Abstract
Background
exposures in childhood and adolescence may impact the development of diseases and symptoms in late life. However, evidence from low- and middle-income countries is scarce. In this cross-sectional study, we examined the association of early life risk factors with frailty amongst older adults using a large, nationally representative cohort of community-dwelling Chinese sample.
Methods
we included 6,806 participants aged  60 years from the China Health and Retirement Longitudinal Study. We measured 13 risk factors in childhood or adolescence through self-reports, encompassing six dimensions (education, family economic status, nutritional status, domestic violence, neighbourhood and health). We used multinomial regression models to examine the association between risk factors and frailty. We further calculated the absolute risk difference for the statistically significant factors.
60 years from the China Health and Retirement Longitudinal Study. We measured 13 risk factors in childhood or adolescence through self-reports, encompassing six dimensions (education, family economic status, nutritional status, domestic violence, neighbourhood and health). We used multinomial regression models to examine the association between risk factors and frailty. We further calculated the absolute risk difference for the statistically significant factors.
Results
persons with higher personal and paternal education attainment, better childhood neighbourhood quality and better childhood health status had lower risk of being frail in old age. Severe starvation in childhood was associated with higher risk of prefrailty. The risk differences of being frail were 5.6% lower for persons with a high school or above education, 1.5% lower for those whose fathers were literate, 4.8% lower for the highest neighbourhood quality and 2.9% higher for worse childhood health status compared to their counterparts.
Conclusions
unfavorable socioeconomic status and worse health condition in childhood and adolescence may increase the risk of late-life frailty amongst Chinese older adults.
Keywords: frailty, lifecourse, China, older people
Key points
Unfavorable SES and worse health condition in childhood and adolescence may increase the risk of late-life frailty.
Chinese people with higher personal and paternal education attainment had lower risk of frailty.
Chinese people with better childhood health status and better neighbourhood quality during childhood had lower risk of frailty.
Introduction
The value of a life course approach, which focuses on linking exposures taking place during life course and the development of disease risks, has been increasingly recognised in the study of ageing [1, 2]. Circumstances in early life stage are hypothesised to exert influence on health in later life through biological, behavioural and psychosocial pathways both independently and synergistically [1]. Researchers have developed a variety of theories to understand the underlying mechanisms linking early life circumstances and later-life health [3–6]. Despite differences in the mechanisms between these conceptual models, there is a general consensus that biological, behavioural, psychosocial and environmental risk factors in early life contribute to health in old age. Additionally, childhood and adolescence may represent a key life stage for designing interventions to preserve cognition and functional independence and improve quality of life in old age. Recently, an increasing number of empirical studies have pointed to the importance of early life risk factors in the development of late-life diseases and syndromes [7–13], including frailty, a clinical syndrome featured with reduced resilience to stressors and increased vulnerability to adverse outcomes [14–16]. Increasing evidence suggests that poor early-life socioeconomic conditions, such as low education and poor housing quality, were associated with higher risk of frailty [9–13]. Although the mechanisms of how early life situations contribute to the development of frailty are not fully understood, recent studies have revealed several factors during adulthood (e.g. health behaviours and social participation) for explaining the associations between disadvantaged conditions of early life, particularly low education, and frailty in old age [12, 13, 17].
Frailty is common amongst older adults and is associated with shorter survival [18], increased risks of hospitalisation and disability [19], and higher health care utilisation and costs [3, 20], placing a substantial burden on older persons, their caregivers [21, 22] and health care resources [18, 20]. However, to our knowledge, few studies to date examined the early life determinants of frailty amongst older adults in low- and middle-income countries [10], and no such studies have been conducted in China—a country that has the largest ageing population in the world, and 7% of those aged ≥60 years are frail [23]. Moreover, most prior studies have used a unidimensional indicator of early life conditions (e.g. education) or a few subdimensions (e.g. housing quality and family’s financial situation during childhood).
Although frailty has been an important concept in the field of geriatrics and gerontology, there is a lack of general consensus on the conceptual basis and operational definition of frailty [24]. Numerous assessments of frailty, guided by distinct frameworks and theories, have been developed to identify frail individuals. In the present study, frailty is conceptualised as a distinct biologic syndrome with specific biological basis and underlying pathophysiology. We operationalised frailty using the Fried’s physical frailty phenotype (PFP) in which five clinical hallmarks are included: slowness, weakness, exhaustion, inactivity and weight loss [15]. The PFP is one of the two most commonly cited frailty assessments, and its construct and predictive validity has been demonstrated [25, 26]. It is also one of the few frailty instruments developed amongst community-dwelling Chinese older adults. In the PFP framework, individuals showing three or more of five clinical signs are defined as frail and those with one or two hallmarks are considered prefrail—an intermediate stage of frailty that is reversible and more susceptible to interventions [27–29]. Identification of prefrail individuals and their risk factor profiles may provide insights into the mechanisms involved in the development and progression of frailty and help design more targeted and effective interventions.
In this cross-sectional study, we examined the association of early life risk factors (childhood and adolescence), including family socio-economic status, domestic violence, health condition and neighbourhood environment, with frailty in old age using a large, nationally representative cohort of community-dwelling Chinese older adults.
Methods
Data and study participants
Data are from the China Health and Retirement Longitudinal Study (CHARLS), an ongoing longitudinal cohort study of a nationally representative sample of community-dwelling adults aged 45 years and older from 28 provinces in China. The baseline survey was conducted between June 2011 and March 2012. A total of 12,740 households were contacted; the response rate was 80.5%, resulting in 17,708 individuals residing in 10,257 households. Follow-up survey is carried out every 2 years thereafter. All alive respondents in the first two waves (2011 and 2013) were invited to participate in the 2014 Life History Survey that included questions about residential history, education history, health and health care history, wealth history and other important childhood and family events. The CHARLS was approved by the Ethical Review Committee at the Peking University. Details about the recruitment strategy and design of the CHARLS can be referred to previous publication [30].
The present study excluded participants who (i) did not participate in the 2014 Life History Survey (n = 3,103); (ii) did not report age (n = 10), (iii) were less than 60 years of age (n = 4,136); or (iv) did not have frailty assessment in 2011 or 2013 (n = 3,653), leading to an analytic sample of 6,806.
Frailty
Frailty was measured by an adapted version of the PFP [15], which was previously constructed and validated in the CHARLS cohort [23]. The PFP includes five criteria: slowness, weakness, exhaustion, inactivity and weight loss. The slowness criterion was met when gait speed, measured as the average of two timed walk tests over a 2.5 m course, was at or below the sex- and height-specific cut-points [23]. The weakness criterion was met when handgrip strength, assessed as the maximum of four readings (two for each hand) by a handheld dynamometer, was at or below the sex- and body mass index (BMI)-specific cut-points [23]. The exhaustion criterion was met if the participant answered ‘A moderate amount of time; 3 to 4 days’ or ‘Most of the time; 5 to 7 days’ when asked ‘How often during the last week did you feel this way’ to either of the two questions from the Center for Epidemiological Studies-Depression scale [23]: ‘I could not get going’ and ‘I felt everything I did was an effort.’ The inactivity criterion was met if the respondent answered ‘No’ when asked ‘During a usual week, did you walk at least 10 minutes continuously?’ The weight loss criterion was met if the respondent self-reported loss of at least five kilograms in the previous year or currently had a BMI  18.5
18.5  (calculated from measured height and weight).
(calculated from measured height and weight).
The frailty level was determined by the number of criteria met. Individuals with none were considered ‘non-frail’, those meeting one or two criteria were considered ‘prefrail’ and those with three to five criteria were defined as ‘frail’. Frailty was missing if two or more of the five criteria were missing (n = 3,653). Frailty was identified in the 2013 wave (n = 5,250); data from the 2011 wave were used for those persons who did not have frailty assessment in 2013 (n = 1,556).
Early life risk factors
We included six dimensions—education, family economic status, nutritional status, domestic violence, neighbourhood and health—from two life stages: childhood and adolescence. Maternal and paternal education level was each classified as low (illiterate) or high (literate). Participant’s education attainment was categorised as illiterate, primary school, middle school or high school or beyond. Family economic status was measured by asking ‘When you were a child before age 17, compared to the average family in the same community/village at that time, how was your family’s financial situation?’ and was classified as low (somewhat or a lot worse than others), average (same as others) or high (somewhat or a lot better off than others). Nutritional status before age 17, reflected by starvation during the Great Leap Forward (1958–1962), was categorised as none (did not experience starvation), moderate (experienced starvation) and severe (family member starved to death). Domestic violence was measured by three questions asking the participants whether female guardian, male guardian and siblings, respectively, ever hit them when they grew up. For each question, participants who responded ‘Often’ or ‘Sometimes’ were considered experiencing domestic violence while those who answered ‘Rarely’ or ‘Never’ were considered not. Childhood neighbourhood quality before age 17 was assessed by four questions asking the participants whether the neighbourhood they lived as a child was safe, willing to help, close-knit and clean and attractive, respectively. Each measure was dichotomised as low (not very or not at all) and high (somewhat or very). The overall neighbourhood quality was measured by the sum score of four measures, ranging from 0 (lowest) to 4 (highest). Childhood health status was assessed by asking ‘Before you were 15 years old (including 15 years old), would you say that compared to other children of the same age, you were?’ and classified as high (somewhat or much healthier), average (about average) or low (somewhat or much less healthy).
Covariates
Socio-demographic and lifestyle factors, including age, sex, marital status (married versus others), current residence (urban versus rural) and smoking status (never, previous and current), were derived from the same wave as the frailty measure. BMI was calculated as body weight divided by height squared and categorised as underweight (<18.5), normal (18.5–23.9), overweight (24.0–27.9) and obese ( 28.0) [31]. Participants were assessed for disability in five activities of daily living (ADL) tasks: dressing, bathing, eating, getting out of bed and toileting. For each task, participants were asked ‘Do you have difficulty in’ performing the task? Those participants who responded, ‘I have difficulty but can still do it’, ‘Yes, I have difficulty and need help’ or ‘I cannot do it’ to one or more tasks were considered having ADL disability. The total number of comorbidities (hypertension, diabetes, cancer [excluding minor skin cancers], cardiac disease [including myocardial infarction, coronary heart disease, angina, heart failure or other heart problems], stroke, chronic lung diseases, liver disease, kidney disease, stomach or other digestive disease and arthritis/rheumatism) was considered as a continuous covariate. Missing covariates for participants who had frailty assessment in 2013 were imputed by observed values in 2011 (n = 865 for smoking status; n = 8 for comorbidity).
28.0) [31]. Participants were assessed for disability in five activities of daily living (ADL) tasks: dressing, bathing, eating, getting out of bed and toileting. For each task, participants were asked ‘Do you have difficulty in’ performing the task? Those participants who responded, ‘I have difficulty but can still do it’, ‘Yes, I have difficulty and need help’ or ‘I cannot do it’ to one or more tasks were considered having ADL disability. The total number of comorbidities (hypertension, diabetes, cancer [excluding minor skin cancers], cardiac disease [including myocardial infarction, coronary heart disease, angina, heart failure or other heart problems], stroke, chronic lung diseases, liver disease, kidney disease, stomach or other digestive disease and arthritis/rheumatism) was considered as a continuous covariate. Missing covariates for participants who had frailty assessment in 2013 were imputed by observed values in 2011 (n = 865 for smoking status; n = 8 for comorbidity).
Statistical analysis
Frailty status and baseline characteristics were compared between participants who had frailty assessment in 2011 and 2013 waves. Next, we compared the characteristics measured at baseline (2011 or 2013) by frailty status (non-frail, prefrail and frail) using a  test for categorical variables and analysis of variance for continuous variables.
test for categorical variables and analysis of variance for continuous variables.
We examined the unadjusted association between each early life risk factor and frailty using a  test. Subsequently, we used a multinomial logistic model to simultaneously examine early life risk factors that were associated with frailty at a significance level of P < 0.20 in the bivariate analysis [32], adjusting for demographic factors (age, sex, residence and marital status), ADL disability and count of comorbidity. To test for potential sex difference, we included a multiplicative interaction term between each risk factor (P < 0.2 in bivariate analysis) and sex in the adjustment model and used a log-likelihood ratio test for interaction. As a sensitivity analysis, multiple imputation with chained equations was used to impute missing values, assuming that data were missing at random [33]. Estimates were combined across 20 imputed datasets based on Rubin’s rules [34].
test. Subsequently, we used a multinomial logistic model to simultaneously examine early life risk factors that were associated with frailty at a significance level of P < 0.20 in the bivariate analysis [32], adjusting for demographic factors (age, sex, residence and marital status), ADL disability and count of comorbidity. To test for potential sex difference, we included a multiplicative interaction term between each risk factor (P < 0.2 in bivariate analysis) and sex in the adjustment model and used a log-likelihood ratio test for interaction. As a sensitivity analysis, multiple imputation with chained equations was used to impute missing values, assuming that data were missing at random [33]. Estimates were combined across 20 imputed datasets based on Rubin’s rules [34].
To improve the interpretability of the estimates, we calculated absolute risk difference (ARD) for each risk factor that showed significance at a level of P < 0.05 from the adjusted model. Specifically, we subtracted the conditional predicted probability of being frail (or prefrail) with the risk factor of interest set equal to a specific value from that with the factor of interest set equal to the reference level when all other categorical variables being set to their reference levels (median for continuous variables) [35]. All analyses were conducted using Stata 13.0 (Stata Corp, College Station, TX).
Results
A total of 1,556 (22.9%) participants with frailty measure in 2011 and 5250 participants with frailty measure in 2013 were included. Persons who had frailty assessment in 2013 were slightly older (69.3 versus 68.0 years) and more likely to be male (52.1 versus 46.5%) and never smokers (70.3 versus 57.9%) than those with frailty measure in 2011 (Table S1). Persons with frailty assessment in 2011 and 2013 had similar level of frailty, disability and comorbidity. Of 6,806 eligible participants, 2,409 (35.4%) were non-frail, 3,849 (56.6%) were prefrail and 548 (8.1%) were frail. Frail participants were older, more often female and not-married, more likely to live in rural area, had more comorbidities and had higher prevalence of ADL disability than those who were non-frail (Table 1).
Table 1 .
Association of baseline characteristics and frailty
| Non-frail (N = 2,409) | Prefrail (N = 3,849) | Frail (N = 548) | Total (N = 6,806) | P-valuea | |
|---|---|---|---|---|---|
| Count (%) or Mean ± SD | |||||
| Age, years | 67.6 ± 6.0 | 69.3 ± 7.0 | 73.0 ± 7.9 | 69.0 ± 6.9 | <0.001 |
| Sex | <0.001 | ||||
| Female | 1,068 (44.3) | 1,994 (51.8) | 285 (52.0) | 3,347 (49.2) | |
| Male | 1,341 (55.7) | 1,856 (48.2) | 263 (48.0) | 3,459 (50.8) | |
| Marital status | <0.001 | ||||
| Marriedb | 2,035 (84.5) | 3,042 (79.0) | 385 (70.3) | 5,462 (80.3) | |
| Othersc | 374 (15.5) | 807 (21.0) | 163 (29.7) | 1,344 (19.8) | |
| Residence | 0.001 | ||||
| Rural | 1,476 (61.3) | 2,513 (65.3) | 374 (68.3) | 4,363 (64.1) | |
| Urban | 933 (38.7) | 1,336 (34.7) | 174 (31.8) | 2,443 (35.9) | |
BMI, kg/
|
<0.001 | ||||
| <18.5 | 0 (0) | 431 (11.2) | 152 (28.4) | 583 (8.6) | |
| 18.5–24.0 | 1,362 (56.6) | 1,968 (51.3) | 218 (40.7) | 3,548 (52.3) | |
| 24.0–28.0 | 769 (32.0) | 1,046 (27.3) | 115 (21.5) | 1,930 (28.5) | |
| ≥28.0 | 276 (11.5) | 394 (10.3) | 51 (9.5) | 721 (10.6) | |
| Smoking status | 0.196 | ||||
| Never | 1,245 (56.1) | 2,152 (59.1) | 296 (56.2) | 3,693 (57.8) | |
| Previous | 304 (13.7) | 471 (12.9) | 76 (14.4) | 851 (13.3) | |
| Current | 672 (30.3) | 1,020 (28.0) | 155 (29.4) | 1,847 (28.9) | |
| Count of comorbidityd | 1.3 ± 1.3 | 1.7 ± 1.5 | 1.9 ± 1.6 | 1.6 ± 1.4 | <0.001 |
| ADL disability (yes versus no) | 250 (10.4) | 844 (21.9) | 250 (45.6) | 1,344 (19.8) | <0.001 |
SD, standard deviation; BMI, body mass index.
Note: There were 24, 415, 108 and 1 missing values for BMI, smoking status, count of comorbidity and ADL disability, respectively.
a P-values were calculated from chi-square tests or analysis of variance.
bIncluding living together.
cIncluding widowed, separated, divorced and never married.
dComorbidity includes hypertension, diabetes, cancer [excluding minor skin cancers], cardiac disease [including myocardial infarction, coronary heart disease, angina, heart failure or other heart problems], stroke, chronic lung diseases, liver disease, kidney disease, stomach or other digestive disease and arthritis/rheumatism.
In the unadjusted models, education (paternal, maternal and self), family’s financial situation, starvation, childhood neighbourhood quality and childhood health status were significantly associated with frailty (P’s < 0.05; Table 2) and were therefore analysed simultaneously in the multinomial logistic regression. None of the domestic violence measures (female guardian, male guardian and siblings) were significantly associated with frailty.
Table 2 .
Bivariate association between early-life risk factors and frailty
| Non-frail | Prefrail | Frail | P-valuea | |
|---|---|---|---|---|
| Count (%) | ||||
| Education (n = 6633) | <0.001 | |||
| Illiterate | 538 (22.7) | 1,242 (33.2) | 231 (44.7) | |
| Primary school | 1,174 (49.4) | 1,740 (46.5) | 228 (44.1) | |
| Middle school | 427 (18.0) | 509 (13.6) | 45 (8.7) | |
| High school or above | 236 (9.9) | 250 (6.7) | 13 (2.5) | |
| Maternal education (n = 6325) | 0.008 | |||
| Illiterate | 2,128 (93.7) | 3,395 (95.1) | 471 (96.7) | |
| Literateb | 142 (6.3) | 173 (4.9) | 16 (3.3) | |
| Paternal education (n = 6036) | <0.001 | |||
| Illiterate | 1,333 (61.7) | 2,273 (66.5) | 336 (74.0) | |
| Literateb | 829 (38.3) | 1,147 (33.5) | 118 (26.0) | |
| Family’s financial situation (n = 6569) | <0.001 | |||
| Better off than others | 207 (8.8) | 332 (9.0) | 31 (6.1) | |
| Same as others | 1,252 (53.1) | 1,799 (48.6) | 229 (45.1) | |
| Worse than others | 900 (38.2) | 1,571 (42.4) | 248 (48.8) | |
| Starvation (n = 6533) | 0.001 | |||
| No | 390 (16.6) | 542 (14.7) | 86 (16.9) | |
| Yes | 1,681 (71.6) | 2,583 (70.3) | 339 (66.7) | |
| Severe | 278 (11.8) | 551 (15.0) | 83 (16.3) | |
| Domestic violence (n = 6806)c | ||||
| Female guardian ever hit | 521 (23.5) | 777 (22.5) | 114 (25.5) | 0.307 |
| Male guardian ever hit | 339 (15.7) | 502 (15.1) | 66 (15.0) | 0.825 |
| Siblings ever hit | 97 (4.2) | 187 (5.2) | 22 (4.7) | 0.218 |
| Neighbourhood (n = 6806)d | ||||
| Very or somewhat safe | 2,142 (92.5) | 3,222 (90.2) | 410 (87.2) | <0.001 |
| Very or somewhat willing to help | 2,038 (87.5) | 2,931 (81.7) | 365 (77.5) | <0.001 |
| Very or somewhat close-knit | 2,239 (95.9) | 3,388 (93.5) | 434 (91.0) | <0.001 |
| Very or somewhat clean and attractive | 1,582 (67.5) | 2,339 (65.4) | 280 (58.8) | 0.001 |
| Childhood health status (n = 6571) | <0.001 | |||
| Healthier than others | 887 (37.7) | 1,220 (32.9) | 132 (5.90) | |
| Same as others | 1,247 (53.0) | 1,980 (53.4) | 271 (53.2) | |
| Worse than others | 220 (9.4) | 508 (13.7) | 106 (20.8) | |
a P-values were calculated from chi-square tests or analysis of variance (bivariate analysis).
bIncluding capable of reading or writing, traditional Chinese school (i.e. Sishu)/home school, elementary school, middle school, high school, vocational school, college or postgraduate.
cCompared with rarely or never hit.
dCompared with low neighbourhood quality (not very or not at all).
In the adjusted model, the risk of being frail versus non-frail was significantly lower amongst participants with higher paternal education levels, higher personal educational attainment, better childhood neighbourhood quality and better childhood health status (Table 3). Persons whose paternal education was literate were 26% (95% CI: 4%, 43%) less likely to be frail than those whose fathers were illiterate, but showed no significant difference for being prefrail. Persons with the highest neighbourhood quality during childhood (score: 4) had significantly lower ratios of being prefrail and frail, than those with the lowest (score: 0). Persons whose childhood health status was healthier than peers of similar ages had a 26% (95% CI: 4%, 43%) lower ratio of being frail than those whose health status was same as others, but showed no statistical significance for prefrailty. Severe starvation in childhood was associated with 30% (95% CI: 4%, 62%) higher likelihood of being prefrail, but showed no statistical significance for frail status. None of the interaction terms between early life risk factors and sex were statistically significant (P’s > 0.1). When we used the multiple imputation with chained equation to deal with missing data, the results did not differ substantively (Table S2).
Table 3 .
Association of early-life risk factors and frailty by multivariate multinomial model (n = 5694)
| Prefrail [RR (95%CI)] | Frail [RR (95%CI)] | P for global test of interactione | |
|---|---|---|---|
| Education | 0.523 | ||
| Illiterate | ref. | ref. | |
| Primary school | 0.77 (0.66, 0.90) | 0.64 (0.49, 0.84) | |
| Middle school | 0.69 (0.56, 0.84) | 0.41 (0.27, 0.63) | |
| High school or above | 0.61 (0.48, 0.78) | 0.23 (0.12, 0.44) | |
| Maternal education | 0.377 | ||
| Illiterate | ref. | ref. | |
| Literatea | 1.05 (0.81, 1.35) | 1.02 (0.53, 1.95) | |
| Paternal education | 0.114 | ||
| Illiterate | ref. | ref. | |
| Literatea | 0.94 (0.83, 1.06) | 0.74 (0.57, 0.96) | |
| Family’s financial situation | 0.599 | ||
| Same as others | ref. | ref. | |
| Better off than others | 1.12 (0.91, 1.38) | 0.81 (0.50, 1.31) | |
| Worse than others | 1.06 (0.94, 1.20) | 1.19 (0.94, 1.51) | |
| Starvation | 0.830 | ||
| No | ref. | ref. | |
| Yes | 1.15 (0.98, 1.35) | 1.07 (0.78, 1.47) | |
| Severe | 1.30 (1.04, 1.62) | 1.24 (0.83, 1.88) | |
| Neighbourhoodb | 0.846 | ||
| 0 | ref. | ref. | |
| 1 | 0.74 (0.41, 1.33) | 0.60 (0.27, 1.34) | |
| 2 | 0.54 (0.32, 0.88) | 0.44 (0.23, 0.88) | |
| 3 | 0.53 (0.33, 0.85) | 0.45 (0.24, 0.84) | |
| 4 | 0.45 (0.28, 0.72) | 0.28 (0.15, 0.52) | |
| Childhood health status | 0.488 | ||
| Same as others | ref. | ref. | |
| Healthier than others | 0.91 (0.80, 1.03) | 0.74 (0.57, 0.96) | |
| Worse than others | 1.32 (1.09, 1.60) | 1.82 (1.33, 2.50) | |
| Age | 1.03 (1.02, 1.04) | 1.09 (1.07, 1.11) | |
| Sex | |||
| Female | ref. | ref. | |
| Male | 0.87 (0.77, 0.99) | 1.00 (0.78, 1.28) | |
| Residence | |||
| Urban | ref. | ref. | |
| Rural | 1.08 (0.95, 1.22) | 0.94 (0.74, 1.20) | |
| Marital status | |||
| Married | ref. | ref. | |
| Others c | 1.09 (0.93, 1.28) | 1.15 (0.87, 1.52) | |
| Count of comorbidityd | 1.16 (1.11, 1.21) | 1.22 (1.13, 1.31) | |
| ADL | 1.96 (1.65, 2.32) | 4.94 (3.83, 6.37) |
RR, risk ratio; CI, confidence interval.
aIncluding capable of reading or writing, traditional Chinese school (i.e. Sishu)/home school, elementary school, middle school, high school, vocational school, college or postgraduate.
b0: lowest quality, 4: highest quality.
cIncluding widowed, separated, divorced and never married.
dComorbidity includes hypertension, diabetes, cancer [excluding minor skin cancers], cardiac disease [including myocardial infarction, coronary heart disease, angina, heart failure or other heart problems], stroke, chronic lung diseases, liver disease, kidney disease, stomach or other digestive disease and arthritis/rheumatism.
eLog-likelihood ratio test for multiplicative interaction between each risk factor and sex, adjusting for age, residence, marital status, ADL disability and count of comorbidity.
Compared to persons who were illiterate, persons who had a primary school education were about 4% less likely to be prefrail, and the risk differences were 5.7 and 7.2% for those with middle school and high school or above education, respectively (Figure 1); the risk difference ranged from 1.7 to 5.6% in being frail by higher levels of personal education (Figure 2). Participants whose fathers were literate had 1.5% lower risk of being frail than those whose fathers were illiterate. Persons with the highest neighbourhood quality (score: 4) had 11.4 and 4.8% lower risk of being prefrail and frail, respectively, than those with the lowest (score: 0). Worse childhood health status was associated with 2.9% higher risk of being frail.
Figure 1 .
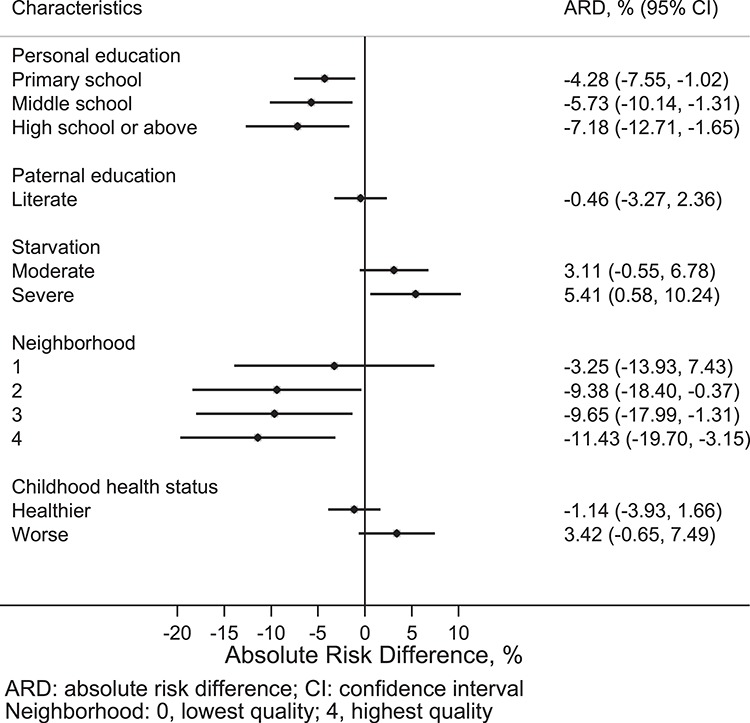
ARDs for early-life risk factors independently associated with prefrailty.
Figure 2 .
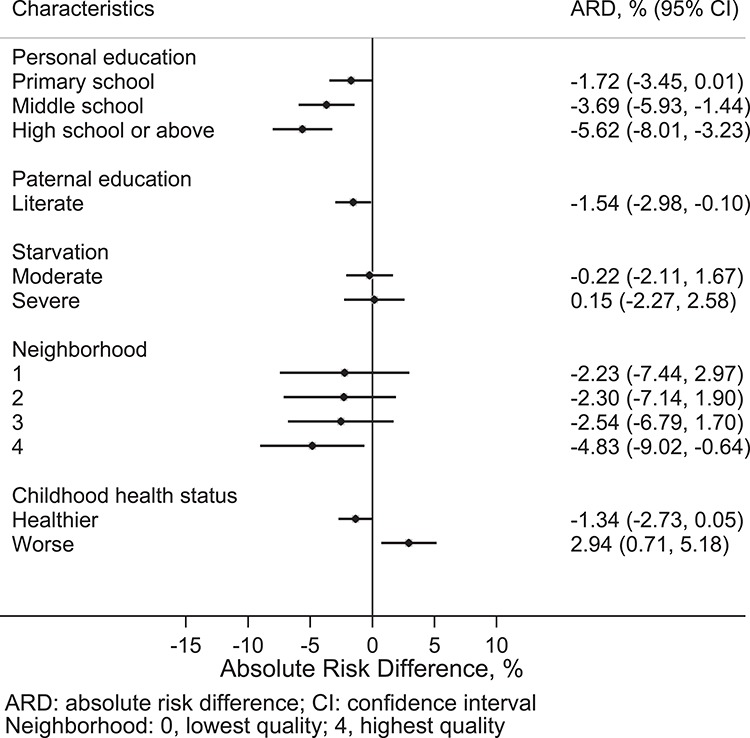
ARDs for early-life risk factors independently associated with frailty.
Discussion
In this nationally representative sample of 6,818 Chinese adults aged 60 years or above, we found that several early life risk factors were significantly associated with frailty in old age. Older persons with higher personal or paternal education attainment, better childhood neighbourhood quality and better childhood health status were less likely to be frail. Exposures taking place in childhood and adolescence may have a long-term influence in health outcomes as expected.
Our findings were consistent with previous studies conducted in other countries, in which persons who were trapped in unfavorable socioeconomic status (e.g. lower social class and experience of hunger) in early life period or worse childhood health conditions were more vulnerable to frailty [7–10]. We found a dose-response relationship between education and level of frailty; Chinese older adults with a primary school, middle school and high school or above education had a 36, 59 and 77% lower risk of frailty, respectively, than those illiterate ones. These results are in line with those of studies focusing on European populations. Using data from a cohort study conducted in Spain, Soler-Vila et al. found that the odds of being frail amongst older women with primary or lower education were approximately two times more than those with university education [12]. Using nationally representative samples of 11 European countries, Etman and colleagues showed that persons with more than 10 years of education had a lower likelihood of frailty than those lower educated (<10 years) [13]. Moreover, our study showed that a lower level of father’s education was associated with a higher risk of frailty. These results were echoed by an earlier study in which Gale et al. found that lower paternal social class was related to higher risk of frailty amongst older adults in Scottland [9]. We did not find the association between higher maternal education level and lower risk of frailty. These findings should be interpreted with caution because over 95% of participants’ mothers were illiterate and, therefore, maternal education level may not be a good indicator of socioeconomic conditions in early life stage for this cohort. Nevertheless, the education level of women in China has improved dramatically over the past decades. Whether maternal education plays a role in the development of frailty might be revisited using more recent birth cohorts in China.
In addition to childhood socioeconomic status and health, we found an association between higher childhood neighbourhood quality and decreased risk of frailty. Neighbourhood quality during childhood may influence health both directly (e.g. dangerous physical environment) and indirectly (e.g. via social connections and social support [36], healthy or unhealthy life styles and access to health and social services) [37]. Residents living in a safer [38, 39], more attractive and more socially cohesive neighbourhood were more likely to report better physical and mental health, regardless of their socioeconomic status [36]. Social cohesion, reflected by two dimensions of neighbourhood quality in our study—willing to help and close-knit—represents the density of social networks, structure of social relationships and personal involvement in society [40], all of which are associated with health outcomes [40, 41]. Perceived neighbourhood environment may be influenced by social norms, lifestyle and physical quality of environment [42] and can further reshape the stressfulness of life events to individuals and the degree of intimacy amongst dwellings within a neighbourhood, which, in turn, influences health [40, 41, 43].
The present study has several strengths. First, to the best of our knowledge, this is the first study to examine early life risk factor for frailty amongst Chinese older adults, providing evidence for making policy-relevant guidelines and developing intervention strategies for preventing frailty amongst the largest ageing population in the world [2]. Second, our sample includes 6,806 individuals residing in 4,843 households, 435 communities and 27 provinces, achieving a nationally representative coverage. Third, the data were collected through face-to-face interview instead of mailed survey, resulting in a high response rate (80.5%) and high data quality [30]. We acknowledge several limitations. First, as early life risk factors were extracted from a life history survey, recall bias is inevitable. However, recall data are the most accessible data type to investigate the health effects of early-life experiences in later life stage, and carefully collected retrospective information on social circumstances during childhood and youth could have a high degree of accuracy [44]. Second, frailty status may vary over time, and we were unable to examine the effect of risk factors on the trajectory of frailty. More longitudinal studies with repeated frailty measures are needed to identify determinants in frailty progression or remission amongst Chinese older adults. Third, numerous definitions and measurements of frailty exist, but we only used one assessment—the PFP approach. Studies have shown that the level of concordance of different frailty assessments is low and different frailty scores are not interchangeable [45, 46]. Our strategy, however, seems most suitable because we developed the PFP approach in the CHARLS cohort and demonstrated its predictive validity. In fact, not many frailty assessments have been developed using a large Chinese sample of older adults. Fourth, the numbers of frail persons in some categories of the early life risk factors, such as maternal education and domestic violence, were quite low. The statistical power for detecting the associations between these early life risk factors and frailty may be, therefore, limited. Future research with larger sample sizes is needed. Fifth, we only included persons who were at least 60 years of age (born after 1951). Therefore, we cannot rule out the possibility that the younger birth cohorts had different levels of frailty and early life risk factors. In an earlier study of older adults in 10 European countries, Stolz et al. [11] revealed that levels and trajectories of frailty were different in distinct birth cohorts. Future research using more recent birth cohorts may elucidate whether there exists cohort differences amongst Chinese older adults. Sixth, because the majority of risk factors examined in this study were collected before the age of 17, we were unable to examine the influence of different stages before adulthood (childhood and adolescence) individually. Lastly, we did not explicitly examine the mediating pathways between early life risk factors and frailty, which may inform interventions to mitigate frailty [47]; this is an important topic that is worth further investigation.
Using a nationally representative sample, we linked early life risk factors with frailty amongst Chinese community-dwelling older adults beyond the common chronic conditions and disability. We showed that greater socioeconomic and health disadvantage in early life could predispose individuals to frailty in late life, suggesting the necessity of incorporating life course approaches to studying the mechanism and interventions of frailty, and expanding the concept of late-life vulnerability beyond diseases and disability. Our findings contribute to the understanding of the origins and development of frailty amongst Chinese older adults.
Supplementary Material
Declaration of Conflicts of Interest
Dr ChenkaiWu provides paid consultant services to Health-KeeperS, a health data analytics company in China.Michelle Odden provides paid consultant services to Cricket Health, Inc, a kidney care company in the USA.
Declaration of Funding
SuzhouMunicipal Science and Technology Bureau (SS2019069), National Institute on Aging (R21-HL135195), U.S. PEPPER Center Scholar Award (P30AG021342) and NIH/NIA grant (K01AG053408).
Reference
- 1. Ben-Shlomo Y, Cooper R, Kuh D. The last two decades of life course epidemiology, and its relevance for research on ageing. Int J Epidemiol 2016; 45: 973–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sayer AA, Gill TM. Commentary: value of the life course approach to the health care of older people. Int J Epidemiol 2016; 45: 1011–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bock J-O, König H-H, Brenner Het al. Associations of frailty with health care costs–results of the ESTHER cohort study. BMC Health Serv Res 2016; 16: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strachan DP, Sheikh A. A life course approach to respiratory and allergic diseases. In: Kuh D, Yoav B-S, eds.A Life Course Approach to Chronic Disease Epidemiology 2004; 2: 240–59. [Google Scholar]
- 5. Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Ann N Y Acad Sci 1999; 896: 85–95. [DOI] [PubMed] [Google Scholar]
- 6. Dannefer D. Cumulative advantage/disadvantage and the life course: cross-fertilizing age and social science theory. J Gerontol B Psychol Sci Soc Sci 2003; 58: S327–37. [DOI] [PubMed] [Google Scholar]
- 7. Haapanen MJ, Perala MM, Salonen MKet al. Early life determinants of frailty in old age: the Helsinki birth cohort study. Age Ageing 2018; 47: 569–75. [DOI] [PubMed] [Google Scholar]
- 8. Herr M, Robine JM, Aegerter P, Arvieu JJ, Ankri J. Contribution of socioeconomic position over life to frailty differences in old age: Comparison of life-course models in a French sample of 2350 old people. Ann Epidemiol 2015; 25: 674–80. [DOI] [PubMed] [Google Scholar]
- 9. Gale CR, Booth T, Starr JM, Deary IJ. Intelligence and socioeconomic position in childhood in relation to frailty and cumulative allostatic load in later life: the Lothian birth cohort 1936. J Epidemiol Community Health 2016; 70: 576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alvarado BE, Zunzunegui MV, Beland F, Bamvita JM. Life course social and health conditions linked to frailty in Latin American older men and women. J Gerontol A Biol Sci Med Sci 2008; 63: 1399–406. [DOI] [PubMed] [Google Scholar]
- 11. Stolz E, Mayerl H, Waxenegger A, Rásky É, Freidl W. Impact of socioeconomic position on frailty trajectories in 10 European countries: evidence from the survey of health, ageing and retirement in Europe (2004-2013). J Epidemiol Community Health 2017; 71: 73–80. [DOI] [PubMed] [Google Scholar]
- 12. Soler-Vila H, García-Esquinas E, León-Muñoz LM, López-García E, Banegas JR, Rodríguez-Artalejo F. Contribution of health behaviours and clinical factors to socioeconomic differences in frailty among older adults. J Epidemiol Community Health 2016; 70: 354–60. [DOI] [PubMed] [Google Scholar]
- 13. Etman A, Kamphuis CB, Cammen TJ, Burdorf A, Lenthe FJ. Do lifestyle, health and social participation mediate educational inequalities in frailty worsening? Eur J Pub Health 2015; 25: 345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381: 752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fried LP, Tangen CM, Walston Jet al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–56. [DOI] [PubMed] [Google Scholar]
- 16. Morley JE, Vellas B, Kan GAet al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14: 392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoogendijk EO, Hout HP, Heymans MWet al. Explaining the association between educational level and frailty in older adults: results from a 13-year longitudinal study in the Netherlands. Ann Epidemiol 2014; 24: 538–44. [DOI] [PubMed] [Google Scholar]
- 18. Cesari M, Prince M, Thiyagarajan JAet al. Frailty: an emerging public health priority. J Am Med Dir Assoc 2016; 17: 188–92. [DOI] [PubMed] [Google Scholar]
- 19. Bandeen-Roche K, Seplaki CL, Huang Jet al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci 2015; 70: 1427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ensrud KE, Kats AM, Schousboe JTet al. Frailty phenotype and healthcare costs and utilization in older women. J Am Geriatr Soc 2018; 66: 1276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ringer TJ, Hazzan AA, Kennedy CCet al. Care recipients' physical frailty is independently associated with subjective burden in informal caregivers in the community setting: a cross-sectional study. BMC Geriatr 2016; 16: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mello JA, Macq J, Van Durme Tet al. The determinants of informal caregivers' burden in the care of frail older persons: a dynamic and role-related perspective. Aging Ment Health 2017; 21: 838–43. [DOI] [PubMed] [Google Scholar]
- 23. Wu CK, Smit E, Xue QL, Odden MC. Prevalence and correlates of frailty among community-dwelling Chinese older adults: the China health and retirement longitudinal study. J Gerontol a-Biol. 2018; 73: 102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walston J, Bandeen-Roche K, Buta Bet al. Moving frailty toward clinical practice: NIA intramural frailty science symposium summary. J Am Geriatr Soc 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bandeen-Roche K, Xue Q-L, Ferrucci Let al. Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol Ser A Biol Med Sci 2006; 61: 262–6. [DOI] [PubMed] [Google Scholar]
- 26. Wu C, Geldhof GJ, Xue QL, Kim DH, Newman AB, Odden MC. Development, construct validity, and predictive validity of a continuous frailty scale: results from 2 large US cohorts. Am J Epidemiol 2018; 187: 1752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walston J, Buta B, Xue QL. Frailty screening and interventions: considerations for clinical practice. Clin Geriatr Med 2018; 34: 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kwon J, Yoshida Y, Yoshida H, Kim H, Suzuki T, Lee Y. Effects of a combined physical training and nutrition intervention on physical performance and health-related quality of life in prefrail older women living in the community: a randomized controlled trial. J Am Med Dir Assoc 2015; 16: 263.e261–8. [DOI] [PubMed] [Google Scholar]
- 29. Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med 2006; 166: 418–23. [DOI] [PubMed] [Google Scholar]
- 30. Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int J Epidemiol 2014; 43: 61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Department of Disease Control Ministry of Health . Guidelines for overweight and obesity of Chinese adult prevention and control. 2003.
- 32. Szklo M, Nieto FJ. Epidemiology: Beyond the Basics. Jones & Bartlett Publishers, 2014. [Google Scholar]
- 33. White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011; 30: 377–99. [DOI] [PubMed] [Google Scholar]
- 34. Rubin DB. Multiple Imputation for Nonresponse in Surveys. vol. 81. John Wiley & Sons, 2004. [Google Scholar]
- 35. Benichou J, Gail MH. Estimates of absolute cause-specific risk in cohort studies. Biometrics 1990; 46: 813–26. [PubMed] [Google Scholar]
- 36. Putrik P, Vries NK, Mujakovic Set al. Living environment matters: relationships between neighborhood characteristics and health of the residents in a Dutch municipality. J Community Health 2015; 40: 47–56. [DOI] [PubMed] [Google Scholar]
- 37. Lang IA, Hubbard RE, Andrew MK, Llewellyn DJ, Melzer D, Rockwood K. Neighborhood deprivation, individual socioeconomic status, and frailty in older adults. J Am Geriatr Soc 2009; 57: 1776–80. [DOI] [PubMed] [Google Scholar]
- 38. Sundquist K, Theobald H, Yang M, Li XJ, Johansson SE, Sundquist J. Neighborhood violent crime and unemployment increase the risk of coronary heart disease: a multilevel study in an urban setting. Soc Sci Med 2006; 62: 2061–71. [DOI] [PubMed] [Google Scholar]
- 39. Wilson-Genderson M, Pruchno R. Effects of neighborhood violence and perceptions of neighborhood safety on depressive symptoms of older adults. Soc Sci Med 2013; 85: 43–9. [DOI] [PubMed] [Google Scholar]
- 40. Gilbert KL, Quinn SC, Goodman RM, Butler J, Wallace J. A meta-analysis of social capital and health: a case for needed research. J Health Psychol 2013; 18: 1385–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murayama H, Fujiwara Y, Kawachi I. Social capital and health: a review of prospective multilevel studies. J Epidemiol 2012; 22: 179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shagdarsuren T, Nakamura K, McCay L. Association between perceived neighborhood environment and health of middle-aged women living in rapidly changing urban Mongolia. Environ Health Prev Med 2017; 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pearce N, Smith GD. Is social capital the key to inequalities in health? Am J Public Health 2003; 93: 122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berney LR, Blane DB. Collecting retrospective data: accuracy of recall after 50 years judged against historical records. Soc Sci Med 1997; 45: 1519–25. [DOI] [PubMed] [Google Scholar]
- 45. Aguayo GA, Donneau AF, Vaillant MTet al. Agreement between 35 published frailty scores in the general population. Am J Epidemiol 2017; 186: 420–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xue QL, Tian J, Walston JD, Chaves PHM, Newman AB, Bandeen-Roche K. Discrepancy in frailty identification: move beyond predictive validity. J Gerontol A Biol Sci Med Sci 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. VanderWeele TJ. A unification of mediation and interaction: a 4-way decomposition. Epidemiology 2014; 25: 749–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


