Abstract
Background
Few studies have examined functional brain changes specifically associated with chemotherapy (CTx) in patients with lung cancer. This prospective longitudinal research aimed to explore the change in intrinsic brain activity by investigating patients with lung cancer after CTx.
Material/Methods
Sixteen patients and 20 healthy individuals were enrolled in this study. The amplitude of low-frequency fluctuation (ALFF), regional homogeneity (ReHo), dynamic amplitude of low-frequency fluctuation (dALFF), and dynamic regional homogeneity (dReHo) were computed. The group differences in resting state functional magnetic resonance imaging (rs-fMRI) parameters were compared. Alterations in the rs-fMRI parameters from before CTx to after CTx were assessed using the paired t-test. We performed correlation analyses between rs-fMRI parameters and Montreal Cognitive Assessment (MoCA) scores.
Results
We found statistically significant differences in MoCA scores before CTx and after CTx. Compared to the healthy group, rs-fMRI values decreased in the frontal regions as well as parietal regions compared to values before CTx. In addition, we found significantly decreased rs-fMRI values in the default-mode network (DMN) region of the brain before CTx compared to after CTx. We found no significant correlations between altered intrinsic activity values and MoCA scores.
Conclusions
The current study indicated that patients with lung cancer after CTx had decreased dynamic brain activity in the DMN region, and the DMN is vulnerable when patients undergoing CTx.
MeSH Keywords: Cognition, Lung Neoplasms, Magnetic Resonance Imaging
Background
In recent years, lung cancer has become the most common cause of cancer morbidity and mortality worldwide [1], with an increasing number of treatment strategies available, including surgery, chemotherapy (CTx), and radiotherapy. CTx is one of the most basic treatment strategies that can prolong patient survival. But CTx can cause severe physical discomfort and can also cause CTx-related cognitive impairment (CRCI) [2]. CRCI is mainly manifested as learning and memory impairment and difficulty in task execution, known as “chemobrain, which can affect a patient’s every daily life. Currently, most neuroimaging studies [3,4] have investigated CRCI in patients with breast cancer. According to the literature, breast cancer patients after CTx were found to have damage in the default-mode network (DMN) region of the brain [5–7], a finding that has been rarely reported in lung cancer patients. The DMN shows increased activities during a resting state and decreased activities during goal-directed cognitive performance [8]. The function of the DMN includes introspective and reflective self-awareness processes, episodic memory retrieval, and mental imagery [9]. Posterior cingulate cortex (PCC), precuneus, medial temporal cortex, as well as medial prefrontal cortex constitute the DMN [8]. Simó et al. first reported the DMN functional connectivity decrease in patients with lung cancer [10]. Bromis et al. demonstrated the possible negative effect of CTx in the DMN region of the brain in small-cell lung cancer populations [11].
At present, neuroimaging techniques can be used to explore “chemobrain” looking at aspects of brain functional activities and brain networks [12]. Resting state functional magnetic resonance imaging (rs-fMRI) is a type of the reliable, non-invasive fMRI method. It is widely used to study cognitive decline in neural and mental illnesses, and there is increasing evidence reporting on close ties between rs-fMRI findings and cognitive function. An advantage of rs-fMRI is the short time required during scanning, making it more likely to be tolerable for lung cancer patients undergoing CTx [13,14]. Rs-fMRI can identify local activity in brain tissue, as well study the activity of neurons by looking at the effect of blood oxygen level dependence (BOLD) [15]. At present, amplitude of low-frequency fluctuation (ALFF) is one of the techniques used to measure the characteristics of a BOLD signal area. It reflects the spontaneous activity intensity of an individual element by using the mean value of amplitude at all frequency points in the low frequency range (0.01–0.08 Hz), so as to reflect the spontaneous activity level of each individual element in the resting state from the perspective of energy [16]. Another method is regional uniformity (ReHo), which is an evaluation parameter of rs-fMRI. ReHo can evaluate the neural activity synchrony of an individual voxel with its adjacent voxels by calculating the Kendall’s coefficient of concordance (KCC) [17]. Many studies of neuropsychiatric diseases have adopted these 2 methods [18,19]. The combined use of ALFF and ReHo is common and is sensitive to detecting local abnormal brain activity. Compared with ALFF or ReHo method alone, ALFF and ReHo together could offer a more extensive pathophysiological evaluation of brain dysfunctions [18,20]. Many studies on brain activity presume that the brain state is stable during the MRI scan. In fact, spontaneous brain activity is dynamic and changes over time. The temporal variability of intrinsic brain activity relates to the functional ability of neural networks, and it can be acquired through dynamic integration or adjustment on multiple time scales [21–23]. So far, no study has been reported on the static or dynamic alteration of intrinsic brain activity for patients with lung cancer treated with CTx or without CTx.
This study aimed to assess whether lung cancer patients after CTx had impairments in the DMN region of the brain by investigating the abnormal alterations of static and dynamic intrinsic brain activity values.
Material and Methods
Patients and clinical data
This study was approved by the Research Ethics Committee of the Nanjing Medical University. All participants provided written informed consent before the study. Nineteen patients with lung cancer and 22 healthy individuals were recruited between June 2017 and August 2019. All participants enrolled in the study were 50 to 70 years of age, right-handed, and had at least 6 years of education. All the patients with lung cancer underwent surgery. The pre-CTx (before CTx) assessment included rs-fMRI scan and cognitive testing. The follow-up assessment (after CTx) was performed within 3 to 6 months after adjuvant CTx. The CTx regimen for patients included pemetrexed+cisplatin (7 patients), pemetrexed+paraplatin (3 patients), pemetrexed (2 patients), gemcitabine (2 patients), gemcitabine+cisplatin (1 patient), gemcitabine+nedaplatin (1 patient), docetaxel+cisplatin (1 patient), docetaxel+nedaplatin (1 patient), and etoposide+nedaplatin (1 patient). The exclusion norm for participants were: 1) proven brain metastatic tumors, 2) a history of receiving PCI, 3) a history of neurological diseases, 4) diagnosis of psychiatric diseases, 5) known major medical diseases. The common cognitive function of the participants including patients before and after CTx, as well as healthy controls was established using Montreal Cognitive Assessment (MoCA) [24].
MR acquisition
All images were obtained using a 3.0 T MRI scanner (Ingenia, Philips Medical Systems, Netherlands) with an 8-channel receiver array head coil; parallel imaging was employed. The rs-fMRI data were collected using gradient echo-planar imaging. The parameters were as follows: repetition time=2000 ms; echo time=30 ms; slices=36; thickness=4 mm; gap=0 mm; flip angle=90°; acquisition matrix=64×64; and field of view=240×240 mm. Participants were told to lie still and close their eyes. Foam padding and ear plugs were used to reduce head motion and scanner noise, respectively. Scans lasted 8 minutes and 8 seconds per participant.
Data pre-processing
We used Data Processing & Analysis for Brain Imaging (DPABI) software to pre-process data [25]. The first 10 time points were deleted from each time series to obtain a stable resting state. Then, the remaining 230 images were obtained by slice-timing correction and realignment. The remaining data were spatially standardized to the Montreal Neurological Institute template and resampled into 3 mm3. In addition, data were smoothed by a 6 mm full width at half maximum (FWHM) isotropic Gaussian kernel, however, for the ReHo analysis, the smoothing was performed after computing ReHo. Time course detrending and band-pass filtering (0.01–0.08 Hz) were used in turn. Data from study participants whose head motion was more than 2 mm were excluded in the analysis.
ALFF and ReHo analyses
We used the REST software to conduct the ALFF analysis [16]. We converted the preprocessed data to frequency domain by Fast Fourier Transform. The power spectra were subjected to the square root transformation and then mean across 0.01–0.08 Hz for each voxel. This averaged square root was the ALFF. We divided whole-brain mean ALFF to make the ALFF map of every voxel standardized. We used the REST software to conduct the ReHo analysis [17]. The spatial smoothing (FWHM=6 mm) was conducted after the ReHo calculation. We divided the whole-brain mean ReHo to make the ReHo map of every voxel standardized.
Dynamic ALFF (dALFF) and dynamic ReHo (dReHo) analyses
A gliding window research approach was used to compute the dynamic ALFF (dALFF) and the dynamic ReHo (dReHo) by using Temporal Dynamic Analysis (TDA) toolkits [26,27]. We applied this approach to study dALFF variability or dReHo variability. In our work, a medium window length was 32 TRs (64 s), and a sliding step size was 1 TR (2 s). This procedure produced 189 windows for each subject. An ALFF and ReHo map was obtained in each sliding window, and we computed the variability of all dALFF and dReHo maps across sliding-windows. We divided the whole-brain mean ALFF and ReHo to make the ALFF and ReHo map of every voxel normalized.
Statistical analysis
Demographic characteristics, including age and years of education, were compared with the 2-sample t-test between the patient group and the healthy group; in addition, gender was compared using the chi-squared test. MoCA scores for the patients with lung cancer were compared using the paired t-test between before CTx and after CTx, and we compared MoCA scores for the patients with lung cancer before CTx and the MoCA scores for healthy controls using the 2-sample t-test. Statistical analyses were conducted with the SPSS version 19 (SPSS Inc, Chicago, IL, USA). We used a statistical significance level of P<0.05.
ALFF, ReHo, dALFF, and dReHo maps were performed for statistical analyses with a voxel-wise one-way ANOVA among the patients with lung cancer before CTx, and after CTx, as well as for the healthy group. We set statistical threshold as P<0.001. we corrected the data by false discovery rate (FDR) norm. If there was statistically significant, we performed further analysis. Namely, we investigated the statistical differences about ALFF, ReHo, dALFF, and dReHo maps with the 2-sample t-test between the patients with lung cancer before or after CTx and healthy controls. For the statistical differences between the patients with lung cancer before and after CTx for the aforementioned maps, we chose the paired t-test. We set statistical threshold as P<0.001 and chose (FDR) criterion to use for correction.
We explored the relationship between abnormal rs-fMRI parameters and MoCA scores with Pearson correlation analyses; we used a statistical significance level of P<0.05.
Results
Demographic characteristics
The research excluded 3 patients and 2 healthy controls due to excessive head movement. Finally, 16 patients and 20 healthy participants were included. Age, duration of education, and gender were not significantly different between the patient group and the healthy group (all P>0.05, Table 1). There was a significant difference between lung cancer patients before and after CTx in terms of MoCA scores (24.69±2.41 and 22.25±4.04, respectively, P=0.001). There was no statistically significant difference in MoCA scores between before CTx and healthy controls (24.69±2.41 and 25.65±2.92, respectively, P=0.297).
Table 1.
Demographic characteristics.
| Characteristics | Control (n=20) | Patients before CTx (n=16) | P value |
|---|---|---|---|
| Demographic characteristic | |||
| Age. year | 59.10±4.64 | 59.19±9.81 | 0.974 |
| Education. year | 10.50±1.40 | 10.88±3.77 | 0.710 |
| Gender: Male/Female | 9/11 | 12/4 | 0.096 |
| Tumor stage | |||
| I | 3 | ||
| IIA | 2 | ||
| IIB | 4 | ||
| IIIA | 3 | ||
| IIIB | 1 | ||
| IV | 3 | ||
CTx – chemotherapy.
ALFF results
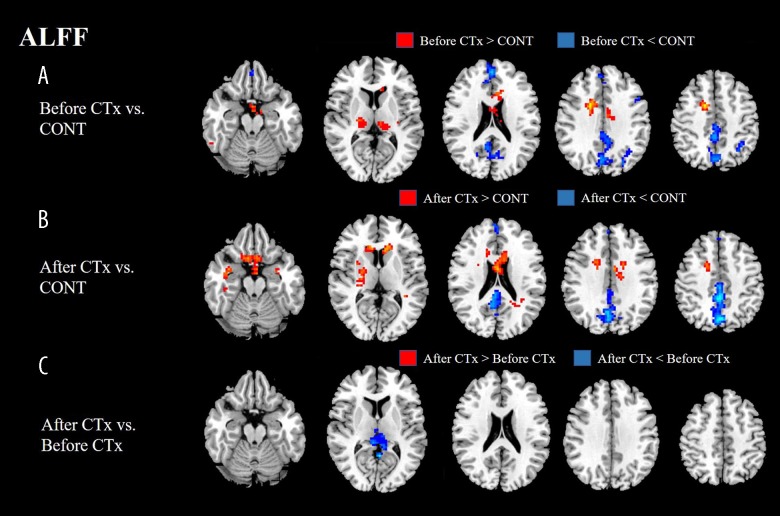
ALFF values of patients before CTx displayed a significant decrease in bilateral frontal, parietal, and occipital regions, while displaying a significant increase in bilateral frontal and thalamus regions compared with ALFF values of the healthy group. ALFF values of the patients after CTx showed a significant decrease in bilateral frontal, parietal, and occipital regions, while displaying a significant increase in bilateral frontal and temporal regions compared with ALFF values of the healthy group. ALFF values of the patients after CTx displayed a significant decrease in the left precuneus compared with ALFF values of the patients before CTx (Figure 1, Table 2).
Figure 1.
(A, B) ALFF values of patients before CTx or after CTx displayed a significant decrease in bilateral frontal, parietal, and occipital regions. (C) ALFF values of patients after CTx displayed a significant decreased in left precuneus compared with ALFF values of patients before CTx. ALFF – amplitude of low-frequency fluctuation; CTx – chemotherapy; CONT – controls.
Table 2.
Regions showing significant differences in rs-fMRI values before and after CTx.
| Analysis | Contrast | Connected area | MNI coordinates x, y, z (mm) | Numbers of voxels | Peak T value |
|---|---|---|---|---|---|
| ALFF | After CTx<before CTx | Left precuneus | −3, −54, 9 | 227 | −5.19 |
| ReHo | After CTx<before CTx | Right PCC | 9, −42, 12 | 282 | −6.18 |
| After CTx>before CTx | Right middle occipital gyrus | 18, −87, 12 | 74 | 5.46 | |
| dALFF | After CTx<before CTx | Right PCC | 6, −33, 6 | 204 | −4.01 |
| dReHo | After CTx<before CTx | Left PCC | −9, −39, 9 | 200 | −6.20 |
rs-fMRI – functional magnetic resonance imaging; CTx – chemotherapy; MNI – Montreal Neurological Institute; ALFF – amplitude of low-frequency fluctuation; ReHo – regional homogeneity; dALFF – dynamic amplitude of low-frequency fluctuation; dReHo – dynamic regional homogeneity; PCC – posterior cingulate cortex.
ReHo results
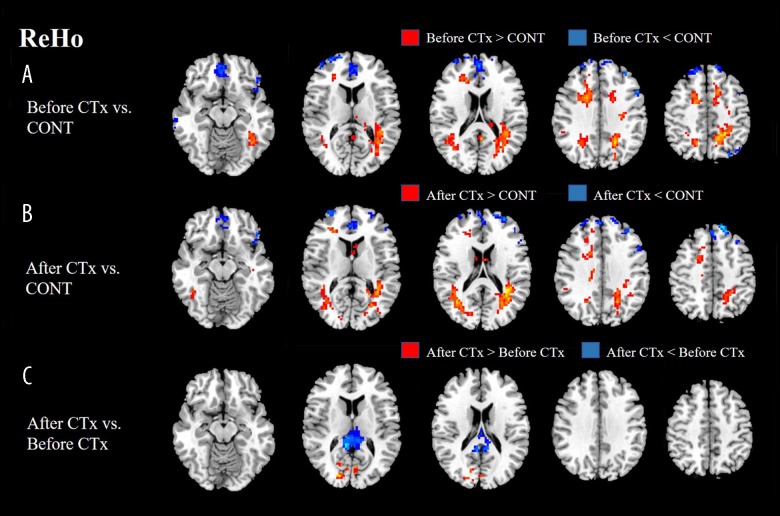
ReHo values of the patients before CTx displayed a significant decrease in bilateral frontal regions, while displaying a significant increase in bilateral frontal, parietal, and occipital regions compared with ReHo values of the healthy group. ReHo values of the patients after CTx displayed a significant decrease in bilateral frontal regions, while displaying a significant increase in right frontal, bilateral parietal, and occipital regions compared with ReHo values of the healthy group. ReHo values of the patients after CTx displayed a significant decrease in the right PCC, while displaying a significant increase in the right middle occipital gyrus compared with ReHo values of the patients before CTx (Figure 2, Table 2).
Figure 2.
(A) ReHo values of patients before CTx displayed a significant decrease in bilateral frontal and left parietal regions. (B) Patients after CTx showed a significantly decreased ReHo in the bilateral frontal regions. (C) ReHo values of patients after CTx displayed a significant decrease in right PCC, while displaying significant increase in right middle occipital gyrus compared with ReHo values of patients before CTx. ReHo – regional homogeneity; CTx – chemotherapy; CONT – controls.
dALFF results
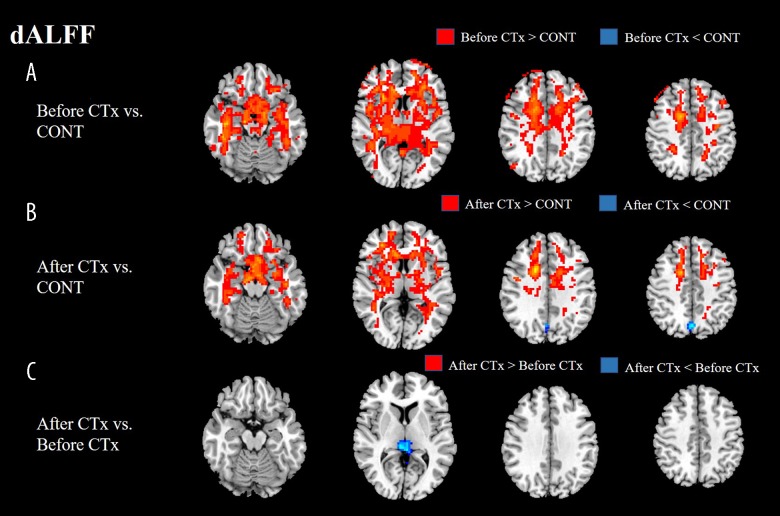
dALFF variability of the patients before CTx displayed a significant increase in bilateral cerebral hemispheres compared with dALFF variability of the healthy group, however, there was no significant decrease in comparison of dALFF variability between the 2 groups. dALFF variability of the patients before CTx displayed a significant increase in bilateral cerebral hemispheres, while displaying a significant decrease in left precuneus compared with dALFF variability of the healthy group. dALFF variability of the patients after CTx displayed a significant decrease in the right PCC compared with dALFF variability of the patients before CTx (Figure 3, Table 2).
Figure 3.
(A, B) Before and after CTx, dALFF variability of patients mainly showed a significant increase in bilateral cerebral hemisphere regions compared with normal control. However, dALFF variability of patients after CTx displayed a significant decrease in left precuneus compared with normal control. (C) dALFF variability after CTx displayed a significant decrease in right PCC in directly comparison of dALFF variability between before and after CTx. dALFF – dynamic amplitude of low-frequency fluctuation; CTx – chemotherapy; CONT – controls.
dReHo results
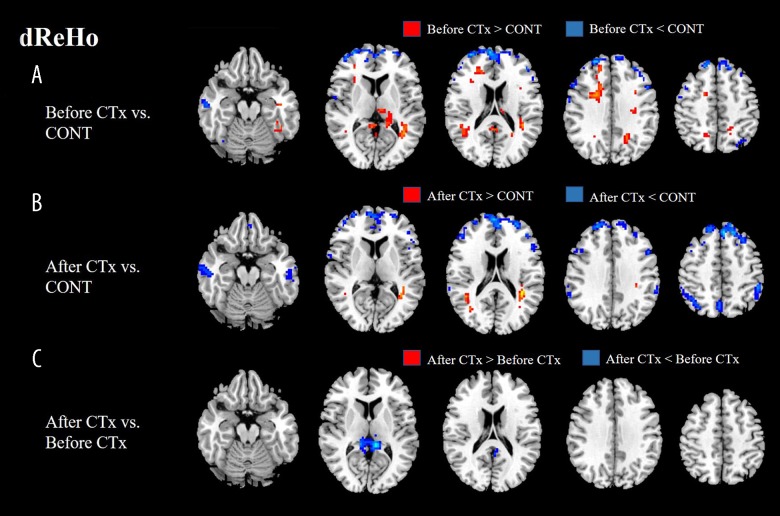
dReHo variability of the patients before CTx displayed a significant decrease in bilateral frontal, left parietal, and right temporal regions, while displaying a significant increase in bilateral frontal, parietal, and temporal regions compared with dReHo variability of the healthy group. dReHo variability of the patients after CTx displayed a significant decrease in bilateral frontal, parietal, and temporal regions, while displaying a significant increase in bilateral temporal regions compared with dReHo variability of the healthy group. dReHo variability of the patients after CTx displayed a significant decrease in the left PCC compared with dReHo variability of the patients before CTx (Figure 4, Table 2).
Figure 4.
(A, B) dReHo variability of patients before CTx mainly displayed a significant decrease in bilateral frontal areas compared with normal control, while dReHo variability of patients after CTx displayed a significant decrease in broader areas including bilateral frontal, parietal, and temporal regions. (C) dReHo variability after CTx showed a significant decrease in left PCC compared with before CTx. dReHo – dynamic regional homogeneity; CTx – chemotherapy; CONT – controls.
Correlation analysis
No significant correlations were found between MoCA scores and altered intrinsic brain activity parameters, which showed significant differences in the comparison of before and after CTx.
Discussion
After CTx, the patients had poor MoCA scores compared to values before CTx. In directly comparison of intrinsic brain activity parameters between before and after CTx, we observed changes in 4 parameters: decreased ALFF in the precuneus, decreased ReHo in right PCC, while increased ReHo in the right middle occipital gyrus, decreased dALFF variability in right PCC as well as decreased dReHo variability in left PCC. However, further analysis displayed that no significant correlations were found between altered intrinsic activity parameters and MoCA scores.
Although several different tests are considered useful for detecting cognitive impairment, the Mini-Mental State Examination (MMSE) is commonly used. However, it is difficult to detect early cognitive impairment using MMSE. But MoCA can screen patients with early cognitive impairment, and Nasreddine et al. reported that the MoCA had a sensitivity of 100% and a specificity of 87% in a mild Alzheimer disease group under the recommended cutoff score of 26 [24]. Yu et al. reported the Beijing version of the MoCA (MoCA-BJ) had a sensitivity of 90.4% and a specificity of 31.3% under the recommended cutoff score of 26, and the MoCA-BJ had a sensitivity of 68.7% and a specificity of 63.9% when the cutoff score was lowered to 22 [28]. MoCA has excellent accuracy and good internal consistency [29]. Besides, it is more likely to be tolerable for lung cancer patients undergoing CTx, because it is simple and does not take long to perform. Thus, we chose MoCA as our cognitive screening instrument.
In our study, we chose 3 to 6 months for the duration of CTx. Joy et al. reported that patients with breast cancer had no obvious cognitive deficits at 1 month after the end of CTx, and had gray matter atrophy recovered on some brain areas at 1 year after CTx [30]. Therefore, to obtain an explicit result as to whether CTx can cause cognitive impairment in lung cancer patients, we chose this same duration. We combined the static and dynamic brain activity changes to detect whether lung cancer patients with CTx had cognitive impairments by analyzing fMRI data. ALFF and ReHo often are used to measure static brain activity, and some previous studies have reported their use in cancer patients treated with CTx [31,32]. However, brain activity is inherently dynamic. Dynamic brain activities describe the temporal alterations in energy expenditure, which can be a result of higher need [33]. These methods are reproducible across time and with different individuals and have shown fine segregation between healthy individuals and patients, suggesting that dynamic properties may provide useful diagnostic and prognostic information [21]. Dynamic brain activities (dALFF variability and dReHo variability) have seldom been reported in cancer patients with CTx. Although Chen et al. studied dALFF variability and dReHo variability in the subacute stroke patients [26]. Therefore, this temporal variability can become a powerful predictor of clinical manifestations of neurological markers.
The precuneus and the PCC are 2 brain regions which constitute the DMN. The precuneus, located on the medial side of the upper parietal lobe, is a key brain region for higher-order cognitive processes, and plays a vital role in visuo-spatial integration, memory and self-awareness [34]. The precuneus represent an important hub in the DMN. It plays different roles according to the individual’s environment and cognitive task status. The precuneus maintains a high metabolic rate in the DMN in the resting state of humans, requiring more glucose than other brain regions to sustain this high metabolic state [35]. Abnormal brain activity in the precuneus may lead to a decline in memory and executive function. In our research, ALFF values of the patients after CTx displayed a significant decrease in the left precuneus compared with patients before CTx. Chen et al. found that breast cancer patients after CTx displayed a significantly decreased fractional ALFF in the left precuneus compared with before CTx [32], which indicated CTx can make the intrinsic brain activity of the precuneus change. Our research again proved that the precuneus played a key place in brain changes after CTx.
The PCC as another component of the DMN is located in the medial part of inferior parietal lobe and lies within the posteromedial cortex, and it is a highly active brain region in terms of connection and metabolism [36]. The PCC is associated with empathy, episodic memory retrieval, visuospatial imagery, and attention. In addition, the PCC plays a key role in altered mood and cognition [37]. In our study, patients after CTx were found to have decreased ReHo at the right PCC, decreased dALFF variability at the right PCC, and decreased dReHo variability at the left PCC compared to before CTx. Thus, abnormal brain activity in the PCC might be related to cognitive impaired in patients with CTx. Zhang et al. revealed that poorer MoCA performance was related to decreased functional connectivity of the PCC [38]. Therefore, our results also support the finding that the PCC can play a key place in CRCI.
Some studies have shown that the DMN is susceptible to CTx-associated alterations in cancer patients. Billiet et al. reported that adult survivors with non-irradiated childhood leukemia showed altered brain functional connectivity of the DMN for CTx-induced brain injury [39]. Kesler et al. showed that there were significant differences in the DMN functional connectivity between breast cancer patients after CTx and before CTx and a normal group [5]. Simo et al. showed that CTx-induced cognitive deficits were related to DMN decreased connectivity abnormalities in lung cancer patients [10]. Our research evaluated intrinsic brain activity in the whole brain instead of investigating DMN functional connectivity. Our results of decreased intrinsic brain activity in the DMN were consistent with previous reports about lower DMN functional connectivity after CTx. Our findings provide additional evidence that the DMN is sensitive to CTx-associated alterations.
Interestingly, an increased ReHo in the right middle occipital gyrus was found in our study. We speculate that this result might be attributed to compensatory efforts which, if brain activity of one region decreases, brain activity of another region will increase. When nerves are damaged, compensation mechanisms might be activated first, and then new neural network will gradually form to produce functional replacement, such that the cognitive function decline can be improved to some extent [40]. However, further work is needed to prove this hypothesis.
We found no significant correlations between altered intrinsic activity values and MoCA scores. Our results were inconsistent with related literatures. Zhang et al. found decreased functional connectivity of lung cancer patients with CTx was positively relevant to reduced MoCA scores [38]. In addition, Kim et al. observed that the executive function of gastric cancer patients after CTx were significantly related to lower ALFF in left inferior frontal gyrus [31]. Based on the aforementioned result, we think that possible associations between altered intrinsic activity values and MoCA scores were not found because of the small sample of our study. In addition, our study only looked at MoCA scores, not more comprehensive neuropsychological testing.
We also observed that the patients before CTx showed altered intrinsic brain activity values in the frontal and parietal regions compared with the healthy group. These results suggest that cancer patients can have cognitive impairment before CTx. Previous literature reported that patients with small cell lung cancer had cognitive impairment before CTx [41]. Hu et al. revealed that altered brain functions in childhood acute lymphoblastic leukemia before CTx were associated with cognitive change and language [42]. According to the other study reports, patients before CTx have cognitive impairment possibly due to psychological distress [43] or effect of surgery [44].
There were several limitations to our pilot study. First, this research was a preliminary study, and the number of patients was small. In addition, the confounding effects of disease stage and CTx regimen might have limited the study findings, such that the correlation analyses did not obtain the desired result. In future studies, a larger sample size should be used to verify these results. A second limitation was that the scales used for neuropsychological assessment were not comprehensive. In future studies, patients should be given comprehensive neuropsychological assessments. The third limitation was that we studied the changes in the brain regions in lung cancer patients with CTx by adopting ALFF and ReHo methods. In future studies, we will use these regions as the seed regions for functional connectivity analysis. Future studies will also aim to investigate the alteration of intrinsic functional connectivity in lung cancer patients with CTx. A fourth limitation was related to frequency band. In a traditional ALFF study, a specific frequency band (0.01–0.08 Hz) is selected, and the frequency band is thought to have great correlation with nerve fluctuation. Zuo et al. found that ALFF had a significance difference in 2 sub-frequency bands groups [45]. Thus, it is important that the ALFF used to analyze the differences among lung cancer patients before and after CTx, and healthy controls, uses different sub-frequency bands group. This would be helpful in future studies to further explore the neural mechanism of cognitive impairment caused by CTx in lung cancer patients. As we did not do this in this study, we plan to carry out a detailed study on subsets of frequency bands in future work. A fifth limitation of our study is related to synchrony. Synchrony is the degree in which fluctuations behave similarly over time, whereas coherence is a way for measuring synchronization in frequency domain [46]. The ReHo methods included KCC-ReHo and coherence regional homogeneity (Cohe-ReHo). Cohe-ReHo has been proposed to measure regional synchronization in frequency domains. In our study, we used KCC-ReHo. Long et al. found that KCC-ReHo in the main areas of DMN was higher than in other brain regions [47]. Whereas, other researchers reported that Cohe-ReHo was superior to KCC-ReHo when there were large random noises due to phase delay among time series [46,48]. In future work, we will compare Cohe-ReHo with KCC-ReHo to explore which one is more suitable in our studies.
Conclusions
ALFF, ReHo, dALFF, and dReHo analyses revealed cognitive impairment associated with CTx for lung cancer patients. Patients with lung cancer after CTx showed alteration of intrinsic brain activity in the DMN compared to the healthy group. Thus, DMN damage could be a potential imaging biomarker to monitor brain changes in lung cancer patients receiving CTx.
Footnotes
Source of support: This work was supported by a grant from the Jiangsu Provincial Special Program of Medical Science (No. BE2017614)
Conflict of interests
None.
References
- 1.Brody H. Lung cancer. Nature. 2014;513(7517):S1. doi: 10.1038/513S1a. [DOI] [PubMed] [Google Scholar]
- 2.Li M, Caeyenberghs K. Longitudinal assessment of chemotherapy-induced changes in brain and cognitive functioning: A systematic review. Neurosci Biobehav Rev. 2018;92:304–17. doi: 10.1016/j.neubiorev.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Deprez S, Amant F, Yigit R, et al. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum Brain Mapp. 2011;32(3):480–93. doi: 10.1002/hbm.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng Y, Zhang XD, Zheng G, et al. Chemotherapy-induced brain changes in breast cancer survivors: Evaluation with multimodality magnetic resonance imaging. Brain Imaging Behav. 2019;13:1799–814. doi: 10.1007/s11682-019-00074-y. [DOI] [PubMed] [Google Scholar]
- 5.Kesler SR, Wefel JS, Hosseini SM, et al. Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proc Natl Acad Sci USA. 2013;110(28):11600–5. doi: 10.1073/pnas.1214551110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumas JA, Makarewicz J, Schaubhut GJ, et al. Chemotherapy altered brain functional connectivity in women with breast cancer: A pilot study. Brain Imaging Behav. 2013;7(4):524–32. doi: 10.1007/s11682-013-9244-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miao H, Chen X, Yan Y, et al. Functional connectivity change of brain default mode network in breast cancer patients after chemotherapy. Neuroradiology. 2016;58(9):921–28. doi: 10.1007/s00234-016-1708-8. [DOI] [PubMed] [Google Scholar]
- 8.Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Ou Y, Lv D, et al. Altered network homogeneity of the default-mode network in drug-naive obsessive-compulsive disorder. Progr Neuropsychopharmacol Biol Psychiatry. 2019;93:77–83. doi: 10.1016/j.pnpbp.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Simo M, Rifa-Ros X, Vaquero L, et al. Brain functional connectivity in lung cancer population: an exploratory study. Brain Imaging Behav. 2018;12(2):369–82. doi: 10.1007/s11682-017-9697-8. [DOI] [PubMed] [Google Scholar]
- 11.Bromis K, Gkiatis K, Karanasiou I, et al. Altered brain functional connectivity in small-cell lung cancer patients after chemotherapy treatment: A resting-state fMRI study. Comput Math Methods Med. 2017;2017 doi: 10.1155/2017/1403940. 1403940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simo M, Rifa-Ros X, Rodriguez-Fornells A, et al. Chemobrain: A systematic review of structural and functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37(8):1311–21. doi: 10.1016/j.neubiorev.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Dou B, Wang J, et al. Dynamic alterations of spontaneous neural activity in Parkinson’s disease: A resting-state fMRI Study. Front Neurol. 2019;10:1052. doi: 10.3389/fneur.2019.01052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu Y, Li Z, Lin Y, et al. Depression affects intrinsic brain activity in patients with mild cognitive impairment. Front Neurosci. 2019;13:1333. doi: 10.3389/fnins.2019.01333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 16.Zang YF, He Y, Zhu CZ, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29(2):83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Zang Y, Jiang T, Lu Y, et al. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22(1):394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 18.Lv Y, Li L, Song Y, et al. The local brain abnormalities in patients with transient ischemic attack: A resting-state fMRI Study. Front Neurosci. 2019;13:24. doi: 10.3389/fnins.2019.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Lu Q, Xiao E, et al. Methamphetamine enhances the development of schizophrenia in first-degree relatives of patients with schizophrenia. Can Psychiatry. 2014;59(2):107–13. doi: 10.1177/070674371405900206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuang C, Zha Y. Abnormal intrinsic functional activity in patients with cervical spondylotic myelopathy: A resting-state fMRI study. Neuropsychiatr Dis Treat. 2019;15:2371–83. doi: 10.2147/NDT.S209952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo J, Biswal BB, Han S, et al. Altered dynamics of brain segregation and integration in poststroke aphasia. Hum Brain Mapp. 2019;40(11):3398–409. doi: 10.1002/hbm.24605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang S, Meng Y, Li J, et al. Temporal dynamic changes of intrinsic brain activity in schizophrenia with cigarette smoking. Schizophr Res. 2019;210:66–72. doi: 10.1016/j.schres.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Yin D, Luo Y, Song F, et al. Functional reorganization associated with outcome in hand function after stroke revealed by regional homogeneity. Neuroradiology. 2013;55(6):761–70. doi: 10.1007/s00234-013-1146-9. [DOI] [PubMed] [Google Scholar]
- 24.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–99. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 25.Yan CG, Wang XD, Zuo XN, et al. DPABI: Data processing & analysis for (resting-state) brain imaging. Neuroinformatics. 2016;14(3):339–51. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Sun D, Shi Y, et al. Dynamic alterations in spontaneous neural activity in multiple brain networks in subacute stroke patients: A resting-state fMRI study. Front Neurosci. 2018;12:994. doi: 10.3389/fnins.2018.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu F, Wang Y, Li M, et al. Dynamic functional network connectivity in idiopathic generalized epilepsy with generalized tonic-clonic seizure. Hum Brain Mapp. 2017;38(2):957–73. doi: 10.1002/hbm.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu J, Li J, Huang X. The Beijing version of the Montreal Cognitive Assessment as a brief screening tool for mild cognitive impairment: A community-based study. BMC Psychiatry. 2012;12:156. doi: 10.1186/1471-244X-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghafar M, Miptah HN, O’Caoimh R. Cognitive screening instruments to identify vascular cognitive impairment: A systematic review. Int J Geriatr Psychiatry. 2019;34(8):1114–27. doi: 10.1002/gps.5136. [DOI] [PubMed] [Google Scholar]
- 30.Perrier J, Viard A, Levy C, et al. Longitudinal investigation of cognitive deficits in breast cancer patients and their gray matter correlates: Impact of education level. Brain Imaging Behav. 2020;14(1):226–41. doi: 10.1007/s11682-018-9991-0. [DOI] [PubMed] [Google Scholar]
- 31.Kim HG, Shin NY, Bak Y, et al. Altered intrinsic brain activity after chemotherapy in patients with gastric cancer: A preliminary study. Eur Radiol. 2017;27(7):2679–88. doi: 10.1007/s00330-016-4578-x. [DOI] [PubMed] [Google Scholar]
- 32.Chen BT, Jin T, Patel SK, et al. Intrinsic brain activity changes associated with adjuvant chemotherapy in older women with breast cancer: A pilot longitudinal study. Breast Cancer Res Treat. 2019;176(1):181–89. doi: 10.1007/s10549-019-05230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu Z, Tu Y, Di X, et al. Characterizing dynamic amplitude of low-frequency fluctuation and its relationship with dynamic functional connectivity: An application to schizophrenia. Neuroimage. 2018;180(Pt B):619–31. doi: 10.1016/j.neuroimage.2017.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruner E, Rangel de Lazaro G, de la Cuetara JM, et al. Midsagittal brain variation and MRI shape analysis of the precuneus in adult individuals. J Anat. 2014;224(4):367–76. doi: 10.1111/joa.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Utevsky AV, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. J Neurosci. 2014;34(3):932–40. doi: 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(Pt 1):12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhi M, Hou Z, We Q, et al. Abnormal spontaneous brain activity is associated with impaired emotion and cognition in hyperthyroidism: A rs-fMRI study. Behav Brain Res. 2018;351:188–94. doi: 10.1016/j.bbr.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Chen YC, Hu L, et al. Chemotherapy-induced functional changes of the default mode network in patients with lung cancer. Brain Imaging Behav. :2019. doi: 10.1007/s11682-018-0030-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Billiet T, Elens I, Sleurs C, et al. Brain Connectivity and cognitive flexibility in nonirradiated adult survivors of childhood leukemia. J Natl Cancer Inst. 2018;110(8):905–13. doi: 10.1093/jnci/djy009. [DOI] [PubMed] [Google Scholar]
- 40.Sun Y, Dai Z, Li Y, et al. Subjective cognitive decline: mapping functional and structural brain changes-a combined resting-state functional and structural MR imaging study. Radiology. 2016;281(1):185–92. doi: 10.1148/radiol.2016151771. [DOI] [PubMed] [Google Scholar]
- 41.Meyers CA, Byrne KS, Komaki R. Cognitive deficits in patients with small cell lung cancer before and after chemotherapy. Lung Cancer. 1995;12(3):231–35. doi: 10.1016/0169-5002(95)00446-8. [DOI] [PubMed] [Google Scholar]
- 42.Hu Z, Zou D, Mai H, et al. Altered brain function in new onset childhood acute lymphoblastic leukemia before chemotherapy: A resting-state fMRI study. Brain Dev. 2017;39(9):743–50. doi: 10.1016/j.braindev.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Berman MG, Askren MK, Jung M, et al. Pretreatment worry and neurocognitive responses in women with breast cancer. Health Psychol. 2014;33(3):222–31. doi: 10.1037/a0033425. [DOI] [PubMed] [Google Scholar]
- 44.Hedayati E, Schedin A, Nyman H, et al. The effects of breast cancer diagnosis and surgery on cognitive functions. Acta Oncol. 2011;50(7):1027–36. doi: 10.3109/0284186X.2011.572911. [DOI] [PubMed] [Google Scholar]
- 45.Zuo XN, Di Martino A, Kelly C, et al. The oscillating brain: complex and reliable. Neuroimage. 2010;49(2):1432–45. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu D, Yan C, Ren J, et al. Using coherence to measure regional homogeneity of resting-state FMRI signal. Front Syst Neurosci. 2010;4:24. doi: 10.3389/fnsys.2010.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Long XY, Zuo XN, Kiviniemi V, et al. Default mode network as revealed with multiple methods for resting-state functional MRI analysis. J Neurosci Methods. 2008;171(2):349–55. doi: 10.1016/j.jneumeth.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 48.Chang J, Yu R. Acute social stress modulates coherence regional homogeneity. Brain Imaging Behav. 2019;13(3):762–70. doi: 10.1007/s11682-018-9898-9. [DOI] [PubMed] [Google Scholar]