Keywords: astrocyte, chronic pain, cognitive impairment, exendin-4, hippocampal dentate gyrus, microglia, Morris water maze, neuroinflammation, spinal nerve ligation
Abstract
Glucagon-like peptide-1 receptor has anti-apoptotic, anti-inflammatory, and neuroprotective effects. It is now recognized that the occurrence and development of chronic pain are strongly associated with anti-inflammatory responses; however, it is not clear whether glucagon-like peptide-1 receptor regulates chronic pain via anti-inflammatory mechanisms. We explored the effects of glucagon-like peptide-1 receptor on nociception, cognition, and neuroinflammation in chronic pain. A rat model of chronic pain was established using left L5 spinal nerve ligation. The glucagon-like peptide-1 receptor agonist exendin-4 was intrathecally injected into rats from 10 to 21 days after spinal nerve ligation. Electrophysiological examinations showed that, after treatment with exendin-4, paw withdrawal frequency of the left limb was significantly reduced, and pain was relieved. In addition, in the Morris water maze test, escape latency increased and the time to reach the platform decreased following exendin-4 treatment. Immunohistochemical staining and western blot assays revealed an increase in the numbers of activated microglia and astrocytes in the dentate gyrus of rat hippocampus, as well as an increase in the expression of tumor necrosis factor alpha, interleukin 1 beta, and interleukin 6. All of these effects could be reversed by exendin-4 treatment. These findings suggest that exendin-4 can alleviate pain-induced neuroinflammatory responses and promote the recovery of cognitive function via the glucagon-like peptide-1 receptor pathway. All experimental procedures and protocols were approved by the Experimental Animal Ethics Committee of Renmin Hospital of Wuhan University of China (approval No. WDRM 20171214) on September 22, 2017.
Chinese Library Classification No. R453; R363; R364
Introduction
Chronic pain is common and can cause an unpleasant sensory and emotional experience (Chen et al., 2019; Li et al., 2019). Patients with chronic pain may have emotional symptoms, such as anhedonia, anxiety, cognitive impairments, and memory deterioration, which in turn can affect individual health and reduce quality of life. Although anatomical (Davies et al., 2006), neurochemical (Doyle et al., 2013), and psychological causes (Byrne et al., 2017) have been identified as the basis of cognitive impairment, the potential mechanisms underlying cognitive impairment in chronic pain remain unclear.
Increasing evidence suggests that the etiology of chronic pain is associated with changes in synaptic plasticity (Geis et al., 2017), decreased neurogenesis (Pawela et al., 2017), and the reduced production of neurotrophic factors (Liu et al., 2017) in both the central and peripheral nervous systems. Glucagon-like peptide-1 (GLP-1) is a hormone that is released from the pancreas after eating, and plays a critical role in maintaining glucose homeostasis. Beyond the pancreas, GLP-1 can also be produced in peripheral tissues such as the lungs, stomach, intestine, kidney, and heart, and can also be produced in the brain, including in the cerebral cortex, hippocampus, amygdala, paraventricular nucleus, and arcuate nucleus. GLP-1 has been confirmed as a neurotrophic factor and is known to participate in the regulation of cognitive and neurological functions (Muscogiuri et al., 2017). Treatment with GLP-1 receptor (GLP-1R) agonists can reduce neuronal damage, ischemia-induced hyperactivity, and cerebral infarct volume in animal models of cerebral ischemia (Gullo et al., 2017; Muscogiuri et al., 2017). Nevertheless, both the role of GLP-1R in long-term chronic pain and its mechanism of regulation remain unclear.
Exendin-4 is a diabetes treatment drug that binds and activates GLP-1R. Recent studies have reported that exendin-4 can traverse the blood-brain barrier and reduce brain inflammatory responses by inhibiting glial cell recruitment and activation (Lau et al., 2010; Kim et al., 2017). The current study investigated the role of the GLP-1R agonist exendin-4 on cognitive function in a rat model of chronic pain, and studied the potential mechanism behind its regulatory pathway.
Materials and Methods
Animals
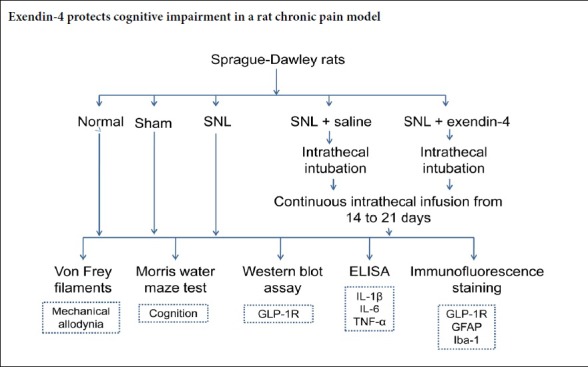
Animal experimental protocols and ethics guidelines were approved by the Animal Use and Care Committee for Research and Education of Wuhan University, China. All protocols were approved by the Experimental Animal Ethics Committee of Renmin Hospital of Wuhan University of China (approval No. WDRM 20171214) on September 22, 2017. Sixty male Sprague-Dawley rats weighing 220–250 g and aged 8–12 weeks were housed in standard conditions in the Hubei Provincial Laboratory Animal Center (SCXK (E) 2015-0018). The experimental procedures followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
All rats were randomly divided into the normal group (n = 12), sham group (n = 12; exposing the left L5 spinal nerve), spinal nerve ligation (SNL) group (left L5 SNL; n = 12), SNL + saline group (left L5 SNL and intrathecal injection of saline; n = 12), or SNL + exendin-4 group (left L5 SNL and intrathecal injection of exendin-4; n = 12).
Exposure
Intrathecal intubation was performed according to a previous protocol (Poon et al., 2005). The rat SNL model was anesthetized using pentobarbital sodium (45 mg/kg, intraperitoneally; NanJing SunShine Biotechnology Co., Nanjing, China). A PE-10 catheter (ID 0.28 mm and OD 0.61 mm) passed caudally from the T8 to the L3 level of the spinal cord of rats. An almost 2 cm length of the free end of the catheter was left at the level of the lumbar enlargement segments, and the other end was subcutaneously placed on the rat’s neck. SNL was then performed after three days of intrathecal intubation. For this procedure, in rats under pentobarbital sodium anesthesia (40–50 mg/kg, intraperitoneally), the left L5 transverse process was dissected to expose the L5 spinal nerves. A 3-0 silk thread was used to ligate the L5 spinal nerve. In the sham group, the surgical procedure was identical, but the spinal nerves were not ligated. A gradually decreased paw withdrawal frequency in the SNL rats from day 1 was used to judge the successful establishment of models.
For exendin-4 treatment, exendin-4 (10 µg/kg; Cayman Chemical, Ann Arbor, MI, USA) was dissolved in 0.9% saline. Exendin-4 or 0.9% saline was then continuously infused intrathecally into the rats using osmotic pumps from 10 to 14 days after SNL (n = 12 per group).
Mechanical allodynia test
The mechanical allodynia test was performed at –1, 1, 3, 7, 10, 14, and 21 days after SNL. Rats were put into inverted plastic boxes, with a volume of 11 cm × 13 cm × 24 cm, on an elevated mesh floor for 30 minutes. Von Frey filaments (Stoelting Co., Wood Dale, IL, USA) were used to test mechanical allodynia in a blinded manner. Stimulus intensity from small to large, each intensity repeatedly stimulate 10 times. When the intensity of the reflex occurs for more than 5 times, the rat is considered to have a response to the mechanical stimulus, and is denoted as the threshold of the reflex. Do this three times and take the average. If the maximum intensity stimulus still does not produce the foot contraction reflex, the value is denoted as 26 g.
Morris water maze test
The Morris water maze was conducted at –1, 7, and 21 days after SNL. The Morris water maze consists of a circular pool with a 180 cm diameter and a height of 60 cm. The pool, filled with opaque water at 22 ± 1°C, contained four equivalent quadrants, visible external cues, and an escape platform (10 cm diameter) submerged 2 cm underwater. On day 0, the rats were placed individually into the pool facing the wall four times and trained to locate and land on the platform (1 minute at each time, in different quadrants) to familiarize them with the pool. At 7 and 21 days, the rats were individually placed into the pool again at different starting points, but not in the target quadrant (containing the hidden platform). Escape latency, swimming speed, and time spent in the target quadrant were collected and analyzed.
Hippocampal tissue homogenate collection
At 21 days after SNL, rats were sacrificed by decapitation under anesthesia. For each rat, the brain was removed and immersed in ice-cold (0.9% w/v) isotonic saline. The right hippocampus was carefully removed and collected (n = 6 per group). Hippocampal tissue homogenate was used for western blot assays and enzyme-linked immunosorbent assays.
Western blot assay
Ice-cold 0.1 M phosphate buffer (pH 7.4) was used to homogenize the right hippocampus. The hippocampus was centrifuged at 16,100 × g for 15 minutes at 4°C. The supernatant was collected for protein detection. Protein concentrations were measured by bicinchoninic acid assay. After concentration measurement and electrophoresis, the protein was electroblotted onto a nitrocellulose membrane. The membrane was incubated overnight at 4°C with the following primary antibodies: mouse anti-GAPDH (1:2000; Santa-Cruz Biotechnology, Santa Cruz, CA, USA) and mouse anti-GLP-1R (1:200; Abcam, Cambridge, UK). The proteins were detected using horseradish peroxidase-conjugated anti-rabbit (1:5000; Cell Signaling Technology) or or anti-mouse (1:5000; Cell Signaling Technology) secondary antibodies at room temperature and visualized using chemiluminescence reagents with the enhanced chemiluminescence kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA). After exposure to film, the grayscale of blots represented the relative protein levels of samples, and these were normalized to the normal group.
Enzyme-linked immunosorbent assay
The dentate gyrus of the right hemisphere was collected and homogenized using RIPA lysis buffer. The supernatant was harvested for protein detection (n = 6 per group). Enzyme-linked immunosorbent assay (ELISA) kits (Prozyme Neobiosciences, Guangdong, China) were used for the detection of IL-1β, IL 6, and TNF-α levels, with a low limit of detection at < 4 pg/mL. Measurements were performed in accordance with kits’ protocols.
Immunofluorescence staining
The right hemisphere of the brain was collected and fixed in 4% paraformaldehyde for 4–6 hours. Samples were then dehydrated in 0.1 M phosphate buffer containing 30% sucrose, and 30 µm sections were cut on a cryostat. These sections were used to measure GLP-1R expression using the following primary antibodies overnight at 4°C: rabbit anti-ionized calcium binding adapter molecule 1 (Iba-1), mouse anti-GLP-1R (1:1000; Cell Signaling Technology), rabbit anti-glial fibrillary acidic protein (GFAP; 1:100; Abcam), and mouse anti-Iba-1 (1:100; Abcam). The sections were then incubated for 2 hours at room temperature with the corresponding secondary antibodies: Alexa 594-conjugated donkey anti-rabbit IgG (1:800; Molecular Probes, Rockford, IL, USA) and fluorescein isothiocyanate-conjugated horse anti-mouse IgG (1:200; Vector, Burlingame, CA, USA). Images were captured with a confocal laser-scanning microscope (FV1000; Olympus, Tokyo, Japan). Relative optical value changes were analyzed using Image-Pro Plus software 6.0 software (Media Cybernetics Corporation, USA), and expressed as percentages.
Statistical analysis
Data were analyzed using SPSS 20.0 software (IBM, Armonk, NY, USA). Results are presented as the mean ± SEM. Data from behavioral and biochemical parameters were subjected to one-way analysis of variance followed by Bonferroni post hoc tests. Values of P < 0.05 were considered statistically significant.
Results
L5 spinal ligation induces mechanical allodynia in rats
Preliminary experiments showed that SNL induced significant mechanical allodynia and hyperalgesia. Behavioral tests revealed that paw withdrawal frequency in the SNL group gradually decreased from days 1 to 21 compared with the normal and sham groups (P < 0.05). With exendin-4 treatment from days 10 to 14, however, paw withdrawal frequency was significantly increased at 14 and 21 days (P < 0.05; Figure 1), indicating that exendin-4 suppresses neuropathic pain behavior.
Figure 1.
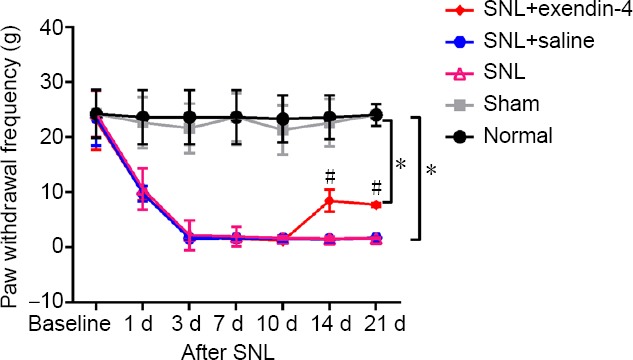
Intrathecal infusion of exendin-4 increases SNL-induced paw withdrawal frequency detected by the Von Frey Filaments.
Baseline values of paw withdrawal frequency were obtained 1 week before SNL. Intrathecal intubation was performed and exendin-4 was infused from days 10 to 14 after SNL. To test mechanical allodynia, paw withdrawal frequency was recorded. *P < 0.05, vs. normal group; #P < 0.05, vs. SNL group (mean ± SEM, n = 12; one-way analysis of variance followed by Bonferroni post hoc test). SNL: Spinal nerve ligation.
Spatial learning and memory are impaired in an SNL-induced rat model of chronic pain
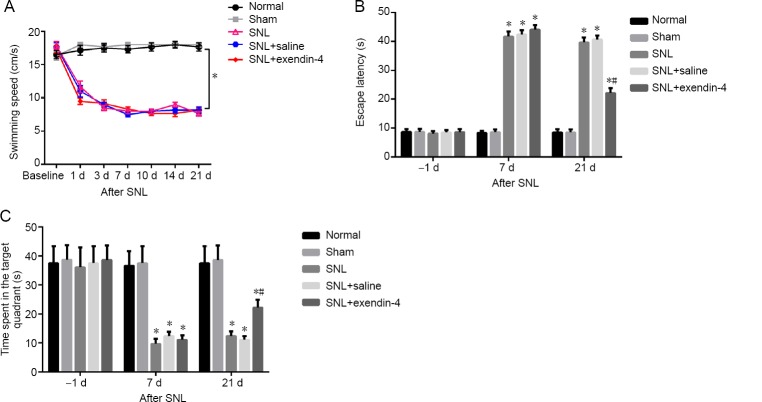
We next investigated the role of exendin-4 in cognitive impairment. Swimming speeds were significantly decreased in the SNL, SNL + saline, and SNL + exendin-4 groups compared with the normal and sham groups (P < 0.05; Figure 2A), indicating that SNL affects hindlimb athletic ability. Next, the Morris water maze test was used to test for cognitive deficits. The latency to reach the platform in the SNL group was significantly increased compared with the normal and sham groups (P < 0.05). However, at 21 days after exendin-4 treatment, this latency was decreased compared with the SNL group (P < 0.05; Figure 2B). Compared with the normal and sham groups, the time spent in the target quadrant was significantly lower in the SNL group. In addition, the time spent in the target quadrant in the SNL + exendin-4 group was significantly higher than that of the SNL group (P < 0.05; Figure 2C).
Figure 2.
Effects of exendin-4 on spatial learning and memory in a rat model of SNL-induced chronic pain in the Morris water maze test.
(A–C) Exendin-4 treatment decreased swimming speed (A), increased escape latency (B), and decreased time spent in the target quadrant (C) in a rat model of SNL-induced chronic pain. *P < 0.05, vs. normal group; #P < 0.05, vs. SNL group (mean ± SEM, n = 12; one-way analysis of variance followed by Bonferroni post hoc test). SNL: Spinal nerve ligation.
GLP-1R is downregulated in the hippocampal dentate gyrus in an SNL-induced rat model of chronic pain
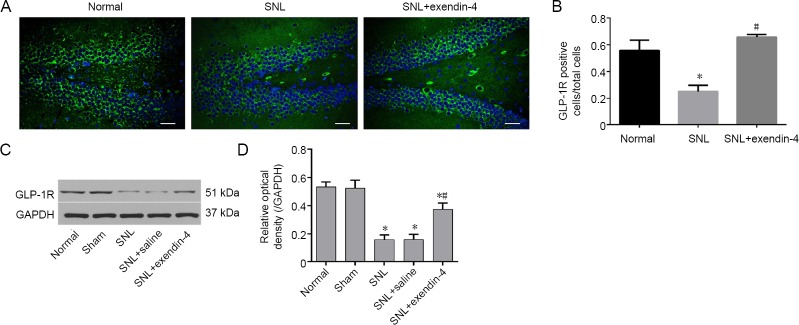
Immunofluorescence staining revealed that the numbers of GLP-1R-immunoreactive cells in the hippocampus were significantly lower in the SNL group than in the normal group. However, the numbers of GLP-1R-immunoreactive cells were increased after exendin-4 treatment (P < 0.05; Figure 3A and B). Western blot assay results indicated that GLP-1R protein expression was downregulated in the SNL group, but that treatment with exendin-4 increased GLP-1R expression (P < 0.05; Figure 3C and D). These data indicate that intrathecal exendin-4 infusion promotes GLP-1R expression in the dentate gyrus in chronic pain.
Figure 3.
Effects of exendin-4 on GLP-1R expression in the hippocampal dentate gyrus.
Exendin-4 attenuated GLP-1R decrease after nerve injury. (A–D) Expressions are shown by immunofluorescence staining (A, B) and western blot assay (C, D). Western blots are expressed as percentages and fold changes compared with GAPDH (D). (A) Green: GLP-1R; blue: DAPI. Scale bars: 200 µm. *P < 0.05, vs. normal group; #P < 0.05, vs. SNL group (mean ± SEM, n = 6; one-way analysis of variance followed by Bonferroni post hoc test). GLP-1R: Glucagon-like peptide-1 receptor; SNL: spinal nerve ligation.
Neuroinflammation is upregulated in the hippocampal dentate gyrus in an SNL-induced rat model of chronic pain
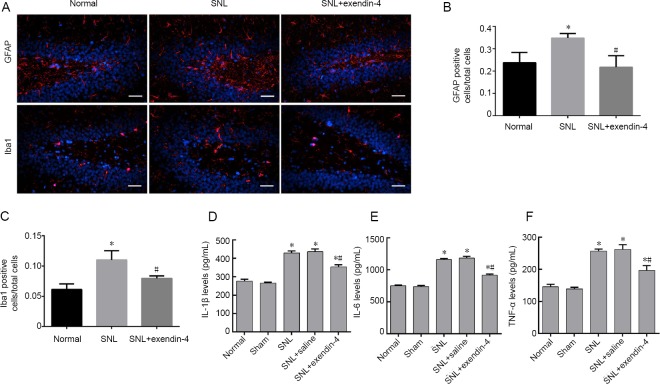
We next investigated the inflammatory response in the dentate gyrus. GFAP staining was used to detect astrocyte activation, while Iba-1 staining was used to detect microglial activation (Figure 4A). Immunofluorescence results revealed that the numbers of GFAP- and Iba-1-immunoreactive cells in the dentate gyrus were significantly higher in the SNL group than in the normal group. However, exendin-4 treatment resulted in a reduction in GFAP- and Iba-1-immunoreactive cells in the dentate gyrus (P < 0.05; Figure 4B and C). ELISA results showed that SNL caused remarkable elevation of proinflammatory cytokines, including TNF-α, IL-1β, and IL-6 levels. After the treatment with exendin-4, the levels of these cytokines decreased (P < 0.05; Figure 4D–F). These data suggest that intrathecal infusion of exendin-4 decreases neuroinflammation in the dentate gyrus in chronic pain.
Figure 4.
Effects of intrathecal exendin-4 injection on glial activation and inflammation in the hippocampal dentate gyrus.
Exendin-4 attenuated neuroinflammation after nerve injury in the hippocampal dentate gyrus. (A) Astrocytes (red: GFAP+, blue: DAPI+) and microglia (red: Iba-1+, blue: DAPI+) are shown by fluorescence immunohistochemistry. Scale bars: 200 µm. (B, C) Ratios of GAFP and Iba1 positive cells. (D–F) IL-1β, IL-6, and TNF-α levels were detected using an enzyme-linked immunosorbent assay (mean ± SEM, n = 6; one-way analysis of variance followed by Bonferroni post hoc test). *P < 0.05, vs. normal group; #P < 0.05, vs. SNL group. DAPI: 4′,6-Diamidino-2-phenylindole; IL: interleukin; SNL: spinal nerve ligation; TNF: tumor necrosis factor.
Discussion
Chronic pain may originate in the body, brain, or spinal cord. Research is needed to explore the potential mechanisms of chronic pain and to develop a treatment or cure. This study revealed that SNL in rats induced a substantial cognitive impairment, as well as the activation of glial cells and microglia, the production of inflammatory cytokines, and a decrease in GLP-1R expression. Treatment with exendin-4 noticeably attenuated pain-induced cognitive impairment and reduced neuroinflammation in the hippocampus.
Various factors including trauma and infection can cause nerve injury and inflammation, stimulate ectopic discharge, promote neurotransmitter release, and generate the perception of pain. Pain is an important clinical sign of inflammation (Guglielmi and Sbraccia, 2017). The SNL has been widely used as an experimental model of both neuropathic and chronic pain. From our data, the pain threshold in the SNL group was decreased from 1 to 14 days; the most prominent hyperalgesia was on day 7, consistent with a previous study (Calsolaro and Edison, 2015). Chronic pain can also induce cognitive deficits (Bushnell et al., 2013). In the present study, platform crossing and target quadrant occupancy were decreased in the SNL group, as detected by the Morris water maze test.
GLP-1, known for its role in the “incretin effect” with proven benefits in terms of improving glacaemic control, has confirmed neuroprotective and anti-inflammatory effects (Lee and Jun, 2016). GLP-1R is widely expressed in the cerebrum, including in the cerebral cortex, substantia nigra, and hippocampus (Li et al., 2009). Previous studies have suggested that GLP-1R plays multiple roles, including in the maintenance of glucose homeostasis and the reduction of oxidative stress, as well as having anti-inflammatory and neuroprotective effects (Hu and Mao, 2016; Kim et al., 2017; Thangavel et al., 2017). Activation of GLP-1R, either by ligands or agonists, can have a neuroprotective effect in Parkinson’s disease and Alzheimer’s disease in rat model (Jia et al., 2015; Liu et al., 2017; L’Episcopo et al., 2018). Exendin-4 is known to affect allodynia after SNL (Cao et al., 2016). In the present study, GLP-1 expression was downregulated in the SNL group, while treatment with its stimulant exendin-4 promoted its expression. Exendin-4 also had a neuroprotective effect, as reflected by behavioral tests and the Morris water maze test.
Chronic pain or nerve injury can induce nociceptor neurons to release inflammatory cytokines into the spinal cord via their central nerve terminals, including neurotransmitters such as calcitonin-gene related peptide, cytokines such as CCL2, CX3CL1, and TNF-α, growth factors such as colony-stimulating factor-1, ATP, and enzymes. Glial and microglial activation is another marker of inflammation, with an important role in the inflammatory response of the central nervous system in conditions including Parkinson’s disease and Alzheimer’s disease (Sharma and Taliyan, 2016; Wang et al., 2016; McKenzie and Klegeris, 2018). The mediators released from nociceptor neurons can activate microglia, which then produce IL-1β, TNF-α, and prostaglandin E2. The activated microglia also produce neurotrophins, such as brain-derived neurotrophic factor, that sensitize second-order pain-mediating interneurons and primary nociceptor neurons (Mangmool et al., 2015). Microglia can also activate inflammatory mediator cells, such as oligodendrocytes and resident astrocytes (Tambuyzer et al., 2009). In the current study, SNL induced microglial and astrocyte activation in the hippocampal dentate gyrus and produced an inflammatory response.
GLP-1R has an anti-inflammatory function and neuroprotective effects (Gao and Ji, 2010). A previous study confirmed that GLP-1R agonists inhibit the activation of glial cells in patients with diabetes (Reeta et al., 2017). In this study, the numbers of activated astrocytes and microglia in the SNL + exendin-4 group were lower than in the SNL group. Furthermore, exendin-4 treatment significantly mitigated the hippocampal levels of inflammatory cytokines compared with the SNL group. These results were consistent with previous studies that reported that exendin-4 reduced expression levels of cytokines such as IFN-γ, TNF-α, IL-1β, and IL-6, and increased nerve growth factor (Pinho-Ribeiro et al., 2017; Thangavel et al., 2017). Neuroinflammatory changes are key pathological components of cognitive diseases (Wang et al., 2015). A dysregulation of incretin balance, especially insulin signaling, is considered to be an essential contributor to many cognitive diseases (Kim et al., 2017). Results from the present study revealed that GLP-1R activation had effects on cognitive protection in a pain-induced cognitive impairment model. These results suggest a potential role of GLP-1R in neuronal inflammatory responses.
However, we recognize that the present study has a number of limitations and unsolved questions. First, the homology between exendin-4 and GLP-1 was only 58%. Therefore, it is unclear whether other factors contributed to the reduction of GLP-1. Second, although the effects of exendin-4 on reducing GLP-1 expression and neuroinflammation were significant, the expression of GLP-1 and neuroinflammation did not completely revert to the baseline value. From these results, we infer that exendin-4 may not be the sole mechanism underlying GLP-1R reduction and neuroinflammation following nerve injury.
In summary, GLP-1R was significantly downregulated in the hippocampal dentate gyrus in rats with cognitive impairment following exposure to chronic pain. Further investigation revealed that a GLP-1R agonist can protect against pain-induced cognitive impairment via suppression of glial activation and the inflammatory response. Our results provide a potential interventional target for the future treatment of chronic pain.
Additional file: Open peer review report 1 (127.4KB, pdf) .
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This work was financially supported by the Special Grant for Scientific and Technological Development Conducted by The Central Government of China in 2016: Quality Test and Operation with Anesthesia Center of Experimental Animal of Hubei Province, No. 2060403 (to BHZ).
Institutional review board statement: All experimental procedures and protocols were approved by the Experimental Animal Ethics Committee of Renmin Hospital of Wuhan University of China (approval No. WDRM 20171214) on September 22, 2017. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Mitsuhiro Enomoto, Tokyo Medical and Dental University, Tokyo, Japan.
Funding: This study was supported by the Special Grant for Scientific and Technological Development Conducted by The Central Government of China in 2016: Quality Test and Operation with Anesthesia Center of Experimental Animal of Hubei Province, No. 2060403 (to BHZ).
P-Reviewer: Enomoto M; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Gardner B, Frenchman B, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14:502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrne C, Coetzer R, Addy K. Investigating the discrepancy between subjective and objective cognitive impairment following acquired brain injury: the role of psychological affect. NeuroRehabilitation. 2017;41:501–512. doi: 10.3233/NRE-162015. [DOI] [PubMed] [Google Scholar]
- 3.Calsolaro V, Edison P. Novel GLP-1 (Glucagon-Like Peptide-1) analogues and insulin in the treatment for alzheimer’s disease and other neurodegenerative diseases. CNS Drugs. 2015;29:1023–1039. doi: 10.1007/s40263-015-0301-8. [DOI] [PubMed] [Google Scholar]
- 4.Cao L, Li D, Feng P, Li L, Xue GF, Li G, Hölscher C. A novel dual GLP-1 and GIP incretin receptor agonist is neuroprotective in a mouse model of Parkinson’s disease by reducing chronic inflammation in the brain. Neuroreport. 2016;27:384–391. doi: 10.1097/WNR.0000000000000548. [DOI] [PubMed] [Google Scholar]
- 5.Chen N, Su W, Cui SH, Guo J, Duan JC, Li HX, He L. A novel large animal model of recurrent migraine established by repeated administration of inflammatory soup into the dura mater of the rhesus monkey. Neural Regen Res. 2019;14:100–106. doi: 10.4103/1673-5374.243715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29:1518–1522. doi: 10.2337/dc05-2228. [DOI] [PubMed] [Google Scholar]
- 7.Doyle T, Esposito E, Bryant L, Cuzzocrea S, Salvemini D. NADPH-oxidase 2 activation promotes opioid-induced antinociceptive tolerance in mice. Neuroscience. 2013;241:1–9. doi: 10.1016/j.neuroscience.2013.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geis C, Geuss E, Sommer C, Schmidt HH, Kleinschnitz C. NOX4 is an early initiator of neuropathic pain. Exp Neurol. 2017;288:94–103. doi: 10.1016/j.expneurol.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Guglielmi V, Sbraccia P. GLP-1 receptor independent pathways: emerging beneficial effects of GLP-1 breakdown products. Eat Weight Disord. 2017;22:231–240. doi: 10.1007/s40519-016-0352-y. [DOI] [PubMed] [Google Scholar]
- 11.Gullo F, Ceriani M, D’Aloia A, Wanke E, Constanti A, Costa B, Lecchi M. Plant polyphenols and exendin-4 prevent hyperactivity and TNF-alpha release in LPS-treated in vitro neuron/astrocyte/microglial networks. Front Neurosci. 2017;11:500. doi: 10.3389/fnins.2017.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu QP, Mao DA. Histone deacetylase inhibitor SAHA attenuates post-seizure hippocampal microglia TLR4/MYD88 signaling and inhibits TLR4 gene expression via histone acetylation. BMC Neruosci. 2016;17:22. doi: 10.1186/s12868-016-0264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia M, Liu WX, Sun HL, Chang YQ, Yang JJ, Ji MH, Yang JJ, Feng CZ. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, attenuates postoperative cognitive dysfunction in aging mice. Front Mol Neurosci. 2015;8:52. doi: 10.3389/fnmol.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S, Jeong J, Jung HS, Kim B, Kim YE, Lim DS, Kim SD, Song YS. Anti-inflammatory effect of glucagon like peptide-1 receptor agonist, exendin-4, through modulation of IB1/JIP1 expression and JNK signaling in stroke. Exp Neurobiol. 2017;26:227–239. doi: 10.5607/en.2017.26.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau WK, Lau YM, Zhang HQ, Wong SC, Bian ZX. Electroacupuncture versus celecoxib for neuropathic pain in rat SNL model. Neruoscience. 2010;170:655–661. doi: 10.1016/j.neuroscience.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 16.Lee YS, Jun HS. Anti-Inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflamm. 2016;2016:3094642. doi: 10.1155/2016/3094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.L’Episcopo F, Tirolo C, Serapide MF, Caniglia S, Testa N, Leggio L, Vivarelli S, Iraci N, Pluchino S, Marchetti B. Microglia polarization, gene-environment interactions and wnt/beta-catenin signaling: emerging roles of glia-neuron and glia-stem/neuroprogenitor crosstalk for dopaminergic neurorestoration in aged Parkinsonian brain. Front Aging Neurosci. 2018;10:12. doi: 10.3389/fnagi.2018.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li SF, Ouyang BS, Zhao X, Wang YP. Analgesic effect of AG490, a Janus kinase inhibitor, on oxaliplatin-induced acute neuropathic pain. Neural Regen Res. 2018;13:1471–1476. doi: 10.4103/1673-5374.235305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Mattson MP, Hoffer BJ, Wang Y, Greig NH. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Wang F, Liu S, Du J, Hu X, Xiong J, Fang R, Chen W, Sun J. Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J Neurol Sci. 2017;381:176–181. doi: 10.1016/j.jns.2017.08.3235. [DOI] [PubMed] [Google Scholar]
- 21.Mangmool S, Hemplueksa P, Parichatikanond W, Chattipakorn N. Epac is required for GLP-1R-mediated inhibition of oxidative stress and apoptosis in cardiomyocytes. Mol Endocrinol. 2015;29:583–596. doi: 10.1210/me.2014-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKenzie JA, Klegeris A. Modulation of microglial functions by methyl jasmonate. Neural Regen Res. 2018;13:1290–1293. doi: 10.4103/1673-5374.235078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muscogiuri G, DeFronzo RA, Gastaldelli A, Holst JJ. Glucagon-like peptide-1 and the central/peripheral nervous system: crosstalk in diabetes. Trends Endocrinol Metab. 2017;28:88–103. doi: 10.1016/j.tem.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Pawela CP, Kramer JM, Hogan QH. Dorsal root ganglion stimulation attenuates the BOLD signal response to noxious sensory input in specific brain regions: Insights into a possible mechanism for analgesia. Neruoimage. 2017;147:10–18. doi: 10.1016/j.neuroimage.2016.11.046. [DOI] [PubMed] [Google Scholar]
- 25.Pinho-Ribeiro FA, Verri WJ, Chiu IM. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol. 2017;38:5–19. doi: 10.1016/j.it.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poon YY, Chang AY, Ko SF, Chan SH. An improved procedure for catheterization of the thoracic spinal subarachnoid space in the rat. Anesth Analg. 2005;101:155–160. doi: 10.1097/00000539-200507000-00029. [DOI] [PubMed] [Google Scholar]
- 27.Reeta KH, Singh D, Gupta YK. Edaravone attenuates intracerebroventricular streptozotocin-induced cognitive impairment in rats. Eur J Neurosci. 2017;45:987–997. doi: 10.1111/ejn.13543. [DOI] [PubMed] [Google Scholar]
- 28.Sharma S, Taliyan R. Epigenetic modifications by inhibiting histone deacetylases reverse memory impairment in insulin resistance induced cognitive deficit in mice. Neuropharmacology. 2016;105:285–297. doi: 10.1016/j.neuropharm.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 29.Tambuyzer BR, Ponsaerts P, Nouwen EJ. Microglia: gatekeepers of central nervous system immunology. J Leukoc Biol. 2009;85:352–370. doi: 10.1189/jlb.0608385. [DOI] [PubMed] [Google Scholar]
- 30.Thangavel R, Kempuraj D, Zaheer S, Raikwar S, Ahmed ME, Selvakumar GP, Iyer SS, Zaheer A. Glia maturation factor and mitochondrial uncoupling proteins 2 and 4 expression in the temporal cortex of Alzheimer’s disease brain. Front Aging Neurosci. 2017;9:150. doi: 10.3389/fnagi.2017.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W, Zhang L, Zhang X, Xue R, Li L, Zhao W, Fu Q, Mi W, Li Y. Lentiviral-mediated overexpression of the 18 kDa translocator protein (TSPO) in the hippocampal dentate gyrus ameliorates LPS-induced cognitive impairment in mice. Front Pharmacol. 2016;7:384. doi: 10.3389/fphar.2016.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang WY, Tan MS, Yu JT, Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med. 2015;3:136. doi: 10.3978/j.issn.2305-5839.2015.03.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.