Keywords: acute cerebral infarction, Barthel Index, cattle encephalon glycoside and ignotin, modified Rankin Scale, National Institutes of Health Stroke Scale, neuroprotectants, recovery rate, stroke
Abstract
Cattle encephalon glycoside and ignotin (CEGI) injection is a compound preparation formed by a combination of muscle extract from healthy rabbits and brain gangliosides from cattle, and it is generally used as a neuroprotectant in the treatment of central and peripheral nerve injuries. However, there is still a need for high-level clinical evidence from large samples to support the use of CEGI. We therefore carried out a prospective, multicenter, randomized, double-blind, parallel-group, placebo-controlled study in which we recruited 319 patients with acute cerebral infarction from 16 centers in China from October 2013 to May 2016. The patients were randomized at a 3:1 ratio into CEGI (n = 239; 155 male, 84 female; 61.2 ± 9.2 years old) and placebo (n = 80; 46 male, 34 female; 63.2 ± 8.28 years old) groups. All patients were given standard care once daily for 14 days, including a 200 mg aspirin enteric-coated tablet and 20 mg atorvastatin calcium, both taken orally, and intravenous infusion of 250–500 mL 0.9% sodium chloride containing 40 mg sodium tanshinone IIA sulfonate. Based on conventional treatment, patients in the CEGI and placebo groups were given 12 mL CEGI or 12 mL sterile water, respectively, in an intravenous drip of 250 mL 0.9% sodium chloride (2 mL/min) once daily for 14 days. According to baseline National Institutes of Health Stroke Scale scores, patients in the two groups were divided into mild and moderate subgroups. Based on the modified Rankin Scale results, the rate of patients with good outcomes in the CEGI group was higher than that in the placebo group, and the rate of disability in the CEGI group was lower than that in the placebo group on day 90 after treatment. In the CEGI group, neurological deficits were decreased on days 14 and 90 after treatment, as measured by the National Institutes of Health Stroke Scale and the Barthel Index. Subgroup analysis revealed that CEGI led to more significant improvements in moderate stroke patients. No drug-related adverse events occurred in the CEGI or placebo groups. In conclusion, CEGI may be a safe and effective treatment for acute cerebral infarction patients, especially for moderate stroke patients. This study was approved by the Ethical Committee of Peking University Third Hospital, China (approval No. 2013-068-2) on May 20, 2013, and registered in the Chinese Clinical Trial Registry (registration No. ChiCTR1800017937).
Chinese Library Classification No. R453; R741; R969.4
Introduction
Acute cerebral infarction (ACI) is one of the most common clinical cardiovascular, cerebrovascular, and neurological diseases, and accounts for about 70% of all stroke (Cheng et al., 2015). It is estimated that ACI may lead to 6.2 million mortalities annually worldwide, and it is fast becoming a major challenge to public health (Inoue et al., 2006; You et al., 2017). An ACI can increase blood viscosity and cause thrombus formation and vascular occlusion in the brain, followed by ischemia and hypoxia in brain tissues, resulting in brain dysfunction (Li et al., 2018; Schregel et al., 2018). Patients with ACI have many health problems that may be associated with age, sex, dementia, stroke severity degree, and other factors (Nakamura et al., 2018).
Currently, conventional therapy with western medicines primarily consist of thrombolysis, controlling the cerebral edema, preventing and treating complications, restoring blood supply to the ischemic area, controlling hypertension, improving microcirculation, reducing blood viscosity, and the use of cerebral protection agents (Cheng et al., 2015). Among these therapies, thrombolytic therapy and cerebral protection are two effective choices for the treatment of ACI. Thrombolytic therapy is generally used as the initial therapy (Tang et al., 2015), and was the Grade-I recommendation in the guide for the diagnosis and treatment of ACI in China in 2010 based on the national conditions and clinical experience (Liu et al., 2018). However, because thrombolysis has a relatively short therapeutic time window, many patients miss this effective time window for thrombolytic treatment. Furthermore, researchers are increasingly demonstrating that revascularization alone does not assure positive clinical outcomes; meanwhile, numerous studies have indicated that neuroprotectants can give beneficial results, at least in animal experiments (Rong et al., 2013; Li et al., 2016).
Cattle encephalon glycoside and ignotin (CEGI) injection (drug approval No. H22025046; Jilin Sihuan Pharmaceutical Co. Ltd., Jilin, China) was approved by the Chinese Food and Drug Administration in 2003. It is a compound preparation formed by a combination of muscle extract from healthy rabbits and brain gangliosides from cattle, and it is used as a neuroprotectant in the treatment of central and peripheral nerve injuries in China. CEGI is mainly composed of polypeptides, a number of gangliosides, free amino acids, and nucleic acids. Monosialotetrahexosy-1 ganglioside (GM-1), an active component in CEGI, is effective in the treatment of nervous system diseases, including in the treatment of ACI (Tang et al., 2006). Furthermore, polypeptides were reported to have a therapeutic effect in mouse models of ischemic brain damage, and these are also a component of CEGI (Baek et al., 2014). However, as yet there have been no high-level clinical studies in large samples that investigate the use of CEGI in ACI (Li et al., 2016). Therefore, the present study was conducted to evaluate the effectiveness and safety of CEGI in the treatment of ACI.
Participants and Methods
Study design
This was a prospective, multicenter, randomized, double-blind, parallel-group, placebo-controlled study. The study was conducted in accordance with the Declaration of Helsinki, and was approved by the Ethical Committee of Peking University Third Hospital, China (approval No. 2013-068-2) on May 20, 2013 (Additional file 1 (1.2MB, pdf) ). Written informed consent (Additional file 2 (238.6KB, pdf) ) was obtained from the patients or their family members. The study was registered at the Chinese Clinical Trial Registry (registration No. ChiCTR1800017937), and followed the CONsolidated Standards Of Reporting Trials (CONSORT) guidelines (Additional file 3).
Additional file 3.
CONSORT checklist of information to include when reporting a randomised trial
| Section/Topic | Item No | Checklist item | Reported on page No |
|---|---|---|---|
| Title and abstract | |||
| 1a | Identification as a randomised trial in the title | 1-2 | |
| 1b | Structured summary of trial design, methods, results, and conclusions (for specific guidance see CONSORT for abstracts) | 1-2 | |
| Introduction | |||
| Background and | 2a | Scientific background and explanation of rationale | 2 |
| objectives | 2b | Specific objectives or hypotheses | 3 |
| Methods | |||
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio | 3 |
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons | 3 | |
| Participants | 4a | Eligibility criteria for participants | 3 |
| 4b | Settings and locations where the data were collected | 3 | |
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | 6 |
| Outcomes | 6a | Completely defined pre-specified primary and secondary outcome measures, including how and when they | 6 |
| were assessed | |||
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | 3 | |
| Sample size | 7a | How sample size was determined | 3 |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | 3 | |
| Randomisation: | |||
| Sequence | 8a | Method used to generate the random allocation sequence | 3 |
| generation | 8b | Type of randomisation; details of any restriction (such as blocking and block size) | 4 |
| Allocation | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | 4 |
| concealment | |||
| mechanism | |||
| Implementation | 10 | Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions | 4 |
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how | 4 |
| 11b | If relevant, description of the similarity of interventions | 7 | |
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | 7 |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | 7 | |
| Results | |||
| Participant flow (a diagram is strongly | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome | 5 |
| recommended) | 13b | For each group, losses and exclusions after randomisation, together with reasons | 7 |
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | 7 |
| 14b | Why the trial ended or was stopped | 7 | |
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | 8 |
| Numbers analysed | 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | 9 |
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) | 10 |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | 11 | |
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing | 12 |
| pre-specified from exploratory | |||
| Harms | 19 | All important harms or unintended effects in each group (for specific guidance see CONSORT for harms) | 13 |
| Discussion | |||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses | 16 |
| Generalisability | 21 | Generalisability (external validity, applicability) of the trial findings | 14 |
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence | 15 |
| Other information | |||
| Registration | 23 | Registration number and name of trial registry | 16 |
| Protocol | 24 | Where the full trial protocol can be accessed, if available | 16 |
| Funding | 25 | Sources of funding and other support (such as supply of drugs), role of funders | 16 |
Sample size calculation
Based on previous literature (Wang et al., 2006), the ratio of good clinical outcome rates (modified Rankin Scale (mRS) scores) between the placebo and CEGI groups (26.76 vs. 50.64%, respectively) was used to determine the ratio of case numbers for the CEGI and placebo groups (3:1). On this basis, the power was set at 80% and α = 0.05. Power Analysis and Sample Size (PASS, 2008, NCSS, LLC., Kaysville, UT, USA) software was used to calculate the sample size of the CEGI group as 198 cases and the placebo group as 66 cases. Based on a shedding rate of approximately 20% and group randomization, it was calculated that approximately 320 cases were needed for this study.
Participants
A total of 319 eligible patients were prospectively recruited from 16 centers (Peking University Third Hospital; The First Affiliated Hospital of Nanchang University; Beijing Chao-Yang Hospital, Capital Medical University; The Second Hospital of Jilin University; Baotou Central Hospital; Jining No.1 People’s Hospital; Affiliated Hospital of Jining Medical University; The First Hospital of Luoyang City; Zhuozhou Hospital; Xuzhou Central Hospital; General Hospital of Xuzhou Mining Group; Huang Gang Central Hospital; The First Hospital of Shijiazhuang City; People’s Hospital of Yichun City; Affiliated Hospital of Jinggangshan University; Jiangxi Hospital of Integrated Chinese and Western Medicine) in China from October 2013 to May 2016. Patients were randomized using a computer-generated randomization sequence (3:1) to either the CEGI group (n = 239) or the placebo group (n = 80). A flowchart for the study population is shown in Figure 1. Treatment assignment was masked from all investigators, study personnel, and patients throughout the trial. Patients eligible for this study (a) were aged between 18 and 75 years old who diagnosed with atherosclerotic cerebral infarction; (b) were within 48 hours after ACI onset; (c) if they had a history of limb paralysis caused by cerebrovascular disease, their mRS score was 0–1 (Uyttenboogaart et al., 2005); (d) had a score of 5–22 points on the National Institutes of Health Stroke Scale (NIHSS) (Fischer et al., 2005); and (e) signed an informed consent form. Patients were excluded if they: (a) were diagnosed with a transient ischemic attack, non-responsible multi lacunar infarction, cerebral hemorrhage after infarction, subarachnoid hemorrhage, and/or post-circulation ischemia; (b) had hemiplegia caused by a brain tumor, brain injury, brain parasitic disease, or metabolic disorder, or a cerebral embolism caused by rheumatic heart disease, coronary heart disease, or other heart disease with atrial fibrillation; (c) had gangliosidoses (such as Tay-Sachs diesease); (d) were thrombolytic patients or peptic ulcer patients who could not take aspirin; (e) had severe primary cardiovascular, liver (alanine aminotransferase or aspartate transaminase exceeding a 1.5-fold upper range value), renal (blood urea nitrogen (BUN) exceeding a 1.2-fold upper range value, creatinine exceeding a 2-fold upper range value), hematopoietic, and/or endocrine system diseases; (f) presented allergies to the study drug or protein; (g) were drug- or alcohol-dependent; (h) were pregnant or lactating; or (i) had participated in other clinical trials within the previous 3 months.
Figure 1.
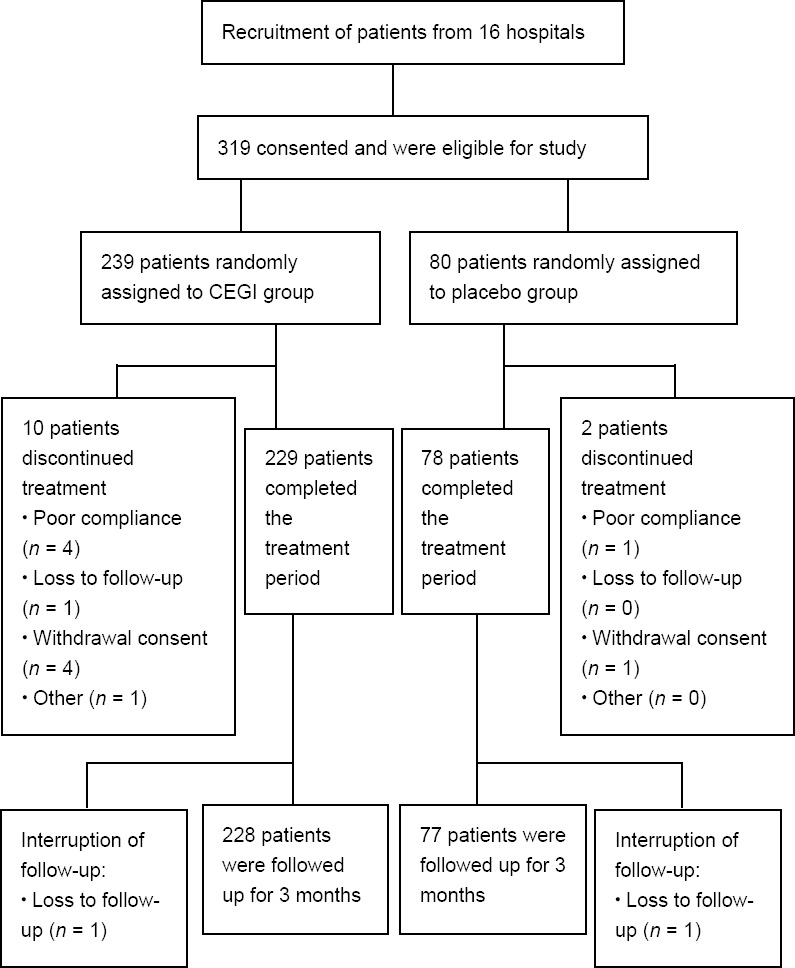
Study design and CONSORT diagram showing the flow of participants.
CEGI: Cattle encephalon glycoside and ignotin; CONSORT: CONsolidated Standards Of Reporting Trials.
Interventions
All patients were given standard care once daily for 14 days, including 200 mg aspirin enteric-coated tablet (drug approval No. J20080078, Bayer Medical and Health Co., Ltd., Beijing, China) and 20 mg atorvastatin calcium (drug approval No. YBH19122005, Pfizer Pharmaceuticals Ltd., Beijing, China), both orally, and intravenous infusion of 250–500 mL 0.9% sodium chloride containing 40 mg sodium tanshinone IIA sulfonate (drug approval No. WS-10001-(HD-1014)-2002, Shanghai First Biochemical Pharmaceutical Co., Ltd., Shanghai, China). Based on conventional treatment, the CEGI and placebo groups were given 12 mL CEGI (1 mL contained 2.56–3.84 mg polypeptides, 40–60 µg gangliosides (measured by lipid-bound sialic acid), 1.40–1.90 mg free amino acids, 0.75–1.10 mg total nitrogen, and 0.24–0.36 mg nucleic acids) or 12 mL sterile water, respectively, in an intravenous drip of 250 mL 0.9% sodium chloride (2 mL/min) once daily for 14 days, with an interval between other intravenous drug drips of 2 hours. During the study, dehydrating agents, diuretics, antihypertensive drugs, lipid-lowering drugs, antidiabetics, vitamin B, and antibiotics were allowed, while heparins, anti-fibrinogen drugs, and neuroprotectants (such as cytophosphate, edaravone, duxil, and piracetam) were prohibited.
Outcome measures
Basic information and disease history of all patients was recorded before treatment. The primary outcome measure was the mRS score at 3 months after treatment. Based on the mRS score, 0–1 was defined as “no symptoms or no significant disability” (0–1, good outcome), 2–3 was defined as “slight/moderate disability”, and 4–6 was defined as “severe disability or dead” (> 1, poor outcome).
The secondary endpoints were the scores from activities of daily living in the Barthel Index (BI) (Quinn et al., 2011) and the NIHSS scores. These were evaluated before treatment and on days 7, 14, and 90 after treatment. BI scores were out of 100, and were classified as follows: (1) 100, independence; (2) 75–95, mild dependence; (3) 50–70, moderate dependence; (4) 25–45, severe dependence; and (5) 0–20, exclusive dependence. The NIHSS score evaluated the degree of neurological deficiency. Based on the NIHSS clinical efficacy standard, the degrees of recovery were classified as follows: (1) 91–100% decrease of NIHSS, recovery; (2) 46–90% decrease, significant progress; (3) 18–45% decrease, progress, (4) ≤ 17% increase or decrease, no change; and (5) > 18% increase, deterioration. In addition, patients were divided into three groups based on their baseline NIHSS scores: mild (NIHSS score < 7), moderate (NIHSS score 8–16), and severe (NIHSS score 17–22) stroke. In this study, there were no patients with severe stroke in the placebo group, so the subgroup analysis for severe stroke could not be performed; thus, only the mild and moderate subgroups were statistically analyzed to evaluate the efficacy of CEGI in different degrees of ACI.
The safety indicators used were the detection of alterations in vital signs, routine blood and urine examinations, liver and kidney function parameters, and electrocardiographic examinations before and during the treatment. Adverse effects were recorded and timely treatment was administered if needed. Patients with adverse effects were followed up until the adverse effects were resolved.
Statistical analysis
Three statistical analysis sets were constructed. The efficacy analysis set was defined as the full analysis set (FAS), which included all patients from the CEGI group (who received at least one dose of the study drug and had at least one follow-up record) and all patients from the placebo group. As a subset of FAS, the per-protocol set (PPS) included all patients who had good compliance, did not take prohibited drugs, and completed the case report form. The safety analysis set (SS) included those patients who received at least one dose of the study drug and had baseline safety data and at least one follow-up safety record. The primary and secondary efficacy indicators were analyzed in the FAS and PPS, while the SS was the main set used for safety assessment in this study.
Statistical analysis was performed using SAS 9.1.3 software (SAS Institute, Inc., Cary, NC, USA). Continuous variables were presented as the number of cases and the mean, standard deviation, median, minimum, and maximum values. For categorical variables, data were expressed as the number of categorical cases or percentages, and analyzed using a Z test. The one-sided Cochran–Mantel–Haenszel chi-squared test was used for the statistical comparison of mRS scores between the two groups. A rank-sum test and independent t-test were performed at baseline and on days 7, 14, and 90 to detect differences in the activities of daily living and NIHSS scores between the two groups. The paired t-test was used to investigate differences in blood pressure before and after treatment in both groups, and the Fisher’s exact test was used to test differences in adverse events between the two groups. A level of P < 0.05 was considered statistically significant.
Results
Compliance analysis and baseline characteristics of ACI patients with CEGI and placebo treatment
Trial medication was given to 319 patients, with 239 receiving CEGI and 80 receiving placebo. Figure 1 shows the patient flow and identifies reasons for dropout from the study. The rate of discontinuation was 3.95% (14/319). The final follow-up rates in the CEGI group and in the placebo group were 95.4% (228/239) and 96.25% (77/80), respectively, with no significant difference between the two groups (P > 0.05). All baseline characteristics were well balanced across the two groups and there were no significant differences in these characteristics (Table 1). Furthermore, there were 307 patients in the FAS analysis (CEGI group, n = 229; placebo group, n = 78), 291 patients in the PPS analysis (CEGI group, n = 218; placebo group, n = 73), and 315 patients in the SS analysis (CEGI group, n = 236; placebo group, n = 79).
Table 1.
Comparison of baseline data of acute cerebral infarction patients between the cattle encephalon glycoside and ignotin (CEGI) group and the placebo group
| Baseline data | CEGI group (n = 239) | Placebo group (n = 80) | Statistics | P-value |
|---|---|---|---|---|
| Age (yr) | 61.2±9.2 | 63.2±8.28 | t=1.74 | 0.083 |
| Height (cm) | 166.3±6.94 | 165.1±7.11 | Z=1.08 | 0.282 |
| Weight (kg) | 66.3±9.52 | 66.3±8.39 | Z=0.222 | 0.824 |
| Sex | χ2=1.39 | 0.234 | ||
| Male | 155 (64.9) | 46 (57.5) | ||
| Female | 84 (35.1) | 34 (42.5) | ||
| Respiratory rate (times/min) | 18.9±1.49 | 18.8±1.57 | Z=0.179 | 0.858 |
| Body temperature (°C) | 36.5±0.33 | 36.5±0.32 | Z=0.304 | 0.761 |
| Systolic blood pressure (mmHg) | 146.5±20.8 | 147.4±23.1 | Z=0.237 | 0.812 |
| Diastolic blood pressure (mmHg) | 86.7±13 | 84.7±12.2 | Z=1.26 | 0.207 |
| Time of progression (h) | 22.5±13.8 | 25.1±14.5 | Z=1.49 | 0.136 |
| Allergic history | χ2=0.316 | 0.854 | ||
| Yes | 7 (2.93) | 3 (3.75) | ||
| No | 220 (92.1) | 72 (90.0) | ||
| Unknown | 12 (5.02) | 5 (6.25) | ||
| Previous medical history | χ2=0.009 | 0.926 | ||
| Yes | 139 (58.2) | 47 (58.8) | ||
| No | 100 (41.8) | 33 (41.2) | ||
| Barthel Index scores | 64.1±18.8 | 63.38±20.2 | Z=0.117 | 0.907 |
| National Institute of Health Stroke Scale scores | 8.10±2.68 | 7.69±2.10 | Z=1.07 | 0.286 |
| Modified Rankin Scale scores | 2.76±0.78 | 2.80±0.85 | Z=0.219 | 0.827 |
Data are expressed as the mean ± SD except sex, allergic history, and previous medical history [n (%)]. The one-sided Cochran–Mantel– Haenszel (CMH) chi-squared test and independent t-test were used.
Efficacy of CEGI treatment in patients with ACI
mRS scores
There was a significant difference between the CEGI and placebo groups in the primary endpoint mRS scores on day 90 after treatment (P < 0.05), in favor of the CEGI group. The rate of patients with good outcomes was higher in the CEGI group than in the placebo group on day 90 after treatment (FAS, P = 0.016; PPS, P = 0.011; Figure 2A). The rate of disability in the CEGI group was lower than in the placebo group on day 90 after treatment (FAS, P = 0.007; PPS, P = 0.005; Figure 2B). For the subgroup of patients with mild stroke, there was no significant difference in the rate of disability between the CEGI and placebo groups on day 90 after treatment (FAS, P = 0.267; PPS, P = 0.232; Figure 3A). In contrast, in the subgroup of patients with moderate stroke, there was significant difference in the rate of disability between the two groups (FAS, P = 0.001; PPS, P = 0.001; Figure 3B).
Figure 2.
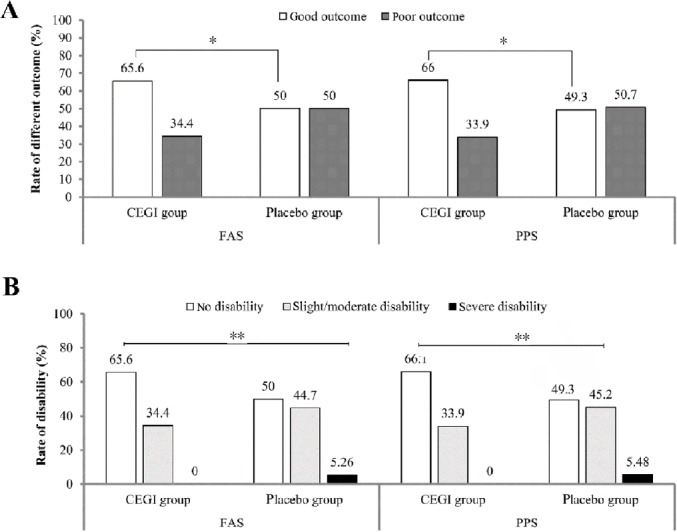
Comparison of modified Rankin Scale scores between the cattle encephalon glycoside and ignotin (CEGI) group and the placebo group on days 90; (A) the rate of good outcomes/poor outcomes and (B) the degree of disability in acute cerebral infarction patients.
The efficacy analysis set was defined as the full analysis set (FAS) (CEGI group, n = 229; placebo group, n = 78) and the per-protocol set (PPS) (CEGI group, n = 218; placebo group, n = 73). The Cochran–Mantel–Haenszel chi-squared test was used to compare the rates of outcomes in the CEGI and placebo groups; the rank-sum test was used to compare the rates of disability in the CEGI and placebo groups. *P < 0.05, **P < 0.01.
Figure 3.
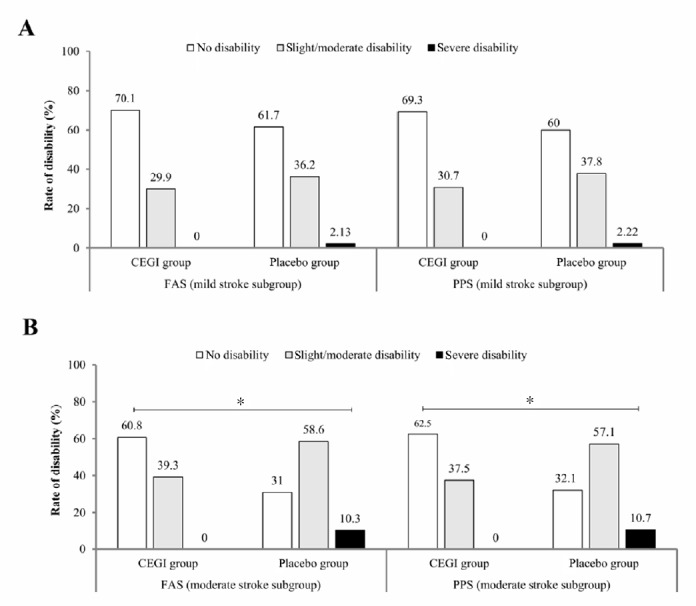
Comparison of the degree of disability using modified Rankin Scale scores of acute cerebral infarction patients between the cattle encephalon glycoside and ignotin (CEGI) subgroup and the placebo subgroup on day 90.
(A, B) Degree of disability in the mild (A) and moderate (B) stroke subgroups in the full analysis set (FAS) (CEGI group, n = 229; placebo group, n = 78) and the per-protocol set (PPS) (CEGI group, n = 218; placebo group, n = 73). The rank-sum test was used to compare the rates of disability between the CEGI and placebo subgroups. *P < 0.05.
NIHSS scores
In the FAS analysis, the NIHSS scores of both groups at baseline and on days 7 and 14 after treatment were not significantly different (P > 0.05), while there was significant difference in the NIHSS scores on day 90 after treatment between the two groups (P = 0.003; Table 2). In the PPS analysis, the NIHSS scores of both groups at baseline and on day 7 were not significantly different, while there were significant differences in the NIHSS scores on days 14 and 90 between the two groups (day 14, P = 0.046; day 90, P = 0.003), meaning that the improvement of neurological disorder became more apparent over time. Furthermore, Table 3 shows the NIHSS scores in the CEGI and placebo subgroups on days 7, 14, and 90 in both the FAS and PPS analyses. For the FAS analysis, when the degree of recovery using NIHSS scores were compared, there were significant differences between the groups on days 14 and 90, the degree of recovery in the CEGI group was higher than in the placebo group (day 14, P < 0.001; day 90, P = 0.003; Figure 4). Similar results were observed in the PPS analysis (day 14, P = 0.005; day 90, P = 0.003; Figure 5).
Table 2.
Comparison of National Institutes of Health Stroke Scale scores of acute cerebral infarction patients between the cattle encephalon glycoside and ignotin (CEGI) and placebo groups at baseline and on days 7, 14, and 90 after treatment
| CEGI group | Placebo group | t-value | P-value | ||
|---|---|---|---|---|---|
| FAS analysis | Before treatment | 8.08±2.66 | 7.68±2.12 | 1.35 | 0.177 |
| After treatment | |||||
| Days 7 | 5.96±2.4 | 6.42±4.99 | –0.79 | 0.433 | |
| Days 14 | 3.69±2.2 | 4.88±5.19 | 1.95 | 0.054 | |
| Days 90 | 2.54±1.9 | 3.53±2.65 | –2.99 | 0.004** | |
| PPS analysis | Before treatment | 8.14±2.67 | 7.74±2.16 | 1.28 | 0.203 |
| After treatment | |||||
| Days 7 | 5.99±2.41 | 5.96±2.35 | 0.08 | 0.933 | |
| Days 14 | 3.71±2.2 | 4.32±2.37 | –2 | 0.046* | |
| Days 90 | 2.51±1.85 | 3.53±2.66 | –3.04 | 0.003** |
Data are expressed as the mean ± SD, and analyzed using the rank-sum test and t-test. *P < 0.05, **P < 0.01. The full analysis set (FAS) consisted of 229 and 78 cases in in the CEGI and placebo groups, respectively. The per-protocol set (PPS) consisted of 218 and 73 cases in the CEGI and placebo groups, respectively.
Table 3.
Comparison of National Institutes of Health Stroke Scale scores of acute cerebral infarction patients between the cattle encephalon glycoside and ignotin (CEGI) group and the placebo group on days 7, 14, and 90 after treatment
| Mild stroke subgroup | t-value | P-value | Moderate stroke subgroup | t-value | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| CEGI group | Placebo group | CEGI group | Placebo group | ||||||
| FAS analysis | Days 7 | 4.85±1.59 | 4.71±1.73 | –0.52 | 0.605 | 7.22±2.55 | 9.17±6.98 | –1.5 | 0.145 |
| Days 14 | 3.04±1.68 | 3.36±1.69 | –1.1 | 0.272 | 4.42±2.48 | 7.27±7.52 | –2.05 | 0.049* | |
| Days 90 | 2.08±1.72 | 2.49±1.83 | –1.36 | 0.174 | 3.05±1.96 | 5.21±2.93 | –3.75 | < 0.001** | |
| PPS analysis | Days 7 | 4.88±1.61 | 4.73±1.75 | 0.5 | 0.621 | 7.2±2.56 | 7.93±1.78 | –1.73 | 0.089 |
| Days 14 | 3.05±1.67 | 3.31±1.68 | –0.88 | 0.38 | 4.42±2.48 | 5.93±2.46 | –2.85 | 0.005** | |
| Days 90 | 2.09±1.7 | 2.49±1.79 | –1.32 | 0.188 | 2.98±1.9 | 5.21±2.99 | –3.76 | < 0.0001** | |
Data are expressed as the mean ± SD. *P < 0.05, **P < 0.01 (rank-sum test and independent t-test). The full analysis set (FAS) in the mild stroke subgroup consisted of 122 and 48 cases in the CEGI and placebo groups, respectively; in the moderate stroke subgroup it consisted of 107 cases in the CEGI group and 30 cases in the placebo group. The per-protocol set (PPS) in the mild stroke subgroup consisted of 114 cases in the CEGI group and 45 in the placebo group; in the moderate stroke subgroup it consisted of 104 cases in the CEGI group and 28 cases in the placebo group.
Figure 4.
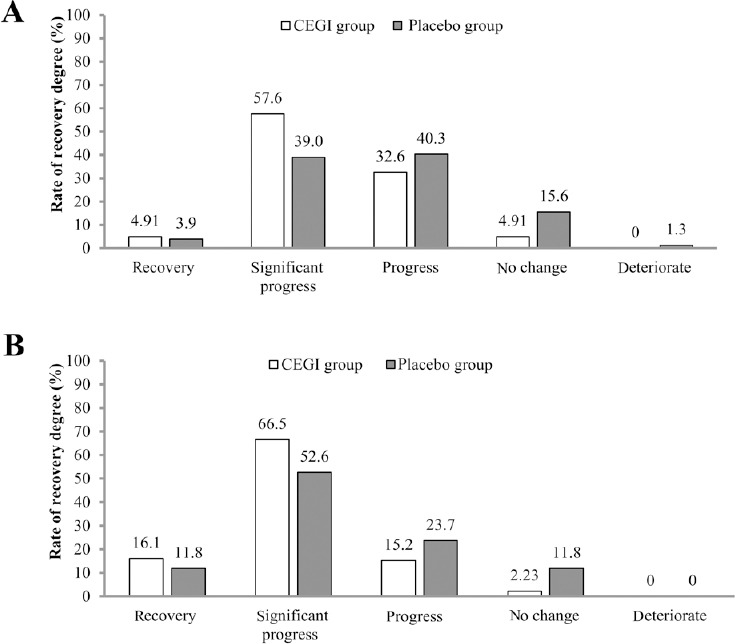
Full analysis set (FAS) analysis of the degree of recovery using the National Institutes of Health Stroke Scale scores of acute cerebral infarction patients in the cattle encephalon glycoside and ignotin (CEGI) group and the placebo group on days 14 (A) and 90 (B) after treatment.
The rank-sum test was used for comparison. The FAS consisted of 229 and 78 cases in the CEGI and placebo groups, respectively.
Figure 5.
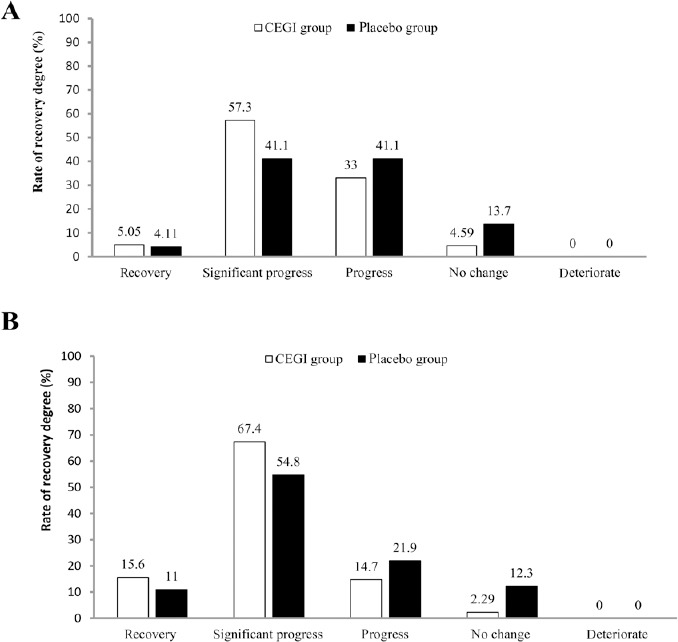
Per-protocol set (PPS) analysis of the degree of recovery using National Institutes of Health Stroke Scale scores of acute cerebral infarction patients in the cattle encephalon glycoside and ignotin (CEGI) group and the placebo group on days 14 (A) and 90 (B) after treatment.
The rank-sum test was used for comparison. The PPS consisted of 218 and 73 cases in the CEGI and placebo groups, respectively.
BI scores
In both the FAS and PPS analyses, there were significant differences in BI scores on days 14 and 90 after treatment between the CEGI and placebo groups (P < 0.05; Table 4).
Table 4.
Comparison of Barthel Index scores of acute cerebral infarction patients between the cattle encephalon glycoside and ignotin (CEGI) group and the placebo group at baseline and on days 7, 14, and 90 after treatment
| CEGI group | Placebo group | t-value | P-value | ||
|---|---|---|---|---|---|
| FAS analysis | Before treatment | 64.2±18.2 | 63.5±19.7 | 0.28 | 0.778 |
| After treatment | |||||
| Days 7 | 72.9±16.2 | 69.5±19.9 | 1.37 | 0.174 | |
| Days 14 | 82.2±14.6 | 76.6±18.2 | 2.45 | 0.016* | |
| Days 90 | 89.8±10.5 | 84.8±16.2 | 2.52 | 0.013* | |
| PPS analysis | Before treatment | 64.0±18.3 | 64.3±18.4 | –0.14 | 0.889 |
| After treatment | |||||
| Days 7 | 72.7±15.9 | 70.8±17.5 | 0.87 | 0.383 | |
| Days 14 | 82.1±14.7 | 77.8±16.1 | 2.1 | 0.037* | |
| Days 90 | 89.9±10.5 | 84.9±16.1 | 2.45 | 0.016* |
Data are expressed as the mean ± SD. *P < 0.05 (rank-sum test and independent t-test). The full analysis set (FAS) consisted of 229 and 78 cases in the CEGI and placebo groups, respectively; the per-protocol set (PPS) consisted of 218 and 73 cases in the CEGI and placebo groups, respectively.
Safety of CEGI treatment in patients with ACI
Analyses of vital signs and laboratory examinations
There were no significant differences between the CEGI and placebo groups in body temperature, respiration rate, systolic blood pressure, and diastolic blood pressure before and after treatment. However, after treatment, blood pressure (both systolic and diastolic) in CEGI and placebo groups was significantly lower than it was before treatment (P < 0.05; Table 5). This lowering of blood pressure may be because: (1) blood pressure was often elevated with cerebral infarction, for various reasons. To protect cerebral tissue in the ischemic area, blood pressure was not treated at the acute stage, so baseline blood pressure was higher. (2) In a more stable condition, stress factors were eliminated and antihypertensive drugs were used, so blood pressure dropped significantly after treatment.
Table 5.
Blood pressure before and after treatment, and the incidence of adverse events in acute cerebral infarction patients between the glycoside and ignotin (CEGI) and placebo groups
| CEGI group (n = 236) | Placebo group (n = 79) | Statistics | P-value | |
|---|---|---|---|---|
| Systolic blood pressure (mmHg) | ||||
| Before treatment | 146±20.8 | 147.3±23.2 | t=–0.29 | 0.768 |
| On days 90 after treatment | 138.2±14.7 | 137.7±13.8 | t=0.27 | 0.787 |
| Change | –8.39±15.4 | –8.74±15.3 | t=0.17 | 0.862 |
| P-value | < 0.05 | |||
| Diastolic blood pressure (mmHg) | ||||
| Before treatment | 86.6±13 | 84.9±12.1 | t=1.02 | 0.31 |
| On days 90 after treatment | 81.8±9.3 | 81.1±9.0 | t=0.63 | 0.528 |
| Change | –4.86±11.7 | –3.49±10.2 | t=–0.93 | 0.354 |
| P-value | < 0.05 | |||
| Adverse event [n (%)] | ||||
| All adverse events | 98 (41.5) | 29 (36.7) | F=0.51 | > 0.05 |
| Serious adverse event | 2 (0.85) | 1 (1.27) | ||
| Drug-related adverse events | 0 | 0 | F=1.0000 | |
| Drug-unrelated adverse events | 98 (41.5) | 29 (36.7) | ||
| Adverse events leading to the suspension of the study | 0 | 0 |
Data are expressed as the mean ± SD, except adverse events. The paired t-test was used to test differences in blood pressure between before and after treatment in both groups, and Fisher’s exact test was used to test differences in adverse events between the CEGI and placebo groups.
There were no significant differences between the two groups in results from routine blood and urine examinations, liver and kidney function parameters, and electrocardiographic examinations (P > 0.05). In the CEGI group, platelet elevation was detected in one patient (0.42%) and urinary erythrocyte elevation was detected in three patients (1.27%). In the placebo group, aspartate aminotransferase elevation was detected in two patients (2.53%), alanine aminotransferase elevation was detected in two patients (2.53%), alkaline phosphatase elevation was detected in one patient (1.27%), and glutamyl transpeptidase elevation was detected in one patient (1.27%).
Adverse effects
Adverse effects were observed in 98 patients (41.5%) in the CEGI group and 29 patients (36.7%) in the placebo group. Among them, serious adverse effects occurred in two patients from the CEGI group (0.85%) and one patient from the placebo group (1.27%). There were no drug-related adverse effects (adverse reactions). Adverse effects did not result in study termination for any patients in the two groups. There was no significant difference between the two groups in adverse effects (Table 5).
Discussion
ACI is a complex disease with high rates of mortality and disability, and represents a significant threat to human life. Its pathophysiological mechanisms are associated with lactate accumulation, free radical release, intracellular calcium overload, inflammatory process, apoptosis and other effects (Del Bene et al., 2012; Zhao et al., 2019). Previously, rapid thrombolysis and early cerebral protection are considered the two most important methods in the treatment of cerebral infarction (Broussalis et al., 2012; Okabe and Miyamoto, 2018). However, thrombolytic therapy for acute stroke is a complex issue, balancing the benefit of the reversal of ischemia against the risk of symptomatic cerebral hemorrhage, which is a major life-threatening complication of thrombolytics (Vidale and Agostoni, 2014). Recently, much research has therefore focused on the use of neuroprotectants in ACI treatment. Furthermore, considering the complex pathophysiological mechanisms of this disease, a multi-target drug therapy may lead to better efficacy in the treatment of ACI.
CEGI is a multi-target neuroprotective agent that includes GM-1, carnosine, free amino acids, and hypoxanthine. By acting on various targets in ACI, CEGI may interrupt the development of the pathophysiological processes that lead to poor outcomes following ACI. Most CEGI components can directly pass through the blood-brain barrier, ensuring a pharmaceutical effect in the treatment of central nervous system diseases. As a member of the ganglioside family, GM-1 is involved in optimal myelin formation and central axon stability (Stevenson et al., 2009), and one study reported that exogenous GM-1 treatment improved myelin sheath damage (Rong et al., 2013). A previous safety evaluation also showed that low-dose and combined use of GM-1 was possibly a better choice than routine medication in clinical practice for treatment of patients with acute ischemic cerebral stroke (Candelise and Ciccone, 2002; Zhao et al., 2016). Carnosine, another important component of CEGI, might reduce brain damage after ischemic stroke by maintaining normal glutathione levels and decreasing levels and activity of matrix metalloproteinase (Rajanikant et al., 2007). Carnosine may also suppress myelin degeneration, as was indicated in a pre-clinical study (Ma et al., 2012). Other components of CEGI, such as free amino acids and hypoxanthine, might contribute to the development and maintenance of the nervous system. Thus, because of its multiple active components, CEGI may perform its neuroprotective effect by interfering with a number of different targets, causing it to be more potent and effective than each component alone would be.
Recently, many researchers have studied the efficacy of neuroprotective reagents in extending the therapeutic time window for thrombolysis, which may inhibit cell death and block reperfusion injury (Xu et al., 2018). CEGI has been widely used in the treatment of central and peripheral nerve injuries with a wide safety margin and few side effects, including in the treatment of Alzheimer’s disease (Gao et al., 2015). Moreover, possible therapeutic mechanisms of CEGI have been reported in pre-clinical studies, suggesting that CEGI might perform multiple forms of cerebral protection by intervening in multiple targets during the occurrence and development of nervous system injury (Li et al., 2016). Therefore, the present study aimed to obtain more clinical evidence for CEGI in the treatment of ACI, as well as to further evaluate the effectiveness and safety of CEGI in ACI treatment. In the current study, 319 patients from 16 centers in China were enrolled. The patient population was homogeneous, as demonstrated by their similar baseline characteristics, minimal premorbid neurological deficits, and similar disease processes.
The results of this study demonstrated the neuroprotective effect of CEGI in ACI. On day 90 after treatment, there were significant differences in term of the decreased of mRS scores between CEGI group and placebo group. The comparison of NIHSS scores between the CEGI and placebo groups indicated a gradual decrease in scores in both groups starting from day 7. Furthermore, the degree of recovery was more pronounced in the CEGI group compared with the placebo group on days 14 and 90 after treatment. These findings indicated that a 2-week treatment of CEGI combined with basic pharmaceutical therapy may contribute to the recovery from neurological impairment and decrease disability rates in patients with ACI, and that this curative effect might be preserved for a long time. In addition, BI scores gradually increased from day 7 after treatment in both groups, and the scores in the CEGI group reached higher levels compared with the placebo group; on day 14 after treatment, there was a significant difference between the groups, suggesting that subjects in the CEGI group achieved greater improvement in functional outcomes than those in the placebo group. CEGI treatment of ACI was beneficial in improving the self-maintenance ability and quality of life of patients. In addition, NIHSS and mRS scores of the subgroups indicated that CEGI led to better outcomes in the treatment of moderate stroke patients compared with mild stroke patients.
In the present study, the enrolled subjects had an ACI less than 48 hours before receiving treatment; therefore, the early administration of CEGI might be a critical factor for the treatment to be successful. In addition, a 14-day course of treatment might help to guarantee its efficacy, which may be maintained for a long time after CEGI administration. All the safety analyses in the present study supported CEGI as a safe treatment for patients with ACI. However, our study had limitations: first, the small sample size; and second, the short duration of follow-up. Therefore, in a future study, we will use a larger sample size and increase the duration of follow-up to confirm the results of the current study.
In conclusion, the present study showed that CEGI treatment of ACI was safe, decreased disability rates and neurological impairments in patients, and improved patients’ daily living activities. CEGI may therefore be a safe and effective treatment for ACI patients, especially in moderate stroke patients.
Additional files:
Additional file 1: Hospital ethics approval document (Chinese).
Additional file 2: Informed consent form (Chinese).
Additional file 3: CONSORT checklist.
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest.
Financial support: None.
Institutional review board statement: The study was performed in strict accordance with the Declaration of Helsinki and relevant ethical requirement of the Ethical Committee of Peking University Third Hospital, China (No. 2013068) on May 20, 2013. The study was registered at the Chinese Clinical Trial Registry (registration No. ChiCTR1800017937).
Declaration of patient consent: The authors certify that they have obtained all appropriate patient consent forms from the patients or their family members. In the forms, the patients or their family members have given their consent for the patients’ images and other clinical information to be reported in the journal. The patients or their family members understand that the patients’ names and initials will not be published and due efforts will be made to conceal the patients’ identity.
Reporting statement: This study followed the CONsolidated Standards Of Reporting Trials (CONSORT) guidance.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatistician of the Peking University Third Hospital, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Individual participant data that underlie the results reported in this manuscript, after deidentification (text, tables, figures, and appendices) will be available indefinitely at ResMan Research Manager (http://www.medresman.org/) within 6 months after the completion of the trial without any charge. Other raw data can be achieved through contact with the corresponding author.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Gardner B, Yu J, Song LP; T-Editor: Jia Y
References
- 1.Baek SH, Noh AR, Kim KA, Akram M, Shin YJ, Kim ES, Yu SW, Majid A, Bae ON. Modulation of mitochondrial function and autophagy mediates carnosine neuroprotection against ischemic brain damage. Stroke. 2014;45:2438–2443. doi: 10.1161/STROKEAHA.114.005183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broussalis E, Trinka E, Killer M, Harrer A, McCoy M, Kraus J. Current therapies in ischemic stroke. Part B.Future candidates in stroke therapy and experimental studies. Drug Discov Today. 2012;17:671–684. doi: 10.1016/j.drudis.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Candelise L, Ciccone A. Gangliosides for acute ischemic stroke. Stroke. 2002;33:2336. doi: 10.1161/01.str.0000029272.13806.46. [DOI] [PubMed] [Google Scholar]
- 4.Cheng J, Zhou ZW, Sheng HP, He LJ, Fan XW, He ZX, Sun T, Zhang X, Zhao RJ, Gu L, Cao C, Zhou SF. An evidence-based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides. Drug Des Devel Ther. 2015;9:33–78. doi: 10.2147/DDDT.S72892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Bene A, Palumbo V, Lamassa M, Saia V, Piccardi B, Inzitari D. Progressive lacunar stroke: review of mechanisms, prognostic features, and putative treatments. Int J Stroke. 2012;7:321–329. doi: 10.1111/j.1747-4949.2012.00789.x. [DOI] [PubMed] [Google Scholar]
- 6.Fischer U, Arnold M, Nedeltchev K, Brekenfeld C, Ballinari P, Remonda L, Schroth G, Mattle HP. NIHSS score and arteriographic findings in acute ischemic stroke. Stroke. 2005;36:2121–2125. doi: 10.1161/01.STR.0000182099.04994.fc. [DOI] [PubMed] [Google Scholar]
- 7.Gao Y, Hu YZ, Li RS, Han ZT, Geng Y, Xia Z, Du WJ, Liu LX, Zhang HH, Wang LN. Cattle encephalon glycoside and ignotin injection improves cognitive impairment in APPswe/PS1dE9 mice used as multitarget anti-Alzheimer’s drug candidates. Neuropsychiatr Dis Treat. 2015;11:537–548. doi: 10.2147/NDT.S78025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue T, Kobayashi M, Uetsuka Y, Uchiyama S. Pharmacoeconomic analysis of cilostazol for the secondary prevention of cerebral infarction. Circ J. 2006;70:453–458. doi: 10.1253/circj.70.453. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Hu XS, Zhou FF, Li S, Lin YS, Qi WQ, Qi CF, Zhang X. Limb remote ischemic postconditioning protects integrity of the blood-brain barrier after stroke. Neural Regen Res. 2018;13:1585–1593. doi: 10.4103/1673-5374.237122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li R, Ma K, Zhao H, Feng Z, Yang Y, Ge H, Zhang X, Tang J, Yin Y, Liu X, Tan L, Feng H. Cattle encephalon glycoside and ignotin reduced white matter injury and prevented post-hemorrhagic hydrocephalus in a rat model of intracerebral hemorrhage. Sci Rep. 2016;6:35923. doi: 10.1038/srep35923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S, Wu JR, Zhang D, Wang KH, Zhang B, Zhang XM, Tan D, Duan XJ, Cui YY, Liu XK. Comparative efficacy of Chinese herbal injections for treating acute cerebral infarction: a network meta-analysis of randomized controlled trials. BMC Complement Altern Med. 2018;18:120. doi: 10.1186/s12906-018-2178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma J, Xiong JY, Hou WW, Yan HJ, Sun Y, Huang SW, Jin L, Wang Y, Hu WW, Chen Z. Protective effect of carnosine on subcortical ischemic vascular dementia in mice. CNS Neurosci Ther. 2012;18:745–753. doi: 10.1111/j.1755-5949.2012.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura Y, Nakajima H, Kimura F, Unoda K, Arawaka S. Preventive effect of cilostazol on pneumonia in patients with acute cerebral infarction. J Stroke Cerebrovasc Dis. 2018;27:2354–2359. doi: 10.1016/j.jstrokecerebrovasdis.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Okabe N, Miyamoto O. Role and limitations of rehabilitation-induced neural network remodeling after stroke. Neural Regen Res. 2018;13:2087–2088. doi: 10.4103/1673-5374.241450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn TJ, Langhorne P, Stott DJ. Barthel index for stroke trials: development, properties, and application. Stroke. 2011;42:1146–1151. doi: 10.1161/STROKEAHA.110.598540. [DOI] [PubMed] [Google Scholar]
- 16.Rajanikant GK, Zemke D, Senut MC, Frenkel MB, Chen AF, Gupta R, Majid A. Carnosine is neuroprotective against permanent focal cerebral ischemia in mice. Stroke. 2007;38:3023–3031. doi: 10.1161/STROKEAHA.107.488502. [DOI] [PubMed] [Google Scholar]
- 17.Rong X, Zhou W, Xiao-Wen C, Tao L, Tang J. Ganglioside GM1 reduces white matter damage in neonatal rats. Acta Neurobiol Exp (Wars) 2013;73:379–386. doi: 10.55782/ane-2013-1944. [DOI] [PubMed] [Google Scholar]
- 18.Schregel K, Behme D, Tsogkas I, Knauth M, Maier I, Karch A, Mikolajczyk R, Bähr M, Schaper J, Hinz J, Liman J, Psychogios MN. Optimized management of endovascular treatment for acute ischemic stroke. J Vis Exp. 2018:e56397. doi: 10.3791/56397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevenson NJ, Addley MR, Ryan EJ, Boyd CR, Carroll HP, Paunovic V, Bursill CA, Miller HC, Channon KM, McClurg AE, Armstrong MA, Coulter WA, Greaves DR, Johnston JA. CCL11 blocks IL-4 and GM-CSF signaling in hematopoietic cells and hinders dendritic cell differentiation via suppressor of cytokine signaling expression. J Leukoc Biol. 2009;85:289–297. doi: 10.1189/jlb.0708394. [DOI] [PubMed] [Google Scholar]
- 20.Tang HL, Chen C, Wang SK, Sun GJ. Biochemical analysis and hypoglycemic activity of a polysaccharide isolated from the fruit of Lycium barbarum L. Int J Biol Macromol. 2015;77:235–242. doi: 10.1016/j.ijbiomac.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Tang XJ, Chen HR, Tan H, Li XG. Effect of GM1 and early acupuncture treatment on the neurological function in patients with acute cerebral infarction. Huaxi Yaoxue Zazhi. 2006;21:496–497. [Google Scholar]
- 22.Uyttenboogaart M, Stewart RE, Vroomen PC, De Keyser J, Luijckx GJ. Optimizing cutoff scores for the Barthel index and the modified Rankin scale for defining outcome in acute stroke trials. Stroke. 2005;36:1984–1987. doi: 10.1161/01.STR.0000177872.87960.61. [DOI] [PubMed] [Google Scholar]
- 23.Vidale S, Agostoni E. Thrombolysis in acute ischaemic stroke. Brain. 2014;137:e281. doi: 10.1093/brain/awu065. [DOI] [PubMed] [Google Scholar]
- 24.Xu F, Lei M, Long L, Gong QH, Gao JM. Mesenchymal stem cell transplantation improves the prognosis of ischemic stroke:a Meta-analysis. Zhongguo Zuzhi Gongcheng Yanjiu. 2018;22:760–765. [Google Scholar]
- 25.Wang YJ, Song XQ, Liu QR, Li XG, Lu H, Zhang GH, Shi ZH, Liu JR. Multicenter and randomized clinical study of cerebroside carnosin treatment in acute ischemic stroke patients. Zhongguo Cuzhong Zazhi. 2006:611–614. [Google Scholar]
- 26.You YN, Cho MR, Kim JH, Park JH, Park GC, Song MY, Choi JB, Han JY. Assessing the quality of reports about randomized controlled trials of scalp acupuncture combined with another treatment for stroke. BMC Complement Altern Med. 2017;17:452. doi: 10.1186/s12906-017-1950-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao HM, Qin WQ, Wang PJ, Wen ZM. Eosinopenia is a predictive factor for the severity of acute ischemic stroke. Neural Regen Res. 2019;14:1772–1779. doi: 10.4103/1673-5374.258411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao L, Zhang LL, Bao H, Yang SM, Pan CS. Randomized clinical trial for treatment of patients with acute ischemic cerebral stroke by acupoint injection of Cobalamin or Gangliosides. Zhen Ci Yan Jiu. 2016;41:347–350. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


