Abstract
Background
Chronic obstructive pulmonary disease (COPD) is characterised by progressive airflow obstruction, worsening exercise performance and health deterioration. It is associated with significant morbidity, mortality and health system burden.
Objectives
To evaluate the effectiveness of outreach respiratory health care worker programmes for COPD patients in terms of improving lung function, exercise tolerance and health related quality of life (HRQL) of patient and carer, and reducing mortality and medical service utilisation.
Search methods
The Cochrane Airways Group Specialised Register of Trials was searched (November 2011). Study references were hand‐searched for additional studies we contacted study authors to identify other unpublished studies.
Selection criteria
We included only randomised controlled trials of COPD patients. We included interventions involving an outreach nurse visiting patients in their homes, providing support, education, monitoring health and liaising with physicians. Studies in which the therapeutic intervention under test was physical training were not included.
Data collection and analysis
Two reviewers independently assessed trial quality and extracted data. We contacted study authors for additional information.
Main results
We pooled mortality data from eight studies and found a non‐significant reduction in mortality at 12 months (OR 0.72, 95% CI 0.45 to, 1.15).
We pooled four studies that assessed disease‐specific heath‐related quality of life (HRQL) and found a statistically significant improvement in HRQL (mean difference ‐2.61, 95% CI ‐4.82 to ‐0.40).
Hospitalisations were reported in five studies. Although there was no statistically significant difference in the number of hospitalisations (OR 1.01, 95% CI 0.71 to 1.44), there was significant heterogeneity. Although this heterogeneity appeared to be caused by one outlying study with a statistically significant decrease in hospitalisations in patients receiving home care, whereas the other studies showed a non‐significant increase in hospitalisations, we could not draw firm conclusions about why this heterogeneity exists. Data on GP visits and emergency department presentations were available, however no consistent effect in these was observed with the intervention. The intervention also incurred higher health care costs than standard care as reported in a single study.
Very few studies provided data on lung function or exercise performance, so there was insufficient evidence to assess impact on these outcomes.
Authors' conclusions
Outreach nursing programmes for COPD improved disease‐specific HRQL. However the effect on hospitalisations was heterogeneous, reducing admissions in one study, but increasing them in others, therefore we could not draw firm conclusions for this outcome.
Plain language summary
Does delivery of home care by outreach nurses improve outcomes for people with chronic obstructive pulmonary disease?
Home visits from nurses for people with chronic lung disease (chronic obstructive pulmonary disease, COPD ‐ a combinations of emphysema and chronic bronchitis) aim to help people maintain their health and reduce the need for hospital stays. The nurses delivering this care aim to help people use their treatments well, provide education about coping strategies, and monitor the lung disease. However, this review of nine randomised controlled trial found that home care resulted in an improvement in people's quality of life, but has an unpredictable effect on the risk of being admitted to hospital. We could only find information on the cost of care from one study, but this indicated that home care was an expensive form of care. More research is needed to confirm the usefulness of home visits for people with COPD.
Summary of findings
Summary of findings for the main comparison. Home care outreach nursing for patients with COPD.
| Home care outreach nursing for patients with COPD | ||||||
| Patient or population: patients with COPD Settings: Intervention: home care outreach nursing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Home care outreach nursing | |||||
| Mortality Follow‐up: 4‐12 months | Study population | OR 0.72 (0.45 to 1.15) | 711 (5 studies) | ⊕⊕⊝⊝ low1,2 |
1 Subjects not blinded due to the nature of the intervention 2 Wide confidence intervals that include the possibility of significant benefit or harm |
|
| 127 per 1000 | 95 per 1000 (61 to 143) | |||||
| SGRQ Total SGRQ (Total) Score. Scale from: 0 to 100. Lower score indicates better quality of life. Follow‐up: 3‐12 months | The mean SGRQ total in the control groups was ‐ 0.1 units | The mean SGRQ total in the intervention groups was 2.60 units lower (4.81 to 0.39 lower) | 587 (4 studies) | ⊕⊕⊝⊝ low1,2 |
1 Subjects not blinded due to the nature of the intervention 2 Wide confidence intervals |
|
| Hospitalisation Follow‐up: 3‐12 months | Study population | OR 1.01 (0.71 to 1.44) | 686 (5 studies) | ⊕⊕⊝⊝ low1,2 |
1 Subjects not blinded due to the nature of the intervention 2 Wide confidence intervals that include the possibility of significant benefit or harm |
|
| 480 per 1000 | 482 per 1000 (396 to 571) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Subjects not blinded due to the nature of the intervention
2 Wide confidence intervals that include the possibility of significant benefit or harm
Background
Chronic obstructive pulmonary disease (COPD) is associated with substantial morbidity and costs to the health care system. The prevalence of COPD is increasing in the community and represents a serious public health issue.
Outreach healthcare delivery in the community, given by a respiratory health worker, may benefit patients with COPD by encouraging self‐management behaviour with education about pulmonary disease, medication (in particular, the correct inhaler technique) and coping strategies. Also, regular visits, including objective measures of lung function, permits greater surveillance of deteriorations. The desired outcome of an outreach care programme is to maintain the patient's optimal respiratory state, thus maintaining health status and reducing hospital admissions. With increasing interest in outreach 'shared care' and 'coordinated care' programmes it is important to evaluate the available evidence as to whether such programmes improve the lives of patients with COPD and those who care for them.
Our aim was to update this systematic review evaluating the impact of outreach nursing care in patients with COPD.
Objectives
Search and critically appraise the relevant literature, in order to determine the strength of the evidence, that outreach respiratory nursing care may:
Improve lung function (as measured by FEV1) and exercise and tolerance;
Improve health related quality of life (HRQL) of patients with COPD;
Reduce mortality;
Reduce health care system costs;
Affect the quality of life of the principal carer at home.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials in which the home visits were provided by a respiratory nurse or similar respiratory health worker to patients with COPD.
Types of participants
We included participants with chronic obstructive pulmonary disease, as defined according to pulmonary function test findings, consistent with British Thoracic Society criteria (BTS 1997).
Types of interventions
We included interventions comprising home visits by a respiratory nurse or similar respiratory health worker, to facilitate health care, provide education, provide social support, identify respiratory deteriorations promptly and reinforce correct technique with inhaler therapy. Eligible control groups were patients who received routine care, without respiratory nurse/health worker input. We considered studies with co‐interventions, with subgroup analysis as necessary. We included only trials with at least three months of follow‐up as it this was considered an appropriate minimum duration of follow‐up to observe any clinically significant benefits of the intervention.
Types of outcome measures
Patient related: Pulmonary function and exercise tolerance, HRQL and mortality.
Costs to health care system: Hospital admissions, emergency department presentations, GP or family doctor visits and medical costs.
Carer related: HRQL and satisfaction.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Airways Group Specialised Register of trials which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane library), MEDLINE, EMBASE, CINAHL, AMED and PsychINFO, and handsearched respiratory journals and meeting abstracts (please see Appendix 1 for further details). The register contains a variety of studies published in foreign languages. We did not exclude trials on the basis of language.
All records in the Register coded as 'COPD' were searched with the following terms:
nurs* or healthcare* or "health care*" or "health provid*" or "health work*" or "health person*" or "home care*" or "home‐care*" or outreach* or out‐reach* or community*
The most recent search was conducted in November 2011.
Searching other resources
We reviewed reference lists of all included studies and of reviews to identify potentially relevant citations. We also made enquiries regarding other published or unpublished studies known to the authors of the included studies
Data collection and analysis
Selection of studies
From the title, abstract, or descriptors, one of us (CXW) independently reviewed the literature searches. We excluded all studies that were clearly not randomised controlled trials or that clearly did not fit the inclusion criteria. Two of us (CXW and KC) reviewed all other citations independently in full text, assessing for inclusion based on study design, population, intervention and outcome.
Data extraction and management
Two authors (CXW and KC) independently extracted data for the trials using a standardised data extraction form before data was entered into The Cochrane Collaboration software program RevMan 5. CXW corresponded with trialists to obtain missing and raw data.
Additional data were obtained from the authors of Littlejohns 1991. Unfortunately the principal author of Bergner 1988 has died. Additional data was sought from the five new studies without success.
Assessment of risk of bias in included studies
We assessed the risk of bias for allocation sequence generation, allocation concealment, blinding, handling of missing data, selective outcome reporting and other threats to validity in the studies. This is in line with the recommendations made in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Data synthesis
Data were entered in to RevMan 5. Continuous data were pooled with a fixed effect model as a weighted mean difference. Dichotomous data were pooled with a Peto Odds Ratio (OR). We prepared a summary of findings table for the 2011 update. Because primary outcomes were not specified, we chose to include mortality, HRQL (as measured by the SGRQ total score) and hospitalisations. This was a post hoc decision.
Results
Description of studies
Results of the search
The literature search returned 489 references. Fourty one references were identified from this search for retrieval and possible inclusion in the review, and 11 studies were obtained from the bibliographies of retrieved articles. One paper was identified through a personal communication with the author (Bergner 1988) and one abstract from conference proceedings was also identified for possible inclusion in the review. From these, nine papers corresponding to nine trials were selected for inclusion in the review. The updated literature search run in November 2011 returned 65 references of which none were eligible for inclusion.
Included studies
The nine included studies were published between 1987 and 2006. Three studies originated from the U.S.A. (Aiken 2006; Bergner 1988; Coultas 2005), two from the U.K. (Cockcroft 1987; Littlejohns 1991), two from Australia (Hermiz 2002; Smith 1999), and one each from Canada (Bourbeau 2003) and Hong Kong (Kwok 2004). For full details of the trials, see Characteristics of included studies.
A total of 1498 participants were included in these nine studies. Participants had moderately severe disease as assessed by inclusion criteria such as patient symptoms, recent exacerbations, hospitalisations and spirometry results. Published details on baseline severity for all the studies are available in Table 3.
1. Baseline lung function (FEV1 %predicted) and inclusion criteria related to exacerbation frequency.
| Study | Baseline FEV1 %predicted | Exacerbation frequency |
| Aiken 2006 | Not reported | Patients had recent exacerbations as evidenced by treatment in an emergency department, urgent care facility, or hospital within the 3 months prior to enrolment. Participants averaged 0.12 emergency department visits per month (SD 0.18) in the previous 6 months. Control participants averaged 0.11 ED visits per month (SD 0.20). |
| Bergner 1988 | 34% | FEV1 <60% predicted Average 11.7 hospital days in previous year |
| Bourbeau 2003 | FEV1 1 L | stable COPD (respiratory symptoms and medication unchanged for at least 4 weeks before enrolment) FEV1 after the use of a bronchodilator between 25% and 70% of the predicted normal value There were approx 1.6 acute exacerbation visits per person in the year previous to study entry |
| Cockcroft 1987 | FEV1 0.8 L | Patients who had been admitted to hospital at least twice during the previous three years and new patients who had been seen during the past year were eligible |
| Coultas 2005 | 40% patients stage IIA (≥50<80%) 44% IIB (≥30<50%) 16% III (<30%) |
FEV1< 80% predicted |
| Hermiz 2002 | Not reported | |
| Kwok 2004 | Intervention PEF 155 L/min Control PEF 51 L/min |
|
| Littlejohns 1991 | Intervention 45.2% (22 4) Control 50.2% (23‐0) |
FEVI < 60% predicted Partcipants were in a stable state as judged by the patient and physician with no change or perceived need for change in medication for at least six weeks before recruitment. |
| Smith 1999 | 33% | Patients required to have a FEV1/FVC ratio of less than 60%, no other active major illnesses at time of entry into study and, be in a stable state. |
In brief, all studies investigated the effects of a supervised, home‐based intervention in patients with COPD using a parallel group RCT design. The home‐based intervention represented a respiratory nurse providing care, education and support in a patient's home. The effects of this was assessed via a variety of outcomes, including patient based outcomes (lung function, exercise testing, HRQL and mortality), health system based outcomes (medical service utilisation), and carer based outcomes (HRQL, satisfaction).
Eight of the nine studies had sample sizes that were moderately large: 96 (Smith 1999), 117 (Hermiz 2002), 152 (Littlejohns 1991), 157 (Kwok 2004), 191 (Bourbeau 2003), 192 (Aiken 2006), 217 (Coultas 2005) and 301 (Bergner 1988). The Cockcroft 1987 study had 75 participants.
Two studies followed‐up the effect of the intervention at three months (Aiken 2006; Hermiz 2002), one at four months (Bourbeau 2003), four at six months (Aiken 2006; Bergner 1988; Coultas 2005; Kwok 2004), one at nine months (Aiken 2006) and five at 12 months (Bergner 1988; Bourbeau 2003; Cockcroft 1987; Littlejohns 1991; Smith 1999).
Coultas 2005 had two intervention groups. Both groups involved a respiratory nurse providing home visits but one group received additional training in specific training aimed at helping people with COPD adopt healthy lifestyle behaviours. Given both intervention groups involved the use of nurse home visits, the primary focus of this review, the data from both these intervention arms were combined and treated as a single intervention for the purpose of meta‐analysis.
Excluded studies
Forty‐eight papers were excluded for the following reasons: predominantly concerned with physical rehabilitation or exercise (n=19), not supervised by a nurse at home (n=15), not a RCT (n=11), data previously reported (n=2) and the intervention was of too short a duration (n=1).
Risk of bias in included studies
Although the nine studies were RCTs, there were important methodological limitations in all studies summarised below. Agreement for assessment of study quality was reached by the reviewers. Full details of our risk of bias judgments can be found in Characteristics of included studies and summaries of our judgments found in Figure 1 and Figure 2.
1.
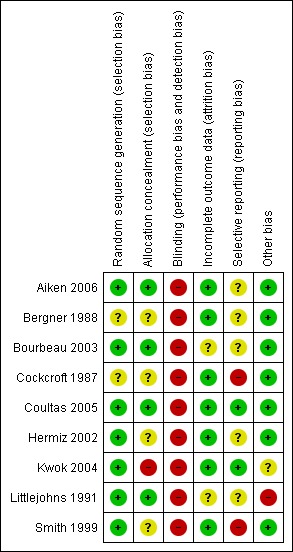
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
2.
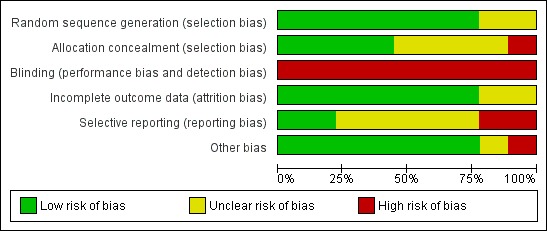
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Allocation
Allocation concealment was unclear in four studies (Bergner 1988; Cockcroft 1987; Hermiz 2002; Smith 1999) and inadequate in one (Kwok 2004).
Blinding
Due to the nature of the studies, it was not possible to blind patients to their assignment group. One study employed a blinded investigator to measure outcomes (Aiken 2006). The remaining eight were unblinded.This may have affected potentially outcome measures dependent on patient factors, such as the health related quality of life questionnaires, effort on lung function testing or effort on exercise performance testing. However, objective outcome measures (hospitalisations, mortality) would have been unlikely to have been affected.
Incomplete outcome data
Incomplete outcome reporting of data could not be excluded in two studies (Bourbeau 2003; Littlejohns 1991).
Selective reporting
Selective reporting, which is defined as the selection of a subset of the original variables recorded, on the basis of the results, for inclusion in publication of trials, was unclear in six studies (Aiken 2006; Bergner 1988; Bourbeau 2003; Hermiz 2002; Littlejohns 1991; Smith 1999), and inadequate in one (Cockcroft 1987).
Other potential sources of bias
Other potential sources of bias could not be excluded in two studies (Kwok 2004; Littlejohns 1991). In Kwok 2004, doctors of patients in the control group were able to refer patients to receive home care visits. Kwok 2004 did not report how often these home care visits occurred or whether patients in the control group who did receive occasional respiratory nurse home visits were excluded from the final analysis. This may have led to a false‐negative result. In Littlejohns 1991, there was evidence of differing baseline severity of disease in the control and intervention group. Finally, although this was not a primary focus of the studies, concomitant pharmacotherapy can have a major impact on outcomes assessed and merits reporting in both trials and the review. Better presentation of data related to baseline and change in pharmacotherapy usage may be informative.
Effects of interventions
See: Table 1
Health Related Quality of Life
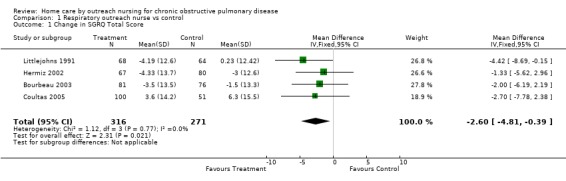
Four studies (Bourbeau 2003; Coultas 2005; Hermiz 2002; Littlejohns 1991) measured HRQL using the St George's Respiratory Questionnaire (SGRQ). This is a disease‐specific questionnaire for COPD. Using this questionnaire, a change of four units is clinically significant. Unpublished data was obtained from one of the authors of Littlejohns 1991. Data for the change in SGRQ total score was available from all four studies, and meta‐analysis demonstrated that HRQL by this questionnaire improved with the intervention (MD ‐2.60; 95% CI ‐4.81 to ‐0.39; Figure 3) and this was a statistically significant difference. Data for the SGRQ sub‐scores; activity (Analysis 1.5); impact (Analysis 1.6) and symptoms (Analysis 1.7); was only available from three studies (Bourbeau 2003; Coultas 2005; Hermiz 2002). The reduction is SGRQ sub‐scores was not statistically significant.
3.
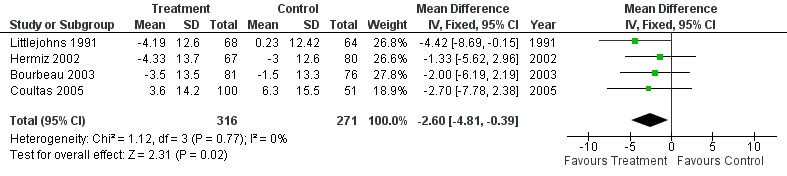
Forest plot of comparison: 1 Respiratory outreach nurse vs control, outcome: 1.3 Change in SGRQ Total Score.
1.5. Analysis.
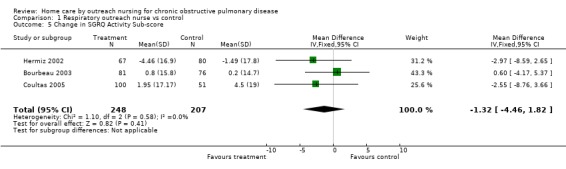
Comparison 1 Respiratory outreach nurse vs control, Outcome 5 Change in SGRQ Activity Sub‐score.
1.6. Analysis.
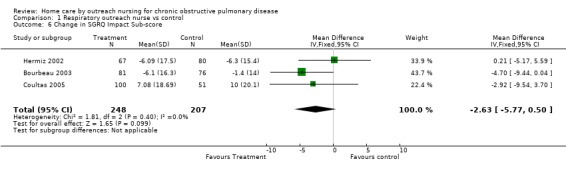
Comparison 1 Respiratory outreach nurse vs control, Outcome 6 Change in SGRQ Impact Sub‐score.
1.7. Analysis.
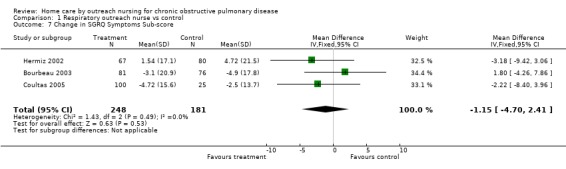
Comparison 1 Respiratory outreach nurse vs control, Outcome 7 Change in SGRQ Symptoms Sub‐score.
Two studies (Bergner 1988; Littlejohns 1991) measured HRQL using the Sickness Impact Profile (SIP). This is a general health measure of HRQL. Standard deviations of the mean change in SIP scores were obtainable only from Littlejohns 1991 who reported that the 'physical score' was significantly improved in the intervention group and so data were not pooled (Analysis 1.8). In contrast, however, Bergner 1988 found no significant difference in the 'physical score' with the intervention.
1.8. Analysis.
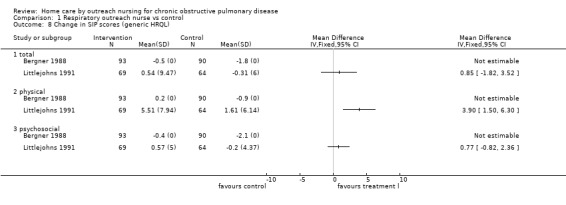
Comparison 1 Respiratory outreach nurse vs control, Outcome 8 Change in SIP scores (generic HRQL).
Two studies measured HRQL using the SF‐36 (Aiken 2006; Coultas 2005). This is a general health measure of HRQL. Sufficient data was not obtainable from any of these studies, however, limiting analysis. Coultas 2005 did not find any change in SF‐36 score with the intervention. Aiken 2006 reported a significant improvement in the linear trajectories of SF‐36 scores with the intervention, however this study included both patients with COPD and congestive heart failure and insufficient disease‐specific data was available for subgroup statistical analysis.
One study (Smith 1999) measured HRQL using a modified Dartmouth Primary Care Co‐operative, (COOP). Sufficient data was not obtainable from this study however, limiting the analysis. When individual items were compared between baseline and twelve months in the intervention arm, three scores were significantly lower, (emotional condition, difficulty doing daily tasks because of physical and emotional health and a general HRQL). The remaining seven items did not show a significant difference between baseline and post‐intervention.
Mortality
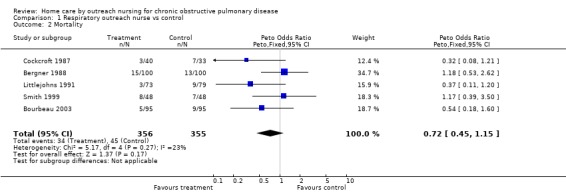
Five studies assessed mortality at 12 months (Bergner 1988; Bourbeau 2003; Cockcroft 1987; Littlejohns 1991; Smith 1999), two at six months (Coultas 2005; Kwok 2004) and one at three months (Hermiz 2002). The decrease in the number of deaths with the intervention was not statistically significant (Peto OR 0.72; 95% CI 0.45 to 1.15; Figure 4).
4.
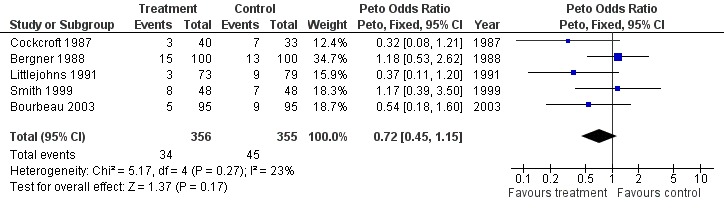
Forest plot of comparison: 1 Respiratory outreach nurse vs control, outcome: 1.9 Mortality.
Medical Service Utilisation
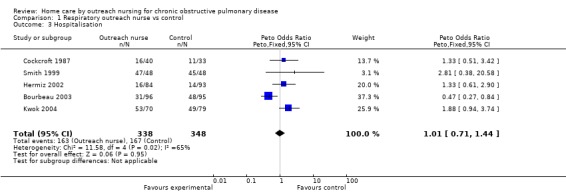
Data regarding hospitalisations was available from five studies (Bourbeau 2003; Cockcroft 1987; Hermiz 2002; Kwok 2004; Smith 1999). Overall, meta‐analysis demonstrated no significant change in the number of hospitalisations with the intervention (Peto OR 1.01; 95% CI 0.71 to 1.44; Figure 5). However, significant statistical heterogeneity was observed (I2 = 65%). Inspection of the forest plot indicated that the heterogeneity was due to one outlying study (Bourbeau 2003), and that sensitivity analysis may be justified although we had no specified subgroup analysis a priori. Subgroup analysis excluding this study demonstrated a statistically significant increase in the number of hospitalisations in patients receiving the intervention (Peto OR 1.59; 95% CI 1.02 to 2.47), and this is considered further in the Discussion.
5.
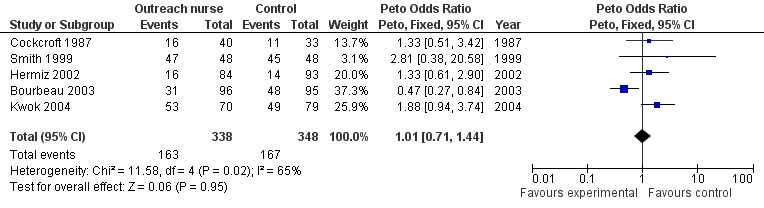
Forest plot of comparison: 1 Respiratory outreach nurse vs control, outcome: 1.10 Hospitalisation.
Data regarding the duration of hospital stay was available from one study (Cockcroft 1987), with this study demonstrating a longer duration of stay in the intervention group.
Data regarding GPor family doctor visits was available from three studies (Bourbeau 2003; Coultas 2005; Hermiz 2002), however insufficient data was available to perform pooled analysis (Analysis 1.4). Bourbeau 2003 reported a significant decrease in the number of unscheduled family doctor visits, but no change in the number of scheduled family doctor visits, with the intervention. Both Coultas 2005 and Hermiz 2002 reported no change in the number of family doctor visits.
1.4. Analysis.
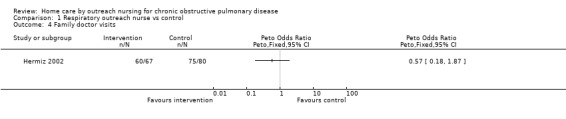
Comparison 1 Respiratory outreach nurse vs control, Outcome 4 Family doctor visits.
Data regarding emergency department presentations was available from four studies (Aiken 2006; Bourbeau 2003; Kwok 2004; Coultas 2005), however insufficient data was available to perform pooled analysis. Bourbeau 2003 reported a significant decrease in the number of patients with one or two emergency department presentations in the intervention group. However, other studies observed no similar difference. Aiken 2006 reported no difference in the average number of emergency department presentations per month. Kwok 2004 reported no difference in the mean number of emergency department presentations per patient. Coultas 2005 reported no difference in the number of mean change in the number of emergency department presentations per patient.
Data regarding costs associated with the intervention was reported in one study (Bergner 1988), which reported significantly higher average annual medical costs with the intervention of $9,768 compared with $5,051 for the control, (P=0.02).
Lung Function and Exercise Testing
A full analysis of the % change from baseline in FEV1 was not possible because sufficient data were obtainable from the authors of one study only (Littlejohns 1991) and therefore data were not pooled. In Littlejohns 1991 there was no significant difference in FEV1. Following the intervention, no significant difference in FEV1 was reported by Bergner 1988, and no significant change in either FEV1 or FVC was reported by Bourbeau 2003.
Data regarding exercise testing was only available from two studies (Littlejohns 1991; Kwok 2004). There was no significant difference in the distance walked in a standard six‐minute walking distance test following the intervention (MD 5.05; 95% CI ‐15.08 to 25.18; Analysis 1.10).
1.10. Analysis.
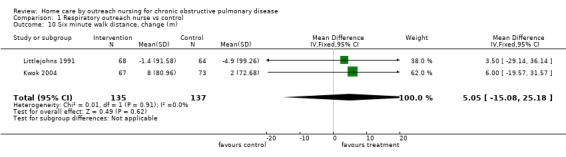
Comparison 1 Respiratory outreach nurse vs control, Outcome 10 Six minute walk distance, change (m).
Carer related HRQL and satisfaction
We did not find any data for carer quality of life or satisfaction.
Discussion
Summary of main results
Nine studies assessed the benefits of outreach nursing care for patients with COPD in 1498 patients. A number of outcomes which were only reported by single studies in the original review were now reported by multiple studies, permitting pooled analysis on a greater number of outcomes.
Whilst there was some methodological variation in the delivery and assessment of the intervention across studies, most notably with regard to study duration, sample size and the frequency of assessment, all investigated the effects of a supervised, home‐based intervention in patients with COPD using a parallel group RCT design. The home‐based interventions represented a respiratory nurse providing care, education and support in a patient's home. Studies employed a variety of outcome measures which allowed some pooled analysis. Whilst the studies were conducted over a wide time range, there did not appear to be any obvious effect related to the year of study.
There was insufficient data available to determine the effect of home care interventions on lung function and exercise capacity.
Quality of life was measure by a number of HRQL questionnaires. Meta‐analysis of data from four studies employing the disease specific SGRQ found a significant improvement in HRQL in patients receiving with outreach nursing care compared to those receiving usual care. Two general health status questionnaires were administered in a number of studies, however insufficient data was available for pooled analysis. However, in contrast to the improvement in disease‐specific health questionnaire the individual results of the general health status questionnaires were mixed.
Mortality data was available from eight studies. Meta‐analysis demonstrated a decrease in the number of deaths with outreach nursing care, but this was not statistically significant.
Data on medical service utilisation was available from a number of studies. Hospitalisations were reported in five studies. Overall, meta‐analysis did not demonstrate a significant change in the number of hospitalisations. However, significant statistical heterogeneity was seen with one outlying study (Bourbeau 2003), which reported a statistically significant decrease in hospitalisations with the intervention, in contrast to the other four studies reporting hospitalisations which reported increases in hospitalisations with the intervention. It was not readily apparent why one study reported results in contrast to the others, however excluding this study revealed a statistically significant increase in the number of hospitalisations in patients receiving the home care intervention in the remaining four studies (Cockcroft 1987; Hermiz 2002; Kwok 2004; Smith 1999). We did not specify sub group analyses to investigate heterogeneity a priori and we do not have an explanation for the conflicting direction of the treatment effects in these studies. The increase in hospitalisations in patients receiving home care in the four studies (Cockcroft 1987; Hermiz 2002; Kwok 2004; Smith 1999) seems to conflict with the improvement in quality of life and mortality found in these same patients. A possible explanation for this apparent discrepancy may be that the educational component of outreach nursing may enable patients to recognise deteriorations promptly, seeking medical service assistance where necessary and thus improving overall quality of life and mortality.
Data on GP or family doctor visits and emergency department presentations was also available from two studies. However, insufficient data was available for pooled analysis and there was no clear trend with the individual results of studies.
Though there were potential improvements in quality of life and mortality, the home care intervention may incur substantially higher health care costs than standard outpatient care for COPD as reported by Bergner 1988, although this is an old study and may not represent the true cost of these interventions today.
Quality of the evidence
Study quality is a potential issue in this review, with some studies being of unclear methodological quality. It is not possible to blind patients to whether or not they received the intervention.
Authors' conclusions
Implications for practice.
Outreach nursing programmes for COPD improved disease‐specific HRQL. However the effect on hospitalisations was heterogeneous, reducing admissions in one study, but increasing them in others, therefore we could not draw firm conclusions for this outcome.
Implications for research.
There is a need for further long term (one year or more) studies, in which the health status and quality of life of patient and carer are measured with appropriate validated instruments. These studies should be of sufficient power and duration to permit further estimation of impact on mortality and medical service utilisation.
What's new
| Date | Event | Description |
|---|---|---|
| 11 November 2011 | New citation required but conclusions have not changed | New literature search run. |
| 11 November 2011 | New search has been performed | New literature search run, no new studies found. Search strategy added to appendix 1. |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 1, 2000
| Date | Event | Description |
|---|---|---|
| 24 January 2011 | New search has been performed | New literature search run (November 2009), five new studies added. |
| 24 January 2011 | New citation required and conclusions have changed | We have been able to draw new conclusions with regards to mortality, health related quality of life and hospital admissions. There was one additional study that contributed mortality data to this update and this did not alter the pooled result. We were able to report data for three additional studies for HRQL as measured by the SGRQ and found a statistically significant improvement among patients receiving home care. We were able to enter data for hospital admissions in this update and although there was no statistically significant difference in admissions in patients receiving home care to those on usual care, there was significant heterogeneity which makes it difficult to draw conclusions. |
| 28 July 2008 | Amended | Converted to new review format. |
| 23 May 2001 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to acknowledge the other authors who contributed to the original review: Sarah Appleton, Robert Adams, Anne‐Marie Southcott and Richard Ruffin.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| CENTRAL (the Cochrane Library) | Quarterly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Hand‐searches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Data and analyses
Comparison 1. Respiratory outreach nurse vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in SGRQ Total Score | 4 | 587 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐4.81, ‐0.39] |
| 2 Mortality | 5 | 711 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.45, 1.15] |
| 3 Hospitalisation | 5 | 686 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.71, 1.44] |
| 4 Family doctor visits | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5 Change in SGRQ Activity Sub‐score | 3 | 455 | Mean Difference (IV, Fixed, 95% CI) | ‐1.32 [‐4.46, 1.82] |
| 6 Change in SGRQ Impact Sub‐score | 3 | 455 | Mean Difference (IV, Fixed, 95% CI) | ‐2.63 [‐5.77, 0.50] |
| 7 Change in SGRQ Symptoms Sub‐score | 3 | 429 | Mean Difference (IV, Fixed, 95% CI) | ‐1.15 [‐4.70, 2.41] |
| 8 Change in SIP scores (generic HRQL) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 total | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 physical | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 psychosocial | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 FEV1 % Change | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Six minute walk distance, change (m) | 2 | 272 | Mean Difference (IV, Fixed, 95% CI) | 5.05 [‐15.08, 25.18] |
1.1. Analysis.
Comparison 1 Respiratory outreach nurse vs control, Outcome 1 Change in SGRQ Total Score.
1.2. Analysis.
Comparison 1 Respiratory outreach nurse vs control, Outcome 2 Mortality.
1.3. Analysis.
Comparison 1 Respiratory outreach nurse vs control, Outcome 3 Hospitalisation.
1.9. Analysis.
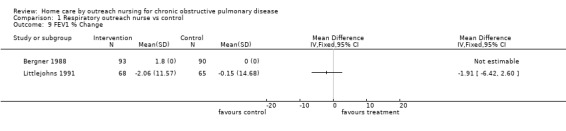
Comparison 1 Respiratory outreach nurse vs control, Outcome 9 FEV1 % Change.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aiken 2006.
| Methods | RCT | |
| Participants | 192 patients with COPD or chronic heart failure who had an estimated two‐year life expectancy. Patients with COPD were required to have oxygen saturations of less than 88% on room air, or baseline pO2 less than 55 on room air, and to be on continuous oxygen. Patients were required to exhibit marked limitation of physical functioning, in that any activity resulted in fatigue, palpitation, dyspnoea or angina. All patients were required to have exhibited recent exacerbation of their conditions. | |
| Interventions | 1. Intervention group (n = 33): Patients in the intervention group received the 'Phoenix Care Program'. This program aimed to increase self‐management of illness and knowledge of health‐related resources by providing information and education, improve patients' preparedness for end of life by promoting acquisition of appropriate legal documents and discussion of these with significant others, and enhance physical and mental functioning by case management and education. 2. Control group (n=28): Patients in the control group received usual care provided by managed care organisations, including medication and technical treatment. The duration of the intervention period was 9 months. |
|
| Outcomes | Patient self‐management of illness and knowledge of resources, preparedness for end of life, physical and mental functioning (including SF‐36), and medical system utilisation (emergency department visits, hospitalisations and associated length of stay). The outcomes of the interventions were assessed at 3 monthly intervals following enrolment. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was carried out within diagnosis in blocks of 30 patients... sealed envelopes, colour‐coded by diagnosis and containing the assignment to condition, were shuffled and assigned to participants in order of shuffling." |
| Allocation concealment (selection bias) | Low risk | "The Enroller, blinded to condition, opened the sealed envelope that identified the patient's study condition." |
| Blinding (performance bias and detection bias) All outcomes | High risk | This study was single‐blinded, with follow‐up measurements assessed by personnel blinded to the study group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Low risk | |
Bergner 1988.
| Methods | RCT | |
| Participants | 301 patients with COPD with enrolled. Patients had to have a clinical diagnosis of COPD, a FEV1 and FEV1/FVC ratio < 60% predicted, be homebound (by US Medicare criteria, for use of public transport), be between 40‐75 years of age, be able to administer aerosolised metaproterenol, be a local resident, be capable of co‐operating with the study. Patients were excluded if there was a primary diagnosis of asthma, a primary diagnosis of other functionally limiting disease which would significantly affect patient mortality, or if they received standard home nursing care during the 6 months prior to study entry. | |
| Interventions | 1. Respiratory home care group (n = 99): Patients in the respiratory home care group received specialised care from trained respiratory nurses at least one a month. 2. Standard home care group (n = 102): Patients in the standard home care group received standard home care from nurses at least once a month. 3: Control group (n = 100): Patients in the control group continued to receive usual care. The duration of the intervention period was 12 months. |
|
| Outcomes | Survival, costs (health care services, travel by patient, cost to family and household, drugs), pulmonary function, everyday function, Sickness Impact Profile (SIP), General Well‐Being Schedule, 10 minute walk test and index of independence in daily living. The outcomes of the interventions were assessed at 6 and 12 months after enrolment. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information regarding sequence generation was not available. |
| Allocation concealment (selection bias) | Unclear risk | Information regarding allocation concealment was not available. |
| Blinding (performance bias and detection bias) All outcomes | High risk | This study was unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Low risk | |
Bourbeau 2003.
| Methods | RCT | |
| Participants | 191 patients who were hospitalised at least once in the preceding year for an acute exacerbation of COPD. Patients had to have stable COPD (respiratory symptoms and medication unchanged for at least 4 weeks prior to enrolment), be at least 50 years of age, be a current or previous smoker, have a FEV1 after use of a bronchodilatory between 25‐70% of the predicted normal value and FEV1:FVC ratio less than 70%, no previous diagnosis of asthma, left congestive heart failure, terminal disease, dementia, or uncontrolled psychiatric illness, no participation in a respiratory rehabilitation program in the past year and no long‐term care facility stays. | |
| Interventions | 1. Intervention group (n = 96): Patients in the intervention group received a disease‐specific self‐management program. This consisted of 1 hour per week of teaching at home for 7 to 8 weeks conducted by health professional case managers (nurses in 4 centres, respiratory therapists in 2 centres, and a physiotherapist in 1 centre). Follow up was then conducted by weekly telephone calls for 8 weeks, and then monthly calls for the remainder of the study. 2. Control group (n=95): Patients in the control group continued to receive usual care managed by their respective specialists or GP. The duration of the intervention period was 12 months. |
|
| Outcomes | Spirometry (FEV1 and FVC), exercise capacity (6‐minute walk test distance), acute exacerbations, medical service utilisation (hospital admissions, emergency department visits, family physician visits) and health related quality of life (St George Respiratory Questionnaire). The outcomes of the interventions were assessed at 4 and 12 months. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients underwent randomisation with the use of a central computed‐generated list of random numbers. Randomization was stratified per centre and in blocks of 6, and patients were assigned to the...intervention group or to usual care”. |
| Allocation concealment (selection bias) | Low risk | "The blocking factor was not known by the investigators or their staff at each participating centre" |
| Blinding (performance bias and detection bias) All outcomes | High risk | This study was unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Explanation for patient attrition was not provided. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Low risk | |
Cockcroft 1987.
| Methods | RCT | |
| Participants | 75 patients with COPD were enrolled. Patients had to have been admitted to hospital at least twice in the previous 3 years or new patients who had been seen within the past year. Patients were excluded if their disability was not caused by a respiratory condition and those unable to understand the questionnaires. | |
| Interventions | 1. Intervention group (n = 42): Patients in the intervention group had a respiratory nurse visit once a month to provide support and goal setting. The intervention was mainly educative for patients to identify problems in activities of daily living and to increase independence in these activities. Patients were encouraged to contact GPs when required. Nurses did not contact doctors except in emergencies. 2. Control group (n = 33): Patients in the control group continued to receive usual care. The duration of the intervention period was 12 months. |
|
| Outcomes | HRQL (General Health Questionnaire), number and duration of admissions to hospital, number of deaths, PEFR and patient knowledge of condition and medicines. The outcomes of the interventions were assessed at the end of the 12 month intervention period. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation was stratified according to the number of admissions to hospital in the previous three years" |
| Allocation concealment (selection bias) | Unclear risk | Information regarding allocation concealment was not available. |
| Blinding (performance bias and detection bias) All outcomes | High risk | This study was single‐blinded, with follow‐up measurements assessed by personnel blinded to the study group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | High risk | "A set of visual analogue scales concerning physical and psychological aspects of the patients' lives, also designed for the study (were used)." These were not reported. |
| Other bias | Low risk | |
Coultas 2005.
| Methods | RCT | |
| Participants | 217 patients with COPD who fulfilled three criteria: were a current or former smoker with at least a 20‐pack‐year smoking history, had at least one respiratory symptom (e.g. cough, shortness of breath, wheeze) during the past 12 months, and had demonstrable airflow obstruction (FEV1/FVC ratio < 70% and FEV1 < 80% predicted). | |
| Interventions | 1. Medical management group (n = 49): Patients in the medical management group received approximately 8 hours of education about the diagnosis of COPD, the assessment of COPD severity, patient self‐management, smoking cessation, follow‐up and the formation of an action plan for exacerbations. 2. Medical and collaborative management group (n = 51): In addition to medical management, patients in the medical and collaborative management group received approximately 8 additional hours of training in 'collaborative care', intended to facilitate the adoption of healthy behaviours such as lifestyle and self‐management skills. 3. Control group (n = 51): Patients in the control group continued to receive usual care. The duration of the intervention period was 6 months. |
|
| Outcomes | Health related quality of life (St George Respiratory Questionnaire, SF‐36 and illness intrusiveness), medical service utilisation (physician office visits, emergency department visits and hospitalisations for lung disease and other conditions). The outcomes of the interventions were assessed at the end of the 6 month intervention period. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned.. using a computer‐generated random list." |
| Allocation concealment (selection bias) | Low risk | As above. |
| Blinding (performance bias and detection bias) All outcomes | High risk | This study was unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Hermiz 2002.
| Methods | RCT | |
| Participants | 117 patients who attended a hospital emergency department or were admitted to hospital with COPD. Patients were excluded if they resided outside the study region, had insufficient English speaking skills, were resident in a nursing home, or were confused or demented. | |
| Interventions | 1. Intervention group (n = 84): Patients in the intervention group received two home visits by a community nurse. These visits included a detailed assessment of the patient's health status and respiratory function, the provision of verbal and written education on disease, advice on stopping smoking, management of activities of daily living, emergency conservation, exercise, understanding and use of drugs, health maintenance, and early recognition of signs that require medical intervention. 2. Control group (n = 93): Patients in the control group continued to receive usual care managed by their respective specialists of GPs. The duration of the intervention period was 1 month. |
|
| Outcomes | Health related quality of life (St George's Respiratory Questionnaire), medical service utilisation (GP visits, emergency department visits and hospital admissions), patient knowledge, GP action and patient behaviour. The outcomes of the interventions were assessed after 3 months. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[We] had intended to use randomised permuted blocks with a block size of four at both sites, but, because of the smaller number of cases at Macarthur Health Service, we used a simple randomisation at that site" |
| Allocation concealment (selection bias) | Unclear risk | Information regarding allocation concealment was not available. |
| Blinding (performance bias and detection bias) All outcomes | High risk | This study was unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Low risk | |
Kwok 2004.
| Methods | RCT | |
| Participants | 157 patients hospitalised for COPD. Patients had to be 60 years or older, be residing locally, and have at least one hospital admission for COPD in the 6 months prior to the current admission. Patients were excluded if they had communication problems, were under institutional care or had a terminal disease with a life expectancy of less than 6 months were excluded. | |
| Interventions | 1. Intervention group (n = 77): Patients in the intervention group had a nurse perform weekly visits for the first 4 weeks, and then monthly visits up to 6 months. The initial visit was to review the patient's condition, give health counselling, provide psychosocial support to the patient and family caregivers, arrange social and health services when required, and to encourage the use of a telephone hotline when symptoms arose. Subsequent visits were to monitor changes in the subjects' physical conditions, to reinforce health counselling, and to encourage the use of the telephone hotline. 2. Control group (n = 80): Patients in the control group continued to receive usual care. The duration of the intervention period was 6 months. |
|
| Outcomes | Exercise capacity (6‐minute walk test distance), General Health Questionnaire, London Handicap Domain scale, Multimensional Health Locus, Cost of Care Index and medical service utilisation (emergency department visits and hospital admissions). The outcomes of the interventions were assessed at the end of the 6 month intervention period. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The other research nurse then allocated research grouping using a random number table." |
| Allocation concealment (selection bias) | High risk | "She then confirmed the recruitment by contacting another research nurse by telephone. The other research nurse then allocated research grouping using a random number table." |
| Blinding (performance bias and detection bias) All outcomes | High risk | This study was unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | "Three control subjects, as opposed to no intervention group subjects, were under an outpatient pulmonary rehabilitation program. This might have slightly favoured the control group." |
Littlejohns 1991.
| Methods | RCT | |
| Participants | 152 patients with COPD were enrolled. Patients had to be 30‐75 years old, have no other major disease, and have a FEV1 < 60% predicted. Patients also had to be in a stable state as judged by the patient and physician with no change or perceived need for change in medication for at least six weeks before recruitment. | |
| Interventions | 1. Intervention group (n = 73): Patients in the intervention group received care from respiratory health worker plus routine outpatient appointments. This included health education, supervision of domiciliary oxygen and correct inhalation techniques, monitoring spirometry and symptoms to enable acute exacerbations and heart failure to be detected and treated, liaison between GP and hospital based services. 2. Control group (n = 79): Patients in the control group continued to receive usual care (outpatient care/chest clinic care only). The duration of the intervention period was 12 months. |
|
| Outcomes | Mortality, FEV1, six minute walk, HRQL: Sickness Impact Profile. The outcomes of the interventions were assessed at the end of the 12 month intervention period. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random numbers were generated by tables in permuted blocks of four stratified by age (55 years and above and below 55) and sex." |
| Allocation concealment (selection bias) | Low risk | "The groups to which successive patients were to be allocated were noted in sealed, numbered envelopes, which were kept centrally. The physician recruiting a patient contacted the controller, who opened the appropriate envelope." |
| Blinding (performance bias and detection bias) All outcomes | High risk | This study was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Incomplete outcome data was not sufficiently described. |
| Selective reporting (reporting bias) | Unclear risk | All stated outcomes were addressed, however the original protocol was not available and we were thus unable to determine if selective reporting occurred. |
| Other bias | High risk | "When the Sickness Impact Profile scores of survivors only are compared at the start of the study the survivors in the intervention group had higher total, physical, and psychosocial SIP scores than those in the non‐intervention group (all significant at the 1% level)" "...whether there is bias in the study design that militates against the achievement of a difference between the two groups. The study was designed to assess the “effectiveness” rather than the “efficacy” of the respiratory health worker, so the clinicians were not given specific instructions regarding changes to their clinical practice." |
Smith 1999.
| Methods | RCT | |
| Participants | 96 patients with COPD were enrolled. Patients had to have a principal diagnosis of COPD, greater than 40 years of age, have a FEV1/FVC < 60%, have no other active major comorbidity, be in a stable state, have a carer involved in their management, and be able to speak and read English. | |
| Interventions | 1. Intervention group (n = 48): Patients in the intervention group received home‐based nursing intervention (HBNI) in addition to usual care from GP and OPD services. Home visits were made at 2‐4 week intervals over 12 months. 2. Control group (n = 48): Patients in the control group were not visited by a nurse but received care from GP and OPD services. The duration of the intervention period was 12 months. |
|
| Outcomes | FEV1, mortality, rate of hospitalisation, number of bed days, OPD attendance, emergency service visits and quality of life (Dartmouth Primary Care Co‐operative Quality of Life questionnaires). Carer quality of life was also measured. The outcomes of the interventions were assessed at the end of the 12 month intervention period. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised as they were enrolled from two lists of randomly computer generated numbers for the intervention and control group." |
| Allocation concealment (selection bias) | Unclear risk | Information regarding allocation concealment was not available. |
| Blinding (performance bias and detection bias) All outcomes | High risk | This study was unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | High risk | "Attempts to perform questionnaires [and spirometry] in the control subjects were unsuccessful due to a combination of (I) these subjects perceived no immediate benefit of the trial; and (ii) the burden of participating in a study, including questionnaires, was greater than expected for those patients who had advanced airways disease." |
| Other bias | Low risk | |
COPD: Chronic obstructive pulmonary disease FEV1: Forced expiratory volume in one second FVC: Forced vital capacity HBNI: HRQL: Health Related Quality of Life Questionnaire OPD: Out patients department PEFR: Peak expiratory flow rate
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aimonino 2008 | The intervention comprised of both physicians and nurse home visits. There was also a significant physical rehabilitation and occupational therapy component to the intervention. |
| Alonso 2004 | The intervention was home hospitalisation. |
| Behnke 2003 | The main intervention was an exercise program. |
| Boxall 2005 | The intervention had a significant physical rehabilitation component. |
| Brown 1997 | RCT, but study duration only 28 days. Readmission rates and costs were the only outcomes measured. |
| Busch 1988 | RCT, main intervention was physical rehabilitation at home. |
| Campbell 1991 | Not a RCT ‐ pre‐post study, not controlled. Patients were selected by their frequency of admissions, i.e. high frequency. Study did not exclude asthma patients. |
| Carrieri‐Kohlman 2005 | The intervention had a significant physical rehabilitation component. |
| Casas 2006 | The intervention was largely by phone. There were few home visits and included physician visits. |
| Cockcroft 1981 | RCT of unsupervised exercise rehabilitation programme, 6 weeks in a rehabilitation centre then four months at home. |
| Cummings 1990 | Not a RCT. Pre/post experimental design. Patients required to have two or more functional impairments or a terminal illness. |
| Diaz 2005 | The intervention was home hospitalisation. |
| Dranove 1985 | Not a RCT. |
| Elliott 2004 | The intervention had a significant exercise component. |
| Enguidanos 2005, 2006 | Not a RCT. |
| Hernandez 2003 | The intervention was home hospitalisation. |
| Hernandez‐Vian 2007 | Not a RCT. |
| Heslop 1988 | Data previously published in Cockcroft 87. |
| Kara 2004 | No home visit component. |
| Lorig 2003 | No home visit component. |
| Man 2004 | No home visit component and the intervention had a significant physical rehabilitation component. |
| McGavin 1977 | Rehabilitation at home was unsupervised. |
| Murphy 2005 | The intervention had a significant physical rehabilitation component. |
| Na 2005 | The intervention had a significant physical rehabilitation component. |
| Neff 2003 | Not a RCT. |
| Nguyen 2008 | No home visit component. |
| Nissen 2007 | The intervention was home hospitalisation. |
| Noonill 2007 | Not a RCT. |
| O'Shea 2007 | The intervention had a significant physical rehabilitation component. |
| Oh 2003 | The intervention had a significant physical rehabilitation component. |
| Pison 2004 | Not a RCT. |
| Rabow 2003 | Not a RCT. |
| Rea 2004 | The intervention did not have a significant home visit component. |
| Resqueti 2007 | The intervention had a significant exercise component. |
| Roselle 1982 | Not a RCT. Pre/post study design. |
| Sinclair 1980 | Intervention group participants not supervised at home by the nurse. Patients selected for intervention/ control group depending on whether they lived in or outside city respectively. |
| Sridhar 2008 | The intervention had a significant physical rehabilitation component. |
| Steele 2008 | The intervention had a significant physical rehabilitation component. |
| Strijbos 1996 | RCT of home care that included a high physiotherapy content designed to improve exercise capacity. |
| Vale 1993 | Not a RCT. Pre/post experimental design. |
| Vrijhoef 2007 | No home visit component. |
| Wedzicha 1998 | RCT of hospital and community based physical rehabilitation programmes. |
| Weinberger 1996 | RCT of 1396 veterans hospitalised with diabetes, congestive heart failure, COPD. Intervention involved close follow‐up by nurse and primary care physician beginning at discharge and continuing for the 6 month study duration. It is not clear what the post discharge intervention is and it appears that the nurse did not make home visits. |
| Wijkstra 1994 | RCT of community based programme with a high physiotherapy and physical training component. |
| Wijkstra 1995 | 18 month RCT of community based programme with a high physiotherapy and physical training component. |
| Wijkstra 1996 | Data previously published in Wijkstra 94 and Wijkstra 95. |
| Xie 2003 | The intervention had a significant exercise component. |
| Zwar 2008 | The intervention did not have a significant home visit component. |
Differences between protocol and review
We have included mortality, hospitalisations and disease specific HRQL (SGRQ) in the summary of findings table. This was a post‐hoc decision; whilst primary outcomes would ordinarily be included in the summary of findings table, the original protocol did not specific primary and secondary outcomes.
Contributions of authors
Christopher X Wong: Protocol, assessment of studies for inclusion, study quality assessment, data extraction and manuscript review. Kristin Carson: Assessment of studies for inclusion, study quality assessment, data extraction and data entry and manuscript review. Brian Smith: Manuscript review.
Sources of support
Internal sources
NHS Research and Development, UK.
External sources
ACAGN (Australasian Cochrane Airways Group Network) Student Scholarship, Australia.
Declarations of interest
CXW: none known KVC: none known BJS: I am lead author on a RCT that was included in this review
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aiken 2006 {published data only}
- Aiken LS, Butner J, Lockhart CA, Volk‐Craft BE, Hamilton G, Williams FG. Outcome evaluation of a randomized trial of the PhoenixCare intervention: program of case management and coordinated care for the seriously chronically ill. Journal of Palliative Care 2006;9(1):111‐26. [DOI] [PubMed] [Google Scholar]
Bergner 1988 {published data only}
- Bergner M, Hudson LD, Conrad DA, Patmont CM, McDonald GJ, Perrin EB, et al. The cost and efficacy of home care for patients with chronic lung disease. Medical Care 1988;26:566‐79. [DOI] [PubMed] [Google Scholar]
Bourbeau 2003 {published data only}
- Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupre A, Begin R, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease‐specific self‐management intervention. Archives of Internal Medicine 2003;163(5):585‐91. [DOI] [PubMed] [Google Scholar]
Cockcroft 1987 {published data only}
- Cockroft A, Bagnall P, Heslop A, Andersson N, Heaton R, Batstone J, et al. Controlled trial of respiratory health worker visiting patients with chronic respiratory disability. BMJ 1987;294:225‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coultas 2005 {published data only}
- Coultas D, Frederick J, Barnett B, Singh G, Wludyka P. A randomized trial of two types of nurse‐assisted home care for patients with COPD. Chest 2005;128(4):2017‐24. [DOI] [PubMed] [Google Scholar]
Hermiz 2002 {published data only}
- Hermiz O, Comino E, Marks G, Daffurn K, Wilson S, Harris M. Randomised controlled trial of home based care of patients with chronic obstructive pulmonary disease. BMJ 2002;325(7370):938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwok 2004 {published data only}
- Kwok T, Lum C M, Chan H S, Ma H M, Lee D, Woo J. A randomized, controlled trial of an intensive community nurse‐supported discharge program in preventing hospital readmissions of older patients with chronic lung disease. Journal of the American Geriatrics Society 2004;52(8):1240‐6. [DOI] [PubMed] [Google Scholar]
Littlejohns 1991 {published data only}
- Littlejohns P, Baveystock CM, Parnell H, Jones PW. Randomised controlled trial of the effectiveness of a respiratory health worker in reducing impairment, disability and handicap due to chronic airflow limitation. Thorax 1987;46:559‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 1999 {published data only}
- Smith B, Appleton S, Bennett P, Roberts G, Fante P, Adams R, et al. The Effect of a Respiratory Nurse Home Intervention in Patients with Chronic Obstructive Pulmonary Disease. Australian and New Zealand Journal of Medicine 1999;29(5):718‐25. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aimonino 2008 {published data only}
- Aimonino Ricauda N, Tibaldi V, Leff B, Scarafiotti C, Marinello R, Zanocchi M, et al. Substitutive "hospital at home" versus inpatient care for elderly patients with exacerbations of chronic obstructive pulmonary disease: A prospective randomized, controlled trial. Journal of the American Geriatrics Society 2008;56(3):493‐500. [DOI] [PubMed] [Google Scholar]
Alonso 2004 {published data only}
- Alonso A. A new model for home care for COPD. Studies in Health Technology and Informatics 2004;103:368‐73. [PubMed] [Google Scholar]
Behnke 2003 {published data only}
- Behnke M, Jorres R A, Kirsten D, Magnussen H. Clinical benefits of a combined hospital and home‐based exercise programme over 18 months in patients with severe COPD. Monaldi Archives for Chest Disease 2003;59(1):44‐51. [PubMed] [Google Scholar]
Boxall 2005 {published data only}
- Boxall A M, Barclay L, Sayers A, Caplan G A. Managing Chronic Obstructive Pulmonary Disease in the Community: A RANDOMIZED CONTROLLED TRIAL OF HOME‐BASED PULMONARY REHABILITATION FOR ELDERLY HOUSEBOUND PATIENTS. Journal of Cardiopulmonary Rehabilitation 2005;25(6):378‐85. [DOI] [PubMed] [Google Scholar]
Brown 1997 {published data only}
- Brown A, Caplan G. A post‐acute respiratory outreach service. Australian Journal of Advanced Nursing 1997;14(4):5‐11. [PubMed] [Google Scholar]
Busch 1988 {published data only}
- Busch AJ, McClements JD. Effects of a supervised home exercise programme on patients with severe obstructive pulmonary disease. Physical Therapy 1988;67:471‐4. [DOI] [PubMed] [Google Scholar]
Campbell 1991 {published data only}
- Campbell‐Hagerty M, Stockdale‐Woolley R, Nair S. An innovative home‐care program for the patient with chronic obstructive pulmonary disease. Chest 1991;100(3):607‐12. [DOI] [PubMed] [Google Scholar]
Carrieri‐Kohlman 2005 {published data only}
- Carrieri‐Kohlman V, Nguyen HQ, Donesky‐Cuenco D, Demir‐Deviren S, Neuhaus J, Stulbarg MS. Impact of brief or extended exercise training on the benefit of a dyspnea self‐management program in COPD. Journal of Cardiopulmonary Rehabilitation 2005;25(5):275‐84. [DOI] [PubMed] [Google Scholar]
Casas 2006 {published data only}
- Casas A, Troosters T, Garcia‐Aymerich J, Roca J, Hernandez C, Alonso A, et al. Integrated care prevents hospitalisations for exacerbations in COPD patients. European Respiratory Journal 2006;28(1):123‐30. [DOI] [PubMed] [Google Scholar]
Cockcroft 1981 {published data only}
- Cockcroft AE, Saunders MJ, Berry G. Randomised controlled trial of rehabilitation in chronic respiratory disability. Thorax 1981;36:200‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cummings 1990 {published data only}
- Cummings JE, Hughes SL, Weaver FM, Manheim LM, Conrad KJ, Nash K, et al. Cost‐effectiveness of Veterans Administration hospital‐based home care. A randomized clinical trial. Archives of Internal Medicine 1990;150:1274‐80. [PubMed] [Google Scholar]
Diaz 2005 {published data only}
- Diaz Lobato S, Gonzalez Lorenzo F, Gomez Mendieta M A, Mayoralas Alises S, Martin Arechabala I, Villasante Fernandez‐Montes C. [Evaluation of a home hospitalization program in patients with exacerbations of chronic obstructive pulmonary disease]. Archivos de Bronconeumologia 2005;41(1):5‐10. [DOI] [PubMed] [Google Scholar]
Dranove 1985 {published data only}
- Dranove D. An empirical study of a hospital‐based home care program. Inquiry 1985;22:59‐66. [PubMed] [Google Scholar]
Elliott 2004 {published data only}
- Elliott M, Watson C, Wilkinson E, Musk A W, Lake F R. Short‐ and long‐term hospital and community exercise programmes for patients with chronic obstructive pulmonary disease. Respirology 2004;9(3):345‐51. [DOI] [PubMed] [Google Scholar]
Enguidanos 2005, 2006 {published data only}
- Enguidanos S M, Cherin D, Brumley R, Finkelstein S M, Speedie S M, Potthoff S. Home‐based palliative care study: site of death, and costs of medical care for patients with congestive heart failure, chronic obstructive pulmonary disease, and cancer, Home telehealth improves clinical outcomes at lower cost for home healthcare. Telemedicine Journal & E‐Health 2005, 2006;1, 12(3, 2):37‐56‐128‐36. [DOI] [PubMed] [Google Scholar]
Hernandez 2003 {published data only}
- Hernandez C, Casas A, Escarrabill J, Alonso J, Puig Junoy J, Farrero E, et al. Home hospitalisation of exacerbated chronic obstructive pulmonary disease patients. European Respiratory Journal 2003;21(1):58‐67. [DOI] [PubMed] [Google Scholar]
Hernandez‐Vian 2007 {published data only}
- Hernandez‐Vian O, Moreno‐Ramos C, Sanchez‐Garcia A, Lopez‐Gomez M J, Ortiz‐Alvarez E, Balboa‐Blanco E. Evaluation of the care of the elderly program in frail elderly individuals with COPD in primary care centers in Sabadell (Spain). Enfermeria Clinica 2007;17(3):109‐16. [DOI] [PubMed] [Google Scholar]
Heslop 1988 {published data only}
- Heslop AP, Bagnall P. A study to evaluate the intervention of a nurse visiting patients with disabling chest disease in the community. Journal of Advanced Nursing 1988;13(1):71‐7. [DOI] [PubMed] [Google Scholar]
Kara 2004 {published data only}
- Kara M, Asti T. Effect of education on self‐efficacy of Turkish patients with chronic obstructive pulmonary disease. Patient Education & Counseling 2004;55(1):114‐20. [DOI] [PubMed] [Google Scholar]
Lorig 2003 {published data only}
- Lorig KR, Ritter PL, Gonzalez VM. Hispanic chronic disease self‐management: a randomized community‐based outcome trial. Nursing Research 2003;52(6):361‐9. [DOI] [PubMed] [Google Scholar]
Man 2004 {published data only}
- Man W D, Polkey M I, Donaldson N, Gray B J, Moxham J. Community pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonary disease: randomised controlled study. BMJ 2004;329(7476):1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
McGavin 1977 {published data only}
- McGavin CR, Gupta SP, Lloyd EL, McHardy GJR. Physical rehabilitation for the chronic bronchitic:Results of a controlled trial of exercises in the home. Thorax 1977;32:307‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2005 {published data only}
- Murphy N, Bell C, Costello R W. Extending a home from hospital care programme for COPD exacerbations to include pulmonary rehabilitation. Respiratory Medicine 2005;99(10):1297‐302. [DOI] [PubMed] [Google Scholar]
Na 2005 {published data only}
- Na JO, Kim DS, Yoon SH, Jegal YJ, Kim WS, Kim ES, et al. A simple and easy home‐based pulmonary rehabilitation programme for patients with chronic lung diseases. Monaldi Archives for Chest Disease 2005;63(1):30‐6. [DOI] [PubMed] [Google Scholar]
Neff 2003 {published data only}
- Neff D F, Madigan E, Narsavage G. APN‐directed transitional home care model: achieving positive outcomes for patients with COPD. Home Healthcare Nurse 2003;21(8):543‐50. [DOI] [PubMed] [Google Scholar]
Nguyen 2008 {published data only}
- Nguyen H Q, Donesky‐Cuenco D, Wolpin S, Reinke L F, Benditt J O, Paul S M, et al. Randomized controlled trial of an internet‐based versus face‐to‐face dyspnea self‐management program for patients with chronic obstructive pulmonary disease: pilot study. Journal of Medical Internet Research 2008;10(2):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nissen 2007 {published data only}
- Nissen I, Jensen M S. [Nurse‐supported discharge of patients with exacerbation of chronic obstructive pulmonary disease]. Ugeskrift for laeger 2007;169(23):2220‐3. [PubMed] [Google Scholar]
Noonill 2007 {published data only}
- Noonill N, Sindhu S, Hanucharurnkul S, Suwonnaroop N. An integrated approach to coordination of community resources improves health outcomes and satisfaction in care of Thai patients with COPD. Thai Journal of Nursing Research 2007;11(2):118‐31. [Google Scholar]
O'Shea 2007 {published data only}
- O'Shea S D, Taylor N F, Paratz J D. A predominantly home‐based progressive resistance exercise program increases knee extensor strength in the short‐term in people with chronic obstructive pulmonary disease: a randomised controlled trial. Australian Journal of Physiotherapy 2007;53(4):229‐37. [DOI] [PubMed] [Google Scholar]
Oh 2003 {published data only}
- Oh EG. The effects of home‐based pulmonary rehabilitation in patients with chronic lung disease. International Journal of Nursing Studies 2003;40(8):873‐9. [DOI] [PubMed] [Google Scholar]
Pison 2004 {published data only}
- Pison C, Cano N, Cherion C, Roth H, Pichard C. Effects of home pulmonary rehabilitation in patients with chronic respiratory failure and nutritional depletion. [French]. Revue des Maladies Respiratoires 2004;21(3 I):573‐82. [DOI] [PubMed] [Google Scholar]
Rabow 2003 {published data only}
- Rabow M W, Petersen J, Schanche K, Dibble S L, McPhee S J. The comprehensive care team: a description of a controlled trial of care at the beginning of the end of life. Journal of Palliative Medicine 2003;6(3):489‐99. [DOI] [PubMed] [Google Scholar]
Rea 2004 {published data only}
- Rea H, McAuley S, Stewart A, Lamont C, Roseman P, Didsbury P. A chronic disease management programme can reduce days in hospital for patients with chronic obstructive pulmonary disease. Internal Medicine Journal 2004;34(11):608‐14. [DOI] [PubMed] [Google Scholar]
Resqueti 2007 {published data only}
- Resqueti V R, Gorostiza A, Galdiz J B, Santa Maria E L, Clara P C, Guell Rous R. Benefits of a home‐based pulmonary rehabilitation program for patients with severe chronic obstructive pulmonary disease. Archivos de Bronconeumologia 2007;43(11):599‐604. [DOI] [PubMed] [Google Scholar]
Roselle 1982 {published data only}
- Roselle S, D'Amico FJ. The effect of home respiratory therapy on hospital readmission rates of patients with chronic obstructive pulmonary disease. Respiratory Care 1982;27(10):1194‐9. [PubMed] [Google Scholar]
Sinclair 1980 {published data only}
- Sinclair DJM, Ingram CG. Controlled trial of supervised exercise training in chronic bronchitis. BMJ 1980;1:519‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sridhar 2008 {published data only}
- Sridhar M, Taylor R, Dawson S, Roberts N J, Partridge M R. A nurse led intermediate care package in patients who have been hospitalised with an acute exacerbation of chronic obstructive pulmonary disease. Thorax 2008;63(3):194‐200. [DOI] [PubMed] [Google Scholar]
Steele 2008 {published data only}
- Steele B G, Belza B, Cain K C, Coppersmith J, Lakshminarayan S, Howard J, et al. A randomized clinical trial of an activity and exercise adherence intervention in chronic pulmonary disease. Archives of Physical Medicine & Rehabilitation 2008;89(3):404‐12. [DOI] [PubMed] [Google Scholar]
Strijbos 1996 {published data only}
- Strijbos JH, Postma DS, Altena R, Gimeno F, Koeter GH. Feasibility and effects of a home‐care rehabilitation programme in patients with chronic obstructive pulmonary disease. Journal of Cardiopulmonary Rehabilitation 1996;16:386‐93. [DOI] [PubMed] [Google Scholar]
Vale 1993 {published data only}
- Vale F, Reardon JZ, Zu Wallak RL. The long‐term benefits of outpatient pulmonary rehabilitation on exercise endurance and quality of life. Chest 1993;103:42‐5. [DOI] [PubMed] [Google Scholar]
Vrijhoef 2007 {published data only}
- Vrijhoef H J, Bergh J H, Diederiks J P, Weemhoff I, Spreeuwenberg C. Transfer of care for outpatients with stable chronic obstructive pulmonary disease from respiratory care physician to respiratory nurse‐‐a randomized controlled study. Chronic Illness 2007;3(2):130‐44. [DOI] [PubMed] [Google Scholar]
Wedzicha 1998 {published data only}
- Wedzicha JA, Bestall‐ JC, Garrod R, Garnham R, Paul EA, Jones PW. Randomized controlled trial of pulmonary rehabilitation in severe chronic obstructive pulmonary disease patients, stratified with the MRC dyspnoea scale. European Respiratory Journal 1998;12:363‐9. [DOI] [PubMed] [Google Scholar]
Weinberger 1996 {published data only}
- Weinberger M, Oddone EZ, Henderson WG. Does increased access to primary care reduce hospital readmissions?. New England Journal of Medicine 1996;334(22):1441‐6. [DOI] [PubMed] [Google Scholar]
Wijkstra 1994 {published data only}
- Wijkstra PJ, Altena R, Kraan J, Otten V, Postma DS, Koeter GH. Quality of life in patients with chronic obstructive pulmonary disease improves after rehabilitation at home. European Respiratory Journal 1994;7:269‐73. [DOI] [PubMed] [Google Scholar]
Wijkstra 1995 {published data only}
- Wijkstra PJ, TenVergert EM, Altena R, Otten V, Kraan J, Postma DS, et al. Long term benefits of rehabilitation at home on quality of life and exercise tolerance in patients with chronic obstructive pulmonary disease. Thorax 1995;50:824‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wijkstra 1996 {published data only}
- Wijkstra PJ, Mark ThW, Kraan J, Altena R, Koeter GH, Postma DS. Effects of home rehabilitation on physical performance in patients with chronic obstructive pulmonary disease (COPD). European Respiratory Journal 1996;9:104‐10. [DOI] [PubMed] [Google Scholar]
Xie 2003 {published data only}
- Xie S L, Zhu M G, Cui H B, Liu H Y. Influence of home‐based training program on patients with COPD. Zhonghua Linchuang Kangfu Zazhi 2003;7(18):2554‐5. [Google Scholar]
Zwar 2008 {published data only}
- Zwar N, Hermiz O, Hasan I, Comino E, Middleton S, Vagholkar S, et al. A cluster randomised controlled trial of nurse and GP partnership for care of chronic obstructive pulmonary disease. BMC Pulmonary Medicine 2008;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
BTS 1997
- British Thoracic Society. Chronic Obstructive Pulmonary Disease Guidelines. Thorax 1997;52(Suppl 5):2‐28. [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, Available from www.cochrane‐handbook.org, 2008. [Google Scholar]
RevMan 5 [Computer program]
- Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan) Version 5.0. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2008.


