Abstract
Background and study aims:
Capsule endoscopy is an attractive alternative to colorectal cancer screening by conventional colonoscopy, but is currently limited by compromised mucosal visibility because of the lack of safe, controlled colonic insufflation. We have therefore developed a novel system of untethered, wireless-controlled carbon dioxide (CO2) insufflation for use in colonic capsule endoscopy, which this study aims to assess in vivo.
Material and methods:
This observational, non-survival, in vivo study used five Yorkshire-Land-race cross swine. A novel insufflation capsule was placed in the porcine colons, and we recorded volume of insufflation, time, force, visualization, and a pathologic assessment of the colon.
Results:
The mean (standard deviation [SD]) diameter of insufflation was 32.1 (3.9)mm. The volume of CO2 produced successfully allowed complete endoscopic visualization of the mucosa and safe proximal passage of the endoscope. Pathologic examination demonstrated no evidence of trauma caused by the capsule.
Conclusions:
These results demonstrate the feasibility of a novel method of controlled colonic insufflation via an untethered capsule in vivo. This technological innovation addresses a critical need in colon capsule endoscopy.
Introduction
Colorectal cancer (CRC) is the third most common cancer in both men and women and the second leading cause of cancer death in the United States [1]. CRC is also one of the most preventable as it has a well-defined premalignant lesion that can be readily detected and removed using endoscopic techniques [2–5]. Colonoscopy remains the “gold standard” for CRC screening because of its safety and effectiveness. Yet its benefits have not been realized for a significant proportion of the population of the United States–in 2009 only a little over 60% had received appropriate screening, compared with more than 70% for breast cancer and 80% or more for cervical cancer [1]. Many factors may account for this, including the disruptive and time-consuming nature of colonoscopy (patients generally have to lose a day of work and must have a companion to help them return home), the potential for pain, and the small risk of adverse events [6]. Thus there is a clear need for improved and more acceptable methods for CRC screening.
In this regard, capsule endoscopy is a very attractive alternative to colonoscopy, and is now an essential tool for evaluation of patients with suspected small-bowel disorders [7,8]. As the technology of small-bowel wireless capsule endoscopy advances, there has been a natural progression towards its adaptation for examination of the colon [9]. Unfortunately, in its current embodiment, the sensitivity and specificity of capsule endoscopy for detection of colon lesions is disappointingly low (sensitivity 73%, specificity 89%) [10]. In conventional colonoscopy, visualization of the mucosa is improved by insufflation of the colon, with a positive correlation between the luminal distension achieved by insufflation and the polyp and adenoma detection rate [11]. One factor contributing to the suboptimal performance of colon capsule endoscopy is the incomplete visualization of the mucosa during the examination. Thus, there is a need for similarly safe, controlled, and reliable insufflation in colon capsule endoscopy.
Our team has been working on a highly innovative approach to address this critical technological gap.We have developed a novel device to achieve untethered controlled carbon dioxide (CO2) insufflation suitable for capsule endoscopy of the colon: the “CO2mfort Cap.” Carbon dioxide has been advocated as a substitute for traditional room air, having the advantages of less post-procedure gas volume because of its rapid absorption, and of better patient comfort because of an overall decrease in intestinal distension [12,13]. Our seminal reports of this technology provided benchtop and ex vivo demonstrations of the ability of the CO2mfort Cap to produce adequate carbon dioxide volumes for complete visualization of the colonic mucosa [14,15]. In this study, we examined the feasibility and safety of this device in vivo.
Materials and methods
The insufflation capsule
The CO2mfort Cap is a novel two-compartment capsule containing reactants that yield carbon dioxide when mixed. The basic chemical reaction uses US Food and Drug Administration (FDA)-approved products [16], sodium bicarbonate (NaHCO3) and citric acid (C6H8O7), to produce sodium citrate (Na3C6H5O7), carbon dioxide (CO2), and water (Fig.1). Fabricated from a polypropylene-like material (Objet DurusWhite RGD430; Billerica, Massachusetts, USA), the CO2mfort Cap is 12mm in diameter and 32 mm in length, comparable in dimensions to the 11mm×31mm Pillcam Colon (Given Imaging, Inc., Yoqneam, Israel). The insufflation capsule has two separate compartments connected by two magnetic valves (Fig.2). When activated, the citric acid solution in the upper compartment reacts with the sodium bicarbonate in the lower compartment, producing carbon dioxide that is released through the exhaust ports along the lower mid-line of the capsule.
Fig.1.
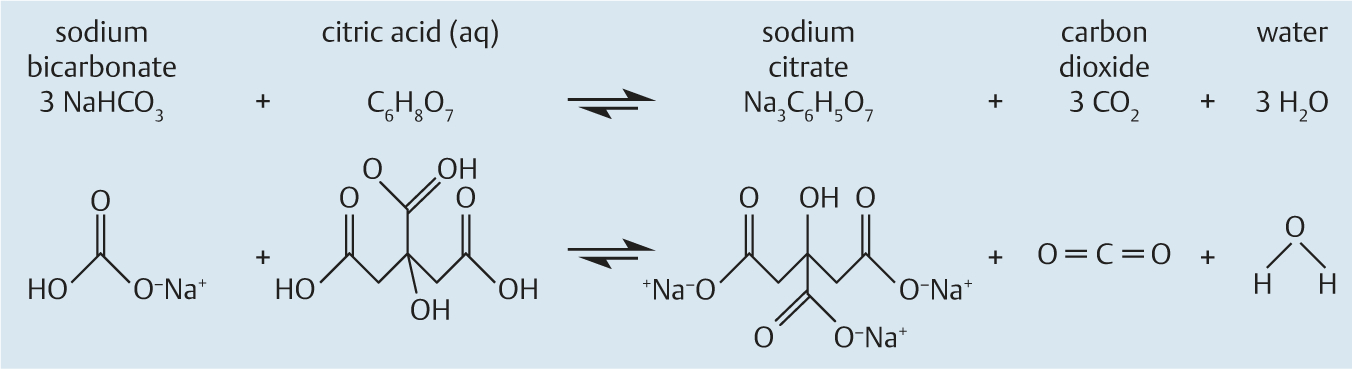
Chemical reaction used in the CO2mfort Cap insufflation capsule, to form carbon dioxide from sodium bicarbonate, citric acid, and water.
Fig.2.
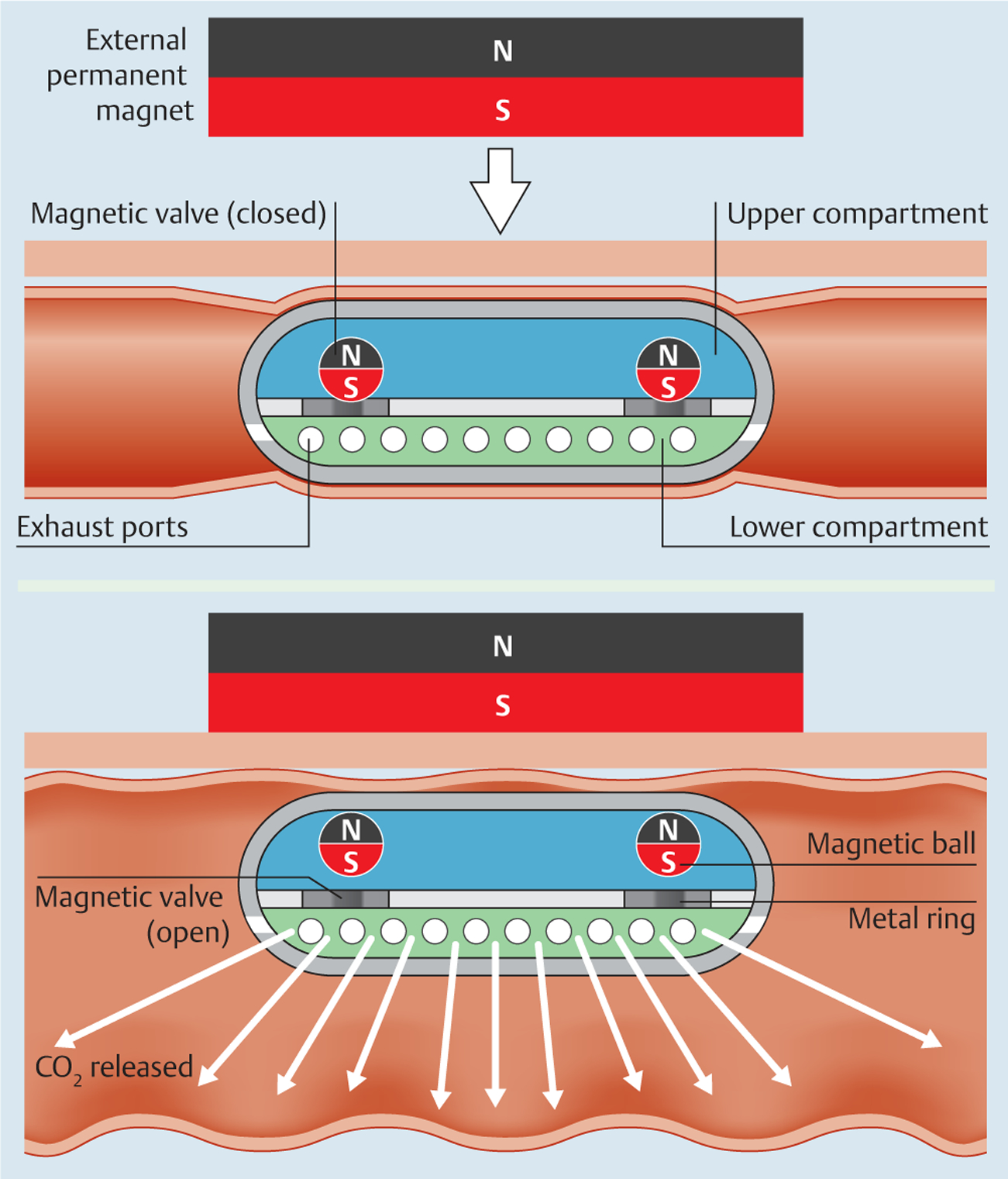
The insufflation capsule has two compartments. In the closed configuration, the magnetic valves form a tight barrier between the compartments. When triggered by an external permanent magnet, the valves open to allow mixture of the two reactants, producing carbon dioxide that is released through exhaust ports along the lining of the lower compartment.
Remote triggering is achieved by means of an external permanent magnet (EPM). This is a disc-shaped neodymium magnet (sintered NdFeB magnets; B&W Technology and Trade GmbH, Jena, Germany) with diameter 50mm, thickness 20mm, and grade N52 axial magnetization.
Benchtop trials to assess reaction kinetics
Previous experiments involving the chemical reaction had been performed at room temperature [18]. To determine the performance at standard body temperature, we conducted ten benchtop trials at 37°C. Each trial used a total of 0.82g sodium bicarbonate and 0.63g citric acid in 0.75mL water. Measured parameters included: volume of carbon dioxide produced, as shown by the water displacement technique; time to reach 50% of the plateau volume of carbon dioxide (t50), and time to reach the carbon dioxide plateau (tplateau).
Benchtop trials to assess force generation and safety
To determine the maximum force that might be exerted on the colonic mucosa by the capsule because of the magnetic couple between the internal and external magnets, we conducted a simple experiment. A CO2mfort Cap insufflation capsule was attached to a load cell (Nano 17; ATI Industrial Automation, Apex, North Carolina, USA) and an N52 EPM measuring 50mm by 20mm was placed 200mm above the center of the capsule. As the EPM was moved toward the insufflation capsule, the force generated by magnetic coupling and the distance between the capsule and the EPM were recorded.
In vivo porcine trials
Based on our prior ex vivo data, we tested the simultaneous use of multiple CO2mfort Caps for optimizing luminal visibility in vivo.
Five separate swine each underwent per rectum placement of three insufflation capsules. Experiments were performed in five female Yorkshire-Landrace cross swine weighing 30–35kg. General anesthesia was induced with Telazol (4.4mg/kg intravenously; Fort Dodge, Ames, Iowa), xylazine (2.2mg/kg intravenously), and ketamine (2.2mg/kg intravenously). Following endotracheal intubation and throughout the procedure, the animals were maintained on a semi-closed circuit inhalation of 1% to 3% isoflurane and ventilated.
The three capsules were placed in close enough proximity to allow simultaneous activation of the reaction by the EPM. All reactions were observed under direct endoscopic visualization (the endoscope was disconnected from air insufflation and suction) and by fluoroscopy for a period of 12 minutes. Fluoroscopic images were acquired to estimate the diameter (in centimeters) of the gas column generated and sustained. These measurements were analyzed by ImageJ software (National Institute of Health, Bethesda, Maryland, USA). Using the known diameter of the endoscope (GIF 160; Olympus, Tokyo, Japan) of 12.9mm, the scale was set at 4.496 pixels/mm. The column of carbon dioxide was modeled as a cylinder, divided into a series of sections at equal intervals, and the diameters were recorded. These were then averaged to estimate the diameter of the gas column generated per trial.
The animal was sacrificed at the end of the procedure. The colon was then explanted and examined by an expert gastrointestinal pathologist.
The study was approved by the local Institutional Animal Care and Use Committee.
Statistical analysis
All continuous data are reported as mean (standard deviation [SD]). Normally distributed continuous variables were analyzed using a t test. Benchtop reaction kinetics were compared with in vivo reaction kinetics using analysis of variance (ANOVA) by Prism6 (GraphPad Software Inc., La Jolla, California, USA). Statistical significance was set at P<0.05.
Results
Carbon dioxide production reaction
We first tested the reaction kinetics and performance characteristics in vitro. The carbon dioxide production reaction was rapid and robust. Benchtop reaction testing at 37°C yielded a mean (SD) carbon dioxide volume of 286.64 (11.80) mL at t50=1 minute and 509.01 (9.35) mL at tplateau=12 minutes.
Magnetic force between the capsule and the external magnet
A modest but measurable force was produced between the external magnet and the capsule. When the distance between the capsule and EPM was 78.7 (6.0) mm, the force generated by the magnetic coupling was sufficient to overcome the weight of gravity on the capsule. When the distance between the capsule and EPM was reduced to 52.5 (2.5) mm, the force generated by the magnet was sufficient to activate the valves. As the distance between the capsule and EPM approached zero, the force generated by the magnet pair approached 1.025 N. If this force is distributed across the upper surface of the capsule, defined as one-third of the total surface area, the maximum pressure placed on the colon is 2458.91 pascals (0.025bar).
Carbon dioxide production in vivo
The carbon dioxide production in vivo occurred in a controlled and effective manner. In all five in vivo porcine trials, the CO2-mfort Caps were successfully triggered and insufflated the porcine colon without evidence of perforation or free air as demonstrated by fluoroscopy. Fluoroscopic image analysis demonstrated experimental reaction kinetics that were similar to those of benchtop trials (P=0.28). The maximum insufflation diameter achieved was 32.1 (3.9) mm by 540 (53.7) seconds (Fig.3).
Fig.3.
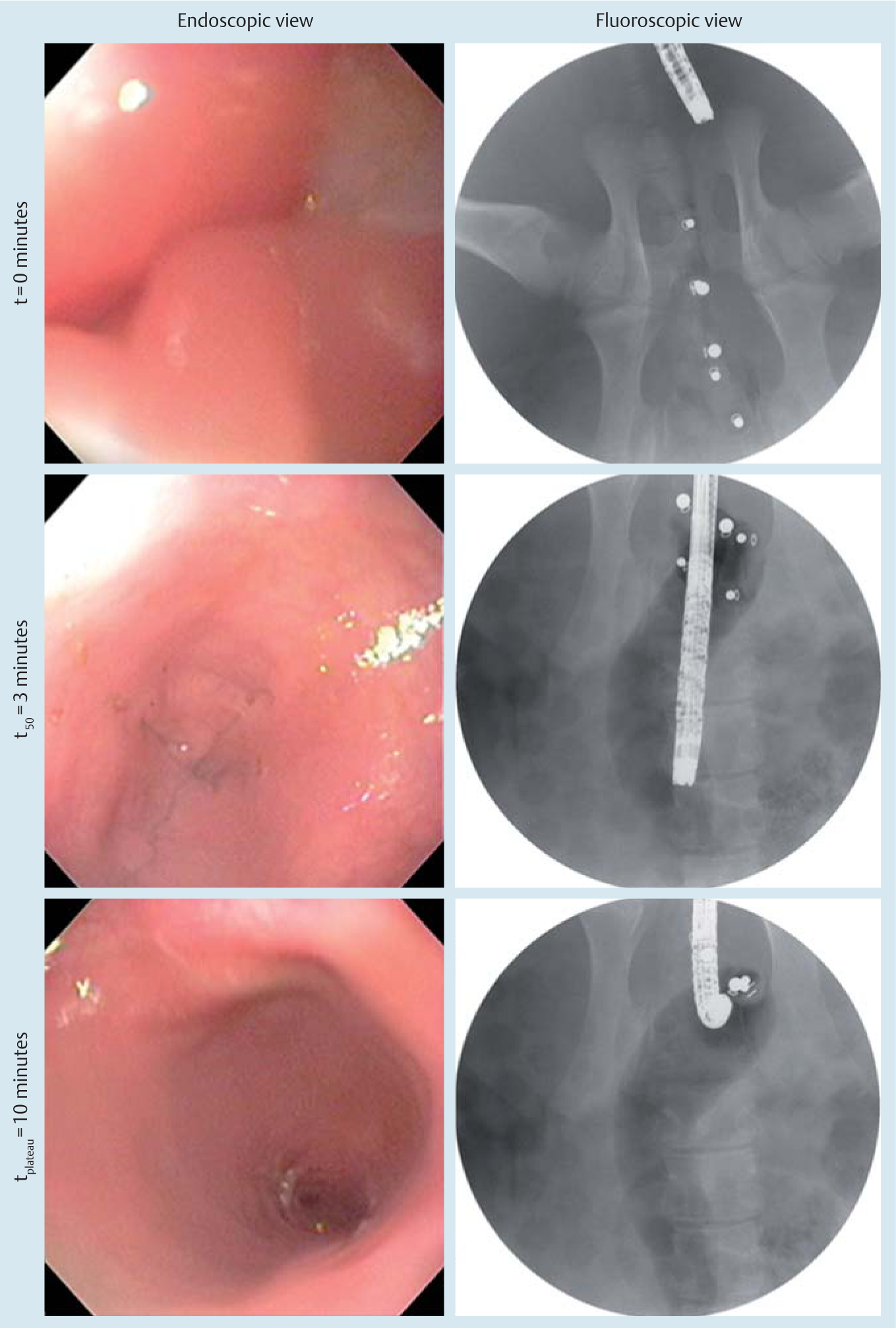
Endoscopic view and corresponding fluoroscopic images over the course of the in vivo trial in animal 3. Fluoroscopic images were acquired and transmitted in real time to a monitor while endoscopic visualization of the distal colon lumen was performed to confirm mucosal visibility.
Visualization of the colon and safety
Complete visualization of the colon was safely enabled by the insufflation capsule. The luminal distension achieved was sufficient for complete endoscopic visualization of the mucosa and safe proximal passage of the endoscope under direct visualization (Video ). All swine survived and there were no intraoperative or immediate post-operative adverse events.
On gross pathological examination, there was no evidence of perforation. There was no evidence of microscopic trauma in three of five specimens (Table1); specimens 2 and 3 each demonstrated a linear red streak that corresponded to mild microscopic submucosal edema and a patchy area of hemorrhage into the lamina propria and submucosa.
Table 1.
Carbon dioxide insufflation using a capsule. Pathology results from in vivo porcine trials.
| Animal no. | Pathological findings | |
|---|---|---|
| Gross | Microscopic | |
| 1 | Focal surface erosion | Patchy accumulation of neutrophils in lamina propria with a few crypt abscesses |
| 2 | Linear red streak | Patchy area of hemorrhage into lamina propria and submucosa, with neutrophils and loss of mucin. Submucosal edema |
| 3 | Linear red streak | Patchy area of hemorrhage into lamina propria and submucosa, with neutrophils and loss of mucin. Submucosal edema |
| 4 | Normal | Small patch of neutrophils in superficial lamina propria (mild) |
| 5 | Normal | Normal |
Discussion
This study has several important results with both technical and clinical implications. We chose to test three CO2mfort Caps simultaneously in our in vivo trials as our benchtop data indicated that a single CO2mfort Cap produces roughly half a liter of insufflation. We attribute the comparable estimated volume produced by three capsules in vivo to loss of carbon dioxide via the rectum and absorption by the colonic mucosa. Nevertheless, in this manner, we were able to demonstrate that when multiple capsules are used in conjunction, they do not stick together and do not self-activate (as the force between the magnetic sphere and metallic ring is stronger than the force arising from any adjacent magnet from other nearby capsules). Thus, if further insufflation is needed during endoscopy, our approach provides the possibility of introducing additional capsules per rectum to achieve the desired result.
We have designed the CO2mfort Cap flow gate (the “magnetic valve” in Fig.2) to allow triggering of the reaction in aliquots. For triggering the reaction, the maximum allowable distance between the EPM and the internal magnets of the insufflation capsule was 52.5 (2.5) mm. Bringing the EPM within this distance from the capsule triggers the reaction by opening the flow gate. When the external magnet is taken away from the capsule, the flow gate closes and the reaction stops. This distance can be easily varied by changing the size and strength of the external magnet. For different patient populations, including those with obesity, larger external magnets can create a stronger magnetic field to allow control of the release trigger. Such a design can assist the physician to optimally control insufflation as needed.
Although two-dimensional measurement approximations from the fluoroscopic images do not allow for an accurate estimate of volume produced per capsule in vivo, we were able to demonstrate that the reaction kinetics based on diameter are similar to benchtop trials for volume. Assuming the in vivo yield was nonetheless lower than in benchtop trials, interestingly, the insufflation capsule could allow full mucosal visualization for the duration of the procedure with relatively little carbon dioxide.
Our system currently has the capacity to exert a maximum pressure of 0.025 bar against the colonic mucosa. Pressure up to 3bar does not result in mucosal damage to the colon [17]. In this study, the colons of two of the five swine demonstrated submucosal edema and a patchy area of hemorrhage into the lamina propria and submucosa (Table1). The diameters of the linear marks were congruent with the diameter of the endoscope; the marks are most consistent with trauma caused by endoscope contact with the colon and not from the insufflation capsule or the reaction itself. These findings can also be seen in colonoscopy and are generally not considered to be clinically significant.
The CO2mfort Cap is designed so that provided the compartments remain tightly sealed; the shelf-life is theoretically determined by the manufacturer’s recommendations regarding care of the reactants. Sigma recommends re-testing sodium bicarbonate (Sigma S5761) every 3 years while citric acid (Sigma C2404) requires no re-testing. Inadvertent or premature exposure to a strong magnetic field has the potential to open the flow gate, allowing reactants to mix. Precautions against this must be taken and are in development (e.g., a shielded container can be used to store the capsules).
Other limitations of our study include the use of an in vivo non-survival porcine model and a small experimental sample size. This design was chosen because of the nature of the study aims: evaluation of in vivo feasibility and safety. We note that just as in conventional colonoscopy, thorough bowel preparation is needed to ensure the efficacy of capsule endoscopy using the insufflation capsule. Finally, while our prototype was manufactured from a polypropylene-like material because of its easy availability, the capsule can be readily fabricated out of a biocompatible material such as polyether ether ketone (PEEK).
The insufflation capsule also has the exciting potential for meeting another critical need in colon capsule endoscopy–the capability to be actively propelled along the colon rather than to move passively, as colonic transit time in healthy people can be 20–56 hours [18]. A future direction for our team is to achieve both insufflation and controllable motion at the same time, as can be obtained with variations in the design of the magnetically controlled valves.
In conclusion, this is the first in vivo demonstration in the literature of a technique that adds an exciting dimension to colon capsule endoscopy: namely, the ability to distend and visualize the colon on demand with an untethered approach. Using a highly innovative design, we safely and reliably remotely triggered a chemical reaction within the CO2mfort Cap. This released carbon dioxide in a controlled fashion and allowed for clear mucosal visibility with a colonoscope. We envisage that such insufflation may allow for better visualization of colonic lesions and increase the diagnostic yield of colon capsule endoscopy. These results have important implications for the future of capsule endoscopy and could potentially be transformative for how we screen patients for CRC.
Supplementary Material
Acknowledgments
The authors would like to gratefully thank Mr. Phil Williams, Director of the Division of Surgical Research at Vanderbilt University Medical Center, and all of the Vanderbilt staff at the Light Surgical Facilities for Animal Trials, for their precious time and assistance during the in vivo experiment.
Support for this work was received from the Center for Compact and Efficient Fluid Power under award #0540834, the Broad Medical Research Program of The Broad Foundation, and from the National Science Foundation under Grant No.CNS-1239355 and IIP-1356639.
Footnotes
Competing interests: None
References
- 1.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2009 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2013: Available at: www.cdc.gov/uscs Accessed: 21 March 2013 [Google Scholar]
- 2.Selby JV, Friedman GD, Quesenberry CP et al. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med 1992; 326: 653–657 [DOI] [PubMed] [Google Scholar]
- 3.Hardcastle JD, Chamberlain JO, Robinson MH et al. Randomised controlled trial of faecal-occult blood screening for colorectal cancer. Lancet 1996; 348: 1472–1477 [DOI] [PubMed] [Google Scholar]
- 4.Kronborg O, Fenger C, Olsen J et al. Randomised study of screening for colorectal cancer with faecal occult blood test. Lancet 1996; 348: 1467–1471 [DOI] [PubMed] [Google Scholar]
- 5.Winawer SJ, Fletcher RH, Miller L et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology 1997; 112: 594–642 [DOI] [PubMed] [Google Scholar]
- 6.Bujanda L, Sarasqueta C, Zubiaurre L et al. Low adherence to colonoscopy in the screening of first-degree relatives of patients with colorectal cancer. Gut 2007; 56: 1714–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fry LC, Vormbrock K, Olano C et al. Small-bowel endoscopy. Endoscopy 2011; 43: 978–84 [DOI] [PubMed] [Google Scholar]
- 8.Spada C, Hassan C et al. Second-generation colon capsule endoscopy compared with colonoscopy. Gastrointest Endosc 2011; 74: 581–589 [DOI] [PubMed] [Google Scholar]
- 9.Van Gossum A, Ibrahim M. Video capsule endoscopy: what is the future? Gastroenterol Clin North Am 2010; 39: 807–826 [DOI] [PubMed] [Google Scholar]
- 10.Spada C, Hassan C, Sturniolo GC et al. Literature review and recommendations for clinical application of colon capsule endoscopy. Dig Liver Dis 2011; 43: 251–258 [DOI] [PubMed] [Google Scholar]
- 11.East JE, Bassett P, Arebi N et al. Dynamic patient position changes during colonoscope withdrawal increase adenoma detection: a randomized, crossover trial. Gastrointest Endosc 2011; 73: 456–463 [DOI] [PubMed] [Google Scholar]
- 12.Dellon ES, Hawk JS, Grimm IS et al. The use of carbon dioxide for insufflation during GI endoscopy: a systematic review. Gastrointest Endosc 2009; 69: 843–849 [DOI] [PubMed] [Google Scholar]
- 13.Maple JT, Banerjee S, Barth BA et al. Methods of luminal distention for colonoscopy. Gastrointest Endosc 2013; 77: 519–525 [DOI] [PubMed] [Google Scholar]
- 14.Gorlewicz JL, Battaglia S, Smith BF et al. Wireless insufflation of the gastrointestinal tract. IEEE Trans Biomed Eng 2013; 60: 1225–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obstein KL, Battaglia S, Smith BF et al. Novel approach for colonic insufflation via an untethered capsule (with video). Gastrointest Endosc 2013; 177: 516–517 [DOI] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration (US FDA). Available at: http://www.accessdata.fda.gov/scripts/fcn/fcnNavigation.cfm?rpt=scogsListing
- 17.Moshkowitz M, Hirsch Y, Carmel I et al. A novel device for rapid cleaning of poorly prepared colons. Endoscopy 2010; 42: 834–836 [DOI] [PubMed] [Google Scholar]
- 18.Wagener S, Shanker KR, Turnock RR et al. Colonic transit time – what is normal? J Pediatr Surg 2004; 39: 166–169; discussion 166–169 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


