Microglial cells are the key innate immune cells in the brain and they are crucial in maintaining brain parenchyma homeostasis. Under physiological conditions, microglial cells assume a ramified morphology with a small cell body and an extensive network of fine processes, which secrete neurotrophic factors and patrol the surroundings in search for pathogens and eliminate cellular debris via phagocytosis. Microglial cells express a repertoire of pattern recognition receptors (PRRs) that enable them to detect diverse danger-associated molecular patterns (DAMPs) released from damaged cells or cells under stress, or pathogen-associated molecular patterns generated by pathogens during infection. PRR activation induces activation of microglial cells, accompanied by retracting the fine processes and adopting an amoeboid morphology, proliferation and migration to the site of tissue damage or infection, where they initiate an innate immune response to clear damaged cells or destroy invading pathogens via generation of proinflammatory mediators as well as via phagocytosis. However, excessive or untimely resolving innate immune activation can damage the surrounding healthy neural tissues. Degenerating neurons release DAMPs that trigger formation of a positive feedback mechanism, leading to chronic microglial cell activation/degeneration and, as a consequence, progressive loss of neurons. There is compelling evidence that microglial cell-mediated neuroinflammation acts as a common mechanism that plays an essential role in the pathophysiology of both neurodegenerative diseases and psychiatric disorders. Therefore, there is an escalating interest in restoring microglial cell function for preventing neuroinflammation in the brain as therapeutic strategies. To this end, it is important to identify the molecular mechanisms and targets in microglial cells that mediate neuroinflammation for drug development.
The P2X7 receptor (P2X7R), a member of the purinergic P2X receptor ATP-gated ion channel family, is highly expressed in microglial cells (Wei et al., 2018). It is activated by submillimolar concentrations of ATP. As such, the P2X7R in microglial cells is well placed as a PRR detecting extracellular ATP, a DAMP molecule that is leaked from dying cells due to brain traumatic injuries or degenerating neurons in neurodegenerative diseases and is also released in a non-cytolytic manner in response to psychological stress. Studies have gathered evidence to suggest an obligatory role of the P2X7R in mediating microglial cell-mediated neuroinflammation in the pathophysiology of neurodegenerative diseases and psychiatric disorders, as exemplified by Alzheimer’s disease (AD) and mood disorders.
P2X7R and AD: AD is an age-related neurodegenerative condition characterized by a progressive decline or loss of cognitive function, and is the most common cause of dementia. Currently, no treatment can stop AD or slow down its progression. The AD brains are manifested with widespread phenotypic changes in microglial cells and prominent neuroinflammation, in addition to the histopathological hallmarks, extracellular deposition of amyloid β (Aβ) peptides in structure known as amyloid senile plaque and intracellular aggregation of hyper-phosphorylated tau proteins to form neurofibrillary tangles. Chronic alterations in microglial cell-mediated innate immune function and ensuing neuroinflammation have gained increasing recognition as an early event driving the pathogenesis and progression of AD (Gutierrez and Vitorica, 2018).
The P2X7R expression is significantly upregulated in human microglial cell cultures after exposure to Aβ42, the most neurotoxic peptide, and also in microglial cells in human AD brains. Upregulation of the P2X7R expression in microglial cells have been observed in transgenic AD mice that recapitulate the pathophysiology of excessive Aβ generation in human AD brains, such as APP/PS1 and J20 mice, and also in rats following intra-hippocampal injection of Aβ42. Furthermore, such upregulation in the P2X7R expression appears to increase with age, occur at the late stage of Aβ-induced neuroinflammation and correlate with synaptic toxicity in the brains of both AD animal models and AD patients (Martínez-Frailes et al., 2019). Pharmacological inhibition of the P2X7R or genetic depletion of the P2X7R expression was effective in preventing Aβ-induced microglial cell activation and neuroinflammation both in vitro and in vivo (Sanz et al., 2009; Chen et al., 2014; Martínez-Frailes et al., 2019). Pharmacological or genetic intervention of the P2X7R also protected ATP-induced reduction in the phagocytosis capacity of microglia cells (Martínez-Frailes et al., 2019). Importantly, pharmacological inhibition of the P2X7R preserved spatial memory and other cognitive functions. More specifically, such intervention stimulated the development of dendritic spines in hippocampal neurons in AD mice induced by intra-hippocampal injection of Aβ42 (Chen et al., 2014). Consistently, genetic depletion of the P2X7R expression ablated cognitive deficits and improved synaptic plasticity in the APP/PS1 mice (Martin et al., 2019). Collectively, these findings provide evidence to support an essential role of the P2X7R in the pathogenesis of AD via mediating Aβ-induced alterations in microglial cell function and neuroinflammation.
It is well established in microglial cells and also peripheral immune cells that activation of the P2X7R induces the assembly and activation of the multi-protein complex nucleotide-binding domain, leucine-rich repeat and pyrin-containing receptor 3 (NLRP3) inflammasome to activate caspase-1, which in turns converts pro-interleukin (IL)-1β into mature IL-1β, the master proinflammatory cytokine. Consistently, genetic depletion of the P2X7R expression ablated Aβ42-induced maturation and release of IL-1β in cultured microglial cells and also in microglial cells in mice with intra-hippocampal injection of Aβ42 (Sanz et al., 2009). Pharmacological and genetic disruption of the NLRP3 inflammasome activation inhibited Aβ42-induced microglial cell activation and neuroinflammation, and increased the phagocytosis capacity of microglial cells to clear Aβ. Similarly, pharmacological and genetic inactivation of the NLRP3 inflammasome also reduced Aβ accumulation and largely restored cognitive function in the APP/PS1 mice (Heneka et al., 2018). The P2X7R is also important for ATP-induced generation of proinflammatory mediators IL-6 and tumour necrosis factor α (TNF-α) (Iwata et al., 2016). These findings are consistent with a critical role of the P2X7R in mediating activation of the NLRP3 inflammasome and caspase-1 and generation of IL-1β, IL-6 and TNF-α. Such a notion would provide a mechanistic explanation for the elevated levels of IL-1β, IL-6 and TNF-α observed in the brain of AD patients.
A recent study has shown that genetic deletion of the P2X7R expression reduced the level of Aβ peptides and reversed the deficiencies in synaptic plasticity and cognitive function in the APP/PS1 mice (Martin et al., 2019). Surprisingly, genetic deletion of the P2X7R expression resulted in no significant effect on the microglial cell phenotypes, generation of IL-1β or the phagocytosis capacity and, instead, attenuated the production of chemokines CCL3, CCL4 and CCL5 from microglial cells and astrocytes induced by exposure to Aβ as well as ATP and subsequent recruitment of CD8+ T cells (Martin et al., 2019). These intriguing findings, while strengthening the importance of the P2X7R in mediating Aβ-induced microglial cell-mediated neuroinflammation, suggest a distinctive mechanism downstream of the P2X7R activation in the pathogenesis of AD (Figure 1A).
Figure 1.
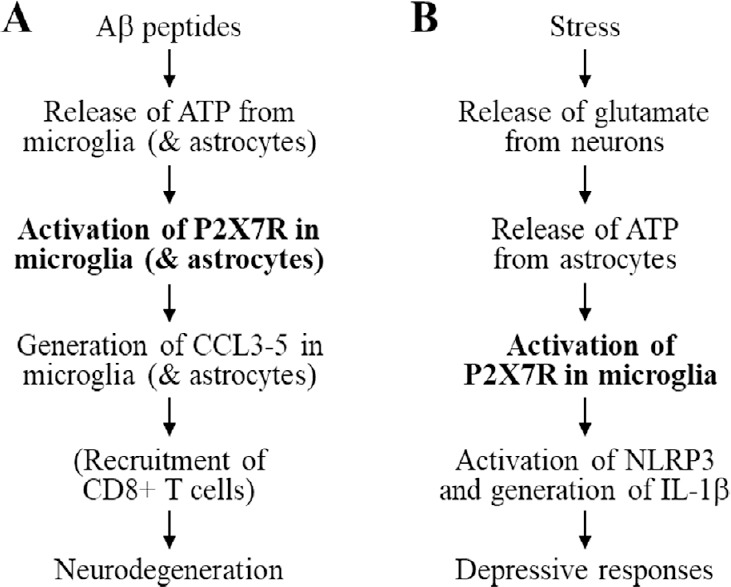
Proposed roles of the P2X7R in microglia in mediating neuroinflammation in the pathophysiology of Alzheimer’s disease and mood disorders.
(A) Aβ peptides induce ATP release from microglia (and astrocytes), and ATP activates microglia (and astrocytes) to further enhance ATP release. ATP-induced activation of the P2X7R in microglia (and astrocytes) triggers generation of chemokines CCL3, CCL4 and CCL5 from microglia (and astrocytes) that cause neurodegeneration directly or indirectly via recruitment of CD8+ T cells. (B) Chronic unpredictable stress evokes glutamate release from neurons in hippocampus, and glutamate in turns activates astrocytes and induces ATP release from astrocytes. ATP-induced activation of the P2X7R in microglia activates the NLRP3 inflammasome (and caspase-1) and subsequent generation of IL-1β from microglia, leading to development of depressive behavioural responses. For more details, consult the text and the references within it. Aβ: Amyloid β; IL: interleukin; NLRP3: nucleotide-binding domain, leucine-rich repeat and pyrin-containing receptor 3; P2X7R: P2X7 receptor.
P2X7R and mood disorders: Mood disorders encompass a group of psychiatric diseases among which, major depressive disorder (MDD) or unipolar depressive disorder (commonly known as depression), bipolar disorder (BPD) and anxiety disorder represent the main types. MDD is predicted by the World Health Organization to become the first leading cause of global disease burdens by 2030. While antidepressant treatments are available, there are still considerable clinical needs for the development of more effective therapeutics. The aetiology of mood disorders remains elusive. The IL-1β level in the cerebrospinal fluid was considerably higher in BPD patients than control subjects and in BPD patients having a recent manic episode. Furthermore, the IL-1β level shows strong correlation with the severity of depression (Wei et al., 2018). Similarly, there was an increase in the IL-1β level in the cerebrospinal fluid and prefrontal cortex in mice and rats subjected to chronic unpredictable or mild stress (Iwata et al., 2016; Wei et al., 2018). An elevated level of TNF-α in hippocampus was also detected in rats exposed to immobilization stress (Iwata et al., 2016). Moreover, the TNF-α level was increased with a noticeable delay relative to the IL-1β level, suggesting that IL-1β stimulates TNF-α generation (Iwata et al., 2016). Pharmacological inhibition of the NLRP3 inflammasome or caspase-1 activation as well as genetic deletion of the NLRP3 protein expression substantially attenuated or reversed depressive-like behaviour responses in mice (Iwata et al., 2016). Mood disorders show significant comorbidity with many different inflammatory conditions. Anti-inflammatory interventions can give rise to the antidepressant phenotypes in BPD and MDD patients and also in rodent models of mood disorders and, conversely, treatments with antidepressants result in significant reduction in the levels of IL-1β, IL-6 or TNF-α in MDD and BPD patients. Collectively, preclinical and clinical studies have gathered strong evidence that leads to the inflammation hypothesis of mood disorders (Wei et al., 2018).
Several studies using various rodent models have examined the role of the P2X7R in determining the development of depressive behavioral responses. Pharmacological inhibition of the P2X7R or genetic deletion of the P2X7R expression strongly suppressed amphetamine-induced manic-like behaviours in mice, and largely reversed chronic unpredictable stress-induced anhedonia, anxiety-like and depressive-like behaviors in rats (Iwata et al., 2016; Yue et al., 2017). The P2X7R-deficient mice became noticeably resilient in the development of learned helplessness behaviors (Wei et al., 2018). Mechanistically, as illustrated in Figure 1B, chronic unpredictable stress is thought to evoke release of glutamate from hippocampal neurons and glutamate in turn stimulates release of ATP from astrocytes. ATP activates the P2X7R in microglial cells to induce activation of the NLRP3 inflammation and caspase-1 and generation of IL-1β. Thus, the P2X7R in microglial cells plays an indispensable role in mediating stress-induced neuroinflammation that drives the depressive behaviours responses (Iwata et al., 2016). In support of such a signaling mechanism, pharmacological inhibition of the P2X7R ablated depressive-like behaviours in rats induced by intra-hippocampal injection of ATP or BzATP, a P2X7 agonist with a greater potency at the P2X7R than ATP (Yue et al., 2017). Furthermore, our recent study shows that pharmacological inhibition of the P2X7R reversed chronic unpredictable-induced microglial cell activation as well as behavioural alterations and neuroendocrine dysregulation in mice (Farooq et al., 2018).
Concluding remarks: Therefore, there is increasing evidence to support that the P2X7R in microglial cells plays a vital role in mediating neuroinflammation in the pathophysiology of AD and mood disorders. It is known that mood disorders exhibit comorbidity with AD (Wei et al., 2018). It is interesting to investigate whether the alteration of P2X7R in mood disorder patients is a risk factor predisposing to AD or vice versa. As discussed above, the current knowledge appears to suggest that P2X7R-dependent neuroinflammation is mediated via different downstream signal transduction pathways, namely, via generation of chemokines in AD, and via activation of the NLRP3 inflammasome and caspase-1 and generation of IL-1β in mood disorders. It is evident that more investigations are needed to understand whether there exists a crosstalk at the P2X7R in microglial cell function alterations and neuroinflammation in the development of these two conditions. Particularly, it remains to be answered whether activation of the NLRP3 inflammasome and caspase-1 contributes to neuroinflammation in AD, independent of generation of IL-1β.
Irrespective, compelling evidence supports the P2X7R as a drug target, opening a new avenue for the development of therapeutic strategies to treat these debilitating conditions. Potent and selective P2X7R antagonists that penetrate the blood-brain barrier become increasingly availability, and some of these compounds have been further developed for use in in vivo imaging to monitor microglial cell activation and brain inflammation. The readers who are interested in the development of the P2X7R antagonists can consult our recent review for details (Wei et al., 2018). Regardless of the signaling mechanisms following the P2X7R activation, it is highly attractive to explore the therapeutic potential of targeting the P2X7R for preventing microglial cell malfunction and neuroinflammation to treat brain pathologies such as AD and mood disorders. 10
This work was supported in part by grants from the Disciplinary Group of Psychology and Neuroscience Xinxiang Medical University (2016PN-KFKT-06) and a visiting professorship from University of Tours (to LHJ).
Additional file: Open peer review report 1 (78.5KB, pdf) .
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Emilio Varea, Universitat de València, Valencia, Spain.
P-Reviewer: Varea E; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Chen X, Hu J, Jiang L, Xu S, Zheng B, Wang C, Zhang J, Wei X, Chang L, Wang Q. Brilliant blue G improves cognition in an animal model of Alzheimer’s disease and inhibits amyloid-β-induced loss of filopodia and dendrite spines in hippocampal neurons. Neuroscience. 2014;279:94–101. doi: 10.1016/j.neuroscience.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 2.Farooq RK, Tanti A, Ainouche S, Roger S, Belzung C, Camus V. A P2X7 receptor antagonist reverses behavioural alterations, microglial activation and neuroendocrine dysregulation in an unpredictable chronic mild stress (UCMS) model of depression in mice. Psychoneuroendocrinology. 2018;97:120–130. doi: 10.1016/j.psyneuen.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 3.Gutierrez A, Vitorica J. Toward a new concept of Alzheimer’s disease models: A perspective from neuroinflammation. J Alzheimers Dis. 2018;64:S329–338. doi: 10.3233/JAD-179914. [DOI] [PubMed] [Google Scholar]
- 4.Heneka MT, McManus RM, Latz E. Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci. 2018;19:610–621. doi: 10.1038/s41583-018-0055-7. [DOI] [PubMed] [Google Scholar]
- 5.Iwata M, Ota KT, Li XY, Sakaue F, Li N, Dutheil S, Banasr M, Duric V, Yamanashi T, Kaneko K, Rasmussen K, Glasebrook A, Koester A, Song D, Jones KA, Zorn S, Smagin G, Duman RS. Psychological stress activates the inflammasome via release of adenosine triphosphate and stimulation of the purinergic type 2X7 receptor. Biol Psychiatry. 2016;80:12–22. doi: 10.1016/j.biopsych.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 6.Martin E, Amar M, Dalle C, Youssef I, Boucher C, Le Duigou C, Brückner M, Prigent A, Sazdovitch V, Halle A, Kanellopoulos JM, Fontaine B, Delatour B, Delarasse C. New role of P2X7 receptor in an Alzheimer’s disease mouse model. Mol Psychiatry. 2019;24:108–125. doi: 10.1038/s41380-018-0108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martínez-Frailes C, Di Lauro C, Bianchi C, de Diego-García L, Sebastián-Serrano Á, Boscá L, Díaz-Hernández M. Amyloid peptide induced neuroinflammation increases the P2X7 receptor expression in microglial cells, impacting on its functionality. Front Cell Neurosci. 2019;13:143. doi: 10.3389/fncel.2019.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanz JM, Chiozzi P, Ferrari D, Colaianna M, Idzko M, Falzoni S, Fellin R, Trabace L, Di Virgilio F. Activation of microglia by amyloid β requires P2X7 receptor expression. J Immunol. 2009;182:4378–4385. doi: 10.4049/jimmunol.0803612. [DOI] [PubMed] [Google Scholar]
- 9.Wei L, Syed Mortadza SA, Yan J, Zhang L, Wang L, Yin Y, Li C, Chalon S, Emond P, Belzung C, Li D, Lu C, Roger S, Jiang LH. ATP-activated P2X7 receptor in the pathophysiology of mood disorders and as an emerging target for the development of novel antidepressant therapeutics. Neurosci Biobehav Rev. 2018;87:192–205. doi: 10.1016/j.neubiorev.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Yue N, Huang H, Zhu X, Han Q, Wang Y, Li B, Liu Q, Wu G, Zhang Y, Yu J. Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. J Neuroinflammation. 2017;14:102. doi: 10.1186/s12974-017-0865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


