Abstract
There is increasing evidence that members of the gut microbiota, especially Fusobacterium nucleatum (F. nucleatum), are associated with Crohn’s disease (CD), but the specific mechanism by which F. nucleatum promotes CD development is unclear. Here, we first examined the abundance of F. nucleatum and its effects on CD disease activity and explored whether F. nucleatum aggravated intestinal inflammation and promoted intestinal mucosal barrier damage in vitro and in vivo. Our data showed that F. nucleatum was enriched in 41.21% of CD tissues from patients and was correlated with the clinical course, clinical activity, and refractory behavior of CD (P < 0.05). In addition, we found that F. nucleatum infection is involved in activating the endoplasmic reticulum stress (ERS) pathway during CD development to promote intestinal mucosal barrier destruction. Mechanistically, F. nucleatum targeted caspase activation and recruitment domain 3 (CARD3) to activate the ERS pathway and promote F. nucleatum-mediated mucosal barrier damage in vivo and in vitro. Thus, F. nucleatum coordinates a molecular network involving CARD3 and ERS to control the CD process. Measuring and targeting F. nucleatum and its associated pathways will provide valuable insight into the prevention and treatment of CD.
Keywords: Fusobacterium nucleatum, intestinal mucosal barrier, endoplasmic reticulum stress, Crohn’s disease, gene regulation
Introduction
Crohn’s disease (CD) is a chronic and disabling disease that can seriously affect quality of life. The natural history of CD can lead to intestinal damage, such as stenosis, fistula, or bowel resection (Malluta et al., 2019). Goals of therapy include resolution of symptoms and mucosal healing. However, many patients have suboptimal responses to currently available therapies. Therefore, understanding the mechanism of CD is critical to optimizing current treatment strategies.
In recent years, it has been determined that the gut microbiota plays a key role in CD (He et al., 2019). The intestinal flora and the intestinal immune system are always in homeostasis. Breaking this balance can trigger an excessive intestinal immune response and cause the damage to the intestinal mucosal barrier (Nishikawa et al., 2009; Allen-Vercoe and Jobin, 2014). Fusobacterium nucleatum (F. nucleatum) is a well-known pro-inflammatory bacterium that has been found in many patients with Crohn’s disease (Kostic et al., 2013). Studies have shown that F. nucleatum is implicated in CD and that strains isolated from inflamed biopsy tissue from CD patients were significantly more invasive than strains that were isolated from healthy tissue from either CD patients or control patients (Cheung and Bellas, 2007; Han et al., 2010; Strauss et al., 2011). However, the effects and mechanisms of F. nucleatum on the CD disease process are not well-defined.
The intact intestinal mucosal barrier can prevent intestinal bacteria, toxins, and antigens from entering immune cells in the lamina propria (Actis et al., 2014). Mucosal healing has been considered the best therapeutic endpoint for CD patients because it is associated with sustained clinical remission followed by a lower incidence of hospitalization and surgery (Malluta et al., 2019). Previous studies have shown that in biofilms, F. nucleatum can penetrate the epithelial/basement membrane barrier and invade the collagen matrix after incubation if the bacterial biofilm is incubated in contact with cells in an organotypic cell culture model (Gursoy et al., 2010). F. nucleatum can invade into mucosa in patients with acute appendicitis and colorectal cancer (Yu et al., 2016). However, the damage to the intestinal mucosa by F. nucleatum has not yet been specifically clarified (Kumar et al., 2016).
Endoplasmic reticulum (ER), as a membrane-bound organelle, plays a crucial role in folding of secreted and membrane proteins (Huang et al., 2019). If the levels of the unfolded and misfolded proteins exceed the processing capacity of the ER, ER stress (ERS) occurs (Li et al., 2019). The ER chaperone protein BIP is a major regulatory factor of ER homeostasis and stress response (Li et al., 2016). Many factors can cause ER homeostasis to be disrupted, including bacterial infection (Ma et al., 2019). Studies have found that microbial infection can trigger ERS, and ERS-activating cells can regulate the expression and activation of ERS-related proapoptotic molecules, ultimately determining whether cells are adaptive or undergo apoptosis (Ma et al., 2019). This response allows pro-inflammatory molecules to be released during the chronic inflammation of the CD, leading to damage to the colon cells, and thereby impairing the integrity of the epithelial barrier. It has been found that the endogenous metabolite acrolein induces ERS, mediates epithelial cell death, leads to impaired intestinal epithelial barrier function and increased permeability, and causes the downregulation and/or redistribution of three representative tight junction proteins (i.e., zonula occludens-1, occludin, and claudin-1) that critically regulate epithelial paracellular permeability (Chen et al., 2017; Odenwald and Turner, 2017). This finding indicates that ERS is closely related to the integrity and function of the intestinal mucosal barrier. However, it is unclear whether F. nucleatum can induce intestinal mucosal damage by inducing ERS.
In this study, we investigated whether and how F. nucleatum affects the integrity of the epithelial barrier in patients with CD. We examined that the F. nucleatum abundance in colon tissue from patients with active CD was increased compared to that in tissues from healthy controls or patients with remitted CD. We then demonstrated that F. nucleatum plays a key role in mediating CD development by upregulating caspase activation and recruitment domain 3 (CARD3) and activating the ERS pathway.
Materials and Methods
Collection of Clinical Samples
The patient materials used in this study were obtained from Wuhan University People’s Hospital (Hubei, China). All participants provided informed consent, and the project was approved by the institutional review board (approval number: 2018K-C089). Inflamed intestinal biopsies were obtained from CD patients undergoing intestinal endoscopy. Normal tissue biopsies were obtained from healthy controls ranging in age from 15 to 65 years (to match the age of patients with CD) who underwent an endoscopy for colon cancer screening without a prior diagnosis of gastrointestinal illness. CD diagnosis was confirmed in conjunction with clinical and histological criteria. Clinical disease activity was assessed by the Harvey-Bradshaw activity index (HBI) and the colitis activity index. CD patients with an HBI of ≤4 were considered to be in remission, those with ≥5 to have active disease. Exclusion criteria included patients with previous inflammatory bowel disease (IBD) treatment, receiving antibiotics or probiotics in the last 12 weeks, receiving biologicals or immunosuppressants within the past 2 years, history of fecal microbiota transplant (FMT), age <15 years, presentation of other known chronic diseases, and pregnant or breastfeeding. Formalin-fixed, paraffin-embedded CD intestinal tissues were obtained from the pathology archives from June 2016 to June 2018. Clinicopathological data for each patient were obtained from hospital records.
Bacterial Strains and Cell Lines
The human normal epithelial cell line NCM460 and the FHC cell line ATCC were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum at 37°C and 5% CO2. F. nucleatum was grown on tryptic soy containing 5% defibrinated sheep blood, under anaerobic conditions (10% H2, 5% CO2, and 85% N2), with a 2-day anaerobic gas filling system at 37°C. Escherichia coli strain (Tiangen, China) was cultured in Luria-Bertani (LB) medium for 16 h at 37°C with shaking at 200–220 rpm. The F. nucleatum suspension was centrifuged at 2,500 × g for 5 min and was then resuspended in antibiotic-free DMEM before being used to infect normal epithelial cells. E. coli-infected cells were used as the control.
Mice
Five- to six-week-old male C57BL/6J CARD3 knockout (KO, CARD3-/-) mice were kindly provided by Dr. Richard Flavell (Howard Hughes Medical Institute, Yale University, New Haven, CT). CARD3 KO mice were backcrossed with C57BL/6 mice for at least six generations to yield CARD3 heterozygous mice. Then, the littermate offspring [card3 KO and wild-type (WT) mice] produced by inbreeding CARD3 heterozygous mice were used for further study. Five- to six-week-old male C57BL/6J WT (CARD3wt) mice were obtained from Nanjing Biomedical Research Institute of Nanjing University (NBRI). Mice were housed and bred in our specific pathogen-free animal facility (Yang et al., 2017). All animal procedures were approved by the Animal Care and Use Committee of Renmin Hospital of Wuhan University, China.
Induction of Colitis
All mice were fed 2 mg/ml streptomycin for 3 days in their drinking water to ensure consistency of the conventional microbiota and promote F. nucleatum colonization. Mice were administered a daily dose of F. nucleatum cells [109 colony-forming units (CFUs)/ml] resuspended in phosphate-buffered solution (PBS) or PBS alone for 2 weeks. To induce colitis, 3% (wt/vol) dextran sulfate sodium (DSS) (wt. 36–50 kDa; MP Biomedicals) was added to the drinking water of the mice (Danese et al., 2007). Animal weight, water/food consumption, morbidity, stool consistency, and the presence of large amounts of blood in the feces or anus were measured or observed once daily. On the seventh day after induction with DSS, animals were quickly euthanized by inhalation of CO2, the colon and cecum were quickly separated, the colon was photographed and used for length measurement, and feces and blood were gently removed with 4°C PBS. A small segment of the colon was fixed in paraformaldehyde for histological staining (H&E) and fluorescence in situ hybridization (FISH), and another portion of the tissue was immediately frozen in liquid nitrogen for PCR or Western blot (WB) analysis.
Inhibition of Endoplasmic Reticulum Stress With 4-Phenyl Butyric Acid
In the 4-phenyl butyric acid (4-PBA) treatment study, mice were injected intraperitoneally with 4-PBA every 3 days at a dose of 100 μg per mouse, and F. nucleatum (109 CFU/ml) resuspended in PBS were administered intragastrically 1 h after 4-PBA injection (Wu et al., 2018). And these mice were treated with DSS and continued for another week. Similarly, NCM460 cells were treated with 4-PBA and were then infected with F. nucleatum (MOI = 100) 1 h later.
RNA Extraction and Real-Time PCR
Total RNA was extracted using TRIpure Total RNA Extraction Reagent (ELK Biotechnology), and real-time PCR with three replicate wells per sample was performed on a StepOne™ Real-Time PCR machine (Life Technologies), using an EnTurbo™ SYBR Green PCR SuperMix kit (ELK Biotechnology, EQ001). Real-time quantitative PCR was performed in triplicate. The Ct values obtained from different samples were compared using the ΔΔCt method. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as the internal reference gene.
Fluorescence in Situ Hybridization
Microbial FISH was performed as described (Yu et al., 2016). Five-micrometer-thick sections were prepared and hybridized following the manufacturer’s instructions (FOCOFISH, Guangzhou, China). The sequence of the “universal bacterial” probe (EUB338; Cy3 labeled) was 5′-GCT GCC TCC CGT AGG AGT-3′. The sequence of the F. nucleatum-targeted probe [FUS664; fluorescein isothiocyanate (FITC) labeled] was 5′-CTT GTA GTT CCG C(C/T) TAC CTC-3′. Slides were examined using a microscope (BX53F; Olympus, Tokyo, Japan). Five random 200× magnification fields per sample were evaluated by three observers blinded to the experimental protocol, and the average number of bacteria per field was calculated. We defined negative, low, or high abundance of F. nucleatum as those cases with <5, between 5 and 20, and >20 visualized FUS664 probes per field on average, respectively. Other bacteria were noted as positive with >5 bacteria per field with EUB 338 probe but negative with FUS664 probe.
High-Throughput Sequencing
DNA or RNA was sent to Adaptive Biotechnology (HuaDa, China) for sequencing. The DNA sequence data have been deposited in the NCBI Sequence Read Archive (SRA) database (accession number: PRJNA541040).
Cell Transfection
siRNA targeting the human CARD3 gene (siCARD3) and nontargeting siRNAs (control siRNAs) were purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China). Cells were cultured and transfected with siRNAs according to the supplier’s instructions. Lipid-based transfections were achieved with Lipofectamine 6000 (Beyotime, China) according to the manufacturer’s protocol. Cells were incubated with the siRNA complex for 72 h, and protein was extracted for assessing transfection efficiency by WB.
Immunohistochemistry and Western Blotting
Immunohistochemistry (IHC) was performed using an UltraSensitive™ SP (mouse/rabbit) IHC kit (Maxib, Fuzhou, China) according to the manufacturer’s instructions. For WB, primary antibodies against the following targets were used: CARD3 (CST), BIP (CST), XBP1 (Abeam), ZO-1 (Abeam), occludin (Abeam), and GAPDH (Bioworld).
Statistical Analyses
Statistical analysis was performed using GraphPad Prism software version 8.0. Data are expressed as the means ± SDs. Normally distributed data were analyzed by Student’s t test. Differences among multiple groups were evaluated for significance using one-way ANOVA combined with Bonferroni’s post hoc test. Statistical significance was defined as P < 0.05.
Results
F. nucleatum Is Abundant in Crohn’s Disease Tissues and Is Linked to Disease Severity
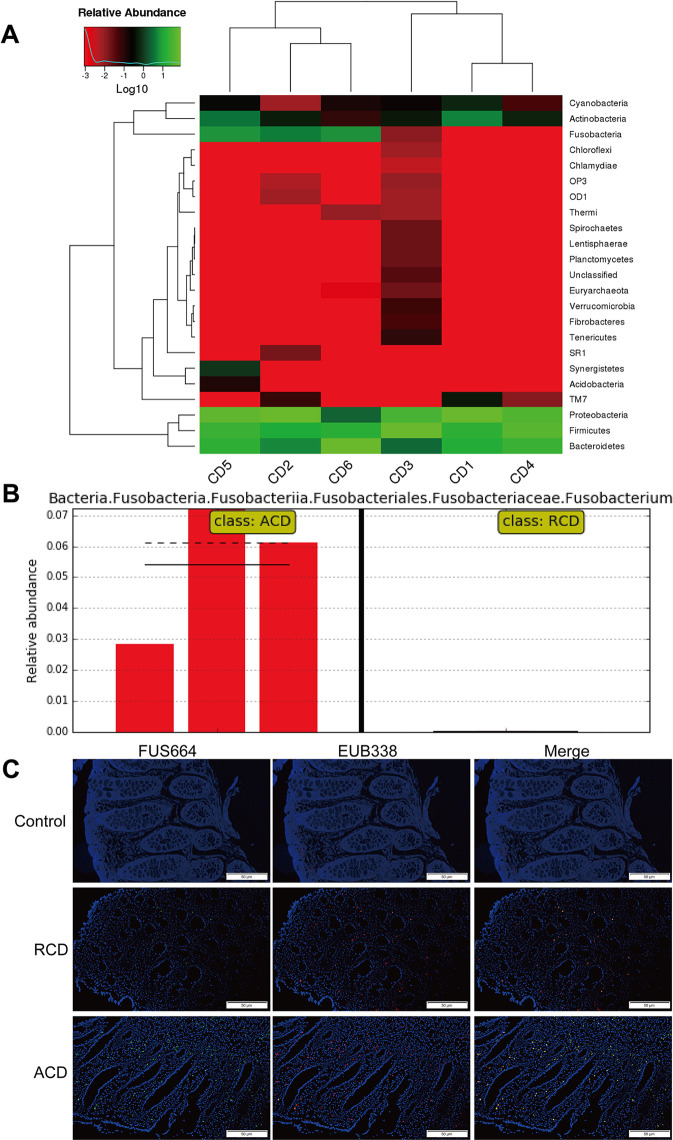
Previous studies have shown that the invasive potential of gut mucosa-derived F. nucleatum positively correlated with CD status of the host (Zhou et al., 2016; Dinakaran et al., 2018). To verify the potential relationship between gut microbiota alterations and CD, we compared three inflamed tissues from patients with active CD (ACD) and three inflamed tissues from patients with remitted CD (RCD) by HiSeq 2500 sequencing. We also found that F. nucleatum was enriched in ACD tissues (P < 0.05; Figures 1A, B ). We further examined the abundance of invasive F. nucleatum in 33 CD tissues (RCD = 11; ACD = 22) from patients and in 10 normal tissues using FISH. F. nucleatum was detected in a significantly higher percentage of CD tissues (41.21%) than control normal tissues (10%; P < 0.05; Figure 1C ). Moreover, F. nucleatum was detected in a significantly higher percentage of ACD tissues (46.71%) than RCD tissues (13.52%; P < 0.05; Figure 1C ). This result suggests that invasive F. nucleatum is present in CD tissues. We then evaluated the relationship between the abundance of F. nucleatum and clinicopathological features as shown in Table 1 . The abundance of F. nucleatum was positively associated with the clinical course, clinical activity, and refractory behavior (P < 0.05). Thus, these data defined the potential value of the abundance of F. nucleatum in predicting CD activity.
Figure 1.
Fusobacterium nucleatum is associated with Crohn’s disease (CD) activity. (A) Hierarchically clustered heat map representing bacterial taxa (genus level) in six CD tissues from patients [active CD (ACD) = 3, remitted CD (RCD) = 3] by 16S rDNA sequencing. The relative percentages of bacteria are indicated by varying color intensities. Species with an abundance of less than 0.5% in all samples were classified as “Others.” ACD: CD2, CD5, CD6; RCD: CD1, CD3, CD4. (B) The LEfSe algorithm was used to identify Fusobacterium in ACD and RCD tissues from patients. (C) Representative fluorescence in situ hybridization (FISH) images to assess the F. nucleatum abundance in ACD (n = 22), RCD (n = 11), and healthy tissues (n = 10). EUB338 (red) is a Cy3-conjugated universal bacterial oligonucleotide probe; FUS664 (green) is a fluorescein isothiocyanate (FITC)-conjugated F. nucleatum-specific oligonucleotide probe. Magnification, 200×. The sequence of the FITC-labeled Fn-targeted probe, FUS664, was: 5′- CTT GTA GTT CCG C(C/T) TAC CTC -3′.
Table 1.
Clinicopathologic characteristics in Fusobacterium. nucleatum-negative vs. F. nucleatum-positive CD.
| Characteristics | F. nucleatum-negative (n = 15) | F. nucleatum-positive (n = 18) | P valuea | |
|---|---|---|---|---|
| Gender | ||||
| Male | 6 | 12 | 0.126 | |
| Female | 9 | 6 | ||
| Age | ||||
| ≤16 | 0 | 1 | 0.505 | |
| ≤40 | 11 | 11 | ||
| >40 | 4 | 7 | ||
| Clinical course | ||||
| Moderate | 7 | 13 | 0.035* | |
| Severe | 0 | 2 | ||
| Remission | 8 | 3 | ||
| Location | ||||
| L1 | 5 | 7 | 0.731 | |
| L2 | 4 | 3 | ||
| L3 | 6 | 7 | ||
| L4 | 0 | 1 | ||
| Behavior | ||||
| B1 | 7 | 5 | 0.495 | |
| B2 | 5 | 7 | ||
| B3 | 3 | 6 | ||
| Perianal disease | ||||
| Yes | 0 | 4 | 0.405 | |
| No | 0 | 14 | ||
| Surgery | ||||
| Yes | 3 | 11 | 0.017* | |
| No | 12 | 7 | ||
Chi-square test, *P < 0.05.
CD, Crohn's disease.
F. nucleatum Destroys Epithelial Barrier Function In Vitro and In Vivo
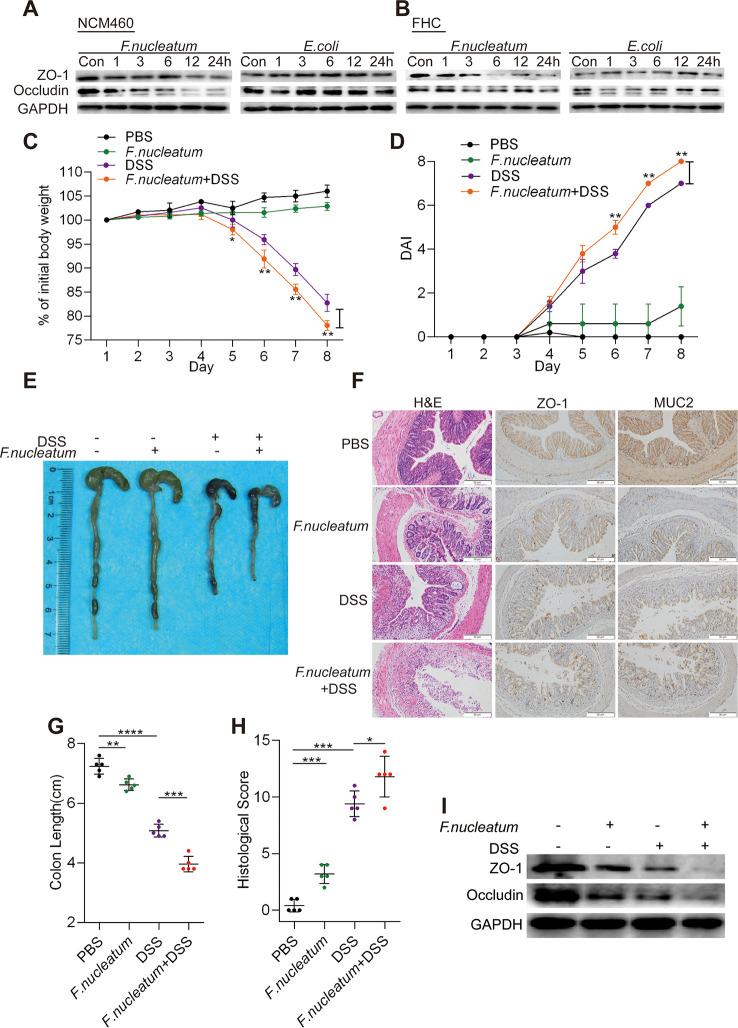
We hypothesized that F. nucleatum infection may enhance the breakdown of intestinal epithelial barrier function in CD. To test this hypothesis, we incubated NCM460 cells ( Figure 2A , Supplementary Figures S1 A–D ) and FHC cells ( Figure 2B , Supplementary Figures S1 E–H ) with F. nucleatum (ATCC10953), DH5α, or PBS (Control, Con). Compared to DH5α and PBS treatment, F. nucleatum treatment downregulated the levels of ZO-1 and occludin in a time-dependent manner, suggesting that F. nucleatum may destroy epithelial barrier formation by interfering with tight junction protein expression at the protein level.
Figure 2.
Fusobacterium nucleatum destroys epithelial barrier function in vitro and in vivo. (A, B) Western blotting was performed to measure the expression of ZO-1 and occludin in NCM460 cells (A) and FHC cells (B) cocultured with F. nucleatum, Escherichia coli, or phosphate-buffered solution (PBS) (Control, Con). (C, D) Mice (n = 5 per group) were administered F. nucleatum or PBS for 2 weeks and treated with 3% dextran sulfate sodium (DSS) for 7 days. Colitis induction was evaluated by body weight loss (C) and the disease activity index (DAI) (D). (*p < 0.05, **p < 0.01, and ***p < 0.001; one-way ANOVA combined with Bonferroni’s post hoc test; the error bars indicate the SDs). (E–G) Representative colon morphology and length in the mice are shown in panel (E) and quantified in panel (G). The sections used for HE, MUC2, and ZO1 staining were from the same mouse in the same group and three sections of the same tissue were stained separately. Representative images of histological analyses are shown in panel (F) and quantified in panel (H). Representative images of MUC2 and ZO-1 expression are shown in panel (F) (*p < 0.05, **p < 0.01, and ***p < 0.001; unpaired Student’s t test; the error bars indicate the SDs; 200× magnification). (I) Western blotting was performed to measure ZO-1 and occludin expression in mouse tissues.
To investigate the roles of F. nucleatum in the development of colitis, we used a DSS-induced colitis model. Mice treated with F. nucleatum + DSS exhibited more severe colitis symptoms, including rapid weight loss ( Figure 2C ) and higher disease activity index (DAI) values ( Figure 2D ), than mice treated with DSS or F. nucleatum alone. The colon length was measured to determine the extent of colonic injury, and we found that the colons of F. nucleatum + DSS group mice were clearly shorter than those of DSS group mice ( Figures 2E, G ). Additionally, F. nucleatum enhanced epithelial damage, including mucosal erosion, crypt loss, and lymphocyte infiltration ( Figure 2F ). Consistent with these observations, histological assessment of the colons revealed a significantly higher histological score (HS) ( Figure 2H ) and more severe disease and disruption of mucosal structures in F. nucleatum + DSS-treated mice than in DSS-treated mice. F. nucleatum-treated mice exhibited a mild inflammatory phenotype ( Figures 2C–H ), suggesting that F. nucleatum may exacerbate the clinical and histological features of DSS-induced colitis. The high abundance of F. nucleatum was often accompanied by low levels of ZO-1 and MUC2 compared with those in normal tissues (PBS group) (P < 0.05; Figure 2F ). In addition, the WB results showed lower levels of ZO-1 and occludin in colitis tissues from F. nucleatum + DSS-treated mice than in tissues from mice in the other groups ( Figure 2I , Supplementary Figures S1I, J ). Taken together, these results indicate that F. nucleatum possibly disrupt the mucosal barrier in vivo and in vitro.
F. nucleatum Damages the Mucosal Barrier via ER Signaling in Intestinal Epithelial Cells
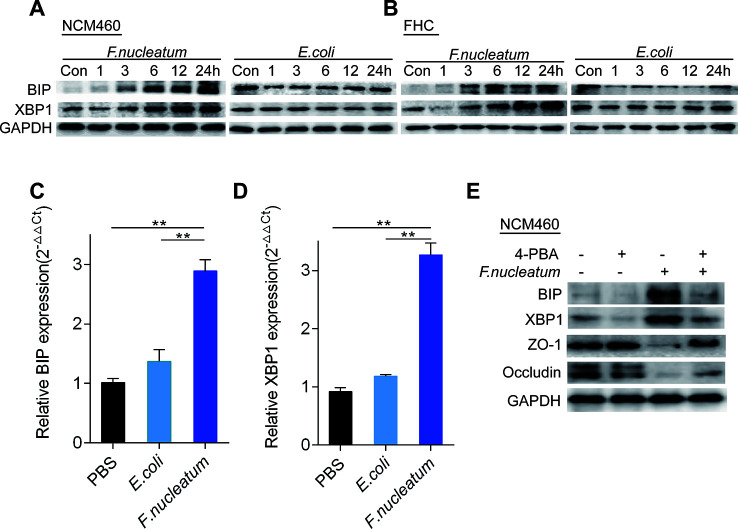
To examine whether the ER signaling pathway could be activated by F. nucleatum infection, we measured the expression levels of BIP and XBP1 using WB. Compared to DH5α and PBS (Control, Con), F. nucleatum treatment upregulated BIP and XBP1 expression in NCM460 cells ( Figure 3A , Supplementary Figures S1 K–N ) and FHC cells ( Figure 3 , Supplementary Figures S2 A–D ) in a time-dependent manner. Additionally, compared to DH5α and PBS treatment, F. nucleatum increased the mRNA levels of BIP ( Figure 3C ) and XBP1 ( Figure 3D ) in NCM460 cells (P < 0.05), suggesting that F. nucleatum may activate the ER pathway in intestinal epithelial cells.
Figure 3.
Fusobacterium nucleatum activates the endoplasmic reticulum (ER) pathway and damages mucosal barrier-associated proteins via ER signaling in intestinal epithelial cell lines. (A, B) Western blotting was performed to measure BIP and XBP1 expression in NCM460 cells (A) and FHC cells (B) cocultured with F. nucleatum, Escherichia coli, or phosphate-buffered solution (PBS) (Control, Con). (C, D) The mRNA expression of BIP (C) and XBP1 (D) was measured in NCM460 cells cocultured with F. nucleatum, E. coli, or PBS (*P < 0.05, **P < 0.01; unpaired Student’s t test; the error bars indicate the SDs). (E) Western blotting was performed to measure BIP, XBP1, ZO-1, and occludin expression in NCM460 cells cocultured with F. nucleatum, 4-phenyl butyric acid (4-PBA), or both.
We hypothesized that F. nucleatum destroys the intestinal mucosal barrier through the ER pathway. To test this hypothesis, we treated NCM460 cells with 4-PBA (10 μg/ml) or PBS 1 h before F. nucleatum (MOI = 100) treatment. Many investigations indicate that 4-PBA acts as a chemical chaperone that attenuates ERS in different cell types (Kolb et al., 2015). Our WB analysis showed that pretreatment with 4-PBA downregulated BIP and XBP1 expression, and blockade of the ER pathway by 4-PBA significantly attenuated the F. nucleatum-mediated decrease in ZO-1 and occludin (P < 0.05; Figure 3E , Supplementary Figures S2 E–H ). These data indicate that F. nucleatum potentially damages the intestinal mucosal barrier via ER signaling in epithelial cells.
F. nucleatum Facilitates Intestinal Mucosal Barrier Destruction Through the ER Pathway in DSS-Induced Mice
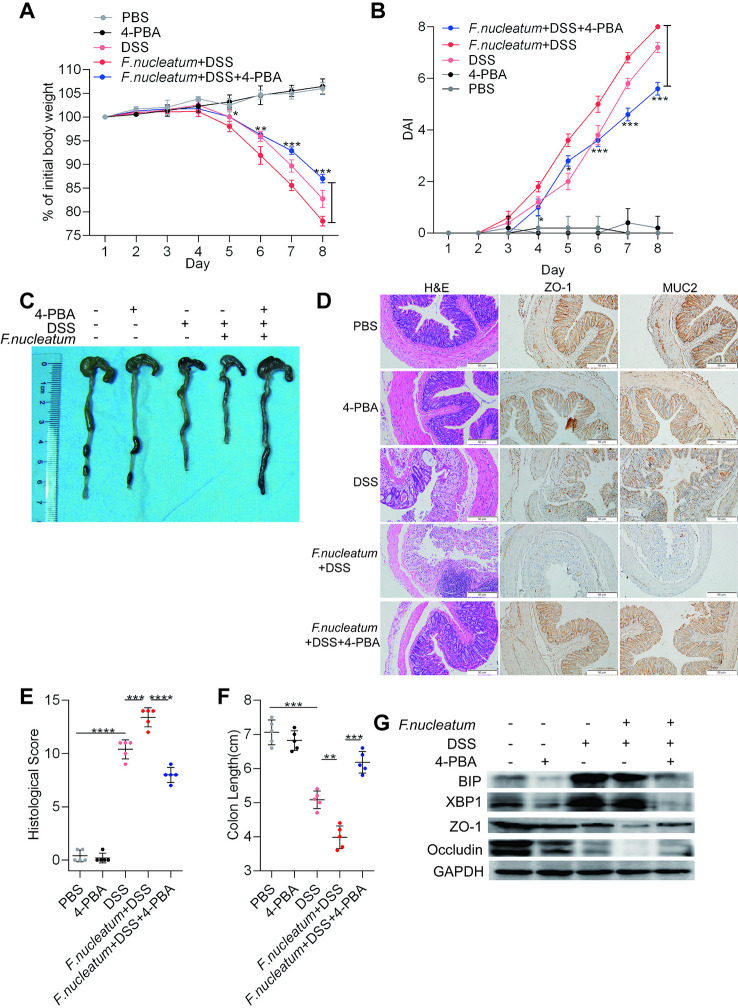
To assess whether the ER pathway contributes to F. nucleatum-induced colitis, 4-PBA was administered to mice with DSS-induced colitis. Mice treated with F. nucleatum + DSS + 4-PBA exhibited a slower decline in body weight (P < 0.05; Figure 4A ), a lower DAI (P < 0.01; Figure 4B ), a significantly lower HS (P < 0.01; Figure 4E ), and milder colitis ( Figure 4D ) than mice treated with F. nucleatum + DSS. DSS-induced or F. nucleatum + DSS-induced colon shortening was mitigated in mice administered 4-PBA (P < 0.01; Figures 4C, F ). Moreover, WB showed that inflamed tissues from F. nucleatum + DSS + 4-PBA-treated mice exhibited lower levels of BIP and XBP1 and higher levels of ZO-1 and occludin than tissues from F. nucleatum + DSS-treated mice ( Figure 4G , Supplementary Figures S2 I–L ). Collectively, these data suggest that F. nucleatum possibly destroy the intestinal mucosal barrier at least in part via the ER pathway in mice.
Figure 4.
Fusobacterium nucleatum facilitates intestinal mucosal barrier destruction through the endoplasmic reticulum (ER) pathway in dextran sulfate sodium (DSS)-induced mice. (A–F) Mice (n = 5 per group) were given 4-phenyl butyric acid (4-PBA) (100 μg per mouse every 3 days) and treated with F. nucleatum or phosphate-buffered solution (PBS) for 2 weeks. Then, these mice were administered DSS along with continued 4-PBA treatment for an additional 7 days. Colitis induction was evaluated by body weight loss (A) and the disease activity index (DAI) (B). (*P < 0.05, **P < 0.01, and ***P < 0.001; one-way ANOVA combined with Bonferroni’s post hoc test; the error bars indicate the SDs). Representative colon morphology and length in the mice are shown in panel (C) and quantified in panel (F). Representative images of histological analyses are shown in panel (D) and quantified in panel (E) (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; unpaired Student’s t test; the error bars indicate the SDs; 200× magnification). (G) Western blotting was performed to measure the levels of BIP, XBP1, ZO-1, and occludin in colon tissues from mice.
F. nucleatum Upregulates CARD3 Expression
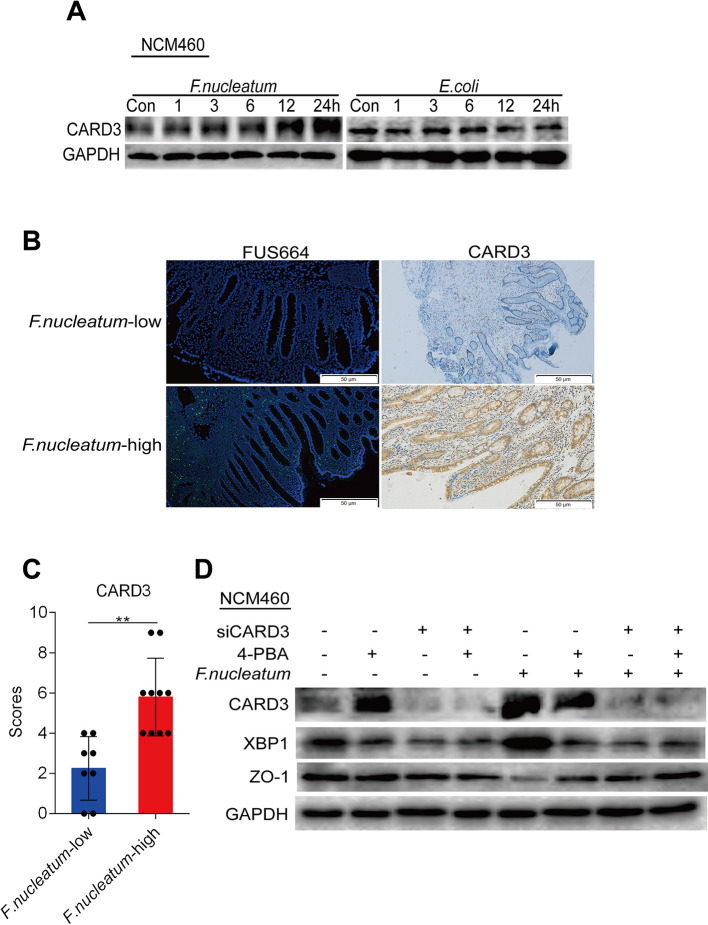
CARD3 is known for its role in inflammation (Irving et al., 2014). Previous studies have found that genetic loss of CARD3 is protective against colitis through decreased epithelial cell apoptosis and consequent enhancement of intestinal epithelial barrier function (Yu et al., 2015). We hypothesized that CARD3 may be a target of F. nucleatum in disease development. To verify this hypothesis, we treated NCM460 cells with F. nucleatum (ATCC10953), DH5α, or PBS (Control, Con). Compared to DH5α and PBS treatment, F. nucleatum treatment increased the levels of CARD3 in a time-dependent manner ( Figure 5A ), suggesting that F. nucleatum may regulate CARD3 expression at the protein level. We next used FISH to visualize the amount of F. nucleatum, as well as the expression of CARD3, in inflamed tissues from CD patients. A high abundance of F. nucleatum in inflamed colon tissues was often accompanied by a high level of CARD3 expression (P < 0.05; Figures 5B, C ). These data support the hypothesis that CARD3 is probably a downstream target of F. nucleatum.
Figure 5.
Fusobacterium nucleatum is associated with caspase activation and recruitment domain 3 (CARD3) expression and activates the endoplasmic reticulum (ER) pathway via CARD3 in NCM460 cells. (A) Western blotting was performed to measure CARD3 protein expression over time in NCM460 cells cocultured with F. nucleatum or Escherichia coli over time. (B, C) Representative images showing that the abundance of invasive F. nucleatum in CD tissues is associated with high expression of CARD3 (**P < 0.01; unpaired Student’s t test; the error bars indicate the SDs; 200× magnification). (D) Western blotting was performed to measure the protein levels of CARD3, XBP1, and ZO-1 in NCM460 cells transfected with nontargeting siRNAs (NC), 4-phenyl butyric acid (4-PBA), or siCARD3 and infected with F. nucleatum.
F. nucleatum Modulates the ER Pathway Via Card3 Upregulation In Vitro and In Vivo
The increase in CARD3 expression in F. nucleatum-infected NCM460 cells led us to hypothesize that CARD3 may regulate F. nucleatum-mediated ER pathway activation. To test this hypothesis, we transfected NCM460 cells with CARD3-targeting siRNA (siCARD3) or nontargeting siRNAs (NC) ( Supplementary Figures S3 A, B ). Knockdown of CARD3 suppressed the F. nucleatum-induced increase in the protein levels of XBP1 and the F. nucleatum-induced decrease in the expression of ZO-1 expression (P < 0.05; Figure 5D , Supplementary Figures S3 C, D ), highlighting the role of CARD3 in F. nucleatum-mediated ER pathway activation in NCM460 cells.
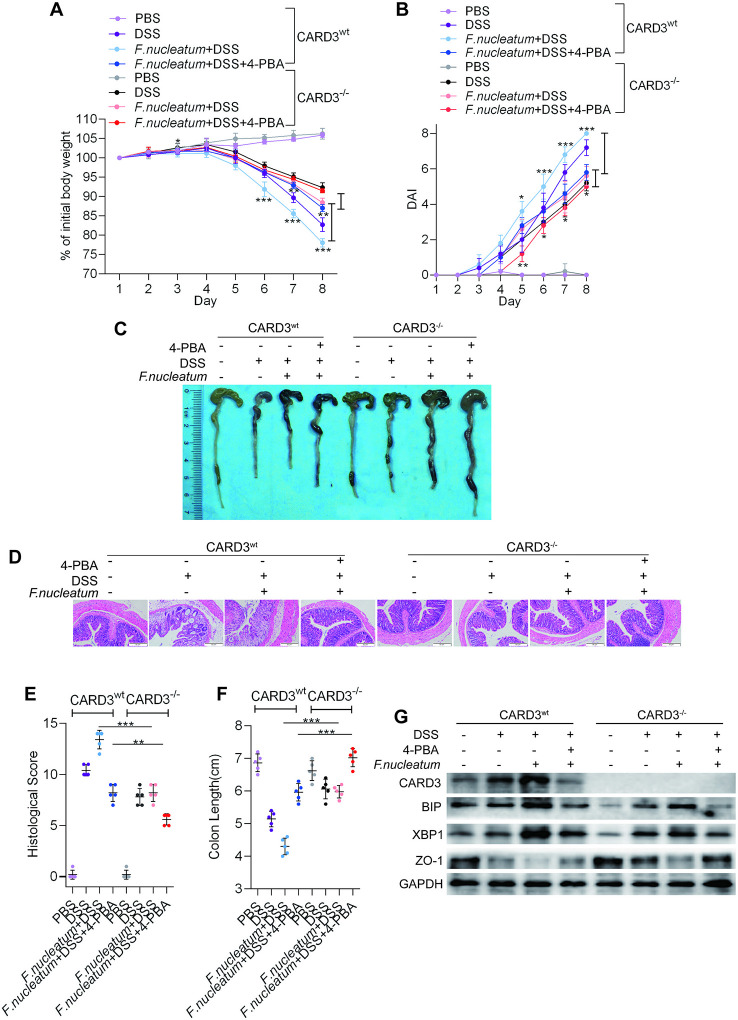
To further confirm our hypothesis that F. nucleatum promotes colitis progression through ER activation via the upregulation of CARD3 expression, we employed CARD3 knockout (KO, CARD3–/–) mice. Both CARD3 WT (CARD3wt) and CARD3–/– mice were initially administered F. nucleatum and were then subjected to DSS treatment. CARD3–/– mice exhibited slower body weight loss (P < 0.01; Figure 6A ), a lower DAI (P < 0.05; Figure 6B ), a significantly lower HS (P < 0.05; Figure 6C, F ), a shorter colon length (P < 0.05; Figures 6C–F ), and milder colitis ( Figure 6D ) than CARD3wt mice. After knockout of CARD3, treatment with 4-PBA or both, DSS-induced cecal edema, colon shortening and colitis were significantly suppressed (P < 0.05; Figures 6C–F ). Within lesions, the levels of BIP and XBP1 were decreased, and the expression of ZO-1 was increased in CARD3–/– mice ( Figure 6G , Supplementary Figures S3 F–I ). Taken together, our data show that CARD3–/– mice are less susceptible to gut inflammation and F. nucleatum infection than CARD3wt mice, suggesting a role for CARD3 in the regulation of F. nucleatum-induced intestinal mucosal barrier destruction.
Figure 6.
Fusobacterium nucleatum activates the endoplasmic reticulum (ER) pathway through the upregulation of caspase activation and recruitment domain 3 (CARD3) in dextran sulfate sodium (DSS)-induced mice. (A–F) CARD3wt mice and CARD3–/– mice (n = 5 per group) were administered intraperitoneal injections of 4-phenyl butyric acid (4-PBA) and treated with F. nucleatum or phosphate-buffered solution (PBS) for 2 weeks. Then, these mice were administered DSS along with continued 4-PBA treatment for an additional 7 days. Colitis induction was evaluated by body weight loss (A) and the disease activity index (DAI) (B). (*P < 0.05, **P < 0.01, and ***P < 0.001; one-way ANOVA combined with Bonferroni’s post hoc test; the error bars indicate the SDs). Representative colon morphology and length in the mice are shown in panel (C) and quantified in panel (F). Representative images of histological analyses are shown in panel (D) and quantified in panel (E) (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; unpaired Student’s t test; the error bars indicate the SDs; 200× magnification). (G) Western blotting was performed to measure the levels of CARD3, BIP, XBP1, and ZO-1 in colon tissues from mice.
Discussion
Recent studies have shown that F. nucleatum in the gut microbiota is involved in the development of colitis. Infection with F. nucleatum is often observed in patients with CD (Allen-Vercoe et al., 2011; Kaakoush et al., 2012; Purcell et al., 2018). Similarly, our high-throughput sequencing has demonstrated an increased abundance of F. nucleatum in colon tissues from CD patients. F. nucleatum infiltrated the epithelium and mucosa and is associated with the clinical activity and severity of colitis (Swidsinski et al., 2011; Strauss et al., 2011). We confirmed that F. nucleatum invades CD tissue and is associated with the degree of clinical activity and refractory behavior of CD. In addition, in DSS-induced colitis models, we used oral administration of F. nucleatum before the induction of acute colitis with DSS to initially assess the precise role of F. nucleatum in IBD. Through a combination of biological methods, in vivo models, and clinical studies, we demonstrated that F. nucleatum was enriched in CD tissues from patients and exacerbated colonic inflammation during CD development.
It has been confirmed that F. nucleatum contributes to the carcinogenesis of colorectal cancer by inducing inflammation and inhibiting host immunity (Feng et al., 2019; Wu et al., 2019). There is evidence that F. nucleatum induces the production of microbial peptides that upregulate the expression of proinflammatory cytokines and tumor necrosis factor and disrupt homeostasis and host defense barriers (Krisanaprakornkit et al., 2000; Repass et al., 2018). An F. nucleatum strain isolated from IBD patients showed upregulation of monocyte chemoattractant protein (MCP)-1 and tumor necrosis factor (TNF)-α in an experimental model (McCoy et al., 2013). However, it is unclear how F. nucleatum mediates intestinal inflammation during the development of CD. We examined whether F. nucleatum induced the downregulation of membrane-associated proteins (ZO-1 and occludin), which are markers of intestinal mucosal barrier function. We found that F. nucleatum infection was negatively correlated with the expression of intestinal mucosal barrier-associated proteins (ZO-1 and occludin) in human epithelial cell lines and human colon tissues and significantly downregulated the expression of intestinal mucosal barrier proteins in a mouse model of DSS-induced colitis. F. nucleatum infection can disrupt intestinal mucosal function and has the potential contribution to induce associated colitis.
Next, we investigated the mechanism by which F. nucleatum causes intestinal mucosal barrier damage. Studies have shown that when ERS occurs, cells initiate a caspase12-dependent apoptosis program, which triggers the destruction of the intestinal mucosal barrier (Ge et al., 2019). We found that F. nucleatum infection significantly increased the expression of ERS-related proteins (BIP and XBP1), which are key regulators of ERS, in vivo and in vitro but that by inhibiting ERS with 4-PBA, the intestinal mucosal barrier function and the severity of colitis were significantly mitigated. Therefore, we concluded that F. nucleatum may induce intestinal mucosal damage partly by activating the ERS pathway to mediate CD, and colitis may be relieved under the treatment of 4-PBA. The majority of investigations suggest that 4-PBA acts as a chemical chaperone that attenuates ERS in different cell types (Kolb et al., 2015). But whether 4-PBA can act via different routes not just as an ERS inhibitor remains to be verified.
In addition, we investigated the mechanism by which F. nucleatum mediates the activation of the ERS pathway. Studies have reported that microbes can induce a CARD-dependent inflammatory response in epithelial cells (McCoy et al., 2013; Wang et al., 2013; Li et al., 2015). Previous studies have found that genetic loss of CARD3 is protective against colitis through decreased epithelial cell apoptosis and consequent enhancement of intestinal epithelial barrier function (Tigno-Aranjuez et al., 2014; Yu et al., 2015). Our previous report has found that the expression of NOD2 was upregulated in intestinal epithelial cells infected with F. nucleatum (Chen et al., 2019). Thus, we choose CARD3 as the downstream molecule of F. nucleatum. In this study, we found that CARD3 expression in CD patients was higher than that in healthy controls and that the abundance of F. nucleatum was positively correlated with the expression of CARD3. Some studies have shown that CARD3 is a nucleotide oligomerization domain (NOD)-independent nodal point of gut inflammation (Panda and Gekara, 2018). Decreased expression of NOD1/2 and interaction between NOD1/2 and CARD3 can decrease the severity of ERS (Zhang et al., 2016). It is clear that NOD1/2 and CARD3 are important mediators of ERS-induced inflammation in mouse and human cells (Keestra-Gounder et al., 2016). In the present study, we demonstrated that knockdown or knockout of the CARD3 gene can alleviate the extent of F. nucleatum-associated colitis and mitigate ERS. We demonstrated for the first time that F. nucleatum activates ERS and promotes CD development by upregulating CARD3 expression. However, we did not explore the mechanism by which F. nucleatum regulates CARD3, and whether other ERS or CARD3 inhibitors can rescue the CD phenotype induced by F. nucleatum + DSS remains to be verified.
From a clinical perspective, since the abundance of F. nucleatum is related to the risk of CD activity, measuring the F. nucleatum abundance may be an effective method for predicting disease activity in patients. In addition, our data raise an important clinical question: Should CD patients with a high abundance of F. nucleatum be treated with conventional treatments against F. nucleatum and/or with ERS or CARD3 inhibitors? Our findings support this view. Therefore, it is important to determine the abundance and related pathways of F. nucleatum and to differentially manage patients with different abundances of F. nucleatum.
Overall, our results demonstrate that F. nucleatum promotes the development of CD by regulating the molecular mechanisms involving CARD3. Moreover, the clinical information that we collected from CD patients also indicated that F. nucleatum and CARD3 are risk factors for a high degree of disease activity in CD patients. Our research provides new evidence demonstrating the pro-inflammatory effects of F. nucleatum in CD and offers new approaches to the assessment of microbial populations and genetic alterations for the treatment and prevention of CD.
Data Availability Statement
The datasets generated for this study can be found in the NCBI Sequence Read Archive (SRA) using the accession number PRJNA541040.
Ethics Statement
All participants provided written informed consent, and the project was approved by the Institutional Review Board (Approval number: 2018K-C089). The animal study was reviewed and approved by the Institutional Review Board of Renmin Hospital of Wuhan University, China.
Author Contributions
Study conception and design: WD, PC, YYC; Specimen provision: NZ, XG; Acquisition of clinical data: PC, YYC, YC, XG; Data analysis and interpretation and statistical analysis: PC, YYC, YC, XG; Animal experiments: PC, YYC, YC, WHS, XG; Manuscript drafting: PC, YYC, WGD.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81870392, No. 81372551, and No. 81572426) and the Guiding Foundation of Renmin Hospital of Wuhan University (No. RMYD2018Z01).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.00106/full#supplementary-material
F. nucleatum destroys epithelial barrier function in vitro and in vivo (A–H) The protein expression of ZO-1 and Occludin in NCM460 cells (A–D) and FHC cells (E–H) cocultured with F. nucleatum, E. coli or PBS (Control, Con) were quantified. (I, J) The protein expression of ZO-1 and Occludin expression in mouse tissues were quantified. (K–N) The protein expression of BIP and XBP1 in NCM460 cells cocultured with F. nucleatum, E. coli or PBS (Control, Con) were quantified. Data are expressed as mean ± SD for three independent experiments. Statistical significance is indicated as follows: *P < 0.05, **P < 0.01, and ***P < 0.001.
F. nucleatum activates the ER pathway and damages mucosal barrier-associated proteins via ER signaling in vitro and in vivo (A–D) The protein expression of BIP and XBP1 in FHC cells cocultured with F. nucleatum, E. coli or PBS (Control, Con) were quantified. (E–H) The protein expression of BIP, XBP1, ZO-1 and Occludin in NCM460 cells cocultured with F. nucleatum, 4-PBA or both were quantified. (I–L) The protein expression of BIP, XBP1, ZO-1 and Occludin in colon tissues from mice were quantified. Data are expressed as mean ± SD for three independent experiments. Statistical significance is indicated as follows: *P < 0.05, **P < 0.01, and ***P < 0.001.
F. nucleatum activates the ER pathway through the upregulation of CARD3 in vitro and in vivo (A–B) The protein expression of CARD3 in NCM460 cells cocultured with PBS (Control, Con), NC, si001, si002 or si003 were detected by immunoblot and quantified. (C–E) The protein expression of CARD3, XBP1 and ZO-1 in NCM460 cells transfected with NC, 4-PBA or siCARD3 and infected with F. nucleatum were quantified. (F–I) The protein expression of CARD3, BIP, XBP1 and ZO-1 in colon tissues from mice were quantified. Data are expressed as mean ± SD for three independent experiments. Statistical significance is indicated as follows: *P < 0.05, **P < 0.01, and ***P < 0.001.
References
- Actis G. C., Pellicano R., Rosina F. (2014). Inflammatory bowel diseases: current problems and future tasks. World J. Gastrointest Pharmacol. Ther. 5 (3), 169–174. 10.4292/wjgpt.v5.i3.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen-Vercoe E., Jobin C. (2014). Fusobacterium and Enterobacteriaceae: important players for CRC? Immunol. Lett. 162 (2 Pt A), 54–61. 10.1016/j.imlet.2014.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen-Vercoe E., Strauss J., Chadee K. (2011). Fusobacterium nucleatum: an emerging gut pathogen? Gut Microbes 2 (5), 294–298. 10.4161/gmic.2.5.18603 [DOI] [PubMed] [Google Scholar]
- Chen W. Y., Wang M., Zhang J., Barve S. S., McClain C. J., Joshi-Barve S. (2017). Acrolein disrupts tight junction proteins and causes endoplasmic reticulum stress-mediated epithelial cell death leading to intestinal barrier dysfunction and permeability. Am. J. Pathol. 187 (12), 2686–2697. 10.1016/j.ajpath.2017.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Chen Y., Cao P., Su W., Zhan N., Dong W. (2019). Fusobacterium nucleatum facilitates ulcerative colitis through activating IL-17F signaling to NF-kappaB via the upregulation of CARD3 expression. J. Pathol. 250 (2), 170–182. 10.1002/path.5358 [DOI] [PubMed] [Google Scholar]
- Cheung W. Y., Bellas J. (2007). Fusobacterium: elusive cause of life-threatening septic thromboembolism. Can. Fam. Physician 53 (9), 1451–1453. [PMC free article] [PubMed] [Google Scholar]
- Danese S., Sans M., Spencer D. M., Beck I., Doñate F., Plunkett M. L., et al. (2007). Angiogenesis blockade as a new therapeutic approach to experimental colitis. Gut 56 (6), 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinakaran V., Mandape S. N., Shuba K., Pratap S., Sakhare S. S., Tabatabai M. A., et al. (2018). Identification of specific oral and gut pathogens in full thickness colon of colitis patients: implications for colon motility. Front. Microbiol 9, 3220. 10.3389/fmicb.2018.03220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y. Y., Zeng D. Z., Tong Y. N., Lu X. X., Dun G. D., Tang B., et al. (2019). Alteration of microRNA-4474/4717 expression and CREB-binding protein in human colorectal cancer tissues infected with Fusobacterium nucleatum. PloS One 14 (4), e0215088. 10.1371/journal.pone.0215088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge C. X., Xu M. X., Qin Y. T., Gu T. T., Lou D. S., Li Q., et al. (2019). Endoplasmic reticulum stress-induced iRhom2 up-regulation promotes macrophage-regulated cardiac inflammation and lipid deposition in high fat diet (HFD)-challenged mice: Intervention of fisetin and metformin. Free Radic. Biol. Med. 141, 67–83. 10.1016/j.freeradbiomed.2019.05.031 [DOI] [PubMed] [Google Scholar]
- Gursoy U. K., Pöllänen M., Könönen E., Uitto V. J. (2010). Biofilm formation enhances the oxygen tolerance and invasiveness of Fusobacterium nucleatum in an oral mucosa culture model. J. Periodontol 81 (7), 1084–1091. 10.1902/jop.2010.090664 [DOI] [PubMed] [Google Scholar]
- Han Y. W., Fardini Y., Chen C., Iacampo K. G., Peraino V. A., Shamonki J. M., et al. (2010). Term stillbirth caused by oral Fusobacterium nucleatum. Obstet Gynecol 115 (2 Pt 2), 442–445. 10.1097/AOG.0b013e3181cb9955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Wang H., Liao W. D., Peng C., Shu X., Zhu X., et al. (2019). Characteristics of mucosa-associated gut microbiota during treatment in Crohn’s disease. World J. Gastroenterol. 25 (18), 2204–2216. 10.3748/wjg.v25.i18.2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Wang Y., Feng Y., Wang P., He X., Ren H., et al. (2019). Role of Endoplasmic Reticulum Stress-Autophagy Axis in Severe Burn-Induced Intestinal Tight Junction Barrier Dysfunction in Mice. Front. Physiol. 10, 606. 10.3389/fphys.2019.00606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving A. T., Mimuro H., Kufer T. A., Lo C., Wheeler R., Turner L. J., et al. (2014). The immune receptor NOD1 and kinase RIP2 interact with bacterial peptidoglycan on early endosomes to promote autophagy and inflammatory signaling. Cell Host Microbe 15 (5), 623–635. 10.1016/j.chom.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Kaakoush N. O., Day A. S., Huinao K. D., Leach S. T., Lemberg D. A., Dowd S. E., et al. (2012). Microbial dysbiosis in pediatric patients with Crohn’s disease. J. Clin. Microbiol 50 (10), 3258–3266. 10.1128/JCM.01396-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keestra-Gounder A. M., Byndloss M. X., Seyffert N., Young B. M., Chávez-Arroyo A., Tsai A. Y., et al. (2016). NOD1 and NOD2 signalling links ER stress with inflammation. Nature 532 (7599), 394–397. 10.1038/nature17631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb P. S., Ayaub E. A., Zhou W., Yum V., Dickhout J. G., Ask K. (2015). The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int. J. Biochem. Cell Biol. 61, 45–52. 10.1016/j.biocel.2015.01.015 [DOI] [PubMed] [Google Scholar]
- Kostic A. D., Chun E., Robertson L., Glickman J. N., Gallini C. A., Michaud M., et al. (2013). Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14 (2), 207–215. 10.1016/j.chom.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisanaprakornkit S., Kimball J. R., Weinberg A., Darveau R. P., Bainbridge B. W., Dale B. A.. (2000). Inducible expression of human beta-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect. Immun. 68 (5), 2907–2915. 10.1128/IAI.68.5.2907-2915.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Thotakura P. L., Tiwary B. K., Krishna R. (2016). Target identification in Fusobacterium nucleatum by subtractive genomics approach and enrichment analysis of host-pathogen protein-protein interactions. BMC Microbiol 16, 84. 10.1186/s12866-016-0700-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Wang X., Chen W., Qi H., Jiang D. S., Huang L., et al. (2015). Regulatory role of CARD3 in left ventricular remodelling and dysfunction after myocardial infarction. Basic Res. Cardiol. 110 (6), 56. 10.1007/s00395-015-0515-4 [DOI] [PubMed] [Google Scholar]
- Li B., Zani A., Lee C., Zani-Ruttenstock E., Zhang Z., Li X., et al. (2016). Endoplasmic reticulum stress is involved in the colonic epithelium damage induced by maternal separation. J. Pediatr. Surg. 51 (6), 1001–1004. 10.1016/j.jpedsurg.2016.02.073 [DOI] [PubMed] [Google Scholar]
- Li P., Fu D., Sheng Q., Yu S., Bao X., Lv Z. (2019). TUDCA attenuates intestinal injury and inhibits endoplasmic reticulum stress-mediated intestinal cell apoptosis in necrotizing enterocolitis. Int. Immunopharmacol 74, 105665. 10.1016/j.intimp.2019.05.050 [DOI] [PubMed] [Google Scholar]
- Ma A. G., Yu L. M., Zhao H., Qin C. W., Tian X. Y., Wang Q. (2019). PSMD4 regulates the malignancy of esophageal cancer cells by suppressing endoplasmic reticulum stress. Kaohsiung J. Med. Sci. 35 (10), 591–597. 10.1002/kjm2.12093 [DOI] [PubMed] [Google Scholar]
- Malluta É. F., Maluf-Filho F., Leite A. Z. A., Ortiz-Agostinho C. L., Nishitokukado I., Andrade A. R., et al. (2019). Pancreatic endosonographic findings and clinical correlation in Crohn’s disease. Clinics (Sao Paulo) 74, e853. 10.6061/clinics/2019/e853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy A. N., Araújo-Pérez F., Azcárate-Peril A., Yeh J. J., Sandler R. S., Keku T. O.. (2013). Fusobacterium is associated with colorectal adenomas. PloS One 8 (1), e53653. 10.1371/journal.pone.0053653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa J., Kudo T., Sakata S., Benno Y., Sugiyama T. (2009). Diversity of mucosa-associated microbiota in active and inactive ulcerative colitis. Scand. J. Gastroenterol. 44 (2), 180–186. 10.1080/00365520802433231 [DOI] [PubMed] [Google Scholar]
- Odenwald M. A., Turner J. R. (2017). The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol 14 (1), 9–21. 10.1038/nrgastro.2016.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S., Gekara N. O. (2018). The deubiquitinase MYSM1 dampens NOD2-mediated inflammation and tissue damage by inactivating the RIP2 complex. Nat. Commun. 9 (1), 4654. 10.1038/s41467-018-07016-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell R. V., Kaakoush N. O., Mitchell H. M., Pearson J. F., Keenan J. I. (2018). Gastrointestinal Pathobionts in Pediatric Crohn’s Disease Patients. Int. J. Microbiol 2018, 9203908. 10.1155/2018/9203908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repass J., Iorns E., Denis A., Williams S. R., Perfito N., Errington T. M. (2018). Replication Study: Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Elife 7, e25801. 10.7554/eLife.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J., Kaplan G. G., Beck P. L., Rioux K., Panaccione R., Devinney R., et al. (2011). Invasive potential of gut mucosa-derived Fusobacterium nucleatum positively correlates with IBD status of the host. Inflammation Bowel Dis. 17 (9), 1971–1978. 10.1002/ibd.21606 [DOI] [PubMed] [Google Scholar]
- Swidsinski A., Dörffel Y., Loening-Baucke V., Theissig F., Rückert J. C., Ismail M., et al. (2011). Acute appendicitis is characterised by local invasion with Fusobacterium nucleatum/necrophorum. Gut 60 (1), 34–40. 10.1136/gut.2009.191320 [DOI] [PubMed] [Google Scholar]
- Tigno-Aranjuez J. T., Benderitter P., Rombouts F., Deroose F., Bai X., Mattioli B., et al. (2014). In vivo inhibition of RIPK2 kinase alleviates inflammatory disease. J. Biol. Chem. 289 (43), 29651–29664. 10.1074/jbc.M114.591388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. A., Deng S., Jiang D., Zhang R., Zhang S., Zhong J., et al. (2013). CARD3 deficiency exacerbates diet-induced obesity, hepatosteatosis, and insulin resistance in male mice. Endocrinology 154 (2), 685–697. 10.1210/en.2012-1911 [DOI] [PubMed] [Google Scholar]
- Wu Y., Liu X., Qin Z., Hu L., Wang X.. (2018). Low-frequency ultrasound enhances chemotherapy sensitivity and induces autophagy in PTX-resistant PC-3 cells via the endoplasmic reticulum stress-mediated PI3K/Akt/mTOR signaling pathway. Onco Targets Ther. 11, 5621–5630. 10.2147/OTT.S176744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Li Q., Fu X. (2019). Fusobacterium nucleatum Contributes to the Carcinogenesis of Colorectal Cancer by Inducing Inflammation and Suppressing Host Immunity. Transl. Oncol. 12 (6), 846–851. 10.1016/j.tranon.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Weng W., Peng J., Hong L., Yang L., Toiyama Y., et al. (2017). Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-kappaB, and Up-regulating Expression of MicroRNA-21. Gastroenterology 152 (4), 851–866.e24. 10.1053/j.gastro.2016.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S. J., Liu Y., Deng Y., Zhu X. Y., Zhan N., Dong W. G.. (2015). CARD3 deficiency protects against colitis through reduced epithelial cell apoptosis. Inflammation Bowel Dis. 21 (4), 862–869. 10.1097/MIB.0000000000000322 [DOI] [PubMed] [Google Scholar]
- Yu J., Chen Y., Fu X., Zhou X., Peng Y., Shi L., et al. (2016). Invasive Fusobacterium nucleatum may play a role in the carcinogenesis of proximal colon cancer through the serrated neoplasia pathway. Int. J. Cancer 139 (6), 1318–1326. 10.1002/ijc.30168 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Wang X., Zheng G., Shan Q., Lu J., Fan S., et al. (2016). Troxerutin Attenuates Enhancement of Hepatic Gluconeogenesis by Inhibiting NOD Activation-Mediated Inflammation in High-Fat Diet-Treated Mice. Int. J. Mol. Sci. 18 (1), E31. 10.3390/ijms18010031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Chen H., He H., Du Y., Hu J., Li Y., et al. (2016). Increased Enterococcus faecalis infection is associated with clinically active Crohn disease. Med. (Baltimore) 95 (39), e5019. 10.1097/MD.0000000000005019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
F. nucleatum destroys epithelial barrier function in vitro and in vivo (A–H) The protein expression of ZO-1 and Occludin in NCM460 cells (A–D) and FHC cells (E–H) cocultured with F. nucleatum, E. coli or PBS (Control, Con) were quantified. (I, J) The protein expression of ZO-1 and Occludin expression in mouse tissues were quantified. (K–N) The protein expression of BIP and XBP1 in NCM460 cells cocultured with F. nucleatum, E. coli or PBS (Control, Con) were quantified. Data are expressed as mean ± SD for three independent experiments. Statistical significance is indicated as follows: *P < 0.05, **P < 0.01, and ***P < 0.001.
F. nucleatum activates the ER pathway and damages mucosal barrier-associated proteins via ER signaling in vitro and in vivo (A–D) The protein expression of BIP and XBP1 in FHC cells cocultured with F. nucleatum, E. coli or PBS (Control, Con) were quantified. (E–H) The protein expression of BIP, XBP1, ZO-1 and Occludin in NCM460 cells cocultured with F. nucleatum, 4-PBA or both were quantified. (I–L) The protein expression of BIP, XBP1, ZO-1 and Occludin in colon tissues from mice were quantified. Data are expressed as mean ± SD for three independent experiments. Statistical significance is indicated as follows: *P < 0.05, **P < 0.01, and ***P < 0.001.
F. nucleatum activates the ER pathway through the upregulation of CARD3 in vitro and in vivo (A–B) The protein expression of CARD3 in NCM460 cells cocultured with PBS (Control, Con), NC, si001, si002 or si003 were detected by immunoblot and quantified. (C–E) The protein expression of CARD3, XBP1 and ZO-1 in NCM460 cells transfected with NC, 4-PBA or siCARD3 and infected with F. nucleatum were quantified. (F–I) The protein expression of CARD3, BIP, XBP1 and ZO-1 in colon tissues from mice were quantified. Data are expressed as mean ± SD for three independent experiments. Statistical significance is indicated as follows: *P < 0.05, **P < 0.01, and ***P < 0.001.
Data Availability Statement
The datasets generated for this study can be found in the NCBI Sequence Read Archive (SRA) using the accession number PRJNA541040.