Conspectus
In addition to nitric oxide and carbon monoxide, hydrogen sulfide (H2S) has been recently recognized as an important biological signaling molecule with implications in a wide variety of processes including vasodilation, cytoprotection, and neuromodulation. In parallel to the growing number of reports highlighting the biological impact of H2S, interest in developing H2S donors as both research tools and potential therapeutics has led to the growth of different H2S-releasing strategies. Many H2S investigations in model systems use direct inhalation of H2S gas or aqueous solutions of NaSH or Na2S, however such systems do not mimic endogenous H2S production. This stark contrast drives the need to develop better sources of caged H2S to be used in biological systems. To address these limitations, different small organosulfur donor compounds have been prepared that release H2S in the presence of specific activators or triggers. Such compounds, however, often lack suitable H2S-depeleted control compounds, which limits the use of these compounds in probing the effects of H2S directly. To address these needs, our group has pioneered the development of carbonyl sulfide (COS) releasing compounds as a new class of H2S donor motifs. Inspired by a commonly-used carbamate prodrug scaffold, our approach utilizes self-immolative thiocarbamates to access controlled release of COS, which is rapidly converted to H2S by the ubiquitous enzyme carbonic anhydrase (CA). In addition, this design enables access to key control compounds that release CO2/H2O rather than COS/H2S, which enables delineation of the effects of COS/H2S from the organic donor byproducts.
In this Account, we highlight a library of first-generation COS/H2S donors based on self-immolative thiocarbamates developed in our lab and also highlight challenges related to H2S donor development. We showcase the release of COS in the presence of specific triggers and activators including biological thiols and bioorthogonal reactants for targeted applications. We also demonstrate the design and development of a series of H2O2 / reactive oxygen species (ROS)-triggered donors and show that such compounds can be activated by endogenous levels of ROS production. Utilizing approaches in bio-orthogonal activation, we establish that donors functionalized with an o-nitrobenzyl photocage can enable access to light-activated donors. Similar to endogenous production by cysteine catabolism, we also prepared a cysteine-selective COS donor activated by a Strongin-ligation mechanism. In efforts to help delineate potential differences in the chemical biology of COS and H2S, we also report a simple esterase-activated donor, which demonstrated fast COS-releasing kinetics and inhibition of mitochondrial respiration in BEAS-2B cells. Additional investigations revealed that COS release rates and cytotoxicity correlated directly within this series of compounds with different ester motifs. In more recent and applied applications of this H2S donation strategy, we also highlight the development of donors that generate either a colorimetric or fluorescence optical response upon COS release. Overall, the work described in this Account outlines the development and initial application of a new class of H2S donors, which we anticipate will help to advance our understanding of the rapidly emerging chemical biology of H2S and COS.
Graphical Abstract

Introduction
Initially disregarded as a toxic and foul-smelling gas,1 hydrogen sulfide (H2S) has recently emerged as an important biological signaling molecule commonly known as a “gasotransmitter”2 with major implications in biological systems.3,4 H2S is produced endogenously by native enzymes including cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST) primarily by cysteine catabolism.5 As a gasotransmitter, H2S can react directly with biological targets or activate specific pathways in the cardiovascular, neuronal, and gastrointestinal systems.6 These signaling pathways have also been observed in diseases states including diabetes,7 atherosclerosis,8 and Parkinson’s disease.9 Notably, H2S-mediated signaling has been implicated in vital life processes including modulation of neurotransmission,10 vasodilation,11 and cytoprotection against reactive oxygen species (ROS) (Figure 1).12 As examples of the role of H2S in different model systems, treatment of murine hippocampal slices with H2S resulted in the enhancement of N-methyl-D-aspartate (NMDA) receptor-mediated responses and induction of long-term potentiation, an important neuronal process during memory formation.13 Similar to nitric oxide, a known vasodilator, administration of H2S to the cardiovascular system directly activates ATP-sensitive potassium (KATP) channels triggering membrane hyperpolarization promoting an overall decrease in arterial blood pressure.14 The addition of H2S to mouse embryonic fibroblasts from a CSE knockout model displaying elevated levels of oxidative stress was shown to halt cellular senescence by persulfidation of Keap1 and activation of Nrf2 leading to increased production of reduced glutathione (GSH), a potent antioxidant.15 In addition to these observations, a continually-growing number of reports of H2S-mediated signaling collectively highlight the biological significance of H2S. This growth has inspired the development of small molecules capable of releasing H2S termed “donors” under physiologically-relevant conditions at rates comparable to endogenous production with goals of harnessing the potential benefits of H2S as both a research and therapeutic tool.16
Figure 1.
Representative physiological processes involving H2S and associated mechanisms of action.
The controlled delivery of H2S has been a long-standing challenge due to the inherent chemical properties of H2S. At physiological pH, the weak acidity of H2S (pKa ~ 7.0) results in a speciation of ~70% hydrosulfide anion (SH–) and ~30% H2S gas. In the presence of oxygen, and especially in the presence of redox-active metals, H2S is readily oxidized and leads to the formation of polysulfides,17 thus complicating the quantification of active H2S concentrations. To avoid the direct use of H2S gas, most biological studies have used sodium hydrosulfide (NaSH) and sodium sulfide (Na2S) as convenient sources of H2S. The addition of these inorganic sulfide salts to a buffered aqueous solution, however, results in a rapid, almost instantaneous increase in H2S concentration followed by a gradual decrease due to volatilization of H2S gas.18 This fast release of H2S is in stark contrast to the rate of H2S production by CBS and CSE measured under similar conditions.19 These factors drive the need to develop alternative sources of H2S which better mimic the rate of endogenous H2S production. To address this problem, a number of synthetic, small molecule H2S donors including diallyl trisulfide (DATS)20 and GYY413721 have been reported. Despite the wide use of these donors, a lack of tunability and appropriate control compounds limits their use in further probing the physiological effects of H2S. To address these concerns, donors that release H2S at varying rates in response to specific stimuli including hydrolysis, thiols, and light have been prepared.22–25
As an alternative approach to previously reported donors that release H2S directly, we were inspired by the conversion of carbonyl sulfide (COS) by carbonic anhydrase (CA) to H2S.26 COS is the most abundant sulfur-containing gas in Earth’s atmosphere, and we as well as others have recently leveraged COS as vehicle for H2S delivery.27 Currently, enzymatic pathways for the mammalian biosynthesis of COS have not been identified, but a number of different metalloenzymes that can convert COS to H2S, most notably, the ubiquitous mammalian enzyme CA. A primary physiological role of CA is regulation of blood pH by conversion of CO2 to bicarbonate (Kcat/KM = 8 × 107 M−1 s−1 for bovine CA-II), but as a relatively promiscuous enzyme, CA can also metabolize COS to H2S and CO2, with high catalytic efficiency (Kcat/KM = 2.2 × 104 M−1 s−1 for bovine CA-II).28–31
In 2016, we reported a new approach to access H2S donors by leveraging the efficient hydrolysis of COS to H2S by CA.32 We drew inspiration from the widely-employed strategy of using triggerable self-immolative carbamates to deliver a payload in response different stimuli (Figure 2a). Because such scaffolds extrude CO2 as a byproduct of the self-immolative decomposition, we reasoned that replacing the carbamate core with a thiocarbamate would result in COS release. In this design, the caged-thiocarbamates can be engineered to respond to specific biologically-relevant stimuli to deliver COS, which in turn is rapidly converted to H2S by CA (Figure 2b,c).
Figure 2.
(a) Design and mechanism of self-immolative carbamates and (b) self-immolative thiocarbamates. (c) Conversion of COS to H2S by CA in the presence of acetazolamide at varying concentrations measured by a sulfide-selective electrode.
The high modularity of this scaffold allows for a “plug and play” approach to H2S donor design, in which both the trigger and the payload can be readily modified to accomplish different goals (Figure 3a). Importantly, the analogous carbamates, which release CO2 rather than COS, serve as key H2S-depleted control compounds that can help to separate the effects of the organic byproducts from that of COS/H2S release. Additionally, the triggerless control, which maintains the thiocarbamate core but lacks the self-immolative triggering group, provides an additional control compound that helps to account for any effects observed as a result of the thiocarbamate moiety. In our first application of this general design, we reported self-immolative thiocarbamates in the development of the first analyte-replacement COS/H2S fluorescent probe (Figure 3b). With an azide as the triggering group and methyl rhodol as the payload, treatment with NaSH yielded both COS/H2S release and a fluorescent turn-on response.32 Importantly, this design provided a first step toward addressing the challenge of analyte consumption in activity-based systems. We have since expanded the strategy of triggered COS/H2S release to encompass a wide range of triggering events and stimuli (Figure 3c).
Figure 3.
(a) Mechanism of self-immolation and subsequent conversion of COS to H2S by CA. (b) Development of analyte-replacement fluorescent probes. (c) Current examples self-immolation-based COS/H2S donors reported to date by our lab.
Stimuli Responsive COS-based H2S Donors
In an early application of stimulus-responsive COS/H2S donors, we developed systems in which a boronate ester, which is converted to a phenol by ROS, was used as the trigger (Figure 4a).33 This system combined the known role of boronate esters as ROS scavengers with the cytoprotective effects of H2S to access enhanced cytoprotection against ROS. Using an H2S-selective electrode, we demonstrated the H2O2-dependent release of COS/H2S from the thiocarbamate, but not from carbamate or triggerless control compounds, in the presence of CA. We also established the ability of these H2O2-activated donors to release COS/H2S in live cell environments. For example, treatment of HeLa cells incubated with an H2S-selective fluorescent probe and the ROS-activated donor exhibited an H2S-derived fluorescence response when treated with exogenous H2O2. Similarly, endogenous levels ROS produced by stimulation with phorbol myristate acetate (PMA) could also trigger COS/H2S release from the donors, as demonstrated in RAW 264.7 cells. Moreover, the thiocarbamate donors showed significant cytoprotective effects against exogenous H2O2 in HeLa cells. The carbamate control compound also proved slightly cytoprotective, although to a much lesser extent than the sulfide-releasing donors (Figure 4b), which was likely due to partial H2O2 consumption by the boronate trigger. These results highlight the benefit of having key control compounds to fully disentangle the observed biological effects of the release H2S from that of other donor components or byproducts. A further systematic study of analogous boronate-containing molecules with variable COS-releasing motifs, including O- and S-alkyl thiocarbamates, O- and S-alkyl thiocarbonates, and dithiocarbonates, demonstrated the broad applicability and tunability of this platform in triggerable COS/H2S delivery.34
Figure 4.
(a) Representative reaction scheme and mechanism for H2O2-triggered COS/H2S release. (b) Cytotoxicity of H2O2 in RAW 264.7 cells, Cytotoxicity of Bpin-triggered thiocarbamate donor, Bpin-triggered carbamate control, and triggerless thiocarbamate control at various concentrations in RAW 264.7 cells when co-incubated with 100 μM H2O2. (c) Imaging ROS-scavenging upon stimulation with PMA in RAW 264.7 cells.
The self-immolative thiocarbamate COS donor scaffold has also been used in common bio-orthogonal activation mechanisms, such as photoactivation. In proof-of-concept studies, we reported the first light-activated COS/H2S donor equipped with a photocleavable o-nitrobenzyl group. Upon irradiation (λ = 365 nm), the benzyl alcohol is cleaved via a Norrish type II mechanism, revealing one equivalent of COS and an aniline payload (Figure 5). The rate of H2S release from this system was found to increase with the addition of electron-donating methoxy substituents on the o-nitrobenzyl group consistent with previous findings.35 Building from this work, light-triggered COS release has also been recently reported using BODIPY-derived photolabile groups.36,37
Figure 5.
Mechanism of self-immolation from photocleavable thiocarbamate COS/H2S donors.
In vivo, H2S is produced primarily through cysteine catabolism. In an effort to better mimic the conditions of endogenous H2S production, we applied the established chemistry of the Strongin ligation to prepare a cysteine-selective COS/H2S donor. The mechanism of COS release proceeds through nucleophilic attack by cysteine into an acrylate, followed by subsequent cyclization by the pendant amine, and finally in elimination to uncage the thiocarbamate moiety (Figure 6). Due to the requirement of a nearby amine in the mechanism, this donor has an inherent selectivity towards cysteine over other biological thiols including reduced glutathione (GSH). The sulfide release of this donor was shown in bEnd.3 cells using an H2S-selective fluorescent probe.38
Figure 6.
Mechanism of COS-release from OA-CysTCM-1 in the presence of cysteine with subsequent hydrolysis of COS to H2S by carbonic anhydrase.
One limitation of many triggerable donor scaffolds is the consumption of biological nucleophiles to initiate release of the desired product, thus perturbing the cellular homeostasis. We envisioned using an enzymatically-triggered reaction as a potential solution, thus enabling activation without depleting the levels of cellular nucleophiles.39,40 By appending a small ester to the 4-hydroxybenzyl alcohol core, exposure to intracellular esterases should reveal the corresponding phenolate, which undergoes the same 1,6-self-immolative cascade to generate COS (Figure 7a). Employing a thiocarbamate with a t-butyl ester trigger and a p-tolyl payload yielded fast COS/H2S donors as determined by a H2S-selective electrode in the presence of porcine liver esterase (PLE). Further studies revealed that this donor led to almost complete cell death even at the low concentration of 10 μM in BEAS2B, whereas neither the triggerless or H2S-depleted control compounds showed any toxicity at those concentrations. Additionally, neither GYY4137 or AP39, two commonly used H2S donors were toxic at these levels. Most surprisingly, the thiocarbamate was much more cytotoxic than Na2S, which is often described to be toxic due to the immediate bolus of H2S released under physiological conditions (Figure 7b). These results highlight the crucial need for adequate control compounds to delineate observed activities of COS/H2S from those attributable to organic byproducts of donor activation.39
Figure 7.
Altered cytotoxicity via steric modulation for esterase-triggered COS/H2S donors. (a) Mechanism of self-immolation of esterase-triggered COS donors. (b) Library of different ester size thiocarbamates prepared. (c) Inhibition of mitochondrial bioenergetics with the tert-Butyl triggered COS donor in BEAS2B cells. (d) The relationship between COS release rate and observed cytotoxicity at 100 μM in HeLa cells for a library of different esterase-triggered COS donors of varying ester size.
The unique toxicity profile of the t-butyl ester thiocarbamate led us to hypothesize that either the specific subcellular localization of the compound caused cell death, or that a build-up of COS itself directly inhibited mitochondrial bioenergetics. To investigate the latter hypothesis, we prepared a series of esterase-triggered COS donors equipped with esters of varying sizes (Figure 7c).41 The rate of small ester cleavage by esterases is likely faster than the rate of COS hydrolysis by CA (up to 5.8 × 105 M–1 s–1 compared to 2.2 × 104 M–1 s–1 for bovine CA II), which could lead to a potentially toxic buildup of COS in the cell.29 Changing the size of an ester significantly changes the rate of cleavage by esterases, and we proposed that donors with small esters would exhibit high levels of cytotoxicity, whereas those with bulkier groups would have little effect on cell viability.42 Consistent with this hypothesis, we demonstrated that the rate of ester hydrolysis directly correlates with the observed cytotoxicity in HeLa cells, supporting the idea that COS may function as more than a simple H2S shuttle (Figure 7d). Despite the toxicity of the small ester donors, this report outlined a suite of COS donors with tunable rates of release, the utility of which was highlighted with fluorescent cell-imaging of the cyclohexyl ester thiocarbamate.41 A similar series of S-alkyl thiocarbamate esterase-triggered COS donors were reported by the Chakrapani group, which display comparable toxicity at 50 μM in human breast cancer MCF-7 cells, however rates of H2S release were found to be slightly slower.40
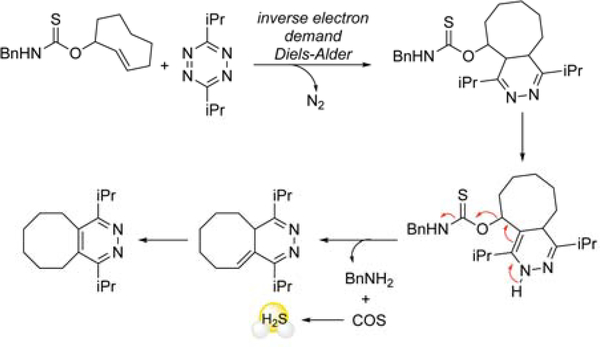
One challenge associated with the 1,6-self-immolative thiocarbamate scaffold is the release of an electrophilic para-quinone methide. As one approach to address this limitation, and also to demonstrate the compatibility of our approach with common biorthogonal chemistry,43 we developed a “click-and-release” donor, in which COS release is triggered through an inverse-electron demand Diels Alder reaction (IEDDA) with a trans-cyclooctene moiety fused to a thiocarbamate (Figure 8). Experiments with both bovine and sheep plasma and blood proved that endogenous levels of CA are sufficient for the hydrolysis of the released COS to H2S.44
Figure 8.
Mechanism of ‘click-and-release’ biorthogonal COS donor, and measured H2S release in the presence of mammalian blood and plasma, without exogenous CA.
COS-based H2S Donors with Optical Readouts
To assess the validity of our donors in vitro, we utilize spectrophotometric methods of H2S detection such as the methylene blue assay.45 In cells, the use of H2S-selective fluorescent probes46–48 consumes the generated H2S and interferes with our ability to fully observe the effect of H2S production on cells. To prevent this undesired analyte consumption, we envisioned coupling a spectroscopic response to COS/H2S release by appending a COS-releasing moiety to a chromophore which would generate a spectroscopic signal concomitantly with the release of COS. This class of donors provides novel chemical tools to visualize COS/H2S release by UV/Vis or fluorescence spectroscopy and allows us to minimize the number of external components needed to visualize H2S release in complex biological systems.
In our initial approach, we designed an analogous self-immolation scaffold which undergoes β-elimination at physiological pH to generate methyl vinyl ketone, COS, and release an amine-based payload (Figure 9a).49 The use of p-nitroaniline (PNA) as the payload allows for optical monitoring of H2S release from γ-KetoTCM1 by measuring the UV/Vis absorbance of PNA (λmax = 381 nm) (Figure 9b). Importantly, UV-Vis spectroscopy showed that PNA formation correlated directly with H2S generation measured using the methylene blue assay, which confirmed that the optical response can be used as a direct proxy for H2S release (Figure 9c).
Figure 9.
(a) Mechanism of COS/H2S release from γ-KetoTCM1 (b) Conditions for measuring H2S release from γ-KetoTCM1 (c) Measurement of PNA formation over time by UV/Vis spectroscopy (d) Correlation between measured [H2S] and PNA formation by the methylene blue assay and UV/Vis spectroscopy.
The incorporation of a spectroscopic handle also allowed us to conduct a series of kinetic experiments to probe the effect of pH on COS/H2S release from this donor by UV/Vis spectroscopy. At physiological pH, this donor releases H2S slowly over 30 h with a measured pseudo-first order rate constant (kobs) of 4.52(2) x10−5 s-1. The rate of H2S release from this donor can be tuned as a function of pH, and the observed rates of H2S release in basic solutions (pH 8.0, kobs = 12.6(2) x10−5 s−1) consistent with a mechanism of β-elimination. Additionally, we were able to decrease the rate of H2S release by installing a methyl group at the β-position resulting in modulation of the β-proton acidity. The addition of bovine serum albumin (5 mg/mL) led to a significant increase in the rate of H2S release at pH 7.4 (kobs = 81(3) x10−5 s−1) suggesting that this donor could function in complex biological environments and benefit from protein microenvironments. In further support of biological compatibility, the addition of biological nucleophiles including cysteine, GSH, and lysine did not impact H2S release. Moreover, H2S release from γ-KetoTCM1 was observed in HeLa cells, as evidenced by the fluorescence response from an H2S-responsive probe.
To improve optical signal to be more compatible with biological samples, we also developed fluorescent turn-on donors that become fluorescent after release of COS/H2S. To accomplish this goal, we relied on the reactivity of sulfenyl thiocarbonates towards thiols to generate a disulfide, COS, and alcohol-based payload (Figure 10a). By a simple, one-step procedure, we prepared a small library of fluorescein-caged sulfenyl thiocarbonates to serve as fluorescent, COS-based H2S donors with FLD-1 serving as the model fluorescent donor (Figure 10b).50 The addition of excess cysteine (100 μM) to 10 μM FLD-1 in the presence of carbonic anhydrase at pH 7.4 resulted in a fluorescent enhancement of over 500-fold consistent with the formation of fluorescein upon consumption of the donor motif (Figure 10c). Using a monofunctionalized sulfenyl thiocarbonate derivative to simplify the reaction kinetics, we demonstrate the formation of fluorescein monitored by fluorescence spectroscopy can be directly correlated to H2S release measured by the methylene blue assay and confirms the validity of this approach to prepare fluorescent H2S/COS donors. The release of H2S from FLD-1 was found to occur exclusively in the presence of thiols including cysteine and GSH over other biological nucleophiles including lysine, serine, and H2O2. To assess the biological compatibility of FLD-1, we examined the activation of this donor by endogenous thiols and concomitant H2S release by use of 7-azido-4-methylcoumarin (C7-Az), an H2S-selective fluorescent probe in HeLa cells. The treatment of HeLa cells with FLD-1 (50 μM) resulted in a fluorescent turn-on of C7-Az and fluorescent signal corresponding to the formation of fluorescein (Figure 10c). An overlay of both channels reveals an even cellular distribution suggesting good uptake of FLD-1 and confirms the compatibility of this donor in live cells. Taken together, γ-KetoTCM-1 and FLD-1 are the first examples of COS-based H2S donors with an incorporated optical readout and provide visual chemical tools for probing the biological effects of H2S.
Figure 10.
(a) Mechanism of thiol-mediated, COS/H2S release from sulfenyl thiocarbonates. (b) Structure of FLD-1 and release of H2S from FLD-1 (10 μM) in the presence of cysteine (100 μM, 10 equiv.) in the presence of carbonic anhydrase in 10 mM PBS (pH 7.4) monitored by fluorescence spectropscopy (λex = 490 nm, λem = 500–650 nm). (c) Imaging of cellular H2S release from FLD-1 (50 μM) in HeLa cells.
Perspective and Outlook
The donors covered in this account can collectively be viewed as a toolbox that chemists, biologists, and physiologists can use to probe the chemical biology of H2S under various conditions and begin to assess the biological impacts of COS. To both further our studies and to advance the field, we envision further investigating the potential roles of COS in sulfur biology. Many of the reported COS-based H2S donors reported by our group and others have been based primarily on the self-immolative thiocarbamate scaffold. Despite the high modularity, the generation of the quinone methide byproduct is often overlooked. The absence of cytotoxicity in our experiments suggests that short-term exposure to this leads to minimal negative effects, but prior reports suggest that chronic exposure may lead to electrophilic stress,51 which adds an additional variable to biological investigations. This potential issue drives the need to develop future generations of COS-based H2S donors that lack electrophilic byproducts. Additionally, further investigation are needed into the potential direct toxicity of COS. We have shown that smaller esterase-triggered COS donors exhibit significant cytotoxicity at concentrations as low as 10 μM, whereas the COS-depleted controls, Na2S, and other small-molecule direct H2S donors have no effect. This observation raises the question of whether COS has activity independent of that of H2S. Although we have demonstrated that the cytotoxicity of small molecule esterase-triggered donors correlates directly with the rate of COS release, fully disentangling the effects of COS delivery from the physiological effects of H2S is a complex problem, and remains an unmet challenge in the field. Furthermore, although there are many different isoforms of CA, little is known about the different reactivity toward COS and CO2, with available data showing that the commonly-used bovine CA-II has a significantly higher catalytic efficiency toward its native substrate CO2 (8 × 107 M−1s−1) than for COS (2.2 × 104 M−1s−1).28,29 There is a relative dearth of knowledge about the activities of different CA isoforms toward COS, and how the subcellular localizations of the enzyme isoforms effects the observed toxicity of different COS donors.
Another outstanding challenge is that of COS detection. Although COS can be detected through GC-MS analysis or other spectroscopic methods, there are currently no simple methods available for the detection of COS directly in aqueous solution, which significantly limits the ability to accurately study COS in biological systems. For example, although COS has been detected in the headspace of porcine coronary artery and cardiac muscle, with the current technology it cannot be conclusively determined whether that COS was from mammalian or bacterial origin.52 To truly advance the field of biological COS research, solution-phase COS probes and detection methods need to be developed. With the rapidly growing interest in COS as both a vehicle for H2S delivery and as a distinct biomolecule, we anticipate that these knowledge gaps will be filled through the collective efforts of the gasotransmitter research community, and that a new category of COS-based targeted tools and therapeutics will emerge.
Acknowledgment
Work described in this manuscript was supported by the NIH (MDP; R01GM113030), NSF/GRFP (CML; DGE-1309047), Dreyfus Foundation, and Sloan Foundation.
Biographies
Carolyn M. Levinn earned her B.S. in chemistry from the State University of New York (SUNY) College at Geneseo in 2014 and her M.S. in chemistry from the University of Illinois at Urbana Champaign in 2017. She is currently pursuing her Ph.D. in organic chemistry at the University of Oregon. Her research centers around the development of small molecule donors and probes for the detection and delivery of H2S and COS.
Matthew M. Cerda received his B.S. in Chemistry from SUNY College at Potsdam in 2015. He is currently a Ph.D. candidate at University of Oregon in lab of Prof. Michael D. Pluth developing chemical tools to study reactive sulfur species including H2S, COS, and organic polysulfides.
Michael D. Pluth is an Associate Professor in the Department of Chemistry and Biochemistry at the University of Oregon. Mike earned his B.S. degree in Chemistry and Mathematics in 2004 from the University of Oregon. He received his Ph.D. in 2008 from UC Berkeley working under the mentorship of Profs. Robert Bergman and Kenneth Raymond. After postdoctoral research at MIT with Prof. Stephen Lippard as an NIH Pathways to Independence fellow, Mike joined the UO faculty in 2011. His lab focuses on different applications of physical organic chemistry and chemical biology to biologically-relevant reactive sulfur species.
References
- (1).Malone Rubright SL; Pearce LL; Peterson J Environmental toxicology of hydrogen sulfide. Nitric Oxide 2017, 71, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Wang R Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 2002, 16, 1792–1798. [DOI] [PubMed] [Google Scholar]
- (3).Wang R Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev 2012, 92, 791–896. [DOI] [PubMed] [Google Scholar]
- (4).Filipovic MR; Zivanovic J; Alvarez B; Banerjee R Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev 2018, 118, 1253–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Hartle MD; Pluth MD A practical guide to working with H2S at the interface of chemistry and biology. Chem. Soc. Rev 2016, 45, 6108–6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Wallace JL; Wang R Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov 2015, 14, 329–345. [DOI] [PubMed] [Google Scholar]
- (7).Wu L; Yang W; Jia X; Yang G; Duridanova D; Cao K; Wang R Pancreatic islet overproduction of H2S and suppressed insulin release in Zucker diabetic rats. Lab Invest 2009, 89, 59–67. [DOI] [PubMed] [Google Scholar]
- (8).Mani S; Li H; Untereiner A; Wu L; Yang G; Austin RC; Dickhout JG; Lhotak S; Meng QH; Wang R Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation 2013, 127, 2523–2534. [DOI] [PubMed] [Google Scholar]
- (9).Hu LF; Lu M; Tiong CX; Dawe GS; Hu G; Bian JS Neuroprotective effects of hydrogen sulfide on Parkinson’s disease rat models. Aging Cell 2010, 9, 135–146. [DOI] [PubMed] [Google Scholar]
- (10).Kimura H Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem. Int 2013, 63, 492–497. [DOI] [PubMed] [Google Scholar]
- (11).Bhatia M Hydrogen sulfide as a vasodilator. IUBMB Life 2005, 57, 603–606. [DOI] [PubMed] [Google Scholar]
- (12).Carballal S; Trujillo M; Cuevasanta E; Bartesaghi S; Moller MN; Folkes LK; Garcia-Bereguiain MA; Gutierrez-Merino C; Wardman P; Denicola A; Radi R; Alvarez B Reactivity of hydrogen sulfide with peroxynitrite and other oxidants of biological interest. Free Radic. Biol. Med 2011, 50, 196–205. [DOI] [PubMed] [Google Scholar]
- (13).Abe K; Kimura H The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci 1996, 16, 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Zhao W; Zhang J; Lu Y; Wang R The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001, 20, 6008–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Yang G; Zhao K; Ju Y; Mani S; Cao Q; Puukila S; Khaper N; Wu L; Wang R Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid. Redox Signal 2013, 18, 1906–1919. [DOI] [PubMed] [Google Scholar]
- (16).Zheng Y; Yu B; De La Cruz LK; Roy Choudhury M; Anifowose A; Wang B Toward Hydrogen Sulfide Based Therapeutics: Critical Drug Delivery and Developability Issues. Med. Res. Rev 2018, 38, 57–100. [DOI] [PubMed] [Google Scholar]
- (17).Giggenbach W Optical spectra of highly alkaline sulfide solutions and the second dissociation constant of hydrogen sulfide. Inorg. Chem 1971, 10, 1333–1338. [Google Scholar]
- (18).DeLeon ER; Stoy GF; Olson KR Passive loss of hydrogen sulfide in biological experiments. Anal. Biochem 2012, 421, 203–207. [DOI] [PubMed] [Google Scholar]
- (19).Whiteman M; Li L; Rose P; Tan CH; Parkinson DB; Moore PK The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid. Redox Signal 2010, 12, 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Benavides GA; Squadrito GL; Mills RW; Patel HD; Isbell TS; Patel RP; Darley-Usmar VM; Doeller JE; Kraus DW Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Li L; Whiteman M; Guan YY; Neo KL; Cheng Y; Lee SW; Zhao Y; Baskar R; Tan CH; Moore PK Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 2008, 117, 2351–2360. [DOI] [PubMed] [Google Scholar]
- (22).Szabo C; Papapetropoulos A International Union of Basic and Clinical Pharmacology. CII: Pharmacological Modulation of H2S Levels: H2S Donors and H2S Biosynthesis Inhibitors. Pharmacol. Rev 2017, 69, 497–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Powell CR; Dillon KM; Matson JB A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol 2018, 149, 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Bora P; Chauhan P; Pardeshi KA; Chakrapani H Small molecule generators of biologically reactive sulfur species. RSC Adv. 2018, 8, 27359–27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Zhao Y; Biggs TD; Xian M Hydrogen sulfide (H2S) releasing agents: chemistry and biological applications. Chem. Commun 2014, 50, 11788–11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Chengelis CP; Neal RA Studies of carbonyl sulfide toxicity: Metabolism by carbonic anhydrase. Toxicol. Appl. Pharmacol 1980, 55, 198–202. [DOI] [PubMed] [Google Scholar]
- (27).Steiger AK; Zhao Y; Pluth MD Emerging Roles of Carbonyl Sulfide in Chemical Biology: Sulfide Transporter or Gasotransmitter? Antioxid. Redox Signal 2018, 28, 1516–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Haritos VS; Dojchinov G Carbonic anhydrase metabolism is a key factor in the toxicity of CO2 and COS but not CS2 toward the flour beetle Tribolium castaneum Coleoptera : Tenebrionidae. Comp. Biochem. Physiol. C-Toxicol. Pharmacol 2005, 140, 139–147. [DOI] [PubMed] [Google Scholar]
- (29).Kernohan JC The activity of bovine carbonic anhydrase in imidazole buffers. Biochim. Biophys. Acta 1964, 81, 346–356. [Google Scholar]
- (30).Lindskog S Structure and mechanism of carbonic anhydrase. Pharmacol. Ther 1997, 74, 1–20. [DOI] [PubMed] [Google Scholar]
- (31).Maren TH Carbonic Anhydrase - Chemistry, Physiology, and Inhibition. Physiol. Rev 1967, 47, 595–781. [DOI] [PubMed] [Google Scholar]
- (32).Steiger AK; Pardue S; Kevil CG; Pluth MD Self-Immolative Thiocarbamates Provide Access to Triggered H2S Donors and Analyte Replacement Fluorescent Probes. J. Am. Chem. Soc 2016, 138, 7256–7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Zhao Y; Pluth M Hydrogen Sulfide Donors Activated by Reactive Oxygen Species. Free Radic. Biol. Med 2016, 100, S28–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Zhao Y; Henthorn HA; Pluth MD Kinetic Insights into Hydrogen Sulfide Delivery from Caged-Carbonyl Sulfide Isomeric Donor Platforms. J. Am. Chem. Soc 2017, 139, 16365–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Zhao Y; Bolton SG; Pluth MD Light-Activated COS/H2S Donation from Photocaged Thiocarbamates. Org. Lett 2017, 19, 2278–2281. [DOI] [PubMed] [Google Scholar]
- (36).Sharma AK; Nair M; Chauhan P; Gupta K; Saini DK; Chakrapani H Visible-Light-Triggered Uncaging of Carbonyl Sulfide for Hydrogen Sulfide (H2S) Release. Org. Lett 2017, 19, 4822–4825. [DOI] [PubMed] [Google Scholar]
- (37).Stacko P; Muchova L; Vitek L; Klan P Visible to NIR Light Photoactivation of Hydrogen Sulfide for Biological Targeting. Org. Lett 2018, 20, 4907–4911. [DOI] [PubMed] [Google Scholar]
- (38).Zhao Y; Steiger AK; Pluth MD Cysteine-activated hydrogen sulfide (H2S) delivery through caged carbonyl sulfide (COS) donor motifs. Chem. Commun 2018, 54, 4951–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Steiger AK; Marcatti M; Szabo C; Szczesny B; Pluth MD Inhibition of Mitochondrial Bioenergetics by Esterase-Triggered COS/H2S Donors. ACS Chem. Biol 2017, 12, 2117–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Chauhan P; Bora P; Ravikumar G; Jos S; Chakrapani H Esterase Activated Carbonyl Sulfide/Hydrogen Sulfide (H2S) Donors. Org. Lett 2017, 19, 62–65. [DOI] [PubMed] [Google Scholar]
- (41).Levinn CM; Steiger AK; Pluth MD Esterase-Triggered Self-Immolative Thiocarbamates Provide Insights into COS Cytotoxicity. ACS Chem. Biol 2019, 14, 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Tian L; Yang YL; Wysocki LM; Arnold AC; Hu A; Ravichandran B; Sternson SM; Looger LL; Lavis LD Selective esterase-ester pair for targeting small molecules with cellular specificity. Proc. Natl. Acad. Sci. USA 2012, 109, 4756–4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Sletten EM; Bertozzi CR Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angewandte Chemie-International Edition 2009, 48, 6974–6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Steiger AK; Yang Y; Royzen M; Pluth MD Bio-orthogonal “click-and-release” donation of caged carbonyl sulfide (COS) and hydrogen sulfide (H2S). Chem. Commun 2017, 53, 1378–1380. [DOI] [PubMed] [Google Scholar]
- (45).Siegel LM A direct microdetermination for sulfide. Anal. Biochem 1965, 11, 126–132. [DOI] [PubMed] [Google Scholar]
- (46).Henthorn HA; Pluth MD Mechanistic Insights into the H2S-Mediated Reduction of Aryl Azides Commonly Used in H2S Detection. J. Am. Chem. Soc 2015, 137, 15330–15336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Hammers MD; Taormina MJ; Cerda MM; Montoya LA; Seidenkranz DT; Parthasarathy R; Pluth MD A Bright Fluorescent Probe for H2S Enables Analyte-Responsive, 3D Imaging in Live Zebrafish Using Light Sheet Fluorescence Microscopy. J. Am. Chem. Soc 2015, 137, 10216–10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Montoya LA; Pluth MD Selective turn-on fluorescent probes for imaging hydrogen sulfide in living cells. Chem. Commun 2012, 48, 4767–4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Zhao Y; Steiger AK; Pluth MD Colorimetric Carbonyl Sulfide (COS)/Hydrogen Sulfide (H2S) Donation from gamma-Ketothiocarbamate Donor Motifs. Angew. Chem. Int. Ed 2018, 57, 13101–13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Zhao Y; Cerda MM; Pluth MD Fluorogenic hydrogen sulfide (H2S) donors based on sulfenyl thiocarbonates enable H2S tracking and quantification. Chem. Sci 2019, 10, 1873–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Monks T; Jones D The Metabolism and Toxicity of Quinones, Quinonimines, Quinone Methides, and Quinone-Thioethers. Curr. Drug Metab 2002, 3, 425–438. [DOI] [PubMed] [Google Scholar]
- (52).Balazy M; Abu-Yousef IA; Harpp DN; Park J Identification of carbonyl sulfide and sulfur dioxide in porcine coronary artery by gas chromatography/mass spectrometry, possible relevance to EDHF. Biochem. Biophys. Res. Commun 2003, 311, 728–734. [DOI] [PubMed] [Google Scholar]