Abstract
Background
Osteogenesis of bone marrow mesenchymal stem cells (BMSCs) is an important research topic in the application of bone tissue engineering. Bone morphogenetic protein-1 (BMP-1) is important in bone formation and stability, but its effects on the osteogenesis of BMSCs are unclear. This study aimed to investigate the association of BMP-1 with the osteogenic capacity of BMSCs.
Material/Methods
Primary rabbit BMSCs were cultured and divided into a BMP-1-overexpressing group, a Green Fluorescent Protein-expressing (GFP) group, and a Control group. The transfection efficiency of BMP-1 was tested by Western blotting. Cell viabilities, alkaline phosphatase (ALP) activities, Ca2+ concentrations, and gross examinations of BMSC sheets were examined at different times. The osteogenic marker collagen I was assessed by immunohistochemical analysis.
Results
The cell viability, ALP activity, and Ca2+ content of the BMP1-overexpressed group were significantly enhanced compared with the GFP group and Control group. Immunohistochemistry staining results showed that BMP-1 promoted the expression of type I collagen in BMSCs sheets.
Conclusions
Our results suggest that the overexpression of BMP-1 can promote the osteogenesis of BMSCs and provides an improved method of cell-based tissue engineering.
MeSH Keywords: Alkaline Phosphatase, Bone Morphogenetic Protein 1, Immunohistochemistry
Background
With the deepening of stem cell and regenerative medicine research, cell-based tissue engineering bone research and development have become the fastest growing and most active field [1]. Bone marrow mesenchymal stem cells (BMSCs) are considered to be clinically valuable tissue-engineered seeding cells due to their multi-directional differentiation potential [2]. Their effects on osteogenesis and bone repair have been extensively studied [3,4]. However, researchers found that BMSCs are only partially transformed into osteoblasts after transplantation, so the osteogenic capacity is weak [5]. Therefore, enhancing the osteogenic differentiation of BMSCs to improve their osteogenic efficiency is an important research focus.
Bone morphogenetic proteins (BMPs), as osteogenic factors, have been verified to play important roles in bone remodeling and regeneration [6]. More than 20 kinds of BMPs have been identified [7], and some of them, like BMP2 and BMP7, have been experimentally confirmed to play an essential role in osteogenesis [8,9]. Bone morphogenetic protein-1 (BMP-1) is an important bone morphogenetic factor. The research interest in BMP-1 is in proteolytic removal of the C-propeptides to make the major fibrillar collagen types I–III stable, and it is essential for the assembly of mature collagen monomers into fibrils [10]. Deficiency of BMP-1 delays cleavage of type I procollagen C-propeptide and hampers the processing of the small leucine-rich proteoglycan [11]. Also, it is importance for bone formation and stability, and the molecular and cellular bases of BMP-1-dependent osteogenesis has been defined [12]. Wang et al. [13] knocked out the BMP-1/mammalian tolloid-like 1 (TLL1) gene of mice and found the crucial roles of BMP-1/TLL1 in root formation and dentin mineralization. Many studies have also reported that BMP-1 is involved in bone diseases [14,15], but the effects of BMP-1 on osteogenesis of BMSCs have been unclear.
In the present study, BMP-1 was overexpressed in a recombinant lentivirus carrying the BMP-1 gene, and the transfection efficiency was verified. Then, cell viability of BMSCs, gross examinations, ALP activity, Ca2+ concentration, and the expression of collagen I were assessed. The role of BMP-1 in the osteogenesis of BMSCs was verified.
Material and Methods
Cell cultures
Rabbit BMSCs were extracted according to published methods with some modification [16]. Briefly, the bilateral tibia of New Zealand rabbits was excised and dissected on a clean bench. The medullary cavity was flushed out with low-glucose Dulbecco’s modified Eagle’s medium (DMEM) containing 200 U/mL heparin. After filtration using a mesh, it was centrifuged at 800 rpm for 5 min. The upper-fat droplets and the supernatant were discarded, and 20 mL of a low-sugar DMEM (containing 10% fetal bovine serum, 100 U/mL penicillin, and 0.27 g/L of L-glutamine) was added. It was gently blown into a single-cell suspension and uniformly inoculated into 2 Petri dishes having a diameter of 10 cm. The culture solution in each dish was added to 12 mL and placed in an incubator at 37°C and 5% CO2. Half of the culture solution was replaced 48 h after incubation. Then, the entire culture solution and floating cells were removed after another 48 h. The remaining adherent cells were defined as BMSCs [17]. Then, every 2 days, the entire culture solution was changed once, and cell growth was observed under an inverted microscope.
BMP-1 transfection
Lentivirus vectors were constructed with the assistance of Genechem (Shanghai, China). Lentivirus packaging was performed using 293 cells according to the manufacturer’s instructions and then screened for the appropriate multiplicity of infection (MOI=10). All cells were divided into 3 groups. The BMP-1 group was transfected with Lenti-BMP-1, the GFP group was transfected with blank lentivirus vectors, and the Control group remained untransfected. The effects of transfection and the cell conditions were observed by fluorescence microscopy.
Transfection efficiency verified by Western blotting
The proteins of the 3 groups were extracted, and Western blotting (WB) was used to verify the transfection efficiency. We took 30 μg of protein from the Control group, GPF group, and BMP-1 group, added 5×protein loading buffer corresponding to 1/4 volume of a protein sample, boiled it for 5 min in a boiling water bath, and then performed SDS-PAGE. Proteins were separated by electrophoresis (5% laminating gel, 10% separation gel, laminated glue voltage 80 V, separation gel voltage 100 V, electrophoresis time 120 min). The protein was transferred to a polyvinylidene difluoride (PVDF) membrane by wet transfer method (constant pressure 100 V, transfer film 150 min, transfer buffer methanol concentration 20%). After the transfer, the PVDF membrane was stained in Ponceau red staining solution for 2–5 min to observe the protein transfer effect. The upper right corner of the PVDF membrane was labeled, and the PVDF membrane was transferred to a TBST (Tween-20 concentration 0.05%) blocking solution containing 5% BSA and blocked at room temperature for 1.5 h. The antibody BMP-1 (ab205394) was diluted 1: 1000, and the antibody β-actin (ab8226) was diluted 1: 500 in a blocking solution. The PVDF membrane was placed in a dish, and a dilution of the primary antibody was added for overnight incubation at 4°C. The next day, the membrane was washed with TBST for 10 min×4 times. The PVDF membrane was placed in a dish containing diluted secondary antibody and incubated for 1 h on a decolorizing shaker. After incubation, the membrane was washed with TBST for 10 min×3 times. In a dark room, the liquid on the membrane was gently blotted with a filter paper, and allowed to react for 2–3 min in the enhanced chemiluminescence reaction mixture, and then developed by tableting.
Cell sheets preparation
The harvested BMSCs were inoculated into a petri dish at a density of 1×105 cells/cm2. Incubation was continued for 2 days (inoculation of cells adherent) with low-glucose DMEM containing 10% fetal bovine serum. Then, the culture solution was changed to a high-glucose DMEM-inducing culture solution containing an osteogenic inducer containing 10% fetal bovine serum, dexamethasone 8 mol/L, β-glycerophosphate 10 mmol/L, and ascorbic acid 50 mg/L. The cultivation was continued at 37°C with 5% CO2. The culture solution was changed once every 2–3 days. After 2 weeks of continuous culture, a translucent milky cell sheet was observed at the bottom of the culture dish. The gross examination of cell sheets was observed in a general view, and the thickness of the cell membrane was measured under a microscope.
Cell viability
Cell viability assays were performed using the Cell Counting Kit-8 (CCK-8) kit. The cell suspension (100 μL/well) was seeded in a 96-well plate and pre-cultured in an incubator (37°C, 5% CO2). After inoculation of cells, 10 μL of CCK solution was added on days 2, 3, 4, 5, 6, 7, 8, 9, and 10, and after 4 h of culture, the absorbance value was measured at 450 nm. The cell viability of each group was calculated by a standard curve.
Alkaline phosphatase activity
The activity of ALP was detected according to the instructions of the alkaline phosphatase assay kit (Beyotime). Cell sheets of the BMP-1 group, GFP group, and Control group were obtained and minced. The cells were rinsed with PBS and lysed with 0.2% Triton X-100. The samples were centrifuged, and the supernatant was removed. The reaction solution was added and allowed to react at 37°C for 30 min. The absorbance of light was measured by an enzyme-linked detector (405 nm wavelength) for quantitative detection of ALP activity. The alkaline phosphatase activity in the sample was calculated with reference to a standard curve. Each sample was measured 3 times.
Calcium concentration examinations
The calcium concentration was detected according to the instructions of the calcium concentration assay kit (Beyotime). Cell sheets of the BMP-1 group, GFP group, and Control group were obtained and minced. The lysis buffer was added in a ratio of 100–200 μl per 20 mg of tissue. The sample was homogenized until fully cleaved. The supernatant was collected after centrifugation at 10 000–14 000 g for 3–5 min at 4°C, then 50 μl of standard (for standard curve) or sample was added to each well in a 96-well plate. We added 150 μl of the test solution and incubated it at room temperature for 5–10 min in the dark. The absorbance was measured at 575 nm. The calcium concentration in the sample was calculated with reference to a standard curve. Each sample was measured 3 times.
Histochemical analysis
Hematoxylin-eosin staining
The cultured sheets were randomly selected and fixed with 4% paraformaldehyde. After gradient alcohol dehydration and conventional paraffin embedding, the sheets were cut into 5-μm-thick sections. Tissue structure was observed using conventional hematoxylin-eosin staining (H&E). At the same time, we selected the “Z” axis profile of the sheets. The thickness of the cell membrane was measured under a microscope at randomly selected points and averaged.
Immunohistochemistry
Cell sheets were fixed with 4% paraformaldehyde and cut into 5-μm paraffin sections. The sections were heated at 60°C for 1 h, and then rehydrated with a gradient of xylene and absolute ethanol, and washed with distilled water for 1 min. The sections were placed in citrate (pH 6.0) antigen retrieval solution for high-pressure repair for 2 min, followed by washing with PBS 3 times for 5 min each time, after which 3% H2O2 was added dropwise, and then the sections were placed in a wet box, allowed to stand at room temperature for 30 min, and washed again with PBS. After the goat serum blocking solution was added dropwise, the sections were placed in a wet box, allowed to stand at room temperature for 1 h, and decanted without washing. The primary antibody diluted 1: 500 was added to the tissue and left overnight in a 4°C wet box. The next day, after rewarming for 30 min, the primary antibody was aspirated and washed with PBS. The biotinylated secondary antibody working solution was added to the tissue and placed in a wet box at room temperature for 30 min. We discarded the secondary antibody and washed the sections 4 times in PBS for 5 min each time. HRP-labeled streptavidin working solution was added to the sections, and the sections were placed in a wet box at room temperature for 20 min. Freshly prepared DAB coloring solution was added dropwise to the sections and sealed with neutral gum. The color development time was controlled under the microscope, and the positive color was brown.
Statistical analysis
All data are expressed as mean values with standard deviation (mean±SD). Differences in the data among each group were analyzed by one-way analysis or two-way analysis of variance (ANOVA) using SPSS version 19.0. P<0.05 was considered statistically significant.
Results
Transfection efficiency
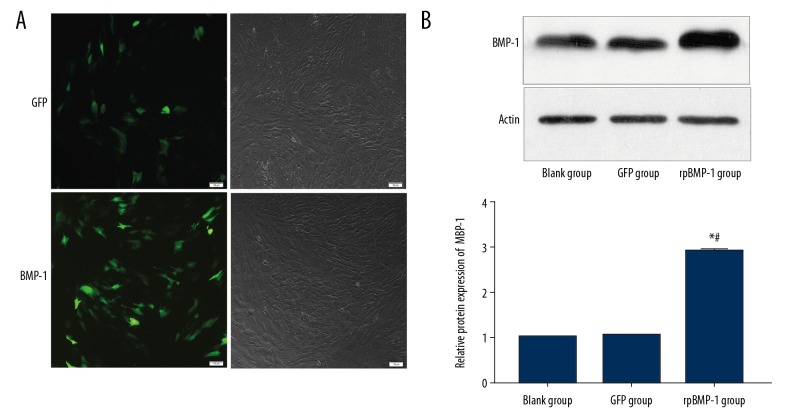
After transfection of the recombined plasmid of BMP-1 or GFP, we verified the transfection efficiencies. The fluorescence pictures in Figure 1A show that the BMP-1 or GFP had been successful transfected. Also, the WB results in Figure 1B show that the expressions of BMP-1 proteins in the BMP-1 group were markedly upregulated compared with the GFP group and Control group.
Figure 1.
Transfection efficiency was assessed by fluorescence microscopy and Western blotting. BMP-1 and GFP genes were transfected successfully (A). The expressions of BMP-1 protein was increased after transfection (B). * p<0.05 compared with the Control group, # p<0.05 compared with the GFP group.
Gross examination of BMSCs sheets
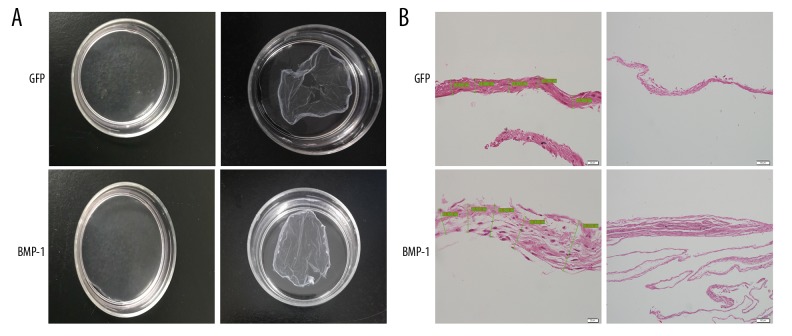
After inoculation into the culture dish, the cells gradually grew over the bottom of the culture dish, the layer became thicker, and the transmittance of the cells was gradually reduced as assessed under the microscope. The gross examination of BMSCs sheets is shown in Figure 2A, demonstrating that the sheet in the BMP-1 group (60.37±8.28) was markedly thicker than in the GFP group (23.81±0.77) (Figure 2B).
Figure 2.
Gross examination of BMSCs sheets. The cells layered multiply and the transmittance of the cells were gradually reduced, as seen under the microscope (A). The sheets were thicker in the BMP-1 group compared with the GFP group (B).
Cell viability, ALP activity, and calcium concentration of BMSCs
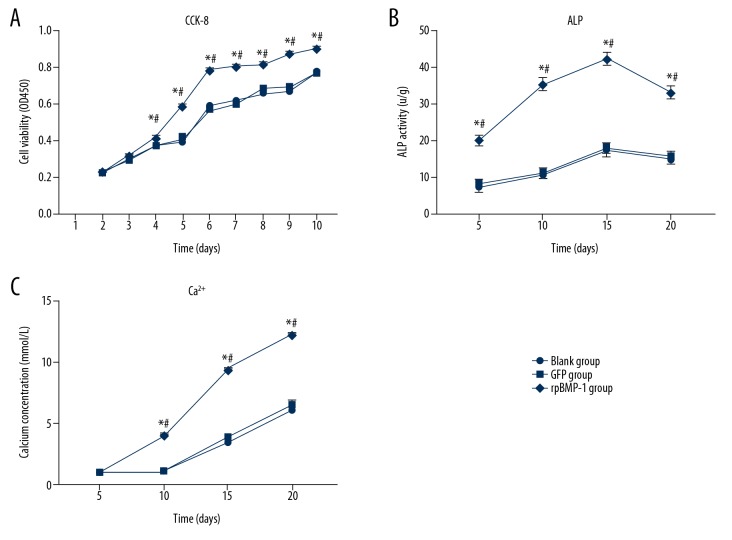
The cell viabilities of 3 groups at the different timepoints are shown in Figure 3A. From day 4, the cell viability of the BMP-1 group significantly increased relative to the GFP group and Control group. There was no difference between the GFP group and Control group.
Figure 3.
Cell viability, ALP activity, and Ca2+ concentration of BMSCs were assessed. The cell viability (A), ALP activity (B), and Ca2+ concentration (C) of the BMP-1 group were significantly higher compared with the GFP group and Control group. The assays were repeated 3 times. * p<0.05 compared with the Control group, # p<0.05 compared with GFP group.
During incubation of the cell sheets, we examined the osteogenic-related indicators ALP, Ca2+, and type I collagen at different timepoints. As shown in Figure 3B, the ALP activity of the BMP-1 group was significantly upregulated compared with the GFP and Control group at all timepoints, which means the overexpression of BMP-1 promotes the differentiation of pre-osteoblasts into mature osteoblasts. There was no difference between the GFP group and Control group.
The Ca2+ concentrations in the 3 groups were assessed, and the results are shown in Figure 3C. From the day 10, the Ca2+ concentration of the BMP-1 group showed significant increases compared with the GFP and Control group, which means that the mineralization of the BMP-1 group was significantly higher than in the other 2 groups.
Collagen I expression of BMSCs sheets
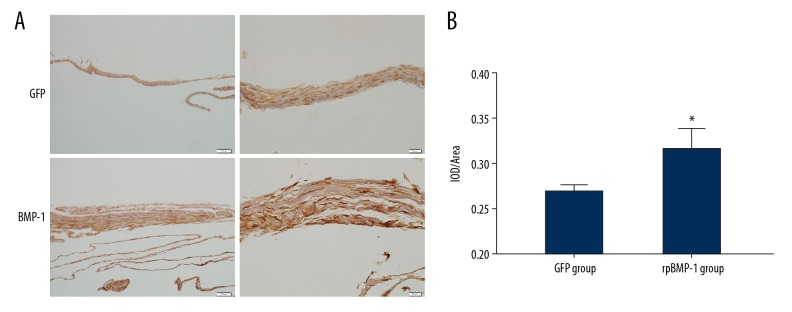
Type I collagen is the most crucial fibrous collagen component in bone matrix. It is a specific and important indicator of osteogenesis. The expression of type I collagen was detected by immunohistochemistry, and the results are presented in Figure 4. The pictures show that the expression of type I collagen of BMP-1 group was markedly increased compared with the GFP group.
Figure 4.
(A, B) Collagen I expression of BMSCs sheets. After transfection of BMP-1, the expression of collagen I was markedly increased compared with the GFP group. * p<0.05 compared with the GFP group.
Discussion
Due to its multiple-differentiation potential, BMSCs are extensively used in cell-based tissue engineering research [18]. BMSCs have been used in cell substitution therapy and tissue engineering, especially in tissue-engineered bone [19,20]. There are also many studies that enhanced the osteogenic efficiency of BMSCs by gene editing [21,22]. In the present study we overexpressed BMP-1 by gene editing and determined its role in the osteogenesis of BMSCs. We extracted BMSCs from rabbit tibia. Then, BMP-1 was overexpressed using lentiviral vectors, and the fluorescence results and WB results showed that the transfection efficiency was high. We compared the gross examinations of cell sheets in the Control group, the GFP group, and the BMP-1 overexpression group, then we examined the osteogenesis-related indicators, ALP, GA2+ concentration, and COL I expression. In general, we demonstrated that overexpression of BMP-1 promoted the osteogenesis of BMSCs.
BMPs (also known as cytokines) regulate osteogenesis during the differentiation of BMSCs to osteoblasts [23,24]. BMP-2, BMP-4, and BMP-7 have been widely studied, showing that recombinant human BMP-4 more strongly promotes spinal fusion than does recombinant human BMP-2 [25,26]. BMP-1 is not a typical BMP and has received little research attention. Some clinical studies showed that mutation of BMP-1 can lead to osteogenesis imperfecta [27], and osteogenesis imperfecta patients with BMP-1 mutations tend to have recurrent fractures, generalized bone deformity, or osteopenia [28]. Moreover, the importance of BMP-1 for bone formation and stability has been established [12]. We hypothesized and demonstrated that overexpressed BMP-1 can promote the osteogenesis of BMSCs.
Lentivirus is a type of retrovirus that has a broad host range and is capable of infecting both dividing cells and non-dividing cells. Compared to other virus tools, lentivirus has the following advantages: a) it can achieve long-term stable expression of the target gene by integrating the foreign gene into the host cell genome, and is not lost as the cell divides and passes; b) it is very safe and has no pathogenicity; and c) it has low immunogenicity and can be directly injected into living tissue to cause an immune reaction, which is suitable for animal experiments. In addition, some studies showed that lentiviral vectors are superior to other vectors [29]. Thus, in the present study, we used lentiviral vectors for transfection. Regarding the other transfection vectors, one study used the Adeno-associated virus (AAV) virus vector system to construct the AAV-BMP-4/7 vector, making the screening of recombinant vectors more straightforward, and they successfully built the recombinant AAV-BMP-4/7 virus that carried the BMP-4/7 fusion gene in a relatively short time [30]. This may provide a new tool for BMPs-related gene research.
As in other studies on BMSCs, obtaining an adequate amount of BMSCs was difficult. The content of BMSCs with osteogenic potential is only 0.001–0.01% of the total amount of bone marrow nucleated cells. Further separation and purification are required to obtain a sufficient concentration of BMSCs, and the proliferation and differentiation abilities of BMSCs are reduced by in vitro culture and expansion [31]. A study suggested that mesenchymal stem cells (MSCs) can be isolated from virtually any tissue and can form cartilage, bone, and fat [32]. However, some researchers pointed out that MSCs are rarely applied to clonal populations, and the effects on bone formation cannot be proven by BMP treatment or genetic engineering [33].
Many studies focus on treating cells with the forced expression of bone morphogenetic proteins by gene editing, but this genetic technic has limits. Stephan et al. [34]analyzed the maximal effects of BMP-2 on osteochondral remodeling, and found that BMP-2 could not induce complete healing despite stable expression. How to ensure the efficiency and stability of gene editing technology application is an important issue. The combination with bone tissue engineering technology had shown potential. Eguchi et al. [35] showed that composite materials containing recombinant human BMP-2 increased the generation of bridging callus and the callus mass in the bone defect. However, the immunogenicity of the carrier material and the toxicity of degradation products are also factors that influence the technical effect. The use of gene editing technology and improvement of the combination of gene editing with bioengineering technology warrant further research.
Conclusions
This study confirmed that the overexpression of BMP-1 can promote the osteogenesis of BMSCs. We showed that BMP-1 has the biological ability to induce BMSCs to form bone, which provides a potential method of cell-based tissue engineering.
Footnotes
Source of support: Departmental sources
Conflict of interests
None.
References
- 1.Knight MA, Evans GR. Tissue engineering: progress and challenges. Plastic and Reconstructive Surgery. 2004;114(2):26e–37e. [Google Scholar]
- 2.Guillot PV, Cui W, Fisk NM, Polak DJ. Stem cell differentiation and expansion for clinical applications of tissue engineering. J Cell Mol Med. 2007;11(5):935–44. doi: 10.1111/j.1582-4934.2007.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El Hadidi YN, El Kassaby M, Ahmed SAEF, Khamis NS. Effect of mesenchymal stem cell application on the distracted bone microstructure: An experimental study. J Oral Maxillofac Surg. 2016;74(7):1463e1–e11. doi: 10.1016/j.joms.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Aykan A, Ozturk S, Sahin I, et al. Biomechanical analysis of the effect of mesenchymal stem cells on mandibular distraction osteogenesis. J Craniofac Surg. 2013;24(2):e169–e75. doi: 10.1097/SCS.0b013e31827c8706. [DOI] [PubMed] [Google Scholar]
- 5.Peters A, Toben D, Lienau J, et al. Locally applied osteogenic predifferentiated progenitor cells are more effective than undifferentiated mesenchymal stem cells in the treatment of delayed bone healing. Tissue Eng Part A. 2009;15(10):2947–54. doi: 10.1089/ten.TEA.2009.0058. [DOI] [PubMed] [Google Scholar]
- 6.Ducy P, Schinke T, Karsenty G. The osteoblast: A sophisticated fibroblast under central surveillance. Science. 2000;289(5484):1501–4. doi: 10.1126/science.289.5484.1501. [DOI] [PubMed] [Google Scholar]
- 7.Wozney JM, Rosen V, Celeste AJ, et al. Novel regulators of bone formation: Molecular clones and activities. Science. 1988;242(4885):1528–34. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 8.Castro-Govea Y, Cervantes-Kardasch VH, Borrego-Soto G, et al. Human bone morphogenetic protein 2–transduced mesenchymal stem cells improve bone regeneration in a model of mandible distraction surgery. J Craniofac Surg. 2012;23(2):392–96. doi: 10.1097/SCS.0b013e318240fe9b. [DOI] [PubMed] [Google Scholar]
- 9.Hu J, Qi M, Zou S, et al. Callus formation enhanced by BMP-7 ex vivo gene therapy during distraction osteogenesis in rats. J Orthop Res. 2007;25(2):241–51. doi: 10.1002/jor.20288. [DOI] [PubMed] [Google Scholar]
- 10.Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 2005;118(7):1341–53. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- 11.Syx D, Guillemyn B, Symoens S, et al. Defective proteolytic processing of fibrillar procollagens and prodecorin due to biallelic BMP1 mutations results in a severe, progressive form of osteogenesis imperfecta. J Bone Miner Res. 2015;30(8):1445–56. doi: 10.1002/jbmr.2473. [DOI] [PubMed] [Google Scholar]
- 12.Asharani P, Keupp K, Semler O, et al. Attenuated BMP1 function compromises osteogenesis, leading to bone fragility in humans and zebrafish. Am J Hum Genet. 2012;90(4):661–74. doi: 10.1016/j.ajhg.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Muir AM, Ren Y, Massoudi D, et al. Essential roles of bone morphogenetic protein-1 and mammalian tolloid-like 1 in postnatal root dentin formation. J Endod. 2017;43(1):109–15. doi: 10.1016/j.joen.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valadares ER, Carneiro TB, Santos PM, et al. What is new in genetics and osteogenesis imperfecta classification? J Pediatr (Rio J) 2014;90(6):536–41. doi: 10.1016/j.jped.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Sakata T, Elalieh HZ, et al. Gender differences in the response of CD-1 mouse bone to parathyroid hormone: potential role of IGF-I. J Endocrinol. 2006;189(2):279–87. doi: 10.1677/joe.1.06351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma D, Zhong C, Yao H, et al. Engineering injectable bone using bone marrow stromal cell aggregates. Stem Cells Dev. 2010;20(6):989–99. doi: 10.1089/scd.2010.0348. [DOI] [PubMed] [Google Scholar]
- 17.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 18.Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. Cell-based bone tissue engineering. PLoS Med. 2007;4(2):e9. doi: 10.1371/journal.pmed.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamashita M, Otsuka F, Mukai T, et al. Simvastatin antagonizes tumor necrosis factor-α inhibition of bone morphogenetic proteins-2-induced osteoblast differentiation by regulating Smad signaling and Ras/Rho-mitogen-activated protein kinase pathway. J Endocrinol. 2008;196(3):601–13. doi: 10.1677/JOE-07-0532. [DOI] [PubMed] [Google Scholar]
- 20.Cancedda R, Giannoni P, Mastrogiacomo M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials. 2007;28(29):4240–50. doi: 10.1016/j.biomaterials.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 21.Zhang WB, Zheng LW, Chua DTT, Cheung LK. Treatment of irradiated mandibles with mesenchymal stem cells transfected with bone morphogenetic protein 2/7. J Oral Maxillofac Surg. 2012;70(7):1711–16. doi: 10.1016/j.joms.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Zeng J, Guo P, Zhou N, et al. Treatment of large bone defects with a novel biological transport disc in non-vascular transport distraction osteogenesis. Int J Oral Maxillofac Surg. 2016;45(5):670–77. doi: 10.1016/j.ijom.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Hermes M, Osswald H, Mattar J, Kloor D. Influence of an altered methylation potential on mRNA methylation and gene expression in HepG2 cells. Exp Cell Res. 2004;294(2):325–34. doi: 10.1016/j.yexcr.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Guo J, Wu G. The signaling and functions of heterodimeric bone morphogenetic proteins. Cytokine Growth Factor Rev. 2012;23(1–2):61–67. doi: 10.1016/j.cytogfr.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Gregory CA, Prockop DJ, Spees JL. Non-hematopoietic bone marrow stem cells: molecular control of expansion and differentiation. Exp Cell Res. 2005;306(2):330–35. doi: 10.1016/j.yexcr.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Hou X, Wu X. [Experimental research on spine fusion induced by tissue engineered bone]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2003;25(1):39–42. [in Chinese] [PubMed] [Google Scholar]
- 27.Pollitt RC, Saraff V, Dalton A, et al. Phenotypic variability in patients with osteogenesis imperfecta caused by BMP1 mutations. Am J Med Genet A. 2016;170(12):3150–56. doi: 10.1002/ajmg.a.37958. [DOI] [PubMed] [Google Scholar]
- 28.Martínez-Glez V, Valencia M, Caparrós-Martín JA, et al. Identification of a mutation causing deficient BMP1/mTLD proteolytic activity in autosomal recessive osteogenesis imperfecta. Hum Mut. 2012;33:343–50. doi: 10.1002/humu.21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson DR. Viral-mediated gene transfer for cancer treatment. Curr Pharm Biotechnol. 2002;3(2):151–64. doi: 10.2174/1389201023378445. [DOI] [PubMed] [Google Scholar]
- 30.Yuan SH, Bi Z. Effect of recombinant adeno-associated BMP-4/7 fusion gene on the biology of BMSCs. Mol Med Rep. 2012;6(6):1413–17. doi: 10.3892/mmr.2012.1090. [DOI] [PubMed] [Google Scholar]
- 31.Morcos MW, Al-Jallad H, Hamdy R. Comprehensive review of adipose stem cells and their implication in distraction osteogenesis and bone regeneration. Biomed Res Int. 2015;2015 doi: 10.1155/2015/842975. 842975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(11):2204–13. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 33.Robey P. “Mesenchymal stem cells”: Fact or fiction, and implications in their therapeutic use. F1000Res. 2017;6 doi: 10.12688/f1000research.10955.1. pii: F1000 Faculty Rev-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogt S, Wexel G, Tischer T, et al. The influence of the stable expression of BMP2 in fibrin clots on the remodelling and repair of osteochondral defects. Biomaterials. 2009;30(12):2385–92. doi: 10.1016/j.biomaterials.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Eguchi Y, Wakitani S, Naka Y, et al. An injectable composite material containing bone morphogenetic protein-2 shortens the period of distraction osteogenesis in vivo. J Orthop Res. 2011;29(3):452–56. doi: 10.1002/jor.21225. [DOI] [PubMed] [Google Scholar]