Abstract
Background
With the increasing frequency and impact of Ebola virus disease (EVD) outbreaks illustrated by recent epidemics, a good understanding of the extent of viral persistance or ribonucleic acid (RNA) detection in body fluids from survivors is urgently needed.
Methods
Ebola viral RNA shedding was studied with molecular assays in semen (n = 1368), urine (n = 1875), cervicovaginal fluid (n = 549), saliva (n = 900), breast milk (n = 168), and feces (n = 558) from EVD survivors in Guinea (PostEbogui cohort, n = 802) at a regular base period until 40 months after inclusion.
Results
Twenty-seven of 277 (9.8%) male survivors tested positive for Ebola RNA in at least 1 semen sample. The probability of remaining positive for Ebola RNA in semen was estimated at 93.02% and 60.12% after 3 and 6 months. Viral RNA in semen was more frequent in patients with eye pain (P = .036), joint pain (P = .047), and higher antibody levels to Ebola virus antigens (nucleoprotein [P = .001], glycoprotein [P = .05], and viral protein-40 [P = .05]). Ebola RNA was only rarely detected in the following body fluids from EVD survivors: saliva (1 of 454), urine (2 of 593), breast milk (2 of 168), cervicovaginal secretions (0 of 273), and feces (0 of 330). Ribonucleic acid was detected in breast milk 1 month after delivery but 500 days after discharge of Ebola treatment unit (ETU) in 1 woman who became pregnant 7 months after discharge from the ETU.
Conclusions
The frequency and potential long-term presence of viral RNA in semen confirmed that systematic prevention measures in male survivors are required. Our observation in breast milk suggests that our knowledge on viral reservoir in immune-privileged sites and its impact are still incomplete.
Keywords: body fluids, breast milk, Ebola, Guinea, semen
On 5,400 body fluid samples from Ebola Virus Disease (EVD) survivors, during 40 months follow-up in Guinea, RNA was observed in semen and breast milk for up to 500 days, illustrating the complexity of the viral reservoir and management of survivors.
Since the first outbreak of Ebola virus disease (EVD) in 1976, in the Democratic Republic of Congo (DRC), 28 outbreaks have been reported across Africa [1]. In general, Ebola virus (EBOV) outbreaks remained restricted to rural or semirural areas and with a limited number of victims. The most important outbreak occurred between December 2013 and March 2016, when the virus spread from a rural area in southeast Guinea to the neighboring countries of Liberia and Sierra Leone and reached the capital cities from each country. More than 28 000 individuals became infected, and at least 11 000 people died [2]. The overall frequency of EBOV outbreaks seems to increase; for example, 3 independent outbreaks occurred between May 2017 and July 2018 in the DRC, and 2 of them also reached major cities [3, 4]. The actual outbreak that started in August 2018 in North Kivu has reached several densely populated cities, and today more than 3200 cases have already been identified, with mortality rate of approximately 60% [5].
The major route of human-to-human transmission of EBOV is direct contact with infected body fluids from symptomatic or deceased patients. However, episodes of EBOV re-emergence and unusual transmission chains have been reported in the West African outbreak [6–10]. The majority of these episodes were associated with viral persistence in semen, but transmission chains through other body fluids (breast milk, cervicovagianl fluids) are also suspected [7, 9, 11–13]. The persistence of EBOV in body fluids was already shown in the outbreak from Kikwit, DRC in 1995, and the large number of EBOV survivors from the West African outbreak increased our knowlegde, especially in semen [14–22]. In this report, we studied in detail viral ribonucleic acid (RNA) shedding in semen and other body fluids at a regular base period over a 4-year time frame in the PostEbogui cohort, which included 800 of 1270 EVD survivors from Guinea.
PATIENTS AND METHODS
Patients
The PostEbogui study is a prospective, multicenter, open cohort study monitoring, on a regular base period, EVD survivors for clinical, biological, and psychosocial parametes as well as presence of viral RNA in different body fluids [23]. Patients were recruited at any time after discharge between March 2015 and July 2016 from an Ebola treatment unit (ETU) at 4 different sites in Guinea: Donka National Hospital (Conakry), Macenta Prefectoral Hospital (Macenta), N’Zérékoré Regional Hospital (N’Zérékoré), and Forécariah Prefectoral Hospital (Forécariah). The median delay after ETU discharge and study enrollment was 350 days (interquartile range [IQR], 223–491). Patients were assessed at inclusion, 1 and 3 months afterwards, and subsequently every 6 months for up to 40 months. Body fluid sampling was proposed at each visit. One year after initiation of the study, no additional body fluids were collected from patients with 2 consecutive negative tests, except for semen. Semen, breast milk, and urine were collected in sterile containers. Saliva and cervicovaginal fluids were collected using a transport device combining a foam swab and placed into 1-mL viral transport medium (Sigma Virocult) or RNAlater. Feces were stored in RNAlater (1/1 volume). All samples were stored at 2–8°C on site and transported within 24 hours to the laboratory where samples were processed within 2 days or stored frozen at −40°C until testing.
Detection of Ebola Virus Ribonucleic Acid in Body Fluids
During the study period, polymerase chain reaction (PCR) tests were performed at 4 different laboratory facilities: the joint Dakar Pasteur Institute and “Laboratoire du Projet des Fièvres Hémorragiques de Guinée” laboratory in Donka National Hospital; the European West African Mobile Laboratory (EUWAM-Lab) and the laboratory of “Institut National de Santé Publique (INSP)”, all located in Conakry, the capital city. The laboratory facility deployed by the Pasteur Institute (Lyon, France) within the ETU of Macenta analyzed samples from this area during the outbreak. The laboratory facilities from Macenta and EUWAM-Lab stopped activities soon after the epidemic ended, and the activities of the laboratory at Donka hospital were interrupted due to renovation of Donka Hospital. All samples have been processed at the INSP since September 2016. Each laboratory used their specific PCR methods, and, depending on availability of assays and laboratories where tests were performed, the following quantitative reverse-transcription PCR (qRT-PCR) assays were used: RealStar Filovirus Screen RT-PCR Kit 1.0 (Altona Diagnostics GmbH, Hamburg, Germany), 2 published in-house techniques targeting the viral nucleoprotein (NP) [24, 25], and 1 unpublished assay. For this latter assay, primers and probes described by Huang et al [25] were adapted as follows: NP2F_ZR (forward primer) 5’-AGTACATGCAGAGCAAGGACTGATACA-3’, NP2R_ZR (reverse primer) 5’-GTTCGCATCAAACGGAAAATCAC-3’, and sNP2_ZR (probe) 5’-FAM-ATCCAACAGCTTGGCAATCAGTAGGACA-BHQ1-3’. For the qRT-PCR assays, RNA was extracted from 100 μL semen, urine, saliva, cervicovaginal fluid, and fecal samples with the QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany). To circumvent PCR inhibition due to seminal compounds, crude semen and seminal fluid were extracted separately when possible, and both extracts were tested undiluted and at 1:10 dilution. The qRT-PCR results are expressed as cycle threshold (Ct) values, with a Ct cutoff for positivity of <41. All other samples were also tested undiluted and at 1:10 dilution to reduce impact of PCR inhibitors.
The BioThreat-E test (BioFire Diagnostics, Salt Lake City, UT) was used on a subset of urine samples (according to the manufacturer’s instructions) using 200 μL urine. Once reports became available on the validation and high sensitivity of the Xpert Ebola Assay (Cepheid, Sunnyvale, CA) on semen [26], this assay was used to detect viral RNA in semen as recommended by the manufacturer. Samples were considered positive for the presence of viral RNA if either target gene (glycoprotein [GP] or NP) was detected. Xpert Ebola Assay was also used on a subset of breast milk samples.
Screening for Ebola Virus Antibodies
Plasma samples were tested for each EVD survivor for Ebola virus antibodies using our previously described serological assay based on Luminex technology [27]. Recombinant proteins of NP, viral protein-40 (VP40), and GP (from Mayinga and Kissoudougou strains) from Zaire EBOV species were used, and plasma samples were tested at 1:1000 dilution as previously reported. Antigen and antibody reactions were subsequently read on BioPlex-200 equipment (BioRad, Marnes-la-Coquette, France), and the results were expressed as median fluorescence intensity (MFI) per 100 beads.
Statistical Analysis
To model the time to test negativity in semen, ie, the period between discharge from ETU and the time when viral RNA was no longer detected with RT-PCR techniques, we used a time-to-event analysis as previously described [28]. All male participants who had at least 1 RT-PCR result (positive or negative) were included in the analysis. In brief, men were assumed to be positive at symptom onset and data were interval-censored; for patients with no negative test result after the last positive result, data were right-censored. The parametric survival model assuming a gamma distribution, associated to the highest likelihood with a survival curve similar to the Turnbull non-parametric approach was used. The Markov chain Monte Carlo algorithm, with noninformative prior for the parameters, was implemented to obtain the 95% confidence intervals (CIs). The relationship between presence of viral RNA in semen and the antibody levels to EBOV antigens (NP, GP, and VP40) or associations with clinical symptoms were studied with a logistic regression model. The effects of the factors were analyzed by using the multivariate models with a 95% CI. All analyses were done using Stata and R softwares. We used Fisher’s exact test to compare percentages.
RESULTS
Ebola Viral Ribonucleic Acid in Semen
The PostEbogui cohort included 360 male survivors, and 277 (77%) donated a semen sample at least once. The median time between discharge from ETU and semen donation was 14.4 months (IQR, 9–18.9; minimum 0.2 and maximum 47.2) for the first sample and 41.7 months (IQR, 24.8–47.5; minimum 2.5 and maximum 56.4) for the last one (Supplementary Figure S1), with a median follow-up of 27.4 months (IQR, 9.2–32.9; minimum 0 and maximum 45.7). A total of 1777 PCR tests have been realized on 1368 samples (mean 4.9 samples/patient; minimum 1 and maximum 12): 620 samples were tested with RealStar Filovirus Screen RT-PCR, 531 samples were tested with an in-house NP qRT-PCR assay, and 626 samples were tested with Ebola Xpert. In 2017, a higher sensitivity was reported for Ebola Xpert assay on semen samples [29], and RNA detection was re-evaluated retrospectively on available leftover samples, most closely to inclusion, for 191 of 273 (70.2%) participants. Ten of 14 patients, with sufficient leftover semen sample and who tested positive with RealStar and/or in-house NP assays, also tested positive with Ebola Xpert (Supplementary Table S1). It is interesting to note that retrospective retesting with Ebola Xpert identified 13 additional patients with viral RNA in semen. When combining the results of all assays, 27 patients tested positive for viral RNA in at least 1 semen sample (Table 1). Moreover, in 9 patients, identified positive by RealStar Filovirus and/or an in-house NP qRT-PCR, EBOV RNA was detected over a longer period in semen with the Xpert assay (Supplementary Table S1).
Table 1.
Ebola Viral RNA Detection in Semen Samples for Different Periods After Discharge From Ebola Treatment Center Using Different RNA Detection Assays
| ETU, EbolaTreatment Unit | In-House NP qRT-PCR | RealStar Filovirus | Ebola Xpert | All Tests Combined | ||||
|---|---|---|---|---|---|---|---|---|
| Months | Samples | Patients | Samples | Patients | Samples | Patients | Samples | Patients |
| N+/Ntested (%) | N+/Ntested (%) | N+/Ntested (%) | N+/Ntested (%) | N+/Ntested (%) | N+/Ntested (%) | N+/Ntested (%) | N+/Ntested (%) | |
| 0–3 | 7/24 (29.2) | 6/19 (31.2) | 2/3 (66.7) | 1/2 (50.0) | 8/8 (100.0) | 7/7 (100.0) | 12/24 (50.0) | 10/19 (52.6) |
| >3–6 | 4/28 (14.3) | 4/26 (15.4) | 2/14 (14.3) | 2/13 (15.4) | 10/14 (71.4) | 9/13 (69.2) | 13/34 (38.2) | 12/29 (41.4) |
| >6–9 | 5/52 (9.6) | 5/47 (10.6) | 0/25 (0.0) | 0/20 (0.0) | 6/21 (28.6) | 5/18 (27.8) | 10/62 (16.1) | 9/54 (16.7) |
| >9–12 | 1/76 (1.3) | 1/62 (1.6) | 0/51 (0.0) | 0/41 (0.0) | 6/37 (15.2) | 6/36 (16.7) | 7/107 (6.6) | 7/81 (8.7) |
| >12–15 | 0/69 (0.0) | 0/61 (0.0) | 0/78 (0.0) | 0/69 (0.0) | 5/45 (11.1) | 5/45 (11.1) | 5/108 (4.6) | 5/93 (5.4) |
| >15–18 | 1/51 (1.9) | 1/49 (2.1) | 1/86 (1.2) | 1/78 (1.3) | 1/45 (2.2) | 1/44 (2.3) | 1/111 (0.9) | 1/95 (1.1) |
| >18–21 | 0/77 (0.0) | 0/76 (0.0) | 0/131 (0.0) | 0/110 (0.0) | 0/41 (0.0) | 0/39 (0.0) | 0/169 (0.0) | 0/137 (0.0) |
| >21–24 | 0/80 (0.0) | 0/74 (0.0) | 0/93 (0.0) | 0/81 (0.0) | 0/9 (0.0) | 0/9 (0.0) | 0/143 (0.0) | 0/122 (0.0) |
| >24–27 | 0/50 (0.0) | 0/50 (0.0) | 0/49 (0.0) | 0/49 (0.0) | 0/10 (0.0) | 0/10 (0.0) | 0/104 (0.0) | 0/101 (0.0) |
| >27–30 | 0/20 (0.0) | 0/19 (0.0) | 0/40 (0.0) | 0/40 (0.0) | 0/18 (0.0) | 0/18 (0.0) | 0/76 (0.0) | 0/75 (0.0) |
| >30–33 | 0/4 (0.0) | 0/4 (0.0) | 0/35 (0.0) | 0/34 (0.0) | 0/25 (0.0) | 0/25 (0.0) | 0/63 (0.0) | 0/63 (0.0) |
| >33–36 | nd | nd | 0/15 (0.0) | 0/15 (0.0) | 0/54 (0.0) | 0/53 (0.0) | 0/69 (0.0) | 0/68 (0.0) |
| >36–39 | nd | nd | nd | nd | 0/54 (0.0) | 0/54 (0.0) | 0/54 (0.0) | 0/54 (0.0) |
| >39–42 | nd | nd | nd | nd | 0/59 (0.0) | 0/59 (0.0) | 0/59 (0.0) | 0/59 (0.0) |
| >42–45 | nd | nd | nd | nd | 0/46 (0.0) | 0/46 (0.0) | 0/46 (0.0) | 0/46 (0.0) |
| >45–48 | nd | nd | nd | nd | 0/65 (0.0) | 0/65 (0.0) | 0/65 (0.0) | 0/65 (0.0) |
| >48–51 | nd | nd | nd | nd | 0/52 (0.0) | 0/52 (0.0) | 0/52 (0.0) | 0/52 (0.0) |
| >51–54 | nd | nd | nd | nd | 0/15 (0.0) | 0/15 (0.0) | 0/15 (0.0) | 0/15 (0.0) |
| >54–57 | nd | nd | nd | nd | 0/7 (0.0) | 0/7 (0.0) | 0/7 (0.0) | 0/7 (0.0) |
| Total | 18/531 (3.4) | 14/225 (6.2) | 5/620 (0.8) | 3/243 (1.2) | 36/626 (5.8) | 23/238 (9.7) | 47/1368 (3.4) | 27/277 (9.8) |
Abbreviations: nd, not done; NP, nucleoprotein; qRT-PCR, quantitative reverse-transcription polymerase chain reaction; RNA, ribonucleic acid.
More important, all samples (8 of 8) from all patients (7 of 7) were positive for viral RNA when tested with Ebola Xpert less than 3 months after discharge from ETU, and 9 of 13 (69.2%) patients were still positive after 3 to 6 months. Only 1 patient tested positive for up to 512 days with the different RT PCR assays, but all of his subsequent samples, starting 1 month later until 39 months for the last sample, were negative (Supplementary Table S1). Nine hundred twenty-two semen samples from 246 patients tested 18 months after discharge from ETU were negative for viral RNA.
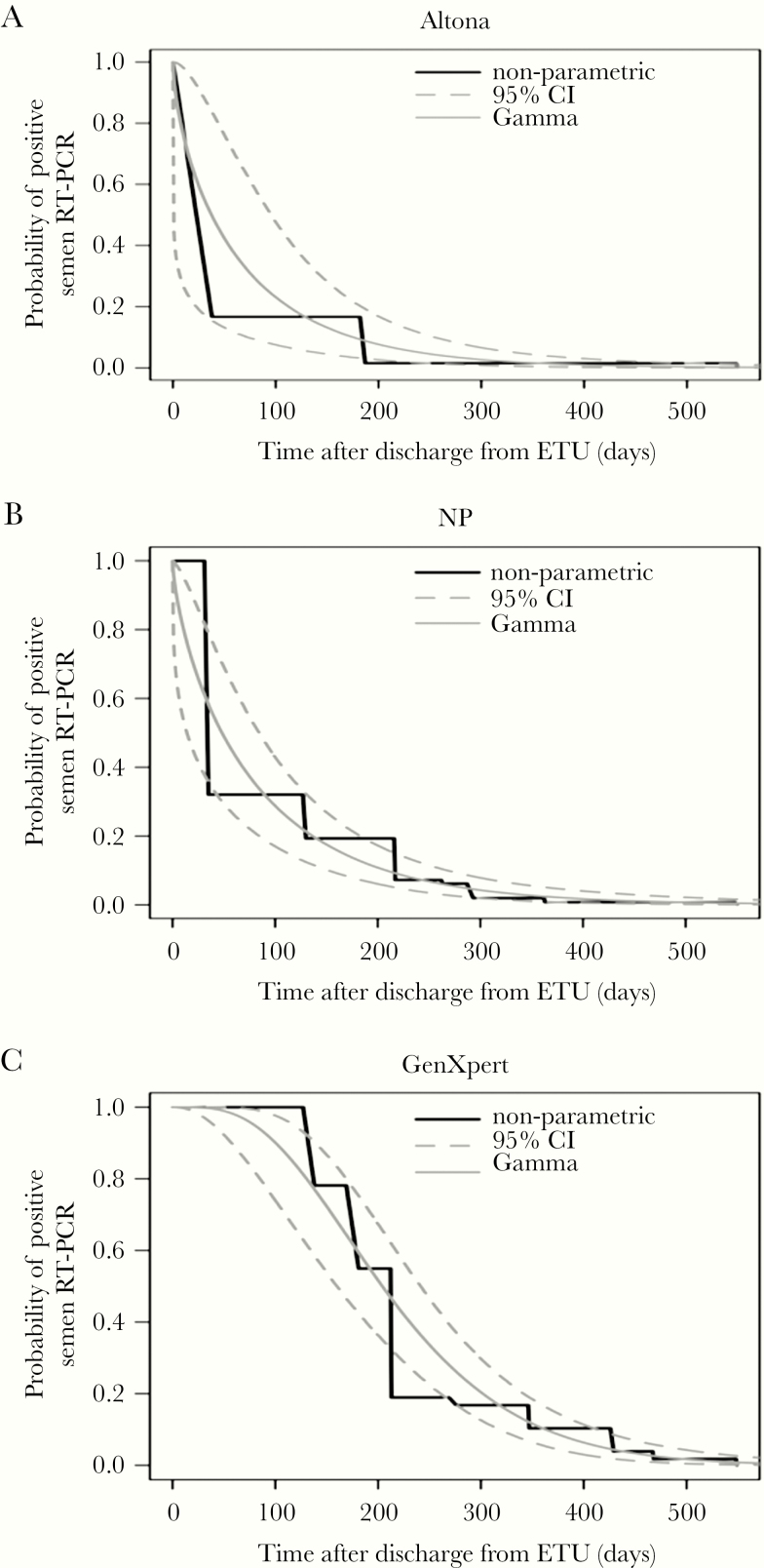
Modeling of viral RNA persistence in semen after discharge from ETU showed a median estimated time of 204.6 days (95% CI, 160.7–240.7) with Ebola Xpert, which is significantly longer than the time estimated with NP qRT-PCR and RealStar Filovirus Screen RT-PCR: 48.22 days (95% CI, 13.82–84.37) and 41.77 days (95% CI, 1.47–98.80), respectively (Figure 1). The probability of remaining positive for Ebola RNA in semen was estimated at 93.02% and 60.12% after 3 and 6 months, respectively, decreasing to 27.68% and 10.32% after 9 and 12 months, respectively, reaching 0.96% after 18 months, and becoming close to zero (0.06%) after 24 months (Table 2).
Figure 1.
Probability for male Ebola virus disease survivors to test positive for Ebola virus in semen by the different reverse-transcription polymerase chain reaction (RT-PCR) test used in our study according to time from discharge from the Ebola treatment unit, for the Turnbull nonparametric method and a parametric survival model using a gamma distribution: (a) RealStar Filovirus assay (Altona Diagnostics); (b) in-house nucleoprotein (NP) quantitative RT-PCR; (c) Ebola Xpert Assay (Cepheid). CI, confidence interval.
Table 2.
Probability in Percentage of Remaining Positive for EBOV RNA in Semen (According to RT-PCR) at 3, 6, 9, 12, 18, and 24 Months for Each Method
| Time After Discharge From ETU | In-House NP qRT-PCR | RealStar Filovirus | Ebola Xpert | All Tests Combined |
|---|---|---|---|---|
| Months | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) |
| 3 | 31.91 (18.84–45.25) | 28.90 (9.74–54.64) | 92.79 (78.12–98.72) | 93.02 (78.19–98.71) |
| 6 | 13.08 (7.46–20.44) | 10.72 (3.64–21.64) | 59.98 (43.14–76.08) | 60.12 (43.15–76.12) |
| 9 | 5.61 (2.64–10.06) | 4.15 (1.23–8.84) | 27.68 (18.01–38.83) | 28.00 (18.16–39.24) |
| 12 | 2.47 (0.87–5.29) | 1.70 (0.34–4.21) | 10.40 (5.74–16.43) | 10.32 (5.81–16.70) |
| 18 | 0.49 (0.08–1.75) | 0.34 (0.02–1.22) | 0.98 (0.23–2.84) | 0.96 (0.22–2.86) |
| 24 | 0.10 (0.006–0.65) | 0.08 (0.009–0.42) | 0.07 (0.004–0.46) | 0.06 (0.004–0.46) |
Abbreviations: CI, confidence interval; EBOV, Ebola virus; ETU, Ebola Treatment Unit; NP, nucleoprotein; qRT-PCR, quantitative reverse-transcription polymerase chain reaction; RNA, ribonucleic acid.
Mean age was comparable in positive versus negative patients (33.45 versus 32.68 years, P = .7), but we observed a positive and significant relationship between older age and the period of viral RNA detection in semen (r = 0.51, P = .0065). Eye pain and joint pain were more often reported in patients with viral RNA in semen; 11 of 27 (40.7%) versus 54 of 246 (21.9%) and 24 of 27 (88.9%) versus 175 of 246 (71.1%), respectively. Multivariate analysis showed that eye pain (adjusted odds ratio [AOR] = 2.56; 95% CI, 1.04–6.20; P = .036) and joint pain (AOR = 3.71; 95% CI, 1.16–16.70; P = .047) were significantly associated with RNA detection in semen.
Higher antibody levels to different EBOV proteins were observed in men who tested positive for Ebola RNA: median MFI of 1560 (IQR, 1060–2468) versus 1204 (IQR, 791–2140) for GP antigens, 2460 (IQR, 1674–3859) versus 1667 (IQR, 857–2681) for VP40, and 9449 (IQR, 6059–11125) versus 4766 (IQR, 2584–8450) for NP. The higher antibody levels in viral RNA-positive patients were significantly different for GP (OR = 1.54; 95% CI, 1.01–2.51; P = .05), VP40 (OR = 1.59; 95% CI, 1.01–2.62; P = .05), and especially to NP (OR = 3.06; 95% CI, 1.64–6.35; P = .001) proteins. All male EVD survivors with positive semen samples were human immunodeficiency virus (HIV) negative.
Ebola Viral Ribonucleic Acid in Other Body Fluids
A total of 4050 samples from other body fluids have also been tested: breast milk (n = 168, 109 patients), saliva (n = 900, 454 patients), cervicovaginal secretions (n = 549, 273 patients), feces (n = 558, 330 patients), and urine (n = 1875, 593 patients) (Table 3). In general, more than 1 sample was tested per patient with a mean number of 1.57 samples/patient for breast milk, 1.98 for saliva, 2.1 for cervicovaginal fluid, 1.7 for feces, and 3.2 for urine. A total of 4637 RT-PCR tests were realized: RealStar Filovirus Screen RT-PCR (n = 997), NP qRT-PCR assays (n = 3312), BioFire (n = 258), and Xpert Ebola (n = 70). For 653 samples, RealStar Filovirus Screen RT-PCR and NP qRT-PCR assays have been tested in parallel with similar results. Ebola viral RNA was detected in 2 saliva samples from a single female patient on samples taken 5 and 34 days after discharge from ETU and in 3 urine samples from 2 male patients on samples taken 7, 43, and 55 days after discharge from ETU (Table 3). In the same male patients, viral RNA was also detected in semen samples (1160 and 1170 in Supplementary Table S1) for up to 6 and 7 months. On 16 breast milk samples, retested with Ebola Xpert assay, 1 (ID1034) was positive on a sample at 58 days (Ct values for GP = 39.8 and NP = 36.4), and subsequent testing of 54 samples, not tested previously, revealed an additional woman (identification [ID] 3082) positive on the first sample taken after 500 days (Ct values for GP = 36.3 and NP = 32.2). Additional samples were only available 2 years later for the first patient (ID 1034), but, for the second patient (ID 3082), the 5 subsequent samples were taken between 1 and 10 months later, and all tested negative. The latter woman (ID 3082) was not pregnant when she developed EVD, and she had 2 children aged 6 and 2.5 years when she was at the ETU. She became pregnant 7 months after discharge from ETU and was included in the PostEbogui study when she attended the hospital for a visit related to complications at 8 months of pregnancy. The breast milk sample, taken 1 month after delivery (ie, 500 days after discharge from ETU), tested positive for EBOV RNA. Because of this unusual and late presence of viral RNA in breast milk, the breast milk samples from both women have been retested on the same and on a duplicate aliquot, confirming the results.
Table 3.
Ebola Viral RNA Detection in Body Fluids for Different Periods After Discharge From Ebola Treatment Center
| Time After Discharge | Breast Milk | Saliva | Cervicovaginal Fluid | Feces | Urine | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| From ETC | Samples | Patients | Samples | Patients | Samples | Patients | Samples | Patients | Samples | Patients |
| Months | N+/Ntested | N+/Ntested | N+/Ntested | N+/Ntested | N+/Ntested | |||||
| 0–3 | 1/1 | 1/1 | 2/84 | 1/60 | 0/32 | 0/26 | 0/77 | 0/59 | 3/128 | 2/79 |
| >3–6 | 0/2 | 0/2 | 0/94 | 0/89 | 0/43 | 0/42 | 0/84 | 0/73 | 0/144 | 0/118 |
| >6–9 | 0/8 | 0/8 | 0/226 | 0/175 | 0/121 | 0/90 | 0/156 | 0/128 | 0/310 | 0/227 |
| >9–12 | 0/11 | 0/9 | 0/252 | 0/201 | 0/132 | 0/115 | 0/153 | 0/130 | 0/378 | 0/291 |
| >12–15 | 0/19 | 0/17 | 0/121 | 0/116 | 0/88 | 0/85 | 0/59 | 0/58 | 0/296 | 0/245 |
| >15–18 | 1/33 | 1/28 | 0/88 | 0/83 | 0/98 | 0/91 | 0/5 | 0/5 | 0/320 | 0/236 |
| >18–21 | 0/42 | 0/34 | 0/26 | 0/26 | 0/24 | 0/23 | 0/4 | 0/4 | 0/201 | 0/161 |
| >21–24 | 0/32 | 0/25 | 0/6 | 0/6 | 0/7 | 0/7 | 0/8 | 0/8 | 0/57 | 0/54 |
| >24 | 0/20 | 0/17 | 0/3 | 0/3 | 0/4 | 0/4 | 0/12 | 0/12 | 0/41 | 0/41 |
| Total | 2/168 | 2/109 | 2/900 | 1/454 | 0/549 | 0/273 | 0/558 | 0/330 | 3/1875 | 2/593 |
Bold numbers highlight when positive samples were observed.
Abbreviations: ETC, Ebola Treatment Center; RNA, ribonucleic acid.
DISCUSSION
Information on presence and persistence of EBOV or Ebola viral RNA in body fluids is essential to evaluate and manage disease transmission from EVD survivors. The majority of our knowledge is from semen and few data are available on other body fluids. In this report, we studied, in a systematic way, the presence of viral RNA in semen over time but also in a large number of other body fluids including saliva, urine, cervicovaginal fluid, breast milk and feces in EVD survivors from the PostEbogui cohort [23]. More than 5400 samples from different body fluids have been tested by PCR, and more than 1 sample per body fluid has been analyzed for the majority of patients. For a large number of patients, semen samples have been analyzed for up to 40 months after discharge from ETU; for the other body fluids, samples have been analyzed for up to 24 months.
Viral persistence in breast milk and cervicovaginal fluids has been suspected in some unusual transmission chains [7, 12, 30]. In this study, we confirm the presence of viral RNA in breast milk in 2 of 99 women, but cervicovaginal fluids from 273 women tested negative. Although data from previous studies suggest that viral persistence and subsequent viral transmission from these body fluids is generally low or only during short periods of time [14, 31, 32], we confirmed the presence of Ebola RNA for up to 500 days in breast milk from 1 female EVD survivor [10]. More important, this woman was not pregnant when she developed EVD, but she became pregnant 7 months after discharge from ETU, and she tested positive in breast milk 1 month after delivery. There is no previous evidence or report on EBOV RNA detection in breast milk in women who become pregnant after they have recovered from EVD. Relapse due to EVD has been reported in a survivor who developed meningitis 9 months after recovery from acute EVD [33]. The reason for the presence of viral RNA in breast milk in this patient remains unclear; virus could have persisted in an immune-privileged site, with pregnancy or lactation being at the origin of relapse with detectable RNA in breast milk. However, RNA was quickly cleared, and no infectious virus made its way into breast milk to infect the newborn, or the infant had an asymptomatic course of illness. Finally, laboratory error causing a false-positive result cannot be excluded.
In this study, we estimated that EBOV RNA can persist for up to 200 days in semen, which is longer than the 46-day estimate from our previous analysis on fewer patients (188 versus 278), fewer follow-up samples (mean of 2.8 versus 4.9), a shorter follow-up period, and, more importantly, using less-sensitive assays [28]. With the more sensitive Ebola Xpert assay, almost all male survivors can be considered to be positive for Ebola RNA for up to 3 months, and 70% can be considered to be positive for up to 6 months. These data are in line with observations from studies in Sierra Leone and Liberia [21, 34]; ie, EBOV RNA was detected in all men within 3 months after ETU discharge in both countries and in 62% at 4 to 6 months in Liberia. No participants were enrolled in this period in Sierra Leone, but 8% and 25% were positive in Sierra Leone and Liberia, respectively, at 7 to 9 months after ETU discharge.
Although the estimated risk of persistance of viral RNA is almost zero after 24 months, 1 patient from Liberia was still positive after 40 months [15, 22]. In our study, all 223 samples from 158 patients were negative after 40 months. The higher total number of positive patients in the Prevail study from Liberia—30% compared with 10% in our study—could be related to the fact that all samples from the Prevail study were tested within 48 hours with Ebola Xpert and not retrospectively on frozen materials for the early samples. The presence of viral RNA does not mean that the virus is still infectious, but it has to be noted that the patient from the PostEbogui cohort who was still positive at 512 days after ETU discharge has been previously reported to be at the origin of a new cluster of EVD in Guinea and Liberia after the declaration of the end of the outbreak [35, 36]. The persisting virus was estimated to have been sexually transmitted approximately 470 days after onset of symptoms [36]. This patient was 55 years old, corresponding to observations from our and other studies that persistence of viral RNA is longer in older male survivors [34]. A previous report suggested that HIV status was associated with presence of viral RNA [17]; however, HIV prevalence in the PostEbogui cohort is 2%, but all positive patients were HIV negative. Similar to the study in Liberia, we found an association between viral RNA shedding in semen with ocular problems and higher antibody levels [22]. In addition, we also found an association with joint pain. Uveitis was shown to be associated with higher viral load during disease, suggesting a higher risk that the virus could remain present in other immune-privileged compartments, which explains the viral presence in semen and the association with joint pain [37]. Higher antibody levels could also suggest persistent immune stimulation with minute viral antigens.
Overall, our study shows that semen seems to be the body fluid at highest risk for additional transmissions after discharge from ETU, as also observed in previous reports [8, 32]. Although the aim of the study was not to compare performance of viral detection assays, we observed important differences among viral RNA detection techniques. Differences can be due to primer and probe sequences but also to sample volumes used in the tests or the viral RNA extraction methods. Moreover, performance and detection limits of the assays can also differ when used in different body fluids compared with blood. Thus, lower rates and absence of viral RNA detection in other body fluids is most likely underestimated depending on the assays used. This is clearly illustrated by retesting of a subset of breast milk and semen samples. Finally, because samples were tested at 3-month intervals, it cannot be excluded that we missed transient or sporadic positivity. However, given the high numbers of samples and patients tested, we expect that at least some samples would have been detected if these other body fluids were important viral reservoirs as was the case for semen in our study.
CONCLUSIONS
Overall, the data from our study clearly confirm the persistence of viral RNA in semen for up to 6 months in a large number of male survivors. More important, we showed that female EBV survivors who become pregnant after the disease can be positive for viral RNA in breastmilk even 500 days after ETU discharge. Similar to other studies, we also show that RNA detection decays over time in semen at variable rates in different survivors, and, as a consequence, transmission risks decrease due to lower inoculum, nonviable virus, or other factors. Nevertheless, these data illustrate the importance of prevention measures and biological monitoring of male EVD survivors. More studies are needed on pregnant women, including when pregnancy occurred after discharge from ETU, to evaluate whether pregnancy can have an impact on relapse of EVD and to educate healthcare workers on this potential risk. The World Health Organization now recommends vaccination for contacts of patients to stop rapidly viral transmission from symptomatic cases, and it can be expected that timely vaccination of contacts will also prevent or reduce sexual transmission. Systematic treatment with an effective antiviral Ebola drug should also be explored for the clearance of virus in body fluids.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the PostEbogui team for their daily work, all of the survivors, the Guinean survivors’ associations for their help in identifying and tracing survivors, the laboratory staff of Institut National de Santé Publique and Centre de Recherche et de Formation en Infectiologie (CERFIG) (Moriba Povogui, Jean-louis Monemou, Joel Ballè Koivogui, Abdoul Karim Soumah, Amara Bamba, and Saran Doumbouya), the laboratory facility from Macenta (Guillain Mikaty), and European West African Mobile Laboratory (Brunel Joanna, Belarbi Essia, Martinez Prudencio, Pannetier Delphine, Picard Caroline, Escudero Beatriz, Mengardi Chloe, Nguyen Xuan-Nhi, Tracqui Elodie, Hamied Lola, Olausson Mikaela, Verner-Carlsson Jenny, Godard Sabine, Moroso Marie, Thomas Damien, Ottmann Michele, Bernard Sandra, Yonga Wansi Gide Martial, Sylla Yahaya, Bocquin Anne, Sautter Carmen-Alexandra, Rittaud Adeline, Carbonnelle Caroline, Mundweiler Stephanie, Calland Noemie, Chevillard Eve, and Juven Fabienne).
Financial support. This work was funded in part by grants from the Institut National de la Santé et de la Recherche Médicale/Ebola Task Force; REACTing (REsearch and ACTion targeting emerging infectious diseases) and the Institut de Recherche pour le Développement and Montpellier Université d’Excellence (EBOHEALTH; I-Site MUSE, ANR-16-IDEX-0006). A. K. K. was supported by a postdoctoral fellowship from Montpellier Université d’Excellence (I-Site MUSE, ANR-16-IDEX-0006).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
PostEbogui Study Group:
Ahidjo Ayouba, Eric Delaporte, Alice Desclaux, Jean François Étard, Bruno Granouillac, Suzanne Izard, Alpha Kabinet Keita, Sandrine Leroy, Laura March, Philippe Msellati, Martine Peeters, Bernard Taverne, Sylvain Baize, Kaba Bangoura, Moumié Barry, Mohammed Cissé, Saliou Bella Diallo, Mamadou Safiatou Diallo, Mariama Sadjo Diallo, Ousmane Faye, Djenaba Kassé, Harissatou Niane, Mamadou Saliou Sow, Fodé Amara Traoré, Thierno Alimou Barry, Cécé Kpamou, Alpha Kabinet Keita, Mariama Djouldé Sall, Abdoulaye Touré, Mamoudou Cissé, Jean-François Delfraissy, Christelle Delmas, Cécile Etienne, Yazdan Yazdanpanah, Ibrahima Fofana, Ibrahima Savané, Sakoba Keita, Lamine Koivogui, Abdoulaye Touré, Falaye Traoré, Christine Lacarabaratz, Claire Levy Marchal, Yves Levy, N’Fally Magassouba, Vincent Mendiboure, Yves-Marie Pers, Hervé Raoul, and Yamoussa Youla
PostEbogui Study Group
IRD/INSERM/Monpellier University, Montpellier, France: Ahidjo Ayouba, Eric Delaporte, Alice Desclaux, Jean François Étard, Bruno Granouillac, Suzanne Izard, Alpha Kabinet Keita, Sandrine Leroy, Laura March, Philippe Msellati, Martine Peeters, and Bernard Taverne. Pasteur Institute/Unit of Biology of Emerging Viral Infections, Lyon, France: Sylvain Baize. Donka National Hospital, Conakry, Guinea: Kaba Bangoura, Moumié Barry, Mohammed Cissé, Saliou Bella Diallo, Mamadou Safiatou Diallo, Mariama Sadjo Diallo, Ousmane Faye, Djenaba Kassé, Harissatou Niane, Mamadou Saliou Sow, and Fodé Amara Traoré. Centre de Recherche et de Formation en Infectiologie de Guinée, Université Gamal Abdel Nasser de Conakry, Conakry, Guinea: Thierno Alimou Barry, Cécé Kpamou, Alpha Kabinet Keita, Mariama Djouldé Sall, and Abdoulaye Touré. Forecariah Prefectoral Hospital, Forecariah, Guinea: Mamoudou Cissé. Reacting, INSERM, Paris, France: Jean-François Delfraissy, Christelle Delmas, Cécile Etienne, and Yazdan Yazdanpanah. Macenta Prefectoral Hospital, Macenta, Guinea: Ibrahima Fofana and Ibrahima Savané. Ministry of Health, Conakry, Guinea: Sakoba Keita. Institut National de la Santé Publique, Conakry, Guinea: Lamine Koivogui, Abdoulaye Touré, and Falaye Traoré. INSERM, Paris, France: Christine Lacarabaratz, Claire Levy Marchal, and Yves Levy. Laboratory of Virology, Conakry University, Projet de Recherche sur les Fièvres Hémorragiques en Guinée, Conakry, Guinea: N’Fally Magassouba. ALIMA, Conakry, Guinea: Vincent Mendiboure. Monpellier University, Montpellier, France: Yves-Marie Pers. Laboratoire P4 Inserm-Jean Mérieux, US003 Inserm, Lyon, France: Hervé Raoul. N’Zérékoré Regional Hospital, N’Zérékoré, Guinea: Yamoussa Youla.
References
- 1. Malvy D, McElroy AK, de Clerck H, et al. . Ebola virus disease. Lancet 2019; 393:936–48. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. 2016 Situation Report: Ebola Virus Disease. Geneva: World Health Organization; 2016. [Google Scholar]
- 3. Mbala-Kingebeni P, Pratt CB, Wiley MR, et al. . 2018 Ebola virus disease outbreak in Équateur Province, Democratic Republic of the Congo: a retrospective genomic characterisation. Lancet Infect Dis 2019; 19: 641–7. [DOI] [PubMed] [Google Scholar]
- 4. Mbala-Kingebeni P, Aziza A, Di Paola N, et al. . Medical countermeasures during the 2018 Ebola virus disease outbreak in the North Kivu and Ituri Provinces of the Democratic Republic of the Congo: a rapid genomic assessment. Lancet Infect Dis 2019; 19: 648–57 [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. Ebola situation reports: Democratic Republic of the Congo. Available at: https://www.who.int/ebola/situation-reports/drc-2018/en/. Accessed 15 October 2019.
- 6. Christie A, Davies-Wayne GJ, Cordier-Lassalle T, et al. ; Centers for Disease Control and Prevention (CDC) Possible sexual transmission of Ebola virus - Liberia, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:479–81. [PMC free article] [PubMed] [Google Scholar]
- 7. Dokubo EK, Wendland A, Mate SE, et al. . Persistence of Ebola virus after the end of widespread transmission in Liberia: an outbreak report. Lancet Infect Dis 2018; 18:1015–24. [DOI] [PubMed] [Google Scholar]
- 8. Brainard J, Pond K, Hooper L, et al. . Presence and persistence of Ebola or marburg virus in patients and survivors: a rapid systematic review. PLoS Negl Trop Dis 2016; 10:e0004475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Subissi L, Keita M, Mesfin S, et al. . Ebola virus transmission caused by persistently infected survivors of the 2014–2016 outbreak in West Africa. J Infect Dis 2018; 218 (suppl_5): S287–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vetter P, Fischer WA 2nd, Schibler M, et al. . Ebola virus shedding and transmission: review of current evidence. J Infect Dis 2016; 214:177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nordenstedt H, Bah EI, de la Vega MA, et al. . Ebola virus in breast milk in an Ebola virus-positive mother with twin babies, Guinea, 2015. Emerg Infect Dis 2016: 759–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sissoko D, Keïta M, Diallo B, et al. . Ebola virus persistence in breast milk after no reported illness: a likely source of virus transmission from mother to child. Clin Infect Dis 2017; 64:513–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mate SE, Kugelman JR, Nyenswah TG, et al. . Molecular evidence of sexual transmission of Ebola virus. N Engl J Med 2015; 373:2448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodriguez LL, De Roo A, Guimard Y, et al. . Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis 1999; 179 (Suppl 1):S170–6. [DOI] [PubMed] [Google Scholar]
- 15. Fischer WA, Brown J, Wohl DA, et al. . Ebola virus ribonucleic acid detection in semen more than two years after resolution of acute Ebola virus infection. Open Forum Infect Dis 2017; 4:ofx155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barnes KG, Kindrachuk J, Lin AE, et al. . Evidence of Ebola virus replication and high concentration in semen of a patient during recovery. Clin Infect Dis 2017; 65:1400–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Purpura LJ, Rogers E, Baller A, et al. . Ebola virus RNA in semen from an HIV-positive survivor of Ebola. Emerg Infect Dis 2017; 23:714–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sissoko D, Duraffour S, Kerber R, et al. . Persistence and clearance of Ebola virus RNA from seminal fluid of Ebola virus disease survivors: a longitudinal analysis and modelling study. Lancet Glob Health 2017; 5:e80–8. [DOI] [PubMed] [Google Scholar]
- 19. Keita AK, Toure A, Sow MS, et al. ; POSTEBOGUI Study Group Extraordinary long-term and fluctuating persistence of Ebola virus RNA in semen of survivors in Guinea: implications for public health. Clin Microbiol Infect 2017; 23:412–3. [DOI] [PubMed] [Google Scholar]
- 20. Sow MS, Etard JF, Baize S, et al. ; Postebogui Study Group New evidence of long-lasting persistence of Ebola virus genetic material in semen of survivors. J Infect Dis 2016; 214:1475–6. [DOI] [PubMed] [Google Scholar]
- 21. Deen GF, Broutet N, Xu W, et al. . Ebola RNA persistence in semen of Ebola virus disease survivors - final report. N Engl J Med 2017; 377:1428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. PREVAIL III Study Group. A longitudinal study of Ebola sequelae in liberia. N Engl J Med 2019; 380:924–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Etard JF, Sow MS, Leroy S, et al. . Postebogui Study Group Multidisciplinary assessment of post-Ebola sequelae in Guinea (Postebogui): an observational cohort study. Lancet Infect Dis 2017; 17:545–52. [DOI] [PubMed] [Google Scholar]
- 24. Weidmann M, Mühlberger E, Hufert FT. Rapid detection protocol for filoviruses. J Clin Virol 2004; 30:94–9. [DOI] [PubMed] [Google Scholar]
- 25. Huang Y, Wei H, Wang Y, et al. . Rapid detection of filoviruses by real-time TaqMan polymerase chain reaction assays. Virol Sin 2012; 27:273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loftis AJ, Quellie S, Chason K, et al. . Validation of the cepheid geneXpert for detecting Ebola virus in semen. J Infect Dis 2017; 215:344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ayouba A, Touré A, Butel C, et al. . Development of a sensitive and specific serological assay based on luminex technology for detection of antibodies to zaire ebola virus. J Clin Microbiol 2017; 55:165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Subtil F, Delaunay C, Keita AK, et al. . Postebogui Study Group Dynamics of Ebola RNA persistence in semen: a report from the Postebogui cohort in Guinea. Clin Infect Dis 2017; 64:1788–90. [DOI] [PubMed] [Google Scholar]
- 29. Pettitt J, Higgs E, Fallah M, et al. . Assessment and optimization of the GeneXpert diagnostic platform for detection of Ebola virus RNA in seminal fluid. J Infect Dis 2017; 215:547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Subissi L. Can Ebola virus re-emerge from survivors’ body fluids other than semen? Lancet Infect Dis 2018; 18:933–4. [DOI] [PubMed] [Google Scholar]
- 31. Liu WJ, Sesay FR, Coursier A, et al. . Comprehensive clinical and laboratory follow-up of a female patient with ebola virus disease: Sierra Leone Ebola virus persistence study. Open Forum Infect Dis 2019; 6:ofz068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Green E, Hunt L, Ross JCG, et al. . Viraemia and Ebola virus secretion in survivors of Ebola virus disease in Sierra Leone: a cross-sectional cohort study. Lancet Infect Dis 2016; 16:1052–6. [DOI] [PubMed] [Google Scholar]
- 33. Jacobs M, Rodger A, Bell DJ, et al. . Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet 2016; 388:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Soka MJ, Choi MJ, Baller A, et al. . Prevention of sexual transmission of Ebola in Liberia through a national semen testing and counselling programme for survivors: an analysis of Ebola virus RNA results and behavioural data. Lancet Glob Health 2016; 4:e736–43. [DOI] [PubMed] [Google Scholar]
- 35. Uyeki TM, Erickson BR, Brown S, et al. . Ebola virus persistence in semen of male survivors. Clin Infect Dis 2016; 62:1552–5. [DOI] [PubMed] [Google Scholar]
- 36. Diallo B, Sissoko D, Loman NJ, et al. . Resurgence of Ebola virus disease in guinea linked to a survivor with virus persistence in seminal fluid for more than 500 days. Clin Infect Dis 2016; 63:1353–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mattia JG, Vandy MJ, Chang JC, et al. . Early clinical sequelae of Ebola virus disease in Sierra Leone: a cross-sectional study. Lancet Infect Dis 2016; 16:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.