Abstract
Background
Outpatient parenteral antimicrobial therapy (OPAT) allows for long-course intravenous treatment of infections without lengthy hospital stays. Upon discharge, antimicrobial therapy may be broadened for “ease” of once-daily administration (EOA). Patients requiring subsequent readmission can be tailored to pre-OPAT regimens to minimize adverse effects. This study assessed continuation of EOA regimens upon hospital readmission during or immediately after OPAT.
Methods
This was a retrospective review of adults enrolled in OPAT and discharged on ertapenem or daptomycin for EOA, defined by the terms “convenience” or “EOA” in OPAT notes or by switching to ertapenem or daptomycin upon OPAT enrollment despite adequate therapy with narrower-spectrum agents. The primary outcome was the percentage of patients readmitted during or after their OPAT course and maintained on an EOA regimen. Secondary outcomes included inpatient therapy cost, rates of Clostridioides difficile infection, and adverse events.
Results
Of 188 patients receiving an OPAT EOA regimen, 71 were readmitted, representing 113 unique readmissions. Patients were mostly males (81%) aged 57 years. The EOA regimens were continued in 27% of hospital readmissions. The Infectious Diseases (ID) team was consulted in 48% of readmissions, and the Antimicrobial Stewardship Program (ASP) intervened in 26%. Combined, this resulted in de-escalation in 28% of cases. Clostridioides difficile infections and adverse events occurred in 7% and 12% of readmissions, respectively. The median acquisition cost of inpatient EOA regimens was $150 per readmission.
Conclusions
The OPAT EOA regimens were continued in 27% of hospital readmissions indicating a role for improved indication documentation and collaboration between ID services, ASPs, and OPAT teams.
Keywords: antimicrobial stewardship, OPAT, readmission
Outpatient parenteral antimicrobial therapy (OPAT) allows for long-course intravenous (IV) antibiotic treatment of infections without lengthy hospital stays [1]. Outpatient parenteral antimicrobial therapy services are readily available in the United States with 1 in 1000 people receiving OPAT annually [2]. Occasionally, antimicrobial therapy may be broadened upon hospital discharge for “convenience” or “ease of administration” [1, 3]. The reason for this practice is that many less-expensive, IV antimicrobials commonly used in the inpatient setting, such as ampicillin-sulbactam and vancomycin, have frequent or variable dosing intervals that may not be feasible in the outpatient setting. Agents such as ertapenem or daptomycin overcome this obstacle with once-daily dosing regimens. However, these agents have broader coverage and higher direct drug costs.
Current literature reports that approximately 17%–27% of OPAT patients are readmitted within 30 days of hospital discharge [4]. From an antimicrobial stewardship standpoint, patients requiring subsequent readmission should have their antimicrobial regimen properly tailored based on susceptibilities, inpatient hospital formularies, and institutional guidelines. Ideally, this should occur during the medication reconciliation process on admission to minimize adverse effects associated with broad-spectrum antibiotics and reduce inpatient cost. However, from an OPAT or transitions of care perspective, frequent switches for short admissions of 24 to 48 hours may complicate therapy and introduce opportunities for error.
Available literature describing the implementation of this practice is limited. Therefore, this study aimed to assess the inpatient continuation of “ease of administration” (EOA) regimens upon hospital readmission during or immediately after OPAT treatment courses.
METHODS
This was a single-center, observational, retrospective chart review of patients aged 18 years or older who were discharged from Beth Israel Deaconess Medical Center (BIDMC), a 673-bed academic medical center in Boston, Massachusetts, between January 1, 2014 and September 30, 2017. Patients were included if they were enrolled in the OPAT clinic upon discharge with ertapenem or daptomycin therapy for EOA and were readmitted during or within 90 days of the documented end date of the OPAT course. Ease of administration was defined by the presence of the terms convenience or “EOA” in the OPAT enrollment note or by broadening of coverage to ertapenem or daptomycin upon OPAT enrollment despite adequate therapy with more narrow-spectrum agents. Patients receiving directed carbapenem or daptomycin therapy before OPAT enrollment were excluded. Ninety days post-OPAT completion was selected to capture patients admitted for reinfection with continuation of previous antimicrobial regimens.
Subjects were identified from the OPAT clinic roster, and medical records were manually reviewed to determine antimicrobial regimens. Inpatient antimicrobials were identified using the inpatient pharmacy system. Data collected included patient demographics, antimicrobial allergies, indication for OPAT, concomitant antimicrobials during OPAT, infecting organisms, reason for hospital readmission, Infectious Diseases (ID) consultation, Antimicrobial Stewardship Program (ASP) intervention, duration of EOA agents, and length of stay.
The primary outcome was the percentage of patients readmitted during or within 90 days of the OPAT course and maintained on an EOA regimen of antimicrobials. This was defined as the receipt of a dose of daptomycin, ertapenem, or meropenem upon hospital readmission. At our institution, meropenem is the only carbapenem on formulary. Therefore, if a patient was receiving ertapenem during OPAT, prescribers may transition to meropenem upon admission to maintain the patient on a carbapenem regimen. Secondary outcomes included inpatient direct drug cost (average wholesale price), incidence of Clostridioides (Clostridium) difficile infection, and adverse drug reactions as documented in the medical record. Clostridioides difficile infection was defined as a positive polymerase chain reaction (PCR) result for C difficile toxin B and binary toxin genes (Cepheid PCR).
Demographics and outcomes were summarized using descriptive statistics. Nominal variables were described as quantities and percentages, and continuous variables were reported as medians and interquartile ranges (IQRs).
Ethical Approval
This study was approved by the Beth Israel Deaconess Medical Center Institutional Review Board.
RESULTS
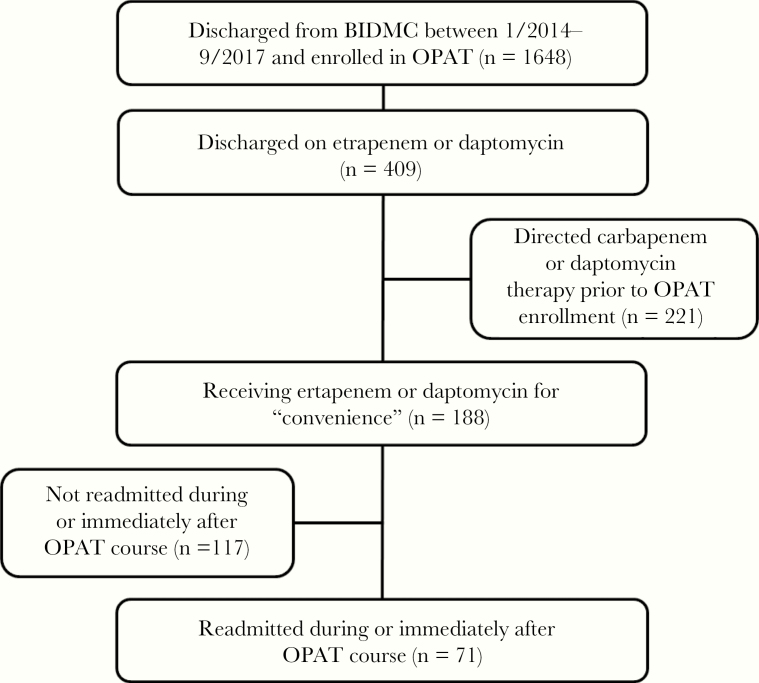
A total of 1648 patients were discharged from the hospital with OPAT enrollment, and 409 were discharged on ertapenem or daptomycin therapy (Figure 1). Of these, 221 patients were receiving directed carbapenem or daptomycin therapy and were excluded. Of the remaining 188 patients receiving an OPAT EOA regimen, 71 were readmitted, representing 113 unique readmissions and 77 unique OPAT courses.
Figure 1.
Flowchart of patient selection.
Of the 113 readmissions, patients were mostly males (71%), of older age (median, 57 years; IQR, 49–68), and a median weight of 83 kg (IQR, 75–97.7). Most were receiving OPAT at home (89%) versus an extended care facility (11%). Antimicrobial allergies were documented in approximately 26% of readmissions with penicillin (35%) and sulfonamide (35%) listed most commonly. Baseline characteristics are listed in Table 1.
Table 1.
Baseline Characteristics
| Characteristic (n = 113)a | n | % |
|---|---|---|
| Age (years) | ||
| Median [IQR] | 57 [49–68] | |
| Sex (male) | 81 | 71.0 |
| Weight (kg) | ||
| Median [IQR] | 83 [75–97.7] | |
| Location of OPAT receipt | ||
| Home | 100 | 88.5 |
| Extended care | 13 | 11.5 |
| Antibiotic Allergy | 29 | 25.7 |
| Penicillins | 10 | 34.5 |
| Sulfonamides | 10 | 34.5 |
| Fluoroquinolones | 5 | 17.2 |
| Other | 9 | 31.0 |
| EOA Regimen | ||
| Daptomycin | 31 | 27.4 |
| Ertapenem | 78 | 69.0 |
| Both | 4 | 3.5 |
| Concomitant agents | 43 | 38.1 |
| OPAT Indication | ||
| Osteomyelitis | 45 | 38.8 |
| Intra-abdominal | 35 | 30.2 |
| Empyema | 11 | 9.5 |
| Prosthetic joint infection | 8 | 6.9 |
| Diabetic foot | 5 | 4.3 |
| Skin and soft tissue | 4 | 3.4 |
| Urinary tract | 2 | 1.7 |
| CLABSI | 1 | 0.9 |
| Other | 5 | 4.3 |
Abbreviations: CLABSI, central-line associated bloodstream infection; EOA, ease of administration; IQR, interquartile range; OPAT, outpatient parenteral antibiotic therapy
a113 unique readmissions from 71 OPAT patients receiving daptomycin and/or ertapenem in 77 unique OPAT courses.
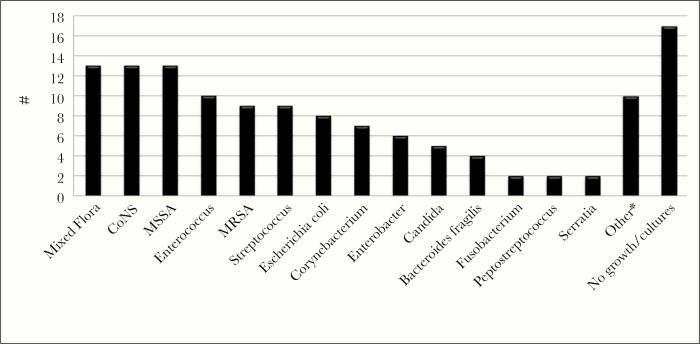
Ertapenem was used more frequently for EOA (69%) than daptomycin (27%), with both agents used concurrently in a small proportion of cases (4%). Approximately 38% received other antimicrobial agents concomitantly as part of their OPAT regimen. Osteomyelitis was the most common indication for therapy (39%), followed by intra-abdominal infection (30%), empyema (10%), prosthetic joint infection (7%), diabetic foot infection (4%), acute bacterial skin and soft tissue infection (3%), urinary tract infection (2%), and central line-associated bloodstream infection (1%). Infections were polymicrobial and monomicrobial in nature in approximately 51% and 26% of readmissions, respectively. Cultures were unable to be obtained or demonstrated no growth in 23% of cases. Infections were largely caused by Gram-positive organisms, including Staphylococcus aureus (n = 22), coagulase-negative Staphylococcus (n = 13), and Enterococcus (n = 10), followed by Gram-negative organisms, including Escherichia coli (n = 8) and Enterobacter (n = 6). Candida species were isolated in 5 patients (Figure 2).
Figure 2.
Infecting organisms isolated from OPAT patients. *Other: Alcaligenes spp. (n=1), Bacillus spp. (n=1), Cutibacterium spp. (n=1), Klebsiella spp. (n=1), Micrococcus spp. (n=1), Pantoea spp. (n=1), Proteus spp. (n=1), Staphylococcus epidermiditis (n=1), S. lugdenensis (n=1), Stenotrophomonas spp. (n=1).
Overall, EOA regimens were continued in 27% of hospital readmissions (Table 2). Approximately 27% of readmissions were preplanned by the medical or surgical team, and 36% were related to the OPAT course in terms of worsening infection or adverse drug event. The ID service was consulted in 48% of cases. These consults prompted de-escalation in 30% of consults and continued the EOA regimen in 19%. The ASP intervened in 26% of cases, prompting de-escalation in 55% of these.
Table 2.
Inpatient Continuation of EOA Regimens
| Characteristic (n = 113) | n/N | % |
|---|---|---|
| Maintenance of EOA regimen upon readmission | 30 | 26.5 |
| Reason for Readmission | ||
| Preplanned admit | 30 | 26.5 |
| Related to OPAT course | 41 | 36.3 |
| ID Consult | ||
| Consultation | 54 | 47.8 |
| Prompted de-escalation | 16/54 | 29.6 |
| Continued “convenience regimen” | 10/54 | 18.5 |
| Antimicrobial Stewardship Intervention | ||
| Documented intervention | 29 | 25.7 |
| Prompted de-escalation | 16/29 | 55.2 |
Abbreviations: EOA, ease of administration; ID, Infectious Diseases; OPAT, outpatient parenteral antimicrobial therapy.
The median length of stay for all readmissions was 5 days (IQR, 3–8). Those who were continued on their EOA regimens upon readmission received the EOA agent for a median of 2 days (IQR, 1–3) before a change in antimicrobial therapy or hospital discharge (Table 3). Of those who were continued on their EOA regimens, 70% received >1 dose of an EOA agent. The cost of inpatient EOA regimens was $15 644, representing a median cost per patient of $150 (IQR, 142–786). If patients had been continued on their pre-OPAT inpatient regimen upon readmission, estimated inpatient cost would have been $3440, or a median of $83 per patient (IQR, 44–144). This would have resulted in an estimated total cost savings of $12 204, or a median of $106 per patient (IQR, 1–685).
Table 3.
Cost of Inpatient EOA Regimens
| Inpatient Convenience | Inpatient Pre-OPAT | Overall | |
|---|---|---|---|
| Length of Stay | |||
| Median [IQR], days | 5 [3–8] | ||
| Length of “convenience” therapy | |||
| Median [IQR], days | 2 [1–3] | ||
| Costa | 15 643.58 | 3439.54 | |
| Additional cost incurreda | 12 204.04 | ||
| Cost per readmissiona Median [IQR] | 150.00 [142.26–786.16] | 82.65 [44.41–144.21] | |
| Additional cost incurreda per readmission, Median [IQR] | 106.32 [0.37–684.84] |
Abbreviations: EOA, ease of administration; IQR, interquartile range; OPAT, outpatient parenteral antimicrobial therapy.
aCost information obtained from Lexicomp.
Adverse events were reported in approximately 12% of all cases (Table 4). Daptomycin led to adverse events in 17% of cases and was most associated with elevated creatine phosphokinase levels (6%). Ertapenem resulted in adverse events in 9% of cases and was most associated with drug-induced fever (2.4%) and seizures (2.4%). In one case, an adverse event attributed to vancomycin occurred in a patient also receiving ertapenem for EOA. No adverse events were documented for other agents used in inpatient regimens after readmission. Clostridium difficile infections occurred in 7% of readmissions, including a 9% infection rate in the daptomycin group and 6% in the ertapenem group.
Table 4.
Adverse Events as Documented in the Medical Record
| Daptomycin (n=35) n (%) | Ertapenem (n=82)\ n (%) | Overall (n=117) n (%) | |
|---|---|---|---|
| Any ADR | - | 13 (11.5)a | |
| Drug-induced fever | - | 2 (2.4) | |
| Seizure | - | 2 (2.4) | |
| Elevated CPK | 2 (5.7) | - | |
| Transaminitis | 1 (2.9) | 1 (1.2) | |
| Hematologic effects | 1 (2.9) | 1 (1.2) | |
| Other | 2 (5.7) | 1 (1.2) | |
| Clostridioides (Clostridium) difficile b | 3 (8.6) | 5 (6.1) |
Abbreviations: ADR, adverse drug reaction; CPK, creatine phosphokinase.
aOne adverse event attributed to vancomycin occurred in the outpatient setting in a patient also receiving ertapenem for ease of administration.
bPolymerase chain reaction positive for C difficile.
Discussion
There is a large pool of existing literature investigating OPAT-related adverse events and readmissions [4–10], but little information is available regarding inpatient OPAT antimicrobial regimens after readmission. To our knowledge, this is the first study to directly examine this matter and identifies an important opportunity for collaboration between ID services, ASPs, and OPAT teams.
Although not the focus of their study, Dela-Pena et al [3] noted inpatient continuation of OPAT regimens as a stewardship intervention opportunity in their retrospective study of the top consumers of their inpatient antimicrobial budget. Approximately 5% of patients were continued on OPAT daptomycin or ertapenem, representing 7% of 71 identified stewardship intervention opportunities. In our study, more than one quarter of OPAT patients were continued on their EOA regimens while readmitted. Ideally, ID services, ASPs, and OPAT teams identify these situations and collaborate on tailoring of antimicrobial regimens in the first 24 hours of readmission. In actuality, the ID service was consulted in almost half of readmissions in this study, but recommendations to de-escalate therapy occurred in less than one third of these consults. Likewise, our ASP, which conducts preprescriptive review of the 3 antimicrobials targeted in this study, documented an intervention in only one quarter of readmissions, prompting de-escalation in half of these cases. Because this was a retrospective study, reasons for maintaining the OPAT regimens were not always readily available. As a preemptive stewardship strategy to reduce inpatient EOA regimens, OPAT providers may choose to adapt notes to include statements clarifying why regimens were changed upon OPAT initiation and how this can be reconciled upon hospital readmission. For example, the note may read, “Patient received vancomycin thrice daily while inpatient for MRSA osteomyelitis; however, he will be converted to daptomycin daily infusions for ease of administration once discharged. If unplanned hospital readmission occurs, thrice daily vancomycin can be restarted.”
However, de-escalation of OPAT EOA regimens upon readmission may not be optimal for all patients. We observed that 27% of readmissions were preplanned for scheduled procedures and usually resulted in short lengths of stay (mean of 4 days vs 7 days for unplanned readmissions). In these cases, frequent switching between antimicrobials may increase the risk for errors during transitions of care or introduce adverse events that could complicate further OPAT regimens. In one study, Shrestha et al [8] observed a 60% lower rate of antimicrobial-associated adverse events with daptomycin versus vancomycin in a propensity score-matched cohort study of OPAT patients. Likewise, in a retrospective study at our institution, Schrank et al [10] found more dosing changes and higher early discontinuation rates (19% vs 7.6%, P < .01) and increased reports of hypersensitivity reactions (22% vs 0%) with vancomycin versus daptomycin therapy. Furthermore, with limited resources, ASPs must prioritize which activities receive dedicated attention. Differentiating between short, planned admissions versus longer, unplanned admissions will be important to maximize the effectiveness and efficiency of already lean ASPs. Therefore, coordination with the OPAT team and ID services is vital to determine an optimal inpatient regimen for readmitted OPAT patients.
Because OPAT patients receive the majority of their care outside the healthcare system, there is a potential for increased or missed adverse events related to EOA agents. Current evidence reports adverse event rates of up to 30% in patients receiving OPAT [7]. In their retrospective chart review, Hale et al [7] observed that 8% of patients experienced an OPAT-related adverse event that resulted in hospital readmission or an emergency department visit. Our observed adverse event rate of 12% is within previously reported ranges. However, because we focused only on patients readmitted to the institution, we potentially missed patients managed in the outpatient setting. Identification of adverse events was retrospective and based on medical record documentation and, therefore, should be interpreted cautiously. Although we observed a higher rate of C difficile infection with daptomycin, one patient was readmitted multiple times with positive testing for C difficile by PCR. In addition, we did not further evaluate for active infection.
Because one of the benefits of OPAT is cost avoidance by decreased hospital length of stay, there is additional incentive to reduce expenditures from inpatient use of OPAT antimicrobials. However, in this study, cost differences between inpatient pre-OPAT and EOA regimens were minimal with a total estimated increased drug cost of $12 200 for 30 readmissions (or $110 per readmission). Of note, this estimate does not include other direct and indirect costs of administering medications in hospitals, such as obtaining drug levels for therapeutic drug monitoring.
There are several limitations of our study. First, it is retrospective and observational in design, which may limit any definitive conclusions. In addition, data collection was dependent on documentation in the medical records, so it is possible that not all outcomes were able to be recorded. However, this study provides important preliminary information in a less studied area of antimicrobial stewardship. Second, we were unable to capture readmissions at other institutions to fully determine readmission and EOA regimen continuation rates. However, it is assumed that these regimens would have been continued at outside institutions because those providers may not have immediate access to patients’ OPAT histories. Third, we were unable to determine resistance rates from the use of broad-spectrum antimicrobials. Antimicrobial resistance is of increasing global concern and is responsible for approximately 2 million infections and 23 000 deaths per year in the United States [11]. In vivo development of resistance has been described for both daptomycin and ertapenem after drug exposure [12–16]. Thus, the potential contribution of broader spectrum, EOA regimens to resistance development remains a concern, but literature demonstrating this phenomenon in the OPAT setting is limited. Fourth, we did not account for all indirect and direct costs of inpatient EOA regimens for the purposes of this study.
Conclusions
In conclusion, our study shows that continuation of EOA regimens upon OPAT patient readmission may occur frequently. Antimicrobial Stewardship Programs and ID services may need to dedicate resources to these cases as part of daily stewardship activities and collaborate with OPAT teams to identify patients in whom to tailor EOA regimens upon hospital readmission. More research is warranted to fully determine the impact of this practice in institutions worldwide.
Acknowledgments
Author contributions. R. S. B. contributed to project design, data collection, statistical analysis, manuscript writing, and review. M. T. L. contributed to project design, data collection, manuscript writing, and review. S. P. contributed to project design, manuscript writing, and review. P. P. contributed to project design, statistical analysis, manuscript writing, and review. C. M. contributed to project design, manuscript writing, and review. M. V. M. contributed to project design, data collection, statistical analysis, manuscript writing, and review.
Potential conflicts of interest. M. V. M is a member of the advisory board for Melinta Therapeutics, Spero Therapeutics, Tetraphase Pharmaceuticals, Inc., QPex Biopharma, and CutisPharma, Inc. and has received research funding from Merck & Co. She also is a member of the Speaker bureau for Tetraphase Pharmaceuticals and Cepheid. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: IDWeek 2018, San Francisco, CA.
References
- 1. Gilchrist M, Seaton RA. Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: challenges and checklists. J Antimicrob Chemother 2015; 70:965–70. [DOI] [PubMed] [Google Scholar]
- 2. Tice AD, Rehm SJ, Dalovisio JR, et al. ; IDSA Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis 2004; 38:1651–72. [DOI] [PubMed] [Google Scholar]
- 3. Dela-Pena J, Kerstenetzky L, Schulz L, et al. Top 1% of inpatients administered antimicrobial agents comprising 50% of expenditures: a descriptive study and opportunities for stewardship intervention. Infect Control Hosp Epidemiol 2017; 38:259–65. [DOI] [PubMed] [Google Scholar]
- 4. Keller SC, Williams D, Gavgani M, et al. Rates of and risk factors for adverse drug events in outpatient parenteral antimicrobial therapy. Clin Infect Dis 2018; 66:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cervera C, Sanroma P, González-Ramallo V, et al. ; DAPTODOM Investigators Safety and efficacy of daptomycin in outpatient parenteral antimicrobial therapy: a prospective and multicenter cohort study (DAPTODOM trial). Infect Dis (Lond) 2017; 49:200–7. [DOI] [PubMed] [Google Scholar]
- 6. Farry JK, Miles CD, Collins CD, Malani PN. Adverse events among renal transplant recipients receiving outpatient parenteral antimicrobial infusion therapy. Transpl Infect Dis 2009; 11:284–5. [DOI] [PubMed] [Google Scholar]
- 7. Hale CM, Steele JM, Seabury RW, Miller CD. Characterization of drug-related problems occurring in patients receiving outpatient antimicrobial therapy. J Pharm Pract 2017; 30:600–5. [DOI] [PubMed] [Google Scholar]
- 8. Shrestha NK, Mason P, Gordon SM, et al. Adverse events, healthcare interventions and healthcare utilization during home infusion therapy with daptomycin and vancomycin: a propensity score-matched cohort study. J Antimicrob Chemother 2014; 69:1407–15. [DOI] [PubMed] [Google Scholar]
- 9. Lee B, Tam I, Weigel B 4th, et al. Comparative outcomes of β-lactam antibiotics in outpatient parenteral antibiotic therapy: treatment success, readmissions and antibiotic switches. J Antimicrob Chemother 2015; 70:2389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schrank GM, Wright SB, Branch-Elliman W, LaSalvia MT. A retrospective analysis of adverse events among patients receiving daptomycin versus vancomycin during outpatient parenteral antimicrobial therapy. Infect Control Hosp Epidemiol 2018; 39:947–54. [DOI] [PubMed] [Google Scholar]
- 11. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep 2016; 65:1–12. [DOI] [PubMed] [Google Scholar]
- 12. Sabol K, Patterson JE. Emergence of daptomycin resistance in Enterococcus faecium during daptomycin therapy. Antimicrob Agents Chemother 2005; 49:1664–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garcia-de-la-Maria C, Pericas JM, del Rio A, et al. Early in vitro and in vivo development of high-level daptomycin resistance is common in mitis group streptococci after exposure to daptomycin. Antimicrob Agents Chemother 2013; 57:2319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Munoz-Price LS, Lolans K, Quinn JP. Emergence of resistance to daptomycin during treatment of vancomycin-resistant Enterococcus faecalis infection. Clin Infect Dis 2005; 41:565–6. [DOI] [PubMed] [Google Scholar]
- 15. Elliott E, Brink AJ, van Greune J, et al. In vivo development of ertapenem resistance in a patient with pneumonia caused by Klebsiella pneumoniae with an extended-spectrum beta-lactamase. Clin Infect Dis 2006; 42:e95–8. [DOI] [PubMed] [Google Scholar]
- 16. Skurnik D, Lasocki S, Bremont S, et al. Development of ertapenem resistance in a patient with mediastinitis caused by Klebsiella pneumoniae producing an extended-spectrum beta-lactamase. J Med Microbiol 2010; 59:115–9. [DOI] [PubMed] [Google Scholar]