Abstract
Objective
In this study, we evaluated the effectiveness of a management bundle for Enterococcus spp bloodstream infection (E-BSI).
Method
This was a single-center, quasi-experimental (pre/post) study. In the prephase (January 2014 to December 2015), patients with monomicrobial E-BSI were retrospectively enrolled. During the post- or intervention phase (January 2016 to December 2017), all patients with incident E-BSI were prospectively enrolled in a nonmandatory intervention arm comprising infectious disease consultation, echocardiography, follow-up blood cultures, and early targeted antibiotic treatment. Patients were followed up to 1 year after E-BSI. The primary outcome was 30-day mortality.
Results
Overall, 368 patients were enrolled, with 173 in the prephase and 195 in the postphase. The entire bundle was applied in 15% and 61% patients during the pre- and postphase, respectively (P < .001). Patients enrolled in the postphase had a significant lower 30-day mortality rate (20% vs 32%, P = .0042). At multivariate analysis, factors independently associated to mortality were age (hazard ratio [HR], 1.03; 95% confidence interval [CI], 1.00–1.05), intensive care unit admission (HR, 2.51; 95% CI, 1.18–3.89), and healthcare-associated (HR, 2.32; 95% CI, 1.05–5.16) and hospital-acquired infection (HR, 2.85; 95% CI, 1.34–4.76), whereas being enrolled in the postphase period (HR, 0.49; 95% CI, 0.32–0.75) was associated with improved survival. Results were consistent also in the subgroups with severe sepsis (HR, 0.37; 95% CI, 0.16–0.90) or healthcare-associated infections (HR, 0.53; 95% CI, 0.31–0.93). A significantly lower 1-year mortality was observed in patients enrolled in the postphase period (50% vs 68%, P < .001).
Conclusions
The introduction of a bundle for the management of E-BSI was associated with improved 30-day and 1-year survival.
Keywords: adequate therapy, bundle, echocardiography, Enterococcus, infectious disease consultation
Regular infectious disease consultation, routine echocardiography, and follow-up blood cultures for enterococcal bloodstream infection are associated with lower mortality. The efficacy of the bundle was confirmed also in critically ill patients and in case of healthcare-associated infection.
Introduction
Enterococcus spp is the fourth most common causative pathogen of bloodstream infection (BSI) in Europe after Escherichia coli, Staphylococcus aureus, and Streptococcus pneumoniae [1, 2]. The incidence of infections caused by Enterococcus spp has increased over the last decades, probably due to aging of the global population and the increasing prevalence of immunocompromised patients [3]. Enterococcus spp is an important cause of infective endocarditis (IE), especially in the elderly, being the third etiological agent after staphylococci and streptococci [4]. Among patients with Enterococcal-BSI, IE is diagnosed in up to 26% [5], with reported higher prevalence of IE among patients presenting with Enterococcus faecalis BSI compared with those affected by BSI caused by other Enterococcal species [4, 6, 7]. Enterococcus spp presents several factors that can contribute to its pathogenicity, including the intrinsic resistance to several pivotal antimicrobials—such as cephalosporines and several carbapenems), the ability to acquire and disseminate determinants of antimicrobial resistance, and the attitude to survive for long periods on surfaces—thus, qualifying as an important cause of hospital-acquired infections [8–10].
Besides antibiotic treatment, the current management of Enterococcus spp bloodstream infection (E-BSI) is unclear. A recent study based on universal echocardiographic screening in all patients with E. faecalis BSI was able to increase significantly the number of IE diagnoses if compared with previous reports [5]. Previous studies focused on the management of S. aureus bacteremia demonstrated that the application of a bundle consisting in early infectious disease (ID) consultation, appropriate therapy, and echocardiography (to rule out the presence of infective endocarditis) is associated with improved outcome [11, 12]. The primary aim of this study was to assess the all-cause 30-day mortality of patients with monomicrobial E-BSI before and after the implementation of a bundle for its management. As a secondary aim, we explored the efficacy of the bundle in the subgroup of patients with BSI caused by E. faecalis or E. faecium, with severe sepsis or septic shock and 1-year mortality. Additionally, we assessed the impact of the bundle on the reduction of E-BSI 90-day recurrence and 90-day newly diagnosed endocarditis.
METHODS
Study Design
We performed a quasi-experimental prephase and postphase study. In the prephase (January 2014 to December 2015), patients with of E-BSI were analyzed retrospectively. During the post- or intervention phase (January 2016 to December 2017), patients with incident E-BSI were prospectively enrolled in the intervention arm.
Study Setting and Population
The study setting was Sant’Orsola-Malpighi Hospital, a 1420-bed teaching hospital in northern Italy, with a catchment population of around 1,000,000 residents. All consecutive adults (aged ≥ 18 years) with E-BSI were screened for inclusion in the study. Polymicrobial infections were excluded. Patients were included only at the first episode of E-BSI during both phases of the study.
Intervention
Intervention included an alert system and a structured ID consultation. An alert system generated from the microbiology laboratory provided a communication to the team of ID consultants (5 staff and 5 ID fellows). The alert system was based on an intranet-shared database that was systematically updated (once daily) by a microbiologist and checked (once daily) by the ID team. Both preliminary results, according to Gram staining, matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF), and definitive results with susceptibility test, were shared by the alert system (see microbiology section).
The structured ID consultation comprised a bedside evaluation performed after the notification of a new E-BSI by a team member. The team member would recommend (1) echocardiography to rule out endocarditis; (2) follow-up blood cultures (BCs) to be performed every 48h during antibiotic treatment until negative results; and (3) early targeted antibiotic treatment.
Advice for additional diagnostic tests, both laboratory and instrumental (eg, abdominal imaging, 18-fluorodeoxyglucose positron emission tomography [18-FDG PET] in case of suspected prosthetic valve endocarditis), and source-control procedures were given during the ID consultation, when deemed clinically necessary.
The antibiotic treatment consisted in combination therapy in case of primary BSI with no obvious other source of infection, and monotherapy in case of secondary BSI (ie, intrabdominal or urinary tract infection). The combination therapy for ampicillin-susceptible E-BSI (mainly E. faecalis) consisted of ampicillin or amoxicillin plus gentamicin or ceftriaxone and daptomycin plus beta-lactam in case of ampicillin-resistant E-BSI (see Supplementary Table 1 for suggested dosages). Patients with isolation of E. faecalis with history of allergy to beta-lactams received vancomycin, teicoplanin, or daptomycin.
Index BCs were performed at the discretion of attending physician and were not mandated by a study protocol.
During the prephase, ID consultation for an E-BSI episode was performed only by request of the attending physician. However, a formalized bundle for the management of E-BSI was not in place in this period. During the postphase, patients were evaluated within 12 hours after the alert awareness. Patients enrolled both in the pre- and postphase were followed up until 1 year after the BSI onset with either inpatient or outpatient visit, medical records review, or a telephone call.
This study was approved by the local ethics committee (Comitato Etico Azienda Ospedaliero-Universitaria di Bologna) and enrolled patients signed an informed consent for study participation.
Endpoints
The primary end point was all-cause 30-day mortality after BSI onset as defined by the day of BC collection. The secondary endpoints comprised relapse of E-BSI within 90 days from the index episode onset and 1-year mortality.
Subgroup analysis of 30-day mortality among patients with E. faecalis and E. faecium BSI, immunocompromised patients, and those with sepsis or septic shock was performed.
Definitions
Immunosuppression included neutropenia (neutrophil count <500/mm3), solid organ transplantation, hematopoietic stem cell transplantation, corticosteroid therapy at a dosage higher than or equivalent to prednisone 16 mg/day for 15 days, and uncontrolled HIV infection (<200 CD4/mm3). Onset of BSI was defined by the day of BC collection. Sources of BSI were established according to the Centers for Disease Control and Prevention criteria [13]. In the absence of a recognized source, BSI was considered as primary. The duration of bacteremia was calculated as the time (days) between the first negative cultures and index BC. Persistent BSI was defined as persistently positive BCs for ≥72 hours after initiation of appropriate antimicrobial therapy [14]. Endocarditis was defined according with modified Duke’s criteria [15]. Only definite endocarditis was considered. Per Friedman’s criteria, the BSI was classified according to the site of acquisition into nosocomial, healthcare-associated, and community-acquired [16]. Because the study was designed before the development of sepsis-3 consensus definitions, severe sepsis was defined as sepsis plus sepsis-induced organ dysfunction or tissue hypoperfusion; septic shock was defined as sepsis-induced hypotension persisting despite adequate fluid resuscitation. These were defined according to the criteria proposed by the 2012 Surviving Sepsis Campaign guidelines [17]. Pitt bacteremia score was collected at BSI onset [18]. Empirical antibiotic treatment was considered as adequate when at least 1 of the following antibiotics was administered at recommended dosages according with pharmacokinetic/pharmacodynamic drug properties: ampicillin, amoxicillin, and piperacillin for E. faecalis only or daptomycin, vancomycin, and teicoplanin for all the pathogens [19–21] Early adequate antibiotic treatment was defined as adequate treatment administered during the first 24 hours after BC collection.
Monotherapy with aminoglycoside, cephalosporines, fluoroquinolones, and carbapenems were defined inadequate. Clinical stability was defined with Halm’s criteria as temperature ≤37.2 °C; heart rate ≤100 beats/min; respiratory rate ≤24 breaths/min; systolic blood pressure ≥90 mm Hg; oxygen saturation ≥90%; or arterial oxygen tension ≥60 mm Hg [22]. Recurrent E-BSI was defined as a new evidence of positive BCs in patients with documented clinical response after completing a course of anti-enterococcal therapy and occurring within 90 days from index episode.
Microbiology
The blood samples were processed following the routine workflow of the microbiology laboratory of Sant’Orsola-Malpighi University Hospital. Samples were inoculated in liquid medium bottles (BD Bactec Plus aerobic/F, BD Bactec Lytic/10 anaerobic/F and BD Bactec Peds Plus/F; Becton Dickinson, Franklin Lakes, NJ, USA) and incubated for 5 days in a Bactec FX blood culture system (Becton Dickinson). Once flagged as positive, the bottles underwent Gram staining and subculturing. In our hospital, an incubator is available for accepting new samples for BC 24 hours a day, 7 days a week. Positive bottles were seeded on horse blood agar (in aerobic and anaerobic conditions) and CHROMagar Orientation (Meus, Paris, France). Moreover, positive samples were inoculated onto chocolate agar plates, which were incubated at 35–37 °C for 3 hours. In order to achieve a rapid and reliable species identification, MALDI-TOF mass spectrometry analysis was performed from microbial growth using Microflex instrument and MALDI Biotyper software (Bruker Daltonik, Bremen, Germany). Antimicrobial susceptibility tests were performed using MicroScan Walkaway-96 (Beckman Coulter, Brea, California, US). Minimal inhibitory concentration (MIC) values were determined for clinically relevant antimicrobials and interpreted following European Committee for Antimicrobial Susceptibility Testing (EUCAST) guidelines.
All results (Gram staining, MALDI-TOF species identification, and susceptibility results) were reported immediately using the Laboratory Information System (DnLab, Dedalus Firenze, Italy). These procedures did not change during the entire study period, which included both pre- and postphase periods. During the prephase period, only attending physicians received the report of both preliminary and definitive results of BC. During the postphase period, the identification of Enterococcus spp from BCs was notified by the system alert to ID consultants.
Statistical analysis
Descriptive statistics were produced for demographic, clinical, and laboratory characteristics of patients. Mean and standard deviation (SD) are presented for normal distribution variables; median and interquartile range (IQR) for nonnormally distributed variables; and number and percentages for categorical variables. Study phases were compared to parametric or nonparametric tests, according to data distribution, for continuous variables and to the χ2 test (or Fisher exact test where appropriate) for categorical variables.
The primary outcome measure was 30-day mortality from the day when the first positive BC was drawn. The exposure of interest was the study phase (prephase vs postphase). In exploratory analyses, patients dying in the first 48 hours were excluded to account for survivor bias.
Cox proportional hazard univariate and multivariate models were used to assess the association between mortality and study phase and other potential risk factors. Variables significantly different between study periods (except bundle components) and those clinically relevant were included in multivariable models; collinear variables were excluded with no further selection.
The effect of study phase on specific subgroups of patients also was explored in univariate models, including patients with severe sepsis, immunosuppression, isolated pathogen at species level (ie, E. faecalis or E. faecium), and healthcare- and hospital- acquired infection. In all cases, 2-tailed tests were used. The cut-off for P value significance was .05. Stata version 15.0 (Stata Corp., College Station, TX) was used for statistical analysis.
RESULTS
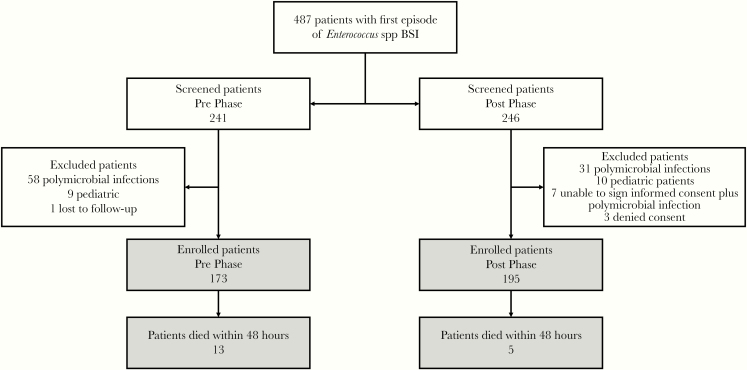
In the study period, 487 patients with E-BSI were screened and 368 were enrolled, with 173 in the prephase and 195 in the postphase. A summary of included and excluded patients is depicted in figure 1.
Figure 1.
Study Flowchart BSI indicates bloodstream infection.
Overall, the mean (SD) age was 70 (±15) years and 230 (62%) patients were male. When comparing the 2 groups (Table 1), differences were found in the presence of metastatic solid malignancies (9% vs 16%, P = .04) and healthcare-associated BSI (9% vs 17%, P = .01), which were more common in the postphase phase group. Similarly, the latter received a concomitant treatment with steroids more frequently (3% vs 9%, P = .03). Several differences were found for management variables between the prephase and postphase (Table 2). Patients enrolled during the latter were more likely to be visited by an ID specialist (45% vs 83%, P < .001), to receive a first line adequate therapy (65% vs 93%, P < .001) or combination therapy (14% vs 23%, P = .02), to undergo at least transthoracic echocardiography (43% vs 73%, P < .001), and to have follow-up BCs performed (42% vs 75%, P < .001). The entire bundle was applied in 15% and 61% patients during the pre- and postphase, respectively (P < .001). The rate of IE was similar between the 2 groups (19% vs 19%, P = 0.99)
Table 1.
Demographics, Comorbidities, and Characteristics of Infection of the Study Population
| Prephase n = 173 (%) | Postphase n = 195 (%) | P value | |
|---|---|---|---|
| Demographic data | |||
| Age (years) (mean [± SD]) | 70 (± 14) | 69 (±15) | .68 |
| Male sex | 99 (57) | 131 (67) | .053 |
| Comorbidities | |||
| Previous miocardial infarction | 12 (32) | 31 (35) | .80 |
| COPD | 9 (24) | 23 (27) | .82 |
| Moderate or severe chronic renal failure | 35 (20) | 56 (29) | .07 |
| Diabetes | 32 (18) | 42 (21) | .51 |
| with organ damage | 15 (9) | 24 (12) | .31 |
| Liver cirrhosis | 24 (14) | 23 (12) | .63 |
| Malignancy | 35 (20) | 52 (27) | .17 |
| Leukemia | 17 (10) | 10 (5) | .11 |
| Metastatic solid malignancy | 16 (9) | 32 (16) | .04 |
| McCabe and Jackson score | .94 | ||
| 1 | 35 (20) | 40 (20) | |
| 2 | 90 (52) | 97 (50) | |
| 3 | 48 (28) | 58 (29) | |
| Charlson comorbidity index, median (IQR) | 7 (5–9) | 7 (5–10) | .17 |
| Immunosuppresion | 41 (23) | 44 (23) | .80 |
| Neutropenia | 23 (13) | 18 (9) | .24 |
| Solid organ transplantation | 12 (7) | 15 (8) | .84 |
| Steroids | 6 (3) | 18 (9) | .03 |
| Biological therapies | 11 (6) | 9 (5) | .49 |
| Ward of attendance | .092 | ||
| Medical ward | 121 (70) | 129 (66) | |
| Surgical ward | 25 (14) | 34 (17) | |
| Hematology | 14 (8) | 7 (4) | |
| ICU | 13 (7) | 25 (13) | |
| Length of in-hospital stay, median (IQR), days | 25 (13–51) | 30 (17–51) | .11 |
| BSI characteristics | |||
| Acquisition | .029 | ||
| Community-acquired | 44 (25) | 33 (17) | |
| Healthcare-associated | 15 (9) | 33 (17) | |
| Hospital-acquired | 116 (65) | 129 (66) | |
| Time between admission and BSI, median (IQR), days | 8 (1–25) | 8 (1–23) | .91 |
| Severity | .088 | ||
| Severe sepsis | 37 (21) | 28 (14) | |
| Septic shock | 14 (8) | 9 (5) | |
| Pitt bacteremia score | 1 (0–2) | 0 (0–2) | .15 |
| Source | |||
| Primary | 49 (28) | 52 (27) | .72 |
| Intra-abdominal | 67 (39) | 67 (34) | .38 |
| Urinary tract | 31 (18) | 34 (17) | .90 |
| Catheter-related | 21 (12) | 32 (16) | .24 |
| Other sourcea | 5 (4) | 10 (5) | .57 |
| Endocarditis | 17 (10) | 19 (10) | .99 |
| Microbiology | |||
| Enterococcus faecalis | 97 (56) | 109 (55) | .97 |
| Enterococcus faecium | 52 (30) | 77 (39) | .06 |
| Other enterococci | 23 (13) | 9 (5) | .01 |
| Ampicillin-resistant strain | 80 (46) | 81 (41) | .40 |
| HLAR | 27 (14) | 20 (10) | .26 |
| VRE | 4 (2) | 1(1) | .19 |
Abbreviations: BSI, bloodstream infection; COPD, chronic obstructive pulmonary disease; HLAR, high-level aminoglycoside resistance; ICU, intensive care unit; IQR, interquartile range; SD, standard deviation; VRE vancomycin-resistant enterococci.
aSurgical site infection (n = 12), skin and soft tissue infection (n = 5).
Table 2.
Differences in Management of Enterococcus spp Bloodstream Infection
| Prephase n = 173 (%) | Postphase n = 195 (%) | P value | |
|---|---|---|---|
| Infectious disease consultation | 79 (45) | 163 (83) | <.001 |
| Appropriate targeted therapy Early (≤24 hours) appropriate therapy | 113 (65) 87 (50) | 182 (93) 131 (67) | <.001 .001 |
| Combination treatment | 25 (14) | 46 (23) | .02 |
| Time to effective therapy, median (IQR), days | 1 (0–2.75) | 0 (0–3) | .44 |
| Duration of antibiotic treatment, median (IQR), days | 6 (0–9) | 10 (1–14) | <.001 |
| Follow-up blood cultures performed | 74 (42) | 147 (75) | <.001 |
| Duration of bacteremia, median (IQR), days | 3 (1–7) | 3 (1–5) | .08 |
| Persistent BSI diagnosed | 14 (8) | 31 (16) | .14 |
| Recurrent BSI within 90 days | 6 (3) | 7 (4) | .91 |
| Echocardiography performed | |||
| Transthoracic | 74 (43) | 143 (73) | <.001 |
| Transesophageal | 16 (9) | 31 (15) | .05 |
| Complete bundle application | 26 (15) | 120 (61) | <.001 |
| Source control | 39 (22) | 57 (29) | .08 |
| Clinical stability | 106 (57) | 140 (72) | .006 |
| Time to clinical stability, median (IQR), days | 2 (1–5) | 2 (1–5) | .35 |
Abbreviations: BSI, bloodstream infection; IQR, interquartile range.
Primary Outcome
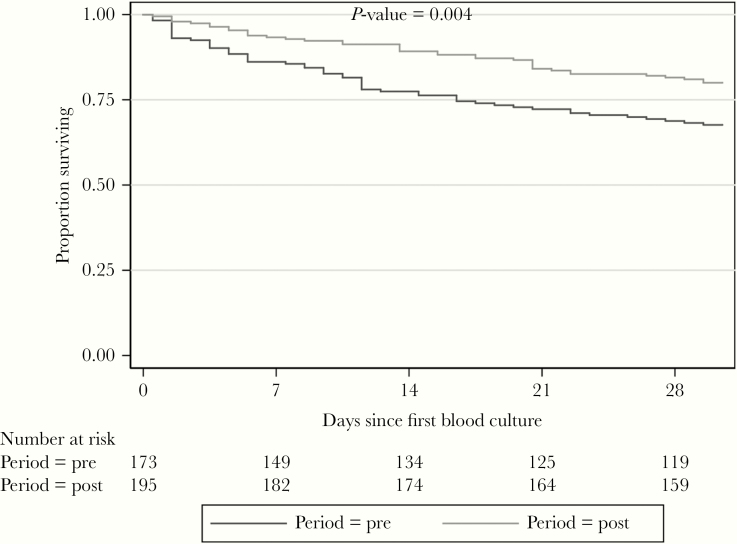
Overall, the 30-day mortality was 26% with a median (IQR) time to death of 3 (0–10) days from BSI onset. Of note, patients enrolled in the postphase had a significantly lower 30-day mortality rate (32% vs 20%, P = .0042; Figure 2), a difference maintained even after excluding patients who died within 2 days from BC (prephase, n = 13; postphase, n =5, P = .035).
Figure 2.
Kaplan-Meier Curves Comparing 30-day Mortality of Patients Enrolled in the Prephase and Postphase Periods
At univariable analysis (Table 3), age (74 [±12] vs 68 [±16], P < .001), Charlson comorbidity index (8 [6–10] vs 7 [5–9], P < .001], severe sepsis or septic shock (46% vs 18%, P > .001), and higher PITT score (2 [0–4] vs 0 [0–1], P < .001) were associated with 30-day mortality, whereas appropriate therapy (71% vs 83%, P = .017), combination therapy (10% vs 22%, P = .02), clinical stability within 7 days from infection onset (21 vs 80%, P < .001), source control (14% vs 30%, P = .004), admission in surgical units (7 % vs 19%, P = .007) and complete bundle application (16% vs 45%, P < .001) were associated with better survival. At multivariate analysis, factors independently associated with mortality were age (HR, 1.04; 95%, CI, 1.02–1.06; P < .001), intensive care unit admission (HR, 2.51; 95% CI, 1.18–3.89; P = .01), and healthcare-associated (HR, 2.32; 95% CI, 1.05–5.16; P = .038) and hospital-acquired infection (HR, 2.85; 95% CI, 1.34–4.76; P = .008), whereas being enrolled in the postphase period (HR, 0.49; 95% CI, 0.32–0.75; P = .001) was associated with improved survival (Table 3).
Table 3.
Univariate and Multivariate Cox Regression Analysis of Risk Factors for All-Cause 30-Day Mortality
| HR (95% CI) | P value | aHR (95% CI) | P value | |
|---|---|---|---|---|
| Demographic data | ||||
| Age | 1.03 (1.01–1.04) | .001 | 1.04 (1.02–1.06) | <.001 |
| Male sex | 0.90 (0.60–1.35) | .62 | ||
| Comorbidities | ||||
| Previous myocardial infarction | 1.09 (0.63–1.89) | .76 | ||
| COPD | 1.39 (0.78–2.29) | .16 | ||
| Moderate or severe chronic renal failure | 0.88 (0.55–1.42) | |||
| Diabetes with organ damage | 1.28 (0.80–2.07) 1.64 (0.93–2.89) | .60 .09 | ||
| Malignancy | 1.06 (0.66–1.69) | .82 | ||
| Leukemia | 1.31 (0.68–2.60) | .44 | ||
| Metastatic solid malignancy | 2.00 (1.21–3.27) | .006 | ||
| Liver cirrhosis | 0.69 (0.35–1.37) | .29 | ||
| Charlson comorbidity index | 1.13 (1.07–1.20) | <.001 | ||
| Immunosuppression | 1.14 (0.71–1.80) | .59 | ||
| Neutropenia | 1.69 (0.97–2.92) | .06 | ||
| Solid organ transplantation | 0.65 (0.71–1.80) | .35 | ||
| Steroids | 1.42 (0.69–2.97) | .34 | ||
| Biological therapy | 0.71 (0.69–2.93) | .51 | ||
| Ward of attendance | ||||
| Medical ward (reference) | 1.00 (1.00-1.00) | |||
| Surgical ward | 0.43 (0.20–0.94) | .03 | 0.22 (0.24–1.18) | .12 |
| Hematology | 1.55 (0.74–3.23) | .27 | 2.09 (0.94–4.63) | .07 |
| ICU | 1.68 (0.96–2.94) | .07 | 2.51 (1.18–3.89) | .01 |
| BSI characteristics | ||||
| Community-acquired (reference) | 1.00 (1.00-1.00) | |||
| Healthcare-associated | 1.85 (0.84–4.05) | .12 | 2.32 (1.05–5.16) | .038 |
| Hospital-acquired | 2.00 (1.08–3.69) | .03 | 2.85 (1.34–4.76) | .004 |
| Severe sepsis | 2.81 (1.56–5.05) | .001 | ||
| Septic shock | 7.29 (3.77–14.01 | <.001 | ||
| PITT bacteremia score | 1.35 (1.25–1.45) | <.001 | ||
| Source | ||||
| Primary | 1.44 (0.93–2.22) | .10 | ||
| Intra-abdominal | 0.92 (0.60–1.40) | .70 | ||
| Urinary tract | 1.00 (0.59–1.68) | .99 | ||
| Catheter-related | 0.59 (0.31–1.01) | .10 | ||
| Other source | 1.25 (0.26–4.38) | .91 | ||
| Endocarditis | 0.37 (0.22–1.08) | .08 | ||
| Microbiology | ||||
| Enterococcus faecalis | 0.84 (0.56–1.26) | .40 | ||
| Enterococcus faecium | 1.32 (0.88-1.99) | .18 | ||
| Other enterococci | 0.68 (0.31–1.46) | .32 | ||
| Ampicillin-resistant strain | 1.09 (0.72–1.62) | .68 | ||
| HLAR | 0.85 (0.44–1.64) | .62 | ||
| VRE | 2.30 (0.73–7.25) | .16 | ||
| Treatment | ||||
| Appropriate therapy Early (≤24 hours) appropriate therapy | 0.55 (0.35–0.85) 0.68 (0.45–1.01) | .008 .05 | ||
| Combination therapy | 0.55 (0.35–0.85) | .02 | ||
| Duration of treatment | 0.99 (0.92–1.05) | .67 | ||
| Source control | 0.39 (0.21–0.70) | .002 | ||
| Transthoracic Echocardiography | 0.34 (0.22–0.51) | <.001 | ||
| Clinical Stability | 0.09 (0.06–0.16) | <.001 | ||
| Time to clinical stability | 1.02 (0.92–1.13) | .65 | ||
| Follow-up blood culturesa | 0.40 (0.27–0.61) | <.001 | ||
| Duration of bacteremia | 0.99 (0.92–1.05) | .67 | ||
| Infectious disease consultation | 0.42 (0.28–0.62) | <.001 | ||
| Complete bundle application | 0.24 (0.14–0.41) | <.001 | ||
| Enrolled during the postphase period | 0.58 (0.39–0.87) | .008 | 0.49 (0.32–0.75) | .001 |
Abbreviations: aHR, adjusted hazard ratio; BSI, bloodstream infection; CI, confidence interval; COPD, chronic obstructive pulmonary disease, HR, hazard ratio; HLAR, high-level aminoglycoside resistance; ICU, intensive care unit; VRE vancomycin-resistant enterococci.
aFollow-up blood cultures were available in 218 (59%) patients (41% in the prephase and 75% in the postphase period).
Secondary Outcomes
During follow up, 90-day recurrence of E-BSI was identified in 13 (3%) patients with a median (IQR) time of 30 (21–48) days. No differences were found in terms of 90-day recurrence between the 2 groups (3% vs 4%, P = .91). Subgroup analysis performed for the time period (prephase vs postphase) revealed that patients with severe sepsis (HR, 0.37; 95% CI, 0.16–0.90) and healthcare-associated infection (HR, 0.53; 95% CI, 0.31–0.93) showed a better outcome when enrolled in the postphase period, respectively. Additionally, a nonstatistically significant lower mortality also was seen among patients with E. faecalis BSI (HR, 0.56; 95% CI, 0.30–1.05) when compared with E. faecium BSI (HR, 0.78; 95% CI, 0.38–1.60). Lastly, all patients completed the pre-established 1-year follow up. All-cause 1-year mortality was 58% for the entire cohort. However, patients enrolled in the postphase had a significantly lower 1-year mortality when compared with those enrolled in the first period (68% vs 50%, P < .001) (Supplementary Figure 1).
Discussion
In this quasi-experimental, prephase/postphase study the application of a bundle for the management of E-BSI consisting of ID consultation, echocardiography, follow-up blood BCs, and targeted antimicrobial treatment was effective in reducing the 30-day mortality. Previous studies evaluating a similar bundle for the management of S. aureus BSI demonstrated its effectiveness [11, 12]. However, none of the aforementioned studies involved cases of Enterococcal BSI.
Enterococcus spp and S. aureus share similar characteristics that may explain the results of our findings. First, both are important causative pathogens of IE. In our study approximately 10% of all BSI had an underlying endocarditis. Second, S. aureus and Enterococcus spp are the first and the second causes of persistent bacteremia, respectively [23]. During the intervention period of our study, 150 out of 197 (76%) patients were tested with follow-up BCs and 31 (21%) had a persistent bacteremia. It is possible that in this subgroup of patients a more intensive screening for source of BSI or metastatic localization has been performed during subsequent visits. In fact, when comparing patients with and without persistent bacteremia, we found higher (although not statistically significant) mortality in those enrolled in the prephase period (43% vs 19%, P = .07), but not in those enrolled in the postphase period (13% vs 14%, P = .83).
Another important finding of our study is that the application of the bundle was able to improve the rate of adequate antibiotic treatment (83% vs 71%, P = .017). Additionally, in a lower but significant proportion of cases, the number of patients receiving adequate therapy in the first 24h was higher after the systematic application of the bundle. Importantly, the use of MALDI-TOF was first introduced in our center in January 2013, so it was available in both study periods. The number of vancomycin-resistant enterococci was very low in our study, and this may have had an impact on the number of erarly adequate treatments in case the complete susceptibility test was not yet available.
Another interesting aspect of our study was that the management of enterococcal BSI by ID consultant increased the rate of combination treatment. This already had been associated with improved outcome in the setting of IE, but, unfortunately, large studies evaluating the role of combination treatment for patients with BSI without endocarditis are lacking.
This study was not able to find a difference in the rate of recurrence of BSI, mainly due to the low rate of events found in both groups. Similarly, we found only 7 new cases of IE diagnosed during follow up. Interestingly, among all patients with recurrent BSI, 53% had an underlying IE. Similarly, the application of an aggressive screening with echocardiography did not result in a higher number of diagnosis of IE in the postphase period (17 [9%] vs 19 [9%], P = .64), despite a higher number of patients undergoing transthoracic echocardiography (TTE) (74 [43%] vs 143 [73%], P < .001].
We also performed an exploratory subgroup analysis. The most interesting finding was that mortality for patients with sepsis or healthcare-associated infections was significantly lower in the postphase period. Both of these factors previously were associated with poor outcome among patients with BSI [24, 25].
The study has several limitations. Patients were not randomized to receive a specific management. Therefore, unobserved biases (eg, improvement of general patients care in the postphase period, Hawthorne effect, and others) may have influenced results. In fact, differences in the 2 groups were observed mainly the rate of metastatic solid cancer found more frequently in the postphase. However, this aspect may be a strength of this study as the application of the bundle we found reduced mortality in the postphase group; on the other hand, the higher rate of patients with metastatic solid cancer may be the result of the adjunctive diagnostic tests requested during ID consultation (ie, the rate of performed 18-FDG-PET increased from 13% and 21% [P = .04] across the study periods).
When we classified patients according to bundle completion, we found that the bundle independently was associated with lower mortality. However, this might be due to survivor bias (ie, only patients with sufficient survival can undergo the intervention of interest, biasing results in favor of the intervention). In fact, in exploratory statistical models we considered each component of the bundle as time-varying covariates (notably, the timing of each bundle component was not predetermined, thus increasing variability and model complexity), but we finally decided to employ study period as a proxy for bundle implementation because survivor bias could not be entirely be ruled out and because the study period was strongly associated with complete bundle application (Table 2). In addition, another potential limitation could be represented by the fact that in some patients during the prephase period, the entire bundle was applied. This was the result of recommendation given during sporadic ID consultations requested by the attending physicians, and it might constitute a bias. However, prephase/postphase study designs applied to quality improvement projects represent, by definition, a real-life clinical experience. In this contest, we were able to detect a significant improvement of outcome in the postphase period. Lastly, being this a single-center study, the external validation should be confirmed in larger trials.
In conclusion, in our study, the introduction of a bundle for the management of patients with E-BSI was associated with improved 30-day and 1-year survival.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Francesco Molà, a medical student, for his help in data collection.
Author contributions. M.B., S.T., M.G., L.S., and P.V. wrote and edited the manuscript. M.B., S.T., M.G., L.S., P.V., and S.A. analyzed the data. M.B., S.T., M.G., P.V., Fa.T., and F.C. created the study concept and design. E.R.d.T, R.P., M.R., L.B., L.M., I.C., Fi.T., S.I., and C.C. collected data. M.B., S.T., S.A., A.B., and M.G. coordinated the study.
Financial support. None declared.
Potential conflicts of interest. All authors: No reported conflict of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Rodríguez-Baño J, López-Prieto MD, Portillo MM, et al. ; SAEI/SAMPAC Bacteraemia Group Epidemiology and clinical features of community-acquired, healthcare-associated and nosocomial bloodstream infections in tertiary-care and community hospitals. Clin Microbiol Infect 2010; 16:1408–13. [DOI] [PubMed] [Google Scholar]
- 2. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309–17. [DOI] [PubMed] [Google Scholar]
- 3. Buetti N, Atkinson A, Marschall J, Kronenberg A; Swiss Centre for Antibiotic Resistance (ANRESIS) Incidence of bloodstream infections: a nationwide surveillance of acute care hospitals in Switzerland 2008-2014. BMJ Open 2017; 7:e013665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet 2016; 387:882–93. [DOI] [PubMed] [Google Scholar]
- 5. Dahl A, Iversen K, Tonder N, et al. . Prevalence of infective endocarditis in Enterococcus faecalis bacteremia. J Am Coll Cardiol 2019; 74:193–201. [DOI] [PubMed] [Google Scholar]
- 6. Bouza E, Kestler M, Beca T, et al. ; Grupo de Apoyo al Manejo de la Endocarditis The NOVA score: a proposal to reduce the need for transesophageal echocardiography in patients with enterococcal bacteremia. Clin Infect Dis 2015; 60:528–35. [DOI] [PubMed] [Google Scholar]
- 7. Anderson DJ, Murdoch DR, Sexton DJ, et al. . Risk factors for infective endocarditis in patients with enterococcal bacteremia: a case-control study. Infection 2004; 32:72–7. [DOI] [PubMed] [Google Scholar]
- 8. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012; 10:266–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller WR, Munita JM, Arias CA. Mechanisms of antibiotic resistance in enterococci. Expert Rev Anti Infect Ther 2014; 12:1221–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dancer SJ. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev 2014; 27:665–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goto M, Schweizer ML, Vaughan-Sarrazin MS, et al. . Association of evidence-based care processes with mortality in Staphylococcus aureus bacteremia at Veterans Health Administration hospitals, 2003-2014. JAMA Intern Med 2017; 177:1489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. López-Cortés LE, Del Toro MD, Gálvez-Acebal J, et al. ; REIPI/SAB group Impact of an evidence-based bundle intervention in the quality-of-care management and outcome of Staphylococcus aureus bacteremia. Clin Infect Dis 2013; 57:1225–33. [DOI] [PubMed] [Google Scholar]
- 13. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36:309–32. [DOI] [PubMed] [Google Scholar]
- 14. Seifert H. The clinical importance of microbiological findings in the diagnosis and management of bloodstream infections. Clin Infect Dis 2009; 48(Suppl 4):S238–45. [DOI] [PubMed] [Google Scholar]
- 15. Habib G, Lancellotti P, Iung B. 2015 ESC Guidelines on the management of infective endocarditis: a big step forward for an old disease. Heart 2016; 102:992–4. [DOI] [PubMed] [Google Scholar]
- 16. Friedman ND, Kaye KS, Stout JE, et al. . Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 2002; 137:791–7. [DOI] [PubMed] [Google Scholar]
- 17. Dellinger RP, Levy MM, Rhodes A, et al. . Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Critical care medicine 2013; 41:580–637. [DOI] [PubMed] [Google Scholar]
- 18. Paterson DL, Ko WC, Von Gottberg A, et al. . International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial infections. Ann Intern Med 2004; 140:26–32. [DOI] [PubMed] [Google Scholar]
- 19. Murray BE. The life and times of the Enterococcus. Clin Microbiol Rev 1990; 3:46–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pea F, Viale P. Bench-to-bedside review: Appropriate antibiotic therapy in severe sepsis and septic shock–does the dose matter? Crit Care 2009; 13:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient–concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev 2014; 77:3–11. [DOI] [PubMed] [Google Scholar]
- 22. Halm EA, Fine MJ, Marrie TJ, et al. . Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA 1998; 279:1452–7. [DOI] [PubMed] [Google Scholar]
- 23. Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis 2016; 16:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shorr AF, Tabak YP, Killian AD, Gupta V, Liu LZ, Kollef MH. Healthcare-associated bloodstream infection: A distinct entity? Insights from a large U.S. database. Crit Care Med 2006; 34:2588–95. [DOI] [PubMed] [Google Scholar]
- 25. Lenz R, Leal JR, Church DL, Gregson DB, Ross T, Laupland KB. The distinct category of healthcare associated bloodstream infections. BMC Infect Dis 2012; 12:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.