Keywords: brain water content, caspase-8, inflammation, Morris water maze, neurological function, neuroprotection, pyrin domain-containing 3, rotarod test, subarachnoid hemorrhage, Z-IETD-FMK
Abstract
Caspase-8 plays an important role in the mediation of inflammation and the effect of its role in subarachnoid hemorrhage remains elusive. The nucleotide-binding oligomerization domain-like receptor protein 3 inflammasome has been postulated to mediate inflammation during SAH. The aim of the present study was to investigate the effects of caspase-8 inhibition on SAH injury and further elucidate the molecular mechanisms. In this study, a subarachnoid hemorrhage model was established by endovascular perforation process in adult male Sprague-Dawley rats. Z-IETD-FMK (0.5, 1, 2 mg/kg; an inhibitor of caspase-8) was delivered via intravenous (tail vein) injection immediately after subarachnoid hemorrhage. After 12 hours of subarachnoid hemorrhage, western blot assay showed that the expression of cleaved caspase-8 was significantly increased at 12 hours, peaked at 24 hours, and then decreased at 72 hours after subarachnoid hemorrhage. Immunofluorescence staining demonstrated that caspase-8 was expressed in microglia after subarachnoid hemorrhage. Z-IETD-FMK significantly improved neurological deficits and reduced brain water content 24 hours after subarachnoid hemorrhage. The Morris water maze and rotarod test confirmed that Z-IETD-FMK significantly improved spatial learning and memory abilities and motor coordination at 21–27 days after subarachnoid hemorrhage. Furthermore, inhibition of caspase-8 activation reduced the expression of pyrin domain-containing 3, caspase-1, and interleukin-1β after subarachnoid hemorrhage. In conclusion, our findings suggest that caspase-8 inhibition alleviates subarachnoid hemorrhage-induced brain injuries by suppressing inflammation. The study was approved by the Institutional Animal Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University, China (approval No. 2016-193) on February 25, 2016.
Chinese Library Classification No. R453; R741; R743.34
Introduction
Subarachnoid hemorrhage (SAH) is a devastating disease that accounts for about 5% of all strokes (Macdonald and Schweizer, 2017; Kanamaru and Suzuki, 2019). A growing body of evidence indicates that neuroinflammation after SAH contributes to the progression and exacerbation of brain injury (Simon et al., 2019; Shiba and Suzuki, 2019). The nucleotide-binding oligomerization domain-like receptor (NLR) family pyrin domain-containing 3 (NLRP3) inflammasome plays a crucial role in innate immunity and inflammation, and can facilitate the production of caspase-1 and interleukin-1β (IL-1β), and increase inflammation (Shao et al., 2016). Several studies have showed that NLRP3 inflammasome plays a pathogenic role in the pathophysiology of SAH, and inhibition of NLRP3 is considered to be a potential therapeutic target against SAH (Shao et al., 2016; Zhang et al., 2017).
Caspases, a family of aspartate-specific cysteine proteases, possess a well-characterized function in apoptotic signaling. Caspase-8 is a cysteine protease that plays a pivotal role in the extrinsic apoptotic signaling pathway via death receptors (Crowder and El-Deiry, 2012). Recently, several non-apoptotic roles of caspase-8 have been reported, especially its contribution to neuroinflammatory responses (Burguillos et al., 2011). Z-IETD-FMK, an inhibitor of caspase-8, has been reported to reduce brain infarction size and seizure activity after middle cerebral artery occlusion (Shabanzadeh et al., 2015). However, the potential anti-neuroinflammation function of caspase-8 inhibition has not been investigated in SAH. In this study, we explored the effect of Z-IETD-FMK on brain injury after SAH and its possible mechanism of action.
Materials and Methods
Experimental animals
All experiments for this study were approved by the Institutional Animal Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University, China (approval No. 2016-193) on February 25, 2016. A total of 177 specific-pathogen-free male Sprague-Dawley rats (weighing 300–320 g and aged 8 weeks) were provided by the Laboratory Animal Center of Zhejiang Province, China (licence No. SCXK (Zhe) 2014-0001) and housed in a light- and temperature-controlled facility with free access to food and water. The investigators were blinded to group assignments during the outcome assessments.
Experimental design
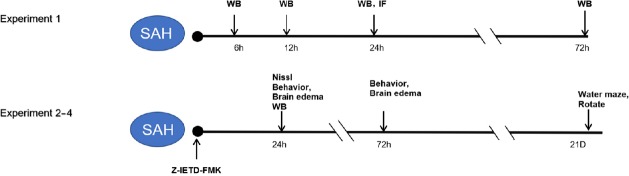
Experiment 1
A total of 30 rats were randomly divided into the following five groups: sham, SAH after 6, 12, 24, and 72 hours (n = 6 per group). The time course of endogenous cleaved caspase-8 expression in the ipsilateral cerebral cortex after SAH was measured using western blot assay. Double immunofluorescence staining was used to test the localization of caspase-8 in microglia in the sham (n = 4) and SAH (24 hours) groups (n = 4) (Figure 1).
Figure 1.
Experimental design and animal group classification.
Experiment 1 was for time course of endogenous cleaved caspase-8 expression after SAH and to test the localization of caspase-8 in microglia. Experiments 2–4 was for exploring the effect of Z-IETD-FMK on brain injury after SAH and its possible mechanism of action. IF: Immunofluorescence; SAH: subarachnoid hemorrhage; WB: western blot.
Experiment 2
A total of 48 rats were randomly divided into the following five groups: sham (n = 12), SAH + vehicle (saline) (n = 12), SAH + Z-IETD-FMK (0.5 mg/kg, n = 6), SAH + Z-IETD-FMK (1 mg/kg, n = 12), and SAH + Z-IETD-FMK (2 mg/kg, n = 6). Neurological scores and brain water content were evaluated at 24 hours after SAH. Based on neurological scores at 24 hours after SAH, Z-IETD-FMK (1 mg/kg) was chosen for western blot assay at 24 hours. The effect of Z-IETD-FMK (1 mg/kg) on brain injuries at 72 hours and long-time neurobehavior after SAH was also assessed. The remaining 18 rats were used to test the effect of Z-IETD-FMK on neuron death in the hippocampus at 24 hours after SAH (Figure 1).
Experiment 3
A total of 18 rats were randomly divided into the following three groups to explore the effect of Z-IETD-FMK on long-term neurological functions at 21 days after SAH: sham, SAH + vehicle, SAH + Z-IETD-FMK (n = 6 per group) (Figure 1).
Experiment 4
A total of 24 rats were randomly assigned to the following four groups to explore the mechanism of caspase-8 inhibition on SAH: sham, SAH + vehicle, SAH + dimethyl sulfoxide, SAH + Z-IETD-FMK (n = 6 per group), and western blot analysis was performed at 24 hours after SAH (Figure 1).
SAH model establishment
The SAH model was induced as previously described (Xie et al., 2017). Rats were intraperitoneally anesthetized with 10% chloral hydrate. The left carotid artery and its branches were surgically exposed. A sharpened 3-cm, 4-0 nylon suture was inserted into the left internal carotid artery and the suture was advanced until resistance was felt, further advanced to puncture the vessel, and then immediately withdrawn to cause SAH. The sham group underwent the same procedure but without perforation. After the operative procedure was completed, the rats were allowed to recover within 1 hour on an electric heating blanket.
Z-IETD-FMK administration
Intravenous (tail vein) administration of the three dosages of Z-IETD-FMK was performed immediately after SAH, as previously described for middle cerebral artery occlusion (Shabanzadeh et al., 2015). Briefly, rats were administered either saline (0.05 mL/kg), dimethyl sulfoxide (50%, 0.05 mL/kg), or Z-IETD-FMK (0.5, 1, and 2 mg/kg).
Measurement of SAH grade
The severity of SAH was assessed using a grading system 24 hours after SAH based on the amount of blood in six segments in the basal cistern, as previously described (Sugawara et al., 2008). The total score (0–18) was calculated and the animals were divided into three groups as follows 0–7: mild SAH, 8–12: moderate SAH, and 13–18: severe SAH. Since mild SAH rats did not show any neurological deficits as compared to sham rats (Sugawara et al., 2008), SAH rats with a score < 8 were excluded from this research.
Neurological score assessment
Neurological scores were assessed at 24 hours or 72 hours after SAH using the modified Garcia test and beam balance tests (Xie et al., 2018). The six items of modified Garcia test were assessed, including spontaneous activity, vibrissae touch, trunk touch, spontaneous movement of the four limbs, stretching of the forelimbs, and climbing capacity.
Each part of the test has a score ranging from 0 to 3, with a maximum score of 18. The beam balance test was used to assess the walking ability of rats (maximum score = 4), whereby a score of 4 indicated normal balance.
Long-term neurological score evaluation
The Morris water maze was performed according to the method of Bromley-Brits et al. (2011) from day 21 to day 27 after SAH, including visible platform tests, hidden platform tests and probe trials. An accelerating rotarod test was used to evaluate forelimb and hindlimb motor coordination and balance. Animals were trained and tested on a rotarod that gradually accelerated. The duration spent on the device was recorded as the time before rats fell off the rungs with beginning speeds of 5 and 10 r/min (Hamm et al., 1994).
Brain water content evaluation
The brain was separated into the left hemisphere, right hemisphere, cerebellum, and brain stem at 24 hours or 72 hours after surgery. Each sample was weighed immediately after removal to obtain the wet weight and then dried in an oven at 105°C for 72 hours to obtain the dry weight. Brain water content (%) for each sample was calculated as (wet weight – dry weight)/wet weight × 100 (Xie et al., 2017).
Double immunofluorescence staining
Double immunofluorescence staining was conducted as described previously (Xie et al., 2018). At 24 hours after SAH, rats were deeply intraperitoneally anesthetized with 10% chloral hydrate. Then, rats were transcardially perfused with cold phosphate-buffered solution (PBS) followed by 10% paraformaldehyde. The whole brains were isolated and fixed in 10% paraformaldehyde for 24 hours then in 30% sucrose solution for 72 hours. Coronal brain sections (10 μm) were sectioned and permeabilized with 0.3% Triton X-100 in PBS for 30 minutes. Blocking was performed by incubating the sections with 5% donkey serum for 1 hour. Primary antibodies against caspase-8 (rabbit, 1:200, Abcam, Cambridge, UK) and ionized calcium binding adaptor molecule 1 (Iba-1; goat, 1:200, Abcam) were added to the sections and incubation occurred overnight at 4°C. After washing with PBS, the sections were then incubated with donkey anti-rabbit secondary antibodies (1:200, Santa Cruz Biotechnology, Dallas, TX, USA) at room temperature for 2 hours. Brain slices were visualized using a fluorescence microscope (Olympus, Tokyo, Japan).
Nissl staining
Neuron death in the hippocampus after SAH was evaluated using Nissl staining, as previously described (Zhang et al., 2016). Brain sections were hydrated in 1% toluidine blue for 10 min. After washing with double-distilled water, sections were dehydrated and mounted onto slides using Permount Mounting Medium. Sections were then photo-graphed by a microscope (Olympus). Neurons with a big cell body, and round and palely stained nuclei were regarded as normal neurons, while those with a shrunken cell body and condensed nuclei were regarded as damaged cells. Images were captured and analyzed at 200× magnification with a light microscope (Leica QWin Plus, Leica Microsystems, Wetzlar, Germany).
Western blot assay
Western blot analysis was performed as previously reported (Kristian et al., 2011). The protein extracted from the left cortex was used for western blot, and equal amounts of protein (50 μg) were loaded in each lane of the sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel. After electrophoresis, the samples were transferred onto a nitrocellulose membrane. Then, the membrane was blocked for 2 hours at room temperature using blocking buffer and incubated at 4°C overnight with the following primary antibodies: rabbit anti-cleaved caspase-8 (1:1000, Abcam), rabbit anti-NLRP3 (1:500, NOVUS, St. Louis, MO, USA), rabbit anti-cleaved caspase-1 (1:1000, NOVUSA), and rabbit anti-IL-1β (1:1000, Abcam). Rabbit anti-beta-actin was used as an internal loading control (1:6000, Santa Cruz Biotechnology). The bands were quantified using densitometry with ImageJ 1.4 software (National Institutes of Health, Bethesda, MD, USA). The membranes were washed with Tris-buffered saline-Tween 20 for 30 minutes, and then incubated with horseradish peroxidase-conjugated secondary anti-rabbit antibody (1:4000, Santa Cruz Biotechnology) at room temperature for 2 hours.
Statistical analysis
The data analysis was performed using GraphPad Prism 6 (GraphPad software, San Diego, CA, USA). Data are showed as the mean ± standard deviation (SD). One-way analysis of variance and then the Tukey’s tests were used to make between-group comparisons of parametric data. The Kruskal-Wallis test followed by Dunn’s post-hoc tests were used to make between-group comparisons of non-parametric data (neurological scores and beam walking). P < 0.05 was considered as significant.
Results
Quantitative analysis of SAH rats
The mortality rates were 14.6% (20/137) in the SAH model group, and no rats from the sham group died. Eleven rats were excluded from this study due to mild SAH. There were no significant differences in SAH grading scores between the SAH groups.
The time course of cortical caspase-8 expression after SAH
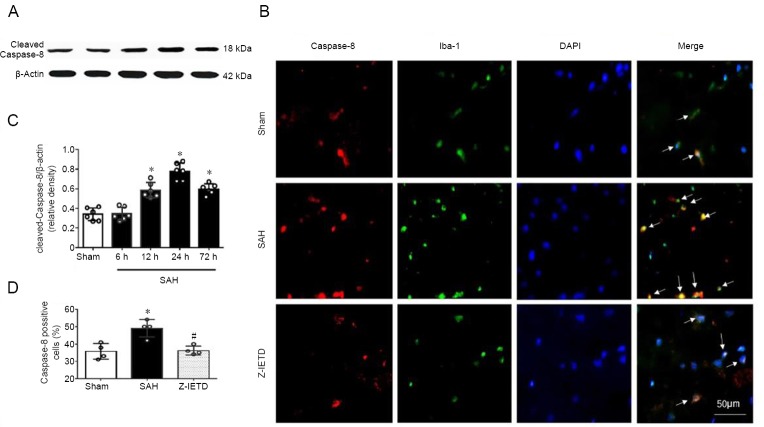
Compared with sham group, the expression of cleaved caspase-8 was significantly higher at 12 hours, peaked at 24 hours, and lower at 72 hours in the left cortex after SAH (P < 0.05; Figure 2A and C).
Figure 2.
Expression of caspase-8 at 24 hours after subarachnoid hemorrhage.
(A, C) The time course of cleaved caspase-8 after SAH was assessed using western blot assay. (B, D) Representative immunofluorescence and quantitative analyses for caspase-8 (red) in Iba-1-positive microglia (green) following SAH. Scale bar: 50 μm. Data are expressed as the mean ± SD (n = 6 per group in A, C; n = 4 per group in B, D). *P < 0.05, vs. sham group; #P < 0.05, vs. SAH group (one-way analysis of variance followed by Tukey’s post hoc test).
Double immunofluorescence staining revealed that the immunopositivity of caspase-8 was upregulated in microglia in the left cortex at 24 hours post-SAH (P < 0.05 versus the sham group) and was decreased in the Z-IETD-FMK group (P < 0.05 versus SAH + vehicle group; Figure 2B and D).
Z-IETD-FMK attenuates brain injury following SAH
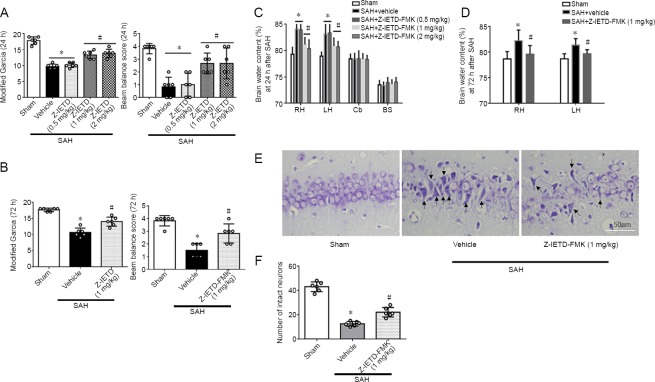
Neurological function was significantly impaired at 24 hours in SAH + vehicle group when compared with sham group (P < 0.05; Figure 3A). However, middle and high dosages of Z-IETD-FMK attenuated neurological deficits compare to SAH + vehicle group (P < 0.05; Figure 3A). SAH induction significantly increased the brain water content compared with the sham group (P < 0.05; Figure 3C). Treatment with middle or high doses of Z-IETD-FMK reduced brain water content (P < 0.05 vs. SAH + vehicle group; Figure 3C). The middle dosage of Z-IETD-FMK also improved neurological outcome and reduced brain edema at 72 hours after brain injury (P < 0.05, vs. SAH + vehicle group; Figure 3B and D). Nissl staining showed that Z-IETD-FMK significantly alleviated neuron injury at 24 hours following SAH (P < 0.05 vs. SAH + vehicle group; Figure 3E and F).
Figure 3.
Protective role of caspase-8 inhibitor Z-IETD-FMK after subarachnoid hemorrhage.
(A, B) Z-IETD-FMK attenuated neurological deficits at 24 hours and 72 hours following SAH. (C, D) Z-IETD-FMK reduced brain water content at 24 and 72 hours following SAH. (E, F) Nissl staining revealed that Z-IETD-FMK reduced the number of damaged hippocampal neurons at 24 hours following SAH. Arrows point to dead neurons. Scale bar: 50 μm. Data are expressed as the mean ± SD (n = 6 per group). *P < 0.05, vs. sham group; #P < 0.05, vs. SAH + vehicle group (Kruskal-Wallis test followed by Dunn’s post-hoc test, or one-way analysis of variance followed by Tukey’s post hoc test). BS: Brain stem; Cb: cerebellum; LH: left hemisphere; RH: right hemi-sphere.
Caspase-8 inhibition improves long-term neurological outcomes post SAH
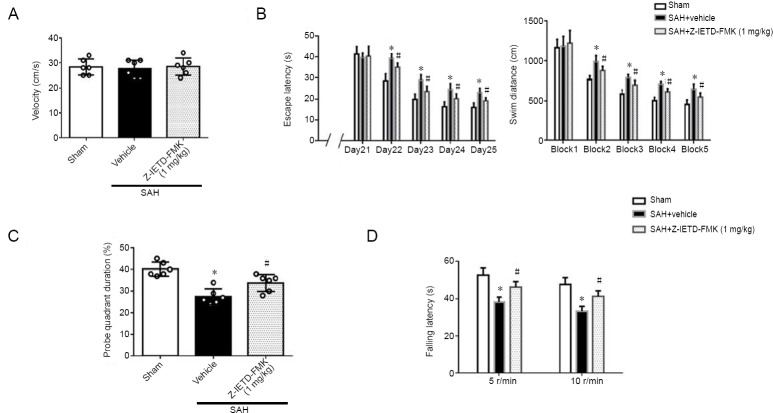
Swimming velocity was similar in all groups in the Morris Water Maze study. Compared with the sham group, the escape latency to find the platform and travel distance was significantly increased in the SAH + vehicle group (P < 0.05; Figure 4B). A decrease in the time to find the platform and a significantly shorter distance were observed in the Z-IETD-FMK treatment group (P < 0.05, vs. SAH + vehicle group; Figure 4B).
Figure 4.
Caspase-8 inhibition improves long-term neurological outcomes after subarachnoid hemorrhage.
(A) Velocity in the Morris water maze at 21 days following SAH. (B) Escape latency and swim distance in the Morris water maze. (C) Probe quadrant duration in the Morris water maze at 26 days following SAH. (D) The latencies to fall on the rod rotating at 5 and 10 r/min at 27 days after SAH. Data are expressed as the mean ± SD (n = 6 per group). *P < 0.05, vs. sham group; #P < 0.05, vs. SAH + vehicle group (one-way analysis of variance followed by Tukey’s post hoc test).
In the probe quadrant trial, the rats spent less time in the third quadrant in the SAH + vehicle group when compared with the sham group. The percentage of time spent in the target quadrant was higher in the treatment groups than in the SAH + vehicle group. (P < 0.05; Figure 4C). In the rotarod test, Z-IETD-FMK treatment group significantly improved motor coordination at both 5 and 10 r/min (P < 0.05, vs. SAH + vehicle group; Figure 4D).
Caspase-8 regulates NLRP3 inflammasome activation
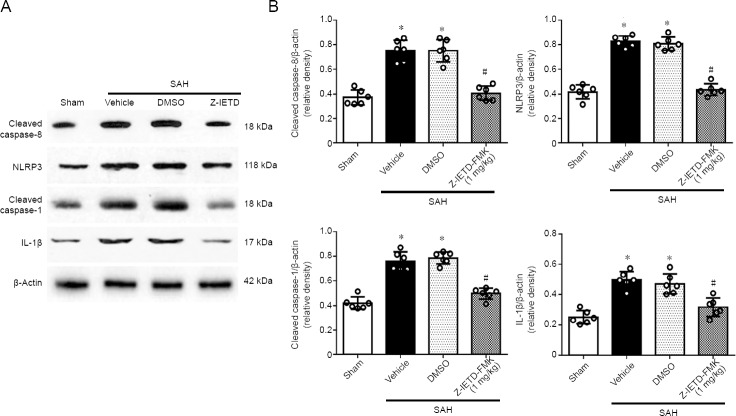
Compared with the sham group, western blot analysis showed that expression of cleaved caspase-8 was increased in left cortex at 24 hours following SAH. IETD-FMK treatment reduced cleaved caspase-8 expression compared to the vehicle group at 24 hours post SAH (P < 0.05; Figure 5). Double immunofluorescence staining also showed that caspase-8 immunopositivity in microglia was decreased after IETD-FMK treatment (Figure 2B and D). Compared with the sham group, the expressions of NLRP3, cleaved caspase-1, and IL-1β were increased dramatically in the SAH group (P < 0.05; Figure 5). However, Z-IETD-FMK attenuated the expressions of NLRP3, cleaved caspase-1 and IL-1β (P < 0.05, vs. SAH + vehicle group; Figure 5).
Figure 5.
Effect of Z-IETD-FMK on the expression of the caspase-8/NLRP3 pathway 24 hours after subarachnoid hemorrhage.
(A) Representative images of the western blot assay. (B) The relative density of each protein. Data are expressed as the mean ± SD (n = 6 per group). *P < 0.05, vs. sham group; #P < 0.05, vs. SAH + vehicle group (one-way analysis of variance followed by Tukey’s post hoc test). DMSO: Dimethyl sulfoxide; IL-1β: interleukin-1β; NLRP3: pyrin domain-containing 3.
Discussion
A previous study demonstrated that inhibition of casepase-8 could reduce ischemic damage after MCAO and anti-neuroinflammation function of inhibiting caspase-8 has not been investigated in SAH (Shabanzadeh et al., 2015). The current results suggest that caspase-8 inhibition decreases the neuropathological consequences of neurological disease. Temporal and spatial activation of caspase-8 in microglia/macrophages have been found in both human stroke and animal models of cerebral ischemia (Rodhe et al., 2016). Shabanzadeh et al. (2015) found that Z-IETD-FMK reduced infarct volumes and brain edema after brain ischemia. Krajewska et al. (2011)demonstrated that in a model of traumatic brain injury, inhibition caspase-8 reduced brain lesion volumes, motor function, spatial learning, and working memory performance. We found that endogenous caspase-8 expression was initially increased during the early stage following SAH. Medium or high dosages of caspase-8 inhibitor alleviated neurological damage and attenuated brain edema post-SAH when compared with SAH group. Furthermore, both Morris water maze and rotarod test water maze test and foot-fault test results demonstrated that long-term neurological functions were improved by inhibition of caspase-8 following SAH. Taken together, our results suggest that caspase-8 inhibition could provide neuroprotection after SAH.
Besides the execution of apoptosis, previous work has found that caspases play an important role in immunity regulation, cell differentiation, and cell fate determination (Crowder and El-Deiry, 2012). The initial inflammatory response after cerebral ischemia is mediated by microglia (Masahito et al., 2015; Yuan et al., 2018). In the present study, double immunofluorescence staining revealed that endogenous caspase-8 was expressed in microglia and upregulated at 24 hours following SAH. Rodhe et al. (2016) found that levels of caspase-8 in Iba1-positive cells in the peri-infarct area were increased at 24 and 48 hours after ischemia in double immunofluorescence staining. Kavanagh et al. (2015) found that caspase-8 deletion in myeloid cells inhibited microglia activation in a mouse model of neurodegeneration. These studies revealed that caspase-dependent signaling was involved in microglia pro-inflammatory activation and associated neurotoxicity.
The NLRP3 inflammasome is a multi-protein complex for caspase-1 activation and IL-1β maturation, and is in-volved in the innate immune responses triggered by cellular stress or infection (Shao et al., 2016). In a model of cerebral ischemia, Yang et al. (2014) reported that NLRP3 inhibition attenuated brain injuries through alleviation of inflammation. In an intracerebral hemorrhage model, the NLRP3 inflammasome amplified the inflammatory response and inflammatory damage, and neurological functions were improved after NLRP3 knockdown (Ma et al., 2014). In our study, the expression of NLRP3 was also increased after SAH. Recent studies have found that the NLRP3 inflammasome contributes to neuroinflammation after SAH injury, and inhibition of the NLRP3 inflam-masome by pharmacological treatment could ameliorate brain damage (Li et al., 2016; Liu et al., 2018). There is growing evidence that caspase-8 contributes to NLRP3 activation. Research has showed that active caspase-8 induces the activation of NLRP3-associated caspase-1 and the maturation of IL-1β (Lawlor et al., 2015). In acute glaucoma, Chi et al. (2014) found that caspase-8 regulated activation of the NLRP3 inflammasome and the production of IL-1β. In the present study, we found that Z-IETD-FMK dramatically reduced the expression of NLRP3, caspase-1, and IL-1β after SAH. Our results indicate that caspase-8/NLRP3 pathway suppression could offer neuroprotection for the treatment of SAH.
This study has some limitations. First, caspase-8 plays an important role in apoptosis and we cannot exclude the possibility that inhibition of caspase-8 had an anti-apoptotic effect on SAH. Further studies should explore the possible underlying mechanisms of neuroprotection after caspase-8 inhibition.
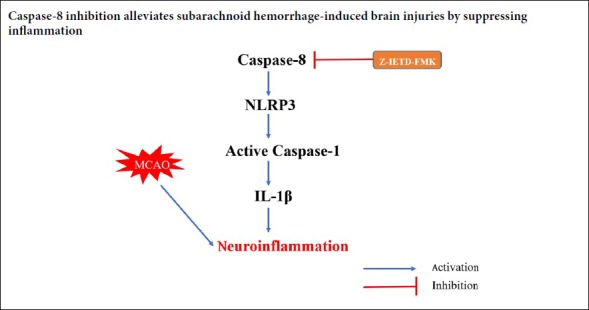
To conclude, we found that casepase-8 elevation occurred after SAH and a caspase-8 inhibitor alleviated neuroinflammation and improved neurological outcome, potentially via suppression of NLRP3 after SAH in rats. This finding supports the role of caspase-8 in alleviating neuroinflammation, and suggests that caspase-8 could represent a therapeutic target for treatment after middle cerebral artery occlusion.
Footnotes
Conflicts of interest: None declared.
Financial support: This study was supported by Clinical Scientific Foundation of Zhejiang Medical Association of China, No. 2018ZYC-A09 (to HL). The funding body played no role in the study design, collection, analysis and in-terpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Institutional review board statement: All experiments for this study were approved by the Institutional Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University, China (approved No. 2016-193) on February 25, 2016.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Min Cheol Chang, University of Ulsan Colleghe of Medicine, Republic of Korea; Anupom Borah, Assam University, India.
Funding: This study was supported by Clinical Scientific Foundation of Zhejiang Medical Association of China, No. 2018ZYC-A09 (to HL).
P-Reviewers: Chang MC, Borah A; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Cason N, Hindle A, Yu J, Song LP; T-Editor: Jia Y
References
- 1.Bromley-Brits K, Deng Y, Song W. Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. J Vis Exp. 2011:2920. doi: 10.3791/2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, Cano J, Brundin P, Englund E, Venero JL, Joseph B. Caspase signalling controls microglia activation and neurotoxicity. Nature. 2011;472:319–324. doi: 10.1038/nature09788. [DOI] [PubMed] [Google Scholar]
- 3.Chi W, Li F, Chen H, Wang Y, Zhu Y, Yang X, Zhu J, Wu F, Ouyang H, Ge J, Weinreb RN, Zhang K, Zhuo Y. Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1beta production in acute glaucoma. Proc Natl Acad Sci U S A. 2014;111:11181–11186. doi: 10.1073/pnas.1402819111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowder RN, El-Deiry WS. Caspase-8 regulation of TRAIL-mediated cell death. Exp Oncol. 2012;34:160–164. [PubMed] [Google Scholar]
- 5.de Oliveira Manoel AL, Macdonald RL. Neuroinflammation as a target for intervention in subarachnoid hemorrhage. Front Neurol. 2018;9:292. doi: 10.3389/fneur.2018.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamm RJ, Pike BR, O’Dell DM, Lyeth BG, Jenkins LW. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma. 1994;11:187–196. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- 7.Kanamaru H, Suzuki H. Potential therapeutic molecular targets for blood-brain barrier disruption after subarachnoid hemorrhage. Neural Regen Res. 2019;14:1138–1143. doi: 10.4103/1673-5374.251190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kavanagh E, Burguillos MA, Carrillo-Jimenez A, Oliva-Martin MJ, Santiago M, Rodhe J, Joseph B, Venero JL. Deletion of caspase-8 in mouse myeloid cells blocks microglia pro-inflammatory activation and confers protection in MPTP neurodegeneration model. Aging (Albany N Y) 2015;7:673–689. doi: 10.18632/aging.100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krajewska M, You Z, Rong J, Kress C, Huang X, Yang J, Kyoda T, Leyva R, Banares S, Hu Y, Sze CH, Whalen MJ, Salmena L, Hakem R, Head BP, Reed JC, Krajewski S. Neuronal deletion of caspase 8 protects against brain injury in mouse models of controlled cortical impact and kainic acid-induced excitotoxicity. PLoS One. 2011;6:e24341. doi: 10.1371/journal.pone.0024341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kristian T, Balan I, Schuh R, Onken M. Mitochondrial dysfunction and nicotinamide dinucleotide catabolism as mechanisms of cell death and promising targets for neuroprotection. J Neurosci Res. 2011;89:1946–1955. doi: 10.1002/jnr.22626. [DOI] [PubMed] [Google Scholar]
- 11.Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D’Cruz AA, Hall C, Kaur Spall S, Anderton H, Masters SL, Rashidi M, Wicks IP, Alexander WS, Mitsuuchi Y, Benetatos CA, Condon SM, Wong WW, Silke J, Vaux DL, Vince JE. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun. 2015;6:6282. doi: 10.1038/ncomms7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Chen J, Mo H, Chen J, Qian C, Yan F, Gu C, Hu Q, Wang L, Chen G. Minocycline protects against NLRP3 inflammasome-induced inflammation and P53-associated apoptosis in early brain injury after subarachnoid hemorrhage. Mol Neurobiol. 2016;53:2668–2678. doi: 10.1007/s12035-015-9318-8. [DOI] [PubMed] [Google Scholar]
- 13.Liu FY, Cai J, Wang C, Ruan W, Guan GP, Pan HZ, Li JR, Qian C, Chen JS, Wang L, Chen G. Fluoxetine attenuates neuroinflammation in early brain injury after subarachnoid hemorrhage: a possible role for the regulation of TLR4/MyD88/NF-kappaB signaling pathway. J Neuroinflammation. 2018;15:347. doi: 10.1186/s12974-018-1388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Q, Chen S, Hu Q, Feng H, Zhang JH, Tang J. NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann Neurol. 2014;75:209–219. doi: 10.1002/ana.24070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet. 2017;389:655–666. doi: 10.1016/S0140-6736(16)30668-7. [DOI] [PubMed] [Google Scholar]
- 16.Rodhe J, Burguillos MA, de Pablos RM, Kavanagh E, Persson A, Englund E, Deierborg T, Venero JL, Joseph B. Spatio-temporal activation of caspase-8 in myeloid cells upon ischemic stroke. Acta Neuropathol Commun. 2016;4:92. doi: 10.1186/s40478-016-0365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shabanzadeh AP, D’Onofrio PM, Monnier PP, Koeberle PD. Targeting caspase-6 and caspase-8 to promote neuronal survival following ischemic stroke. Cell Death Dis. 2015;6:e1967. doi: 10.1038/cddis.2015.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao A, Wu H, Hong Y, Tu S, Sun X, Wu Q, Zhao Q, Zhang J, Sheng J. Hydrogen-rich saline attenuated subarachnoid hemorrhage-induced early brain injury in rats by suppressing inflammatory response: possible involvement of NF-kappaB pathway and NLRP3 inflammasome. Mol Neurobiol. 2016;53:3462–3476. doi: 10.1007/s12035-015-9242-y. [DOI] [PubMed] [Google Scholar]
- 19.Shiba M, Suzuki H. Lessons from tenascin-C knockout mice and potential clinical application to subarachnoid hemorrhage. Neural Regen Res. 2019;14:262–264. doi: 10.4103/1673-5374.244789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon F, Floros N, Ibing W, Schelzig H, Knapsis A. Neurotherapeutic potential of erythropoietin after ischemic injury of the central nervous system. Neural Regen Res. 2019;14:1309–1312. doi: 10.4103/1673-5374.253507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie Z, Enkhjargal B, Reis C, Huang L, Wan W, Tang J, Cheng Y, Zhang JH. Netrin-1 preserves blood-brain barrier integrity through deleted in colorectal cancer/focal adhesion kinase/RhoA signaling pathway following subarachnoid hemorrhage in rats. J Am Heart Assoc. 2017;6:e 005189. doi: 10.1161/JAHA.116.005198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie Z, Huang L, Enkhjargal B, Reis C, Wan W, Tang J, Cheng Y, Zhang JH. Recombinant Netrin-1 binding UNC5B receptor attenuates neuroinflammation and brain injury via PPARgamma/NFkappaB signaling pathway after subarachnoid hemorrhage in rats. Brain Behav Immun. 2018;69:190–202. doi: 10.1016/j.bbi.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang F, Wang Z, Wei X, Han H, Meng X, Zhang Y, Shi W, Li F, Xin T, Pang Q, Yi F. NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J Cereb Blood Flow Metab. 2014;34:660–667. doi: 10.1038/jcbfm.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan ZJ, He XY, Yuan P, Zheng XM, Li XG. Morroniside improves the neurological function in intracerebral hemorrhage rats by inhibiting inflammatory response. Zhongguo Zuzhi Gongcheng Yanjiu. 2018;22:1217–1222. [Google Scholar]
- 26.Zhang X, Wu Q, Zhang Q, Lu Y, Liu J, Li W, Lv S, Zhou M, Zhang X, Hang C. Resveratrol attenuates early brain injury after experimental subarachnoid hemorrhage via inhibition of NLRP3 inflammasome activation. Front Neurosci. 2017;11:611. doi: 10.3389/fnins.2017.00611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang XS, Wu Q, Wu LY, Ye ZN, Jiang TW, Li W, Zhuang Z, Zhou ML, Zhang X, Hang CH. Sirtuin 1 activation protects against early brain injury after experimental subarachnoid hemorrhage in rats. Cell Death Dis. 2016;7:e2416. doi: 10.1038/cddis.2016.292. [DOI] [PMC free article] [PubMed] [Google Scholar]