Abstract
Objective
Machine learning methods may have better or comparable predictive ability than traditional analysis. We explore machine learning methods to predict the likelihood of acute kidney injury after liver cancer resection.
Methods
This is a secondary analysis cohort study. We reviewed data from patients who had undergone resection of primary hepatocellular carcinoma between January 2008 and October 2015.
Results
The analysis included 1,173 hepatectomy patients, 77 (6.6%) of whom had AKI and 1,096 (93.4%) who did not. The importance matrix for the Gbdt algorithm model shows that age, cholesterol, tumor size, surgery duration and PLT were the five most important parameters. Figure 1 shows that Age, tumor size and surgery duration had weak positive correlations with AKI. Cholesterol and PLT also had weak negative correlations with AKI. The models constructed by the four machine learning algorithms in the training group were compared. Among the four machine learning algorithms, random forest and gbm had the highest accuracy, 0.989 and 0.970 respectively. The precision of four of the five algorithms was 1, random forest being the exception. Among the test group, gbm had the highest accuracy (0.932). Random forest and gbm had the highest precision, both being 0.333. The AUC values for the four algorithms were: Gbdt (0.772), gbm (0.725), forest (0.662) and DecisionTree (0.628).
Conclusions
Machine learning technology can predict acute kidney injury after hepatectomy. Age, cholesterol, tumor size, surgery duration and PLT influence the likelihood and development of postoperative acute kidney injury.
Keywords: Machine learning, AKI, Hepatectomy, Postoperative, Secondary analysis
Introduction
Acute kidney injury (AKI) is a common postoperative complication among surgical patients. The incidence of postoperative AKI accounts for 18%–47% of total hospitalized AKI patients (Tang & Murray, 2004). Postoperative AKI can prolong the hospitalization period and increase the risk of both in-hospital mortality and chronic kidney disease. Clinically, postoperative AKI is easy to overlook, and the diagnostic rate is low (Moore et al., 2010; Bennet et al., 2010).
Hepatectomy is the most common aggressive treatment for primary liver cancer. In order to control hemorrhaging during surgery, it is often necessary to block the hepatic portal. This can disturb liver microcirculation. Hepatic ischemia-reperfusion injury occurs after the hepatic portal is opened, releasing a large amount of inflammatory media and oxygen free radicals, thus inhibiting liver function. At the same time, due to surgical trauma, decreased circulation in the liver and kidneys, the release of granulocyte elastase and other factors, postoperative renal damage is also common (Miranda et al., 2010). Therefore, although progress has been made on hepatectomy, the occurrence of AKI remains an important factor influencing prognosis (Nadeem et al., 2014).
Many studies have used classical regression methods to identify risk factors and construct risk prediction models. However, a non-linear relationship between explanatory variables and outcome variables cannot be ruled out (Chen et al., 2011; Vives et al., 2016; Jun et al., 2018). However, compared with conventional analysis methods, machine learning techniques minimize these limitations and may perform better. Studies have shown that machine learning can predict AKI after liver transplant, cardiac surgery, severe burns and percutaneous coronary intervention (Lee et al., 2018a; Lee et al., 2018b; Tran et al., 2019; Huang et al., 2018). Other studies have shown that decision tree algorithms can predict hospitalized patients’ AKI risk after surgery (Thottakkara et al., 2016). Studies have also shown that support vector machines can be used as risk prediction models for postoperative AKI in septic patients (Mohamadlou et al., 2018) . This study investigated the preoperative risk factors associated with secondary AKI after hepatectomy. It used machine learning techniques (logistic regression, decision tree and GradientBoosting) to construct a predictive model of secondary AKI after hepatectomy, thus providing guidance for clinical therapies, and improving surgical patient prognosis.
Materials and Methods
Contributions of previous research
Study design
This is a secondary analysis cohort study. After this retrospective observational study was approved by the ethics committee of the Asan Medical Center, data for patients who had undergone primary hepatocellular carcinoma resection between January 2008 and October 2015 were reviewed. Since this research was retrospective, informed consent was waived. All surgical procedures were performed continuously by the same surgeon. Among the 1,184 identified patients, those with stage 3 or later serious chronic kidney disease (CKD) were excluded by a consulting nephrologist (n = 11). As serum creatinine level examination was part of the routine preoperative assessment, we referred patients with serum creatinine >1.5 mg/dL or patients with a history of CKD, to a consulting nephrologist for preoperative risk stratification. The final cohort included 1,173 patients.
Anesthesia and surgical technique
General anesthesia was performed with thiopental, fentanyl and rocuronium. Anesthesia was maintained with 2–4% sevoflurane in 50% air/oxygen. Routine invasive arterial blood pressure monitoring and central venous pressure monitoring were also conducted. Crystals and colloids were infused as well. The total hydroxyethyl starch volume did not exceed 20 mL/kg. When the patient’s hemoglobin was <8 mg/dL, red blood cells were infused. For patients with a history of ischemic heart disease, hemoglobin levels were maintained >10 mg/dL. Central venous pressure was maintained <5 mmHg. Vasoactive drugs were administered if the mean arterial blood pressure was <65 mmHg.
Indicator collection
The primary endpoint was AKI, based on the definition of the Kidney Disease: Improving Global Outcomes (KDIGO) Guidelines. Postoperative AKI was defined as an increase in serum creatinine ≥0.3 mg/dL within 2 days after surgery, or an increase ≥1.5-fold in serum creatinine within 7 days after surgery (Tran et al., 2019).
Patients’ baseline characteristics, laboratory variables and perioperative variables were collected. The baseline characteristics included age, sex, body mass index (BMI) and diabetes. Variables associated with tumor characteristics included, for example, alpha-fetoprotein. Laboratory data included hemoglobin, platelets, creatinine, white blood cell (WBC) count, glucose and total cholesterol. Intraoperative data included crysta and operative time.
The methods were applied by the authors
The Python programming language (Python Software Foundation, version 3.6) was used for our analysis. The Scikit-learn package (Scikit Learning (https://github.com/scikit-learn/scikit-learn)) (Huang et al., 2018; Teles et al., 2016) was used for machine learning. This included forest, gbm, decision tree and Gbdt. The programming analysis code used in our research is shown in Appendix S1.
The sample was randomly divided into a training set and a test set, at a ratio of 7:3. The coefficients for the machine learning technique were trained with the training set and tested with the test set. Evaluation and comparison were completed with the prediction accuracy of a model constructed by machine learning and the area under the receiver operating characteristic curve. We also compared MSE, accuracy and recall rate. Missing data were estimated through multiple imputations.
F1-Measure evaluation indicators are often used in information retrieval and natural language processing. They constitute a comprehensive evaluation index based on precision rate and recall rate, and their specific definitions are as follows:
where R is the recall and P is the precision.
Precision rate indicates the proportion of correctly classified cases among the sample.
Accuracy rate indicates the number of paired cases divided by the total number of cases.
Recall rate indicates how many positive cases in the sample were predicted correctly.
Machine learning algorithm
In machine learning, a random forest (forest) is a classifier that includes multiple decision trees. The categories of its output are determined by the modes of categories output by individual trees.
The LightGBM (gbm) algorithm is a lifting machine learning algorithm. It is a fast, distributed and high-performing gradient lifting framework based on a decision tree algorithm. It can sort, classify, run regressions, and perform many other machine learning tasks.
The construction of a decision tree model has two steps: induction and pruning. Induction is the step of constructing a decision tree (tr) by setting all hierarchical decision boundaries based on data at hand. However, the tree model is subject to severe over-fitting due to the nature of the training decision tree, and this is when pruning is required. Pruning is the process of removing unnecessary branch structures from the decision tree, simplifying the process of overcoming over-fitting and making it easier to interpret.
Elevation is a machine learning technique that can be used for regression and classification problems. It produces a weak prediction model (like a decision tree) at each step and weights it into the total model. If the weak prediction model of each step generates consistent loss function gradient direction, then it is called gradient boosting (Gbdt).
Results
The pandas_profiling package was applied to data exploration (see attachment Appendix S1 for the results) with Python. The analysis included 1,173 hepatectomy patients, including 77 patients (6.6%) with AKI and 1,096 (93.4%) without. The BMI values of the two groups were different, and the difference was statistically significant (P < 0.040). Neither age nor tumor size showed statistically significant difference between the two groups (see Table 1).
Table 1. Clinical basic characteristic information.
| AKI | NO | Yes | P-value |
|---|---|---|---|
| N | 1,096 | 77 | |
| AGE (years) | 55.7 ± 10.3 | 55.7 ± 9.3 | 0.789 |
| BMI (kg/m2) | 24.2 ± 2.8 | 25.0 ± 3.2 | 0.040 |
| TUMOR SIZE (cm) | 4.5 ± 3.7 | 5.1 ± 4.2 | 0.510 |
| AFP | 9057.7 ± 59451.3 | 18930.6 ± 105276.9 | 0.046 |
| WBC (×103/µL) | 5.4 ± 1.8 | 5.2 ± 1.5 | 0.365 |
| HB (mg/dL) | 14.0 ± 1.6 | 13.6 ± 1.6 | 0.059 |
| PLT (×103/µL) | 165.1 ± 66.5 | 147.2 ± 68.1 | 0.002 |
| CR (mg/dL) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.135 |
| ALB (g/dL) | 3.8 ± 0.4 | 3.7 ± 0.4 | 0.008 |
| AST (IU/L) | 39.0 ± 28.9 | 51.6 ± 47.6 | 0.002 |
| ALT (IU/L) | 36.6 ± 27.8 | 44.2 ± 31.5 | 0.010 |
| GLU (mg/dL) | 117.8 ± 45.8 | 128.1 ± 63.1 | 0.626 |
| CHOLESTEROL (mg/dL) | 163.7 ± 34.6 | 160.8 ± 43.3 | 0.138 |
| PRBC (units) | 0.2 ± 1.0 | 0.6 ± 2.4 | 0.001 |
| CRYSTALLOID (mL) | 2242.5 ± 934.7 | 2562.5 ± 1491.9 | 0.140 |
| Duration of surgery (min) | 268.2 ± 79.5 | 311.9 ± 93.9 | <0.001 |
| SEX | 0.048 | ||
| Female | 214 (19.5%) | 8 (10.4%) | |
| Male | 882 (80.5%) | 69 (89.6%) | |
| OPEN_LAP | <0.001 | ||
| No | 853 (77.8%) | 73 (94.8%) | |
| Yes | 243 (22.2%) | 4 (5.2%) | |
| DM | 0.085 | ||
| No | 1,030 (94.0%) | 68 (88.3%) | |
| Yes | 66 (6.0%) | 9 (11.7%) | |
| RAS | 0.023 | ||
| No | 932 (85.0%) | 58 (75.3%) | |
| Yes | 164 (15.0%) | 19 (24.7%) |
Notes.
- WBC
- white blood cell
- HB
- Hemoglobin
- DM
- Diabetes
- BMI
- Body index
- CR
- Creatinine
- GLU
- Glucose
- RAS
- Renin-angiotensin system (RAS) blocker
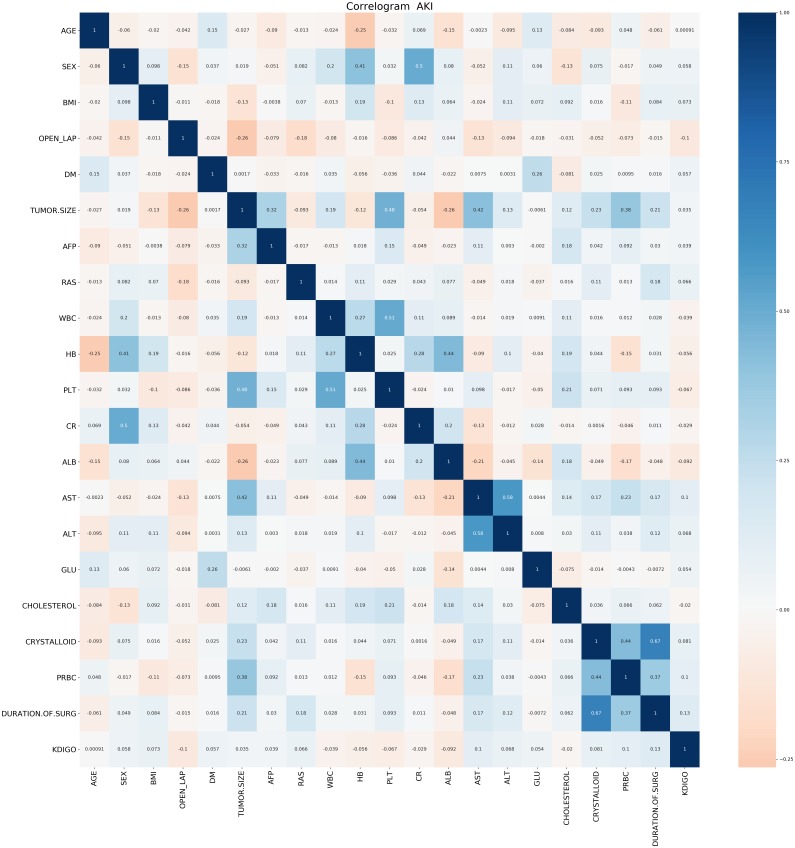
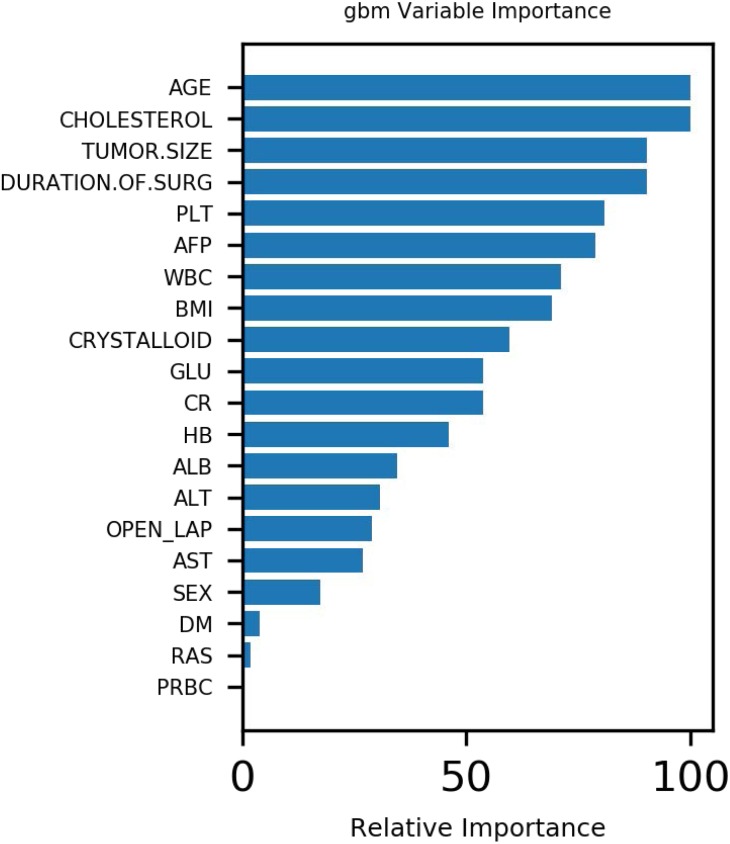
Figure 1 demonstrates that age, tumor size and surgery duration have weak positive correlations with AKI. Cholesterol and PLT each had weak negative correlations with AKI.The Gbdt algorithm model importance matrix is shown in Fig. 2. Age, cholesterol, tumor size, surgery duration and PLT are the five most influential factors.
Figure 1. Correlation Analysis of various factors.
Figure 2. Variable importance of features included in Gbdt algorithm for prediction of AKI.
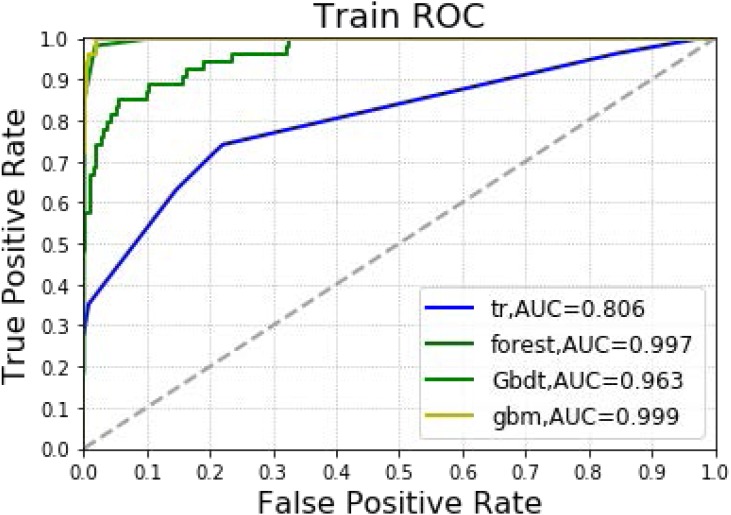
In Table 2 and Fig. 3, the models constructed by the four machine learning algorithms in the training group are compared. Among the four machine learning algorithms, random forest and gbm have the highest accuracy, 0.989 and 0.970 respectively. The precision of four of the five algorithms is 1, with random forest as the lone exception. The highest recall rate was that of the random forest algorithm (0.852). Among the four algorithms, random forest had the highest recall rate and f1 score, 0.852 and 0.911, respectively. The AUC values for the four algorithms were: gbm (0.999), forest (0.997), Gbdt (0.963) and DecisionTree (0.806). Among the four algorithms, random forest had the lowest MSE value (0.011).
Table 2. Forecast results of training group.
| Accuracy | Precision | Recall | f1_score | Auc | MSE | |
|---|---|---|---|---|---|---|
| Decision Tree | 0.952 | 1.000 | 0.278 | 0.435 | 0.806 | 0.048 |
| forest | 0.989 | 0.979 | 0.852 | 0.911 | 0.997 | 0.011 |
| Gbdt | 0.946 | 1.000 | 0.185 | 0.312 | 0.963 | 0.054 |
| gbm | 0.970 | 1.000 | 0.537 | 0.699 | 0.999 | 0.030 |
Figure 3. Machine learning algorithm for prediction of AKI in training group.
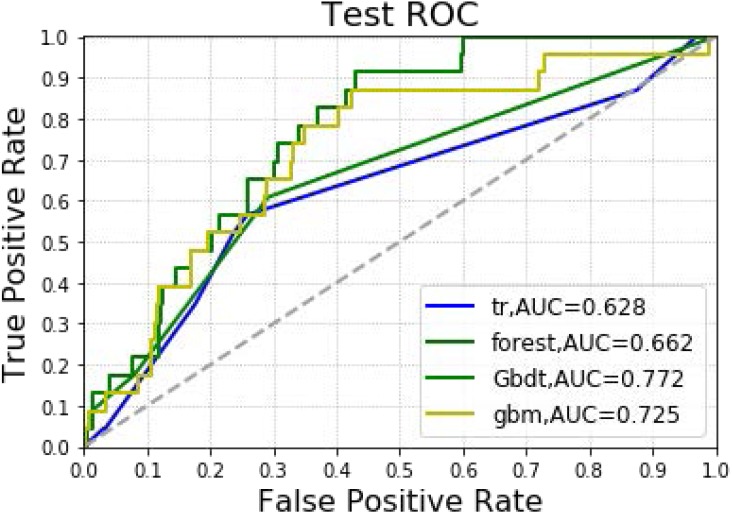
In Table 3 and Fig. 4, the models constructed by four machine learning algorithms in the test group are compared. Gbm had the highest accuracy (0.932). Random forest and gbm had the highest precision, both being 0.333. The recall rate for the random forest algorithm was 0.087. The lowest f1 score was that of decision tree at 0.059. The AUC values of the four algorithms were: Gbdt (0.772), gbm (0.725), forest (0.662) and DecisionTree (0.628). Among the four algorithms, gbm had the lowest MSE value at 0.068.
Table 3. Forecast results of testing group.
| Accuracy | Precision | Recall | f1_score | Auc | MSE | |
|---|---|---|---|---|---|---|
| Decision Tree | 0.909 | 0.091 | 0.043 | 0.059 | 0.628 | 0.091 |
| forest | 0.929 | 0.333 | 0.087 | 0.138 | 0.662 | 0.071 |
| Gbdt | 0.929 | 0.250 | 0.043 | 0.074 | 0.772 | 0.071 |
| gbm | 0.932 | 0.333 | 0.043 | 0.077 | 0.725 | 0.068 |
Figure 4. Machine learning algorithm for prediction of AKI in the testing group.
Discussion
Hepatectomy is an effective therapy for primary liver cancer. To block interoperative bleeding, it is often necessary to block the hepatic hilum. This can induce hepatic ischemia-reperfusion injury. It can cause not only liver dysfunction, but also kidney injury (Sheridan et al., 2016; Gao et al., 2018). At the same time, due to surgical trauma, decreased blood flow in the liver and decreased kidney circulation, granulocyte elastase release and other factors, postoperative renal damage can also occur (Fonseca-NetoI et al., 2012). In this study, machine learning techniques compared the predictive accuracy of AKI predictions after hepatectomy. The Gbdt algorithm indicated that age, cholesterol, tumor size, surgery duration and PLT were the five most important weights for AKI. The results showed that Gbdt had the highest AUC in both training and test groups. Thus, it could predict the likelihood of AKI. All four machine learning algorithms could predict the likelihood of AKI as well. The accuracy was greater than 90%, and the MSE values were less than 0.1.
Studies (Craig et al., 2001; Yim et al., 2000; Amar et al., 2007) have shown that laparoscopic surgery can reduce postoperative inflammatory response indicator levels, including C-reactive protein, interleukin and reactive oxygen species in neutrophils. These inflammatory mediators have been shown to be identical to the inflammatory mediators in AKI (Wu et al., 2014). In addition, triglyceride deposition around the renal tubules can cause high levels of free fatty acids around the kidney cells. This can impair kidney function (Levine et al., 1997). Zhang et al. (2011) analyzed 3,336 patients from 19 related studies covering 11 countries and found that blood CysC is a good predictor of acute kidney injury. It also has high specificity and accuracy for early kidney injury. These findings are similar to those of the present study.
Ongoing studies (Slankamenac et al., 2009) also show that diabetes, high BMI and low postoperative albumin are risk factors for postoperative AKI. Diabetes is a well-known risk factor for various postoperative AKIs, including hepatectomy. Low serum albumin concentrations have recently been associated with various postoperative AKIs (Cho et al., 2014). Moreover, studies (Wu et al., 2020) have also shown that the lowest platelet count over the first 48 h is a new biomarker for AKI. This study’s findings support these views.
The goal of logistic regression in statistics is different from that of logistic regression in machine learning. By default, there is a potential law in statistics. There are various restrictions in adjusting the model to meet the assumption conditions to find the potential law. However, machine learning is different; it is only concerned with the deviation between predicted and real values. Moreover, the integration algorithm adopted in this study considers more information gain when calculating. Thus, it naturally eliminates linear correlation, and also prevents non-linear correlation.
In addition, variables are often screened with principal component analysis (Zhang & Castelló, 2017). However, principal component analysis is not always required in machine learning algorithms. It is used excessively to screen features. Doing so can omit important factors for outcome variables. In the real world, no clinical factor affecting prognosis should be ignored.
This study has several limitations. Firstly, not all confounding factors could be controlled, as this was a retrospective study. Secondly, caution should be exercised in interpreting the study’s results since it was a single-center study in which all surgeries were performed by an experienced surgeon. Thirdly, the machine learning techniques’ performance may vary when applied to larger samples with different covariate distributions. This study only performed internal, and not external, verification. In addition, different parameters in machine learning can result in different AUC values. Corresponding models are needed for different occasions according to needs, and should not excessively prioritize AUC values. Furthermore, an exorbitant AUC value may be unsuitable, as the accuracy, precision and recall rates may fall to unacceptable levels. This would make models unreliable in real world applications when the AUC value is high. Although most of the important reported variables are not clinically modifiable, appropriate measures could be taken to personalize prevention based on AKI risk.
Conclusion
This study shows that all four machine learning techniques can predict AKI likelihood, among which GradientBoosting performs the best. At the same time, the Gbdt algorithm suggests that age, cholesterol, tumor size, surgery duration and PLT are the five most important weights for the likelihood of acute kidney injury after liver cancer resection.
Supplemental Information
Acknowledgments
We are grateful to Professor Jun-Gol Song for disclosing his data (Moon et al., 2017) and allowing us to use them for research. We are also very grateful to the BioStudies (public) database for including and providing Professor Song’s original data.
Funding Statement
This study was supported by grants from the National Natural Science Foundation of China (Nos., 81600950, 81771156, 81772126). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Cheng-Mao Zhou, Email: zhouchengmao187@foxmail.com.
Jian-Jun Yang, Email: yjyangjj@126.com.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Lei Lei, Ying Wang, Cheng-Mao Zhou and Jian-Jun Yang conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Qiong Xue and Jianhua Tong performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
This previous study was approved by institutional review board of Asan Medical Center (Moon et al., 2017). Now the data are included in BioStudies database, which is a public health database. Our study did not require the approval of an ethics committee as it is a secondary analysis of BioStudies database of a public database and of free access (Alarconruiz, Heredia & Tayperondan, 2019; Sarkans et al., 2018). This article does not contain any studies with human participants or animals performed by any of our authors.
Data Availability
The following information was supplied regarding data availability:
The programming analysis code is available as Supplemental File.
Data is available at BioStudies database: S-EPMC5640237.
References
- Alarconruiz, Heredia & Tayperondan (2019).Alarconruiz CA, Heredia P, Tayperondan A. Association of waiting and consultation time with patient satisfaction: secondary-data analysis of a national survey in Peruvian ambulatory care facilities. BMC Health Services Research. 2019;19:439. doi: 10.1186/s12913-019-4288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amar et al. (2007).Amar D, Zhang H, Park B, Heerdt PM, Fleisher M, Thaler HT. Inflammation and outcome after general thoracic surgery. European Journal of Cardio-Thoracic Surgery. 2007;32(3):431–434. doi: 10.1016/j.ejcts.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Bennet et al. (2010).Bennet SJ, Berry OMB, Goddard J, Keating JF. Acute renal dysfunction following hip fracture. Injury-international Journal of the Care of the Injured. 2010;41(4):335–338. doi: 10.1016/j.injury.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2011).Chen J, Singhapricha T, Hu KQ, Hong JC, Steadman RH, Busuttil RW, Xia VW. Postliver transplant acute renal injury and failure by the RIFLE criteria in patients with normal pretransplant serum creatinine concentrations: a matched study. Transplantation. 2011;91(3):348–353. doi: 10.1097/TP.0b013e31820437da. [DOI] [PubMed] [Google Scholar]
- Cho et al. (2014).Cho E, Kim SC, Kim MG, Jo SK, Cho WY, Kim HK. The incidence and risk factors of acute kidney injury after hepatobiliary surgery: a prospective observational study. BMC Nephrology. 2014;15(1):169. doi: 10.1186/1471-2369-15-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig et al. (2001).Craig SR, Leaver HA, Yap PL, Pugh GC, Walker WS. Acute phase responses following minimal access and conventional thoracic surgery. European Journal of Cardio-Thoracic Surgery. 2001;20(3):455–463. doi: 10.1016/S1010-7940(01)00841-7. [DOI] [PubMed] [Google Scholar]
- Fonseca-NetoI et al. (2012).Fonseca-Neto OCL, Miranda LEC, Batista TP, Sabat BD, Melo PSVD, Amorim AG, Lacerda CM. Postoperative kidney injury does not decrease survival after liver transplantation. Acta Cirurgica Brasileira. 2012;27(11):802–808. doi: 10.1590/s0102-86502012001100010. [DOI] [PubMed] [Google Scholar]
- Gao et al. (2018).Gao C, Sun H, Wang T, Tang M, Bohnen NI, Muller ML, Herman T, Giladi N, Kalinin AA, Spino C, Dauer WT, Hausdorff JM, Dinov ID. Model-based and model-free machine learning techniques for diagnostic prediction and classification of clinical outcomes in Parkinson’s disease. Scientific Reports. 2018;8(1):7129. doi: 10.1038/s41598-018-24783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. (2018).Huang C, Murugiah K, Mahajan S, Li S, Dhruva SS, Haimovich JS, Wang Y, Schulz WL, Testani JM, Wilson FP, Mena C, Masoudi FA, Rumsfeld JS, Spertus JA, Mortazavi B, Krumholz HM. Enhancing the prediction of acute kidney injury risk after percutaneous coronary intervention using machine learning techniques: a retrospective cohort study. PLOS Medicine. 2018;15(11):e1002703. doi: 10.1371/journal.pmed.1002703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun et al. (2018).Jun IG, Kwon H, Jung K, Moon J, Shin W, Song J, Hwang G. The impact of postreperfusion syndrome on acute kidney injury in living donor liver transplantation: a propensity score analysis. Anesthesia and Analgesia. 2018;127:369–378. doi: 10.1213/ANE.0000000000003370. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2018a).Lee H, Yoon H, Nam K, Cho YJ, Kim TK, Kim WH, Bahk J. Derivation and validation of machine learning approaches to predict acute kidney injury after cardiac surgery. Journal of Clinical Medicine. 2018a;7(10):e7100322. doi: 10.3390/jcm7100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2018b).Lee H, Yoon SB, Yang S, Kim WH, Ryu H, Jung C, Suh K, Lee KH. Prediction of acute kidney injury after liver transplantation: machine learning approaches vs. logistic regression model. Journal of Clinical Medicine. 2018b;7(11):e7110428. doi: 10.3390/jcm7110428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine et al. (1997).Levine JS, Koh JS, Triaca V, Lieberthal W. Lysophosphatidic acid: a novel growth and survival factor for renal proximal tubular cells. American Journal of Physiology. 1997;273(4):575–585. doi: 10.1152/ajprenal.1997.273.4.F575. [DOI] [PubMed] [Google Scholar]
- Miranda et al. (2010).Miranda LEC, Capellini VK, Reis GS, Celotto AC, Carlotti CG, Evora PRB. Effects of partial liver ischemia followed by global liver reperfusion on the remote tissue expression of nitric oxide synthase: lungs and kidneys. Transplantation Proceedings. 2010;42:1557–1562. doi: 10.1016/j.transproceed.2010.02.097. [DOI] [PubMed] [Google Scholar]
- Mohamadlou et al. (2018).Mohamadlou H, Lynnpalevsky A, Barton C, Chettipally UK, Shieh L, Calvert J, Saber N, Das R. Prediction of acute kidney injury with a machine learning algorithm using electronic health record data. Canadian Journal of Kidney Health and Disease. 2018;5:2054358118776326. doi: 10.1177/2054358118776326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon et al. (2017).Moon YJ, Jun IG, Kim KH, Kim S, Song J, Hwang G. Comparison of acute kidney injury between open and laparoscopic liver resection: propensity score analysis. PLOS ONE. 2017;12(10):e0186336. doi: 10.1371/journal.pone.0186336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore et al. (2010).Moore EM, Simpson JA, Tobin A, Santamaria JD. Preoperative estimated glomerular filtration rate and RIFLE-classified postoperative acute kidney injury predict length of stay post-coronary bypass surgery in an Australian setting. Anaesthesia & Intensive Care. 2010;38(1):113–121. doi: 10.1177/0310057X1003800119. [DOI] [PubMed] [Google Scholar]
- Nadeem et al. (2014).Nadeem A, Salahuddin N, Hazmi AE, Joseph M, Bohlega B, Sallam H, Sheikh Y, Broering D. Chloride-liberal fluids are associated with acute kidney injury after liver transplantation. Critical Care. 2014;18:e625. doi: 10.1186/s13054-014-0625-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkans et al. (2018).Sarkans U, Gostev M, Athar A, Behrangi E, Melnichuk O, Ali A, Minguet J, Rada J, Snow C, Tikhonov A, Brazma A, Mcentyre J. The BioStudies database-one stop shop for all data supporting a life sciences study. Nucleic Acids Research. 2018;46:1266–1270. doi: 10.1093/nar/gkx1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan et al. (2016).Sheridan RP, Wang WM, Liaw A, Ma J, Gifford EM. Extreme gradient boosting as a method for quantitative structure-activity relationships. Journal of Chemical Information and Modeling. 2016;56(12):2353–2360. doi: 10.1021/acs.jcim.6b00591. [DOI] [PubMed] [Google Scholar]
- Slankamenac et al. (2009).Slankamenac K, Breitenstein S, Held U, Beckschimmer B, Puhan MA, Clavien P. Development and validation of a prediction score for postoperative acute renal failure following liver resection. Annals of Surgery. 2009;250(5):720–728. doi: 10.1097/SLA.0b013e3181bdd840. [DOI] [PubMed] [Google Scholar]
- Tang & Murray (2004).Tang IY, Murray PT. Prevention of perioperative acute renal failure: what works? Best Practice and Research: Clinical Anaesthesiology. 2004;18(1):91–111. doi: 10.1016/j.bpa.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Teles et al. (2016).Teles F, Uchoa JV, Mendonca DM, Costa AF. Acute kidney injury in leptospirosis: the Kidney Disease Improving Global Outcomes (KDIGO) criteria and mortality. Clinical Nephrology. 2016;86(12):e108865. doi: 10.5414/CN108865. [DOI] [PubMed] [Google Scholar]
- Thottakkara et al. (2016).Thottakkara P, Ozrazgat-Baslanti T, Hupf BB, Rashidi P, Pardalos PM, Momcilovic P, Bihorac A. Application of machine learning techniques to high-dimensional clinical data to forecast postoperative complications. PLOS ONE. 2016;11(5):e0155705. doi: 10.1371/journal.pone.0155705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran et al. (2019).Tran NK, Sen S, Palmieri TL, Lima K, Falwell S, Wajda J, Rashidiet HH. Artificial intelligence and machine learning for predicting acute kidney injury in severely burned patients: a proof of concept. Burns. 2019;45(6):1350–1358. doi: 10.1016/j.burns.2019.03.021. [DOI] [PubMed] [Google Scholar]
- Vives et al. (2016).Vives M, Callejas R, Duque P, Echarri G, Wijeysundera DN, Hernandez A, Sabate A, Besrastrollo M, Monedero P. Modern hydroxyethyl starch and acute kidney injury after cardiac surgery: a prospective multicentre cohort. British Journal of Anaesthesia. 2016;117(4):458–463. doi: 10.1093/bja/aew258. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2014).Wu C, Ke L, Tong Z, Li B, Zou L, Li WQ, Li N, Li JS. Hypertriglyceridemia is a risk factor for acute kidney injury in the early phase of acute pancreatitis. Pancreas. 2014;43(8):1312–1316. doi: 10.1097/MPA.0000000000000180. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2020).Wu M, Luan Y, Lu J, Li H, Zhan H, Chen Y, Zhang F, Tian Y, Yang Z, Yao Y, Feng Y. Platelet count as a new biomarker for acute kidney injury induced by hemorrhagic shock. Platelets. 2020;31(1):94–102. doi: 10.1080/09537104.2019.1581921. [DOI] [PubMed] [Google Scholar]
- Yim et al. (2000).Yim AP, Wan S, Lee TW, Arifi AA. VATS lobectomy reduces cytokine responses compared with conventional surgery. Annals of Thoracic Surgery. 2000;70(1):243–247. doi: 10.1016/S0003-4975(00)01258-3. [DOI] [PubMed] [Google Scholar]
- Zhang & Castelló (2017).Zhang Z, Castelló A. Principal components analysis in clinical studies. Annals of Translational Medicine. 2017;5(17):e351. doi: 10.21037/atm.2017.07.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2011).Zhang Z, Lu B, Sheng X, Jin N. Cystatin C in prediction of acute kidney injury: a systemic review and meta-analysis. American Journal of Kidney Diseases. 2011;58(3):356–365. doi: 10.1053/j.ajkd.2011.02.389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The programming analysis code is available as Supplemental File.
Data is available at BioStudies database: S-EPMC5640237.