecting the risk for alcohol dependence. Am J Med Genet C Semin Med Genet, 81(3), 207–215. doi:doi: [DOI] [PubMed] [Google Scholar]
- Reilly MT, Noronha A, Goldman D, & Koob GF (2017). Genetic studies of alcohol dependence in the context of the addiction cycle. Neuropharmacology, 122, 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, & Greenland S (Eds.). (1998). Modern epidemiology. Philadelphia, PA: Lippincott Williams and Wilkins. [Google Scholar]
- Rothman KJ GS, Lash TL. (2008). Modern Epidemiology (3rd ed.): Lippincott, Williams, & Wilkins. [Google Scholar]
- Sahi T (1994). Genetics and epidemiology of adult-type hypolactasia. Scandinavian Journal of Gastroenterology, 29(sup202), 7–20. [DOI] [PubMed] [Google Scholar]
- Sahi T, Isokoski M, Jussila J, & Launiala K (1972). Lactose malabsorption in Finnish children of school age. Acta Pædiatrica, 61(1), 11–16. [DOI] [PubMed] [Google Scholar]
- Sanchez-Roige S, Fontanillas P, Elson SL, Gray JC, de Wit H, Davis LK, et al. Genome-wide association study of alcohol use disorder identification test (AUDIT) scores in 20 328 research participants of European ancestry. Addiction biology, 0(0). doi:doi: 10.1111/adb.12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez J, Palou A, & Pico C (2009). Response to carbohydrate and fat refeeding in the expression of genes involved in nutrient partitioning and metabolism: striking effects on fibroblast growth factor-21 induction. Endocrinology, 150(12), 5341–5350. [DOI] [PubMed] [Google Scholar]
- Sasakabe T, Wakai K, Kawai S, Hishida A, Naito M, Suzuki S, et al. (2018). Modification of the Associations of Alcohol Intake With Serum Low-Density Lipoprotein Cholesterol and Triglycerides by ALDH2 and ADH1B Polymorphisms in Japanese Men. J Epidemiol, 28(4), 185–193. doi: 10.2188/jea.JE20160189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Limpar M, Sata F, Kobayashi S, & Kishi R (2017). Interaction between maternal caffeine intake during pregnancy and CYP1A2 C164A polymorphism affects infant birth size in the Hokkaido study. Pediatr Res, 82(1), 19–28. doi: 10.1038/pr.2017.70 [DOI] [PubMed] [Google Scholar]
- Savolainen MJ, Baraona E, & Lieber CS (1987). Acetaldehyde binding increases the catabolism of rat serum low-density lipoproteins. Life Sci, 40(9), 841–846. [DOI] [PubMed] [Google Scholar]
- Schumann G, Coin LJ, Lourdusamy A, Charoen P, Berger KH, Stacey D, et al. (2011). Genome-wide association and genetic functional studies identify autism susceptibility candidate 2 gene (AUTS2) in the regulation of alcohol consumption. Proc Natl Acad Sci U S A, 108(17), 7119–7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G, Liu C, O’Reilly P, Gao H, Song P, Xu B, et al. (2016). KLB is associated with alcohol drinking, and its gene product β-Klotho is necessary for FGF21 regulation of alcohol preference. Proceedings of the National Academy of Sciences, 113(50), 14372–14377. doi: 10.1073/pnas.1611243113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemura N, Shirosaki S, Sanematsu K, Yoshida R, & Ninomiya Y (2009). Genetic and molecular basis of individual differences in human umami taste perception. PLoS ONE, 4(8), e6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin CM, Kim N, Cho SI, Kim JS, Jung HC, & Song IS (2011). Association between alcohol intake and risk for gastric cancer with regard to ALDH2 genotype in the Korean population. Int J Epidemiol, 40(4), 1047–1055. doi: 10.1093/ije/dyr067 [DOI] [PubMed] [Google Scholar]
- Shyam S, & Smith HE Towards Sustainable Food and Nutrition-View Points from Early Career Researchers.
- Singh GM, Micha R, Khatibzadeh S, Shi P, Lim S, Andrews KG, et al. (2015). Global, regional, and national consumption of sugar-sweetened beverages, fruit juices, and milk: a systematic assessment of beverage intake in 187 countries. PLoS ONE, 10(8), e0124845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siraki AG, & Shangari N (2005). Aldehyde Sources, Metabolism, Molecular Toxicity Mechanisms, and Possible Effects on Human Health AU - O’Brien, Peter J. Critical Reviews in Toxicology, 35(7), 609–662. doi: 10.1080/10408440591002183 [DOI] [PubMed] [Google Scholar]
- Smith CE, Coltell O, Sorli JV, Estruch R, Martinez-Gonzalez MA, Salas-Salvado J, et al. (2016). Associations of the MCM6-rs3754686 proxy for milk intake in Mediterranean and American populations with cardiovascular biomarkers, disease and mortality: Mendelian randomization. Sci Rep, 6, 33188. doi: 10.1038/srep33188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith QR (2000). Transport of glutamate and other amino acids at the blood-brain barrier. The Journal of nutrition, 130(4), 1016S–1022S. [DOI] [PubMed] [Google Scholar]
- Sobczyk-Kopciol A, Broda G, Wojnar M, Kurjata P, Jakubczyk A, Klimkiewicz A, et al. (2011). Inverse association of the obesity predisposing FTO rs9939609 genotype with alcohol consumption and risk for alcohol dependence. Addiction, 106(4), 739–748. [DOI] [PubMed] [Google Scholar]
- Soedamah-Muthu SS, Ding EL, Al-Delaimy WK, Hu FB, Engberink MF, Willett WC, et al. (2011). Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Am J Clin Nutr, 93(1), 158–171. doi: 10.3945/ajcn.2010.29866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer S, Minică C, Verweij KJ, Mbarek H, Bernard M, Derringer J, et al. (2017). Genome-wide association study of lifetime cannabis use based on a large meta-analytic sample of 32 330 subjects from the International Cannabis Consortium. Transl Psychiatry, 6(3), e769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulem P, Gudbjartsson DF, Geller F, Prokopenko I, Feenstra B, Aben KK, et al. (2011). Sequence variants at CYP1A1-CYP1A2 and AHR associate with coffee consumption. Hum Mol Genet, 20(10), 2071–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Wang L, Ma Q, Cui Q, Lv Q, Zhang W, et al. (2017). Association between tea consumption and osteoporosis: A meta-analysis. Medicine (Baltimore), 96(49), e9034. doi: 10.1097/MD.0000000000009034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow DM (2003). Genetics of Lactase Persistence and Lactose Intolerance. Annual review of genetics, 37(1), 197–219. doi: 10.1146/annurev.genet.37.110801.143820 [DOI] [PubMed] [Google Scholar]
- Tabara Y, Ueshima H, Takashima N, Hisamatsu T, Fujiyoshi A, Zaid M, et al. (2016). Mendelian randomization analysis in three Japanese populations supports a causal role of alcohol consumption in lowering low-density lipid cholesterol levels and particle numbers. Atherosclerosis, 254, 242–248. doi: 10.1016/j.atherosclerosis.2016.08.021 [DOI] [PubMed] [Google Scholar]
- Tabatabaie L, Klomp L, Berger R, & De Koning T (2010). L-serine synthesis in the central nervous system: a review on serine deficiency disorders. Molecular genetics and metabolism, 99(3), 256–262. [DOI] [PubMed] [Google Scholar]
- Takeuchi F, Isono M, Nabika T, Katsuya T, Sugiyama T, Yamaguchi S, et al. (2011). Confirmation of ALDH2 as a Major locus of drinking behavior and of its variants regulating multiple metabolic phenotypes in a Japanese population. Circ J, 75(4), 911–918. [DOI] [PubMed] [Google Scholar]
- Talukdar S, Owen BM, Song P, Hernandez G, Zhang Y, Zhou Y, et al. (2016). FGF21 regulates sweet and alcohol preference. Cell Metab, 23(2), 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka F, Yamamoto K, Suzuki S, Inoue H, Tsurumaru M, Kajiyama Y, et al. (2010). Strong interaction between the effects of alcohol consumption and smoking on oesophageal squamous cell carcinoma among individuals with ADH1B and/or ALDH2 risk alleles. Gut, 59(11), 1457–1464. doi: 10.1136/gut.2009.205724 [DOI] [PubMed] [Google Scholar]
- Taubes G (1995). Epidemiology faces its limits. Science, 269(5221), 164–169. [DOI] [PubMed] [Google Scholar]
- Tawa EA, Hall SD, & Lohoff FW (2016). Overview of the Genetics of Alcohol Use Disorder. Alcohol and alcoholism, 51(5), 507–514. doi: 10.1093/alcalc/agw046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AE, Davey Smith G, & Munafò MR (2018). Associations of coffee genetic risk scores with consumption of coffee, tea and other beverages in the UK Biobank. Addiction, 113(1), 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper BJ (2008). Nutritional implications of genetic taste variation: the role of PROP sensitivity and other taste phenotypes. Annu Rev Nutr, 28, 367–388. [DOI] [PubMed] [Google Scholar]
- Teucher B, Skinner J, Skidmore PM, Cassidy A, Fairweather-Tait SJ, Hooper L, et al. (2007). Dietary patterns and heritability of food choice in a UK female twin cohort. Twin Res Hum Genet, 10(5), 734–748. [DOI] [PubMed] [Google Scholar]
- Thomas D (2010). Gene--environment-wide association studies: emerging approaches. Nat Rev Genet, 11(4), 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn CF, Aklillu E, Klein TE, & Altman RB (2012). PharmGKB summary: very important pharmacogene information for CYP1A2. Pharmacogenet. Genomics, 22(1), 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognon G, Nilsson LM, Shungin D, Lissner L, Jansson JH, Renstrom F, et al. (2017). Nonfermented milk and other dairy products: associations with all-cause mortality. Am J Clin Nutr, 105(6), 1502–1511. doi: 10.3945/ajcn.116.140798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma A, Pare G, & Leong DP (2017). Alcohol and Cardiovascular Disease: How Much is Too Much? Curr Atheroscler Rep, 19(3), 13. doi: 10.1007/s11883-017-0647-0 [DOI] [PubMed] [Google Scholar]
- Trepanowski JF, & Ioannidis JPA (2018). Perspective: Limiting Dependence on Nonrandomized Studies and Improving Randomized Trials in Human Nutrition Research: Why and How. Advances in Nutrition, 9(4), 367–377. doi: 10.1093/advances/nmy014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treur JL, Taylor AE, Ware JJ, Nivard MG, Neale MC, McMahon G, et al. (2017). Smoking and caffeine consumption: a genetic analysis of their association. Addiction biology, 22(4), 1090–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Cichon S, Ridinger M, Wodarz N, Soyka M, Zill P, et al. (2009). Genome-wide association study of alcohol dependence. Arch Gen Psychiatry, 66(7), 773–784. doi: 10.1001/archgenpsychiatry.2009.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Frank J, Streit F, Reinbold C, Juraeva D, Degenhardt F, et al. (2017). Genetic Contribution to Alcohol Dependence: Investigation of a Heterogeneous German Sample of Individuals with Alcohol Dependence, Chronic Alcoholic Pancreatitis, and Alcohol-Related Cirrhosis. Genes, 8(7), 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer E, D’Onofrio BM, Maes HH, & Eaves LJ (2005). Analysis and Interpretation of Twin Studies Including Measures of the Shared Environment. Child Development, 76(6), 1217–1233. doi:doi: 10.1111/j.1467-8624.2005.00845.x-i1 [DOI] [PubMed] [Google Scholar]
- Ugai T, Kelemen LE, Mizuno M, Ong JS, Webb PM, Chenevix-Trench G, et al. (2018). Ovarian cancer risk, ALDH2 polymorphism and alcohol drinking: Asian data from the Ovarian Cancer Association Consortium. Cancer Sci, 109(2), 435–445. doi: 10.1111/cas.13470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga T, Czimmerer Z, & Nagy L (2011). PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1812(8), 1007–1022. doi: 10.1016/j.bbadis.2011.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij KJ, Vinkhuyzen AA, Benyamin B, Lynskey MT, Quaye L, Agrawal A, et al. (2013). The genetic aetiology of cannabis use initiation: a meta-analysis of genome-wide association studies and a SNP-based heritability estimation. Addict Biol, 18(5), 846–850. doi: 10.1111/j.1369-1600.2012.00478.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink JM, Staphorsius AS, & Boomsma DI (2009). A genetic analysis of coffee consumption in a sample of Dutch twins. Twin Res Hum Genet, 12(2), 127–131. [DOI] [PubMed] [Google Scholar]
- von Holstein-Rathlou S, BonDurant LD, Peltekian L, Naber MC, Yin TC, Claflin KE, et al. (2016). FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab, 23(2), 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Zhou PY, Liu P, & Zhang Y (2014). ALDH2 and ADH1 genetic polymorphisms may contribute to the risk of gastric cancer: a meta-analysis. PLoS ONE, 9(3), e88779. doi: 10.1371/journal.pone.0088779 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang JC, Hinrichs AL, Bertelsen S, Stock H, Budde JP, Dick DM, et al. (2007). Functional variants in TAS2R38 and TAS2R16 influence alcohol consumption in high-risk families of African-American origin. Alcohol Clin Exp Res, 31(2), 209–215. doi: 10.1111/j.1530-0277.2006.00297.x [DOI] [PubMed] [Google Scholar]
- Wang JC, Hinrichs AL, Stock H, Budde J, Allen R, Bertelsen S, et al. (2004). Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Human molecular genetics, 13(17), 1903–1911. [DOI] [PubMed] [Google Scholar]
- Wang KS, Liu X, Zhang Q, Pan Y, Aragam N, & Zeng M (2011). A meta-analysis of two genome-wide association studies identifies 3 new loci for alcohol dependence. J Psychiatr Res, 45(11), 1419–1425. doi: 10.1016/j.jpsychires.2011.06.005 [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhou S, Wang L, Lei M, Wang Y, Miao C, et al. (2013). ALDH2 rs671 Polymorphism and coronary heart disease risk among Asian populations: a meta-analysis and meta-regression. DNA Cell Biol, 32(7), 393–399. [DOI] [PubMed] [Google Scholar]
- Wang Y, Harvey CB, Hollox EJ, Phillips AD, Poulter M, Clay P, et al. (1998). The genetically programmed down-regulation of lactase in children. Gastroenterology, 114(6), 1230–1236. [DOI] [PubMed] [Google Scholar]
- Wardle J, Carnell S, & Cooke L (2005). Parental control over feeding and children’s fruit and vegetable intake: how are they related? Journal of the American dietetic association, 105(2), 227–232. [DOI] [PubMed] [Google Scholar]
- Ware JJ, Tanner JA, Taylor AE, Bin Z, Haycock P, Bowden J, et al. (2017). Does coffee consumption impact on heaviness of smoking? Addiction, 112(10), 1842–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr H, Rodo M, Lieber CS, & Baraona E (1993). Acetaldehyde adducts and autoantibodies against VLDL and LDL in alcoholics. J Lipid Res, 34(7), 1237–1244. [PubMed] [Google Scholar]
- Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, et al. (2009). Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet, 41(1), 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett WC (1998). Nutritional Epidemiology. New York: Oxford University Press. [Google Scholar]
- Wise PM, Hansen JL, Reed DR, & Breslin PA (2007). Twin study of the heritability of recognition thresholds for sour and salty taste. Chem Senses, 32(8), 749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Krystal JH, Farrer LA, Zhao H, & Gelernter J (2014). Deep resequencing of 17 glutamate system genes identifies rare variants in DISC1 and GRIN2B affecting risk of opioid dependence. Addiction biology, 19(5), 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Kranzler HR, Sherva R, Sartor CE, Almasy L, Koesterer R, et al. (2015). Genomewide association study for maximum number of alcoholic drinks in European Americans and African Americans. Alcoholism: clinical and experimental research, 39(7), 1137–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Palmer AA, & de Wit H (2010). Genetics of caffeine consumption and responses to caffeine. Psychopharmacology (Berl), 211(3), 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mao Q-X, Xu H-X, Ma X, & Zeng C-Y (2014). Tea consumption and risk of type 2 diabetes mellitus: a systematic review and meta-analysis update. BMJ open, 4(7), e005632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Lin SL, Au Yeung SL, Kwok MK, Xu L, Leung GM, et al. (2017). Genetically predicted milk consumption and bone health, ischemic heart disease and type 2 diabetes: a Mendelian randomization study. Eur J Clin Nutr, 71(8), 1008–1012. doi: 10.1038/ejcn.2017.8 [DOI] [PubMed] [Google Scholar]
- Yang S, Lee J, Choi IJ, Kim YW, Ryu KW, Sung J, et al. (2017). Effects of alcohol consumption, ALDH2 rs671 polymorphism, and Helicobacter pylori infection on the gastric cancer risk in a Korean population. Oncotarget, 8(4), 6630–6641. doi: 10.18632/oncotarget.14250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SJ, Yokoyama A, Yokoyama T, Huang YC, Wu SY, Shao Y, et al. (2010). Relationship between genetic polymorphisms of ALDH2 and ADH1B and esophageal cancer risk: a meta-analysis. World J Gastroenterol, 16(33), 4210–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Lu X, Wang L, Chen S, Li J, Cao J, et al. (2013). Common variants at 12q24 are associated with drinking behavior in Han Chinese. The American journal of clinical nutrition, 97(3), 545–551. doi: 10.3945/ajcn.112.046482 [DOI] [PubMed] [Google Scholar]
- Yokoyama A, Tsutsumi E, Imazeki H, Suwa Y, Nakamura C, Mizukami T, et al. (2008). Salivary Acetaldehyde Concentration According to Alcoholic Beverage Consumed and Aldehyde Dehydrogenase-2 Genotype. Alcoholism: clinical and experimental research, 32(9), 1607–1614. doi:doi: 10.1111/j.1530-0277.2008.00739.x [DOI] [PubMed] [Google Scholar]
- Zaitlen N, & Kraft P (2013). Heritability in the genome-wide association era. Hum Genet, 131(10), 1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Mai R, & Huang B (2010). ADH1B Arg47His Polymorphism Is Associated with Esophageal Cancer Risk in High-Incidence Asian Population: Evidence from a Meta-Analysis. PLoS ONE, 5(10), e13679. doi: 10.1371/journal.pone.0013679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GH, Mai RQ, & Huang B (2010). Meta-analysis of ADH1B and ALDH2 polymorphisms and esophageal cancer risk in China. World J Gastroenterol, 16(47), 6020–6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LL, Wang YQ, Fu B, Zhao SL, & Kui Y (2015). Aldehyde dehydrogenase 2 (ALDH2) polymorphism gene and coronary artery disease risk: a meta-analysis. Genet Mol Res, 14(4), 18503–18514. doi: 10.4238/2015.December.23.38 [DOI] [PubMed] [Google Scholar]
- Zhang SY, Chan SW, Zhou X, Chen XL, Mok DK, Lin ZX, et al. (2015). Meta-analysis of association between ALDH2 rs671 polymorphism and essential hypertension in Asian populations. Herz, 40 Suppl 2, 203–208. doi: 10.1007/s00059-014-4166-2 [DOI] [PubMed] [Google Scholar]
- Zhao C, Liu Y, Xiao J, Liu L, Chen S, Mohammadi M, et al. (2015). FGF21 mediates alcohol-induced adipose tissue lipolysis by activation of systemic release of catecholamine in mice. Journal of lipid research, jlr. M058610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Wang C, Shen L, Gu D, Xu Z, Zhang X, et al. (2015). Clinical significance of ALDH2 rs671 polymorphism in esophageal cancer: evidence from 31 case-control studies. Onco Targets Ther, 8, 649–659. doi: 10.2147/OTT.S76526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Baird D, Borges M-C, Bowden J, Hemani G, Haycock P, et al. (2017). Recent developments in Mendelian randomization studies. Current epidemiology reports, 4(4), 330–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong V, Kuang A, Danning R, Kraft P, van Dam R, Chasman D, et al. (2018). A genome-wide association study of habitual bitter and sweet beverage consumption. submitted. [DOI] [PMC free article] [PubMed]
- Zuk O, Hechter E, Sunyaev SR, & Lander ES (2012). The mystery of missing heritability: Genetic interactions create phantom heritability. Proc Natl Acad Sci U S A, 109(4), 1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, Gelernter J, Zhang CK, Zhao H, Lu L, Kranzler HR, et al. (2012). Genome-wide association study of alcohol dependence implicates KIAA0040 on chromosome 1q. Neuropsychopharmacology, 37(2), 557–566. doi: 10.1038/npp.2011.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, Tan Y, Zhang X, Wang X, Krystal J, Tabakoff B, et al. (2015). A New Genomewide Association Meta-Analysis of Alcohol Dependence. Alcoholism: clinical and experimental research, 39(8), 1388–1395. doi:doi: 10.1111/acer.12786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, Wang K, Zhang XY, Krystal JH, Li CS, Zhang F, et al. (2013). NKAIN1-SERINC2 is a functional, replicable and genome-wide significant risk gene region specific for alcohol dependence in subjects of European descent. Drug Alcohol Depend, 129(3), 254–264. doi: 10.1016/j.drugalcdep.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]


