Abstract
Background and Aims:
Supraglottic airway devices have several roles including maintenance of a clear upper airway during general anesthesia. We primarily compared the efficacy of Baska mask (BM) and laryngeal mask airway supreme (LMAS) for the rate of first time successful placement and the seal pressure. The secondary outcome measures included laryngopharyngeal morbidity and the correct positioning of the gastric port.
Material and Methods:
A sample size of 30 was calculated in each study group. A total of 70 study participants were included in the statistical analysis of which 36 patients were in the BM group and 34 patients were in the LMAS group.
Results:
The BM was successfully inserted in 28 patients (77.8%), whereas LMAS was successfully inserted in 33 patients (97.1%) in the first attempt (P = 0.028). The mean oropharyngeal seal pressure in the BM group was higher (33.28 ± 6.80 cm H2O) than compared to the LMAS group (27.47 ± 2.34 cm H2O) with a P value <0.001. There was no significant difference between the two groups in the incidence of postoperative laryngopharyngeal morbidity both in the immediate postoperative period (P = 0.479) and that seen 24 hours post operatively (P = 0.660). The nasogastric tube could easily be inserted in the entire study population.
Conclusion:
From the present study, it is concluded that the BM creates a higher oropharyngeal seal pressure than the LMAS. However, the BM is more difficult to insert. The incidence of postoperative laryngopharyngeal morbidity is similar in both groups.
Keywords: Baska mask, efficacy, Laryngeal mask airway supreme, seal pressure
Introduction
Supraglottic airway devices (SADs) have several roles including maintenance of a clear upper airway, which is of prime importance in the practice of general anesthesia.[1]
The Baska mask (BM) (Logikal Health Products PTY. Ltd, Morisset, NSW, Australia) incorporates many features of the second-generation SADs with several novel features.[2] It is characterized by an advanced self sealing variable pressure cuff, which produces an oropharyngeal seal that increases proportionately with increasing airway pressure. Its self-recoiling cuff has a dorsal slit made possible by preformed flaps keeping it semi distended in the resting state. With positive pressure ventilation, the mask distends increasing the pharyngeal seal and as the pressure is released, the mask partially deflates to its resting state. It also has a gastric reflux drainage system with its large distal aperture located at the upper oesophagus, which opens into the integral sump cavity. The soft, oval airway orifice at the distal end provides patency of seal against gastric overflow.[1,2]
The laryngeal mask airway supreme (LMAS) (The Laryngeal Mask Company Limited, Le Rocher, Victoria, Mahe, Seychell) is a second generation gastric access device, which has a soft, elongated cuff designed to form an effective first seal with the oropharynx permitting higher glottic seal pressures and an innovative second seal with the upper esophageal sphincter. This maintains the patency of the drain tube reducing the risk of insufflation during ventilation and the risk of regurgitated gastric contents leaking around the tip of the mask.[3]
Currently, there is no available literature comparing the efficacy and safety of BM with LMAS. In our study, we hypothesized that BM is not superior to LMAS for short surgical procedures. There were two primary outcomes, namely the rate of first time successful placement and the seal pressure created by the SAD. The secondary outcome measures include laryngopharyngeal morbidity and the correct positioning of gastric port of the device.
Material and Methods
The institutional ethical committee approval was obtained on 02/12/2016 and the study was registered under the Clinical Trial Registry India (Reg no: CTRI/2017/08/009255). After obtaining written informed consent, low-risk adult patients (ASA 1 and ASA 2) who underwent surgeries of duration less than 2 h over a period of 1 year from February 2017 to February 2018 were recruited into the study. The exclusion criteria included patients with neck pathology, previous or anticipated problem with upper airway or upper gastrointestinal tract, laparoscopic surgeries, pregnancy, and patients at increased risk of aspiration.
Patients were randomized into either the BM group or the LMAS group by computer-generated randomization chart and sealed envelope technique. The study was single-blinded with the patient being informed that one of these SADs will be used. All device insertions were done by consultant anesthesiologists with more than 3 years experience. The size of the SAD was selected according to the body weight as per the manufacturer's recommendations.
A standard anesthesia sequence was followed in the operation theater. ECG, Non invasive blood pressure (NIBP), pulse oximetry, end tidal CO2, and temperature monitoring were done during the entire surgical procedure. Injection glycopyrrolate 0.2 mg, injection midazolam 0.03 mg/kg, and fentanyl 2 mcg/kg were given intravenously 5 min before induction of anesthesia. After pre-oxygenation anesthesia was induced using propofol 2–2.5 mg/kg till loss of responsiveness was noted. After confirming that adequate bag and mask ventilation was possible, injection atracurium 0.5 mg/kg was given and gently ventilated for 3 min using a facemask. Anesthesia was considered adequate for device insertion when the patient was unresponsive with no response to anterior jaw thrust. The selected airway device was inserted and confirmation of correct positioning was done in strict accordance to the manufacturer's recommendations. After connecting the airway device to the breathing circuit, adequate placement and ventilation were confirmed by chest wall movement, auscultation of breath sounds, and by square wave capnography. If the device placement was considered inadequate, as judged by poor capnographic curve or delivery of inadequate tidal volumes (fractional loss of >20% of set tidal volume) the following manipulations were performed in sequence: the depth of insertion was increased, the device was rotated or the device was withdrawn slightly. If the device failed to work effectively in spite these maneuvers the device was removed and reinserted for a maximum of two attempts. If this failed tracheal intubation was performed. After successful placement, a well lubricated gastric tube of 14 French size was inserted through the drain channel of both the device.[4] In LMAS group, the cuff pressure was checked using a hand-held pressure gauge (Covidien 1200694, Germany) and adjusted if necessary to keep cuff pressure <60 cm H2O.
The ease of insertion was graded as easy or difficult. The insertion was considered “easy” if it was successfully inserted in a single attempt not requiring any manipulation. The device insertion was considered difficult if manipulation was required on a single attempt or required more than one attempt for successful insertion. The device insertion was considered “successful in the first time” only if this was an easy insertion.[4]
Anesthesia was maintained with sevoflurane 1.0% to 2.0% in a mixture of 60% nitrous oxide and oxygen. Positive pressure ventilation was done using volume control mode (Penlon Prima 450) at a tidal volume of 6–8 ml/kg, and the ventilator frequency set at a rate of 12 to 14 breaths per minute to maintain an end-tidal carbon dioxide around 35 mmHg.
The seal pressure test was performed to assess the oropharyngeal leak pressure. While the patient was apnoeic and following confirmation of adequate ventilation, the adjustable pressure limiting valve was set at 70 cm of water, the fresh gas flow set at 6 l/min, and the airway pressure was measured on the breathing system pressure gauge. Leak pressure was defined as the plateau airway pressure that was achieved or the pressure at which leak was audible. In those patients whose airway pressure reached 40 cm of water, the test was interrupted and a value of 40 cm of water was recorded.[4]
Intraoperatively, monitoring was done for audible leak, gastric insufflation by visual inspection of the abdomen, and regurgitation by visual inspection of the appearance of enteric contents in the drain tube. After completion of the procedure, anesthesia was discontinued and the patient was reversed with neostigmine (0.05 mg/kg) and glycopyrrolate (5–10 mcg/kg). The device was removed after the return of spontaneous ventilation, and when the patient was fully awake responding to verbal commands. The postoperative laryngopharyngeal morbidity was assessed by looking for the presence of any of the complications such as sore throat, hoarseness of voice or dysphagia in the postoperative recovery room and also 24 h postoperatively.
In an earlier study, comparing BM with ProSeal LMA, a mean difference of 5.84 mmHg was noted in the oropharyngeal seal pressure. To detect a similar difference in our study population with 95% confidence interval, alpha error of 5%, and a power of 80%, a sample size of 30 was calculated in each study group.[5] We generated 80 computer-generated random samples to account for any loss during the study period. A total of 73 patients were recruited during the study period. Three patients who required tracheal intubation were excluded and a total of 70 patients were included in the final analysis.
Student t test (two–tailed and independent) had been used to find the significance of study parameters on continuous scale between the two groups on metric parameters. The Chi-square test was used to find the significance of study parameters on a categorical scale between two or more groups. The Fisher exact test was used, when cell samples are very small. The statistical software namely SPSS 18.0 and R environment ver. 3.2.2 were used for the analysis of the data and Microsoft word, and Excel had been used to generate graphs, tables, etc.
Results
A total of 70 study participants were included in the statistical analysis, of which 36 patients were in the BM group and 34 patients were in the LMAS group. There was no statistically significant difference between the two groups regarding age, gender, height, weight, body mass index [Table 1], and American society of anesthesiologists (ASA) grade [Table 2].
Table 1.
Patient characteristics
| Parameter | BASKA (n=36) | LMAS (n=34) | P |
|---|---|---|---|
| Age (years) Mean±SD | 41.64±4.52 | 46.97±15.77 | 0.145 |
| Gender (male/female) | 20/16 | 26/8 | 0.100 |
| Height (cm) Mean±SD | 163.11±8.95 | 162.09±8.02 | 0.617 |
| Weight (kg) Mean±SD | 60.53±10.32 | 62.29±9.69 | 0.464 |
| BMI (kg/m2) | 22.66±2.62 | 23.66±2.99 | 0.137 |
Student t test, Chi square test
Table 2.
ASA grading
| ASA class | BASKA | LMAS | Total |
|---|---|---|---|
| 1 | 21 (58.3%) | 16 (47.1%) | 37 (52.9%) |
| 2 | 15 (41.7%) | 18 (52.9%) | 33 (47.1%) |
| Total | 36 (100%) | 34 (100%) | 70 (100%) |
P=0.345, Chi square test
The BM was successfully inserted in 28 patients (77.8%) in the first attempt not requiring any manipulation, whereas LMAS was successfully inserted in 33 patients (97.1%) in the first attempt not requiring any manipulation. This difference was found to be statistically significant (P = 0.028) [Figure 1]. In the BM group, seven patients required manipulation during the first attempt for successful insertion and one patient required two attempts for successful insertion. Two attempts were required in only one patient of the LMAS group.
Figure 1.
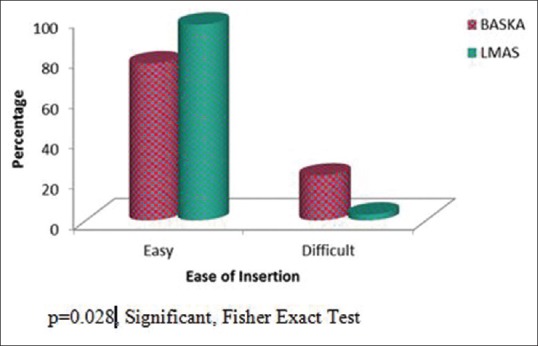
Ease of insertion
The mean oropharyngeal seal pressure in the BM group was 33.28 ± 6.80 cmH2O and that of the LMAS group is 27.47 ± 2.34 cmH2O. BM created a significantly higher oropharyngeal seal pressure than the LMAS group (P < 0.001) [Figure 2]. The maximum oropharyngeal seal pressure of 40 cm of water was achieved in 10 patients (27.7%) in the BM group.
Figure 2.
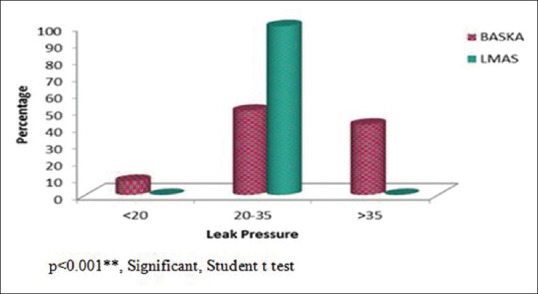
Seal pressure
Intraoperatively, leak was noted in four (5.7%) cases that were distributed in both groups equally. Gastric insufflation and regurgitation were not observed with the usage of both the devices. Two patients (5.6%) in the BM group had transient cough on the removal of the device. Blood staining on mask removal was noticed in two patients (5.6%) in the BM group and in three patients (8.8%) in the LMAS group. There was no significant difference between the two groups in the incidence of these complications. We did not notice any other complications such as vomiting, regurgitation and lip or dental trauma in any of our study population.
There was no significant difference between the two groups in the incidence of postoperative laryngopharyngeal morbidity both in the immediate postoperative period (P = 0.479) [Table 3] and that seen 24 h postoperatively (P = 0.660) [Table 4]. Sore throat was noticed in 6 (16.7%) patients in BM group and 3 (8.8%) patients in LMAS group in the postoperative recovery room and 9 (25%) patients in BM group and 7 (20.6%) patients in LMAS group 24 h postoperatively. Hoarseness of voice, dysphonia, and dysphagia was not seen in any of the patients. The nasogastric tube could easily be inserted in the entire study population.
Table 3.
Laryngopharyngeal morbidity (post-operative recovery room)
| Laryngopharyngeal morbidity post-op recovery room | BASKA (n=36) | LMAS (n=34) | Total (n=70) | P |
|---|---|---|---|---|
| No | 30 (83.3%) | 31 (91.2%) | 61 (87.1%) | 0.479 |
| Yes | 6 (16.7%) | 3 (8.8%) | 9 (12.9%) |
Chi-Square/Fisher Exact Test
Table 4.
Laryngopharyngeal morbidity (24 h post-operatively)
| Laryngopharyngeal morbidity 24 h post-operatively | BASKA (n=36) | LMAS (n=34) | Total (n=70) | P |
|---|---|---|---|---|
| No | 27 (75%) | 27 (79.4%) | 54 (77.1%) | 0.660 |
| Yes | 9 (25%) | 7 (20.6%) | 16 (22.9%) |
Chi-Square/Fisher Exact Test
Discussion
In our study, the first attempt successful placement without the need of any manipulation was found to be significantly higher in the LMAS group than the BM group.
The difficulty encountered in the insertion of BM was the need for manipulation even on a single attempt to achieve optimal ventilation. The probable difficulty encountered while inserting BM is the difficulty to negotiate the palatopharyngeal curve. To overcome this difficulty, the preformed insertion tab intended to manually curve the mask has to be pulled during insertion.
Similarly, BM had shown a significantly lower first-time successful insertion success rate than the LMA classic in low-risk female patients undergoing ambulatory surgery (73% versus 98% respectively, P < 0.001).[4] The insertion of BM was more difficult than the LMA classic, requiring longer insertion time, more insertion attempts, and had a higher user-rated device difficulty scores. However, the overall insertion success rates of BM and LMA classic were not different.[4]
When the BM was compared with the LMA ProSeal in adult patients, the mean insertion time was significantly shorter in the BM group than the LMA ProSeal group (16.43 ± 4.54 versus 21.45 ± 6.13 s, P = 0.001). However, there was no significant difference in the mean number of attempts required for SAD placement in either group.[5]
However, when the BM airway was compared with Igel for controlled ventilation in obese patients undergoing ambulatory surgery, both the devices were easily inserted with high success rates (76.67% versus 73.3%, no statistically significant difference). Successful insertion time was significantly shorter in Igel group.[6]
A significantly higher first attempt insertion success rate and a significantly shorter mean insertion time were found in the LMAS group than the LMA classic.[7,8] There was no significant difference in the insertion time, number of insertion attempts, and the success rate of insertion when LMAS was compared with I gel.[9,10,11] However, although one study had shown that Igel is better than LMAS in terms of faster insertion time (11.07 ± 1.93 versus 12.50 ± 2.35 s, P = 0.01), the overall insertion success rate was comparable in both the groups.[12] When the LMAS was compared with LMA ProSeal in patients undergoing laparoscopic cholecystectomy, the first attempt success rate and the ease of insertion grading were found to be significantly higher in the LMAS group.[13] The mean duration for a successful insertion was also significantly shorter in the LMAS group than the LMA ProSeal.[13,14,15]
The second primary outcome of our study was to assess the seal pressure created by both the devices during positive pressure ventilation. BM produced a significantly higher oropharyngeal seal pressure than the LMAS group (P < 0.001). The oropharyngeal leak pressure was more than 35 cmH2O in 41.7% of the BM group. All the patients in the LMAS group had seal pressures less than 35 cmH2O. This finding of higher oropharyngeal seal pressure in the BM group is similar to that of the pre-existing studies.
An initial observational study of the BM in low risk female patients also found a mean airway leak pressure of 35.7 ± 13.3 cm H2O.[16] Similarly, when the performance of BM was evaluated for use in anesthesia in adult patients undergoing a variety of surgical interventions, the oropharyngeal leak pressure was above 30 cm H2O in all patients and the maximum of 40 cm H2O was achieved in 82% of the patients.[17] When BM was compared with laryngeal mask ProSeal also they observed a significantly higher mean seal pressure in the BM group (29.98 ± 8.51 versus 24.50 ± 6.19 cm H2O, P = 0.001). The seal pressure ranged from 15–40 cm H2O in the BM group to 14–32 cm H2O in the LMA ProSeal group, which was similar to the findings from our study.[5] The oropharyngeal seal pressure was significantly higher in the BM than the Igel for controlled ventilation in obese patients and in laparoscopic surgeries.[6,18]
When the LMAS was evaluated during gynecological laparoscopic surgeries, the mean oropharyngeal leak pressure at the level of 60 cm H2O cuff pressure was 28.2 ± 5.1 cm H2O.[19] The mean oropharyngeal leak pressure was significantly lower in the LMAS group than in LMA ProSeal during laparoscopic cholecystectomy.[13] However, in a similar study, the oropharyngeal leak pressure was similar in these two groups. The oropharyngeal leak pressure values did not change during the induction and throughout pneumoperitoneum.[14] The LMA ProSeal achieved a slight but significantly higher airway seal pressure than LMAS in patients anesthetized in prone position.[20] There was no significant difference in airway leak pressures when LMAS was compared to LMA ProSeal in children.[21] Igel demonstrated a higher oropharyngeal leak pressure than LMAS in anesthetized children.[22] The seal pressure was significantly higher during neck flexion than the neutral position and significantly lower during neck extension than the neutral position in both Igel and LMAS.[23] When LMAS was compared with Igel during laparoscopic cholecystectomy, the oropharyngeal leak pressure changed following carbon dioxide pneumoperitoneum in both groups without any significant difference between the two groups.[10] When LMAS was compared with LMA ProSeal and Igel in laparoscopic surgeries, the oropharyngeal leak pressure was lower in the Igel group initially, but it was higher than the ProSeal group and supreme group at 30 min of surgery after trendelenburg position and at the 60 min of surgery.[24]
The other studies also noticed a higher incidence of sore throat than dysphagia and hoarseness of voice in both groups,[4,5,6,18] whereas in our study, the only laryngopharyngeal morbidity present was sore throat, with no significant difference between the two groups. The occurrence of postoperative complications was not significantly different when LMAS was compared with LMA classic, I gel and LMA ProSeal.[8,10,13,14]
The nasogastric tube could easily be inserted in all the study population without any resistance. Gastric tube insertion was easier and achieved more quickly with the LMAS when compared to Igel.[11,12] Gastric tube insertion was successful in all patients when LMAS was compared to LMA ProSeal with no difference in mean insertion time.[13] However, in a similar study, although the first attempt success rate for insertion of the drainage tube was similar, they were inserted more quickly with LMAS than LMA ProSeal.[14]
Our study was single-blinded, however there was an inherent possibility of observer bias. In an attempt to reduce this limitation, all insertions were done by experienced anesthesia consultants not involved in the study. In our system, blinding the investigator in the immediate postoperative was difficult and was not done. However, 24 h data collection was done by a blinded observer. There was no post hoc power analysis done for this study. In our study, we compared the oropharyngeal seal pressure created by the device only once immediately after insertion. We did not know whether the seal pressure remained the same throughout the surgical procedure and also in different head and neck positions. We also did not include obese patients or laparoscopic surgeries in the study to evaluate the performance of BM at higher airway pressures.
Conclusion
From the present study, it is concluded that the BM creates a higher oropharyngeal seal pressure and thus provides a better airway seal than the LMAS. However, the BM is more difficult to insert than the LMAS. The incidence of postoperative laryngopharyngeal morbidity is similar in both the groups. The gastric port was correctly positioned over the esophagus in both the devices reducing the chances of aspiration.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Jubilee Mission Medical College and Research Institute.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cook T, Hagberg C. Non–laryngeal mask airway supraglottic airway devices. In: Benumof J, Hagberg CA, editors. Benumof and Hagberg's Airway Management. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2013. pp. 466–506. [Google Scholar]
- 2.Baskamask.com.au [homepage on the Internet] Sydney: BVLM Pty Ltd; [Last cited on 2018 Nov 07]. Available from: http://www.baskamask.com.au/ [Google Scholar]
- 3.LMA Supreme [homepage on the Internet]. Teleflex. com. Pennsylvania: Teleflex Incorporated; [Last cited on 2018 Nov 08]. Available from: https://www.teleflex.com/emea/documentLibrary/documents/940689-000001_31817-LMA-TF-Supreme-A4_1403_PDF.pdf . [Google Scholar]
- 4.Alexiev V, Ochana A, Abdelrahman D, Coyne J, McDonnell J, O'Toole D, et al. Comparison of the Baska mask with the single-use laryngeal mask airway in low-risk female patients undergoing ambulatory surgery. Anaesthesia. 2013;68:1026–32. doi: 10.1111/anae.12356. [DOI] [PubMed] [Google Scholar]
- 5.Al-Rawahi SAS, Aziz H, Malik AM, Khan RM, Kaul N. A comparative analysis of the Baska mask vs. Proseal laryngeal mask for general anesthesia with IPPV. Anaesth Pain Intensive Care. 2013;17:233–6. [Google Scholar]
- 6.Aziz ARA, Osman YM. Comparison of I-gel with Baskamask airway for controlled ventilation in obese patients undergoing ambulatory surgery: A prospective randomized trial. J Anaesthesiol. 2017;5:29–35. [Google Scholar]
- 7.Ali A, Canturk S, Turkmen A, Turgut N, Altan A. Comparison of the laryngeal mask airway supreme and laryngeal mask airway classic in adults. Eur J Anaesthesiol. 2009;26:1010–4. doi: 10.1097/EJA.0b013e3283313fdd. [DOI] [PubMed] [Google Scholar]
- 8.Chavan T, Budhakar A, Agaskar R. Comparative study of laryngeal mask airway supreme and laryngeal mask airway classic in paralyzed patients. Int J Biomed Adv Res. 2016;7:502–7. [Google Scholar]
- 9.Radhika K, Sripriya R, Ravishankar M, Hemanth Kumar V, Jaya V, Parthasarathy S. Assessment of suitability of I-gel and laryngeal mask airway-supreme for controlled ventilation in anesthetized paralyzed patients: A prospective randomized trial. Anesth Essays Res. 2016;10:88–93. doi: 10.4103/0259-1162.167849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SY, Rim JC, Kim H, Lee JH, Chung CJ. Comparison of I-gel and LMA supreme during laparoscopic cholecystectomy. Korean J Anesthesiol. 2015;68:455–61. doi: 10.4097/kjae.2015.68.5.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teoh WHL, Lee KM, Suhitharan T, Yahaya Z, Teo MM, Sia AT. Comparison of the LMA Supreme vs the I-gel in paralysed patients undergoing gynaecological laparoscopic surgery with controlled ventilation. Anaesthesia. 2010;65:1173–9. doi: 10.1111/j.1365-2044.2010.06534.x. [DOI] [PubMed] [Google Scholar]
- 12.Gupta V, Mehta N, Gupta S, Mahotra K. Comparative evaluation of supraglottic airway devices I-gel versus LMA-supreme in patients undergoing surgery under general Anaesthesia. Indian J Clin Anaesth. 2015;2:86–91. [Google Scholar]
- 13.Anand LK, Goel N, Singh M, Kapoor D. Comparison of the Supreme and the ProSeal laryngeal mask airway in patients undergoing laparoscopic cholecystectomy: A randomized controlled trial. Acta Anaesthesiol Taiwanica. 2016;54:44–50. doi: 10.1016/j.aat.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Hoşten T, Yildiz TŞ, Kuş A, Solak M, Toker K. Comparison of Supreme laryngeal mask airway and Pro Seallaryngeal mask airway during cholecystectomy. Balkan Med J. 2012;29:314–9. doi: 10.5152/balkanmedj.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill R, Tarat A, Pathak D, Dutta S. Comparative study of two laryngeal mask airways: Proseal laryngeal mask airway and Supreme laryngeal mask airway in anesthetized paralyzed adults undergoing elective surgery. Anesth Essays Res. 2017;11:23–7. doi: 10.4103/0259-1162.177184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexiev V, Salim A, Kevin L, Laffey J. An observational study of the Baska mask: A novel supraglottic airway. Anaesthesia. 2012;67:640–5. doi: 10.1111/j.1365-2044.2012.07140.x. [DOI] [PubMed] [Google Scholar]
- 17.Gatt S, Zundert T. The Baska Mask®-A new concept in Self-sealing membrane cuff extraglottic airway devices, using a sump and two gastric drains: A critical evaluation. J Obstet Anaesth Critical Care. 2012;2:23–30. [Google Scholar]
- 18.Shanmugavelu G, Kanagarajan T. Comparing the functional analysis of I-gel with Baska mask in laparoscopic surgeries : An observational study. Int J Res Med Sci. 2018;6:1440–3. [Google Scholar]
- 19.Beleña JM, Núñez M, Gracia JL, Pérez JL, Yuste J. The laryngeal mask airway supreme: Safety and efficacy during gynaecological laparoscopic surgery. S Afr J Anaesth Analg. 2012;18:143–7. [Google Scholar]
- 20.López AM, Valero R, Hurtado P, Gambs P, Pons M, Anglada T. Comparison of the LMA supreme with the LMA Proseal for airway management in patients anaesthetized in prone position. Br J Anaesth. 2011;107:265–71. doi: 10.1093/bja/aer104. [DOI] [PubMed] [Google Scholar]
- 21.Jagannathan N, Sohn LE, Sawardekar A, Gordon J, Langen KE, Anderson K. A randomised comparison of the LMA SupremeT and LMA Pro Seal in children. Anaesthesia. 2012;67:632–9. doi: 10.1111/j.1365-2044.2012.07088.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Lee JY, Lee SY, Park SY, Lee SC, Chung CJ. A comparison of I-gel and lma supreme in anesthetized and paralyzed children. Korean J Anesthesiol. 2014;67:317–22. doi: 10.4097/kjae.2014.67.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta S, Dogra N, Chauhan K. Comparison of I-gel and Laryngeal Mask Airway Supreme in different head and neck positions in spontaneously breathing pediatric population. Anaesth Essays Res. 2017;11:647–50. doi: 10.4103/aer.AER_238_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukadder S, Zekine B, Erdogan KG, Ulku O, Muharrem U, Saim Y, et al. Comparison of the proseal, supreme, and I-gel SAD in gynecological laparoscopic surgeries. Scientific World Journal. 2015;2015:634320. doi: 10.1155/2015/634320. [DOI] [PMC free article] [PubMed] [Google Scholar]


