Abstract
Background and Aims:
Surgery for pheochromocytoma (PCC) can cause excessive catecholamine release with severe hypertension. Alpha blockade is the mainstay of preoperative management. The aim of this study was to evaluate the efficacy and tolerance of intra-venous (IV) urapidil, a competitive short acting α1 receptor antagonist, in the prevention of peri-operative hemodynamic instability of patients with PCC.
Material and Methods:
This retrospective observational study included 75 patients (79 PCC) for PCC removal surgery from 2001 to 2017 at the Bordeaux University Hospital. They received, 3 days before surgery, continuous intravenous infusion of urapidil with stepwise increase to the maximum tolerated dose. Urapidil was maintained during the procedure and stopped after clamping the adrenal vein. Plasma catecholamine concentrations were measured during surgery. Hypertensive peaks (SAP >160 mmHg) and tachycardia >100 beats/min were treated with boluses of nicardipine 2 mg and esmolol 0.5 mg/kg.
Results:
We recorded 20/79 (25%) cases with systolic arterial pressure (SAP) >180 mmHg. Only 11/79 (14%) had hypotension with SAP <80 mmHg. Peaks of catecholamine secretions were observed preferentially during peritoneal insufflation and tumor dissection (P < 0.05). A correlation was found between tumor size (mm) and the highest norepinephrine levels [r = 0.288, P = 0.015], and between hypertensive peaks (mmHg) and the highest norepinephrine levels [r = 0.45, P = 0.017]. No mortality was reported. The median [range] postoperative hospital stay was 4 [2–9] days.
Conclusion:
IV urapidil limits hypertensive and hypotensive peaks during PCC surgery, and corresponds to surgical imperatives allowing a short hospital stay, due to its “on–off” effect.
Keywords: Anesthesia, catecholamine, hypertension, pheochromocytoma, urapidil
Introduction
Pheochromocytoma (PCC) is a rare catecholamine-secreting neuroendocrine tumor. The estimated annual incidence is two to eight patients per million in the general population, and its estimated prevalence among hypertensive patients is 0.1–0.6%.[1,2] Management of PCC exposes patients to uncontrolled massive catecholamine release, especially during anesthesia induction, pneumoperitoneum insufflation and surgical removal. Life-threatening complications such as myocardial infarction, coronary spasms, arrhythmias, acute cardiomyopathy, shock, stroke and pulmonary edema may occur.[3]
To prevent these potentially fatal events, pre-operative management is necessary. Scholten et al.[4] demonstrated that emergency surgery in untreated patients leads to more post-operative complications, intensive care unit admissions and longer hospital stay than treated patients. As a result, European and Americans endocrine societies[1] have issued recommendations for the peri-operative management of PCC in 2014 with mandatory initiation of α blocker, even in normotensive patients, to prevent an unpredictable per-operative hemodynamic instability.[5] The therapeutic goals are to normalize blood pressure and heart rate, to restore volume depletion and to prevent surgery-induced catecholamine release.[1,2] However, the alpha blocker pharmacologic class is heterogeneous with covalent or competitive action, non-selective α1 α2 or selective α1 blockade and oral or intra-venous (IV) administration. But studies are retrospective, small sized, and the results are conflicting. Based on current data, there are no prospective, randomized studies showing the most effective alpha-blocker for hemodynamic instability control during surgery.[6,7]
The main objective of our retrospective observational study was to evaluate the efficacy of pre-operative preparation by continuous IV infusion of Urapidil in the prevention of hypertensive peaks (HTN) (SAP >180 mmHg), and tolerance, represented by hypotension (SAP <80 mmHg) during the peri-operative management of 79 cases (75 patients) of PCC ablation. Our secondary objectives were to determine the levels of catecholamine secretion at specific surgical time points and to find a correlation between tumor size and intra-operative hypertension.
Material and Methods
The publication group of the Bordeaux University Hospital ethics committee approved the conduct of this study. The study population underwent PCC surgery from January 2001 to June 2017 through a standard institutional management protocol. This included the diagnosis of PCC, in all patients, which was based on their clinical symptoms, elevated urine or serum levels of meta-normetanephrine. The adrenal tumor was identified by computed tomography (CT)-scans and meta-iodo-benzyl-guanidine (MIBG)-scintigraphy or PET scan. Final selection of each patient data relied on histological confirmation.
All the patients had their pre-operative blood pressure stabilized using oral prazosin (5 mg/day) and oral bisoprolol if necessary (10–20 mg/day). The patients were hospitalized 3 days before planned surgery in the cardiology department.[8] These two drugs were stopped and replaced by a continuous infusion of Urapidil, initiated at a dose of 5 mg/h and increased in steps of 1 mg/h every hour. The dose was increased until the appearance of dizziness or orthostatic hypotension [orthostatic systolic arterial pressure (SAP) <90 mmHg]. In the event of these signs of poor tolerance, the Urapidil infusion was reduced to previous tolerated dose, which was maintained throughout anesthesia until surgical clamp of the adrenal vein.
Radial artery cannulation was first performed under local anesthesia for continuous monitoring of blood pressure. General anesthesia was standardized using propofol, sufentanil, atracrium/cisatracrium, and end-tidal sevoflurane 2–3%. Ventilation was adjusted to keep end-tidal PaCO2 between 4.7 and 6 kPa. Pulse pressure variation, used to monitor volemia, was maintained <10% as stipulated by our institution's protocol. Isotonic saline solution was infused intra-operatively at the rate of 5–10 ml/kg/ h. Intra-peritoneal pressure was maintained below 15 mmHg.
Arterial blood samples were collected for the determination of plasma concentrations of norepinephrine 5 min before induction of anesthesia (control T0), 5 min after oro-tracheal intubation sequence (T1), at the end of peritoneal insufflation (T2), during adrenal gland dissection (T3), just after clamping of the adrenal vein (T4), and in the recovery room 10 min after tracheal extubation and the patient hemodynamically stable, i.e. SAP <150 mmHg (T5). The blood samples were taken in current practice and not only for research purposes. HTN with SAP >160 mmHg was treated by IV boluses of nicardipine 2 mg. Boluses of esmolol 0.5 mg/kg were used when heart rate exceeded 100 beats/min. Hypotensive episodes (SAP <80 mmHg) were treated by IV boluses of ephedrine 3–9 mg and a crystalloid or colloid infusion.
We collected demographic, clinical, histologic, intra-operative and hospital stay data from the institutional patient care registry retrospectively. We performed data protection and anonymization in accordance with the French law on computerization of data.
Data are expressed as mean ± SD, or as median [25–75% interquartile or range] in case of non-parametric distribution. Catecholamine plasma levels at the specific surgical time points were compared using the Kruskal–Wallis one-way repeated-measures ANOVA on ranks. A Spearman rank correlation was used to test the relationship between the different parameters. Receiver operating characteristic (ROC) curves were applied to the correlated parameters to define the cut off values (when the sensitivity and specificity were very close to each other). A P < 0.05 was considered statistically significant. The Sigmaplot 10 (Systat Inc Chicago, USA) and the MedCalc 16.8.4 (Ostend, Belgium) software packages were used to perform the statistical analysis.
Results
Seventy-five patients ASA II/III were enrolled and seventy-nine PCC were resected. Baseline patient characteristics and operative data are summarized in Table 1.
Table 1.
Baseline patient characteristics and operative data
| Characteristic | Value |
|---|---|
| Male | 32 (42.6%) |
| Female | 43 (57.3%) |
| Age* | 50.7±16.5 years |
| Right PCC | 39 |
| Left PCC | 40 |
| Tumor size* | 49±20 mm |
| Associated pathologies | 8 (10.6%) |
| MEN type 2 | 5 (6.6%) |
| Cardiopathy | 6 (8%) |
| Coronary disease | 4 (5.3%) |
| Pre-operative FEVG <45% | 0 (0%) |
| Recklinghausen disease | 3 (4%) |
| Morbid obesity | 2 (2.6%) |
| Beckwith Wiedermann syndrome | 1 (1.3%) |
| Chronic renal failure | 1 (1.3%) |
| Pre-operative hypertension (SAP >160 mmHg) | 0 (0%) |
| Duration of pneumoperitoneum* | 76.3±32 min |
| Urapidil infusion rate* | 42.4±30 mg/h |
| Sufentanyl total dose* | 114±40 μg |
| Median per-operative plasma levels of norepinephrine** (normal value <510 pg/ml) | 3216[1487-8645] pg/ml |
| Surgical approach | |
| Laparoscopic trans-peritoneal | 69 (87.3%) |
| Laparoscopic retro-peritoneal | 8 (10.1%) |
| Conversion to laparotomy | 2 (2.5%) |
| Average blood loss | 200 (150-340) ml |
*Mean±SD, **Median [interquartile range]. PCC=Pheochromocytoma; MEN: multiple endocrine neoplasia; SAP=Systolic arterial pressure
The incidence of HTN with SAP >180 mmHg was 25% (20/79). The incidences of HTN with 160 mmHg ≤SAP ≤180 mmHg and with SAP <160 mmHg were 19% (15/79) and 56% (44/79) respectively. Incidence of tachycardia (>100 bpm) was 19% (15/79). HTN and sinus tachycardia were transient (<30 s) and easily treated with 2 mg IV bolus nicardipine and 0.5 mg/kg IV bolus of esmolol respectively. 11 (14%) hypotensive episodes (SAP <80 mmHg) were noted after clamping of the adrenal vein (T4). The variations in plasma norepinephrine concentration at the specific surgical time points showed that peritoneal insufflation (T2) and adrenal gland dissection (T3) were significantly associated with norepinephrine secretion peak [Figure 1].
Figure 1.
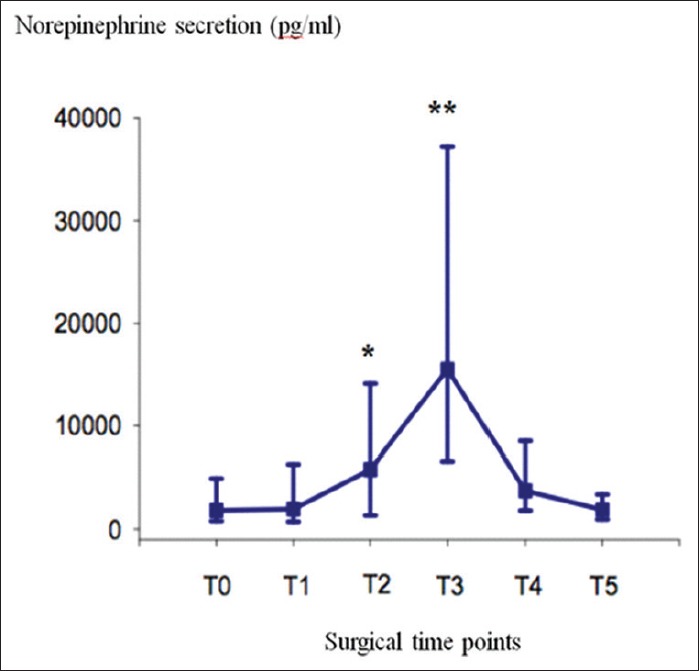
Median [range] of plasma norepinephrine concentration at the different surgical time points. T0: Before induction of anesthesia = control; T1: After oro-tracheal intubation sequence; T2: At the end of peritoneal insufflation; T3: During adrenal gland dissection; T4: After clamping of the adrenal vein; T5: In the recovery room after extubation; *P < 0.05 (vs T0); **P < 0.05 (vs T0, T1, T2, T4, and T5)
We observed a correlation between tumor size (mm) and the highest intraoperative norepinephrine levels [r = 0.288, R2 = 0.0083, (95%CI; 0.0416–0.475), P = 0.015] and a correlation between HTN (mmHg) and the highest intraoperative norepinephrine levels [r = 0.45, R2 = 0.208, (95%CI; 0.05276–0.4840), P = 0.017] [Figures 2 and 3]. No correlation exits between HTN and tumor size [r = 0.143 (95% CI; −0.0788 to 0.352), P = 0.204], between IV Urapidil dose and the highest SAP during surgery [r = 0.014 (95%CI; −0.206 to 0.233), P = 0.901], between duration of pneumoperitoneum and tumor size [r = 0.125 (95%CI; −0.0976 to 0.335), P = 0.270]. Regarding the correlation between tumor size and HTN (SAP >160 mmHg), a cutoff value of 41 mm (sensitivity 58% and specificity 66%) was obtained. The cut off value for intra-operative norepinephrine levels was 16,013 pg/ml with a sensitivity of 62% and specificity of 61% for HTN [Figure 4].
Figure 2.
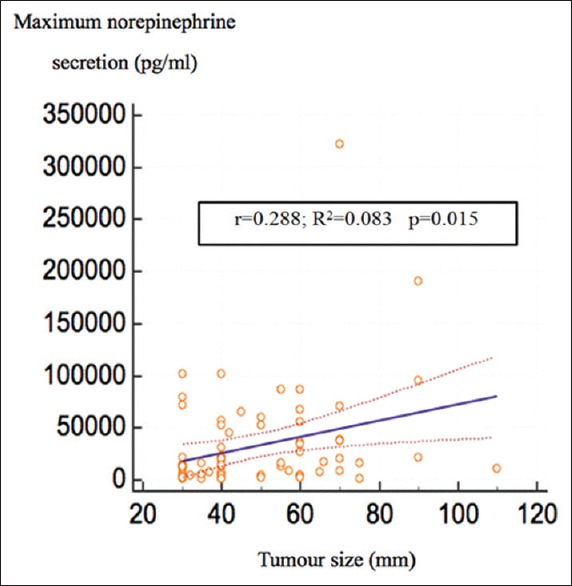
Correlation between maximum norepinephrine secretion and tumor size. r: correlation coefficient; R2: coefficient of determination significant
Figure 3.
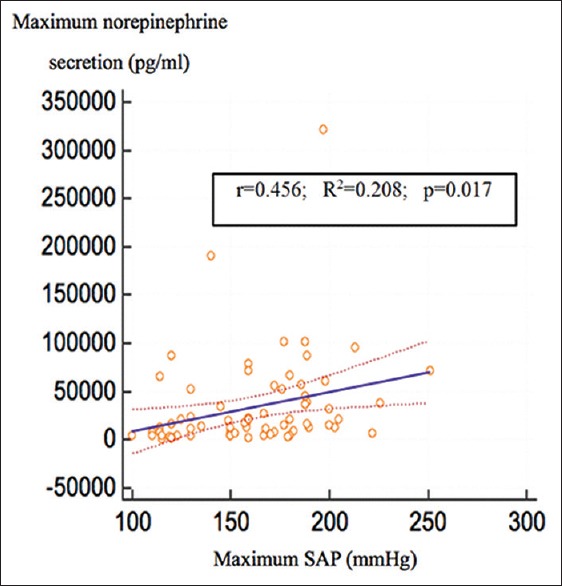
Correlation between maximum norepinephrine secretion and maximum systolic arterial pressure. r: correlation coefficient; R2: coefficient of determination significant
Figure 4.
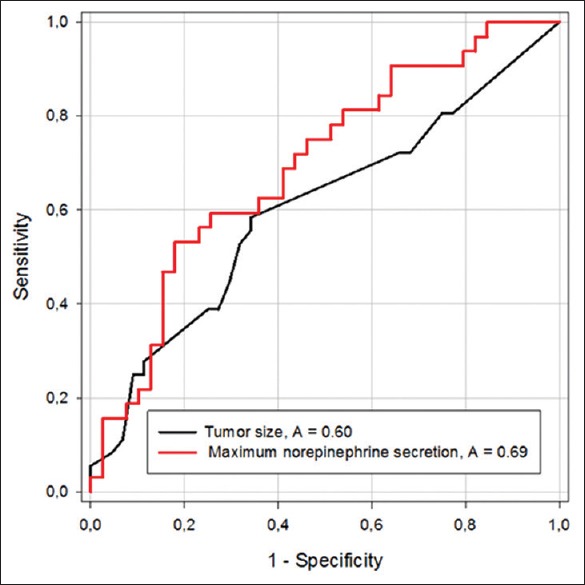
Receiver operating characteristic (ROC) curves for maximum norepinephrine secretion and tumor size. A: area under the ROC curve
Two conversions (to open surgery) were necessary. These patients were included in the statistical analysis because their norepinephrine levels were within the mean of the study population. Post-operative course was uneventful. After 24 h in the intensive care unit, patients were discharged in the surgical ward. The median [range] post-operative hospital stay was 4 [2–9] days.
Discussion
The main findings of this study are: (1) the peri-operative management of patients with PCC by urapidil provides efficient blood pressure control without complication, (2) norepinephrine secretion peaks occur mainly during the creation of pneumoperitoneum and tumor manipulations, and (3) tumor size >41 mm is correlated with the highest catecholamine secretion rate, which leads to intra-operative HTN.
Many studies have reported the use of three groups of drugs in the peri-operative management of PCC. First, phenoxybenzamine (PBZ) is non-competitive, irreversible inhibitor that covalently binds to α1 and α2 receptor widely used for blood pressure control. Its half-life is long (24–30 h). This oral drug must be initiated for at least 21 days. Reflex tachycardia secondary to inhibition of presynaptic α2 blockade often requires pre-operative β blockade. The main adverse effect is prolonged hypotension after tumor resection. Second, prazosin and doxazosin are selective α1 competitive blockers with a pharmacological half-life of 3 and 22 hours respectively. Thirdly, Urapidil, a competitive short acting (half-life of 2.7 h) α1 blocker, is also an antagonist of central serotoninergic receptor with a weak β- action, explaining the absence of tachycardia.[9] Our study confirms its many advantages: (a) Preparation with Urapidil alone led to pre-operative blood pressure control in all our cases. (b) Treatment of HTN was easy and rapidly effective. The ultra-short elimination half-life of plasma catecholamines and the continuous competitive α block produced by urapidil explained transient hypertension observed. Moreover, IV boluses of nicardipine are potentiated by end-tidal sevoflurane concentration of 2.5% explaining the low doses (2 mg) required.[10] (c) Hypotension after tumor resection was due to the combined effects of pre-operative preparation, the acute decrease of plasma catecholamine and surgical hemorrhage. Residual effects of Urapidil are limited because of its pharmacologic profile.
It is difficult to compare our results with Urapidil to other cohorts studying other alpha blockers. First, the thresholds of hypertension and hypotension are varied according to the articles, which limit the possibilities of comparing cohorts using the same threshold. Secondly, the studies differ from one another by other confounding factors which may affect the risk of intraoperative hemodynamic instability: the surgical approach, anesthetic management, sedative and vasoconstrictor drugs. Thirdly, most of them are retrospective trials with small sample sizes comparing PBZ with doxazosin or prazosin. They have provided conflicting results[6] which do not permit to confirm the superiority of a molecule.[11,12,13] Only one study published by Habbe et al.[14] compared Urapidil versus PBZ (11 and 19 patients, respectively) with no significant difference between intra-operative HTN or hypotension. The incidence of HTN (SAP >180 mmHg) was 25% in our study, lower than those reported by Kocac et al.[15]81% in the PBZ group, 73% in the prazosin group and 82% in the doxazosin group (NS). Agrawal et al.[16] described 57% in the PBZ group and 84% in the prazosin group (NS). The incidence of hypotensive peaks (SAP <80 mmHg) was 14% in our study, lower than those reported by Randle et al.[17]35% in the PBZ group and 66% in the doxazosin group and by Agrawal et al.[16]57% in the PBZ group and 77% in the prazosin group.
Our results confirm the feasibility of PCC surgical management by a laparoscopic approach with a low laparotomy conversion rate of 2.5%. Besides, we had found no correlation between duration of pneumoperitoneum and tumor size. The proven benefits of laparoscopic surgery include mild hemodynamic changes, reduced catecholamine release and faster recovery.[18] A prospective study comparing open versus laparoscopic adrenalectomy confirm these data even for large tumor (>6 cm).[19] Moreover, our median [range] hospital stay was 4 [2–9 days]. In the study by Habbe et al.[14], it was 17 days in the PBZ group in contrast to 11 days in the Urapidil group (P < 0.009). Patients with IV Urapidil spent significantly fewer days in hospital prior to surgery [median 3 (range 3–7 days) versus 9 (range 3–21 days); P < 0.001]. The total cost of hospitalization was significantly lower with Urapidil.
Larger tumor size (>40 mm), high pre-operative norepinephrine levels are considered as predisposing factors to intra-operative hypertensive peaks.[20] Our results demonstrate that a tumor size >41 mm is correlated with high catecholamine secretion leading to HTN. This finding corroborates with the results of Kwon et al.[21] who reported a critical tumor size of 42.5 mm. We could suggest that patients with PCC >41 mm receive continuous IV administration of nicardipine at the beginning of surgery to prevent HTN. But further studies are warranted in this situation.
Pheochromocytoma crisis is a life-threatening complication due to massive per-operative release of catecholamine. The triggering factors are surgical manipulations,[22] biopsies, tumor hemorrhage and general anesthesia. The pathophysiology involves intense arterial vasoconstriction, reduced intravascular volume, reduced end-organ perfusion and tissue ischemia leading to organ failure. Whitelaw et al.[23] in a review of 106 case reports a higher mortality rate of 28% in type B crisis (shock and two or more organ failure) compared with 6% in type A crisis (hemodynamic instability and one or more organ failure). Lack of PCC crisis reported after per-operative α blockade signifies a protective effect.[24] We chose as primary outcome SAP >180 mmHg because, according to recommendation of the French national agency of the security of drugs and medical products (ANSM), a hypertensive crisis requiring an immediate treatment is defined by SAP >180 mmHg or DAP >110 mmHg with one or more organ failure.[25]
The limitations of this study are certainly its retrospective design and the lack of comparison with others α blockers, but a prospective therapeutic trial to analyze, with sufficient power, different strategies for blood pressure management seems unrealistic for such a rare pathology.
Conclusion
The relatively large sample size of our cohort with the same anesthetic and surgical management shows that the use of Urapidil in the peri-operative period of these patients is safe and efficient to prevent HTN and hypotension. As compared to other α blockers given orally, the IV route is an interesting alternative. It permits patients to have controlled blood pressure and heart rate with a single drug upon arrival in the operating room. It facilitates the treatment of hemodynamic disorders, fits the surgical imperatives by allowing a shorter hospital stay. Further studies are warranted to confirm these data.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, et al. Pheochromocytoma and paraganglioma: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915–42. doi: 10.1210/jc.2014-1498. [DOI] [PubMed] [Google Scholar]
- 2.Pacak C. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069–79. doi: 10.1210/jc.2007-1720. [DOI] [PubMed] [Google Scholar]
- 3.Prejbisz A, Lenders JW, Eisenhofer G, Januszewicz A. Cardiovascular manifestations of phaeochromocytoma. J Hypertens. 2011;29:2049–60. doi: 10.1097/HJH.0b013e32834a4ce9. [DOI] [PubMed] [Google Scholar]
- 4.Scholten A, Cisco RM, Vriens MR, Cohen JK, Mitmaker EJ, Liu C, et al. Pheochromocytoma crisis is not a surgical emergency. J Clin Endocrinol Metab. 2013;98:581–91. doi: 10.1210/jc.2012-3020. [DOI] [PubMed] [Google Scholar]
- 5.Lafont M, Fagour C, Haissaguerre M, Darancette G, Wagner T, Corcuff JB, et al. Per-operative hemodynamic instability in normotensive patients with incidentally discovered pheochromocytomas. J Clin Endocrinol Metab. 2015;100:417–21. doi: 10.1210/jc.2014-2998. [DOI] [PubMed] [Google Scholar]
- 6.van der Zee PA, de Boer A. Pheochromocytoma: A review on preoperative treatment with phenoxybenzamine or doxazosin. Neth J Med. 2014;72:190–201. [PubMed] [Google Scholar]
- 7.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–75. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 8.Gosse P, Tauzin-Fin P, Sesay M-B, Sautereau A, Ballanger P. Preparation for surgery of phaeochromocytoma by blockade of a-adrenergic receptors with urapidil: What dose? J Hum Hypertens. 2009;23:605–9. doi: 10.1038/jhh.2008.172. [DOI] [PubMed] [Google Scholar]
- 9.Doolay M, Doa KI. Urapidil: A reappraisal of its use in the management of hypertension. Drugs. 1998;56:929–55. doi: 10.2165/00003495-199856050-00016. [DOI] [PubMed] [Google Scholar]
- 10.Nishiyama T, Matsukawa T, Hanaoka R, Conway C. Interactions between nicardipine and enflurae, isoflurazne and sevorane. Can J Anaesth. 1997;44:1071–6. doi: 10.1007/BF03019228. [DOI] [PubMed] [Google Scholar]
- 11.Ramachandran R, Rewari V. Current perioperative management of pheochromocytomas. Indian J Urol. 2017;33:19–25. doi: 10.4103/0970-1591.194781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naranjo J, Dodd S, Martin YN. Peroperative management of pheochromocytoma. J Cardiothorac Vasc Anesth. 2017;3:1427–39. doi: 10.1053/j.jvca.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Van der Horst-Schrivers AN, Kerstens MN, Wolffenbuttel BH. Preoperative pharmacological management of phaeochromocytoma. Neth J Med. 2006;64:290–5. [PubMed] [Google Scholar]
- 14.Habbe N, Ruger F, Bojunga J, Bechstein WO, Holzer K. Urapidil in the preoperative treatment for pheochromocytoma: A safe and cost-effective method. World J Surg. 2013;37:1141–6. doi: 10.1007/s00268-013-1933-9. [DOI] [PubMed] [Google Scholar]
- 15.Kocak S, Aydintug S, Canakci N. Alpha blockade in preoperative preparation of patients with pheochromocytoma. Int Surg. 2002;87:191–4. [PubMed] [Google Scholar]
- 16.Agrawal R, Mishra SK, Bhatia E, Mishra A, Chand G, Agarwal G, et al. Prospective study to compare peri-operative hemodynamic alterations following preparation for pheochromocytoma surgery by phenoxybenzamine or prazosin. World J Surg. 2014;38:716–23. doi: 10.1007/s00268-013-2325-x. [DOI] [PubMed] [Google Scholar]
- 17.Randle RW, Balentine CJ, Pitt SC, Schneider DF, Sipple RS. Selective versus non-selective α blockade prior to laparoscopic adrenalectomy for pheochromocytoma. Ann Surg Oncol. 2017;24:244–50. doi: 10.1245/s10434-016-5514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toniato A, Boschin IM, Opocher G, Guolo A, Pelizzo M, Mantero F. Is the laparoscopic adrenalectomy for pheochromocytoma the best treatment? Surgery. 2007;141:723–7. doi: 10.1016/j.surg.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Li P, Wang Y, Wang Y, Ma Z, Wang G, et al. Effectiveness and safety of laparoscopic adrenalectomy of large pheochromocytoma: A prospective, nonrandomized, controlled study. Am J Surg. 2015;210:230–5. doi: 10.1016/j.amjsurg.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Bruynzeel H, Feelders RA, Groenland TH, van den Meiracker AH, van Eijck CH, Lange JF, et al. Risk factors for hemodynamic instability during surgery for pheochromocytoma. J Clin Endocrinol Metab. 2010;95:678–85. doi: 10.1210/jc.2009-1051. [DOI] [PubMed] [Google Scholar]
- 21.Kwon SY, Lee KS, Lee JN, Ha YS, Choi SH, Kim HT, et al. Risk factors for hypertensive attack during pheochromocytoma resection. Investig Clin Urol. 2016;57:184–90. doi: 10.4111/icu.2016.57.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tauzin-Fin P, Hilbert G, Krol-Houdek M, Gosse P, Maurette P. Mydriasis and acute pulmonary oedema complicating laparoscopic removal of phaeochromocytoma. Anaesth Intensive Care. 1999;27:646–9. doi: 10.1177/0310057X9902700615. [DOI] [PubMed] [Google Scholar]
- 23.Whitelaw BC, Prague JK, Mustafa OG, Schulte KM, Hopkins PA, Gilbert JA, et al. Phaeochromocytoma crisis. Clin Endocrinol. 2014;80:13–22. doi: 10.1111/cen.12324. [DOI] [PubMed] [Google Scholar]
- 24.Tauzin-Fin P, Sesay M, Gosse P, Ballanger P. Effects of perioperative alpha1 block on haemodynamic control during laparoscopic surgery for phaeochromocytoma. Br J Anaesth. 2004;92:512–7. doi: 10.1093/bja/aeh083. [DOI] [PubMed] [Google Scholar]
- 25.Agence Française de Sécurité Sanitaire des Produits de Santé (AFSSAPS) Hypertensive events in adults High blood pressure without organ involvement and hypertensive events requiring emergency care. Guidelines J Mal Vasc. 2002;27:234–8. [PubMed] [Google Scholar]


