Abstract
Human parainfluenza virus 3 (HPIV3) and respiratory syncytial virus (RSV) are leading causes of lower respiratory tract infections. There are currently no vaccines or antiviral therapeutics to treat HPIV3 or RSV infections. We recently reported a peptide (VIQKI), derived from the C-terminal heptad repeat (HRC) domain of the HPIV3 fusion (F) glycoprotein that inhibits infection by both HPIV3 and RSV. The dual inhibitory activity of VIQKI is due to its unique ability to bind to the N-terminal heptad repeat (HRN) domains of both HPIV3 and RSV F, thereby preventing the native HRN-HRC interactions required for viral entry. Here we describe the structure-guided design of dual inhibitors of HPIV3 and RSV fusion with improved efficacy. We show that VIQKI derivatives possessing one (I456F) or two (I454F/I456F) phenylalanine substitutions near the N-terminus exhibit more stable assemblies with RSV-HRN domain and enhanced antiviral efficacy against both HPIV3 and RSV infection. Co-crystal structures of the new Phe-substituted inhibitors co-assembled with HPIV3 or RSV-HRN domains reveal that the I456F substitution makes intimate hydrophobic contact with the core trimers of both HPIV3 and RSV F.
Graphical Abstract
Respiratory syncytial virus (RSV) and human parainfluenza virus 3 (HPIV3) are major causes of lower respiratory tract infections in young children, resulting in thousands of deaths each year.13-14 There is no vaccine for RSV or HPIV3 and no treatment for these infections, which present with similar symptoms although the viruses are quite different. To be most effective, treatment would have to start soon after the appearance of symptoms, and before the causative agent had been identified. Therefore, a single agent that could address both RSV and HPIV3 is desirable.
Inhibition of viral fusion is a potential therapeutic strategy4-14, and we recently described a 36-residue peptide derived from the HPIV3 fusion (F) glycoprotein that displays significant inhibition of RSV infection while maintaining anti-HPIV3 potency.15 This peptide, designated VIQKI, appears to block viral infection by interfering with F-mediated fusion of the HPIV3 or RSV envelope and the target cell membrane. Fusion is essential for infection and is driven by the formation of a six-helix bundle assembly within the F protein trimer (Figure 1B,C).16-18 The three inner helices are formed by the N-terminal heptad repeat (HRN) domain of F, and the three outer helices are formed by the C-terminal heptad repeat (HRC) domain. VIQKI is a variant of HPIV3-HRC. Crystallographic data revealed that that the N-terminal portion of VIQKI displays an unexpected ability to adapt itself to two distinct assemblies: a native-like extended conformation when co-assembled with HPIV3-HRN, but an unexpected helical conformation upon co-assembly with RSV-HRN. Presumably this structural adaptability underlies the dual activity of VIQKI against HPIV3 and RSV.
Figure 1.
Domain architecture and structures of HPIV3 and RSV F. (A) Diagram of HPIV3 and RSV F with fusion peptide (FP), N-terminal (HRN) and C-terminal (HRC) heptad repeat, and transmembrane (TM) domains. Structures of pre-fusion (B, PDB:4MMV) and post-fusion (C, PDB:3RRR) conformations of RSV F. (D) Peptide sequences for HPIV3-HRC, RSV-HRC, VIQKI-I456F, and VIQKI-I454F/I456F (Phe-substitutionsv in red).
Here we ask whether efficacy against RSV can be enhanced without sacrificing efficacy against HPIV3 by modifying the N-terminal region of VIQKI. Because this portion of VIQKI uses two different sets of residues to engage HPIV3-HRN vs. RSV-HRN, it was not clear at the outset that our goal could be achieved. Nevertheless, an experimental design based on careful inspection of F protein structures from both viruses delivered enhanced dual inhibitors.
Our recent crystal structure of VIQKI co-assembled with HPIV3-HRN16 revealed a six-helix bundle that corresponds closely to the HRC/HRN assembly in the post-fusion form of the HPIV3 F protein (Figure 2A,B).19 Co-crystallization of VIQKI with the RSV-HRN peptide led to a comparable hexameric assembly, but the N-terminal portion of VIQKI displayed substantial conformational variation between these two structures (Figure 2B,D). In the HPIV3-HRN co-assembly, the N-terminal segment of VIQKI is extended and projects three aliphatic side chains (L451, I454 and I456; numbering based on the full-length HPIV3 F protein) to make native hydrophobic contacts with the HRN trimer core. In the RSV-HRN co-assembly, the N-terminal segment of VIQKI forms an additional helical turn, and the side chain carboxylates of D452 and D455 form salt bridges with the RSV-HRN trimer core. The corresponding region of the RSV-HRC domain in the post-fusion state of the RSV F protein does not form salt bridges with the HRN trimer; instead, the Phe483 and Phe488 side chains make hydrophobic contacts with the HRN trimer (Figure 2C).20,21
Figure 2.
HRC domain N-terminal segments in six-helix bundle assemblies. X-ray structures of (A) the full 6HB HPIV3-HRN+VIQKI (PDB:6NRO) with magnified images of (B) HPIV3-HRN+VIQKI, (C) post-fusion RSV F (PDB:3RRR), and (D) RSV-HRN+VIQKI (PDB:6NTX).
We predicted that the anti-RSV potency of VIQKI could be enhanced via replacement of one residue or more in the N-terminal region with Phe, if an appropriately positioned Phe side chain engaged the complementary pocket on the RSV-HRN trimer that is occupied by the F483 or F488 side chain in the post-fusion RSV F structure (Figure 2C). By mimicking these native interactions, we hypothesized that we could maintain specificity for the RSV-HRN trimeric core while limiting non-specific engagement of unintended targets. A key uncertainty, however, was the impact of new Phe residue(s) on anti-HPIV3 potency; enhancing activity against RSV would be most valuable if activity against HPIV3 were not compromised.
We conducted a “Phe scan” of the first eight positions of VIQKI, corresponding to HPIV3 F residues V449-I456, to identify favorable Phe substitutions. The impact of each Phe modification was assessed by circular dichroism (CD) measurements. Combining VIQKI with a peptide corresponding to the RSV-HRN domain or the HPIV3-HRN domain leads to a strong α-helical CD signature, which is attributed to helix-bundle formation. Thermal disruption of the helix bundle can be detected by monitoring the characteristic CD minimum at 222 nm as the sample is heated, and the midpoint of the transition provides an apparent melting temperature (Tm,app). Table 1 shows Tm,app values for VIQKI and each of the eight single-site Phe variants in combination with RSV-HRN. At most positions, Phe substitution led to a destabilization of the helix-bundle assembly (ΔTm,app<0). However, increases in assembly stability were observed for modifications L451F, I454F or I456F, with the most substantial enhancement for the last of these three variants. Two double Phe substitutions were examined, L451F/I456F and I454F/I456F. ΔTm,app values suggested that only the latter combination was favorable.
Table 1.
Apparent Melting Temperatures (Tm,app) of Co-assemblies Formed Between VIQKI Variants and HPIV3-HRN or RSV-HRN
| HPIV3-HRN | RSV-HRN | |||
|---|---|---|---|---|
| Inhibitor |
Tm,appa (°C) |
ΔTm,app†b |
Tm,appa (°C) |
ΔTm,app††c |
| VIQKI | 88.6 | - | 64.3 | - |
| VIQKI-V449F | - | - | 41.9 | −22.4 |
| VIQKI-A450F | - | - | 45.3 | −19.0 |
| VIQKI-L451F | - | - | 65.9 | +1.6 |
| VIQKI-D452F | - | - | 42.2 | −22.1 |
| VIQKI-P453F | - | - | 63.0 | −1.3 |
| VIQKI-I454F | - | - | 65.1 | +0.8 |
| VIQKI-D455F | - | - | 55.6 | −8.7 |
| VIQKI-I456F | 84.9 | −3.7 | 70.0 | +5.7 |
| VIQKI-L451F/I456F | 83.1 | −5.5 | 67.2 | +2.9 |
| VIQKI-I454F/I456F | 85.1 | −3.5 | 72.8 | +8.5 |
1:1 mixture of Inhibitor (50 μM) and HRN (50 μM) peptides.
ΔTm,app†=Tm,app(HPIV3-HRN+Inhibitor)–Tm,app(HPIV3-HRN+VIQKI).
ΔTm,app††=Tm,app(RSV-HRN+Inhibitor)–Tm,app(RSV-HRN+VIQKI).
We employed a plaque-reduction assay to measure the efficacies of selected Phe-containing variants of VIQKI at inhibiting RSV infection. Monolayers of Hep2 cells were exposed to an engineered recombinant RSV that expresses green fluorescent protein (GFP), which allows the extent of infection to be quantified via fluorescence high content image screening. Previous data demonstrate that VIQKI is approximately as effective as RSV-HRC and much more effective than HPIV3-HRC at inhibiting RSV infection.15 The substitutions in VIQKI enhance inhibition of HPIV3 infection relative to HPIV3 HRC .10,14,15 Here, we asked whether we could improve upon VIQKI's anti-RSV potency without decreasing its efficacy against HPIV3. Four peptides were evaluated, new compounds VIQKI-I456F and VIQKI-I454F/I456F, along with RSV-HRC and VIQKI as reference compounds (Figure 3A); peptide was added to the cells along with the virus. RSV-HRC and VIQKI displayed comparable efficacies in this assay, with IC50 values of ~1 μM. Both Phe-containing variants, VIQKI-I456F and VIQKI-I454F/I456F, were significantly more effective inhibitors than either RSV-HRC or VIQKI, with IC50 values ≥10-fold lower for the Phe-containing variants (Table S1). Thus, the stabilization of six-helix bundle assembly suggested by CD measurements for the two new peptides relative to VIQKI (Table 1) correlates with enhanced efficacy in blocking RSV infection.
Figure 3.
Antiviral efficacy of VIQKI-I456F and VIQKI-I454F/I456F against RSV or HPIV3. Peptide activity against (A) RSV or (B) HPIV3 was determined by plaque-reduction assay in infected Hep-2 cell monolayers. Data are expressed as mean ± standard deviation (n=3 separate experiments).
An analogous plaque-reduction assay was performed to compare the anti-HPIV3 activities of the two new Phe-containing VIQKI variants with the activity of VIQKI itself. We have previously demonstrated that RSV-HRC does not block infection by HPIV3.15 Monolayers of Hep2 cells were exposed to an engineered recombinant HPIV3 that expresses GFP along with variable concentrations of VIQKI, VIQKI-I456F or VIQKI-I454F/I456F (Figure 3B). Both of the new Phe-containing peptides were modestly superior to VIQKI as inhibitors of HPIV3 infection. Thus, both peptides are potent dual inhibitors of RSV and HPIV3.
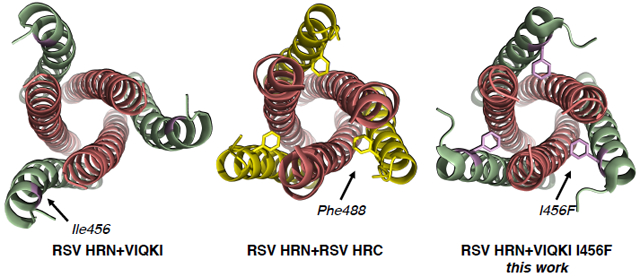
In order to elucidate Phe side chain locations within helix-bundle assemblies containing the new dual inhibitors, we sought to co-crystallize VIQKI-I456F and VIQKI-I454F/I456F with either RSV-HRN or HPIV3-HRN. Two of the four pairings produced high-quality crystals that led to atomic-resolution structures, VIQKI-I456F+RSV-HRN and VIQKI-I454F/I456F+HPIV3-HRN (Figure 4).
Figure 4.
X-ray structures of (A) 6HB segment of RSV F in post-fusion conformation (PDB:3RRR) and co-crystal structures of (B) RSV-HRN+VIQKI-I456F (PDB:6OJ7) and (C) HPIV3-HRN+VIQKI-I454F/I456F (PDB:6O40). The phenylalanine-substituted VIQKI analogs adopt a binding mode similar to that of RSV-HRC. Colors correspond to RSV-HRN (salmon), HPIV3-HRN (orange), RSV-HRC (yellow), and VIQKI/VIQKI-I456F/VIQKI-I454F/I456F (green with Phe-substitutions in violet). Electrostatic potential maps at the binding site of (D) Phe488 (RSV F), (E) Phe456 (RSV-HRN+VIQKI-I456F), and (F) Phe456 (HPIV3-HRN+VIQKI-I454F/I456F) with anionic (red), neutral (gray), or positive (blue) charge density.
The VIQKI-I456F+RSV-HRN co-crystal structure revealed that the newly introduced Phe residue induced a reorientation of the N-terminal region of the VIQKI derivative relative to the trimeric HRN core, as intended. Although salt bridges involving D455 and D452 form when VIQKI co-assembles with the RSV-HRN trimer (Figure 2D), comparable salt bridges are not found in the new complex; instead, the side chain of F456 occupies the pocket that accommodates the F488 side chain in the structure of post-fusion RSV F (Figure 4D,E, Figure S30).20,21 Other critical residues of VIQKI that engage the RSV-HRN trimer, such as L467, I474, and L481, maintain their contacts in the new VIQKI-I456F+RSV-HRN structure (Figure S31).
The VIQKI-I454F/I456F+HPIV3-HRN co-crystal structure displayed a reorientation of the VIQKI derivative N-terminal region comparable to that observed for VIQKI-I456F+RSV-HRN (Figure 4E,F). Helical secondary structure extends further toward the N-terminus of VIQKI-I454F/I456F than was observed in the VIQKI+HPIV3-HRN co-crystal structure. The side chain of F456 occupies the pocket that accommodates I456 in the structure of VIQKI+HPIV3 (Figure 4F vs. 2B).19 We were surprised, however, to observe that the side chain of F454 is oriented away from the HPIV3-HRN trimeric core; we had speculated that this side chain would occupy the pocket that is naturally filled by the side chain of I454. Inspection of interactions involving neighboring six-helix bundles within the co-crystal lattice revealed that F454 from one assembly stacks against W473 from an adjacent assembly (Figure S32). It is possible that this lattice contact distorts the positioning of F454 relative to an isolated six-helix bundle in solution. On the other hand, the Tm,app values are indistinguishable for co-assemblies with HPIV3-HRN formed by VIQKI-I456F and VIQKI-I456F/I454F, which suggests that the side chain of F454 does not contribute to helix-bundle stability in the latter case. This observation is consistent with the location of the F454 side chain in the VIQKI-I454F/I456F+HPIV3-HRN co-crystal structure (Figure 4C,F).
We have shown that a structure-guided strategy can deliver new dual inhibitors of HPIV3 and RSV that manifest enhanced anti-RSV potency relative to the previously reported peptide VIQKI, which is very effective against HPIV3 but less effective against RSV. Comparisons involving available crystal structures led us to hypothesize that introduction of a Phe residue in the N-terminal segment of VIQKI would improve binding to the RSV-HRN trimer if the new Phe residue could mimic Phe488 in the RSV-HRC domain. Because we could not predict the best modification site in VIQKI, we evaluated a set of variants in which each of the eight residues nearest the N-terminus was replaced, individually, by Phe. Three sites of modification stabilized the assembly with RSV-HRN; the largest benefit observed was for substitution at I456. VIQKI-I456F is substantially superior as an inhibitor of RSV infection relative to VIQKI itself or the RSV-HRC domain. In addition, VIQKI-I456F is comparable or possibly superior to VIQKI as an inhibitor of HPIV3 infection. Crystal structures of six-helix bundles formed between new inhibitors and either RSV-HRN or HPIV3-HRN show that in both assemblies the Phe456 side chain makes intimate hydrophobic contacts with the core trimer. VIQKI-I456F is a promising therapeutic candidate for both RSV and HPIV3 infections.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH/NIAID R01AI114736 to AM and NIH/NIAID R01AI121349 to MP. VKO was funded in part by a Ruth L. Kirschstein National Research Service Award from the NIH (F32GM122263). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Use of the LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (Grant 085P1000817). We thank Craig Bingman of the University of Wisconsin Crystallography Core facility for assistance with x-ray data collection, Dale F. Kreitler for advice on x-ray data processing, and the Morgan Stanley Global Alliance–Morgan Stanley Children’s Hospital and Beijing Children’s Hospital Pediatric Initiative for support of Y. Zhu.
REFERENCES
- 1.Nair H; Nokes DJ; Gessner BD; Dherani M; Madhi SA; Singleton RJ; O'Brien KL; Roca A; Wright PF; Bruce N; Chandran A; Theodoratou E; Sutanto A; Sedyaningsih ER; Ngama M; Munywoki PK; Kartasasmita C; Simões EA; Rudan I; Weber MW; Campbell H Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. The Lancet 2010, 375, 1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Englund JA; Moscona A Paramyxoviruses: Parainfluenza viruses In Viral Infections of Humans Kaslow RA; Stanberry LR; Le Duc JW Eds., Springer, New York: 2014, 579–600. [Google Scholar]
- 3.Shi T; McAllister DA; O'Brien KL; Simoes EAF; Madhi SA; Gessner BD; Polack FP; Balsells E; Acacio S; Aguayo C; Alassani I; Ali A; Antonio M; Awasthi S; Awori JO; Azziz-Baumgartner E; Baggett HC; Baillie VL; Balmaseda A; Barahona A; Basnet S; Bassat Q; Basualdo W; Bigogo G; Bont L; Breiman RF; Brooks WA; Broor S; Bruce N; ruden D; Buchy P; Campbell S; Carosone-Link P; Chadha M; Chipeta J; Chou M; Clara W; Cohen C; de Cuellar E; Dang D-A; Dash-Yandag B; Deloria-Knoll M; Dherani M; Eap T; Ebruke BE; Echavarria M; de Freitas Lázaro Emediato CC; Fasce RA; Feikin DR; Feng L; Gentile A; Gordon A; Goswami D; Goyet S; Groome M; Halasa N; Hirve S; Homaira N; Howie SRC; Jara J; Jroundi I; Kartasasmita CB; Khuri-Bulos N; Kotloff KL; Krishnan A; Libster R; Lopez O; Lucero MG; Lucion F; Lupisan SP; Marcone DN; McCracken JP; Mejia M; Moisi JC; Montgomery JM; Moore DP; Moraleda C; Moyes J; Munywoki P; Mutyara K; Nicol MP; Nokes DJ; Nymadawa P; da Costa Oliveira MT; Oshitani H; Pandey N; Paranhos-Baccalà G; Phillips N; Picot VS; Rahman M; Rakoto-Andrianarivelo M; Rasmussen ZA; Rath BA; Robinson A; Romero C; Russomando G; Salimi V; Sawatwong P; Scheltema N; Schweiger B; Scott JAG; Seidenberg P; Shen K; Singleton R; Sotomayor V; Strand TA; Sutanto A; Sylla M; Tapia MD; Thamthitiwat S; Thomas ED; Tokarz R; Turner C; Venter M; Waicharoen S; Wang J; Watthanaworawit W; Yoshida L-M; Yu H; Zar HJ; Campbell H; Nair H; RSV Global Epidemiology Network. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children in 2015: a Systematic Review and Modelling Study. Lancet 2017, 390 (10098), 946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert DM; Barney S; Lambert AL; Guthrie K; Medinas R; Davis DE; Bucy T; Erickson J; Merutka G; Petteway SR Peptides From Conserved Regions of Paramyxovirus Fusion (F) Proteins Are Potent Inhibitors of Viral Fusion. Proc. Natl. Acad. Sci USA 1996, 93 (5), 2186–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wild C; Dubay JW; Greenwell T; Baird T; Oas TG; McDanal C; Hunter E; Matthews T Propensity for a leucine zipper-like domain of human immunodeficiency virus type 1 gp4l to form oligomers correlates with a role in virus-induced fusion rather than assembly of the glycoprotein complex. Proc. Natl. Acad. Sci. USA 1994, 91 (26), 12676–12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao Q; Compans RW Peptides corresponding to the heptad repeat sequence of human parainfluenza virus fusion protein are potent inhibitors of virus infection. Virology 1996, 223, 103–112. [DOI] [PubMed] [Google Scholar]
- 7.Rapaport D; Ovadia M; Shai Y A synthetic peptide corresponding to a conserved heptad repeat domain is a potent inhibitor of Sendai virus-cell fusion: an emerging similarity with functional domains of other viruses. EMBO J. 1995, 14, 5524–5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolarinwa O; Zhang M; Mulry E; Lu M; Cai J Sulfono-ɣ-AA modified peptides that inhibit HIV-1 fusion. Org. Biomol. Chem 2018, 16 (42), 7878–7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews T; Salgo M; Greenberg M; Chung J; DeMasi R; Bolognesi D Enfuvirtide: The first therapy to inhibit the entry of HIV-1 into host CD4 lymophocytes. Nat. Rev. Drug Discov 2004, 3 (3), 215–225. [DOI] [PubMed] [Google Scholar]
- 10.Porotto M; Carta P; Deng Y; Kellogg GE; Whitt M; Lu M; Mungall BA; Moscona A Molecular determinants of antiviral potency of paramyxovirus entry inhibitors. J. Virol 2007, 81 (19), 10567–10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathieu C; Huey D; Jurgens E; Welsch JC; DeVito I; Talekar A; Horvat B; Niewiesk S; Moscona A; Porotto M Prevention of measles virus infection by intranasal delivery of fusion inhibitor peptides. J. Virol 2015, 89 (2), 1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figueira TN; Palermo LM; Veiga AS; Huey D; Alabi CA; Santos NC; Welsch JC; Mathieu C; Horvat B; Niewiesk S; Moscona A; Castanho MARB; Porotto M In vivo efficacy of measles virus fusion protein-derived peptides is modulated by the properties of self-assembly and membrane residence. J. Virol 2017, 91 (1), e01554–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathieu C; Augusto MT; Niewiesk S; Horvat B; Palermo LM; Sanna G; Madeddu S; Huey D; Castanho MARB; Porotto M; Santos NC; Moscona A Broad spectrum antiviral activity for paramyxoviruses is modulated by biophysical properties of fusion inhibitory peptides. Sci. Rep 2017, 7, 43610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porotto M; Rockx B; Yokoyama CC; Talekar A; DeVito I; Palermo LM; Liu J; Cortese R; Lu M; Feldmann H; Pessi A; Moscona A Inhibition of Nipah virus infection in vivo: Targeting an early stage paramyxovirus fusion activation during viral entry. PLoS Pathog. 2010, 6 (10), e1001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Outlaw VK; Bottom-Tanzer S; Kreitler DF; Gellman SH; Porotto M; Moscona A Dual Inhibition of Human Parainfluenza Type 3 and Respiratory Syncytial Virus Infectivity with a Single Agent. J. Am. Chem. Soc 2019, 141 (32), 12648–12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison SC Viral membrane fusion. Nat. Struct. Mol. Biol 2008, 15, 690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang A; Dutch RE Paramyxovirus fusion and entry: Multiple paths to a common end. Viruses 2012, 4, 613–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kielian M Mechanisms of virus membrane fusion proteins. Ann. Rev. Virol. 2014, 1, 171–189. [DOI] [PubMed] [Google Scholar]
- 19.Yin H-S; Paterson RG; Wen X; Lamb RA; Jardetzky TS Structure of the uncleaved ectodomain of the paramyxovirus (hPIV3) fusion protein. Proc. Natl. Acad. Sci. USA 2005, 102 (26), 9288–9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao X; Singh M; Malashkevich VN; Kim PS Structural characterization of the human respiratory syncytial virus fusion protein core. Proc. Natl. Acad. Sci. USA 2000, 97 (26), 14172–14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLellan JS; Yang Y; Graham BS; Kwong PD Structure of Respiratory Syncytial Virus Fusion Glycoprotein in the Postfusion Conformation Reveals Preservation of Neutralizing Epitopes. J. Virol 2011, 85 (15), 7788–7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






