Abstract
Objective:
To test the hypothesis that adjunctive inhaled NO would improve RV function and viability in acute PE.
Methods:
This was a randomized, placebo-controlled, double blind trial conducted at four academic hospitals. Eligible patients had acute PE without systemic arterial hypotension but had RV dysfunction and a treatment plan of standard anticoagulation. Subjects received either oxygen plus 50 parts per million nitrogen (placebo) or oxygen plus 50 ppm NO for 24 hours. The primary composite endpoint required a normal RV on echocardiography and a plasma troponin T concentration <14 pg/mL. The secondary endpoint required a blood brain natriuretic peptide concentration < 90 pg/mL and a Borg dyspnea score <=2. The sample size of N=76 tested if 30% more patients treated with NO would achieve the primary endpoint with 80% power and alpha=5%.
Results:
We randomized 78 patients and after two withdrawals, 38 were treated per protocol in each group. Patients were well matched for baseline conditions. At 24 hours, 5/38 (13%) of patients treated with placebo and 9/38 (24%) of patients treated with NO reached the primary endpoint (P=0.375). The secondary endpoint was reached in 34% with placebo and 13% of the NO (P=0.11). In a pre-planned post-hoc analysis, we examined how many patients with RV hypokinesis or dilation at enrollment resolved these abnormalities; 29% more patients treated with NO resolved both abnormalities at 24 hours (P=0.010, Cochrane’s Q test).
Conclusions:
In patients with severe submassive PE, inhaled nitric oxide failed to increase the proportion of patients with a normal troponin and echocardiogram but increased the probability of eliminating RV hypokinesis and dilation on echocardiography.
Keywords: nitric oxide, randomized trial, pulmonary embolism, heart failure, troponin, brain natriuretic peptide, echocardiography, pulmonary hypertension
Introduction
Acute pulmonary embolism (PE) causes direct thrombotic occlusion of pulmonary arteries, which increases pulmonary vascular resistance and right ventricular (RV) afterload, ultimately causing RV contractile dysfunction and inflammatory damage.[1, 2] As a result, PE impairs RV contraction on transthoracic echocardiography, and produces elevated circulating cardiac troponin proteins and brain natriuretic peptides (BNP).[3] Severe RV afterload and dysfunction clearly increases the risk of death and contributes to ventilation-perfusion abnormalities that cause dyspnea and hypoxemia, which may be long-lasting.[4–7]
The rationale for an inhaled vasodilator as a treatment for PE stems from evidence that non-mechanical processes contribute to increased pulmonary vascular resistance. The percentage of radiographically-evident pulmonary thrombotic vascular occlusion often fails to predict severity of RV dysfunction, biomarker abnormalities and clinical outcome.[6, 8, 9] Acute PE triggers pulmonary vasoconstriction, platelet hyperactivation and microvascular obstruction, caused in part by reduced nitric oxide availability to the pulmonary vasculature.[10–12] Drugs that increase cyclic guanosine monophosphate, such as inhaled NO, acutely decrease pulmonary arterial pressures in experimental models of hemolysis- and PE-induced pulmonary hypertension as well as in patients with chronic pulmonary hypertension.[13–16] Several case reports and a small, non-randomized clinical trial of PE showed temporal improvement in dyspnea from PE with NO treatment.[15, 17]
We therefore hypothesized that inhaled NO would reduce both RV dysfunction and injury from acute PE seen on echocardiography and high precision plasma troponin concentrations, respectively. Our secondary aim tested if inhaled NO would reduce RV strain, evidenced by a reduction in BNP and patient perception of dyspnea.
Study design
The inhaled Nitric Oxide for Pulmonary Embolism (iNOPE) trial was a multicenter randomized, double-blind, controlled trial of inhaled NO + oxygen versus nitrogen + oxygen in subjects with acute PE with normotension but with RV dysfunction (intermediate risk or submassive PE). The iNOPE protocol and methods have been described in detail and the trial was registered on clinicaltrials.gov ().[18] The protocol was approved by the Institutional Review Boards at participating centers.
Funding
The trial was sponsored by the National Institutes of Health (UM1HL113203–01) and by an investigator initiated grant from Mallinckrodt Pharmaceuticals and Roche Diagnostics in 2015. The sponsors had no role in the protocol design, data analysis or manuscript writing.
Patient selection
All patients were >17 years old, normotensive, had image-proven proximal PE and were intermediate-high risk using the European Society of Cardiology definition (sPESI≥1 and RV dysfunction).[19] The definition of image proven was a filling defect suggestive of acute PE in a contrast-enhanced pulmonary artery. RV dysfunction was defined by an elevated BNP (>90 pg/mL) or troponin I (>0.1 ng/mL), and evidence of RV dilation (RV >42 mm in diastole, or RV:LV ratio >1.0 on CT scan measured as part of usual care by a board certified radiologist who was not affiliated with the study) or RV hypokinesis on echocardiography.[6, 19] Exclusions included pregnancy, vasopressor use, inability to tolerate a nasal cannula, altered mental status, written plan to not administer anticoagulation, written plan for fibrinolysis, and the use of drugs known to increase cyclic guanosine monophosphate.
Treatment protocol
All patients received treatment doses of either unfractionated or low molecular weight heparin. Antiplatelet drugs were discontinued. Study drug or placebo was administered with a commercially available, FDA-cleared device (Inovent,® Mallinckro dt Pharmaceuticals, Clinton, NJ). Blinded canisters of NO or placebo were supplied by Mallinckrodt. The method of randomization and blinding, blood collection and echocardiography has been described in detail.[18] NO or nitrogen placebo was titrated at 2 ppm/min over 25 min to a dose of 50 ppm via nasal cannula, which was maintained for 24 hours. The rationale for the 2 ppm per minute titration and weaning rate was based upon considerations of patient safety versus the potential feasibility and generalizability of the protocol. As previous described,, we believed the 2 ppm per minute rate of change allowed research personnel enough time to observe the patient for changes (symptoms and vital signs), but would not excessively prolong the protocol.[18] After 24 hours (+/− 3h) of treatment, the NO or nitrogen was weaned at 2ppm per minute to 0 ppm NO over 25 min. To investigate integrity of blinding, the research coordinator asked the primary physician in charge of the patient’s care during hospitalization for his or her opinion whether the patient received NO or Placebo.
Patient safety concerns
Vital signs and methemoglobin, carboxyhemoglobin with a Masimo Rainbow® Cooximeter device (Masimo Corporation, Irving, CA), were monitored hourly. An unblinding envelope and emergency treatment algorithm were posted at the bedside to inform the healthcare team how to handle acute deterioration.
Study measurements
The Borg dyspnea score (0–10) was measured hourly. A study-funded transthoracic Doppler-echocardiogram was performed after weaning NO, always within 3 hours. All patients had blood specimensdrawn for troponin, BNP and nitrate measurements within one hour of starting NO at baseline and then immediately prior (< 1 hour in all cases) to weaning, 24 hours later. Plasma concentrations of nitrite and nitrate were measured with high performance liquid chromatography (EiCOM ENO-30, San Diego, CA); troponin T (TnT) was measured with a high precision instrument (Roche COBAS, e411, Indianapolis, IN) and BNP was measured with iSTAT®, (Abbott, Princeton, NJ).
Outcome measurements
The primary efficacy outcome was normal RV function on echocardiography and a TnT concentration <14 pg/mL[3] at 24 hours. Normal RV function required normal RV size (<42 mm in diastole) and tricuspid annular plane systolic excursion (TAPSE) >16 mm and normal right ventricular index of myocardial performance (RIMP) (<0.40 using spectral Doppler or < 0.55 using tissue Doppler) and normal fractional area of contraction (FAC) (>33%); All echocardiograms, including those obtained at enrollment, were read by a cardiologist and echocardiography expert (author RAM) who was blinded to group assignment. Missing echocardiographic parameters were analyzed as if they were normal. The secondary efficacy outcome required a Borg dyspnea score ≤2 and a BNP level <90 pg/mL. We also measured mortality rate and perceived perception of wellness with a standardized survey at 3 months after treatment (via SF36 and PEmb QoL).[20] Lastly, we a preplanned secondary analysis, assessed the frequency of resolution of two commonly measured abnormalities on echocardiography, RV hypokinesis and dilation. We compared the proportion of patients between treatment groups who had neither abnormality at 24 hours, and we compared the frequency with which each treatment was associated with resolution of both RV hypokinesis and dilation (i.e., conversion from having one or both abnormalities at diagnosis to having neither at 24 hours).
Sample size and analytical methods
The minimum sample size of N=72 treated per protocol was predicated on detection of a 30% difference in the proportions of patients in each group who reached the primary efficacy endpoint. Means, medians and frequency data were compared between groups using a two-tailed unpaired t-test, a Mann Whitney U, or Fisher’s exact test as appropriate. In the post-hoc analysis, we use a Cochrane’s Q test whether changes in paired frequencies differ by randomization. Because this was a phase II study with a physiological endpoint, and we did not anticipate an group imbalance in patients crossing over or withdrawing, we did not use an intent to treat design.[21]
Results
As shown in Figure 1, from March 2014 to October 2016, we screened 1550 patients with diagnosed PE and obtained informed consent from 86 patients. After 8 screen failures and two voluntary withdrawals after starting drug (n=1) or placebo (n=1), we treated 76 patients treated per protocol (n=38 per group) for 24 hours. Regarding timing of enrollment relative to the qualifying CT scan performance, we enrolled 32 patients within 12 hours of CT scanning, 39 patients within 12–20 hours, 8 between 20 and 24 hours and one 48 hours later.
Figure 1.
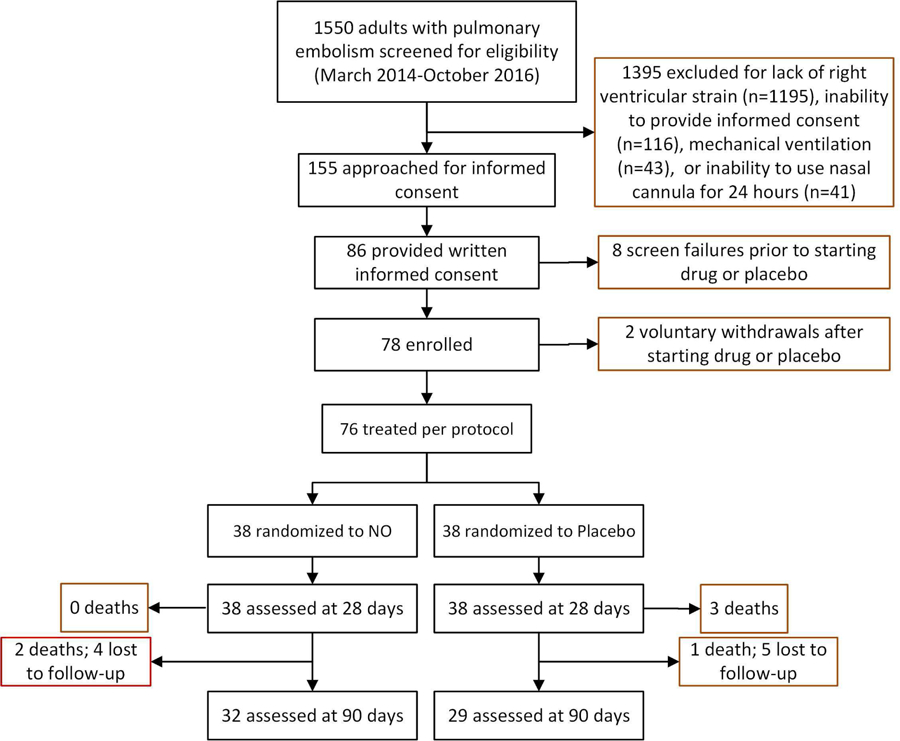
Flow diagram showing the numbers of patients screened, consented, enrolled and treated per protocol
Table 1 shows that patients were well matched for baseline vital signs, prior medical conditions and indexes of PE severity. The mean (SD) RV/LV ratio was 1.32 (0.39) [median 1.11, 1st-3rd quartiles 0.89–1.69] and all 78 patients had one or more filling defects in a lobar or more proximal pulmonary artery. After one patient in each group withdrew after enrollment, 38 patients completed the entire protocol in each group (N=76 total).
Table 1.
Clinical characteristics of enrolled patients.
| Age and vital signs | NO treated (N = 39)a | Placebo treated (N = 39)a | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 55 | 15 | 59 | 16 |
| Body mass index (kg/mˆ2) | 36 | 12 | 33 | 11 |
| Heart rate (beats/min) | 86 | 12 | 93 | 13 |
| Systolic blood pressure (mm Hg) | 125 | 20 | 127 | 20 |
| Pulse oximetry (%) | 97 | 3 | 96 | 6 |
| Patient features and past medical history | N | % | N | % |
| Female gender | 21 | 54% | 20 | 51% |
| Caucasian race | 28 | 72% | 21 | 54% |
| Creatinine > 1.7 mg/dL | 2 | 6% | 1 | 3% |
| Hemoglobin < 10.0 g/dL | 9 | 26% | 5 | 14% |
| Surgery in past 4 weeks | 7 | 18% | 4 | 10% |
| Trauma in past 4 weeks | 4 | 10% | 5 | 13% |
| Coronary artery disease | 3 | 8% | 5 | 13% |
| Heart failure | 6 | 15% | 1 | 3% |
| Chronic obstructive pulmonary disease | 7 | 18% | 4 | 10% |
| Prior venous thromboembolism | 10 | 26% | 12 | 31% |
| Active cancer | 3 | 8% | 5 | 13% |
| Connective tissue disease | 6 | 15% | 7 | 18% |
| Post partum (within 4 weeks) | 1 | 3% | 1 | 3% |
| Never smoked | 25 | 64% | 20 | 51% |
| Markers of RV strain | Median or proportion | 1st-3rd quartile or percentage | Median or proportion | 1st-3rd quartile or percentage |
| Troponin concentration (pg/mL) | 23 | 12–71 | 51 | 18–101 |
| Brain natriuretic peptide (pg/mL) | 206 | 83–386 | 234 | 84–556 |
| Right ventricular systolic pressure (mm Hgb) | 54.5 | 50–62 | 50 | 47–52.5 |
| Right ventricular/Left ventricular ratio | 1.12 | 0.89–1.69 | 1.10 | 0.85–1.68 |
| Right ventricular dilation (> 43 mm) on echocardiography | 14/24 | 58% | 13/26 | 50% |
| Right ventricular hypokinesis on echocardiography | 15/24 | 63% | 20/26 | 77% |
These data are for enrolled patients; one patient in each group withdrew after enrollment.
Estimated from equation Vˆ2*4 + 10 mm Hg where V = tricuspid regurgitant jet velocity (meters/second).
Primary and secondary outcomes
Table 2 presents the primary outcome and shows that 9/38 (24%) of patients treated with NO and 5/38 (13%) of patients treated with placebo had both normal RV function on echocardiography and a normal TnT after 24 hours of treatment (P=0.375, Fisher’s exact test). Analysis of the components of the primary outcome failed to show any salient difference between treatments; Table 2 shows relatively equal distribution of normal results for each the four echocardiographic variables used to define RV function. Figure 2, which plots the pre- and post-treatment TnT values for all patients, shows similar changes with each treatment from enrollment to 24 hours. Likewise, Table 3 shows no difference in the secondary outcome of a BNP<90 pg/mL and the Borg dyspnea score between patients, and no difference in the fraction of patients who scored favorably on the psychometric tests for health related quality of life. Figure 3 plots the pre- and post-treatment BNP values for each patient at enrollment. Similarly, no difference was found in the fraction of patients who scored favorably on the psychometric tests for health related quality of life after 90 days (Supplemental Figure 1). Heart failure, kidney disease and coronary artery disease may increase steady-state concentrations of troponins and natriuretic peptides. To assess this possible confounding effect, we removed patients with prior heart failure, kidney disease (creatinine >1.7 mg/dL), and coronary artery disease, which excluded eight patients from NO treatment and five from placebo (supplemental Tables 1 and 2). At enrollment, mean troponin and BNP concentrations were elevated in patients with these comorbidities. However, as Supplemental Tables 3 and 4 show, their removal had no effect on the probability of normalization on either troponin T (Troponin T <14 pg/mL at wean: 12/30 [40%] after NO treatment versus 10/33 [30%] after placebo, P = 0.46, Fisher’s exact), or BNP (BNP<90 pg/mL at wean: 7/23 after NO treatment versus 10/23 after placebo, P=0.55).
Table 2.
The primary outcome.
| Measurement | Definitions | NO | Placebo | P value | ||
|---|---|---|---|---|---|---|
| n/38* | % | n/38* | % | |||
| Primary outcome | Normal right ventricular function on echocardiography and normal troponin T** | 9 | 24% | 5 | 13% | 0.375 |
| Components of primary outcome | Right ventricular diastolic diameter < 43 mm | 31 | 82% | 27 | 71% | |
| Tricuspid annular plane systolic excursion > 16 mm | 29 | 76% | 28 | 74% | ||
| Right ventricular index of myocardial performance normal | 22 | 58% | 22 | 58% | ||
| Right ventricular fractional area of contraction > 33% | 23 | 61% | 21 | 55% | ||
| Plasma troponin T concentration < 14 pg/mL | 14 | 37% | 11 | 29% | ||
Figure 2.
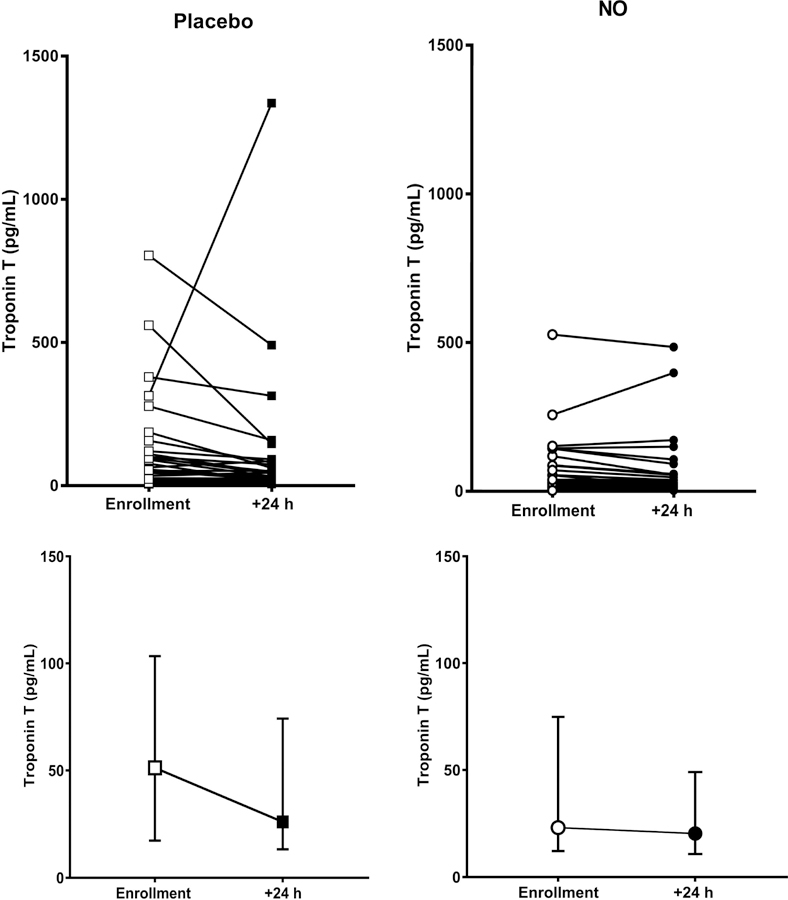
Dot plot showing plasma concentration of troponin T at enrollment, and then again after 24 hours of treatment for patients each treated with either placebo (n=38, squares), or 50 parts per million of inhaled nitric oxide (n=38, circles). The bottom panels show the median and interquartile ranges. The cutoff for abnormal was 14 pg/mL
Table 3.
Secondary outcome and mortality.
| Measurement | Definitions | NO | Placebo | P value | ||
|---|---|---|---|---|---|---|
| n/38 | % | n/38 | % | |||
| Secondary outcome | Brain natriuretic peptide < 90 pg/mL and Borg dyspnea score ≤ 2 | 6 | 16% | 13 | 34% | 0.111 |
| Components of secondary outcome | Brain natriuretic peptide < 90 pg/mL | 13 | 34% | 15 | 39% | |
| Borg dyspnea score ≤ 2 | 27 | 71% | 32 | 84% | ||
| Health related quality of life (90 days) | Favorable PEmb QoL scorea | 11 | 29% | 14 | 37% | 0.450 |
| Favorable SF36b | 15 | 39% | 15 | 39% | 1.000 | |
| Survival | Mortality at 28 days | 0 | 0% | 3 | 8% | 0.210 |
| Mortality at 90 days | 2 | 5% | 4 | 11% | 0.670 | |
Score < 40%.
Normalized score > 50%; N = 33, NO and N = 32, Placebo; see Fig. 1 for additional detail.
Figure 3.
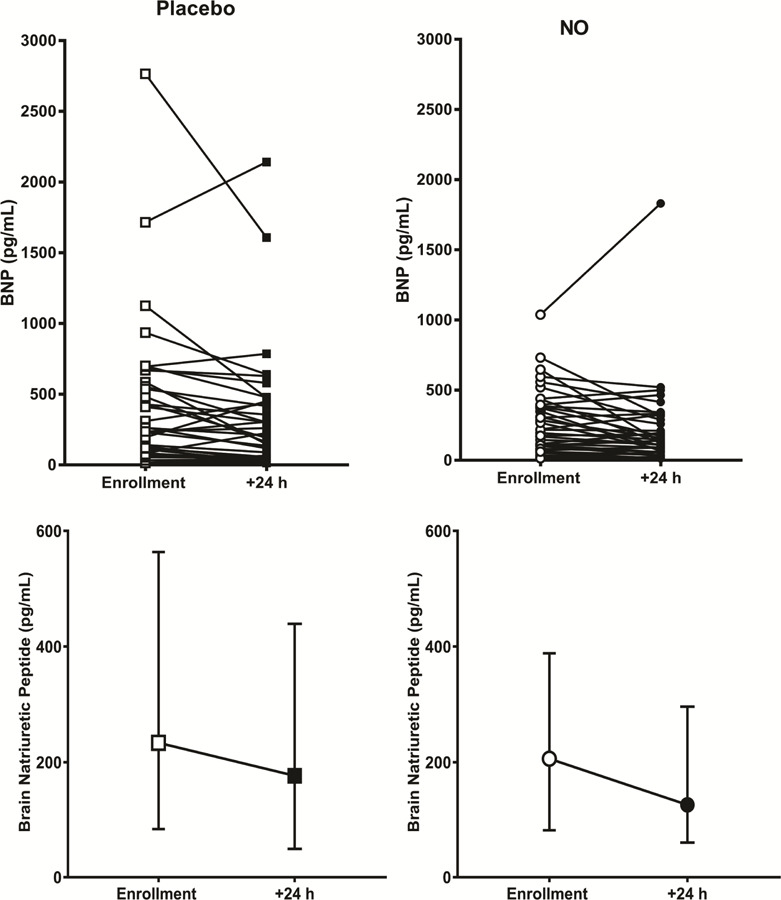
Dot plot showing plasma concentration of brain natriuretic peptide at enrollment, and then again after 24 hours of treatment for patients each treated with either placebo (n=38, squares), or 50 parts per million of inhaled nitric oxide (n=38, circles). The bottom panels show the median and interquartile ranges. The cutoff for abnormal was 90 pg/mL.
Safety measurements (Table 4)
Table 4.
Safety endpoints during treatment.
| Event | NO | Placebo | P value | ||
|---|---|---|---|---|---|
| n/39a | % | n/39a | % | ||
| Unblinding | 0 | 0% | 1 | 3% | 1.000 |
| Escalated to thrombolysis | 0 | 0% | 3 | 8% | 0.240 |
| Escalated to thrombectomy | 0 | 0% | 1 | 3% | 1.000 |
| Bleedingb | 0 | 0% | 1 | 3% | 1.000 |
| Hypotension | 2 | 5% | 1 | 3% | 1.000 |
| Respiratory distress requiring intervention | 1 | 3% | 1 | 3% | 1.000 |
| METH018 – Supportive Rx: continued O2 support | |||||
| METH027 – Supportive Rx: Cpap continuation at night. | |||||
| Hypoxemia requiring intervention | 3 | 8% | 1 | 3% | 0.615 |
| ESKE002 – Supportive Rx: O2 evaluation for home oxygen. Increase in NC flow from 4L (rest) - 6L (exertion) of O2. | |||||
| ESKE008 - Supportive Rx: O2 evaluation for home oxygen. Increase in NC flow from 1L (rest) - 2L (exertion) of O2. | |||||
| METH019 - Supportive Rx: O2 evaluation for home oxygen & discharged with home O2. | |||||
| METH028 – Supportive Rx: Bipap use at night for untreated sleep apnea | |||||
| Arrhythmia requiring intervention | 2 | 5% | 1 | 3% | 1.000 |
| ESKE003 – Supportive Rx: Patient restarted on home medication, Diltiazem 30 mg PO QID, due to reported A-fib with RVR with HR = 124. | |||||
| METH014 – Supportive Rx: metoprolol started | |||||
| METH049 – Supportive Rx: Amniodarone increased from 200 mg to 600 mg PO | |||||
| Death | 0 | 0% | 0 | 0% | 1.000 |
| Other adverse event requiring medication or supportive carec | 12 | 31% | 10 | 26% | 0.802 |
N = 39 used in each group for denominator despite voluntary withdrawal of one subject in each group prior to completion of the 24 h protocol because 39 in each group receive NO for some time period.
Vaginal bleeding without need for transfusion or procedural intervention.
Zero instances of vasopressor use, cardiopulmonary resuscitation, death bronchospasm, neurological deterioration, gastrointestinal distress or methemoglobi-nemia > 10% for 1 h during the 24 h treatment period.
No patient was discontinued from NO for any safety reason and no serious adverse event was attributed to study drug. No patient had sustained methemoglobinemia (see Supplemental Figure 2). After receiving intravenous promethazine and morphine, two NO-treated patients developed hypotension that required saline infusion. Care providers opened the blinding envelope during treatment in one placebo treated patient who developed worsened dyspnea.
Additional clinical outcomes
Mortality at 28 days for patients treated with NO and Placebo respectively was 0/38 and 3/38 (P=0.24, Fisher’s exact) and 2/38 and 4/38 at 90 days (P=0.67). Causes of death included one death from complications associated with rescue embolectomy on day 3, another from respiratory failure on day 69, in two, the patient died at home and the cause of death was unknown but were not ascribed to recurrent PE, and the other two died from progression of cancer. No patient had an autopsy at death. One patient, treated with placebo, had a recurrent DVT, and was the only case of VTE recurrence within 90 days. Supplemental figures 3–6 show the, Borg score, heart rate, SpO2%, and systolic blood pressure values at enrollment, 1, 8 and 24 hours of treatment. The salient observations include no apparent difference in any of these measurements, except for a consistently higher Borg score for NO treated patients.
Effectiveness of study procedures
Regarding the integrity of blinding, the clinical care physicians thought 32 patients received NO, and were correct in 14/32 (43%) instances; they thought 31 patients received Placebo, and were correct in 17 (54%) instances; physicians had no opinion of treatment assignment in 13 patients. We confirmed evidence of NO delivery with doubling of the plasma nitrate concentration in patients treated with NO (Figure 4). The median nitrate concentrations (μg/mL, 1st-3rd quartiles) in the Placebo group were 15 (9–19) and 15 (11–20) (P=0.113, sign-rank test) at enrollment and 24 hours, respectively and the median nitrate concentrations in the NO group were 12 (8–16) and 27 (22–38) (P<0.001, sign rank-test). Nitrite concentrations were not significantly changed from enrollment to 24 hours in either group.
Figure 4.
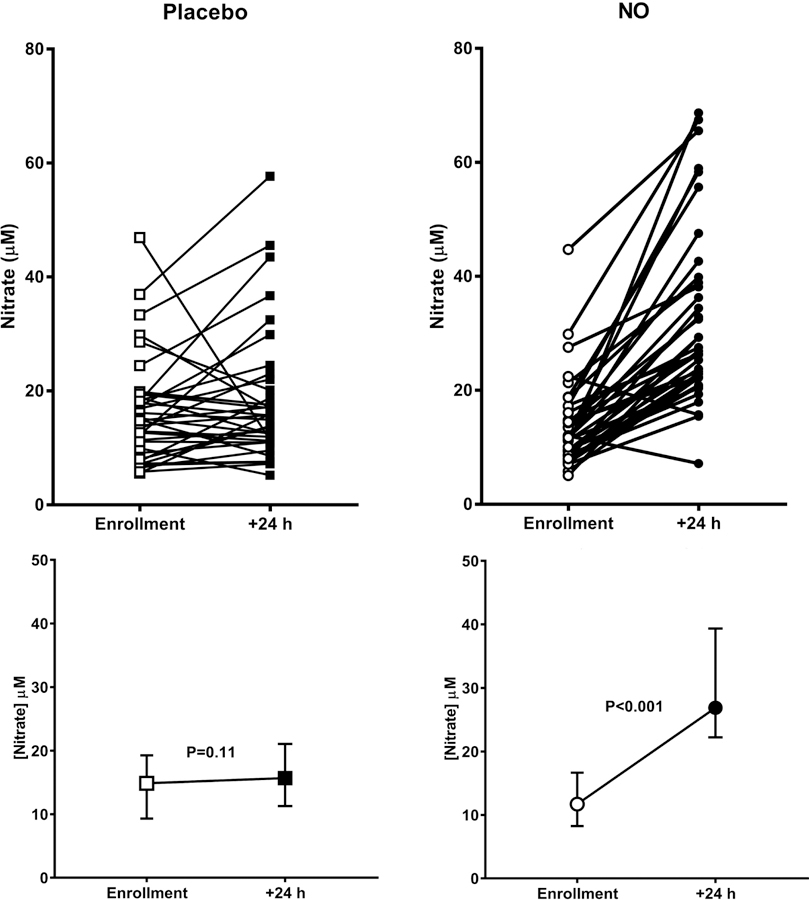
Dot plot showing plasma concentration of nitrates at enrollment, and then again after 24 hours of treatment for patients each treated with either placebo (n=38, squares), or 50 parts per million of inhaled nitric oxide (n=38, circles). The bottom panels show the median and interquartile ranges.
Frequency of resolution of RV hypokinesis and dilation
This study used several echocardiographic measurements of RV function that are not commonly used in routine medical practice. However, we believe that RV hypokinesis and dilation are more commonly reported in routine echocardiographic interpretations. Accordingly, in a post-hoc analysis using only the 71 study echocardiograms with adequate imaging to assess both RV hypokinesis and dilation, 19/35 (54%) of NO-treated and 10/36 (28%) of placebo-treated patients were free of either abnormality (P=0.03, Fisher’s exact). Furthermore, as part of usual care, 22/38 (58%) and 24/38 (63%) of NO and Placebo patients, respectively had a transthoracic echocardiogram performed within 12 hours prior to enrollment with inadequate imaging to assess both RV hypokinesis and dilation. At enrollment, 4/22 (18%) and 4/24 (17%) of NO and Placebo patients had neither abnormality; at 24 hours, 11/22 (50%) and 5/24 (21%) of patients treated with NO or Placebo, respectively, were free of both abnormalities (P=0.010, Cochrane’s Q test).
Discussion
In this randomized, double-blind, placebo-controlled trial, the proportion of patients randomized to 24 hours of inhaled NO who had normal RV function on echocardiography, and no evidence of myocardial injury (hs TnT) was not significantly higher than patients treated with placebo. Likewise, NO treatment did not lower BNP concentrations, patient perceived dyspnea (Borg score), and did not improve patient reported health related quality of life on psychometric testing at 90 days (PEmb QoL and SF 36). The serial measurements of methemoglobin, dyspnea perception, heart rate, and oxygenation were not different between groups over the 24 hour treatment period. Study procedures appeared to reduce bias reasonably well. Randomization produced two groups that were similar in the distributions of prior cardiopulmonary disease. However, the troponin T concentrations tended to be higher in the placebo treated group, possibly introducing bias in favor of NO. The Borg dyspnea score appeared to be higher at baseline in the NO treated group, possibly introducing bias in favor of placebo. We confirmed effective delivery of NO, based upon increase in plasma nitrate concentrations, similar to those measured in a previous trial of inhaled NO for acute chest syndrome in sickle cell disease.[22] The mechanism of blinding appeared effective based upon the balanced percentage of the time that clinicians correctly guessed which treatment the patient received.
The rationale for the components of each outcome were well justified.[18] The four echocardiographic parameters assessed the global function of the RV pumping against an acutely increased afterload: 1. The RV diameter to measure volume overload, 2. The TAPSE to measure longitudinal shortening, 3. The FAC to reflect circumferential contraction, and 4. The RIMP to indicate speed of stroke volume ejection.[23] The high precision troponin T was intended to measure degree of myocyte necrosis from shear effect and inflammatory injury, both of which increase with higher RV afterload.[24] Regarding troponin assays, we used troponin I as an eligibility criterion, whereas we used troponin T in the primary outcome. The reason for this difference was that during the time of enrollment, we used the FDA cleared troponin assays that were used in usual clinical practice at the enrolling hospitals, which all measured troponin I. The preplanned primary outcome required high precision troponin, and the device we used for this measures troponin T. In the time since our study was completed, this device has been cleared to market in the US. Data in Table 1 show that 14/38 (39%) of patients treated with NO normalized their troponin values, compared with 11/38 (29%), which if analyzed independently would have yielded P=0.63 (Fisher’s exact test). We do acknowledge the possibility that had we measured troponin concentrations later, for example at 48 hours, we may have seen wider separation in the frequency of normalization of troponin measurements between groups.
In the secondary outcome, the plasma BNP and dyspnea severity were intended to measure RV muscle strain and a patient-oriented measurement of ventilation-perfusion mismatching from increased alveolar deadspace; moreover, these two measurements.[25] While BNP values could have been altered by coincident heart failure, kidney disease or coronary artery disease, their removal had no effect on the proportion of patients who normalized BNP (Supplemental Tables 1–4). The two psychometric tests are self-evident as measures of patient perceived health wellness. We used four physiologically-oriented echocardiographic indexes that are not always used in usual care and can be difficult to obtain because of limitations in acoustic windows. For this reason, we conducted a post-hoc analysis of RV hypokinesis and dilation. Our rational for choosing these two variables between NO-treated and Placebo-treated in the post-hoc analysis is because both variables were assessable in 93% of study echocardiograms, and RV hypokinesis and dilation are often reported on clinical echocardiogram interpretations. These post-hoc analyses suggest that NO may have improved the probability of correcting RV hypokinesis and dilation more than chance alone would predict.
Taken together, these data provide some evidence of clinical value of inhaled NO for PE. On one hand, inhaled NO did not significantly increase the proportion of patients with good primary or secondary outcomes, and did not improve symptoms, heart rate, oxygenation or perception of wellness. However, this study does not rule out the possibility of modest improvements in RV function with inhaled NO. As a phase II study, the sample size was predicated on a relatively large 30% difference in primary outcome to justify progression to a large, expensive phase III trial. After study completion, we found an only an 11% difference in the primary outcome. By comparison, the only two randomized controlled trials of fibrinolysis versus placebo that repeated echocardiograms at 24 hours after treatment both found only a 10% increase in rate of RV resolution compared with placebo.[26, 27] Moreover, no NO-treated patient required escalation of therapy while on protocol, compared with four Placebo-treated patients, including two who underwent both catheter directed fibrinolysis and then surgical embolectomy (Table 4). Third, the post-hoc analysis suggests NO was more likely than Placebo at correcting RV hypokinesis and dilation at 24 hours. Finally, NO was not associated with any serious adverse events. These findings may leave open the possibility of future work with higher doses of NO or the use of drugs that directly stimulate or activate guanylate cyclase.[28] It could be speculated that NO may benefit patients with more severe PE, such as those with hypotension or requiring mechanical ventilation, but we believe the rarity of patients with this severe form of PE, combined with real world difficulty of obtaining their written informed consent constitute an insurmountable barrier to conducting such a trial.
Conclusions
Compared with placebo, 24 hours of nasally inhaled NO did not increase the proportion of patients with intermediate-high risk PE who normalized their RV function, circulating troponin T or BNP concentrations after 24 hours of treatment. A secondary analysis suggests that inhaled NO may increase likelihood of resolving echocardiographically-observed RV hypokinesis and dilation. Future studies should focus on this endpoint.
Supplementary Material
Highlights.
Patients with intermediate risk pulmonary embolism were randomized to 24 hours of inhaled nitric oxide (N), delivered by nasal cannula, or oxygen placebo. The primary outcome was a normal right ventricle on echocardiography and a normal high sensitivity troponin measurement.
Patients who received NO were not more likely to reach the primary outcome. NO treatment also did not increase the proportion of patients with a normal brain natriuretic peptide.
A post-hoc analysis showed that patients who had RV hypokinesis on echocardiography at enrollment who were treated with NO were more likely to resolve their hypokinesis, compared with patients treated with placebo.
Acknowledgments
Funding provided by an investigator initiated research by Mallinckrodt, Roche and National Institutes of Health (UM1HL113203) to JAK.
Abbreviations
- BNP
brain natriuretic peptide
- CT
computed tomography
- FAC
fractional area of contraction
- LV
left ventricle
- PE
pulmonary embolism
- PEmb QoL
pulmonary embolism quality of life survey
- RIMP
right ventricular index of myocardial performance
- SF 36
standard form 36 quality of life survey
- TAPSE
tricuspid annual plane systolic excursion
- NO
nitric oxide
- RV
right ventricle
Footnotes
Conflicts of interest: No other authors have a conflict to report
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical trial registration
Contributor Information
Jeffrey A. Kline, Indiana University School of Medicine, Dept. of Emergency Medicine, 720 Eskenazi Avenue, Fifth Third Faculty Office Building, 3rd Floor Emergency Medicine Office, Indianapolis, IN 46202.
Michael A. Puskarich, Department of Emergency Medicine, Hennepin County Medical Center
Alan E. Jones, Department of Emergency Medicine, University of Mississippi Medical Center.
Ronald A. Mastouri, Indiana University School of Medicine, Department of Medicine, Division of Cardiology.
Cassandra L. Hall, Indiana University School of Medicine, Dept. of Emergency Medicine.
Anthony Perkins, Indiana University Center for Health Innovation and Implementation Science, Indiana Clinical and Translational Sciences Institute.
Emily Gundert, Department of Emergency Medicine, University of Texas Southwestern.
Tim Lahm, Division of Pulmonary, Allergy, Critical Care, Occupational and Sleep Medicine, Indiana University School of Medicine.
References
- 1.McIntyre KM, Sasahara AA: Determinants of right ventricular function and hemodynamics after pulmonary embolism. Chest 1974, 65(5):534–543. [DOI] [PubMed] [Google Scholar]
- 2.Watts JA, Zagorski J, Gellar MA et al. : Cardiac inflammation contributes to right ventricular dysfunction following experimental pulmonary embolism in rats. J Mol Cell Cardiol 2006, 41(2):296–307. [DOI] [PubMed] [Google Scholar]
- 3.Lankeit M, Jimenez D, Kostrubiec M et al. : Predictive value of the high-sensitivity troponin T assay and the simplified pulmonary embolism severity index in hemodynamically stable patients with acute pulmonary embolism: a prospective validation study. Circulation 2011, 124(24):2716–2724. [DOI] [PubMed] [Google Scholar]
- 4.Elliott CG: Pulmonary physiology during pulmonary embolism. Chest 1992, 101(4 Suppl):163s–171s. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez O, Trinquart L, Colombet I et al. : Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. EurHeart J 2008, 29(12):1569–1577. [DOI] [PubMed] [Google Scholar]
- 6.Meinel FG, Nance JW Jr., Schoepf UJ et al. : Predictive Value of Computed Tomography in Acute Pulmonary Embolism: Systematic Review and Meta-analysis. Am J Med 2015, 128(7):747–759.e742. [DOI] [PubMed] [Google Scholar]
- 7.Kahn SR, Akaberi A, Granton JT et al. : Quality of Life, Dyspnea, and Functional Exercise Capacity Following a First Episode of Pulmonary Embolism: Results of the ELOPE Cohort Study. Am J Med 2017, 130(8):990.e999–990.e921. [DOI] [PubMed] [Google Scholar]
- 8.Sharma GV, McIntyre KM, Sharm S et al. : Clinical and hemodynamic correlates in pulmonary embolism. Clin Chest Med 1984, 5(3):421–437. [PubMed] [Google Scholar]
- 9.Agterof MJ, Schutgens RE, Verzijlbergen JF et al. : No firm association between N-terminal pro-brain natriuretic peptide and percentage of pulmonary vascular obstruction in patients with acute pulmonary embolism. ThrombRes 2011, 127(6):547–550. [DOI] [PubMed] [Google Scholar]
- 10.Kline JA, Marchick MR, Hogg MM: Reduction in plasma haptoglobin in humans with acute pulmonary embolism causing tricuspid regurgitation. JThrombHaemost 2009, 7(9):1597–1599. [DOI] [PubMed] [Google Scholar]
- 11.Kline JA, Watts J, Courtney D et al. : Severe pulmonary embolism decreases plasma L-arginine. EurRespirJ 2013. [DOI] [PubMed]
- 12.Rezania S, Puskarich MA, Petrusca DN et al. : Platelet hyperactivation, apoptosis and hypercoagulability in patients with acute pulmonary embolism. Thromb Res 2017, 155:106–115. [DOI] [PubMed] [Google Scholar]
- 13.Blood AB, Schroeder HJ, Terry MH et al. : Inhaled nitrite reverses hemolysis-induced pulmonary vasoconstriction in newborn lambs without blood participation. Circulation 2011, 123(6):605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watts JA, Gellar MA, M.B. F et al. : Pulmonary vascular reserve during experimental pulmonary embolism: Effects of a soluble guanylate cyclase stimulator, BAY 41–8543. Crit Care Med 2011, 39(12):2700–2704. [DOI] [PubMed] [Google Scholar]
- 15.Bloch KD, Ichinose F, Roberts JD Jr. et al. : Inhaled NO as a therapeutic agent. Cardiovasc Res 2007, 75(2):339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ichinose F, Roberts JD Jr., Zapol WM: Inhaled nitric oxide: a selective pulmonary vasodilator: current uses and therapeutic potential. Circulation 2004, 109(25):3106–3111. [DOI] [PubMed] [Google Scholar]
- 17.Bhat T, Neuman A, Tantary M et al. : Inhaled nitric oxide in acute pulmonary embolism: a systematic review. Reviews in cardiovascular medicine 2015, 16(1):1–8. [DOI] [PubMed] [Google Scholar]
- 18.Kline JA, Hall CL, Jones AE et al. : Randomized trial of inhaled nitric oxide to treat acute pulmonary embolism: The iNOPE trial. Am Heart J 2017, 186:100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konstantinides SV, Torbicki A, Agnelli G et al. : 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Endorsed by the European Respiratory Society (ERS). EurHeart J 2014.
- 20.van EJ, den Exter PL, Kaptein AA et al. : Quality of life after pulmonary embolism as assessed with SF-36 and PEmb-QoL. ThrombRes 2013, 132(5):500–505. [DOI] [PubMed] [Google Scholar]
- 21.Gupta SK: Intention-to-treat concept: A review. Perspectives in Clinical Research 2011, 2(3):109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gladwin MT, Kato GJ, Weiner D et al. : Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial. JAMA 2011, 305(9):893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudski LG, Lai WW, Afilalo J et al. : Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am SocEchocardiogr 2010, 23(7):685–713. [DOI] [PubMed] [Google Scholar]
- 24.Jimenez D, Uresandi F, Otero R et al. : Troponin-based risk stratification of patients with acute nonmassive pulmonary embolism: Systematic review and metaanalysis. Chest 2009, 136(4):974–982. [DOI] [PubMed] [Google Scholar]
- 25.Klok FA, Mos IC, Huisman MV: Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. AmJRespirCrit Care Med 2008, 178(4):425–430. [DOI] [PubMed] [Google Scholar]
- 26.Goldhaber SZ, Haire WD, Feldstein ML et al. : Alteplase versus heparin in acute pulmonary embolism: Randomized trial assessing right-ventricular function and pulmonary perfusion. Lancet 1993, 341(8844):507–511. [DOI] [PubMed] [Google Scholar]
- 27.Becattini C, Agnelli G, Salvi A et al. : Bolus tenecteplase for right ventricle dysfunction in hemodynamically stable patients with pulmonary embolism. ThrombRes 2010, 125(3):e82–e86. [DOI] [PubMed] [Google Scholar]
- 28.Dasgupta A, Bowman L, D’Arsigny CL et al. : Soluble guanylate cyclase: a new therapeutic target for pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Clinical pharmacology and therapeutics 2015, 97(1):88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


