Abstract
Background
Recent studies in gram-negative bacteremia (GNB) suggest that intravenous (IV) to oral (PO) switch and short treatment durations yield similar clinical outcomes and fewer adverse events. Antimicrobial stewardship program (ASP) bundled initiatives have been associated with improved clinical outcomes for bloodstream infections.
Methods
This single-center retrospective cohort evaluation included inpatient adults from 11/2014–10/2015 and 10/2017–9/2018 with GNB. The pre-ASP period was before the establishment of an ASP program. In the post period, the ASP promoted IV-to-PO switches, avoidance of repeat blood cultures, and short treatment durations for patients with uncomplicated GNB. The primary outcome was duration of antibiotic therapy. Secondary outcomes included process measures associated with the bundle and clinical outcomes.
Results
One hundred thirty-seven patients met criteria for inclusion, with 51 patients in the pre group and 86 patients in the post group. Background characteristics were similar between groups. The median duration of therapy (interquartile range) was 14 (10–16) days in the pre group and 10 days (7–14) in the post group (P < .001). The median day of IV-to-PO switch was day 5 (4–6) in the pre group vs day 4 (3–5) in the post group (P = .046). The average total hospital cost per case decreased by 27% in the post group (P = .19). Mortality rates and bacteremia recurrence were not significantly different between groups.
Conclusions
An ASP bundle for uncomplicated GNB was associated with reduced durations of therapy and earlier PO switch. These findings highlight the synergistic role of ASPs in optimizing antibiotic use and promoting patient safety.
Keywords: antimicrobial stewardship, bacteremia, bundle
The burden of gram-negative bloodstream infections is high, accounting for almost 50% of community-onset bacteremias and approximately 30% of all nosocomial bacteremias [1, 2]. The literature regarding optimal treatment of uncomplicated gram-negative bacteremia (GNB) has evolved significantly in recent years. Previously, longer treatment durations of up to 2 weeks were preferred; however, multiple retrospective studies have demonstrated similar patient outcomes, fewer adverse effects, and less development of drug-resistant pathogens (DRPs) with shortened treatment duration [3–5]. Support for shorter durations of therapy was recently cemented by a randomized multicenter open-label trial of 7 vs 14 days of therapy for GNB among patients who achieved clinical stability (afebrile and hemodynamically stable for at least 48 hours) before day 7. In this large study (n = 604), noninferiority was achieved in their composite end point of 90-day all-cause mortality, relapse, suppurative or distant complications, and readmission or extended hospitalization (>14 days). Together, these data suggest shortening duration of therapy in GNB as a desirable antimicrobial stewardship intervention.
The literature also demonstrates that oral (PO) therapy following initial intravenous (IV) therapy results in similar outcomes compared with a full course of IV therapy in GNB [3, 5, 6]. In the largest study to date on oral step-down vs intravenous therapy in GNB, a propensity score–matched cohort of 1478 patients observed a similar 30-day mortality of 13.1% and 13.4% among their oral step-down and IV groups, respectively [6]. Additionally, PO therapy resulted in an average 2-day decrease in length of stay. Finally, similar outcomes were observed among high and low-bioavailable oral step-down agents. Although a recent meta-analysis of 2289 patients has suggested no difference in mortality with low-bioavailability step-down agents in GNB, there was a higher rate of recurrence of infection (defined as primary site and/or recurrent bacteremia) [7]. However, absolute event rates were low, and elsewhere it has been pointed out that these data had significant loss to follow-up bias [8]. In contrast, the largest study to date, with 4090 patients and robust follow-up within a single health system, reflected no difference in outcomes with low bioavailability step down therapy [8–10]. These data further encourage the potential of antimicrobial stewardship in streamlining GNB management, particularly using agents with improved safety profiles.
Antimicrobial stewardship program (ASP) bundled initiatives for bacteremias utilizing rapid diagnostic testing (RDT) have been associated with improved outcomes [11, 12]. Although bundles for Staphylococcus aureus bacteremia are well described, the integration of RDT with a bundled approach of ASP interventions in GNB is absent in the literature. The purpose of this quality improvement project was to evaluate the impact of ASP interventions on treatment of GNB through a targeted bundle approach of promoting IV-to-PO switches, discouraging repeat blood cultures, avoiding fluoroquinolones when possible, and encouraging short treatment durations.
METHODS
Patients
This was a retrospective evaluation of adult patients admitted to the University of Utah Hospital with at least 1 blood culture positive for GNB from 11/2014–10/2015 and 10/2017–9/2018. If patients had multiple admissions for bacteremia, only the first encounter was included. We also excluded patients with polymicrobial infections and patients who received a transplant (solid organ or hematopoietic stem cell) at any time during their lives. These patients were excluded due to the increased complexity of treatment, which has been associated with clinical inertia and less streamlined management [8, 13] along with less evidence in support of shorter treatment durations in immunosuppressed populations. Additionally, we excluded patients without source control (eg, lack of drainage of infected fluid collections, central line removal, or intervention for obstruction for biliary and urinary sources) and those treated for extended durations (>21 days) due to unresolved source control issues or indications for prolonged durations of therapy (eg, osteomyelitis). This evaluation also excluded pregnant women, patients who died before the end of therapy, patients who enrolled in hospice by day 7, or patients without in vitro active therapy in the first 24 hours. Although our ASP routinely performs interventions on all bacteremic patients, including facilitating early appropriate therapy based on RDT results, patients without in vitro active therapy in the first 24 hours were excluded as previous data have reflected downstream management impacts of delayed appropriate therapy in GNB [14].
Interventions
The pre period represents the use of RDT without active intervention by the ASP. The post period represents the use of RDT combined with an ASP bundled approach for GNB. BioFire blood culture identification (BCID) RDT was implemented during 11/2014; however, ASP review and intervention on results did not begin until 10/2016, when a 0.5 full-time equivalent (FTE) MD began intervening on BCID results, focusing on shortening time to appropriate therapy and de-escalation of unnecessary additional IV therapy. There was less focus at this time on IV-to-PO switches and shortened durations of therapy given lack of available resources and literature to support expanded recommendations. The ASP expanded in 10/2017 with the addition of 1 FTE antimicrobial stewardship pharmacist. Also beginning in 10/2017, the ASP began to fully implement a targeted bundle approach for GNB, actively promoting IV-to-PO switches, 7-day treatment durations, and recommending against repeat cultures for all patients with GNB without indications for extended treatment duration. These recommendations were communicated daily (M–F 8am–5pm) directly to the primary medical team in person or via electronic medical record (EMR) note, in addition to a page to the on-call provider from the antimicrobial stewardship pharmacist or physician. Most bundle recommendations were provided in person in the first 6 months by both the antimicrobial stewardship pharmacist and physician, as data suggest that face-to-face stewardship communication is most effective [15]. Formal infectious diseases consultations were recommended if the patient was deemed complicated by the ASP; however, most patients with uncomplicated bacteremia were managed through antimicrobial stewardship review.
Outcomes
The primary outcome was duration of antibiotic therapy. Secondary outcomes included definitive therapy agent, length of stay, change in number of CVC placements, 30-day all-cause readmission, recurrence of bacteremia with the same infecting organism within 30 days, 30-day mortality, and costs. We collected demographic and clinical information by manual chart review. We also collected patient antibiotic therapy for total duration of treatment, evaluating discharge orders for discharge durations to be included in the total duration. IV-to-PO switch day was documented and rounded to nearest 24-hour period from antibiotic start time. For all applicable secondary end points (30-day readmission, bacteremia recurrence, and 30-day mortality), day 0 was the last day of antibiotic therapy. Additionally, we performed analyses to identify factors predictive of long treatment durations for uncomplicated GNB. We also evaluated cost changes. We calculated cost data as mean cost per case, as previously described [16, 17], including cost centers of imaging, supply, pharmacy, lab, other services, and facility utilities. Finally, we evaluated appropriate use of deep venous thrombosis (DVT) prophylaxis (heparin, enoxaparin, low-dose aspirin, and sequential compression devices or full anticoagulation) as a nonequivalent dependent variable to assess possible systematic improvements in medication management that could have occurred over time at our institution and thus impacted outcomes [18, 19].
Statistical Analysis
We evaluated categorical variables using the Pearson chi-square or Fisher exact test and continuous variables using the Wilcoxon rank-sum test. We considered a P value of <.05 to be statistically significant. The evaluation adjusted cost data for inflation. Additionally, we evaluated predictors of long-course therapy using a median dichotomized duration and backwards stepwise selection for relevant predictors. Collinearity and model fit were assessed with variance inflation factor and the Hosmer-Lemeshow test, respectively. Candidate predictors were selected based on clinical judgement and previous characteristics associated with differences in duration among GNB [20–25]. Finally, we performed all analyses in R, version 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria). The Institutional Review Board classified this project as quality improvement and did not require review or oversight.
RESULTS
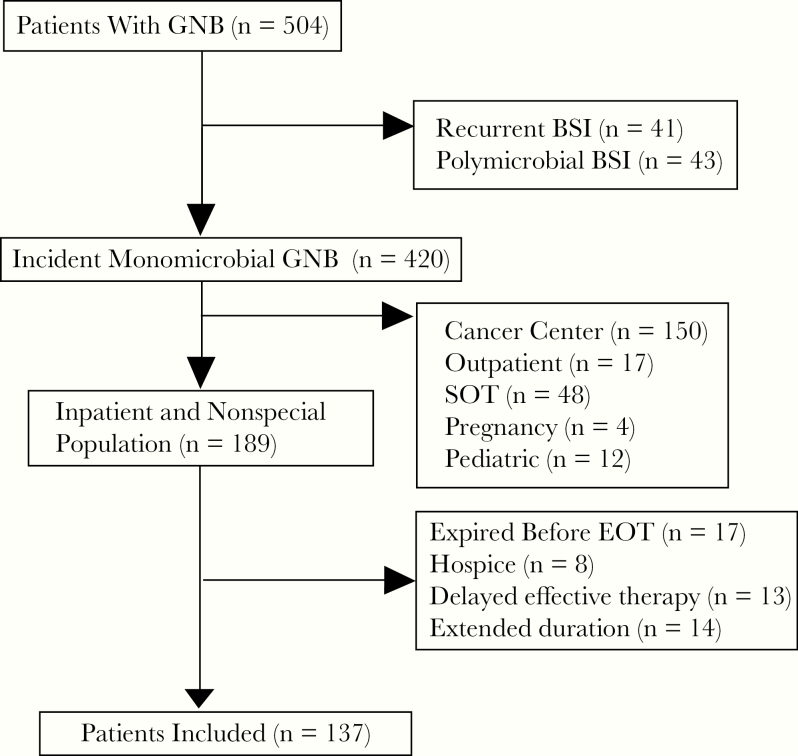
Of 510 patients, 137 patients met criteria for inclusion, with 51 patients in the pre–ASP intervention group and 86 patients in the post–ASP intervention group (Figure 1). Background characteristics were similar between groups (Table 1). The median duration of therapy (interquartile range) was 14 days (10–16) in the pre group and 10 days (7–14) in the post group (P < .001) (Table 2). Patients in the pre group switched from IV to PO therapy on day 5 (4–6), vs day 4 (3–5) in the post group (P = .046). Patients in the pre group also had higher 30-day readmission rates than patients in the post group (39.2% vs 23.3%; P = .047). A lower proportion of patients completed therapy on oral beta-lactams in the pre group than in the post group (19.6% vs 44.2%); consequently, more patients received definitive therapy with oral fluoroquinolones in the pre vs post group (49% vs 39.5%). CVC placement was not significantly different between groups, although more patients required CVC placement in the pre group vs the post group (15.7% vs 8.1%; P = .171). Thirty-day mortality and bacteremia recurrence were not significantly different between groups (0% vs 2.3%; P = .273; and 0% vs 2.3%; P = .273, respectively). Cost per case decreased 27% from the pre period to the post period (P = .19). In multivariable analysis, factors associated with long treatment (>11 days) duration included repeat blood cultures, urinary obstructions, the pre–ASP intervention period, and infection with an organism other than E. coli (Table 3). Medication management quality, assessed with appropriate proportions of DVT prophylaxis, did not significantly change between periods (P = .14).
Figure 1.
Flow diagram for inclusion and exclusion. Abbreviations: BSI, bloodstream infection; EOT, end of therapy; GNB, gram-negative bacteremia; SOT, solid organ transplant.
Table 1.
Baseline Characteristics
| Pre-ASP (n = 51) | Post-ASP (n = 86) | P | |
|---|---|---|---|
| Age, y | 60 (40–74) | 65 (54–75) | .13 |
| Female gender | 55 (64) | 26 (51) | .14 |
| BMI | 29 (22–35) | 29 (24–34) | .52 |
| Charlson Comorbidity Score | 1 (0–2) | 1 (0–3) | .94 |
| Comorbidities | |||
| Urinary abnormalitya | 11 (21.6) | 12 (14.0) | .25 |
| Diabetes | 16 (31.4) | 32 (37.2) | .49 |
| Liver disease | 2 (3.9) | 7 (8.1) | .34 |
| Renal disease | 7 (13.7) | 8 (9.3) | .42 |
| Immunosuppressive medications | 1 (2.0) | 5 (5.8) | .29 |
| Admitting service | |||
| Internal medicine | 52 (60.5) | 31 (60.8) | .97 |
| Other | 34 (39.5) | 20 (39.2) | |
| ICU admission | 16 (31.4) | 30 (34.9) | .67 |
| Pitt Bacteremia Score | 3 (2–4) | 3 (2–4) | .72 |
| Source of bacteremia | |||
| CLABSI | 2 (3.9) | 2 (2.4) | .02 |
| Intra-abdominal | 14 (27.5) | 13 (15.3) | |
| Pneumonia | 6 (11.8) | 1 (1.2) | |
| Skin and soft tissue | 0 (0) | 4 (4.7) | |
| Urinary tract | 25 (49.0) | 58 (68.2) | |
| Other | 4 (7.8) | 8 (9.3) | |
| Organism | |||
| E. coli | 37 (72.5) | 61 (70.9) | .40 |
| Non–E. coli EB | 14 (27.5) | 22 (25.6) | |
| Non-EB | 0 (0) | 3 (3.5) | |
| Infectious diseases consult | 4 (7.8) | 10 (11.6) | .48 |
Data are presented as number (percentage) or median (interquartile range).
Abbreviations: ASP, antimicrobial stewardship program; BMI, body mass index; CLABSI, central line–associated bloodstream infection; EB, Enterobacteriaceae; ICU, intensive care unit.
aUrinary abnormalities included presence of calculi, obstruction, or structural abnormalities.
Table 2.
Outcomes
| Pre-ASP | Post-ASP | P | |
|---|---|---|---|
| Treatment duration, d | 14 (10–16) | 10 (7–14) | <.01 |
| IV to PO, d | 5 (4–6) | 4 (3–5) | .046 |
| CVC placementa | 8 (15.7) | 7 (8.1) | .17 |
| Repeat blood cultures | 34 (66.7) | 38 (44.2) | .01 |
| Length of stay, d | 5 (3–7) | 4 (3–8) | .54 |
| Definitive therapyb | |||
| Beta-lactam/SMX PO | 10 (19.6) | 38 (44.2) | <.01 |
| Beta-lactam/SMX IV | 14 (27.5) | 14 (16.3) | |
| Fluoroquinolone PO | 25 (49.0) | 34 (39.5) | |
| Fluoroquinolone IV | 0 (0) | 0 (0) | |
| Other | 2 (3.9) | 0 (0) | |
| 30-d all-cause readmission | 20 (39.2) | 20 (23.3) | .047 |
| 30-d infection-related readmission | 4 (7.8) | 9 (10.5) | .61 |
| 30-d recurrence of bacteremia | 0 (0) | 2 (2.3) | .27 |
| 30-d mortality | 0 (0) | 2 (2.3) | .27 |
Data are presented as number (percentage) or median (interquartile range). Costs per case are not reported, per institutional policy.
Abbreviations: ASP, antimicrobial stewardship program; CVC, central venous catheter; IV, intravenous; PO, oral; SMX, sulfamethoxazole-trimethoprim.
aCVC placement after onset of bacteremia.
bFinal therapy of treatment.
Table 3.
Predictors of Long Treatment Duration
| Univariate OR (95% CI) | P | Multivariate OR (95% CI) | P | |
|---|---|---|---|---|
| Age ≥65 y | 1.09 (0.59–1.50) | .80 | ||
| Pre-ASP intervention period | 3.06 (1.50–6.43) | <.01 | 3.67 (1.60–8.44) | <.01 |
| Charlson Comorbidity Index ≥2 | 1.19 (0.55–2.61) | .66 | ||
| Immunosuppression | 0.49 (0.07–2.61) | .42 | ||
| E. coli | 0.27 (0.12–0.59) | <.01 | 0.27 (0.11–0.68) | <.01 |
| Pitt Bacteremia Score >3 | 1.51 (0.75–3.07) | .26 | ||
| Repeat blood cultures | 3.46 (1.73–7.09) | <.01 | 3.54 (1.57–7.98) | <.01 |
| Urinary source | 0.79 (0.40–1.58) | .51 | ||
| Genito-urinary tract obstruction | 3.84 (1.28–14.25) | .03 | 8.03 (2.06–31.28) | <.01 |
Abbreviations: ASP, antimicrobial stewardship program; CI, confidence interval; OR, odds ratio.
DISCUSSION
Our project examined the impact of antimicrobial stewardship bundled interventions on the treatment of patients with uncomplicated GNB. Overall, we found that patients with uncomplicated GNB who had the ASP bundled intervention had significantly shorter treatment durations, earlier IV-to-PO switches, and lower readmission rates. The decrease in readmission rates may have been due to fewer CVC-related complications or drug-related adverse events; however, this needs to be studied further. Additionally, the ASP bundled intervention was associated with numerically lower rates of CVC placement, decreased fluoroquinolone therapy, and cost per case decreases. Our data suggest that focusing ASP resources on bundled interventions for GNB could result in improved management, improved downstream clinical outcomes, and potential cost avoidance.
Although most other studies evaluating the impact of ASPs on GNB have focused on general RDT implementation, few have evaluated specific interventions. Multiple recent studies have investigated the role of RDT implementation in combination with ASP intervention for patients with GNB [26–28]. These studies found that ASP intervention decreased time to effective therapy, time to appropriate de-escalation, length of stay, infection-related mortality, and overall mortality while increasing the proportion of patients on optimal therapy after BCID. Although all of these studies highlighted the benefits of general ASP intervention on patient care, our evaluation targets specific interventions made by an ASP program, including interventions on durations of therapy and IV-to-PO switch. Our project also examined additional outcomes, including CVC placement, readmission rates, and bacteremia recurrence. Given the progressing data on management of GNB, we believe our data are useful to ASPs in targeting their activities and streamlining management during the hospital stay for GNB. Moreover, given the impact observed and that the present population only reflected approximately a quarter of GNB patients at our institution, we believe this is an impactful approach for other institutions that requires minimal resources.
Besides emerging literature regarding ASP bundled interventions for BSIs, recent literature surrounding GNB treatment supports the ASP interventions made in this evaluation. Multiple recent studies have investigated clinical outcomes in the setting of short treatment durations and early IV-to-PO switches for uncomplicated GNB. These studies have shown similar or improved patient outcomes, including mortality, bacteremia recurrence, and readmission rates [3–6, 29]. Similarly, our evaluation did not find a significant difference between mortality or bacteremia recurrence between groups. The results of this evaluation add to the growing body of literature demonstrating the lack of patient harm from shorter durations of therapy and early IV-to-PO switches. These interventions may also decrease DRP emergence, medication adverse effects, and hospital costs, as described in previous studies [4]. Although we did not systematically evaluate challenges with our bundle implementation, anecdotally, providers’ most frequent concerns were related to step-down therapy decisions. Given the evolving data in this area, it seems the majority of providers now find step-down therapy agreeable [7, 9, 30].
Our evaluation had the unique strength of evaluating the impact of our interventions on cost. With our local institutional policy, we are only able to present percent decreases in costs per case, and we observed a 27% decrease in costs per case. Furthermore, based on published median estimates of GNB costs [31], our observed decreases would translate to a cost avoidance estimate of $3800 per case. This further translates into an estimate of $326 800 in cost avoidance for the post-ASP period. Though our cost-per-case analysis did not achieve significance, likely related to the limited size of our sample, larger cohorts have reflected 2-day decreases in length of stay associated with oral step-down for GNB [6]. Our observed decreased cost trend was likely related to our median 1-day decrease in length of stay among patients in the post period. Additionally, overall cost avoidance could be due to decreased lab and pharmacy costs related to avoidance of repeat cultures and changes to oral therapy, respectively. Finally, we observed a decrease in CVC placement (15.7% vs 8.1%; P = .17), which could have contributed. Focusing on these bundled interventions in GNB should be considered among ASPs, as it may yield a cost avoidance benefit.
Finally, our project identified a variety of factors associated with long treatment duration for GNB. Although previous studies on duration of therapy in GNB have not formally evaluated predictors for duration, these studies have shown associations for longer duration of therapy among patient baseline characteristics including age, comorbidities, source of infection, severity of illness, appropriate empiric therapy, and infectious diseases consult [20–24]. Notably, we found an increase in duration of therapy among patients with renal obstructions. In the setting of a postobstruction intervention, the literature on antimicrobial therapy remains scant, and further literature in this area would be of great benefit in informing clinical decision-making on durations of therapy [32, 33]. Additionally, we found that patients treated before ASP bundle promotion who had repeat cultures and who were infected with bacteria other than E. coli were more likely to receive longer treatment. As a minority of patients were febrile when repeat blood cultures were obtained (data not shown), repeat cultures could potentially reflect a surrogate for outliers in clinical practice, as our bundle discouraged this practice. Since the implementation of our bundled approach, additional data have reinforced short durations in non-lactose-fermenting GNB such as Pseudomonas aeruginosa and therefore should be of benefit in discouraging longer durations in patients clinically meeting considerations for short durations [34]. Future evaluations should consider focusing on durations of therapy among populations with these factors.
This evaluation has limitations that should be considered. First, the retrospective nature of this evaluation lends to the potential for confounding. Similarly, baseline differences in the groups could have been associated with treatment duration, though we attempted to address this with regression modeling and still found our temporal intervention was associated with decreased duration. However, temporal changes in practice between the pre and post periods may have also impacted outcomes. In particular, this may be true with our choice of comparison periods, which were chosen to reflect the incremental changes of the program. However, given our outcome measures, when we initiated the bundle, and the availability of evidence to support these changes, our choice of comparison periods is pragmatic. Additionally, the results of our nonequivalent dependent variable analysis did not suggest changes in our general quality of medication management, and thus add to the robustness of our conclusion regarding the impact of our intervention. We also excluded transplant patients and patients at our cancer hospital, which limits the generalizability of our evaluation to transplant and oncology patients, although previous studies have included low numbers of patients in these populations, suggesting that similar interventions in these populations may be reasonable [4, 29]. We only evaluated readmissions, reinfection, and mortality within our system, which could have impacted those secondary outcomes given potential loss to follow-up. Barriers to oral step-down (susceptibilities, allergies, and drug–drug interactions) were not evaluated and could have biased results. Finally, we did not evaluate time for or acceptance rates of ASP interventions for this bundle. Therefore, the cost-effectiveness of this specific bundle is unclear. However, other data support the cost-effectiveness of active ASP interventions for bacteremic patients [35].
CONCLUSIONS
An ASP bundle for uncomplicated GNB was associated with reduced durations of therapy, earlier PO switch, decreased fluoroquinolone therapy, and lower readmission rates without any adverse clinical outcomes. This evaluation also identified factors associated with longer treatment durations, including renal obstruction, repeat blood cultures, lack of ASP intervention, and infecting organism other than E. coli. Although warranting further study, these findings highlight the synergistic role of ASPs in optimizing antibiotic use and promoting patient safety.
Acknowledgments
Financial support. This work was unfunded.
Potential conflicts of interest. Tristan Timbrook has served as a consultant to BioFire Diagnostics, GenMark Diagnostics, Roche Diagnostics, and Wolters Kluwer. All other authors have no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Prior presentation: This project was presented in part at the 29th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID); April 13–16 2019; Amsterdam, the Netherlands (Abstract P2104).
References
- 1. Diekema DJ, Beekmann SE, Chapin KC, et al. . Epidemiology and outcome of nosocomial and community-onset bloodstream infection. J Clin Microbiol 2003; 41:3655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sligl WI, Dragan T, Smith SW. Nosocomial gram-negative bacteremia in intensive care: epidemiology, antimicrobial susceptibilities, and outcomes. Int J Infect Dis 2015; 37:129–34. [DOI] [PubMed] [Google Scholar]
- 3. Mercuro NJ, Stogsdill P, Wungwattana M. Retrospective analysis comparing oral stepdown therapy for Enterobacteriaceae bloodstream infections: fluoroquinolones versus β-lactams. Int J Antimicrob Agents 2018; 51:687–92. [DOI] [PubMed] [Google Scholar]
- 4. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. ; Antibacterial Resistance Leadership Group Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis 2018; 66:172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sutton JD, Sayood S, Spivak ES. Top questions in uncomplicated, non-Staphylococcus aureus bacteremia. Open Forum Infect Dis 2018; 5:ofy087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tamma PD, Conley AT, Cosgrove SE, et al. ; Antibacterial Resistance Leadership Group Association of 30-day mortality with oral step-down vs continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med 2019; 179:316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Punjabi C, Tien V, Meng L, Deresinski S, Holubar M. Oral fluoroquinolone or trimethoprim-sulfamethoxazole vs. ss-lactams as step-down therapy for Enterobacteriaceae bacteremia: systematic review and meta-analysis. Open Forum Infect Dis 2019; 6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hughes MA, Beganovic M. Introduction of selection biases due to loss to follow-up in infectious diseases retrospective outcomes studies. Antimicrob Agents Chemother 2019; 63:e01722–19.30559137 [Google Scholar]
- 9. Sutton JD, Chang NC, Stevens VW, Timbrook TT, Spivak ES. Low-bioavailability versus high-bioavailability oral antibiotics for the definitive treatment of Enterobacteriaceae bacteremia from suspected urine source in hospitalized veterans. Abstract presented at: 2–6 October 2019; Washington, DC. [Google Scholar]
- 10. Dominitz JA, Maynard C, Boyko EJ. Assessment of vital status in Department of Veterans Affairs national databases. Comparison with state death certificates. Ann Epidemiol 2001; 11:286–91. [DOI] [PubMed] [Google Scholar]
- 11. Wenzler E, Wang F, Goff DA, et al. . An automated, pharmacist-driven initiative improves quality of care for Staphylococcus aureus bacteremia. Clin Infect Dis 2017; 65:194–200. [DOI] [PubMed] [Google Scholar]
- 12. Timbrook TT, Morton JB, McConeghy KW, et al. . The effect of molecular rapid diagnostic testing on clinical outcomes in bloodstream infections: a systematic review and meta-analysis. Clin Infect Dis 2017; 64:15–23. [DOI] [PubMed] [Google Scholar]
- 13. Rosa R, Suarez JF, Bravo G, et al. . Challenges in antimicrobial stewardship: rapid diagnostics and optimization of therapy among immunocompromised patients. Open Forum Infect Dis 2019; 6:ofz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raman G, Avendano E, Berger S, Menon V. Appropriate initial antibiotic therapy in hospitalized patients with gram-negative infections: systematic review and meta-analysis. BMC Infect Dis 2015; 15:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. MacBrayne CE, Williams MC, Levek C, et al. . Sustainability of handshake stewardship: extending a hand is effective years later. Clin Infect Dis. 2019:ciz650. [DOI] [PubMed] [Google Scholar]
- 16. Kawamoto K, Martin CJ, Williams K, et al. . Value Driven Outcomes (VDO): a pragmatic, modular, and extensible software framework for understanding and improving health care costs and outcomes. J Am Med Inform Assoc 2015; 22:223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee VS, Kawamoto K, Hess R, et al. . Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA 2016; 316:1061–72. [DOI] [PubMed] [Google Scholar]
- 18. Harris AD, Bradham DD, Baumgarten M, et al. . The use and interpretation of quasi-experimental studies in infectious diseases. Clin Infect Dis 2004; 38:1586–91. [DOI] [PubMed] [Google Scholar]
- 19. Musgrove MA, Kenney RM, Kendall RE, et al. . Microbiology comment nudge improves pneumonia prescribing. Open Forum Infect Dis 2018; 5:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Swamy S, Sharma R. Duration of treatment of gram-negative bacteremia. Infect Dis Clin Pract 2016; 24(3):155–160. [Google Scholar]
- 21. Sousa A, Pérez-Rodríguez MT, Suárez M, et al. . Short- versus long-course therapy in gram-negative bacilli bloodstream infections. Eur J Clin Microbiol Infect Dis 2019; 38:851–7. [DOI] [PubMed] [Google Scholar]
- 22. Giannella M, Pascale R, Toschi A, et al. . Treatment duration for Escherichia coli bloodstream infection and outcomes: retrospective single-centre study. Clin Microbiol Infect 2018; 24:1077–83. [DOI] [PubMed] [Google Scholar]
- 23. Doi A, Morimoto T, Iwata K. Shorter duration of antibiotic treatment for acute bacteraemic cholangitis with successful biliary drainage: a retrospective cohort study. Clin Microbiol Infect 2018; 24:1184–9. [DOI] [PubMed] [Google Scholar]
- 24. Nelson AN, Justo JA, Bookstaver PB, et al. . Optimal duration of antimicrobial therapy for uncomplicated gram-negative bloodstream infections. Infection 2017; 45:613–20. [DOI] [PubMed] [Google Scholar]
- 25. Sayood S, Sutton J, Baures T, Spivak E. The utility of repeat blood cultures for bacteremic urinary tract infections and associated durations of therapy. Open Forum Infect Dis 2017; 4(Suppl 1):S344–5. [Google Scholar]
- 26. Bookstaver PB, Nimmich EB, Smith TJ 3rd, et al. Cumulative effect of an antimicrobial stewardship and rapid diagnostic testing bundle on early streamlining of antimicrobial therapy in gram-negative bloodstream infections. Antimicrob Agents Chemother 2017; 61:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rivard KR, Athans V, Lam SW, et al. . Impact of antimicrobial stewardship and rapid microarray testing on patients with gram-negative bacteremia. Eur J Clin Microbiol Infect Dis 2017; 36:1879–87. [DOI] [PubMed] [Google Scholar]
- 28. Perez KK, Olsen RJ, Musick WL, et al. . Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant gram-negative bacteremia. J Infect 2014; 69:216–25. [DOI] [PubMed] [Google Scholar]
- 29. Yahav D, Franceschini E, Koppel F, et al. ; Bacteremia Duration Study Group Seven versus 14 days of antibiotic therapy for uncomplicated gram-negative bacteremia: a noninferiority randomized controlled trial. Clin Infect Dis 2019; 69:1091–8. [DOI] [PubMed] [Google Scholar]
- 30. Hospenthal DR, Waters CD, Beekmann SE, Polgreen PM. Practice patterns of infectious diseases physicians in transitioning from intravenous to oral therapy in patients with bacteremia. Open Forum Infect Dis 2019: ofz386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thaden JT, Li Y, Ruffin F, et al. . Increased costs associated with bloodstream infections caused by multidrug-resistant gram-negative bacteria are due primarily to patients with hospital-acquired infections. Antimicrob Agents Chemother 2017; 61:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reyner K, Heffner AC, Karvetski CH. Urinary obstruction is an important complicating factor in patients with septic shock due to urinary infection. Am J Emerg Med 2016; 34:694–6. [DOI] [PubMed] [Google Scholar]
- 33. Hamasuna R, Takahashi S, Nagae H, et al. . Obstructive pyelonephritis as a result of urolithiasis in Japan: diagnosis, treatment and prognosis. Int J Urol 2015; 22:294–300. [DOI] [PubMed] [Google Scholar]
- 34. Fabre V, Amoah J, Cosgrove SE, Tamma PD. Antibiotic therapy for Pseudomonas aeruginosa bloodstream infections: how long is long enough? Clin Infect Dis. 2019; 69:2011–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pliakos EE, Andreatos N, Shehadeh F, Ziakas PD, Mylonakis E. The cost-effectiveness of rapid diagnostic testing for the diagnosis of bloodstream infections with or without antimicrobial stewardship. Clin Microbiol Rev 2018; 31:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]