Abstract
Background
5-Fluorouracil (5-Fu) has been applied to treat pancreatic cancer, which is one of the most common types of digestive system tumors. Evidence has shown that miR-486-5p could promote the proliferation of pancreatic cancer cells. Therefore, this study aimed to investigate whether downregulation of miR-486-5p could enhance the anti-tumor effect of 5-Fu on pancreatic cancer cells.
Methods
Cell Counting Kit 8 assay, flow cytometry and wound healing assays were used to detect proliferation, apoptosis and migration in PANC-1 cells. The expressions of Bcl-2, Bax, cleaved caspase 3, PTEN, p-Akt and p-ERK in PANC-1 cells were detected with Western blot assay.
Results
In this study, the inhibitory effects of 5-Fu on the proliferation, migration and invasion of PANC-1 cells were significantly enhanced following transfection with miR-486-5p antagonist. In addition, downregulation of miR-486-5p markedly enhanced the pro-apoptosis effect of 5-Fu on PANC-1 cells. Moreover, bioinformatics analysis and luciferase reporter assay identified that PTEN was the directly binding target of miR-486-5p. Meanwhile, downregulation of miR-486-5p markedly enhanced the anti-tumor effect of 5-Fu in PANC-1 cells via upregulation of the level of PTEN, and downregulation of the expressions of p-ERK and p-Akt. In vivo experiments confirmed that knockdown of miR-486-5p could enhance the anti-tumor effect of 5-Fu in PANC-1 xenograft model.
Conclusion
We found that the downregulation of miR-486-5p could enhance the anti-tumor effect of 5-Fu on pancreatic cancer cells. Therefore, miR-486-5p antagonist plus 5-Fu might be considered as a potential therapeutic strategy for the treatment of pancreatic cancer.
Keywords: pancreatic cancer, 5-fluorouracil, miR-486-5p, PTEN, apoptosis
Introduction
Pancreatic cancer is one of the most common malignancies in human worldwide.1 Pancreatic ductal adenocarcinoma (PDAC) accounts for more than 85% of all pancreatic tumors, and the overall 5-year survival rate of patients with PDAC less than 7%.2,3 Clinically, surgery, chemotherapy, radiation therapy and immunotherapy are the main treatment methods for pancreatic cancer.4 However, the survival rate and prognosis of patients with pancreatic cancer remain worse.5 Previous study found that the absence of druggable targets and chemotherapy resistance are the main causes of poor prognostic factors for patients with pancreatic cancer.6 It has been shown that 5-Fluorouracil (5-Fu) is a first-line chemotherapy drug for the treatment of pancreatic cancer.7 However, 5-Fu resistance is one of the major obstacles affecting the effect of chemotherapy.7 In addition, 5-Fu also exhibits some side effects in some elementary functions, such as memory, executive function and sensory gating.8 Therefore, novel effective therapies for the treatment of pancreatic cancer are imminently needed.
MicroRNAs (miRNAs), short non-coding RNA molecules, which could bind to the 3′-untranslated region (3′-UTR) of their target mRNAs to reduce the expression of proteins.9,10 Meanwhile, miRNAs play important roles in the mediation of gene expression.11–13 It has been shown that miRNAs have emerged as key regulators during the development of cancer initiates, and play vital roles in tumor growth, biosynthesis, and drug resistance.14 Zhao et al indicated that miRNAs acted as novel chemo-resistance regulators, which could regulate the levels of drug resistance-related genes.15 Meanwhile, recent studies indicated that miR-486-5p served as a biomarker in various kinds of tumors, including non-small cell lung cancer, prostate cancer.16,17 Ali et al found that the level of miR-486-5p was significantly upregulated in patients with pancreas cancer.18 However, the role of miR-486-5p in pancreatic cancer cells remain poorly explored.
In the present study, we aimed to determine whether miR-486-5p could enhance the anti-tumor effect of 5-Fu on pancreatic cancer cells with the purpose of providing a promising therapeutic direction for patients with pancreatic cancer.
Materials and Methods
Cell Culture and Transfection
The human pancreatic cancer cell lines PANC-1 and MiaPaCa-2 were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA), and 1% penicillin/streptomycin, and maintained at 37°C in a humidified incubator (5% CO2).
MiR-486-5p agonist (5ʹ-UCCUGUACUGAGCUGCCCCGAG-3ʹ) and miR-486-5p antagonist (5ʹ-CUCGGGGCAGCUCAGUACAGGA-3ʹ) and negative control (NC) were purchased from GenePharma (Shanghai, China) with a stock concentration of 20 μM. PANC-1 and MiaPaCa-2 cells were transfected with 10 nM miR-486-5p agonist, 10 nM miR-486-5p antagonist, or 10 nM NC for 48 h, using lipofectamine 2000 reagent (Thermo Fisher Scientific) following the manufacture’s instruction.
Quantitative Real‑time Polymerase Chain Reaction (RT‑qPCR)
A miRNeasy Mini Kit (Qiagen, Valencia, CA, USA) was used to extract total RNAs from cells according to the manufacturer’s instruction. The complementary DNA (cDNA) was carried out using the PrimeScript RT Master Mix (TaKaRa, Otsu, Shiga, Japan). After that, the following qPCR was performed using SYBR Green premix Ex TaqTM (TaKaRa) on an Applied Biosystems 7500 Real Time PCR System (Applied Biosystems, Foster City, CA, USA). The PCR conditions were as follows: 95°C for 5 min, then 40 cycles consisting of 95°C for 30 s and 60°C for 30 s. The sequences of the primers were: PTEN, Forward: 5ʹ-ATTCCCAGTCAGAGGCGCTAT-3ʹ; Reverse; 5ʹ- GAACTTGTCTTCCCGTCGTGT-3ʹ. Actin, Forward: 5ʹ- GTCCACCGCAAATGCTTCTA-3ʹ; Reverse: 5ʹ-TGCTGTCACCTTCACCGTTC-3ʹ. MiR-486-5p, Forward: 5ʹ- TGTACTGAGCTGCCCCGAG-3ʹ; Reverse: 5ʹ- CTCAACTGGTGTCGTGGAGTC-3ʹ. U6, Forward: 5ʹ- CTCGCTTCGGCAGCACAT-3ʹ; Reverse: 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ. The level of miR-486-5p was normalized to the internal control U6 using the 2−ΔΔCT method. The level of PTEN was normalized to the internal control β-actin using the 2−ΔΔCT method.
Cell Counting Kit 8 Assay
Cell Counting Kit-8 (Dojindo Molecular Technologies, Gaithersburg, MD, USA) was applied to determine the viability of pancreatic cancer cells. PANC-1 or MiaPaCa-2 cells (5 × 103 cells/well) were plated into 96-well plates, respectively, followed by incubation at 37°C overnight. After that, cells were transfected with miR-486-5p antagonist, and then exposed to various doses (0, 0.01, 0.05, 0.1, 0.2, 0.4 or 0.8 μg/mL) of 5-Fu, respectively. After 48 h of incubation, the culture medium was replaced by fresh DMEM medium containing 10% Cell Counting Kit-8 reagent, and then incubated for another 2 h at 37°C. The absorbance was measured using a microplate reader (Bio-Rad, Hercules, CA, USA) at 450 nm.
Ki67 Immunofluorescence Staining
PANC-1 cells were fixed by 4% paraformaldehyde and then incubated with rabbit anti-Ki67 antibody (1:100, Abcam Cambridge, MA, USA) overnight at 4°C. Then, the cells were incubated with the anti-rabbit IgG secondary antibody at room temperature for 1 hr. Later on, cells were stained with DAPI for 5 min. Subsequently, cells were observed using a fluorescence microscope (Olympus CX23 Tokyo, Japan).
Cell Apoptosis Assay
Apoptosis of PANC-1 cells was assessed by an Annexin V-FITC apoptosis detection kit (Thermo Fisher Scientific) on a flow cytometer (FACScan™, BD Biosciences, Franklin Lakes, NJ, USA). PANC-1 cells were collected in pre-cold PBS (Thermo Fisher Scientific) and then resuspended in 100 μL binding buffer from the Annexin V-FITC apoptosis detection kit. Later on, the cells were stained with propidium iodide (PI) and Annexin V-FITC for 15 min in darkness at room temperature according to the recommended protocols. Then, the percentage of apoptotic cells was analyzed.
Western Blot
Cells were lysed in RIPA buffer (Thermo Fisher Scientific), and the contents of proteins were quantified using the BCA protein Assay kit (Beyotime Institute of Biotechnology). Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by blotting to the polyvinylidene difluoride membrane (PVDF, Thermo Fisher Scientific). After that, the membrane was blocked in 5% skim milk powder at room temperature. Then, the membrane was incubated in a solution containing the primary antibodies at 4°C overnight, including anti-Bax (1:1000, Abcam), anti-Bcl-2 (1:1000, Abcam), anti-cleaved caspase 3 (1:1000, Abcam), anti-PTEN (1:1000, Abcam), anti-p-Akt (1:1000, Abcam), anti-Akt (1:1000, Abcam), anti-p-ERK (1:1000, Abcam), anti-ERK (1:1000, Abcam) and anti-β-actin (1:1000, Abcam). Later on, the membrane was incubated with goat anti-rabbit secondary antibody (1:5000, Abcam) for 1 hr at the room temperature. After that, the membrane was visualized using an ECL Chemiluminescent Substrate Reagent Kit (Thermo Fisher Scientific). β-actin was acted as the internal control.
Wound Healing Assay
PANC-1 cells (2×105 cells per well) were seeded into 12-well culture plates overnight at 37°C in 5% CO2. At 80% confluent cells, a wound area was made in the cell monolayer using a sterile micropipette tip. Then, cells were transfected with 10 nM miR-486-5p antagonist, and then exposed to 0.1 μg/mL 5-Fu for 48 h at 37°C. The width of the wound area was observed at 0 h and 48 h using the fluorescence microscope (Olympus). Image J software was used for the width measurements.
Transwell Invasion Assay
A membrane coagulated with 100 μL of Matrigel (BD Biosciences) was added into the upper chamber. Then, the PANC-1 cells (5 × 104 cells suspended in 200 µL serum-free media) were seeded into the upper compartment of the chamber, and DMEM medium (600 μL) containing 10% FBS was added into the lower compartment of each well. After 24 h of incubation, the cells attached to the upper compartments were removed. After that, the cells that invaded through the transwell membrane were fixed with 4% paraformaldehyde, and then stained with 0.2% crystal violet. After washing twice with PBS, cells were photographed using a laser confocal microscope (Olympus Corp., Tokyo, Japan) at 400 × magnification. Five random fields of each membrane were counted.
Dual-Luciferase Reporter Assay
The 3ʹ UTR of PTEN sequences containing the wild-type (WT) or mutant (MT) miR-486-5p binding sites were ligated into the pmirGLO luciferase reporter vector (Promega, Madison, WI, USA) respectively. PANC-1 cells were co-transfected with PTEN reporter construct (WT or MT) and miR-486-5p mimics and NC, respectively, using Lipofectamine 2000respectively. Forty-eight hours later, the luciferase activities were measured using a Dual-Luciferase Reporter Assay System (Promega) with renilla luciferase activity as endogenous control.
Animal Study
4-6-week-old BALB/c nude mice (15–20 g) were purchased from the Shanghai SLAC Animal Center (Shanghai, China) and animals were maintained following the guidelines of the Institutional Animal Care and Use Committee (No. 20190410135). All animal experiments were performed according to the rules approved by the Institutional Ethical Committee of the Henan Provincial People’s Hospital. Animals were randomized into four groups: blank, 5-Fu, miR-486-5p antagonist and 5-Fu + miR-486-5p antagonist group. 1 × 107 PANC-1 cells (in 100 μL of PBS) were injected subcutaneously into the left flank of nude mice (3 per group). When the tumors reach 180 mm3, 50 nM miR-486-5p antagonist was directly injected into the tumors twice a week. Meanwhile, the mice were intraperitoneal administration of 10 mg/kg 5-Fu weekly. Tumor volume was monitored every week with a caliper and analyzed using the formula V = (length x width2)/2. After 28 days of treatment, all nude mice were sacrificed and the entire tumors were dissected out and weighed.
Statistical Analysis
All data were repeated in triplicate. Data are presented as mean ± standard deviation (S. D.). All statistical analyses were performed using GraphPad Prism software (version 7.0, La Jolla, CA, USA). One-way analysis of variance (ANOVA) and Tukey’s tests were carried out for multiple group comparisons. A P-value < 0.05 was considered as statistically significant.
Results
Downregulation of miR-486-5p Enhanced the Cytotoxic Effect of 5-Fu in Pancreatic Cancer Cells
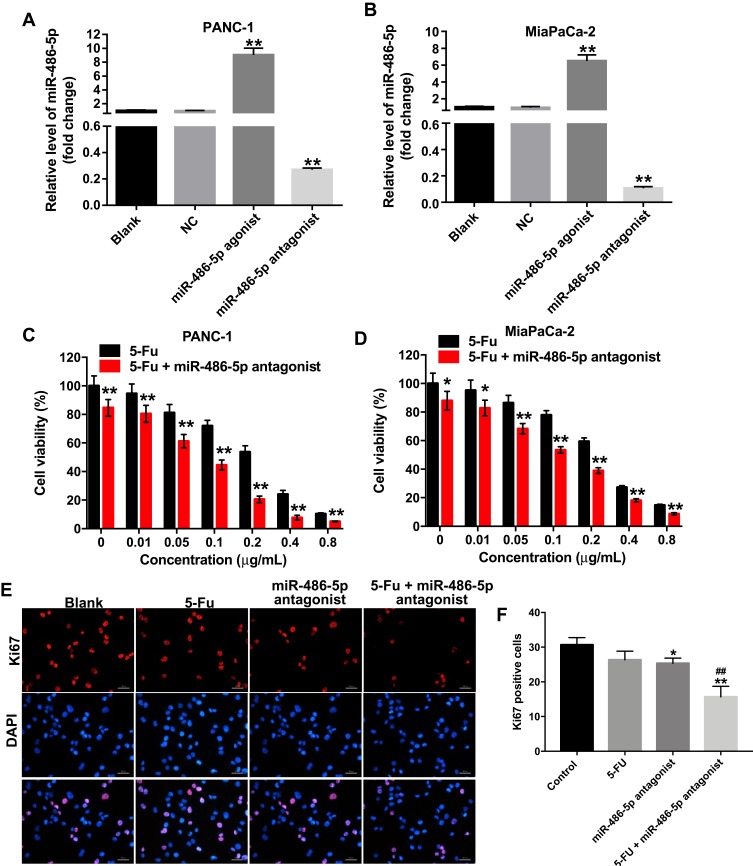
To investigate the role of miR-486-5p in pancreatic cancer cells, PANC-1 and MiaPaCa-2 cells were treated with miR-486-5p agonist or antagonist, respectively. As shown in Figure 1A and B, Supplementary Figure 1A and B, 10 nM miR-486-5p agonist significantly increased the level of miR-486-5p in PANC-1 and MiaPaCa-2 cells, while 10 nM miR-486-5p antagonist markedly decreased the level of miR-486-5p in cells.
Figure 1.
Downregulation of miR-486-5p enhanced the cytotoxic effect of 5-Fu in pancreatic cancer cells. (A) PANC-1 and (B) MiaPaCa-2 cells were transfected with 10 nM miR-486-5p agonist or 10 nM miR-486-5p antagonist for 48 hrs. RT-qPCR was used to detect the level of miR-486-5p in PANC-1 and MiaPaCa-2 cells. (C) PANC-1 and (D) MiaPaCa-2 cells were transfected with 10 nM miR-486-5p antagonist, and then exposed to 5-Fu for 48 hrs. Cell Counting Kit 8 assay was used to determine the cell viability. (E, F) PANC-1 cells were transfected with 10 nM miR-486-5p antagonist and then exposed to 5-Fu for 48 hrs. Meanwhile, PANC-1 cells were transfected with 10 nM miR-486-5p antagonist or exposed to 5-Fu for 48 hrs, respectively. Relative fluorescence expressions were quantified by Ki67 and DAPI staining. *P<0.05, **P<0.01 vs NC group; ##P<0.01 vs 5-FU group.
In addition, the proliferation of pancreatic cancer cells was detected using Cell Counting Kit 8 assay. As shown in Figure 1C and D, Supplementary Figure 2A and B, 5-Fu inhibited the viability of PANC-1 and MiaPaCa-2 cells in a dose-dependent manner. In addition, the IC50 value of 5-Fu was 0.251 μg/mL and 0.241 μg/mL in PANC-1 and in MiaPaCa-2 cells, respectively. When 5-Fu was combined with miR-486-5p agonist (10 nM), the IC50 value of 5-Fu was increased to 0.419 μg/mL and 0.533 μg/mL in PANC-1 and in MiaPaCa-2 cells, respectively (Supplementary Figure 2A and B). These data indicated that the upregulation of miR-486-5p could attenuate the cytotoxic effect of 5-Fu in pancreatic cancer cells.
In contrast, when 5-Fu was combined with miR-486-5p antagonist (10 nM), the IC50 value of 5-Fu was decreased to 0.065 μg/mL and 0.178 μg/mL in PANC-1 and in MiaPaCa-2 cells, respectively (Figure 1C and D). PANC-1 cells were more sensitive to miR-486-5p antagonist compared with MiaPaCa-2 cells. Therefore, PANC-1 cells were utilized in the following studies. Meanwhile, 0.1 μg/mL 5-Fu induced 28% growth inhibition of PANC-1 cells, which was considerably increased to 56% in the presence of miR-486-5p antagonist (Figure 1C). Therefore, 0.1 μg/mL 5-Fu was utilized in the following experiments.
Furthermore, the results of Ki67 immunofluorescence staining assay indicated that combination with 5-Fu and miR-486-5p antagonist significantly reduced the number of Ki67-positive PANC-1cells, compared with the 5-Fu group (Figure 1E and F). These data indicated that the downregulation of miR-486-5p could enhance the cytotoxic effect of 5-Fu in pancreatic cancer cells.
Knockdown of miR-486-5p Enhanced the Pro-Apoptotic Effect of 5-Fu in PANC-1 Cells
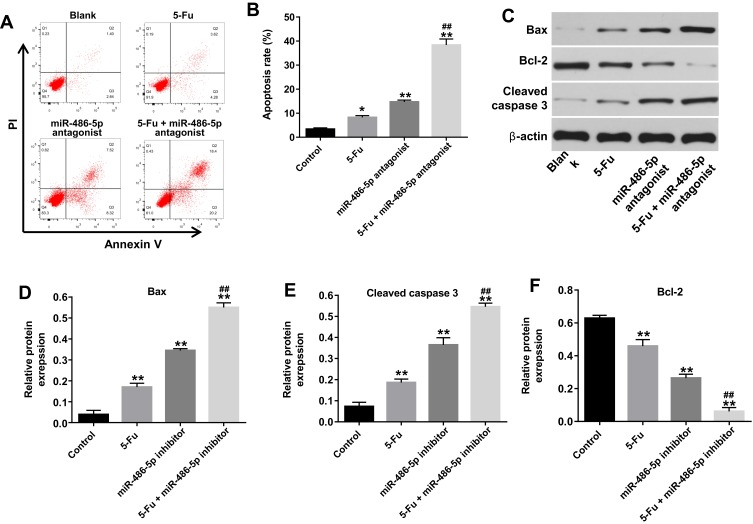
To investigate the biological effect of miR-486-5p plus 5-Fu on the apoptosis of PANC-1 cells, flow cytometry was used. As shown in Figure 2A and B, Supplementary Figure 2B and 2C, 5‑Fu alone treatment increased the apoptosis rate of PANC-1 cells, compared with the control group. However, 5-Fu-induced cell apoptosis was markedly reversed following transfection with miR-486-5p agonist (Supplementary Figure 2C and D). In contrast, combination of 5-Fu with miR-486-5p antagonist significantly induced the apoptosis in PANC-1 cells, compared with the 5-Fu group (Figure 2A and B). In addition, 5-Fu alone treatment could upregulate the levels of Bax and cleaved caspase 3 and downregulated the expression of Bcl-2 in PANC-1 cells. These effects were markedly enhanced following transfection with miR-486-5p antagonist (Figure 2C–F). These results suggested that the downregulation of miR-486-5p could enhance 5-Fu-induced apoptosis in PANC-1 cells.
Figure 2.
Knockdown of miR-486-5p enhanced 5-Fu-induced apoptotic rate in PANC-1 cells. PANC-1 cells were transfected with 10 nM miR-486-5p antagonist and then exposed to 5-Fu for 48 hrs. Meanwhile, PANC-1 cells were transfected with 10 nM miR-486-5p antagonist or exposed to 5-Fu for 48 hrs, respectively. (A, B) Apoptotic cells were detected with Annexin V and PI double staining. (C) Expression levels of Bax, cleaved caspase 3 and Bcl-2 in PANC-1 cells were detected with Western blotting. (D–F) The relative expression of Bax, cleaved caspase 3 and Bcl-2 in PANC-1 cells were quantified via normalization to β-actin. *P<0.05, **P<0.01 vs control group; ##P<0.01 vs 5-Fu group.
miR-486-5p Antagonist Enhanced the Inhibitory Effects of 5-Fu on the Migration and Invasion of PANC-1 Cells
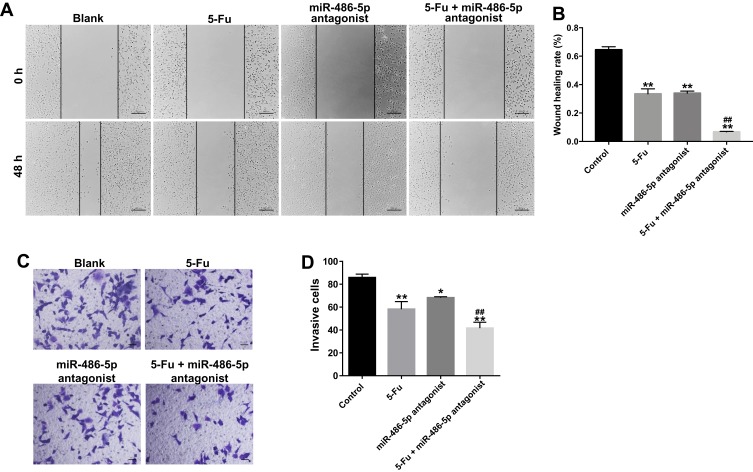
In order to further explore the biological role of the miR-486-5p and 5-Fu on the migration and invasion of PANC-1 cells, wound healing and transwell invasion assays were applied. As shown in Figure 3A and B, 5‑Fu or miR-486-5p antagonist alone treatment could inhibit the migratory ability of PANC-1 cells. As expected, combination treatment exhibited better inhibitory effect the migration ability of PANC-1 cells, compared with the 5-Fu or miR-486-5p antagonist group (Figure 3A and B). In consistency, the combination treatment exhibited better inhibitory effect on the invasion ability of PANC-1 cells compared with the 5-Fu or miR-486-5p antagonist group (Figure 3C and D). These data suggested that miR-486-5p antagonist significantly enhances the inhibitory effects of 5-Fu on the migration and invasion of PANC-1 cells.
Figure 3.
Downregulation of miR-486-5p enhanced the inhibitory effects of 5-Fu on the migration and invasion of PANC-1 cells. PANC-1 cells were transfected with 10 nM miR-486-5p antagonist and then exposed to 5-Fu. Meanwhile, PANC-1 cells were transfected with 10 nM miR-486-5p antagonist or exposed to 5-Fu, respectively. (A, B) Wound healing assay was used to detect the cell migration. Representative images captured at 0 and 48 h are shown (magnification x 400). (C, D) Transwell invasion assay was used to measure the invasive ability of PANC-1 cells. *P<0.05, **P<0.01 vs control group; ##P<0.01 vs 5-Fu group.
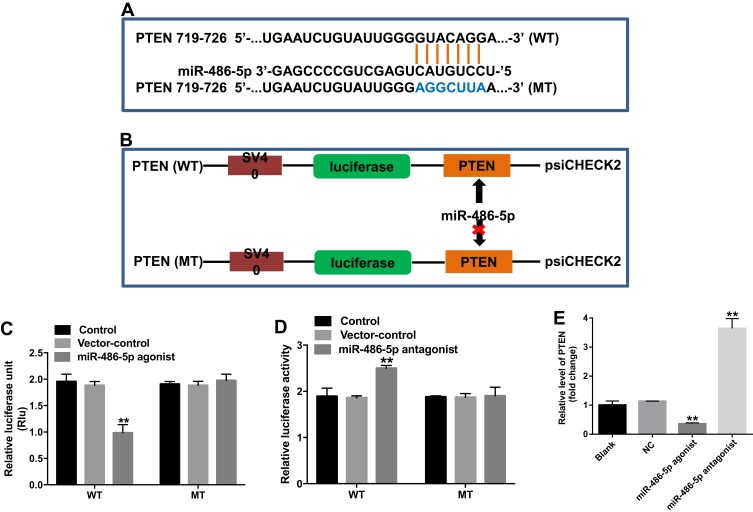
PTEN Was a Direct Target of miR-486-5p
Online bioinformatics tool TargetScan was used to search target genes of miR-486-5p. The data showed that PTEN was a potential target gene of miR-486-5p (Figure 4A). In addition, dual-luciferase reporter assay was used to validate whether PTEN is a direct target of miR-486-5p. The results indicated that miR-486-5p agonist significantly inhibited the luciferase activity of PTEN-WT, but it did not affect the luciferase activity of PTEN-MT (Figure 4B and C). In contrast, the increased luciferase activity was observed in PANC-1 cells following transfection with PTEN-WT and miR-486-5p antagonist (Figure 4D). Moreover, qRT-PCR assay indicated that the upregulation of miR-486-5p significantly decreased the level of PTEN in PANC-1 cells, while downregulation of miR-486-5p markedly increased the level of PTEN in PANC-1 cells (Figure 4E). These findings suggested that PTEN was a direct target of miR-486-5p.
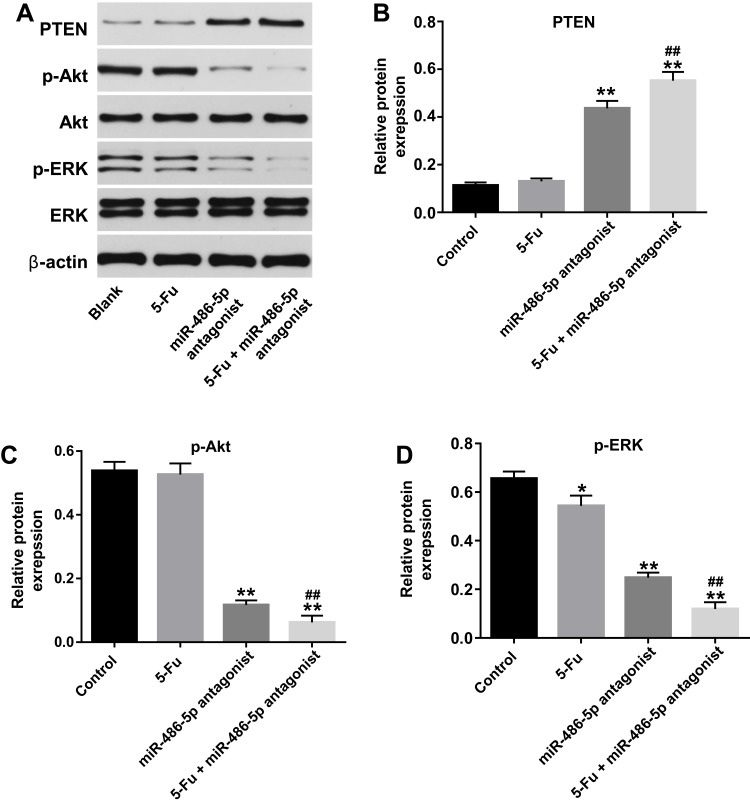
Figure 5.
Downregulation of miR-486-5p enhanced the anti-tumor effect of 5-Fu in PANC-1 cells via upregulation PTEN expression. PANC-1 cells were transfected with 10 nM miR-486-5p antagonist and then exposed to 5-Fu for 48 hrs. Meanwhile, PANC-1 cells were transfected with 10 nM miR-486-5p antagonist or exposed to 5-Fu for 48 hrs, respectively. (A) Expression levels of PTEN, p-Akt, p-ERK in PANC-1 cells were detected with Western blotting. (B–D) The relative expression of PTEN, p-Akt, p-ERK in PANC-1 cells were quantified via normalization to β-actin, Akt and ERK, respectively. *P<0.05, **P<0.01 vs control group; ##P<0.01 vs 5-Fu group.
Figure 4.
PTEN was a direct target of miR-486-5p. (A) Sequence alignment of miR-586-5p with the putative binding sites within the WT or MT regions of PTEN. (B, C) The luciferase activity in PANC-1 cells following co-transfecting with PTEN-WT/MT 3ʹ-UTR plasmid and miR-486-5p agonist was detected using dual luciferase reporter assay. (D) The luciferase activity in PANC-1 cells following co-transfecting with PTEN-WT/MT 3ʹ-UTR plasmid and miR-486-5p antagonist was detected using dual luciferase reporter assay. (E) PANC-1 cells were transfected with miR-486-5p antagonist for 48 hrs. RT-qPCR was used to detect the level of PTEN in PANC-1 cells. **P<0.01 vs control group.
Downregulation of miR-486-5p Enhanced the Anti-Tumor Effects of 5-Fu in PANC-1 Cells via Upregulation PTEN Expression
To investigate the pro-apoptotic mechanisms of miR-486-5p antagonist in PANC-1 cells, the expressions of apoptosis‑associated proteins were detected using Western blot. As indicated in Figure 5A–D, combination of 5‑Fu with miR-486-5p antagonist markedly upregulated the level of PTEN and downregulated the expressions of p-Akt and p-ERK in PANC-1 cells, compared with the 5-Fu group. These data indicated that knockdown of miR-486-5p notably enhances the anti-tumor effects of 5-Fu in PANC-1 cells via upregulating the expression of PTEN and suppressing the Akt/ERK pathway.
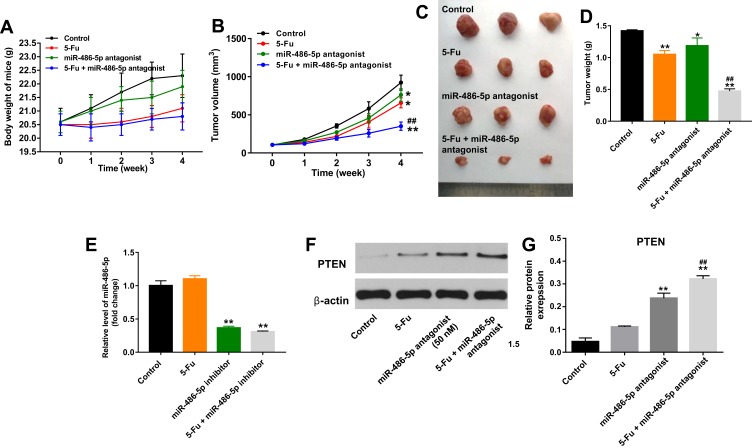
Downregulation of miR-486-5p Enhanced the Anti-Tumor Effect of 5-Fu in PANC-1 Pancreatic Cancer Xenograft Model in vivo
To evaluate whether miR-486-5p could enhance the anti-tumor effect of 5-Fu in PANC-1 xenograft model in vivo, tumor xenograft model was established. As shown in Figure 6A, 5-Fu or/and miR-486-5p had no system toxicity on mice. In addition, 5‑Fu alone or miR-486-5p antagonist alone treatment could inhibit the tumor volume and tumor weight, compared with the control group (Figure 6B–D). As expected, when the xenografts were treated with 5‑Fu plus miR-486-5p antagonist, the unfavorable role of 5-Fu on tumor growth was significantly enhanced by miR-486-5p antagonist (Figure 6B–D). In addition, combination of 5‑Fu with miR-486-5p antagonist obviously decreased the level of miR-486-5p in tumor tissues (Figure 6E). Moreover, the combination treatment markedly upregulated the level of PTEN in tumor tissues (Figure 6F and G). These data indicated that the downregulation of miR-486-5p enhanced the anti-tumor effect of 5-Fu in PANC-1 xenograft model in vivo via upregulating the expression of PTEN.
Figure 6.
Downregulation of miR-486-5p enhanced the anti-tumor effect of 5-Fu in PANC-1 xenograft model in vivo. (A) 1 × 107 PANC-1 cells were injected subcutaneously into the left flank of nude mice. 50 nM miR-486-5p antagonist was directly injected into the tumors twice a week. Meanwhile, the mice were intraperitoneal administration of 5-Fu weekly. The body weight of mice was monitored. (B) The xenograft tumor volumes were monitored weekly. (C) Xenografts tumors were photographed after 4 weeks. (D) The weights of the tumors were calculated. (E) RT-qPCR was used to detect the level of miR-486-5p in tumor tissues. (F) Expression level of PTEN in tumor tissues was detected with Western blotting. (G) The relative expression of PTEN in tumor tissues was quantified via normalization to β-actin. *P<0.05, **P<0.01 vs control group; ##P<0.01 vs 5-Fu group.
Discussion
Pancreatic cancer is one of the most aggressive malignancies worldwide, and the treatment of pancreatic cancer is extremely difficult.19 Evidence has illustrated that miRNAs act as novel therapeutic targets in cancer, and differential miRNA expression exhibits a functional role in the progression of pancreatic cancer.20,21 A previous study showed that miR-486 function as an oncogenic miRNA in pancreatic cancer, suggesting that miR-486 might serve as a biomarker in patients with pancreatic cancer.22 In this study, we found that the downregulation of miR-486-5p could inhibit the migration and invasion, and induce the apoptosis in PANC-1 cells, which was consistent with the previous study.22
Previous study indicated that miRNAs are associated with chemo-sensitivity in advanced bladder cancer.23 Korourian et al indicated that the upregulation of miR-31 could increase the sensitivity of gastric cancer cells to 5-Fu in gastric adenocarcinoma.24 Qin et al found that miR-106a could decrease the sensitivity of colorectal cancer cells to 5-Fu.25 In the present study, we found that knockdown of miR-486-5p enhanced the inhibitory effects of 5-Fu on the migration and invasion of PANC-1 cells. In addition, downregulation of miR-486-5p enhanced 5-Fu-induced apoptosis in PANC-1 cells in vitro. Furthermore, miR-486-5p had very limited system toxicity on mice. However, downregulation of miR-486-5p could enhance anti-tumor effect of 5-Fu in vivo. These data indicated that the downregulation of miR-486-5p could enhance anti-tumor effect of 5-Fu in vitro and in vivo.
These data indicated that the downregulation of miR-486-5p could enhance the anti-tumor effect of pancreatic cancer cells to 5-Fu. To investigate the mechanisms by which miR-486-5p regulates the apoptosis and invasion of pancreatic cancer, as well as the function of miR-486-5p in the chemo-sensitivity of pancreatic cancer to 5‑Fu, we explored the target genes of miR-486-5p. Bioinformatics analysis and luciferase reporter assay indicated that PTEN was identified as a validated target of miR‑486-5p. Meanwhile, downregulation of miR-486-5p markedly increased the level of PTEN in PANC-1 cells, along with downregulated p‑Akt and p‑ERK expression. PTEN functions as a tumor suppressor gene, which could suppress cells from dividing and growing too rapidly.26 Loss of PTEN function could promote the development and progression of multiple human cancers, such as glioblastoma, lung, breast and prostate cancers.27 Evidence has been shown that PTEN exerts its tumor-suppressing role via inhibiting downstream oncogenic AKT/ERK-mediated signaling pathway.28,29 Shen et al found that knockdown of miR-147 enhanced the sensitivity of gastric cancer cells to 5-Fu by targeting PTEN and regulating the AKT signaling.30 Meanwhile, miR-21 could enhance drug resistance to 5-Fu in PANC-1 cells by downregulation of PTEN.31 In this study, we found that the downregulation of miR-486-5p markedly enhanced the anti-tumor effects of 5‑Fu in PANC-1 cells via upregulating the expression of PTEN and suppressing the activation of AKT/ERK signaling pathway. These data suggested that the downregulation of miR-486-5p could enhance anti-tumor effect of 5-Fu on pancreatic cancer cells via upregulating the level of PTEN.
In addition, gemcitabine is the first-line single chemotherapy agent for pancreatic cancer, while the severe systemic toxicity of gemcitabine limits the application of gemcitabine.32 Therefore, it will be necessary to investigate whether miR-486-5p could enhance the anti-tumor effect of gemcitabine on pancreatic cancer cells in the future.
Conclusion
In the present study, we have demonstrated that downregulation of miR‑486-5p could enhance the anti-tumor effects of 5‑FU in PANC-1 cells via upregulation of PTEN, and then blocking the Akt/ERK pathway. These data suggested that miR-486-5p antagonist plus 5-Fu might be considered as a potential therapeutic strategy for the treatment of pancreatic cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sasajima J, Okamoto K, Taniguchi M. Hematogenous gastric metastasis of pancreatic cancer. Case Rep Gastroenterol. 2016;10(1):75–80. doi: 10.1159/000444249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039–1049. doi: 10.1056/NEJMra1404198 [DOI] [PubMed] [Google Scholar]
- 3.Mohan S, Ayub M, Rothwell DG, et al. Analysis of circulating cell-free DNA identifies KRAS copy number gain and mutation as a novel prognostic marker in Pancreatic cancer. Sci Rep. 2019;9(1):11610. doi: 10.1038/s41598-019-47489-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreau M, Ibeh U, Decosmo K, et al. Flavonoid derivative of cannabis demonstrates therapeutic potential in preclinical models of metastatic pancreatic cancer. Front Oncol. 2019;9:660. doi: 10.3389/fonc.2019.00660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403 [DOI] [PubMed] [Google Scholar]
- 6.Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144(6):1316–1326. doi: 10.1053/j.gastro.2013.01.078 [DOI] [PubMed] [Google Scholar]
- 7.Wang W, Zhao L, Wei X, et al. MicroRNA-320a promotes 5-FU resistance in human pancreatic cancer cells. Sci Rep. 2016;6:27641. doi: 10.1038/srep27641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wigmore PM, Mustafa S, El-Beltagy M, et al. Effects of 5-FU. Adv Exp Med Biol. 2010;678:157–164. [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Yang B, Zhang Y, et al. miR-424-5p represses the metastasis and invasion of intrahepatic cholangiocarcinoma by targeting ARK5. Int J Biol Sci. 2019;15(8):1591–1599. doi: 10.7150/ijbs.34113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Liu J, Xu L, et al. MicroRNA-17 promotes cell proliferation and migration in human colorectal cancer by downregulating SIK1. Cancer Manag Res. 2019;11:3521–3534. doi: 10.2147/CMAR.S191087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macfarlane LA, Murphy PR. MicroRNA: biogenesis, function and role in cancer. Curr Genomics. 2010;11(7):537–561. doi: 10.2174/138920210793175895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou L, Jia S, Ding G, et al. Down-regulation of miR-30a-5p is associated with poor prognosis and promotes chemoresistance of gemcitabine in pancreatic ductal adenocarcinoma. J Cancer. 2019;10(21):5031–5040. doi: 10.7150/jca.31191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan Y, Qin D, Hu B, et al. Deletion of miR-126a promotes hepatic aging and inflammation in a mouse model of cholestasis. Mol Ther Nucleic Acids. 2019;16:494–504. doi: 10.1016/j.omtn.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin RF, Zhang J, Huo HR, Yuan ZJ, Xue JD. MiR-205 mediated APC regulation contributes to pancreatic cancer cell proliferation. World J Gastroenterol. 2019;25(28):3775–3786. doi: 10.3748/wjg.v25.i28.3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao L, Zou D, Wei X, et al. MiRNA-221-3p desensitizes pancreatic cancer cells to 5-fluorouracil by targeting RB1. Tumour Biol. 2016;37:16053–16063. doi: 10.1007/s13277-016-5445-8 [DOI] [PubMed] [Google Scholar]
- 16.Tian F, Wang J, Ouyang T, et al. MiR-486-5p serves as a good biomarker in nonsmall cell lung cancer and suppresses cell growth with the involvement of a target PIK3R1. Front Genet. 2019;10:688. doi: 10.3389/fgene.2019.00688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, Ji C, Guo S, et al. The miR-486-5p plays a causative role in prostate cancer through negative regulation of multiple tumor suppressor pathways. Oncotarget. 2017;8(42):72835–72846. doi: 10.18632/oncotarget.20427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali S, Saleh H, Sethi S, Sarkar FH, Philip PA. MicroRNA profiling of diagnostic needle aspirates from patients with pancreatic cancer. Br J Cancer. 2012;107(8):1354–1360. doi: 10.1038/bjc.2012.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan J, Jia Y, Chen H, Chen W, Zhou X. Long non-coding RNA PXN-AS1 suppresses pancreatic cancer progression by acting as a competing endogenous RNA of miR-3064 to upregulate PIP4K2B expression. J Exp Clin Cancer Res. 2019;38(1):390. doi: 10.1186/s13046-019-1379-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catela Ivkovic T, Voss G, Cornella H, Ceder Y. microRNAs as cancer therapeutics: a step closer to clinical application. Cancer Lett. 2017;407:113–122. doi: 10.1016/j.canlet.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 21.Yin Z, Ma T, Huang B, et al. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-beta signaling pathway. J Exp Clin Cancer Res. 2019;38(1):310. doi: 10.1186/s13046-019-1313-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia L, Song M, Sun M, Chen W, Yang C. miR-486 promotes capan-2 pancreatic cancer cell proliferation by targeting phosphatase and tensin homolog deleted on chromosome 10 (PTEN). Front Genet. 2019;10:541. doi: 10.3389/fgene.2019.00541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nordentoft I, Birkenkamp-Demtroder K, Agerbaek M, et al. miRNAs associated with chemo-sensitivity in cell lines and in advanced bladder cancer. BMC Med Genomics. 2012;5:40. doi: 10.1186/1755-8794-5-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korourian A, Madjd Z, Roudi R, Shariftabrizi A, Soleimani M. Induction of miR-31 causes increased sensitivity to 5-FU and decreased migration and cell invasion in gastric adenocarcinoma. Bratisl Lek Listy. 2019;120(1):35–39. doi: 10.4149/BLL_2019_005 [DOI] [PubMed] [Google Scholar]
- 25.Qin Y, Chen X, Liu Z, Tian X, Huo Z. miR-106a reduces 5-Fluorouracil (5-FU) sensitivity of colorectal cancer by targeting Dual-Specificity Phosphatases 2 (DUSP2). Med Sci Monit. 2018;24:4944–4951. doi: 10.12659/MSM.910016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu EC, Tarnawski AS. PTEN regulatory functions in tumor suppression and cell biology. Med Sci Monit. 2004;10(10):Ra235–241. [PubMed] [Google Scholar]
- 27.Lee MS, Jeong MH, Lee HW, et al. PI3K/AKT activation induces PTEN ubiquitination and destabilization accelerating tumourigenesis. Nat Commun. 2015;6:7769. doi: 10.1038/ncomms8769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fata JE, Debnath S, Jenkins EC Jr, Fournier MV. Nongenomic mechanisms of PTEN regulation. Int J Cell Biol. 2012;2012:379685. doi: 10.1155/2012/379685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butler DE, Marlein C, Walker HF, et al. Inhibition of the PI3K/AKT/mTOR pathway activates autophagy and compensatory Ras/Raf/MEK/ERK signalling in prostate cancer. Oncotarget. 2017;8(34):56698–56713. doi: 10.18632/oncotarget.v8i34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen J, Niu W, Zhang H, Jun M, Zhang H. Downregulation of MicroRNA-147 inhibits cell proliferation and increases the chemosensitivity of gastric cancer cells to 5-fluorouracil by directly targeting PTEN. Oncol Res. 2018;26(6):901–911. doi: 10.3727/096504017X15061902533715 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Wei X, Wang W, Wang L, et al. MicroRNA-21 induces 5-fluorouracil resistance in human pancreatic cancer cells by regulating PTEN and PDCD4. Cancer Med. 2016;5(4):693–702. doi: 10.1002/cam4.626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y, Zhou Y, Wang K, et al. ROCK2 confers acquired gemcitabine resistance in pancreatic cancer cells by upregulating transcription factor ZEB1. Cancers (Basel). 2019;11:12. doi: 10.3390/cancers11121881 [DOI] [PMC free article] [PubMed] [Google Scholar]