Abstract
Female reproductive aging is characterized by a rise in follicle stimulating hormone (FSH) levels during peri-menopause. N-linked glycans are co-translationally attached to the Asn7 and Asn24 residues on the FSHβ subunit. Differences in the number of N-glycans on the FSHβ subunit result in distinct glycoforms: hypo-glycosylated (FSH21/18, glycans on either Asn24 or Asn7, respectively) or fully-glycosylated (FSH24, glycans on both Asn7 and Asn24). The relative abundance of FSH glycoforms changes with advanced reproductive age, shifting from predominantly FSH21/18 in younger women to FSH24 in older women. Previous in vitro studies in granulosa cell lines and in vivo studies using Fshb null mice showed these glycoforms elicit differential bioactivities. However, the direct effects of FSH glycoforms on the mouse ovarian follicle have not yet been determined. In this study, we isolated secondary follicles from pre-pubertal mice and treated them with 20- or 100 ng/mL purified recombinant FSH glycoforms for one-hour or 18-20 hours. Analysis of phosphorylated PKA substrates showed that glycoforms were bioactive in follicles following one-hour treatment, although differential bioactivity was only observed with the 100 ng/mL dose. Treatment of follicles with 100 ng/mL of each glycoform also induced distinct expression patterns of FSH-responsive genes as assessed by qPCR, consistent with differential function. Our results, therefore, indicate that FSH glycoforms are bioactive in isolated murine follicles.
Keywords: ovarian follicle, follicle-stimulating hormone, glycosylation, aging
Introduction
Reproductive aging is universal, affects all females, and occurs decades prior to the decline of any other human organ system (Broekmans et al., 2007, Broekmans et al., 2009, Banerjee et al., 2014). One of the earliest signs of aging is increasing basal levels of circulating follicle stimulating hormone (FSH), associated with a shortened menstrual cycle and follicular phase (McTavish et al., 2007). This sharp rise in FSH is thought to result from decreased inhibin B levels due to the decreasing number of small antral follicles as the ovarian reserve is depleted with age (Klein et al., 1996). FSH is a glycoprotein hormone secreted by the anterior pituitary gonadotrope cells which is essential for ovarian folliculogenesis. In adult females, functional FSH receptors (FSHR) located on granulosa cells respond to FSH, leading to follicle maturation. Similar to other pituitary and placental hormones, FSH consists of two non-covalently associated heterodimeric α- and β-subunits, of which the β-subunit is hormone-specific and confers biological activity (Hunzicker-Dunn and Mayo, 2015, Das and Kumar, 2018). FSH signaling is regulated in part via N-glycosylation. This is a multi-enzyme driven process wherein the multi-subunit oligosaccharyl transferase (OST) enzyme complex co-translationally attaches GlcNAC2 Man9Glc3 precursors onto conserved Asn24 and/or Asn7 residues of the human FSH β-subunit (Pierce and Parsons, 1981, Baenziger and Green, 1988, Wang et al., 2016b). Previous studies have established the presence of different N-glycosylated FSH glycoforms in human pituitaries and/or urine based on the presence of one or two N-glycans (Dalpathado et al., 2006, Davis et al., 2014, Bousfield et al., 2014b, Bousfield et al., 2015) in the FSHβ subunit. FSHβ with N-glycans on both Asn7 and Asn24 residues is detected as a 24 kDa band on immunoblots, and the heterodimer consisting of this subunit is designated fully-glycosylated FSH24. Two additional glycoform variants exist that are classified as hypo-glycosylated - FSH21 and FSH18 based on the absence of Asn24 or Asn7 glycans, resulting in 21 and 18 kDa size immunoreactive bands, respectively. These band sizes correspond to the nomenclature of the glycoforms. Purified recombinant hypo-glycosylated FSH21 and FSH18 exist as a mixture, which is designated FSH21/18 (Wang et al., 2016b, Davis et al., 2014, Bousfield et al., 2007).
Changes in the relative abundance of FSH glycoforms occur with age (Davis et al., 2014, Bousfield et al., 2007, Anobile et al., 1998, Creus et al., 1996, Loreti et al., 2009, Padmanabhan et al., 1999, Jiang et al., 2015). Hypo-glycosylated FSH21/18 is the predominant form in younger women with ovulatory cycles, and fully-glycosylated FSH24 is predominant in older women (Davis et al., 2014, Bousfield et al., 2007, Bousfield et al., 2014a, Jiang et al., 2015, Bousfield et al., 2014b, Butnev et al., 2015, Green et al., 1986). This change in glycoform abundance as a function of age is significant in the context of female reproductive aging not only due to the overall rise in levels of FSH at peri-menopause but also because of the differences in bioactivity between these glycoforms. In vitro studies using radio-ligand and radio-receptor assays (Bousfield et al., 2014b, Butnev et al., 2015, Bousfield et al., 2014a, Bousfield et al., 2007, Dalpathado et al., 2006, Bousfield et al., 2015), and a study conducted on an FSH-responsive KGN granulosa tumor cell line (Jiang et al., 2015) showed that the hypo-glycosylated FSH21/18 glycoform preparation was more bioactive than the fully-glycosylated FSH24 glycoform. The FSH21/18 glycoform exhibits enhanced receptor occupancy and faster kinetics, and lower doses of FSH21 are needed for cAMP, estradiol, and progesterone production in granulosa cell lines compared to FSH24. In vivo studies using immature Fshb null mice in a pharmacological rescue approach investigated the biological effects of these glycoforms and further demonstrated that hypo-glycosylated FSH21/18 and fully-glycosylated FSH24 glycoforms act via distinct biological pathways to regulate differential effects in target tissues (Wang et al., 2016b). Although these studies suggest unique age-specific actions of FSH glycoforms, the in vitro studies were conducted in isolated granulosa cells from a tumor cell line (Jiang et al., 2015), and the in vivo studies interrogated the whole ovary, precluding analysis of specific FSH-responsive sub-compartments within the ovary (Wang et al., 2016b). Therefore, the goal of this study was to investigate the effects of recombinant FSH glycoforms directly on the follicle, which is the functional unit of the ovary and primary target of FSH.
The ovarian follicle provides a unique organotypic system in which to investigate the impact of FSH glycoforms without any systemic side effects of the body or of surrounding ovarian cells (Xu et al., 2016). In the follicle, FSH drives granulosa cell proliferation and production of estrogen, which in turn promotes follicle growth, follicle and oocyte maturation and ovulation (Hunzicker-Dunn and Maizels, 2006). Here we examined the biological activity of these age-specific glycoforms on known FSH responsive protein and gene pathways. We hypothesized that individual FSH glycoforms would induce downstream signaling pathways differentially in the ovarian follicle. To this end, we isolated murine secondary follicles and first validated FSH dose responsiveness using a commercially available recombinant human FSH (rec. hFSH) preparation. We then treated isolated follicles with recombinant hypo-glycosylated FSH21 and fully-glycosylated FSH24 at two concentrations at one-hour and 18-20 hour exposure time-points. In response to these FSH glycoforms, we specifically examined the activation of the PKA signaling pathway and several known FSH-responsive genes in the ovarian follicle. Our data confirm that age-specific FSH glycoforms are bioactive in vitro and stimulate different downstream signaling pathways in the follicle.
Materials and Methods
Mice
Pre-pubertal 12-14-day-old CD1 female mice were obtained from a breeding colony. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Northwestern University and were performed in accordance with the guidelines and requirements of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice were housed in a controlled barrier facility in the Center for Comparative Medicine at Northwestern University (Chicago, IL). Food and water were provided ad libitum. Temperature, humidity, and photo-period (14L:10D) were kept constant. Ovaries were harvested from mice and removed from the bursa according to standard procedure.
Follicle isolation
Two-layered secondary follicles (mean size 129.56 μm ± 10.48 SD) were mechanically isolated using insulin-gauge needles in dissection medium (Leibovitz 15 [L15] media (Life Technologies Corporation, Grand Island, NY) supplemented with 1% fetal bovine serum (FBS, Life Technologies Corporation) and 50 U/mL Pen Strep (Thermo Fisher Scientific, Waltham, MA). Healthy two-layered secondary follicles were identified based on their diameter (measured using ImageJ) and morphology (immature oocyte surrounded by two layers of granulosa cells) as previously described (Xu et al., 2006). After isolation, the follicles were allowed to recover from the isolation procedure for a minimum of one hour in maintenance medium (minimum essential medium [α-MEM] (Life Technologies Corporation) supplemented with 1% FBS and 50 U/mL Pen Strep at 37°C in a humidified atmosphere of 5% CO2 in air.
Recombinant FSH preparations and treatment
Recombinant hFSH was obtained from Organon (Oss, The Netherlands, a gift from Dr. Irving Boime), and predominantly consists of fully-glycosylated FSH24. Individual recombinant human FSH glycoforms – fully-glycosylated FSH24 and hypo-glycosylated FSH21 – were purified from rat pituitary GH3 cells co-expressing fully-glycosylated FSH24 and hypo-glycosylated FSH21 and FSH18 as a 1:1 mixture. The purification and characterization of the hormones from GH3-conditioned media was described in detail elsewhere (Butnev et al., 2015, Bousfield et al., 2014a, Jiang et al., 2015). Secondary follicles in groups of 10 were treated with rec. hFSH at concentrations of 50, 100 and 250 ng/mL concentration diluted in growth media for one-hour and 18-20 hour time-points. These doses correspond to ~ 5, 10 and 25 mIU/mL respectively of the standard reference human FSH preparation (Wang et al., 2016b). Growth medium was composed of α-MEM supplemented with 3 mg/ml bovine serum albumin (MP Biomedicals, Santa Ana, California), 1 mg/ml bovine fetuin (Sigma-Aldrich, St. Louis, MO), and 5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml selenium (Thermo Fisher Scientific). Follicles maintained in growth media alone served as controls. All cultures were performed in 96-well Ultra-Low Attachment plates (Corning, Corning, New York). Secondary follicles were also treated with of 20- and 100 ng/mL of purified recombinant human FSH glycoforms FSH24 and FSH21 in growth medium for one-hour and 18-20 hours. These concentrations and times were chosen based on in vitro studies where differences in glycoform bioactivity were documented (Jiang et al., 2015). After treatment, follicles were imaged using 10X and 20X long working distance objectives on an EVOS FL Auto microscope (Life Technologies Corporation). Follicles were then either snap-frozen on dry ice for Western blot and qPCR or fixed for immunofluorescence analysis. For Western blots, sets of 10 follicles of uniform sizes were rinsed through L15 supplemented with 50 U/mL Pen Strep and 3 mg/ml polyvinylpyrolidine (Sigma-Aldrich) (L15/PVP/PS) to remove exogenous protein supplements and snap-frozen in minimal volume.
Western blot analysis
The snap-frozen follicles were thawed on ice and lysates were prepared in the presence of 1X phosphatase inhibitor cocktail (Thermo Fisher Scientific) to preserve protein phosphorylation. The follicle lysates were then separated on 4-15% SDS-PAGE polyacrylamide gels (Bio-Rad, Hercules, CA) according to standard procedure and transferred onto Amersham Hybond P 0.45 PVDF membranes (GE Healthcare Life Sciences, Pittsburg, PA). The membranes were blocked in 3% Amersham ECL Blocking Agent (GE Healthcare Life Sciences) diluted in Tris buffered saline containing 0.1% v/v Tween-20 (TBS-T) (block) for 3-4 hours at room temperature. Blots were probed with primary antibody diluted in block at 4°C 18-20 hours. The primary antibody used was phospho-PKA substrates (RRXS*/T*) (1:1000 dilution) (Cell Signaling Technologies, Danvers, MA) (catalog number 9624S). Blots were rinsed three times in TBS-T and incubated in horse radish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (1:10,000) (GE Healthcare Life Sciences) diluted in block for 1 hour at room temperature. Following secondary antibody incubation, the blots were washed three times in TBS-T and developed with a chemiluminescent substrate, ECL Prime (GE Healthcare Life Sciences) according to the manufacturer’s instructions. Blots were stripped using Restore Western Blot Stripping Buffer according to manufacturer’s instructions (Thermo Fisher Scientific), blocked for one hour and re-probed with 1:2500 dilution of an antibody against MSY2 (generous gift of R. Schultz, University of Pennsylvania), 18-20 hours at 4°C. MSY2 is a highly abundant oocyte-specific protein (Yu et al., 2001) and was used as a loading control to ensure equal numbers of follicles were loaded onto the gel. The amount of total protein in small numbers of isolated follicles is very low, and thus there is no reliable way to accurately measure total protein levels. Therefore, we loaded equal number of follicles per lane, included follicles only of similar diameters and morphology and normalized the data based on the expression of MSY2. Each follicle contains a single oocyte and if the follicles are at the same developmental stage, the expression of MSY2 should be constant across groups. By normalizing to the follicle number (and keeping follicle number and starting sizes consistent) our analysis assessed PKA phosphorylation directly in the follicle as a response to FSH. The secondary antibody used and detection methods were the same as for phospho-PKA substrates. Densitometry analysis was performed using ImageJ software (National Institutes of Health, Bethesda, MD). For the phospho-PKA substrate antibody, the most distinct band at ~70 KDa was used for quantification purposes. Mean pixel intensities for p-PKA substrate and MSY2 bands were determined following background subtraction. Because equal numbers of follicles were loaded per lane, the mean pixel intensity values of the band of interest were normalized to MSY2 mean pixel intensity values. The fold change value was determined by dividing the normalized phospho-PKA substrate intensities of FSH-treated follicles over normalized phospho-PKA substrate intensities of control follicles.
Immunofluorescence analysis
Follicles were fixed in 3.8% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) for 1 hour at room temperature and then washed in PBS (Life Technology Corporation) containing 0.3% BSA (Sigma-Aldrich), 0.01% Tween-20 (Sigma-Aldrich) and 0.02% NaN3 (Sigma-Aldrich) (blocking buffer). Follicles were permeabilized for 30 minutes in buffer containing 1X PBS, 0.3% BSA, 0.1% Triton X-100 (Sigma-Aldrich), and 0.02% sodium azide. Following permeabilization, follicles were incubated in 20 μg/mL Proteinase K (Sigma-Aldrich) for 5 minutes at 37°C. Proteinase K has been used previously for unmasking antigens in histological sections (Svistunova et al., 2012). Following two more 5-minute washes in blocking buffer, follicles were incubated in the phospho-PKA substrate antibody diluted in blocking buffer (0.717μg/μL) at 4°C 18-20 hours. Non-immune rabbit IgG used at the same concentration (Vector Laboratories, Burlingame, CA) was used as an isotype control to show that staining was specific even following Proteinase K treatment (Supplemental Figure 1). Follicles were rinsed in blocking buffer three times and then incubated in an Alexa-Fluor-488 conjugated goat anti-rabbit secondary antibody diluted in blocking buffer (1:100) (Life Technologies) for 1-hour at room temperature. Staining for F-actin was performed simultaneously using rhodamine-phalloidin (R415, Life Technologies) at a 1:50 dilution in blocking buffer. Follicles were rinsed again in blocking buffer three times and mounted in Vectashield containing DAPI (4’,6-diamidino-2-phenylindole; Vector Laboratories) to detect DNA. Follicles were imaged with a Leica SP5 inverted laser-scanning confocal microscope using a 40x objective (Leica Microsystems). Z-stacks of 1μm optical section thickness were taken. Images were processed and quantified using ImageJ. For quantification of phospho-PKA substrates fluorescence intensity, the optical section containing the middle of the follicle (including the granulosa cells and the oocyte with a visible nucleus) was used. The ratio of fluorescence intensity of the follicle relative to the total area of the follicle based on pixel intensity was calculated and reported for follicles within each treatment group.
RNA isolation, cDNA synthesis and Taqman Real-time qPCR assays
Isolated murine follicles were treated as above with 100 ng/mL FSH glycoforms and then snap-frozen in groups of 50. Total RNA was isolated from 100 snap-frozen mouse ovarian follicles treated with 100 ng/mL FSH glycoforms using RNEasy micro columns (Qiagen), and the RNA was quantified using a NanoDrop spectrophotometer (ThermoScientific) at 260 nm. Total RNA was reverse transcribed by the oligo dT method using the SuperScript III kit (ThermoScientific) as described (Wang et al., 2014, Wang et al., 2015, Wang et al., 2016a). Taqman real time qPCR assays were performed on the cDNA samples in triplicate using custom-made or pre-inventoried primer/combo mixes (ThermoScientific or IDT). Expression of Ppil1 was used as an internal control and the relative amounts of mRNA expression were calculated as described (Wang et al., 2014, Wang et al., 2015, Wang et al., 2016a). A list of genes interrogated is provided in Supplemental Table 1.
Statistical analysis
All experiments were performed with a minimum of two or three biological and technical replicates unless specified. Graphs were created and data were analyzed using GraphPad version 7 for Mac OS X (GraphPad Software, La Jolla, CA, www.graphpad.com). A one-way ANOVA with Tukey’s post-hoc test was used to compare responses across treatment groups. A P-value of <0.05 was considered significant.
Results
Isolated murine secondary follicles are responsive to rec. hFSH in a dose-responsive fashion at the one-hour time point
To validate our model system, we first confirmed that isolated secondary follicles were responsive to rec. hFSH following one-hour and 18-20 hours of treatment using phosphorylated PKA substrates as an endpoint because PKA is a well-known target of FSH in the ovarian follicle (Figure 1). FSHR, expressed on granulosa cells is a G-protein-coupled receptor (GPCR), to which FSH binds and signals through multiple downstream signaling pathways, including the canonical Gsα/cAMP/PKA pathway. FSHR couples to Gsα and produces cyclic AMP (cAMP) which binds and activates Protein Kinase A (PKA). The catalytic subunits of PKA phosphorylate other downstream proteins (Das and Kumar, 2018, Hunzicker-Dunn and Mayo, 2015). The blots for phosphorylated PKA substrates in isolated follicles therefore show multiple bands (Figure 1 A, B). Although we do not know the specific protein identity of these bands, immunoblot analysis to assess PKA substrate phosphorylation is standard practice that has been utilized previously to assess the effects of FSH glycoforms (Jiang et al., 2015, Wang et al., 2016b). Known PKA downstream targets include CREB, PI3K/Akt, Histone H3 and p38 MAPK (Hunzicker-Dunn and Maizels, 2006).
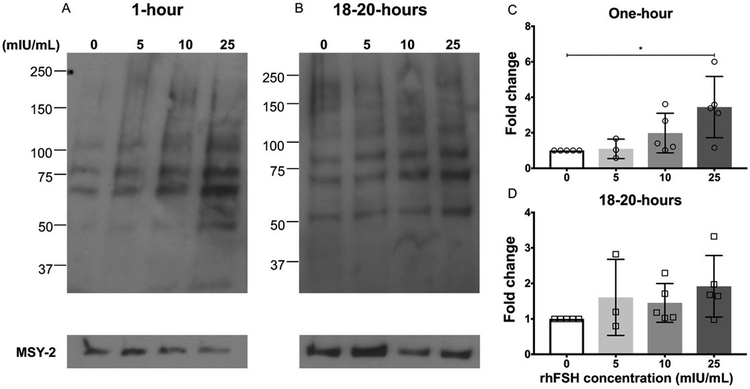
Figure 1. Rec. hFSH treatment induces phosphorylated PKA substrates in a dose-dependent manner in secondary follicles.
(A, B) Immunoblot analysis of phosphorylated PKA substrates in murine follicles following rec. hFSH treatment for one-hour (A) and 18-20 hours (B). Densitometry analysis of phospho-PKA substrate protein levels were performed and values indicated (C, D) were normalized to MSY-2 levels. The experiment was repeated 5 times (3 times for 5mIU/mL concentration) with 10 follicles in each group. Representative images are shown. The arrow indicates the phospho-PKA substrate band that was chosen for densitometry. Data are expressed as mean ± S.D. * P<0.05.
At the one-hour timepoint, there was a dose-dependent increase in the fold change of PKA substrate phosphorylation to rec. hFSH treatment which was significant relative to untreated control at the 25 mIU/mL dose (P<0.05) (Figure 1C). This trend was not observed at the 18-20-hour time point (Figure 1D). These findings indicate that the canonical PKA signaling pathway is activated in a dose-responsive manner to rec. hFSH in the follicle, but the signaling activation is time-dependent and occurs rapidly. These results also confirm that isolated ovarian follicles provide us with a reliable system to study the direct effects of individual FSH glycoforms.
Recombinant FSH glycoforms induce phosphorylation of PKA substrates in secondary follicles
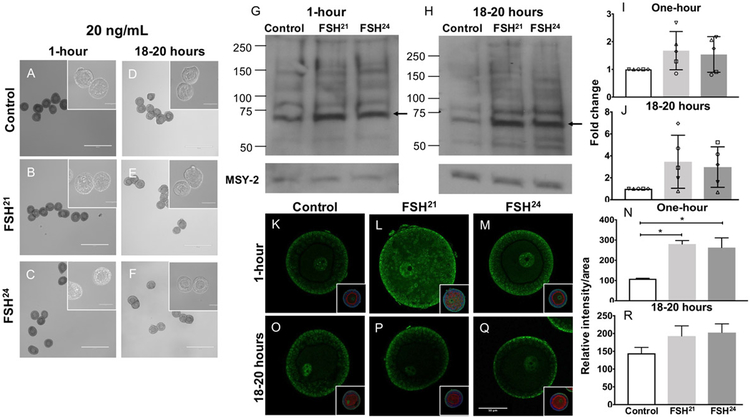
To determine whether recombinant human fully-glycosylated FSH21 and hypo-glycosylated FSH24 glycoforms activate the PKA signaling pathway, we treated isolated secondary follicles with 20- and 100 ng/mL FSH glycoforms at one-hour and 18-20 hour timepoints and examined the phosphorylation of PKA substrates. Treatment of follicles with 20 ng/mL of either purified FSH glycoform did not affect their morphology at any time point (Figure 2 A-F). By western blot, each glycoform tended to stimulate PKA substrate phosphorylation relative to the untreated control at both time points but this did not reach statistical significance (Figure 2 G-J). In addition to assessing PKA substrate phosphorylation by Western blot, we also performed wholemount immunocytochemistry on intact follicles. Within the follicle, phosphorylated PKA substrates localized primarily to the outer granulosa cells but were also observed within the oocyte with particular enrichment in the nucleus (Figure 2K-Q). The staining was specific as it was not observed in the non-immune IgG isotype control (Supplemental Figure 1). Based on immunofluorescence, phospho-PKA substrate expression increased significantly following the one-hour treatment with 20 ng/mL FSH21 and FSH24 (P<0.05) (Figure 2 K-N). No increase was observed at the 18-20-hour time point (Figure 2 O-R). These results indicate that follicles respond to FSH glycoform concentrations as low as 20 ng/mL, but this bioactivity is significant only at the one-hour time point.
Figure 2. Treatment of follicles with 20ng/mL individual purified FSH glycoforms for one-hour results in upregulation of phospho-PKA substrates.
(A-F) Follicles were morphologically intact with healthy oocytes following treatment with 20 ng/mL FSH glycoforms at the one-hour and 18-20 hour timepoints. Scale bar = 400μm (100μm for insets). (G, H) Immunoblot analysis of phosphorylated PKA substrates in murine follicles following FSH glycoform treatment for one-hour (G) and 18-20 hours (H). (I, J) Densitometry analysis of phospho-PKA substrate protein levels were performed and values were normalized to MSY-2 levels. Experiment was repeated 5 times with 10 follicles per group. (K-R) Immunofluorescence analysis of phosphorylated PKA substrates in murine follicles following FSH glycoform treatment for one-hour (K-M) and 18-20 hours (O-Q). Single optical sections are shown. Insets show DNA in blue and F-actin in red. Images corrected to +20% brightness and −20% contrast. (N, R) Relative intensity per area of all follicles examined is plotted for each time point. Between 10-21 follicles were examined at each time point. Data expressed as mean ± S.D. Scale bar = 50μm. * P<0.05.
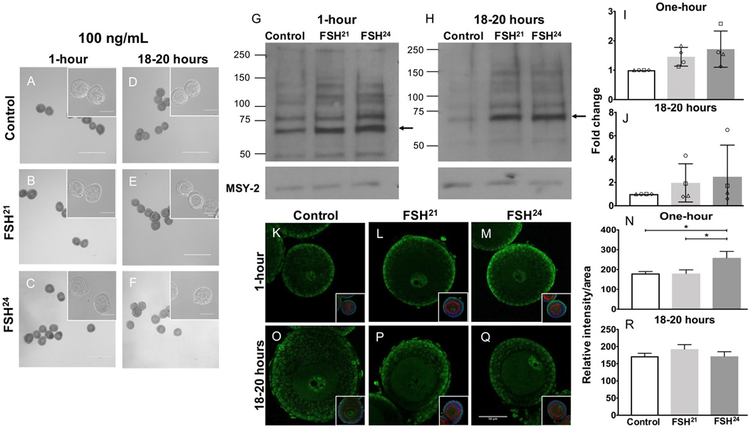
Treatment with the 100 ng/mL dose of purified FSH glycoforms was similar to the treatment with 20 ng/mL in that follicle morphology was unaffected at both the one-hour and 18-20 hour time points (Figure 3 A-F), and Western blot analysis indicated a consistent but not significant trend of increased PKA substrate phosphorylation relative to the untreated control at both time points (Figure 3 G-J). However, by immunofluorescence we detected a significant increase in phospho-PKA substrate expression following one-hour treatment with 100 ng/mL FSH24 relative to untreated control (P<0.05). Unlike treatment with 20 ng/mL, there was a significant difference between FSH21 and FSH24 action following treatment with 100 ng/ml of the FSH glycoforms at the one-hour time point (P<0.05) (Figure 3 K-N). No differences were observed at the 18-20 hour time point (Figure 3 O-R). These results indicate that treatment of follicles with 100 ng/mL of FSH glycoforms activates differential patterns of PKA signaling in the ovarian follicle, and this activation is time-dependent and most robust at the one-hour time point.
Figure 3. Treatment of follicles with 100ng/mL individual purified FSH glycoforms for one-hour results in differential regulation of phospho-PKA substrates.
(A-F) Follicles were morphologically intact with healthy oocytes following treatment with 100 ng/mL FSH glycoforms at the one-hour and 18-20-hour time points. Scale bar = 400μm (100μm for insets). (G, H) Immunoblot analysis of phosphorylated PKA substrates in murine follicles following FSH glycoform treatment for one-hour (G) and 18-20 hours (H). (I, J) Densitometry analysis of phospho-PKA substrate protein levels were performed and values were normalized to MSY-2 levels. Experiment was repeated 4 times with 10 follicles per group. (K-R) Immunofluorescence analysis of phosphorylated PKA substrates in murine follicles following FSH glycoform treatment for one-hour (K-M) and 18-20 hours (O-Q). Single optical sections are shown. Insets show DNA in blue and F-actin in red. Images corrected to +20% brightness and −20% contrast. (N, R) Relative intensity per area of all follicles examined is plotted for each time point. Between 13-20 follicles were examined at each time point. Data expressed as mean ± S.D. Scale bar = 50μm. * P<0.05.
Hypo- and fully-glycosylated FSH glycoforms elicit time-dependent changes in gene expression in isolated follicles
Phosphorylation of PKA substrates, while a well-accepted readout of FSH action, is only a single endpoint which occurs at the protein and post-translational level. Our findings indicate that the response to the FSH glycoforms occurs rapidly within one hour. Therefore, we wanted to take a broader approach by examining the expression of well-known FSH-responsive genes – Apaf1, Cyp19a1, Caps3, Cepbp, Esr1, Ereg, Fshr, Inha, Inhbb, Kcnj8, Ki67, S100a6 and Tagln. (Table 1) (Wang et al., 2016b). These genes were shown previously to be differentially regulated by FSH glycoforms in vivo in whole ovaries (Wang et al., 2016b). To test the in vitro bioactivity of recombinant human hypo-glycosylated FSH21 and fully-glycosylated FSH24 glycoforms in regulating gene expression, isolated secondary follicles were treated with 100 ng/mL glycoforms. At one-hour and 18-20 hour time points, RNA was isolated and reverse-transcribed, and Taqman real-time qPCR assays were performed for these particular genes of interest. We specifically focused on the 100 ng/mL doses because this concentration elicited a differential response in PKA signaling within the follicle as assessed by immunofluorescence (Figure 3 K-N).
Table 1.
Summary of gene expression in ovarian follicles following treatment with FSH glycoforms (100 ng/ml) and comparison to in vivo gene expression results in whole ovaries.
| Gene | One-hour (in vitro) | One-hour (in vivo) 1 | 18-20 hours (in vitro) | |||
|---|---|---|---|---|---|---|
| Up or down regulated relative to control |
Up or down regulated between glycoforms |
Up or down regulated relative to control |
Up or down regulated between glycoforms |
Up or down regulated relative to control |
Up or down regulated between glycoforms |
|
| Apaf1 | 21 | NS | 21, 24 | 21 > 24 | NS | NS |
| Casp3 | 24 | 24 > 21 | 21, 24 | 21 > 24 | 21 | NS |
| Cebpb | 24 | 24 > 21 | NS | NS | NS | 21 > 24 |
| Cyp19a1 | 21, 24 | 24 > 21 | 21, 24 | NS | 21, 24 | NS |
| Ereg | 21 | NS | 21, 24 | NS | 21, 24 | NS |
| Fshr | 24 | NS | 21, 24 | 21 > 24 | 21, 24 | NS |
| Inhbb | 24 | NS | 24 | NS | NS | NS |
| Ki67 | NS | NS | 21, 24 | NS | 24 | NS |
| S100a6 | NS | NS | NS | NS | 21, 24 | NS |
| Tagln | 21 | NS | 21 | NS | 21, 24 | NS |
In vivo data from WANG, H., MAY, J., BUTNEV, V., SHUAI, B., MAY, J. V., BOUSFIELD, G. R. & KUMAR, T. R. 2016. Evaluation of in vivo bioactivities of recombinant hypo- (FSH(21/18)) and fully- (FSH(24)) glycosylated human FSH glycoforms in Fshb null mice. Mol Cell Endocrinol, 437, 224-236.
Bolded text indicates upregulation of gene expression and text not bolded denotes downregulation of gene expression. Data indicated as “NS” was not significant.
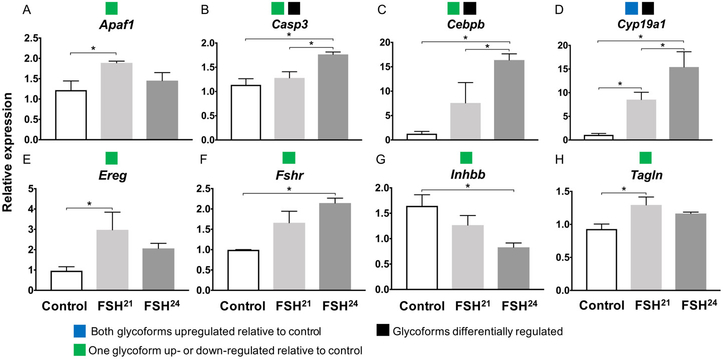
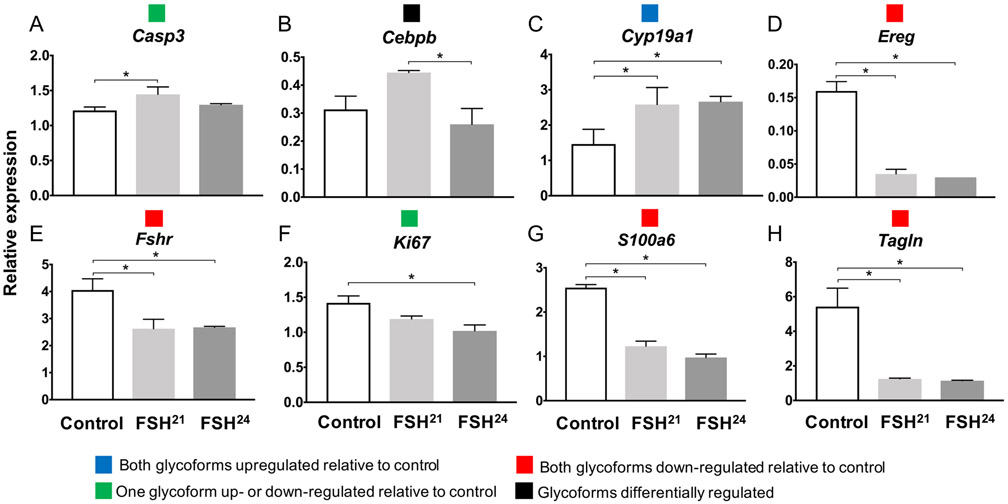
Expression of Esr1, Inha and Kcnj did not differ between untreated controls or between glycoforms at either timepoints (Supplemental Figure 2). At the one-hour time point, seven genes (Apaf1, Casp3, Cebpb, Ereg, Fshr, Inhbb and Tagln) had expression patterns in which one of the glycoforms caused a significant up- or down-regulation relative to control (Figure 4). Only one gene, Cyp19a1, showed upregulation relative to the untreated control when treated with both FSH21 and FSH24 (Figure 4D). Three genes, (Casp3, Cebpb and Cyp19a1) were differentially regulated by the two glycoforms, with FSH24 inducing higher expression compared to FSH21 for all three genes (Figure 4).
Figure 4. One-hour treatment with 100ng/mL FSH glycoforms shows gene expression changes in secondary follicles.
Gene expression was assayed by Taqman real time qPCR assays following one-hour of glycoform treatment. Groups of 100 follicles were analyzed in triplicate. * P<0.05. Colored boxes in legend indicates the four gene expression patterns observed for both one-hour and 18-20-hour exposure time points.
At the 18-20-hour timepoint, four genes (Ereg, Fshr, S100a6 and Tagln) were significantly down-regulated following treatment with both glycoforms relative to control (Figure 5 D, E, G, H). Two genes showed a significant up-regulation (Casp3) (Figure 5A) or down-regulation (Ki67) (Figure 5F) by one of the glycoforms relative to control. Similar to the one-hour response, Cyp19a1 showed upregulation by both FSH21 and FSH24 relative to the untreated control (Figure 5C). Cebpb was also the only gene at the 18-20-hour time point to have differential regulation between glycoforms with FSH21 treatment resulting in higher expression relative to FSH24 (Figure 5B). These data demonstrate that treatment of follicles with 100 ng/mL of FSH glycoforms results in significant up-regulation as well as differential regulation at the one-hour time point compared to the 18-20-hour timepoint.
Figure 5. 18-20-hour treatment with 100ng/mL FSH glycoforms shows a decreased trend in gene expression in secondary follicles.
Gene expression was assayed by Taqman real time qPCR assays following 18-20-hour glycoform treatment. Groups of 100 follicles were analyzed in triplicate. * P<0.05. Colored boxes in legend indicates the four gene expression patterns observed for both one-hour and 18-20-hour exposure time points.
Discussion
We tested the effects of individual purified hypo-glycosylated FSH21 and fully-glycosylated FSH24 glycoforms on murine secondary follicles. Recombinant GH3 cell-derived FSH glycoform preparations were biologically active at the level of the ovarian follicle and directly regulated signaling responses within the follicle. Previously, hypo-glycosylated FSH21/18 and fully-glycosylated FSH24 glycoform preparations were tested in vitro in radio-ligand and radio-receptor assays (Bousfield et al., 2014a, Bousfield et al., 2014b, Butnev et al., 2015) and in immortalized granulosa cell tumor lines (Jiang et al., 2015). In these studies, hypo-glycosylated FSH21/18 was more active than the fully-glycosylated FSH24. Pharmacological rescue studies testing the recombinant GH3 cell-derived FSH glycoform preparations using an immature Fshb null mouse model indicated that these preparations were biologically active in vivo (Wang et al., 2016b) and acted via different downstream signaling pathways. We used the unique features of the isolated ovarian follicle as an organotypic system consisting of the female gamete and the supporting somatic cells to investigate the effects of these glycoforms without contributions from other ovarian somatic tissue within the stroma. Additionally, the responses to FSH seen in this study are innate to the follicle as the mice used were prepubertal (sexually immature) and were not primed prior to follicle isolation. Therefore, no external source of FSH action was introduced into the system, confirming that the follicle is indeed directly responsive to the glycoforms.
Our findings demonstrate that the ovarian follicle responds to FSH glycoforms and activates signaling pathways in a time-dependent fashion, as seen by the increase in PKA substrate phosphorylation following one-hour treatment. These results are consistent across lower (20 ng/mL) and higher (100 ng/mL) concentrations of fully- and hypo-glycosylated FSH glycoforms. The PKA signaling pathway is known to act rapidly and transiently within the ovarian follicle. For example, phosphorylation of CREB, a transcription factor regulated by PKA, can be detected in granulosa cells within one minute of FSH addition (Cottom et al., 2003). Our results at the one-hour timepoint confirm the rapid nature by which the PKA signaling pathway responds to FSH stimulation to induce downstream targets. Although previously published in vivo data have demonstrated responses to FSH glycoforms in whole ovarian extracts at 30 minutes (Wang et al., 2016b), we chose one-hour as our earliest time point to examine due to the technical challenges of manipulating multiple groups of follicles within a shorter time interval. We expect, however, that we would likely also observe effects at shorter time points because seven genes were upregulated in response to either glycoform at the 1 hour timepoint compared to the 18-20-hour timepoint. For example, at the one-hour time point FSH24 increased Fshr mRNA expression relative to control, but at the 18-20 hour time point both FSH21 and FSH24 decreased Fshr mRNA expression relative to control. There are several explanations as to why there is attenuation of PKA substrate phosphorylation and gene expression changes following 18-20-hour treatment with the glycoforms. First, the hormones may not be stable over the course of the longer-term treatment. Second, the FSHR, like most other GPCRs, is known to exhibit desensitization with prolonged exposure to an agonist (Hunzicker-Dunn and Mayo, 2015).
Although increased trends of PKA substrate phosphorylation were observed following FSH glycoform treatment relative to untreated control, only the one-hour time point following glycoform treatment indicated a differential response between hypo- and fully-glycosylated glycoforms. These results support the in vivo studies on Fshb null mice (Wang et al., 2016b) that showed hypo- and fully-glycosylated FSH glycoforms differentially regulate FSH-responsive gene expression within the whole ovary following one-hour of hormone injection. FSH-responsive genes in the ovary were differentially regulated and therefore implicated in distinct cell signaling events (cell cycle, apoptosis and cell adhesion events for FSH21 compared to cell differentiation pathways and transcription factor-mediated events for FSH24) (Wang et al., 2016b). There are several key differences when comparing our gene expression findings to those obtained in vivo from whole ovarian extracts (Wang et al., 2016b) (Table 1). In vivo both FSH21 and FSH24 treatment resulted in upregulation of six genes (Apaf1, Casp3, Cyp19a1, Ereg, Fshr, Ki67). Differential responses between glycoforms coincided in vivo and in vitro only for the Casp3 gene, but FSH21 resulted in upregulation compared to FSH24. This was also seen in Apaf1 and Fshr where differential responses were observed in vivo. Overall, although the glycoforms elicited differential patterns of gene expression in the follicle, the general patterns were different from those observed in vivo. These differences are important because they suggest that the glycoforms activate unique downstream signaling pathways in individual compartments of the ovary (for example, the stroma, theca cells, and granulosa cells) either directly or indirectly. The microenvironment is different between the in vivo and in vitro studies in which follicles were isolated and directly treated with FSH glycoforms. Thus the glycoforms may have indirect effects on cells besides the granulosa cells in vivo. It is important to note that our conclusions are based on analysis of a select signaling pathway and group of FSH-responsive genes. An unbiased large-scale gene expression profiling by RNASeq analysis will be required in the future to further identify the downstream effects of FSH glycoforms on the ovarian follicle.
Although our data clearly indicate that FSH glycoforms are directly active on the murine follicle, isolated ovarian follicles exposed to short term treatments may not accurately reflect the in vivo physiology of a normally cycling ovary. During follicular maturation, a cohort of immature follicles is recruited by FSH but only a subset of these follicles survive to full maturity with most becoming atretic during the cycle (Hunzicker-Dunn and Maizels, 2006, Hunzicker-Dunn and Mayo, 2015). The follicles that were selected for these experiments were morphologically healthy, but their quality as it would be in vivo is unknown. In vivo studies also showed that estradiol production is increased within ovaries from Fshb null mice injected with hypo-glycosylated FSH21/18 (Wang et al., 2016b). Although we measured steroid hormone production of follicles following FSH glycoform treatment, the levels were too low to detect in the culture media via standard ELISA techniques likely due to the small number of isolated follicles combined with the short nature of our cultures. We also do not know whether treatment using these glycoforms have downstream effects on follicle growth dynamics and oogenesis outcomes. In vitro follicle growth studies testing the efficacy of these glycoforms on follicle growth, maturation and survival in addition to investigating oocyte outcomes will, therefore, be important in validating the current results (Kreeger et al., 2006, Xu et al., 2006). However, the amount of hormone required to sustain these long-term cultures is substantial and beyond the capacity of the volumes used in the present study.
The hypo-glycosylated human FSH variant and its changing abundance with age is well documented (Bousfield et al., 2014a, Bousfield et al., 2014b). Younger women undergoing ovulatory cycles require rapid hormone action and clearance rates (Wide and Eriksson, 2013) and FSH21 (more abundant in younger women) has been shown to possess these characteristics (Bousfield et al., 2014a, Jiang et al., 2015, Butnev et al., 2015). Because the abundance of FSH24 increases with aging when ovarian responses are diminished and based on in vitro biochemical data which demonstrated that FSH24 exhibits less affinity for FSHRs in terms of binding and other kinetic parameters (Bousfield et al., 2014a, Jiang et al., 2015, Butnev et al., 2015), it has been speculated that FSH24 is ineffective in maintaining ovarian function in old age. However, changes in FSHR number or FSH ligand-receptor recycling dynamics cannot be ruled out as other potential reasons for defective follicle maturation and oocyte development in old age. This issue has been long debated and newer technological advances in reliably measuring FSHRs will hopefully resolve this issue in the future. Defective follicle maturation and oocyte development could possibly be associated with the loss of hypo-glycosylated FSH glycoforms due to aging, manifesting in reduced ovarian function (Wang et al., 2016b, Jiang et al., 2015, Bousfield et al., 2014a). This does not exclude the ability of FSH24 to elicit responses when directly injected or added to follicle cultures. Our published in vivo data (Wang et al., 2016b) and the present vitro studies indicate that FSH24 and FSH21 have distinct gene expression signatures. Because these glycoforms elicit distinct pathways both in in vitro and in vivo models, it is possible that these glycoforms or the ratio of glycoforms will have significant physiological effects, and this warrants further investigation. Additional studies including longer cultures and use of human tissue samples to analyze folliculogenesis and oogenesis outcomes specifically will be required to draw decisive conclusions regarding clinical implications of these glycoforms. Additionally, this study was conducted only on follicles from young, prepubertal mice. Further analysis of these glycoforms on follicles from older mice would provide necessary insights into FSH glycoform responsiveness on ovarian follicles with age.
In conclusion, our studies show that FSH glycoform treatment for one-hour elicits bioactivity within the follicle as measured by an induction of PKA signaling and modulating gene expression. Future studies investigating the effects of these glycoforms in human ovarian follicles will be of considerable interest in understanding clinical benefits of the hormone.
Supplementary Material
Supplemental Figure 1. Permeabilization by proteinase K treatment of formalin-fixed follicles allows antibody binding. Fixed follicles incubated with 20μg/mL Proteinase K at 37°C showed greater localization of PKA substrates (B) in comparison to untreated control follicles (A). (C) Non-immune IgG control shows that PKA substrate expression was specific. Insets show rhodamine phalloidin (red) and DAPI (blue). N=5 follicles per condition group. Scale bar = 50μm.
Supplemental Figure 2. Expression patterns of genes that did not show a response to glycoform treatment. Gene expression was assayed by Taqman real time qPCR assays following one-hour and 18-20-hour glycoform treatment. Groups of 100 follicles were analyzed in triplicate.
Acknowledgements
We acknowledge Dr. Richard Schultz (Department of Biology, University of Pennsylvania) for his generous gift of the MSY2 primary antibody and Dr. Irving Boime (Washington University School of Medicine) for a gift of recombinant human FSH. We also acknowledge Dr. Teresa K. Woodruff and Sarah Wagner (Northwestern University) for resource support and Dr. Kelly Mayo and Dr. Mary Ellen Pavone (Northwestern University) for their insightful discussions that have helped to advance this work.
Funding information
This work was supported in part by Northwestern University startup funds from the Department of Obstetrics and Gynecology (FED) and the Center for Reproductive Health After Disease (P50 HD076188) from the National Centers for Translational Research in Reproduction and Infertility, the Master of Science in Reproductive Science and Medicine Program, the Makowski Endowment Funds at the University of Colorado Anschutz Medical Campus and NIH funds (AG056046 to TRK and AG029531 to GRB and TRK).
List of abbreviations
- ART
Assisted reproductive technology
- cAMP
Cyclic adenosine 5′-monophosphate
- CREB
Cyclic AMP-responsive element binding protein
- FSH
Follicle stimulating hormone
- FSHR
FSH receptor
- GPCR
G-protein coupled receptor
- MAPK
Mitogen-activated protein kinases
- PKA
Protein kinase-A
- PVDF
Polyvinylidine difluoride
- rec. hFSH
Recombinant human FSH
Footnotes
Declaration of Interest
The authors have nothing to disclose and no conflict of interest.
References
- ANOBILE CJ, TALBOT JA, MCCANN SJ, PADMANABHAN V & ROBERTSON WR 1998. Glycoform composition of serum gonadotrophins through the normal menstrual cycle and in the post-menopausal state. Molecular Human Reproduction, 4, 631–639. [DOI] [PubMed] [Google Scholar]
- BAENZIGER JU & GREEN ED 1988. Pituitary glycoprotein hormone oligosaccharides-Structure, synthesis and function of the asparagine-linked oligosaccharides on lutropin, follitropin and thyrotropin. Biochimica et Biophysica Acta (BBA), 947, 287–306. [DOI] [PubMed] [Google Scholar]
- BANERJEE S, BANERJEE S, SARASWAT G, BANDYOPADHYAY SA & KABIR SN 2014. Female reproductive aging is master-planned at the level of ovary. PLoS One, 9, e96210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUSFIELD GR, BUTNEV VY, HIROMASA Y, HARVEY DJ & MAY JV 2014a. Hypo-glycosylated human follicle-stimulating hormone (hFSH(21/18)) is much more active in vitro than fully-glycosylated hFSH (hFSH(24)). Mol Cell Endocrinol, 382, 989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUSFIELD GR, BUTNEV VY, RUEDA-SANTOS MA, BROWN A, HALL AS & HARVEY DJ 2014b. Macro- and Micro-heterogeneity in Pituitary and Urinary Follicle-Stimulating Hormone Glycosylation. J Glycomics Lipidomics, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUSFIELD GR, BUTNEV VY, WALTON WJ, NGUYEN VT, HUNEIDI J, SINGH V, KOLLI VSK, HARVEY DJ & RANCE NE 2007. All-or-none N-glycosylation in primate follicle-stimulating hormone β-subunits. Molecular and Cellular Endocrinology, 260-262, 40–48. [DOI] [PubMed] [Google Scholar]
- BOUSFIELD GR, BUTNEV VY, WHITE WK, HALL AS & HARVEY DJ 2015. Comparison of Follicle-Stimulating Hormone Glycosylation Microheterogenity by Quantitative Negative Mode Nano-Electrospray Mass Spectrometry of Peptide-N Glycanase-Released Oligosaccharides. J Glycomics Lipidomics, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROEKMANS FJ, KNAUFF EA, TE VELDE ER, MACKLON NS & FAUSER BC 2007. Female reproductive ageing: current knowledge and future trends. Trends Endocrinology and Metabolism, 18, 58–65. [DOI] [PubMed] [Google Scholar]
- BROEKMANS FJ, SOULES MR & FAUSER BC 2009. Ovarian aging: mechanisms and clinical consequences. Endocr Rev, 30, 465–93. [DOI] [PubMed] [Google Scholar]
- BUTNEV VY, BUTNEV VY, MAY JV, SHUAI B, TRAN P, WHITE WK, BROWN A, SMALTER HALL A, HARVEY DJ & BOUSFIELD GR 2015. Production, purification, and characterization of recombinant hFSH glycoforms for functional studies. Mol Cell Endocrinol, 405, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTTOM J, SALVADOR LM, MAIZELS ET, REIERSTAD S, PARK Y, CARR DW, DAVARE MA, HELL JW, PALMER SS, DENT P, et al. 2003. Follicle-stimulating Hormone Activates Extracellular Signalregulated Kinase but Not Extracellular Signal-regulated Kinase Kinase through a 100-kDa Phosphotyrosine Phosphatase. J Biol Chem, 278, 7167–7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREUS S, PELLIZZARI E, CIGORRAGA SB & CAMPO S 1996. FSH isoforms: bio and immuno‐activities in post‐menopausal and normal menstruating women. Clinical Endocrinology, 44, 181–189. [DOI] [PubMed] [Google Scholar]
- DALPATHADO DS, IRUNGU J, GO EP, BUTNEV VY, NORTON K, BOUSFIELD GR & DESAIRE H 2006. Comparative Glycomics of the Glycoprotein Follicle Stimulating Hormone- Glycopeptide Analysis of Isolates from Two Mammalian Species. Biochemistry, 45, 8665–8673. [DOI] [PubMed] [Google Scholar]
- DAS N & KUMAR TR 2018. Molecular regulation of follicle-stimulating hormone synthesis, secretion and action. J Mol Endocrinol, 60, R131–R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS JS, KUMAR TR, MAY JV & BOUSFIELD GR 2014. Naturally Occurring Follicle-Stimulating Hormone Glycosylation Variants. J Glycomics Lipidomics, 4, e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN ED, BOIME I & BAENZIGER JU 1986. Differential processing of Asn-linked oligosaccharides on pituitary glycoprotein hormones: implications for biologic function. Molecular and Cellular Biochemistry, 72, 81–100. [DOI] [PubMed] [Google Scholar]
- HUNZICKER-DUNN M & MAIZELS ET 2006. FSH signaling pathways in immature granulosa cells that regulate target gene expression- Branching out from protein kinase A. Cell Signaling, 18, 1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNZICKER-DUNN M & MAYO K 2015. Gonadotropin Signaling in the Ovary. Knobil and Neill’s Physiology of Reproduction. [Google Scholar]
- JIANG C, HOU X, WANG C, MAY JV, BUTNEV VY, BOUSFIELD GR & DAVIS JS 2015. Hypoglycosylated hFSH Has Greater Bioactivity Than Fully Glycosylated Recombinant hFSH in Human Granulosa Cells. J Clin Endocrinol Metab, 100, E852–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN NA, ILLINGWORTH PJ, GROOME NP, MCNEILLY AS, BATTAGLIA DE & SOULES MR 1996. Decreased inhibin B secretion is associated with the monotropic FSH rise in older, ovulatory women- a study of serum and follicular fluid levels of dimeric inhibin A and B in spontaneous menstrual cycles. Journal of Clinical Endocrinology and Metabolism, 81, 2742–2745. [DOI] [PubMed] [Google Scholar]
- KREEGER PK, DECK JW, WOODRUFF TK & SHEA LD 2006. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials, 27, 714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORETI N, AMBAO V, JULIATO CT, MACHADO C, BAHAMONDES L & CAMPO S 2009. Carbohydrate complexity and proportion of serum FSH isoforms reflect pituitary-ovarian activity in perimenopausal women and depot medroxyprogesterone acetate users. Clin Endocrinol (Oxf), 71, 558–65. [DOI] [PubMed] [Google Scholar]
- MCTAVISH KJ, JIMENEZ M, WALTERS KA, SPALIVIERO J, GROOME NP, THEMMEN AP, VISSER JA, HANDELSMAN DJ & ALLAN CM 2007. Rising follicle-stimulating hormone levels with age accelerate female reproductive failure. Endocrinology, 148, 4432–9. [DOI] [PubMed] [Google Scholar]
- PADMANABHAN V, LEE JS & BEITINS IZ 1999. Follicle-stimulating isohormones: regulation and biological significance. Journal of Reproduction and Fertility Supplement, 54, 87–99. [PubMed] [Google Scholar]
- PIERCE JG & PARSONS TF 1981. Glycoprotein hormones: Structure and function. Annual Review of Biochemistry, 50, 465–495. [DOI] [PubMed] [Google Scholar]
- SVISTUNOVA DM, MUSINOVA YR, POLYAKOV VY & SHEVAL EV 2012. A simple method for the immunocytochemical detection of proteins inside nuclear structures that are inaccessible to specific antibodies. J Histochem Cytochem, 60, 152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG H, GRAHAM I, HASTINGS R, GUNEWARDENA S, BRINKMEIER ML, CONN PM, CAMPER SA & KUMAR TR 2015. Gonadotrope-specific deletion of Dicer results in severely suppressed gonadotropins and fertility defects. J Biol Chem, 290, 2699–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG H, HASTINGS R, MILLER WL & KUMAR TR 2016a. Fshb-iCre mice are efficient and specific Cre deleters for the gonadotrope lineage. Mol Cell Endocrinol, 419, 124–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG H, LARSON M, JABLONKA-SHARIFF A, PEARL CA, MILLER WL, CONN PM, BOIME I & KUMAR TR 2014. Redirecting intracellular trafficking and the secretion pattern of FSH dramatically enhances ovarian function in mice. Proc Natl Acad Sci U S A, 111, 5735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG H, MAY J, BUTNEV V, SHUAI B, MAY JV, BOUSFIELD GR & KUMAR TR 2016b. Evaluation of in vivo bioactivities of recombinant hypo- (FSH(21/18)) and fully- (FSH(24)) glycosylated human FSH glycoforms in Fshb null mice. Mol Cell Endocrinol, 437, 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIDE L & ERIKSSON K 2013. Dynamic changes in glycosylation and glycan composition of serum FSH and LH during natural ovarian stimulation. Ups J Med Sci, 118, 153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU M, WEST E, SHEA LD & WOODRUFF TK 2006. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod, 75, 916–23. [DOI] [PubMed] [Google Scholar]
- XU Y, DUNCAN FE, XU M & WOODRUFF TK 2016. Use of an organotypic mammalian in vitro follicle growth (IVFG) assay to facilitate female reproductive toxicity screening. Reproduction, Fertility and Development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YU J, HECHT NB & SCHULTZ RM 2001. Expression of MSY2 in Mouse Oocytes and Preimplantation Embryos. Biology of Reproduction, 65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Permeabilization by proteinase K treatment of formalin-fixed follicles allows antibody binding. Fixed follicles incubated with 20μg/mL Proteinase K at 37°C showed greater localization of PKA substrates (B) in comparison to untreated control follicles (A). (C) Non-immune IgG control shows that PKA substrate expression was specific. Insets show rhodamine phalloidin (red) and DAPI (blue). N=5 follicles per condition group. Scale bar = 50μm.
Supplemental Figure 2. Expression patterns of genes that did not show a response to glycoform treatment. Gene expression was assayed by Taqman real time qPCR assays following one-hour and 18-20-hour glycoform treatment. Groups of 100 follicles were analyzed in triplicate.