Abstract
Background
This study aims to estimate the disease burden of vertically acquired hepatitis C virus (HCV) in a large Midwestern hospital and to identify factors associated with HCV diagnostic testing among high-risk infants.
Methods
This is a retrospective analysis of an infant cohort (n = 58 427) born from 2014 to 2016 in the Greater Cincinnati region, where universal maternal urine testing is conducted at delivery to assess for intrauterine drug exposure (IUDE). Demographics and birth characteristics were analyzed among high-risk infants to identify factors associated with receiving HCV testing. A nested, matched, case-control analysis examined the association of pediatric HCV infection and IUDE.
Results
The HCV prevalence rate among high-risk infants who received testing was 3.6%–5.2% of births. Approximately 66.7% of maternally acquired HCV infections may be missed using current testing recommendations. Prenatal care had no significant effect (adjusted odds ratio [aOR], 1.2; 95% confidence interval [CI], 0.4–3.5) on the odds of a high-risk infant receiving HCV testing. Opioid-exposed cases had a more than 6-fold increase in the odds of HCV infection (aOR, 6.2; 95% CI, 2.3–16.6]) compared with nonopioid exposed infants.
Conclusions
The IUDE was significantly associated with increased odds of pediatric HCV infection in this population. The gaps in pediatric HCV testing identified in this study, despite known risk level and maternal infection, suggest the need for increased focus on HCV identification in the pediatric population.
Keywords: diagnostics, hepatitis C virus, intrauterine opioid exposure, pediatrics
The current opioid epidemic in the United States has changed the demographics of the population with chronic hepatitis C virus (HCV) infection, and it has impacted the dynamics of HCV transmission [1–3]. Between 2004 and 2014, the rate of acute HCV infection in women increased almost 4-fold to 0.8 per 100 000 and in men over 2-fold to 0.7 per 100 000, predominantly among persons 18 to 39 years old [4]. This is largely due to injection drug use. More than 75% of acute HCV infections reported to the National Notable Disease Surveillance System cited injection drug use as a risk factor every year between 2011 and 2014 [3, 4]. This increase of HCV infection among women of childbearing age puts infants at increased risk of infection through viral transmission during pregnancy. Among HCV-infected pregnant women, mother-to-child transmission of infection has been reported in 2.8%–10.8% of cases [5, 6].
However, significant challenges continue to prevent a large proportion of children from being identified as HCV-infected. A meta-analysis of research from 2003 to 2013 indicated that only 50% (95% confidence interval [CI], 43%–57%) of adults with chronic HCV are aware of their status [7]. In addition, less than half of women in Ohio with medical records indicating past or present HCV infection were tested for HCV during pregnancy [2]. Such findings have contributed to newly issued recommendations from the American Association for the Study of Liver Diseases (AASLD)/Infectious Diseases Society of America (IDSA) to introduce universal HCV testing in pregnancy [8].
This recommendation alone may not be sufficient in capturing the pediatric population born to mothers with HCV infection. Among children born to an HCV-infected mother, reported rates of HCV testing ranged between 16% and 68% [1, 3, 5] despite consistent recommendations from the American Academy of Pediatrics and the Centers for Disease Control and Prevention (CDC) to test all infants with a known HCV exposure [9, 10]. Therefore, the present study aims to estimate the pediatric disease burden of vertically acquired HCV attributable to injection drug use and assess limitations that may be contributing to gaps in pediatric HCV testing.
This study was conducted in a large health center where universal maternal urine drug testing at birth is regionally implemented [11], which provides a unique opportunity to capture an otherwise poorly documented risk factor for pediatric HCV infection and allows for a proxy measurement of evidence of intrauterine drug exposure (IUDE) at time of delivery.
METHODS
Setting
A retrospective analysis was conducted using data from Cincinnati Children’s Hospital Medical Center (CCHMC). Eight counties within the Greater Cincinnati region are considered the primary service area for CCHMC pediatricians. Within this area, CCHMC pediatricians see 75%–80% of infants born in the hospital delivery setting. Only those births geocoded to one of the counties that comprises the primary service area were considered in this study.
A universal maternal urine drug testing policy was implemented at all hospitals in the Greater Cincinnati region on September 1, 2013 [11]. Women provided consent to urine drug testing on admission to labor and delivery, as part of standard hospital admission throughout the region. Urine samples were obtained before delivery to support timely identification and treatment of infants at risk for withdrawal and to rule out iatrogenic exposure. Urine was analyzed first with immunoassay, and confirmatory testing was done with mass spectrometry technology to test for amphetamines, benzodiazepines, barbiturates, buprenorphine, cannabinoids, cocaine, heroin, methadone, phencyclidine, and prescription pain medication. Although women may opt out of testing, more than 98% consented to testing. Confirmatory test results, either by mass spectrometry or maternal confirmation, resulted in standard International Classification of Diseases (ICD) coding within the newborn electronic health record (EHR) indicating in utero opioid exposure.
Study Population
For this study, all newborns from the primary service area who were seen by a CCHMC pediatrician between January 1, 2014 and December 31, 2016 were included. Infant records were abstracted on June 30, 2018 to allow for sufficient follow-up for all infants to be at least 18 months of age. After 18 months of age, the false-positive rate for both methods of HCV testing (HCV antibody or ribonucleic acid [RNA]) is minimized.
Structured data elements from infant medical records and physician billing records, including diagnoses, demographics, laboratory results, and other observational data, were abstracted using automated queries. These data are maintained in the Maternal and Infant Data Hub (MIDH) at CCHMC [12]. Infants were classified as HCV-exposed if (1) the ICD-9 or ICD-10 codes in their medical record indicated an HCV infection, or (2) their most recent HCV antibody or RNA laboratory test results were positive, regardless of age at time of testing, indicating exposure. The ICD-9 and ICD-10 codes used to define each variable are presented in Supplementary Table 1. An HCV test was considered confirmatory if it was administered according to the timeframe recommended by the CDC (minimum 30 days of life for polymerase chain reaction testing, and minimum 18 months old for antibody testing). Infants were considered HCV-infected if they had received confirmatory HCV antibody and/or HCV RNA testing indicating seropositivity. Those HCV-infected infants with at least 1 subsequent positive HCV RNA test result were considered current infections. The MIDH records indicated opioid-specific prenatal exposure as well as exposure to any 1 or more of the tested substances coded as a single “any-drug” exposure. This allowed for accurate identification of a subset of high-risk infants. An infant was considered high-risk for HCV infection if they had a documented IUDE or confirmed hepatitis B virus (HBV) and/or human immunodeficiency virus (HIV) infection.
Cohort Analysis
Analyses compared differences between the cohort population (for which HCV status is either unknown or negative) and HCV-infected infants. Differences in sociodemographic characteristics, health behaviors, and birth characteristics of the HCV-infected patients were assessed using t tests for continuous variables and Fisher’s exact test for categorical variables.
A diagnostic cascade was used to examine 5 levels of available data that are included in the MIDH to confirm HCV infection status. The cascade captures and monitors the following: (1) the number of infants born in the CCHMC primary service area considered high risk, (2) the number of infants tested for HCV, (3) the number of tested infants tested in the timeframe recommended by the CDC, (4) the number of appropriately tested children who are HCV seropositive at the end of the study period, and (5) the number of HCV seropositive children who have known current HCV infection. A case that meets all criteria is considered a confirmed case of pediatric HCV.
Ranges for the prevalence rates of pediatric HCV infection in the populations defined as high risk and low risk were estimated using only the subset of infants who received HCV testing according to CDC recommendations. The lower limit of the range was calculated using the fraction of infants that had positive HCV RNA test results, indicating current HCV infection. The upper limit of the range was calculated using the fraction of HCV-infected infants who were seropositive, indicating current or resolved infection.
To calculate the estimated number of missed HCV-infected cases within the high-risk population, the number of infants with current HCV infection whose maternal infection status was previously unknown was divided by all infants who received testing in the recommended timeframe. This fraction was multiplied by the number of infants at high risk for HCV infection who did not receive HCV testing (ie, “Missing Labs’, Figure 1, Line C”). The upper estimate of missed high-risk cases was calculated using the same methodology, but it included all HCV-infected infants, regardless of previously known HCV infection in the mother. These calculations were repeated within the low-risk population to estimate the range of potentially missed cases.
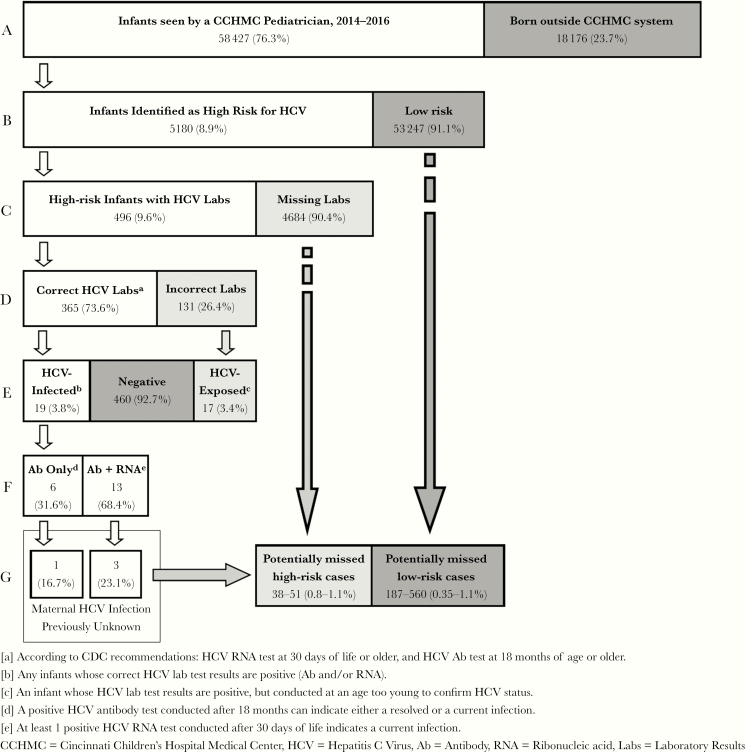
Figure 1.
Diagnostic cascade for determining hepatitis C virus (HCV) infection rates among high-risk infants, defined as having an intrauterine drug exposure and/or confirmed hepatitis B virus or human immunodeficiency virus infection. Percentages in lines B–G are calculated as the proportion of infants who completed the previous step of the diagnostic cascade. The lowest estimated number of missed high-risk cases of HCV infections was calculated as the proportion of infants with a current HCV infection born to a woman whose HCV infection status was previously unknown ([G] Ab + RNA, Previously Unknown) over all of the high-risk infants who were tested according to Centers for Disease Control and Prevention (CDC) recommendations ([D] Correct HCV Labs) multiplied by the number of high-risk infants who were not tested ([C] Missing Labs). The highest estimated number of missed HCV infections was calculated using the same methodology but used the proportion of all HCV-infected infants (E), regardless of previously known maternal infection, in the numerator. The estimated range of potentially missed low-risk cases was calculated using the same methodology (data not shown).
Factors associated with HCV diagnostic testing among high-risk infants were examined using univariable and multivariable logistic regression. High-risk infants identified in the diagnostic cascade who received HCV testing were compared with high-risk infants who did not receive HCV testing. Variables included in the final multivariable logistic model were specified a priori and also selected based on univariate analyses. The model was adjusted for race, birth weight, insurance type, IUDE, limited or no prenatal care, foster care, any neonatal intensive care unit (NICU) stay, confirmed HBV infection, and follow-up over 18 months. The interaction between opioid exposure and NICU stay was assessed using the Wald test for an interaction term and test of homogeneity for the Mantel-Haenszel adjusted odds ratios (aORs). This interaction was adjusted for in the model.
Nested Case-Control Analysis
Due to the rarity of HCV exposure as our outcome of interest in the study population, a nested case-control analysis was conducted to measure the association between HCV seropositivity and an IUDE. Cases and controls were matched 1:3 on race, ethnicity, birth weight (±50 grams), and gestational age. Cases were defined as all HCV-exposed infants. Controls were defined as individuals with confirmed negative HCV test results or those without known positive HCV test results. Controls were randomly selected from the full cohort population using R package dplyr. Differences between the matched controls and the full cohort population were examined using univariate analysis.
Predictors of pediatric HCV exposure were examined using a univariable and multivariable logistic regression comparing cases and controls from the nested analysis. Variables included in the final multivariable logistic regression model were those specified a priori and those that were significantly associated at the univariable level. The final model was analyzed using 3 separate predictor variables for drug exposure (any drug exposure, opioid exposed, and neonatal abstinence syndrome [NAS] diagnosed) while adjusting for the same variables in each of the 3 models. Each full model adjusted for insurance type, known maternal HCV infection, limited or no prenatal care, foster care, any NICU stay, and initial hospital length of stay. All analyses were conducted using R version 3.3.2. This study was approved by the Institutional Review Board of Cincinnati Children’s Hospital Medical Center.
RESULTS
Demographics
Between 2014 and 2016, there were 76 603 births in CCHMC’s primary service area (Perinatal Institute, unpublished data, August 2018). Among these newborns, 58 427 (76.3%) were seen by a CCHMC pediatrician and included in the current study. There were 114 (0.2%) infants with documented HCV exposure. Among those with HCV exposure, 90 (90 of 114, 78.9%) had both HCV test results and ICD-9 or ICD-10 codes indicating HCV infection. Of these 90, 17 infants had HCV test results confirming HCV infection, 14 had positive HCV antibody tests before 18 months of age and ICD-9 or ICD-10 codes indicating infection after 18 months, 19 infants had HCV antibody tests before 18 months of age indicating HCV-exposure but no ICD-9 or ICD-10 codes, and 40 were initially coded as HCV exposed but later test results indicated no infection. An additional 24 infants had ICD-9 or ICD-10 codes indicating HCV exposure but no accessible laboratory results.
Infants with HCV exposure were more likely to be white (70.2% vs 45.7%, P < .001) and to be born to mothers with public insurance or no insurance (86.8% vs 51.2%, P < .001) compared with the rest of the study cohort population (Table 1). In addition, HCV-exposed infants were more likely to born earlier (37.7 ± 2.4 weeks vs 38.4 ± 2.1 weeks, P = .002) and have lower birth weights (2951 ± 627 grams vs 3252 ± 616 grams, P < .001) compared with the general population.
Table 1.
Demographics of HCV-Exposed Infants Compared to Cohort Population
| Characteristic | HCV Exposed n = 114 (%) | Cohorta n = 58 313 (%) | P b |
|---|---|---|---|
| Race | |||
| Black/African American | 27 (23.7) | 12 990 (22.2) | <.001 |
| White/Caucasian | 80 (70.2) | 26 621 (45.7) | |
| Otherc,d | 0 (0) | 2074 (3.6) | |
| Any Drug Exposuree | 92 (80.7) | 5071 (8.70) | <.001 |
| Opioid Exposure | 78 (68.4) | 2431 (4.17) | <.001 |
| NAS Diagnosis | 39 (34.2) | 774 (1.33) | <.001 |
| Known Maternal HCV Infection | 91 (79.8) | 874 (1.5) | <.001 |
| HIV Infection | 1 (0.88) | 11 (0.02) | .023 |
| HBV Infection | 1 (0.88) | 11 (0.02) | .023 |
| High Riskf | 92 (80.7) | 5088 (8.7) | <.001 |
| Foster Care | 17 (14.9) | 456 (0.78) | <.001 |
| Insurance | |||
| Public or Self-Pay | 99 (86.8) | 29 860 (51.2) | <.001 |
| Private | 15 (13.2) | 28 453 (48.8) | |
| Any NICU Admittance | 49 (43.0) | 5 748 (9.86) | <.001 |
| No Prenatal Care | 3 (2.63) | 358 (0.61) | .034 |
| Birth Weightg (grams) | 2951 ± 627 | 3252 ± 616 | <.001 |
| Gestational Ageg (weeks) | 37.7 ± 2.4 | 38.4 ± 2.1 | .002 |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; NAS, neonatal abstinence syndrome; NICU, neonatal intensive care unit.
aRepresents general population; includes HCV-uninfected infants as well as untested infants, regardless of risk level.
bFisher’s exact test, except for birth weight and gestational age; t test.
cIn the cohort, “Other” includes Hispanic/Latino (1199, 2.1%), Asian (765, 1.31%), Middle Eastern (14, 0.02%), American Indian and/or Alaska Native (19, 0.03%), and Native Hawaiian and/or other Pacific Islander (77, 0.13%).
dData are missing or excluded for race if it was unreported, parent/guardian was not present, or declined to answer among HCV-exposed infants (n = 7, 6.1%) and cohort population (n = 16 628, 28.5%).
eAny infant born to a mother whose urine tested positive at time of delivery for one of the following drugs: amphetamines, benzodiazepines, barbiturates, buprenorphine, cannabinoids, cocaine, heroin, methadone, phencyclidine, and prescription pain medications (illicit or prescribed use cannot be differentiated).
fDefined as having an intrauterine drug exposure, confirmed by urine drug testing at time of delivery, or confirmed HIV or HBV infection.
gMissing data: HCV-infected infants, n = 1; cohort population, n = 2785.
Prevalence of Hepatitis C Virus Infection
All infants were evaluated for completion of each step along the diagnostic cascade in Figure 1. Due to positive maternal drug screening results or confirmed HIV or HBV infection, 5180 (8.9%) infants were considered high-risk for HCV infection. Of the high-risk infants, 496 (9.6%) had documented HCV test results. However, only 365 high-risk infants (73.6%) were tested at the ages specified by CDC recommendations. Nineteen infants were tested according to CDC recommendations and were found to be seropositive and were considered confirmed HCV-infected cases (19 of 496, 3.8%). Among the 131 HCV tests that were conducted before of the CDC age recommendation, an additional 17 infants (17 of 496, 3.4%) tested positive for HCV antibody. These infants were considered HCV-exposed cases, but testing to differentiate between circulating maternal antibody or pediatric infection was not completed before the end of the study period.
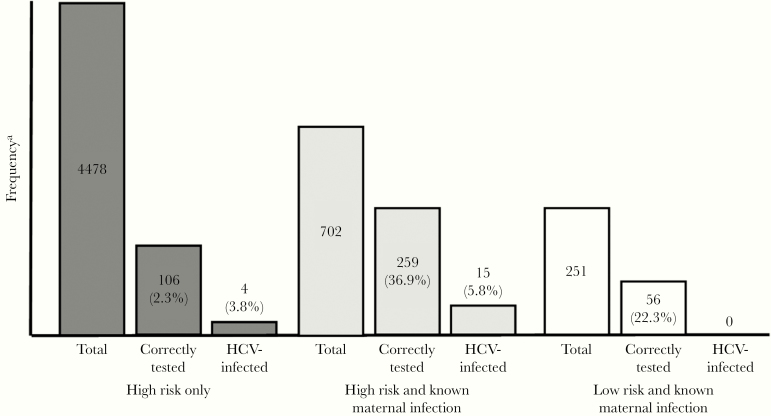
Of the HCV-infected cases, 13 (68.4%) were current infections and 6 (31.6%) did not have HCV RNA testing to determine whether the infection was current or resolved. Thus, the prevalence rate of pediatric HCV infection among high-risk infants was estimated to be 3.6%–5.2%. Four HCV-infected infants were born to women unaware of their HCV infection (4 of 19, 21.1%) (Figure 2). Using the rates of infection (HCV antibody and RNA tests positive) among infants without recorded maternal HCV infection (3 of 365 to 4 of 365), an additional 38–51 HCV cases are estimated to have occurred among the 4684 untested high-risk infants. If there are 19 identified HCV-infected infants and 38 missed HCV-infected infants, approximately 66.7% (38 of 57) of HCV cases are being missed in this pediatric setting.
Figure 2.
Frequency of hepatitis C virus (HCV)-infected infants within 3 subsets: (1) high-risk infants, defined as any intrauterine drug exposure, and/or human immunodeficiency virus or hepatitis B virus infection; (2) high-risk infants with a known intrauterine HCV exposure, determined by International Classification of Diseases (ICD)-9 or ICD-10 codes on infant medical records indicating maternal infection; and (3) low-risk and known maternal infection. Within each subset, the total population is progressively broken down into 2 steps: (1) those infants who received HCV antibody or HCV ribonucleic acid testing according to Centers for Disease Control and Prevention recommendations (“Correctly Tested”) and (2) those tested according to recommended testing schedule who tested positive for HCV (“HCV-Infected”). Percentages are calculated as the proportion of the previous step in each subset.
Among the 53 247 low-risk infants, 460 (0.9%) were tested for HCV, 285 (62%) of which were tested according to the CDC recommendations. Three low-risk infants (3 of 285, 1.1%) were identified as HCV infected. One of the HCV-infected infants was confirmed to be a current infection (1 of 285, 0.35%). Thus, the prevalence rate within the low-risk population is estimated to be between 0.35% and 1.1%. In addition, 4 low-risk infants (4 of 459, 0.87%) were HCV exposed, but infection status was not confirmed. The medical records of these low-risk HCV-infected and HCV-exposed infants did not indicate previously known maternal HCV infection. Using the range of current infections within the low-risk population (1 of 285) and all HCV-infected low-risk infants (3 of 285), there may be an estimated 187–560 missed cases of pediatric HCV infection. Analysis of loss to follow-up was approximately equal between groups (Table 2).
Table 2.
Number of Infants Lost to Follow-up Between High-Risk and Low-Risk Infantsa
| Age at Last CCHMC Provider Interaction | |||
|---|---|---|---|
| Risk Factor | 1 Month of Age n (%) | 1–18 Months of Age n (%) | 18+ Months of Age n (%) |
| Neonatal Abstinence Syndrome Diagnosis n = 813 | 178 (21.9) | 259 (31.9) | 376 (46.2) |
| Opioid Exposure (Without NAS) n = 1696 | 700 (41.3) | 347 (20.5) | 649 (38.3) |
| “Other” Drug Exposureb n = 2654 | 994 (37.5) | 528 (19.9) | 1132 (42.7) |
| High Risk n = 5180 | 1873 (36.2) | 1139 (22.0) | 2168 (41.9) |
| Low Risk n = 53 247 | 24 653 (46.3) | 10061 (18.9) | 18 533 (34.8) |
Abbreviations: CCHMC, Cincinnati Children’s Hospital Medical Center; CDC, Centers for Disease Control and Prevention; NAS, neonatal abstinence syndrome.
aInfants were considered lost to follow-up if they did not have 1 or more provider interactions with a CCHMC pediatrician after initial hospital discharge by at least 18 months of age. Percentages are calculated as the proportion within each row. The cohort was analyzed for loss to follow-up in 2 time periods: before 1 month of age and between 1 and 18 months of age, because those older than 1 month can receive conclusive hepatitis C virus ribonucleic acid testing, according to Centers for Disease Control and Prevention recommendations. High-risk infants are considered any infant with either an intrauterine drug exposure (IUDE) and/or human immunodeficiency virus or hepatitis B virus infection. Loss to follow-up in high-risk infants is further divided by type of IUDE
bInfants classified as having “Other Drug Exposure” includes any infant who had an intrauterine exposure to amphetamines, benzodiazepines, barbiturates, cannabinoids, cocaine, and phencyclidine.
Pediatric Hepatitis C Virus Diagnostic Testing
High-risk infants who received HCV testing (n = 522) and high-risk infants who had no documented testing (n = 4658) were evaluated to identify whether maternal receipt of prenatal care, during which time HCV-exposure could be determined, was associated with HCV diagnostic testing. There were 9 HCV-tested infants and 198 untested infants excluded from multivariate analysis due to missing birth weight data. Infants tested for HCV were more likely to be in foster care compared with those without HCV testing (32.0% vs 1.1%, P < .001). However, less than half of the infants in foster care were considered high risk (220 of 473, 46.5%) by the definition used in this study. In adjusted analyses, only infants who continued to see their pediatrician until at least 18 months of age (aOR, 18.1; 95% CI, 6.34–51.5) had statistically significantly increased odds of receiving HCV testing (Table 3).
Table 3.
Association of Point of Care and Likelihood of Receiving HCV Testing Among High-Risk Infantsa
| Point of Care | HCV Tested (n = 522) n (%) | HCV Untested (n = 4658) n (%) | P | OR (95% CI) | aORb (95% CI) |
|---|---|---|---|---|---|
| Race–White/Caucasian | 107 (20.5) | 1511 (32.4) | <.001 | 0.54 (0.19–1.53) | - |
| Birth Weight (grams) | 2823.3 ± 650.0 | 2989.8 ± 604.0 | <.001 | 1.00 (0.35–2.85) | - |
| Public Insurance or Self-Pay | 475 (91.0) | 4059 (87.1) | .012 | 1.49 (0.52–4.3) | - |
| Intrauterine Opioid Exposure | 387 (74.1) | 2122 (45.6) | <.001 | 3.43 (1.20–9.8) | - |
| “Other” Drug Exposurec | 132 (25.3) | 2522 (54.1) | <.001 | 0.29 (0.10–0.82) | - |
| Limited or No Prenatal Care | 27 (5.2) | 108 (2.3) | <.001 | 2.30 (0.81–6.55) | 1.22 (0.43–3.48) |
| Confirmed HBV Infection | 5 (1.0) | 7 (0.2) | .002 | 6.43 (2.25–18.3) | - |
| Foster Care | 167 (32.0) | 53 (1.1) | <.001 | 40.9 (14.3–116.5) | - |
| Any NICU Stay | 227 (43.5) | 914 (19.6) | <.001 | 3.15 (1.11–9.0) | 1.03 (0.36–2.93) |
| Age at Last Interaction ≥18 Months | 469 (89.8) | 1735 (37.2) | <.001 | 14.9 (5.23–42.5) | 18.1 (6.34–51.5) |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; NICU, neonatal intensive care unit; OR, odds ratio.
aNine HCV-tested and 198 untested infants were excluded in multivariate analysis due to missing data.
bAdjusted for race, birth weight, insurance type, any drug exposure, and confirmed HBV coinfection. Interactions between opioid exposure and NICU stay were also adjusted for in the model.
cInfants classified as having “Other Drug Exposure” includes any infant who had an intrauterine exposure to amphetamines, benzodiazepines, barbiturates, cannabinoids, cocaine, and phencyclidine.
Association of Pediatric Hepatitis C Virus Exposure and Drug Exposure
Among 114 HCV-exposed cases, 7 were excluded due to unique birth characteristics (ie, very low birth weight, very preterm) or because their race/ethnicity was not reported. Of the 291 control infants, there were no HCV test results for 283 infants. The statistical differences between the entire cohort population and the randomly selected cohort subset used as the control group in the nested analysis are shown in Table 4.
Table 4.
Association of Pediatric HCV Exposure and Varying Definitions of Intrauterine Drug Exposure Comparing Matched Cases and Controls (n = 388)
| Characteristic | HCV Exposed n = 97 (%) | Matched Controlsa n = 291 (%) | Cohort n = 58 313 (%) | P b | OR [95% CI] | Any Drug aORc [95% CI] | Opioid Exposure aOR [95% CI] | NAS Diagnosis aOR [95% CI] |
|---|---|---|---|---|---|---|---|---|
| Race | ||||||||
| White/Caucasian | 72 (74.2) | 216 (74.2) | 26 621 (45.7) | Reference | ||||
| Black/African American | 21 (21.6) | 63 (21.6) | 12 990 (22.2) | -- | 1.00 [0.57–1.8] | -- | -- | -- |
| Otherd,e | 4 (4.1) | 12 (4.1) | 2074 (3.56) | 1.00 [0.31–3.2] | ||||
| Any Drug Exposuref | 79 (81.4) | 37 (12.7) | 5071 (8.70) | .02 | 30.1 [16.2–55.9] | 4.2 [1.7–10.7] | -- | -- |
| Opioid Exposure | 63 (64.9) | 19 (6.5) | 2239 (3.84) | .3 | 26.0 [14.2–47.9] | -- | 6.2 [2.3–16.6] | -- |
| NAS Diagnosis | 33 (34.0) | 11 (3.78) | 774 (1.33) | .002 | 13.1 [6.3–27.4] | -- | -- | 2.6 [0.7–9.2] |
| Known Maternal HCV | 78 (80.4) | 8 (2.7) | 874 (1.5) | .09 | 145.2 [61.3–344.3] | 55.3 [20.2–151.3] | 72.0 [26.5–195.9] | 92.4 [35.3–242.0] |
| HIV Infection | 1 (1.03) | 0 (0.0) | 11 (0.02) | 1.00 | -- | -- | -- | -- |
| HBV Infection | 1 (1.03) | 0 (0.0) | 11 (0.02) | 1.00 | -- | -- | -- | -- |
| High Risk | 79 (81.4) | 37 (12.7) | 5088 (8.7) | .02 | 115.4 [44.2–301.4] | -- | -- | -- |
| Foster Care | 16 (16.5) | 3 (1.03) | 456 (0.78) | .50 | 19.0 [5.4–66.7] | 12.5 [1.9–82.6] | 11.5 [1.7–77.7] | 11.5 [1.9–68.3] |
| Insurance | ||||||||
| Private | 11 (11.3) | 158 (54.3) | 28 453 (48.8) | .06 | Reference | Reference | Reference | Reference |
| Public or Self-Pay | 86 (88.7) | 133 (45.7) | 29 860 (51.2) | 9.3 [4.8–18.1] | 1.5 [0.5–4.4] | 1.5 [0.51–4.1] | 2.1 [0.80–5.6] | |
| Any NICU Admittance | 41 (42.3) | 49 (16.8) | 5748 (9.86) | <.001 | 3.6 [2.8–6.0] | 2.1 [0.80–5.5] | 1.7 [0.63–4.7] | 2.1 [0.80–5.6] |
| Limited or No Prenatal Care | 3 (3.09) | 3 (1.03) | 358 (0.61) | .27 | 3.1 [0.61–15.4] | 0.16 [0.01–2.4] | 0.12 [0.01–1.9] | 0.10 [0.01–1.2] |
| Birth Weightg (grams) | 2954.6 ± 608 | 2954.8 ± 606 | 3252 ± 616 | -- | 1.00 [1.00–1.00] | -- | -- | -- |
| Gestational Ageg (weeks) | 37.8 ± 2.3 | 37.8 ± 2.3 | 38.4 ± 2.1 | -- | 1.00 [0.91–1.1] | -- | -- | -- |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; NAS, neonatal abstinence syndrome; NICU, neonatal intensive care unit; OR, odds ratio.
aIncludes infants with confirmed negative HCV test results (n = 8) as well as untested infants (n = 283).
bFisher’s exact test, except for birth weight and gestational age; t test. The variables that the cases and controls were matched on were excluded in analysis.
cAdjusted for insurance type, known maternal exposure, prenatal care, foster care, and NICU stay.
d“Other” includes infants identified as Hispanic/Latino, Asian, Middle Eastern, American Indian and/or Alaska Native, and Native Hawaiian and/or other Pacific Islander.
eData are missing or excluded for race if it was unreported, parent/guardian was not present, or declined to answer among cohort population (n = 16 628).
fAny infant born to a mother whose urine tested positive at time of delivery for one of the following drugs: amphetamines, benzodiazepines, barbiturates, buprenorphine, cannabinoids, cocaine, heroin, methadone, phencyclidine, and prescription pain medications (illicit or prescribed use cannot be differentiated).
gMissing data in cohort population, n = 2785.
Intrauterine drug exposure to any drug was associated with a 4.5-fold increase in the odds of HCV exposure compared to controls without drug exposure in adjusted analyses (aOR, 4.2; 95% CI, 1.7–10.7) (Table 4). Cases with an intrauterine opioid exposure had more than a 6-fold increase in the odds of HCV exposure (aOR, 6.2; 95% CI, 2.3–16.6) compared with controls after adjusting for insurance type, known maternal HCV infection, prenatal care, foster care, NICU stay, initial hospital length of stay, and HIV or HBV coinfection. Opioid-exposed infants who were diagnosed with severe opioid withdrawal requiring pharmacologic treatment (or NAS) were approximately 3 times more likely to be HCV-exposed (aOR, 2.6; 95% CI, 0.7–9.2), but this difference was not statistically significant.
Discussion
The prevalence of pediatric HCV exposure among all newborn children in this study (0.2%), as well as the prevalence of pediatric HCV infection when stratified by risk (low risk 0.35%–1.1% and high risk 3.6%–5.2% of live births), are consistent with previously reported rates [5, 13]. These findings support the idea that the population at high risk for vertical HCV transmission during pregnancy is largely captured in the healthcare system during the perinatal period. Therefore, this could be a key time period for practitioners to implement policies for perinatal HCV diagnostic testing to better capture vertical HCV transmission [1, 5].
It is alarming that two thirds of HCV infection cases among high-risk infants (38 cases) are estimated to have been missed due to inconsistent protocols for HCV infection identification. The diagnostic cascade indicates that this may be partially attributable to a lack of consensus regarding a pediatric HCV testing schedule. More than one quarter of HCV tests conducted did not yield conclusive diagnoses by the definition used in this study, due to testing before the age recommended by the CDC.
Moreover, to the best of our knowledge, this study is the first of its kind to address perinatal HCV testing in a setting where universal drug testing at the time of delivery has been implemented. This policy was implemented to improve the identification of infants at high risk for developing NAS. Wexelblatt et al [11] showed that 20% of opioid-positive urine tests were recorded in mothers with no screening risk factors. Likewise, this study found that 21.2% of the high-risk cases and 100.0% of the low-risk cases identified in the diagnostic cascade were not coded as having been prenatally HCV-exposed in their medical records. These findings support recent urges for improving consistency in HCV testing, predominantly through the implementation of universal HCV testing [1–3, 5].
However, previous recommendations have largely focused on implementing universal prenatal HCV testing. Our study found that mothers of high-risk infants were significantly less likely to receive prenatal care (P = .034). Prenatal care also had no significant effect on the likelihood of high-risk infants receiving HCV testing. These data indicate that universal prenatal HCV testing alone may not be sufficient in capturing the pediatric population at risk.
In contrast, an infant was more likely to receive testing if they were maintained in care by a CCHMC pediatrician. High-risk infants who were at least 18 months of age, entered the foster care system, or were admitted to the NICU were significantly more likely to have received HCV testing in univariate analysis. In adjusted analysis, only age at last interaction was still a statistically significant predictor. These findings indicate the importance of establishing continuity of care and accurate transfer of medical information between providers in care of vertically transmitted diseases, as has been demonstrated in studies of vertical HIV transmission [14]. These are challenges that institutions may face without a universal policy.
This was a retrospective study that used EHR data and thus has several limitations. The possibility for variation between EHR codes used does exist. This could be aggravated by the switch from ICD-9 to ICD-10 codes during the study period. For this reason, the ICD codes used in this analysis were confirmed with clinicians and billing code specialists to ensure consistency. The ICD codes are grouped by type of drug and do not differentiate between method of drug administration. Therefore, the route of newborn drug exposure (eg, through maternal intravenous drug use) could not be determined. Furthermore, the urine drug testing does not differentiate between illicit or appropriately prescribed and consumed opioid-based medications. Finally, we did not have access to maternal HCV laboratory results, so our classification of known maternal infection relied on accurate coding. Testing for pediatric HCV is more likely to occur among children with known risk factors (ie, maternal HCV infection) whom we designated as high-risk infants. Thus, our analyses may be subject to misclassification that may bias our effect estimate in either direction. Furthermore, not all infants included as controls in the nested case-control analysis were tested for HCV. This could not be directly controlled for through selective matching, but the regression models were adjusted for variables associated with receipt of testing to control for confounding factors in the analysis. Finally, more than half of the births attended by the CCHMC pediatrician do not continue to see a CCHMC pediatrician by 18 months, resulting in loss to follow-up; however, the analysis showed that follow-up was approximately equal between low- and high-risk infants. This rate of loss to follow-up is similar or lower than the rates reported by other large Midwestern hospitals [1].
Conclusions
The opioid epidemic is driving increases in HCV infection nationwide. Our results confirm the significant association between intrauterine opioid exposure and pediatric exposure. The availability of a large representative pediatric cohort combined with universal drug testing results for all pregnant women seeking care in the region uniquely positioned this study to assess HCV prevalence in a population that is otherwise difficult to identify. These findings indicate that current testing recommendations may not properly address the barriers to HCV testing among high-risk infants, which likely contributes to the number of missed HCV infections. New policies for pediatric HCV testing, such as universal pediatric HCV testing, may help address the gaps in maternal HCV testing and identify HCV-infected populations where universal drug screening is not implemented.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. S. P. designed the study, conducted the data analysis, and drafted the initial manuscript; E. S. H. and S. L. W. conceptualized and designed the study; L. B. M. was involved in the epidemiological design of the study; J. T. B. contributed hepatitis C virus-specific expertise; and all authors reviewed and revised the manuscript and approved the contents of the final manuscript as submitted.
Financial support. This work was funded by the National Center for Advancing Translational Sciences of the National Institutes of Health through the Center for Clinical and Translational Science and Training at the University of Cincinnati (Grant 5UL1TR001425-02) and divisional support from the Perinatal Institute at Cincinnati Children’s Hospital Medical Center.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Chappell CA, Hillier SL, Crowe D, et al. . Hepatitis C virus screening among children exposed during pregnancy. Pediatrics 2018; 141:e20173273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gowda C, Kennedy S, Glover C, et al. . Enhanced identification of maternal hepatitis C virus infection using existing public health surveillance systems. Paediatr Perinat Epidemiol 2018; 32:401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuncio DE, Newbern EC, Johnson CC, Viner KM. Failure to test and identify perinatally infected children born to hepatitis C virus-infected women. Clin Infect Dis 2016; 62:980–5. [DOI] [PubMed] [Google Scholar]
- 4. Zibbell JE, Asher AK, Patel RC, et al. . Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health 2018; 108:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Epstein RL, Sabharwal V, Wachman EM, et al. . Perinatal transmission of hepatitis C virus: defining the cascade of care. J Pediatr 2018; 203:34–40.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis 2014; 59:765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yehia BR, Schranz AJ, Umscheid CA, Lo Re V III. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One 2014; 9:e101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. AASLD-IDSA HCV Guidance Panel. Hepatitis C guidance 2018 Update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clin Infect Dis 2018; 67:1477–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Academy of Pediatrics. Committee on Infectious Diseases. Red Book for PDA: Report of the Committee on Infectious Diseases. Elk Grove Village, IL: American Academy of Pediatrics; 2003. CD-ROMs. [Google Scholar]
- 10. Division of Viral Hepatitis, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Centers for Disease Control and Prevention. Viral Hepatitis: Testing Recommendations: U.S. Department of Health and Human Sciences 2015. Available at: https://www.cdc.gov/hepatitis/hcv/guidelinesc.htm. Accessed 15 October 2015.
- 11. Wexelblatt SL, Ward LP, Torok K, et al. . Universal maternal drug testing in a high-prevalence region of prescription opiate abuse. J Pediatr 2015; 166:582–6. [DOI] [PubMed] [Google Scholar]
- 12. Hall ES, Greenberg JM, Muglia LJ, et al. . Implementation of a regional perinatal data repository from clinical and billing records. Matern Child Health J 2018; 22:485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang CHT, Yoo ER, Ahmed A. The role of direct-acting antivirals in the treatment of children with chronic hepatitis C. J Clin Transl Hepatol 2017; 5:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gill MJ, Ody M, Lynch T, et al. . Maintaining the continuity of HIV-care records for patients transferring care between centers: challenges, workloads, needs and risks. AIDS Care 2016; 28:1073–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.