Neurodegenerative diseases are a group of neuronal disorders caused by progressive neuronal cell death in different regions of the human brain. Alzheimer’s disease (AD) and Parkinson’s disease (PD) are the most common types of neurodegenerative diseases that affect millions of people worldwide. There is no cure available for either disease. One of the pathological hallmarks of neurodegenerative diseases is the abnormal protein aggregation in the central nervous system. Although this protein aggregation is the major pathological event, innate immunity-mediated neuroinflammation actively contributes to the onset and progression of neurodegenerative diseases (Labzin et al., 2018). Furthermore, the activation of inflammasomes, critical components of innate immunity, has an important role in neuroinflammation and neurodegenerative diseases (Heneka et al., 2018). In this perspective, we discuss the latest developments on the NLR family pyrin domain containing 3 protein (NLRP3) inflammasome, its role in AD and PD, and the therapeutic implications of targeting the NLRP3 inflammasome.
Innate immunity and inflammasomes: The innate immune system is the first line of host defense against pathogens and tissue injury. Innate immune cells, including macrophages, monocytes, and neutrophils, are the major players during innate immune responses. They express pattern recognition receptors (PRRs) to recognize the pathogen-associated molecular patterns and host- or environment-derived danger-associated molecular patterns. Engagement of PRRs activates a variety of inflammatory signaling pathways to eliminate infection and repair damaged tissue. PRRs include Toll-like receptors, C-type lectin receptors, RIG-1 like receptors, and nucleotide-binding oligomerization domain-like receptors (NLRs). A subset of PRRs can form cytosolic multiprotein complexes called inflammasomes (Broz and Dixit, 2016). An inflammasome commonly consists of a member of PRRs as the sensor, the adaptor apoptosis-associated speck-like protein containing a caspase-activation and recruitment domain (ASC) and caspase-1. Once assembled, inflammasomes trigger caspase-1 activation through the proximity-induced self-cleavage of capase-1. Activated caspase-1 mediates the maturation and secretion of pro-inflammatory cytokines, such as interleukin (IL)-1β and IL-18. In addition, active caspase-1 also cleaves the protein gasdermin D to induce a proinflammatory form of cell death termed pyroptosis. Currently, there are five members of PRRs confirmed to form inflammasomes: absent-in-melanoma 2, pyrin, and nucleotide-binding oligomerization domain-like receptor family members NLRP1, NLRP3, NLRC4 (Broz and Dixit, 2016).
Mechanism of NLRP3 inflammasome activation and regulation: The NLRP3 inflammasome has become the most studied inflammasome over the years due to its activation by a diverse range of stimuli and contribution to the pathology of inflammatory diseases. Despite extensive investigation, the mechanism of NLRP3 inflammasome activation remains unclear. A two-signal model has been proposed for NLRP3 inflammasome activation (Kelley et al., 2019). The priming signal (Signal 1), activated by ligands for Toll-like receptors, NLRs or cytokine receptors, leads to the upregulation of pro-IL-1β and NLRP3 through the nuclear factor-κB-mediated signaling pathway. This priming signal is not sufficient to trigger NLRP3 inflammasome activation. A second signal (Signal 2), provided by stimuli such as ATP, pore-forming toxins, or particulate matter, triggers NLRP3 inflammasome assembly and activation. Since NLRP3 stimuli are structurally and chemically dissimilar, it is thought that they induce an intracellular stress signal that is sensed by NLRP3. However, the nature of this stress signal remains elusive. Potassium efflux, mitochondrial dysfunction and reactive oxygen species (ROS), and lysosomal disruption have each been proposed as the upstream signaling event for NLRP3 inflammasome activation. Most NLRP3 stimuli can trigger the efflux of intracellular potassium. Conversely, inhibition of potassium efflux by high extracellular potassium concentrations blocks NLRP3 inflammasome activation by most stimuli. Thus, potassium efflux is a key upstream signaling event for NLRP3 inflammasome activation. The roles of mitochondria and ROS in NLRP3 inflammasome activation remain controversial. Many studies support a role for mitochondrial dysfunction and associated ROS in NLRP3 inflammasome activation. However, other studies indicate that ROS is only required for priming the NLRP3 inflammasome and mitochondrial dysfunction is dispensable for NLRP3 inflammasome activation. Lysosomal damage is only required for NLRP3 inflammasome activation by particulate matter. Several studies have proposed that members of the cathepsin family released from damaged lysosomes mediate NLRP3 inflammasome activation. Besides these upstream events, a number of NLRP3-interacting partners and posttranslational modifications of NLRP3 also regulate NLRP3 inflammasome activation (Kelley et al., 2019).
Role of the NLRP3 inflammasome in AD: NLRP3 inflammasome activation has been implicated in the pathogenesis of neurodegenerative diseases, especially AD and PD (Figure 1). AD, the most common age-related neurodegenerative disease, is the leading cause of dementia among people 65 years and older. The deposition of misfolded amyloid-β (Aβ) in the brain is a key pathological event for AD. Recent studies have indicated that the NLRP3 inflammasome-mediated neuroinflammation plays an important role in the pathogenesis of AD. Microglia, the predominant CNS-resident innate immune cells, express NLRP3, ASC, and caspase-1. Halle et al. (2008) first found that fibrillar Aβ induced the secretion of IL-1β from microglia (Halle et al., 2008). In their findings, Aβ-induced secretion of IL-1β was dependent on NLRP3, ASC, and caspase-1 activity, and required cathepsin B released from damaged lysosomes. Importantly, they also found that ASC- and caspase-1 knockout mice, as well as IL-1 receptor or MyD88 deficient mice, had less recruitment of microglia and mononuclear phagocytes to Aβ in the brain as compared to the wild type control after the injection of Aβ into the striatum. In a later study with APP/PS1 mice that model AD, genetic deletion of NLRP3 or caspase-1 significantly reduced hippocampal and cortical Aβ deposition, and ameliorated spatial memory impairment and hyperactive behavior in mice (Heneka et al., 2013). Furthermore, another study also shows that inhibition of the NLRP3 inflammasome reduced Aβ deposition, neuroinflammation, and cognitive impairment in an AD mouse model (Dempsey et al., 2017). Thus, these findings suggest that NLRP3 inflammasome activation induced by Aβ promotes the pathogenesis of AD by triggering the release of proinflammatory cytokines and neuroinflammation. In agreement, brain samples from human AD patients showed increased caspase-1 activity as compared to non-demented and age-matched controls (Heneka et al., 2013).
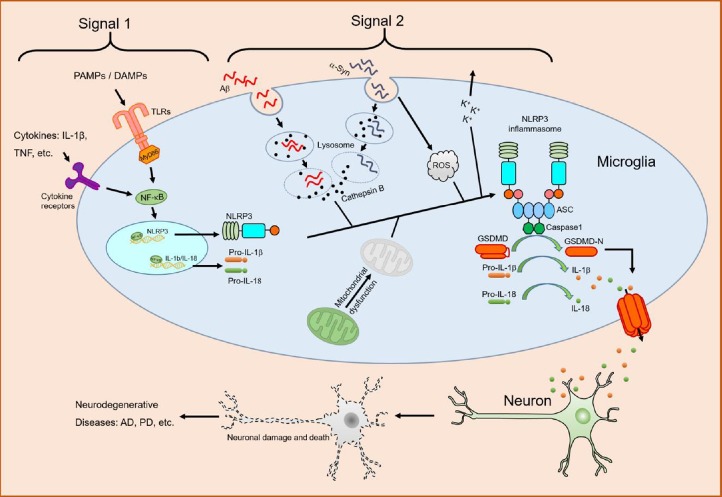
Figure 1.
Role of NLRP3 inflammasome in neurodegenerative diseases.
Cytokines, pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) engage their receptors, leading to activation of transcription factor nuclear factor-κB (NF-κB) and subsequent upregulation of NLR family pyrin domain containing 3 protein (NLRP3) and pro-IL-1β (Signal 1). Phagocytosis of misfolded amyloid-β (Aβ) or α-synuclein (α-Syn) induces lysosomal damage and subsequent release of cathepsin B and ROS accumulation, which trigger NLRP3 inflammasome assembly and caspase1 activation (Signal 2). Activated caspase1 cleaves pro-IL-1β, pro-IL-18 and gasdermin D (GSDMD) into their bioactive forms. Mitochondrial dysfunction, K+ efflux, or both might also contribute to NLRP3 inflammasome activation by Aβ or α-Syn. Proinflammatory cytokines, such as IL-1β and IL-18, or other inflammatory mediators induce neuronal damage and death, leading to neurodegenerative diseases. AD: Alzheimer’s disease; IL: interleukin; NLRP3: NLR family pyrin domain containing 3 protein; PD: Parkinson’s disease; ROS: reactive oxygen species; TLR: Toll-like receptor.
Role of the NLRP3 inflammasome in PD: Recent studies have also indicated a role for the NLRP3 inflammasome in PD. PD is a type of neurodegenerative disease characterized by the progressive loss of dopaminergic neurons in the substantia nigra region of the human brain. The pathological hallmark of this disease is the formation of Lewy bodies that consist of intra-neuronal aggregates of α-synuclein. Similar to Aβ, the fibrillar form of α-synuclein, but not its monomeric form, induced caspase-1-mediated IL-1β secretion in human monocytes and BV2 microglial cells (Codolo et al., 2013). This process was dependent on the NLRP3 inflammasome, and required the phagocytosis of α-synuclein, ROS production, and cathepsin B activity. In the α-synuclein A53T mouse model of PD, caspase-1 deficiency significantly reduced the activation of microglia (Zhou et al., 2016). In a rat PD model, inhibition of caspase-1 through microinjection increased the number of dopaminergic neurons, which associated with reduced expression of NLRP3 inflammasome components (Mao et al., 2017). Recently, activated caspase-1 was shown to directly cleave α-synuclein, which further promoted the aggregation and neuronal toxicity of α-synuclein (Wang et al., 2016).
Therapeutic implications of targeting the NLRP3 inflammasome: The important role of the NLRP3 inflammasome in these diseases makes targeting this pathway an attractive therapeutic strategy. There are three approved therapies that target the inflammasome downstream effector IL-1β: anakinra (IL-1 receptor antagonist), canakinumab (IL-1β neutralizing antibody), and rilonacept (soluble decoy receptor for IL-1β and IL-1α) (Dinarello et al., 2012). Although their efficacies have been demonstrated in treating autoinflammatory diseases, there is no report of their use in clinical trials on neurodegenerative diseases like Alzheimer’s disease and Parkinson’s disease. One reason may be due to their poor penetration of the blood-brain barrier. Furthermore, IL-18 and other inflammatory mediators released by pyroptosis might also contribute to the pathology of these diseases. Therefore, development of small molecules that target the pathway upstream of NLRP3 inflammasome activation might represent a better therapeutic strategy to treat NLRP3-driven neurodegenerative diseases. Indeed, NLRP3 inhibitors, such as MCC950 and the caspase-1 inhibitor Ac-YVAD-CMK, have been shown to alleviate the disease progression in animal models (Dempsey et al., 2017; Mao et al., 2017). Furthermore, as a number of NLRP3 regulators have been shown to regulate NLRP3 inflammasome activation, it would be of great value to investigate whether these regulators contribute to disease progression and therefore be used to develop new therapeutic strategies for treating neurodegenerative diseases.
The work on the NLRP3 inflammasome in the He laboratory was funded by NIH grant K22AI120988 and Wayne State University Startup.
Additional file: Open peer review report 1 (75.4KB, pdf) .
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Alessandra Bigi, University of Florence, Italy.
P-Reviewer: Bigi A; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 2.Codolo G, Plotegher N, Pozzobon T, Brucale M, Tessari I, Bubacco L, de Bernard M. Triggering of inflammasome by aggregated alpha-synuclein, an inflammatory response in synucleinopathies. PLoS One. 2013;8:e55375. doi: 10.1371/journal.pone.0055375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dempsey C, Rubio Araiz A, Bryson KJ, Finucane O, Larkin C, Mills EL, Robertson AAB, Cooper MA, O’Neill LAJ, Lynch MA. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-beta and cognitive function in APP/PS1 mice. Brain Behav Immun. 2017;61:306–316. doi: 10.1016/j.bbi.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heneka MT, McManus RM, Latz E. Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci. 2018;19:610–621. doi: 10.1038/s41583-018-0055-7. [DOI] [PubMed] [Google Scholar]
- 8.Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019 doi: 10.3390/ijms20133328. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labzin LI, Heneka MT, Latz E. Innate immunity and neurodegeneration. Annu Rev Med. 2018;69:437–449. doi: 10.1146/annurev-med-050715-104343. [DOI] [PubMed] [Google Scholar]
- 10.Mao Z, Liu C, Ji S, Yang Q, Ye H, Han H, Xue Z. The NLRP3 inflammasome is involved in the pathogenesis of Parkinson’s disease in rats. Neurochem Res. 2017;42:1104–1115. doi: 10.1007/s11064-017-2185-0. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Nguyen LT, Burlak C, Chegini F, Guo F, Chataway T, Ju S, Fisher OS, Miller DW, Datta D, Wu F, Wu CX, Landeru A, Wells JA, Cookson MR, Boxer MB, Thomas CJ, Gai WP, Ringe D, Petsko GA, et al. Caspase-1 causes truncation and aggregation of the Parkinson’s disease-associated protein alpha-synuclein. Proc Natl Acad Sci U S A. 2016;113:9587–9592. doi: 10.1073/pnas.1610099113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Lu M, Du RH, Qiao C, Jiang CY, Zhang KZ, Ding JH, Hu G. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol Neurodegener. 2016;11:28. doi: 10.1186/s13024-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.