Currently, despite the use of a preventive vaccine for several decades as well as the use of effective and well-tolerated viral suppressive medications since 1998, approximately 250 million people remain infected with the virus that causes hepatitis B worldwide. Hepatitis C virus (HCV) and hepatitis B virus (HBV) are the leading causes of liver cancer and overall mortality globally, surpassing malaria and tuberculosis. Linkage to care is estimated to be very poor both in developing countries and in high-income countries, such as the United States, countries in Western Europe, and Japan.
KEYWORDS: antiviral therapy, hepatitis B diagnosis, hepatitis B management, hepatitis B treatment
SUMMARY
Currently, despite the use of a preventive vaccine for several decades as well as the use of effective and well-tolerated viral suppressive medications since 1998, approximately 250 million people remain infected with the virus that causes hepatitis B worldwide. Hepatitis C virus (HCV) and hepatitis B virus (HBV) are the leading causes of liver cancer and overall mortality globally, surpassing malaria and tuberculosis. Linkage to care is estimated to be very poor both in developing countries and in high-income countries, such as the United States, countries in Western Europe, and Japan. In the United States, by CDC estimates, only one-third of HBV-infected patients or less are aware of their infection. Some reasons for these low rates of surveillance, diagnosis, and treatment include the asymptomatic nature of chronic hepatitis B until the very late stages, a lack of curative therapy with a finite treatment duration, a complex natural history, and a lack of knowledge about the disease by both care providers and patients. In the last 5 years, more attention has been focused on the important topics of HBV screening, diagnosis of HBV infection, and appropriate linkage to care. There have also been rapid clinical developments toward a functional cure of HBV infection, with novel compounds currently being in various phases of progress. Despite this knowledge, many of the professional organizations provide guidelines focused only on specific questions related to the treatment of HBV infection. This focus leaves a gap for care providers on the other HBV-related issues, which include HBV’s epidemiological profile, its natural history, how it interacts with other viral hepatitis diseases, treatments, and the areas that still need to be addressed in order to achieve HBV elimination by 2030. Thus, to fill these gaps and provide a more comprehensive and relevant document to regions worldwide, we have taken a global approach by using the findings of global experts on HBV as well as citing major guidelines and their various approaches to addressing HBV and its disease burden.
INTRODUCTION
Chronic hepatitis B (CHB) virus infection is a well-known threat to global health (1, 2). CHB is transmitted through exposure to infected blood and body secretions. Currently, the main route of transmission remains from mother to neonate (vertical transmission) or from mother to child or child to child (horizontal), but inadequate sterilization of health care instruments and the administration of contaminated blood products also remain major modes of transmission, especially in poorer countries. In addition, with the global opioid crisis, intravenous drug use has again become a more common mode of transmission. Finally, male-to-male sex and heterosexual sexual contact by an individual with many partners remain major modes of transmission (1, 2) (Table 1).
TABLE 1.
Geographic prevalence of hepatitis B surface antigen carriage in the general population and possible routes of transmission
| Prevalence | Geographic area | Age at infection | Possible routes of transmission |
|---|---|---|---|
| Low (<2%) | North America, Western Europe | Early adulthood | Sexual, percutaneous, others |
| Moderate (2–8%) | Mediterranean, Eastern Europe | Childhood | Horizontal |
| High (>8%) | East Asia, Africa | Birth, toddler stage, preschool | Perinatal, horizontal |
Due to the various modes of transmission, the geographic prevalence of this infection varies widely as well and is categorized as high, intermediate, or low (Table 1). Before the universal implementation of vaccination for hepatitis B, the prevalence of hepatitis B surface antigen (HBsAg) globally ranged from 2% to 20%. A review of published data from 161 countries that were reported between 1965 and 2013 estimated the worldwide prevalence of HBsAg to be 3.61%, with the highest rates being in Africa (8.83%) and the Western Pacific regions (5.26%). Within the World Health Organization (WHO) territories, the prevalence of the virus ranged from 0.20% (Mexico) to 13.55% (Haiti) in the Americas, 0.48% in the Seychelles, and 22.38% in the African (South Sudan) region (3). When combined, this prevalence corresponded to 248 million people globally in 2010.
In 2016, an updated estimate indicated that the total global hepatitis B virus (HBV) infection prevalence increased to 3.9% (95% confidence interval, 3.4 to 4.6%), corresponding to 292 million people globally, suggesting that the presence of HBV was not decreasing. Furthermore, only approximately 29 million (10%) were diagnosed with HBV infection. In addition, it was found that only 4.8 million (5%) of those eligible for treatment had actually been treated.
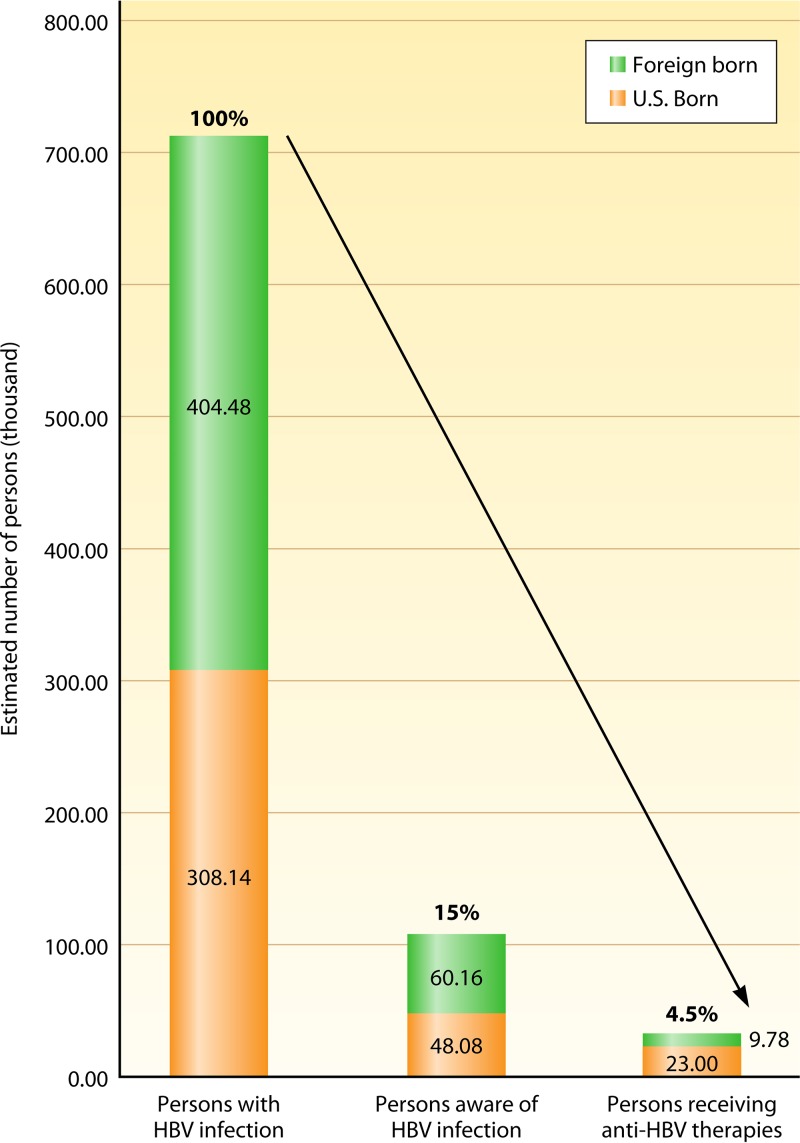
In the United States, as reported by a study using data from the National Health and Nutrition Examination Survey (NHANES; 1999 to 2016), there are considerable racial/ethnic disparities in HBV infection rates (4). From a total of 47,628 persons who underwent HBV serology testing, the overall prevalence of chronic hepatitis B (CHB) was 0.35%. However, the prevalence among Asians was 3.41%, while it was only 0.69% among non-Hispanic blacks and less than 0.2% among non-Asian, non-black individuals (4). These differences in CHB prevalence rates in the United States are attributed to the fact that the majority of CHB cases are considered imported (5), such that foreign-born Asians and blacks have a higher prevalence than their U.S.-born counterparts (3.85% versus 0.79%, respectively, for foreign-born Asians and 1.94% versus 0.52%, respectively, for blacks) (4). However, these numbers may still underestimate the number of persons infected with HBV due to the limitations of using NHANES data, which do not adequately account for institutionalized populations, such as those in prisons, or other populations at higher risk of HBV infection, such as immigrant populations (Fig. 1).
FIG 1.
Disease awareness and treatment care cascade of persons with HBV infection in the United States.
Other changes in the status of global CHB that have been noted were recently reported. A real-world study of 44,026 CHB patients from the United States found that the median age of the population with CHB had increased from 48 years in 2006 to 52 years in 2015. Alongside the increase in age, the number of non-liver-related comorbidities also increased, and these, combined with CHB, have also increased the disease burden of CHB (6). Other real-world studies have also reported findings similar to those described above on the increasing rates of liver comorbidities as well as the increased health care utilization by individuals with CHB and the cost of CHB (7, 8).
Another factor that complicates the elimination of CHB is the difference in the distribution of HBV genotypes throughout the world (9). Currently, there are 10 HBV genotypes, identified by the letters A to J (Table 2), which, through genetic mutations and the lack of proofreading in reverse transcriptase, have evolved over the long term, creating challenges to their elimination.
TABLE 2.
Global distribution of hepatitis B virus genotypes A to J
| HBV genotype | Geographic location |
|---|---|
| A | Sub-Saharan Africa, India, northern Europe, Western Africa, Gambia, Nigeria |
| B | Japan, East Asia, Taiwan, China, Indonesia, Vietnam, Philippines, Alaska, northern Canada, Greenland |
| C | Taiwan, China, South Korea, Japan, Southeast Asia, Australia, Philippines, Vietnam, Indonesia |
| D | Africa, Europe, Mediterranean countries, India, Indonesia, Australia |
| E | Western and central Africa, Saudi Arabia |
| F | Central and South America |
| G | France, Germany, United States |
| H | Central America |
| I | Vietnam and Laos |
| J | Japan |
PREVENTION AND CURRENT LINKAGE TO CARE
In 2011, the World Health Organization passed a resolution recognizing viral hepatitis as a global health concern. In October 2015, that body released its first official strategy for the control of viral hepatitis, which aims to significantly reduce the considerable morbidity and mortality found in individuals with chronic hepatitis B and chronic hepatitis C (CHC) virus infections by the year 2030 (10). Governmental agencies (n = 194 countries) have signed on to this strategy, which includes ambitious targets of a 90% reduction in the incidence of viral hepatitis, an 80% treatment uptake by eligible patients, and a 65% reduction in mortality.
The global elimination of HBV can become a reality thanks to the use of an effective and low-cost vaccine, which has been available for almost 30 years. The hepatitis B vaccine has been shown to prevent hepatocellular carcinoma (HCC) (11, 12). Currently, most countries have implemented universal neonatal HBV vaccination programs, which are inexpensive and which may eradicate HBV infection within the next century (13). Improving the rates of vaccination at birth and providing prepartum therapy to highly viremic mothers to prevent transmission from mother to child could accelerate this eradication of hepatitis B.
Recent data demonstrate that immunoprophylaxis with hepatitis B immunoglobulin and the hepatitis B vaccine in newborns can reduce the rate of mother-to-child transmission (MTCT) from 90% to 10% (14). However, if the mother has an HBV DNA level of greater than 200,000 IU/ml, immunoprophylaxis has a failure rate of 10 to 30% in infants born to such mothers. Transmission can be effectively eliminated by the administration of antiviral therapy to the mother during the third trimester, as shown by a controlled and randomized study from China (15, 16). The analysis showed that the transmission rate dropped from 7% in the control group to 0% in the treatment group at the 28th postpartum week. Thus, currently, major liver societies in the United States and Europe recommend that all pregnant women with HBV DNA levels greater than 200,000 IU/ml be considered for treatment with tenofovir disoproxil fumarate (TDF) starting toward the end of the second trimester and at the beginning of the third trimester (24 to 28 weeks of pregnancy). Importantly, breast-feeding and TDF treatment can continue postpartum in HBsAg-positive untreated women (17).
Unfortunately, universal neonatal vaccination and the elimination of transmission from mother to child do not affect the projected morbidity/mortality for the hundreds of millions of adults living with CHB, such that by 2030 there are expected to be 17 million deaths attributable to CHB. Currently, the cascade of care is poor, with only about 15% of the estimated 712,000 infected persons in the United States being aware of their infection and only 4.5% receiving antiviral therapies (Fig. 1) (4).
The 2010, the United States Institute of Medicine (IOM) found many areas in which unmet needs regarding hepatitis existed, including health care providers’ education, vaccination, and prevention programs, as well as accurate diagnosis and treatment (18). Following publication of that report, in 2011 the U.S. Department of Health and Human Services (HHS) announced an action plan for the prevention, care, and treatment of viral hepatitis to assist with and accelerate the elimination of HBV in the United States. The strategic plan covers the following areas: the education of all affected parties to reduce health disparities among members of the community and the populations seen by health care providers, increase testing and treatment by increasing surveillance to detect transmission of the disease, increase the rate of vaccination for viral hepatitis, reduce drug use behaviors that contribute to the transmission of hepatitis, and prevent health care-associated viral hepatitis (19).
However, despite these programs, multiple surveys have shown that complete knowledge of care for CHB is lacking, even among primary care physicians and specialists, who sometimes do not know the most current management recommendations (20, 21). In fact, one study found that patients who were evaluated by specialists were more likely to have a complete laboratory evaluation than those examined by only primary care providers, but even then, 40% of those who received specialty care did not receive a thorough evaluation that included continued follow-up of untreated patients to determine when or if treatment becomes necessary (22–24).
The lack of appropriate patient education and cultural barriers are also variables that limit interaction with the health care setting. A recent study of 1,000 African immigrants to the United States found that culturally targeted patient navigators achieved very high rates of continued care (97%) and adherence (25). However, a major obstacle to the initiation and continuation of care for CHB, despite any extra efforts at patient engagement, was the lack of health insurance (26).
Due to treatment deferral, provider-requested further observation, patient loss to follow-up, and/or patient refusal of treatment, up to half of the patients in the United States eligible for treatment were not treated within the first year after their diagnosis (27). Specialists were more likely to initiate both treatment and treatment with the most appropriate drug therapy. However, even for university-based liver clinics, only 59% to 73% of eligible patients were treated as recommended by professional practice guidelines (28). Once treatment is initiated, adherence to treatment is often poor due to the asymptomatic nature of CHB during the pre-end-stage liver disease period. In one study, all the entecavir treatment failures were accounted for through nonadherence rates of 10% to 12% (over 4 years) (29).
Thus, trying to effectively screen, diagnose, and treat patients requires a multifaceted approach. Provider education, especially at the level of the primary care practitioner, is needed. Information technology is needed to obtain complete medical evaluations, improve the referral process, and improve the guidelines driving the delivery of patient care. Efforts need to be continued to raise awareness among the public as well as provide culturally sensitive and stigma-free education on HBV infection, its routes of transmission, and its prevention through vaccination. Interpreters and language-specific materials are other proven methods that can be used to improve rates of referral and follow-up with the health care system. Effective program delivery depends upon adequate financial support, accessibility, and promotion by community networks, all of which are vital to the meet the WHO goal of the elimination of viral hepatitis by 2030.
DIAGNOSIS AND CLINICAL EVALUATION
In order to accurately screen, diagnose, and then treat patients who are infected with HBV, an understanding of the current diagnostic tests is necessary. An indication of CHB virus infection is the presence of a positive HBsAg result for over 6 months. Seroclearance, identified by a qualitative test for HBsAg (i.e., the loss of HBsAg, which is equal to <0.05 IU/ml in serum) with or without the appearance of antibodies (anti-HBs), is regarded as a functional cure (17, 30, 31). Protective immunity is acknowledged when the anti-HBs level is greater than 10 IU/ml.
Serological, Molecular, and Genomic Testing
Quantitative HBsAg.
HBsAg levels and the source of HBsAg production change over the different phases of CHB. In the immune-tolerant phase, HBsAg concentrations are high, while in the inactive phase they are low (32–34). The source of HBsAg production also changes from being predominantly covalently closed circular DNA (cccDNA) transcription in the young hepatitis B e antigen (HBeAg)-positive patient to being from HBV integrants in the older HBeAg-negative patient (35). An HBsAg level of <100 IU/ml in Asian HBeAg-negative patients is predictive of spontaneous HBsAg seroclearance within 6 to 8 years (36). Meanwhile, higher HBsAg levels may mean a lower likelihood of spontaneous clearance; as a result, the HBsAg level has recently been incorporated into HCC risk scores (37).
The HBsAg level can also be useful when trying to predict and/or monitor a patient’s response to therapy with peginterferon (PEG-IFN) (38), such that in HBeAg-positive patients, an HBsAg level of >20,000 IU/ml at week 24 confers a 96% negative predictive value (NPV) for genotype A HBV and a 100% NPV for genotypes B, C, and D. A positive response to treatment for HBV infection is defined as the loss of HBeAg and an HBV DNA level of <2,000 IU/ml at 6 months posttreatment (39). The best-validated model for prediction of the impact of treatment in HBeAg-negative patients is to note a decline in both HBV DNA and HBsAg levels at week 12. The NPV for patients who fail to achieve a 2-log decline in the HBV DNA level and/or any decline in HBsAg is 95 to 100% when defined as an HBV DNA level of <2,000 IU/ml with a normal alanine aminotransferase (ALT) level at 24 weeks posttreatment (40). Nucleos(t)ide analogue (NA) therapy, which is associated with a very slow decrease in HBsAg levels, regardless of a strong suppression of HBV DNA, indicates that immune clearance is weak as well as the continued transcription of integrated viral genomes not affected by chain terminators (41). Studies from Hong Kong and Taiwan conducted with HBeAg-negative patients reported that a sustained response and HBsAg seroclearance after the cessation of lamivudine treatment can be predicted when the serum HBsAg level is <100 to 200 IU/ml (42, 43).
HBeAg.
Since the discovery of HBeAg in the early 1970s, it has long been seen as an indicator of viral replication and infectivity. With the introduction of HBV DNA testing, infection and replication are more effectively measured by the measurement of HBV DNA in serum. The definition of a patient’s phase in the history of chronic HBV infection is currently measured by the HBeAg level (17, 44). The seroconversion of HBeAg indicates an important phase in immune clearance. In spite of this, some HBeAg-negative patients may contract active hepatitis with high HBV DNA concentrations. This state is often characterized as HBeAg-negative disease. Concomitant mutations in the basal core promoter and/or the precore stop codon can appear (45). Current international opinion includes recommendations regarding the status of HBeAg (17, 30, 31) (also see Treatment below).
HBeAg seroconversion can occur on peginterferon treatment, with a rapid decline in HBeAg levels. On the other hand, a high HBeAg level can be used to predict a nonresponse and terminate treatment early, when appropriate (46). Unfortunately, HBeAg quantification is not yet standardized, which hinders its application in clinical practice, while measurement of HBeAg levels is not recommended for patients who have HBeAg-negative HBV.
Anti-HBc.
The core antibody to hepatitis B virus (anti-HBc) can be detected through immunoassays for total anti-HBc, which is able to detect both anti-HBc IgG and anti-HBc IgM. Anti-HBc IgM is the determinant of acute hepatitis B and is often the only sign that may be detected during the period of acute hepatitis B when HBsAg has become undetectable. Patients are also positive for anti-HBc when they have severe and acute flare-ups of chronic hepatitis B (47). However, anti-HBc IgG may indicate current or previous HBV infection. In patients with cryptogenic hepatocellular carcinoma (HCC), a negative result for HBsAg and a positive result for anti-HBc may indicate HBV infection or possible occult HBV infection (48). Patients receiving potent immunosuppression and cancer chemotherapy (for example, rituximab) may have a reactivation of occult HBV infection (49). The amount of HBV DNA detectable in patients with occult HBV infection is usually <200 IU/ml.
HBV DNA.
Current guidelines suggest antiviral therapy for patients with increased ALT levels and HBV DNA levels of 2,000 to 20,000 IU/ml, as well as in those with HBV DNA at any detectable levels in the presence of cirrhosis, since HBV DNA can be a marker of viral replication and is the main target of antiviral therapy (17, 30, 31). This tactic is based on data that demonstrate a strong relationship between high HBV DNA levels and the future development of cirrhosis and HCC (50, 51). Suppression of HBV DNA to an undetectable level on testing is associated with a reduced risk of cirrhosis, HCC, and decompensation (52, 53).
Patients on treatment with nucleos(t)ide analogs (NAs) need to be tested for HBV DNA to assess the treatment response and guide treatment. Failure to suppress HBV DNA to achieve undetectable levels by the 6th month of treatment with telbivudine and lamivudine or by 12 months of treatment with adefovir (all of these drugs have a low barrier to resistance) is associated with an increased risk of therapeutic resistance. Therefore, the use of a more potent agent to which the virus is not resistant is usually recommended (17, 30, 31). The baseline or on-treatment HBV DNA level also predicts the response to peginterferon therapy (40, 54).
HBcrAg.
Hepatitis B core-related antigen (HBcrAg) is a new indicator that measures an amino acid sequence common to HBeAg and hepatitis B core antigen (HBcAg), as well as a putative 22-kDa precore protein. Since HBcrAg positivity correlates with intrahepatic HBV DNA and pregenomic RNA (pgRNA) levels among patients on NA treatment, HBcrAg may be a good serum marker of the active transcriptional activity of liver cccDNA (55). In patients treated or not treated with NA, higher HBcrAg levels may be associated with an increased risk of liver cancer (55, 56). Therefore, among NA-treated subjects, the level of serum HBcrAg associates with the serum HBV DNA level but not the serum HBsAg level (57). The higher that the HBcrAg level is prior to stopping NA treatment, the higher the risk for hepatitis reactivation is, reflecting a possible role of HBcrAg in residual viral replication during NA therapy (58, 59). Hence, HBcrAg may be an effective indicator of the presence of cccDNA in the liver and may be useful for surveillance and an indicator of the efficacy of treatment and the clinical course for CHB patients.
POC Diagnostics
A rapid point-of-care (POC) assay for HBsAg provides a plausible diagnostic strategy in low-resource areas. A recent meta-analysis summarizing the findings of 27 studies evaluated 49 rapid POC assays (60). Despite a robust specificity close to 100% (range, 90% to 100%), the reliability of individual testing varied greatly and was heterogeneous in a range of from 43.5% to 99.8%. Study location, reference standard, and study score were the three key factors most dependably aligned with the estimates and the heterogeneity (60). However, another study tested three POC tests for HBsAg in the field and in labs in the Western Africa country of the Gambia. They found that the sensitivity and specificity ranged from 88.5% to 90.0% and 99.8% to 100%, respectively, in the field and from 93.9% to 95.3% and 93.3% to 94.7%, respectively, in the laboratory setting (61). Though further research is indicated, POC tests for HBsAg may prove to be accurate, rapid, and less expensive choices than laboratory serological screening for HBV in the field.
Genomic and Novel Molecular Markers
The HBV genotype has been associated with both the response to antiviral therapy and disease outcomes (62). There are currently 10 HBV genotypes, with each genotype being classified by an 8% or more divergence in the nucleotide sequence of the genome (63). Among them, genotypes A to D are the four predominant genotypes (64). Genotypes B and C are the most common in eastern and southeastern Asia (65, 66), while genotypes A and D are most commonly found in North America, Africa, and Europe (67). Genotype E has been reported from West Africa. Genotypes A and B appear to have a greater response to interferon therapy than genotypes C and D (68). In contrast, the various HBV genotypes do not have different responses to the nucleos(t)ide analogues. Delayed HBeAg seroconversion and a higher risk of reactivation in the HBeAg-negative phase are associated with genotype C, and hence, those infected with genotype C have more advanced fibrosis and more severe liver damage than those infected with genotype B (69). In a meta-analysis of 14,545 patients, a greater risk of HCC was seen in those infected with genotype C than in those infected with the other major genotypes (70, 71). Patients with genotype C infection also account for more cases of fibrosis, cirrhosis, and liver cancer than those with genotype B infection. However, studies in Hong Kong and Taiwan have noted the development of HCC in young noncirrhotic genotype B-infected patients (72, 73). In contrast, reports from different parts of the world have indicated that individuals infected with HBV genotypes C, D, and F are more likely to have a greater cumulative cirrhosis and HCC risk than those infected with genotypes A and B (74). In southern Africa, HCC occurs at a younger age in those infected with subtypes of genotype A.
In HBV, host genomic factors that interfere with the viral replication cycle could indicate additional targets for therapy to reduce viral loads (75). Interferon lambda 3 (IFN-λ3), formerly interleukin-28B (IL-28B), creates a polymorphism in the human leukocyte antigen (HLA) locus and thus is a promising single nucleotide polymorphism (SNP) that may indicate a virological response to peginterferon (76). HLA-DPA1, another HLA locus, is often linked to HBeAg seroconversion (77). Hepatic disease progression in Chinese Han hepatitis B patients has been found to be linked to the G-201A allele, in the promoter region of the interferon-inducible IP-10 gene, through the upregulation of IP-10 expression (78). This phenomenon should be studied in patients of other ethnicities.
Since HBV DNA is suppressed to low levels in most patients on NA therapy, the HBV DNA level does not accurately reflect the levels of viral cccDNA, RNA, or antigen production in the livers of treated patients. Other groups have studied the role of HBV RNA as a potential biomarker (79). Since mRNA exists in an unstable state in the serum, most HBV RNA found in the serum is thought to be pregenomic RNA. Previous studies have suggested that the measurement of HBV RNA may be helpful in predicting HBeAg seroconversion in patients on NA therapy and is deserving of further evaluation (80).
Recommendations for Screening and Diagnosis
AASLD.
The American Association for the Study of Liver Diseases (AASLD) currently suggests that all persons born in countries with a HBsAg seroprevalence of 2%, U.S.-born persons not vaccinated as infants whose parents were born in regions with high rates of HBV endemicity (8%), pregnant women, persons needing immunosuppressive therapy, and individuals at high risk for exposure to HBV (for example, blood recipients, blood donors, individuals with male-to-male sexual contact, prisoners, people with a history of liver disease; refer to the guidelines for a full list) be screened for HBV using tests for both HBsAg and anti-HBs. Screened persons who are anti-HBs negative should be vaccinated.
Other guidelines.
At present, the European Association for the Study of the Liver (EASL) does not have specific guidelines on who to screen and what tests to use for diagnosis. The guidelines of the Asian Pacific Association for the Study of the Liver (APASL) follow the AASLD recommendations on who would benefit the most from screening. Testing should include a serological assay for HBsAg (subgenotype A1), anti-HBs (subgenotype B2), and total anti-HBc (subgenotype B2). However, APASL emphasizes that screening should be linked to appropriate counseling and referral for further care, including clinical evaluation of the need for treatment and vaccination. The World Health Organization (WHO) also recognizes the same groups of people that need to be screened for HBV noted by AASLD. However, for testing, WHO recommends using a single quality-assured serological in vitro diagnostic test (IVD; i.e., either a laboratory-based immunoassay [an enzyme immunoassay or a chemiluminescence immunoassay] or rapid diagnostic test [RDT]) to detect HBsAg and hepatitis C virus (HCV) antibody. The RDTs used should meet minimum performance standards and should be delivered at the point of care to improve access and a linkage to care and treatment. When a person is found to have a reactive HBsAg serological test result, HBV DNA nucleic acid testing should be conducted to help further guide who to treat or not treat, if there is no evidence of cirrhosis, and to monitor for the treatment response.
Assessment of Liver Necroinflammation and Fibrosis
Serum alanine aminotransferase.
ALT concentrations generally correlate with hepatic necroinflammation in CHB patients. Cutoffs for ALT should be lowered to 30 IU/liter for males and 19 IU/liter for females (81), as high-normal ALT levels ranging from 40 to 70 IU/liter are linked to cirrhosis (82) and liver-related deaths (83). However, a current AASLD 2018 guidance update suggested that the ALT cutoffs should be 35 U/liter for males and 25 U/liter for females (84).
Assessment of hepatic fibrosis by invasive versus noninvasive tests.
Percutaneous biopsy of the liver is traditionally the best diagnostic measurement to use when assessing liver fibrosis in clinical trials (85), as well as for determination of patient prognosis and interventions (86). However, its use in routine clinical practice is limited due to its invasive nature, the potential for serious complications, difficulty with selection of the correct location for the biopsy, selection of a sample that is large enough, as well as heterogeneity among pathologist readings (86).
Recently, noninvasive tests for the staging of fibrosis have become available. Such tests can be classified as physical tests or tests for serum biomarkers, and each has its own advantages and disadvantages (Table 3). There are two groups of physical tests: shear wave elastography (including transient, acoustic radiation force impulse, or multidimensional shear wave elastography) as well as magnetic resonance elastography (MRE). Among these tests, FibroScan transient elastography is one of the best applicable tools worldwide. The standard M probe transmits a shear wave followed by an ultrasound wave through a probe placed near the liver parenchyma to assess liver stiffness measurement (LSM) (87). Doppler calculates the velocity of the wave passing through the liver. This technique can discriminate between severe fibrosis, no or minimal fibrosis, and cirrhosis (88), and it is useful and accurate across liver diseases, such as CHB, CHC, and autoimmune hepatitis (87). However, measuring LSM by transient elastography is not as efficient in obese patients (89), unless an XL probe is used (90). On the other hand, MRE assesses liver stiffness with a phase-contrast imaging method that uses mechanical wave propagation (91). Generally, obesity or the severity of the ascites does not affect MRE, and it is not as operator dependent or prone to technical failure as other tests. MRE can be used to stage even mild fibrosis (92). However, MRE is less cost-effective, slower to perform, and less well tolerated than an ultrasound-based approach.
TABLE 3.
Different noninvasive approachesa
| Noninvasive test | Features | Advantages | Disadvantages | Sensitivity (reference) | Specificity (reference) |
|---|---|---|---|---|---|
| Physical | |||||
| Shear wave elastography | |||||
| Transient elastography | Ultrasound-based liver stiffness measurement by shear wave velocity determination with a specific probe | Useful across different liver disease entities; special probes designed for different body builds; measures liver fat at the same time with CAP, can identify no or minimal fibrosis | Less reliable in obese patients, in severe acute exacerbations of hepatitis, and in posttreatment fibrosis stages in CHB or CHC patients | ≥F2, 0.716; ≥F3, 0.790; =F4, 0.800 (265) | ≥F2, 0.816; ≥F3, 0.846; =F4, 0.866 (265) |
| Point shear wave elastography (pSWE) | Uses the force of the ultrasound beam (ARFI) to assess the longitudinal displacement | No additional apparatus except an ultrasound machine is needed; can reflect disease progression; results are available in real time; fewer technical difficulties are associated with it; it is accurate with overweight or obese patients; results can be expressed in kilopascals or meters per second | Difficult to define a cutoff; experience in ultrasound B mode imaging is needed | ≥F3, 0.84; =F4, 0.86 (266) | ≥F3, 0.90; =F4, 0.84 (266) |
| Multidimensional shear wave elastography (2D-SWE, 3D-SWE) | Ultrasound measurement of shear wave velocity | No additional apparatus except an ultrasound machine is needed; elasticity can be reflected by numbers or colors; sensitive for early-stage fibrosis; results can be expressed in kilopascals or meters per second | Limited studies on its clinical application | NA | NA |
| Magnetic resonance elastography | Phase-contrast imaging depending on mechanical wave propagation | Less operator dependent and fewer technical failures; limited effect by obesity or ascites; can assess complications; sensitive for detection of early-stage fibrosis; reproducible results | High cost; limited availability in some countries or regions; more time-consuming; not applicable with patients with iron overload or hemochromatosis; limited studies on its clinical application | ≥F2, 0.928; ≥F3, 0.896; =F4, 0.895 (265) | ≥F2, 0.937; ≥F3, 0.932, =F4, 0.920 (265) |
| Serum test formulas | |||||
| Common laboratory parameters | APRI (AST, platelet), Forns index (platelet, GGT, cholesterol), FIB-4 (ALT, AST, platelet), Fibro index (platelet, AST, gamma globulin), Hui index (bilirubin, albumin, platelet) | Results from routine liver function tests; convenient to perform; no interobserver variations | Cannot be used for all chronic liver diseases | APRI, ≥F2, 0.700; ≥F3, 0.500; =F4, 0.369 (267) | APRI, ≥F2, 0.600; ≥F3, 0.830; =F4, 0.925 (267) |
| FibroTest | Consists of GGT, total bilirubin, α2-macroglobulin, apolipoprotein A1, and haptoglobin | Useful in different chronic liver diseases; reliable; applicable; accurate with overweight or obese patients | Suboptimal for early-stage fibrosis | ≥F2, 0.61; =F4, 0.62 (268) | ≥F2, 0.80; =F4, 0.91 (268) |
| FibroMeter | For the first 2 generations of the device, consists of platelets, prothrombin index, AST, α2- macroglobulin, hyaluronate, urea, and age; the third generation (3G) does not take into account hyaluronate | High fibrosis classification accuracy; good predictive value for severe fibrosis in different liver disease entities | High cost | NA | NA |
| Enhanced liver fibrosis | Consists of 3 direct blood markers: procollagen III amino-terminal peptide, hyaluronic acid, and tissue inhibitor of metalloproteinase I | Good prognostic factor for clinical outcomes in patients with chronic liver diseases; similar results are obtained by using fresh blood or cryopreserved blood; sensitive for detection of advanced fibrosis or cirrhosis | Not sensitive for early stages of fibrosis; age, low CD4+ T-cell count, and other factors can affect ELF results | ≥F3, 0.97; =F4, 0.86 (269) | ≥F3, 0.86; =F4, 0.98 (269) |
ALT, alanine aminotransferase; AFRI, acoustic radiation force impulse; APRI, aspartate aminotransferase (AST)-to-platelet ratio index; AST, aspartate aminotransferase; CAP, controlled attenuation parameter; CHB, chronic hepatitis B; CHC, chronic hepatitis C; ELF, enhanced liver fibrosis; F2, stage 2 of liver fibrosis; F3, stage 3 of liver fibrosis; F4, stage 4 of liver fibrosis (cirrhosis); FIB-4, fibrosis 4 index; GGT, gamma-glutamyltransferase; NA, not applicable; 2D-SWE and 3D-SWE, two-dimensional and three-dimensional shear wave elastography, respectively.
Serum biomarkers for liver fibrosis are often used in combination. The two most well-known indices which are based on routinely available laboratory markers are the aspartate aminotransferase (AST)-to-platelet ratio index (APRI) (93) and the Forns index (94), though caution is warranted if the APRI is used in patients from Africa, as its sensitivity is low for this group. Other tests include those that test for biochemical markers specifically related to fibrinolysis or fibrinogenesis (95). FibroTest (BioPredictive, Paris, France) and FibroSure (LabCorp, Burlington, NC, USA) test for gamma-glutamyltransferase (GGT), total bilirubin, α2-macroglobulin, apolipoprotein A1, and haptoglobin (96–98). They have been validated for use in patients with CHB and are widely used. Other specific serum-based noninvasive fibrosis tests or measures are FibroMeter (Echosens, Paris, France) (99) and the enhanced liver fibrosis (ELF) score (100, 101).
To enhance the accuracy of fibrosis assessment, different approaches combining physical tests and biochemical markers have been proposed. A significant proportion of patients can avoid liver biopsy if the ELF-LSM algorithm is used (100). An additional well-validated combination approach is the Forns index, which does not use the ALT level in the LSM algorithm (102).
PATHOGENESIS AND NATURAL HISTORY
HBV Life Cycle and HBV Cure Targets
HBV is a DNA virus of the Hepadnaviridae group. The virion contains a circular DNA genome of 3,200 bp. An RNA pregenome replicates DNA by reverse transcription within the capsid. The virion is internalized into the cell by attaching to a cellular receptor, sodium taurocholate cotransporting polypeptide (NTCP). The first replicative event is the change of the relaxed circular DNA (rcDNA) into a covalently closed circular DNA (cccDNA) minichromosome inside the hepatocytes. Conversion of the relaxed circular DNA to the closed circular DNA requires disassembly of the HBV capsid, deproteinization of the rcDNA, and the ligation of rcDNA to cccDNA. Notably, cccDNA is synthesized from rcDNA either from infecting virions or from subsequent intracellular nucleocapsids via a cccDNA shuttle amplification pathway, thus providing a critical mechanism for persistence as a minichromosome in the center of infected cells. The transcriptional template used for the transcription of major viral mRNA is cccDNA. HBsAg, HBcAg, HBeAg, the DNA polymerase, and the HBx protein are encoded by conserved open reading frames. HBsAg is also produced following the transcription of integrated viral DNA (103). Human RNA polymerase II mediates the transcription of cccDNA to generate pgRNA. This polymerase attaches to a secondary structure, epsilon, at the end of the pregenome. The capsid of the virus is constructed into core particles containing pgRNA and the viral polymerase (reverse transcriptase). HBV replication occurs within capsids and the nucleocapsid, where, through reverse transcription, the pgRNA forms incomplete rcDNA, where the HBV capsids become mature virus particles after being coated with HBsAg (104). The capsid-containing rcDNA alternatively shuttles to the nucleus to replenish cccDNA or becomes covered to form the infectious virions, which are then released from the cell (105, 106).
Complete and incomplete viral particles are then secreted. Numerous empty non-DNA-containing virions are exuded; importantly, recent findings have shown that RNA-containing particles are released. As described above, inhibition of cccDNA synthesis within the nucleus is not directly affected by current nucleoside analogue therapy, as only minus- and plus-strand DNA synthesis within the cytoplasm is targeted. We know that HBx is needed to transcribe cccDNA via epigenetic regulation (107, 108). We also know that fragments of HBV DNA become part of the genome of hepatocytes but that integration is not necessary for the replication of HBV. A soluble, dimeric protein, HBeAg, is secreted from hepatocytes. HBeAg is processed from the precore protein: the bulk of amino acids are shared with HBcAg, but HBeAg possesses an N-terminal extension of 10 amino acids and a C-terminal truncation of 34 amino acid residues. Both HBV and hepatitis D virus (HDV) have on their surfaces the large envelope glycoprotein, which plays an important role in the entry of the virus. The antigenic loop on the S protein mediates the attachment of heparin sulfate proteoglycans (HSPGs) to the cell surface (109, 110). The entry receptor has recently been discovered to be the sodium taurocholate cotransporting polypeptide (NTCP) (111–113). Based on nucleotide divergence, a sequence variation (variation of up to 12% of nucleotides) between isolates of HBV can produce up to 10 different genotypes (genotypes A to J) as a result of this divergence (111–113).
Natural History
Since the pathophysiology of HBV is so complex, the history and phases of infection are still being studied. Most children infected in infancy or childhood will develop chronic HBV infection (114–116). Different age-dependent phases of the disease have long been recognized and generally consist of an immunotolerant phase (or high-replication, low-inflammation phase), an immunoactive phase, an inactive carrier state (low replication levels and normal/nearly normal serum aminotransferase levels), and reactivated disease (Table 4). These have been renamed in the recent EASL HBV clinical practice guidelines as HBeAg-positive infection, HBeAg-positive hepatitis, as well as HBeAg-negative infection and HBeAg-negative hepatitis (17).
TABLE 4.
Phases of chronic hepatitis Ba
| Characteristics of the following phase: | ||||
|---|---|---|---|---|
| Immune-tolerant phase | Immune activation | Low replication | Reactivation | Remission |
| Occurs in patients with perinatally acquired infection | High or fluctuating HBV DNA levels | Low or nondetectable HBV DNA levels | Fluctuating ALT and HBV DNA levels | After many years, some patients may enter a remission phase |
| Minimal liver inflammation | High or fluctuating ALT levels | Normal ALT levels | Usually older patients with more advanced liver disease | Not considered a cure because trace HBV DNA is still present |
| May last from 1 year to decades | Active inflammation and liver damage | Mild hepatitis and minimal fibrosis | Chronic hepatitis | |
| Can lead to chronic infection | Can lead to chronic infection | Cirrhosis may be present due to previous liver damage | HBsAg positive | |
| ALT levels normal | HBeAg positive → HBeAg negative and anti-HBe positive | Chronic infection | HBeAg negative, anti-HBe positive | |
| HBV DNA levels high | HBsAg positive | HBsAg positive | HBeAg negative, anti-HBe positive | |
| HBeAg positive | HBeAg negative, anti-HBe positive | HBsAg negative | ||
| HBsAg positive | ||||
In children and young adults, the high-replication, low-inflammation phase is characterized by detectable serum HBsAg and HBeAg concentrations and high serum HBV DNA concentrations but only slightly increased serum ALT levels, and liver histology is often relatively benign (i.e., minimal to no inflammation or fibrosis). However, in this phase, disease is under way, with expansion of hepatocytes and HBV integration that may ultimately progress to active disease.
Progression to cirrhosis in HBeAg-positive patients occurs at a rate of 2 to 5.5% per year, becoming 8 to 20% in 5 years. The high-replication phase may be followed by active HBV infection with developing necroinflammation (also referred to as HBeAg hepatitis, or the immunoactive phase) or by HBeAg seroconversion and remission in a proportion of patients (HBeAg-negative infection, or the inactive carrier state). Inactive carriers characteristically exhibit normal aminotransferase levels, and the HBV DNA level is generally found to be less than 2,000 IU/ml. A small proportion of patients experience disease regression at a rate of from 0.5 to 2% per year. Anti-HBe early seroconversion before the onset of marked hepatic fibrosis may signal remission and may indicate a good prognosis, depending upon the degree of liver damage.
Conversely, HBeAg-negative anti-HBe-positive disease (also called HBeAg-negative hepatitis, or the reactivation phase) is a progressive stage of chronic disease. Though HBeAg is not apparent in these patients due to mutant precore HBV, HBcAg is detected in liver cells and evidence of active disease is still present. Patients with anti-HBe-positive CHB are usually older, have more continuing inflammatory changes, and experience variations in their liver disease course, with inconsistent serum aminotransferase levels and different HBV DNA concentrations. Anti-HBe-positive patients experience a quicker progression to cirrhosis at a yearly rate of 8 to 20%, and the levels of HBsAg and HBV DNA in these patients tend to be lower than those in patients who are HBeAg positive.
Patients with cirrhosis experience hepatic failure at a rate of 16% over 5 years. In 366 HBsAg-positive patients with compensated cirrhosis, the cumulative probability of survival was 84% and 68% at 5 and 10 years, respectively (117–120). It was determined that risk factors for a high rate of progression and shorter survival were age, male sex, high liver enzyme levels, high HBV DNA levels, high HBsAg levels, infection with a genotype C strain, as well as basal core promoter expression. The REVEAL studies delineated the relationship between HCC risk and high HBV DNA concentrations (37, 121).
TREATMENT
The ultimate goals of treatment are to improve the patients’ quality of life and their survival. Therefore, treatment is geared toward the prevention of liver disease progression to cirrhosis, decompensated cirrhosis, hepatocellular carcinoma, liver transplantation, and death. These goals can be achieved through the elimination of HBV replication, which results in the normalization of liver enzyme levels and the resolution of histologic necroinflammatory activity. Sustained viral suppression prevents fibrosis progression and leads to the regression of fibrosis even for patients with established cirrhosis. In the tenofovir registration study, at 5 years of follow-up, paired liver biopsy results demonstrated that 348 patients experienced an 87% regression of fibrosis, including 71 out of the 96 cirrhotic patients (74%) (122). Sustained viral suppression also reduced the risk for hepatocarcinogenesis (13). The following briefly describes the recommendations for initiating treatment per professional society guidelines and the current regimens available for the treatment of HBV infection (Fig. 2).
FIG 2.
Universal HBV screening and care action items for persons residing in and immigrants from areas where HBV is endemic (an HBsAg prevalence of 2% or higher). US, ultrasound; AFP, alpha-fetoprotein; HAV, hepatitis A virus; CBC, complete blood count; INR, international normalized ratio.
Summary of Current Recommendations for Initiating Antiviral Therapy
Treatment guidelines are regularly updated to incorporate the new developments in the field. The key features of the treatment recommendations from major guidelines available worldwide are found in Table 5.
TABLE 5.
Summary of latest international guidelines for management of chronic hepatitis Ba
| Management step | Guidelines of the following: |
||||
|---|---|---|---|---|---|
| EASL 2017 | AASLD 2018 | ATA 2015 | APASL 2015 | WHO 2015 | |
| When to start treatment | HBV DNA level of >2,000 IU/ml, ALT level >ULN,b and/or at least moderate liver necroinflammation or fibrosis | ALT level >2× ULNc and HBV DNA level of >2,000 IU/ml (HBeAg negative) or >20,000 IU/ml (HBeAg positive) | ALT level >ULN,d HBV DNA level of >2,000 IU/ml | ALT level >2× ULN and HBV DNA level of >2,000 IU/ml (HBeAg negative) or >2,000 IU/ml (HBeAg-positive) | Compensated or decompensated cirrhosis (or APRI >2 in adults) |
| Compensated or decompensated cirrhosis with detectable HBV DNA | Cirrhosis with HBV DNA level of >2,000 IU/ml | Compensated or decompensated cirrhosis with detectable HBV DNA | Compensated or decompensated cirrhosis with detectable HBV DNA | Age >30 yr, persistently abnormal ALT levels, and HBV DNA level of >20,000 IU/ml | |
| HBV DNA level of >20,000 IU/ml and ALT level >2× ULN | Other factors include age >40 yr, family history of HCC, previous treatment, and presence of extrahepatic manifestations | If no HBV DNA, persistently abnormal ALT levels | |||
| HBeAg positive, high HBV DNA level, and age >30 yr | |||||
| Family history of HCC or cirrhosis and extrahepatic manifestations | |||||
| Treatment | ETV, TDF, TAF, PEG-IFN-α | ETV, TDF, PEG-IFN-α | ETV, TDF, PEG-IFN-α | ETV, TDF, and PEG-IFNα or LAM, ADV, and LdT (less preferred) | ETV, TDF |
| When to stop treatment | Confirmed HBsAg loss | HBsAg loss | HBsAg loss for >6–12 mo | Confirmed HBsAg loss for at least 12 mo | Lifelong treatment if the patient has cirrhosis |
| Noncirrhotic HBeAg positive with seroconversion and undetectable HBV DNA after 12 mo of consolidation therapy | Not recommended in patients with cirrhosis | Noncirrhotic HBeAg positive with seroconversion and undetectable HBV DNA after at least 1 yr (preferably 3 yr) of consolidation therapy | Stop treatment if there is no cirrhosis, HBeAg seroconversion, or persistently normal ALT levels with or without undetectable HBV DNA | ||
| Noncirrhotic HBeAg negative with undetectable HBV DNA for ≥3 yr | Noncirrhotic HBeAg negative and undetectable HBV DNA for ≥2 yr | Stop treatment if there is persistent HBsAg loss with 1 yr of consolidation therapy | |||
| In cirrhotic individuals, if possible, implement a careful off-therapy monitoring plan | |||||
| When to retreat | Similar to treatment-naive patients | No specific recommendation | If the patient has a relapse in terms of HBV DNA and ALT (no specific levels mentioned) | No specific recommendation | In the case of reactivation (HBsAg or HBeAg becomes positive, ALT levels increase, or HBV DNA reappears) |
ADV, adefovir dipivoxil; ALT, alanine aminotransferase; APRI, aspartate aminotransferase (AST)-to-platelet ratio index; ATA, American treatment algorithm; ETV, entecavir; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; LAM, lamivudine; LdT, telbivudine; PEG-IFN-α, peginterferon alpha; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumurate.
The ULN for ALT is 40 U/liter.
The ULN for ALT in healthy adults is 30 U/liter for males and 19 U/liter for women.
The ULN for ALT in healthy adults is 30 U/liter for males and 19 U/liter for females.
EASL 2017 clinical practice guidelines.
One of the most frequently updated guidelines is from the European Association for the Study of the Liver (EASL), which appeared online in April 2017. The key features in the new EASL guidelines included updated definitions of the different CHB phases using the status of HBeAg, as well as the recommendation for the use of tenofovir alafenamide (TAF) as a first-line antiviral agent and discussion of the need for antiviral prophylaxis for patients with hepatitis B and C virus coinfection treated with direct-acting antivirals (DAAs) (17).
AASLD 2018 guidelines and ATA 2015.
There are two major practice guidelines or treatment algorithms in the United States (30, 123). The American Association for the Study of Liver Diseases (AASLD) last updated is practice guidelines in 2018 (84). One major update was the increase in the ALT threshold for treatment (2× the upper limit of normal [ULN]), with the upper limit of normal being changed to 35 U/liter for males and 25 U/liter for females.
In addition, a very practical algorithm, comprehensive review, and recommendations on screening for HBV, pretreatment evaluation, treatment, and vaccination were published by a group of experts and are collectively known as the American treatment algorithm (ATA). The last update was in 2015 (123), and the ALT threshold treatment recommendation was 1× the upper limits of normal (30 U/liter males and 25 U/liter females).
APASL clinical practice guidelines 2015.
The practice guidelines developed by the Asian Pacific Association for the Study of the Liver (APASL) have had a major influence on the management of CHB in the Asia-Pacific region, which represents the area with the largest HBV disease burden (31). As NAs with low genetic barriers are still commonly used in some Asia-Pacific countries, a detailed drug resistance monitoring and management recommendation is presented along with a very detailed section on screening for hepatocellular carcinoma (HCC) and HCC risk factors.
WHO 2015.
The World Health Organization (WHO) issued its first guidelines for the management of CHB in March 2015. With a worldwide perspective and coverage (including Africa), the 2015 guidelines provided practical suggestions, with special attention being given to settings with limited resources. As an example, APRI was suggested to be the noninvasive test that should be used for assessment of the presence of cirrhosis, using a cutoff value of 2 for adults in monetarily limited areas. More advanced noninvasive tests, such as transient elastography or the FibroTest, were recommended for use in wealthier settings. An age greater than 30 years was also set as a condition to consider antiviral treatment, in particular, for patients with persistently abnormal ALT levels. Detailed recommendations about who should receive treatment, as well as when to end treatment and how to monitor and prevent hepatitis B virus infection, are described below. However, a recent publication from Ethiopia suggests a low performance of the WHO criteria in the correct selection of CHB patients who could benefit from therapy (124, 125).
Current Treatment Regimens and Treatment Durations
Current therapies for the management of CHB include peginterferon (PEG-IFN) and orally administered nucleos(t)ide analogs (NAs). NAs are the most widely chosen option globally.
For NAs, all regional associations agree that first-line therapy should be with an oral antiviral with a strong barrier to resistance: either entecavir, TDF, or TAF (17, 30, 31). Short-term treatment with NAs is feasible for those HBeAg-positive patients who experience seroconversion to anti-HBe during treatment. After HBeAg seroconversion occurs, treatment should continue for at least 1 year and, it is hoped, an additional 3 years in order to achieve a long-lasting response once therapy is discontinued. Therapy continuation for at least 3 years lowers relapse rates to less than 30% and hastens the subsequent loss of HBsAg. Higher relapse rates following therapy discontinuation occur among older patients and those with HBV genotype C infection.
The recommended therapy cutoff criteria for NAs in HBeAg-negative patients vary by region. Given the very high rate of relapse following treatment withdrawal, AASLD recommends that NAs be withdrawn from HBeAg-negative patients only after confirmation of the loss of HBsAg, with or without seroconversion. Recent reports from Asian and European countries suggest that NAs may be stopped in HBeAg-negative patients who have undetectable HBV DNA at three different times when the testing times are 6 months apart, although the duration of on-therapy HBV DNA undetectability remains important (31, 126). The disease remains inactive, defined as an HBV DNA level of <2,000 IU/ml and a normal ALT level, in approximately half of the patients at 3 years after therapy withdrawal. Treatment discontinuation is not recommended for patients with cirrhosis, due to the risk of life-threatening hepatitis flares following virological relapse (127). In the near future, newer markers, such as HBV RNA and HBcrAg, may guide treatment decisions.
In contrast to NA therapy, the use of peginterferon as primary therapy in patients with CHB has been limited by its poor efficacy and tolerability. However, unlike NA therapy, PEG-IFN has a finite duration. For HBeAg-positive and HBeAg-negative patients who have a good chance of HBeAg seroconversion, 48 weeks of PEG-IFN is recommended. In phase 3 clinical studies, only one third of HBeAg-positive patients and <10% of HBeAg-negative patients achieved a sustained response after 48 weeks of treatment with peginterferon. The efficacy seemed to be improved in those patients who had already developed an ostensible immune response against HBV, such as HBeAg-positive patients with an HBV DNA level of <9 log IU/ml and an ALT level >5× the ULN. Monitoring of an on-treatment HBsAg decline (at 12 or 24 weeks) identifies nonresponders, allowing the early cessation of treatment, which improves both the cost-effectiveness and the tolerability of this treatment (128, 129). Effectiveness can also be found for HBeAg-negative patients, as it may be the only option that may produce an opportunity for a sustained response off treatment after a limited course of therapy.
Combinations of peginterferon with NAs or add-on or sequential therapy with NAs followed by peginterferon treatment have been demonstrated to be safe and to have improved seroconversion rates compared to those of single therapies (130, 131). Larger studies of these options need to be performed before they can be recommended. It should be noted that peginterferon should not be used in patients with decompensation, but it can be used with caution in patients with compensated cirrhosis. In contrast, oral NA therapy improves and restores function even in patients with severe decompensated liver disease (132).
Disadvantages of Oral Antiviral Therapy and Future HBV Cure Therapies
Viral suppression difficulties.
Although maintained viral suppression improves the outcomes in most patients with CHB, long-term NA therapy has a number of disadvantages. First, a mere one-fifth of patients with CHB meet the current criteria for beginning treatment with antivirals. Second, the rate of seroclearance (functional cure) with oral antiviral therapies does not appear to be significantly higher than the spontaneous rate of about 1.0%; therefore, treatment is often lifelong (133). Also, with long-term therapy, the HBV transmission risk may decrease, but the risk is not completely eliminated. A recent in vitro study using new highly sensitive molecular assays for HBV DNA demonstrated a residual ability of HBV DNA to transmit HBV infection in an experimental model (134).
Development of liver cancer.
In addition, long-term therapy may reduce but not eliminate the risk of liver cancer (135). NAs prevent the formation of a covalent bond with the adjoining nucleotide by competing at the HBV catalytic site during the formation of nascent HBV, causing chain termination of the elongating DNA, but they do not act on cccDNA (136). The stable conservation and functioning of the cccDNA in the nuclei of HBV-infected cells in nucleoside analogue-treated patients provide a continual source of viral RNA transcripts but generally result in only minimal rates of HBsAg loss and prohibit cure in most patients. Integrated viral genomes are not directly affected by NA therapy (136).
Oral antiviral toxicity.
Although NAs have been proven to be safe and well tolerated, cumulative toxicity may develop in some patients after long-term use. Elderly patients and those with HIV infection have developed bone disease and renal tube injury with the use of tenofovir. Preclinical studies have shown carcinogenesis (cytotoxicity in human lymphocytes and tumors in rats) with the use of entecavir, and consequently, it is not indicated for use in women of childbearing age or in children. Finally, the risk of liver cancer may increase following sequential lamivudine-adefovir dipivoxil therapy, due to A181T variant selection and the development of the cytoplasmic accumulation of S proteins which have been truncated, causing activation of the c-Raf-1/mitogen-activated protein kinase pathway (137).
Future Directions for Therapy
Therefore, new therapies for HBV that can achieve sustained suppression and HBsAg loss after a limited course of therapy are of high interest. Such therapies will permit the discontinuation of NAs after short periods of therapy and provide a so-called HBV cure. There are three defined endpoints: (i) partial cure, which is HBV DNA suppression and normal ALT levels after the end of treatment without a loss of HBsAg; (ii) functional cure, which is HBV DNA suppression and ALT level normalization after the end of treatment with a loss of HBsAg but without cccDNA elimination (which does not retain the risk of late HBV reactivation during immunosuppression); and (iii) complete cure, which is permanent HBV DNA suppression and normal ALT levels after treatment with the loss of HBsAg and the elimination of cccDNA. None of these strategies will target integrated HBV DNA and, hence, will not prevent HCC unless they are administered early in the HBV infection process, before integration has occurred. Thus, HBV cure will require a combination of novel antivirals that will reduce the levels of HBV DNA (or the level of protein production) and immunomodulators which boost the exhausted natural immune responses found in chronic hepatitis B (137, 138).
Therapeutic HBV vaccines.
The individual patient’s own immune response to HBV infection is important and foreshadows the clinical outcome following an acute HBV infection. The spontaneous clearance of HBV infection requires a vigorous, polyclonal antigen-specific, adaptive immune response against HBV proteins. A broad CD8+ T-cell response is also required for spontaneous clearance (138). Activation of antiviral immunity against HBV includes (i) the generation of new T cells via therapeutic vaccines; (ii) stimulation of antiviral effector cells, such as T cells, B cells, and dendritic cells; and (iii) reduction of the T-cell exhaustion that accompanies chronic HBV infection. To date, the therapeutic vaccines have been disappointing. Although immunogenic vaccines and T-cell peptide vaccines generate sustainable and protective HBV-specific B- and T-cell responses in HBV-naive patients and reduce hepatitis B virus replication in animals with chronic hepadnaviral infection, they have absolutely no efficacy in patients with chronic hepatitis B when they are used either alone or with oral antiviral therapy (139, 140). A promising vaccine developed using the targeted molecular immunogen platform (the GS-4774 vaccine) uses recombinant Saccharomyces cerevisiae yeast to express surface, core, and X proteins. Weekly or monthly GS-4774 vaccines given to health volunteers produced HBV-specific T-cell-mediated responses in almost all the volunteers (90%), with two subjects developing low-level anti-HBs (141). GS-4774 was well tolerated in virally suppressed patients, but it did not produce significant reductions in HBsAg levels, even though almost half (40%) had an increase in HBV-specific T cells affecting HBV core and HBx proteins (142).
Pharmacological stimulation of the innate immune response.
There is also evidence that HBV proteins are recognized by pathogen recognition receptors, including Toll-like receptor 2 (TLR2), TLR4, and RIG-I (143–145). The virus may have evolved mechanisms to inhibit pathogen recognition receptor pathway signaling. Pharmacological stimulation of the innate immune response with Toll-like receptor 7, 8, or 9 is being studied. Toll-like receptor 7 affects multiple arms of the immune system with its pattern recognition receptor, including both innate and adaptive effector cells and antiviral cytokine responses. Additionally, TLR7 is expressed by B lymphocytes, and upon activation, TLR7 results in polyclonal expansion and differentiation toward immunoglobulin-producing plasma cells, providing a humoral component to the adaptive immune response. Agonist-induced activation of TLR7 may provide a new treatment avenue for CHB patients through its effects on the innate immune effectors and HBV-specific T- and B-cell responses (143–145).
TLR7.
Oral vesatolimod (GS-9620) is an oral TLR7 agonist being developed at the current time. The administration of vesatolimod to mammals for 1 to 2 months has led to HBV DNA suppression, the loss of HBsAg, and a reduced rate of development of HCC (143–145). In two randomized controlled studies comparing virally suppressed and treatment-naive CHB patients, weekly vesatolimod was found to be safe, well tolerated, and associated with the production of peripheral interferon-stimulated gene 15 (ISG15) without significant systemic interferon alpha (IFN-α) levels or related symptoms but had no effect on HBsAg levels (146, 147). This discrepancy between chimp and human responses may reflect the lower dosing used in humans because of dose-related toxicity. Several other potent TLR7 agonists are now entering clinical trials (148).
TLR8.
In contrast to TLR7, myeloid cells (myeloid dendritic cells, monocytes, and Kupffer cells) are where the expression of TLR8 receptors is found. Stimulation by TLR8 agonists should trigger the maturation of professional antigen cells, noted to be in gut lymphoid tissue or the liver. A variety of cytokines which can stimulate or rescue antigen-specific T-cell responses to improve anti-HBV activity can result from such stimulation. TLR8 agonism should produce a more effective antiviral immune response and lead to the functional cure of CHB. In animal models, TLR8 agonism can induce an innate immune response in circulating blood cells without inducing adverse systemic IFN-α. The first TLR8 agonist, GS-9688, has recently entered phase 1 clinical development (148).
STING.
Another innate immunity-related target is STING (stimulator of the IFN gene). STING is a pathogen recognition receptor that activates downstream signaling and the expression of interferons and is also the adapter protein of multiple cytoplasmic DNA receptors. Pharmacological activation of the innate immune response may use STING as a potential target (149). STING agonists are in preclinical development (150–154).
RIG-1.
The HBV polymerase may counteract IFN-β production in humans (155). RIG-I (retinoic acid-inducible protein) is a cytosolic sensor of RNA that can promote inflammatory signals through activation of interferon regulatory factor 3 (IRF-3) and NF-κB. Importantly, RIG-I has been proven to recognize and bind the epsilon stem-loop of HBV pgRNA and interfere with HBV replication, as well as induce IFN and cytokine production. Study data suggest that the mechanisms of action for RIG-I are 2-fold, whereby RIG-I interferes with the HBV polymerase in human liver cells and RIG-I performs as an HBV sensor that begins an innate signaling process (156). The experimental drug SB 9200, an oral prodrug relative of the dinucleotide SB 9000, then activates RIG-1 and nucleotide-binding oligomerization for protein 2, resulting in an IFN-mediated antiviral immune response in infected cells (157).
siRNA/LNA.
It is likely that to enhance the weakened innate and immune responses specific to HBV in patients with lifelong CHB, new immunomodulators need to be used along with other modalities to ameliorate T-cell exhaustion. The small interfering RNA (siRNA)/locked nucleic acid (LNA) approach (see below) could help reconstitute immune responses specific to HBV through the rapid and effective knockdown of hepatitis B proteins. However, due to the overexpression of T-cell receptors in patients with chronic hepatitis B, T-cell effector function is limited (158).
PD-1 and PD-1 inhibitors.
Among the T-cell receptors, PD-1 is expressed the most on HBV-specific T cells within the liver, while at the same time, PD-L1 expression is increased in hepatocytes (159). For this reason, PD-1 and PD-1 inhibitors are currently being studied as possible treatments for chronic hepatitis B. Experiments on woodchucks with chronic woodchuck hepatitis virus infection have shown that a blockade of PD-L1, in addition to DNA vaccination, proved to effectively control viremia (160). In the first clinical trial of anti-PD-1 in patients with CHB, a low dose (a single dose of 0.3 mg/kg of body weight; in comparison, monthly doses of 3 mg/kg are used in patients with melanoma) induced significant reductions of HBsAg, and a single patient achieved complete HBsAg seroconversion (161). Further studies will need to be cautious in determining the dosage to avoid stimulating autoimmune conditions, such as pneumonitis, colitis, and hepatitis, and immune-mediated HBV flares.
HBV life cycle.
In addition, the HBV life cycle provides many different access points for antiviral therapies, and clinical development is focused on small molecules that block HBV entry (myrcludex) (113), HBV protein synthesis (siRNAs ARC520 and ARC521, ALN-HBV, ARB-001467, and LNAs), core synthesis (NVR3-778, GLP-2, BAY41-410, AB-423, ABI-H0731), and finally, the release and formation of virions (REP-2139) (162). Researchers have discovered an allosteric agent, a synthesized derivative of the large HBsAg protein myrcludex B, that retards NTCP. The peptide irreversibly blocks the receptor to block the transport of bile salts and the introduction of HBV in cellular experiments. This protein is currently being studied in chronic hepatitis B and hepatitis D infections (163).
cccDNA target.
The hepatitis B core protein performs multiple crucial roles in virus replication, cccDNA maintenance, and downward progression of the host’s inborn immune response. As the core is preserved in all HBV genotypes, it is thus a promising target for inhibitors consisting of small molecules that attach to the core protein, causing allosteric modulation that prevents dimerization and assembly of the nucleocapsid (164). The use of small molecules that target capsid assembly represents a promising antiviral strategy. These molecules include heteroaryldihydropyrimidines, phenylpropenamides, pyridazinone derivatives, and sulfamoyl benzamides. As capsid formation is an indispensable step in the generation of HBV virions but also in the augmentation and persistence of cccDNA in the nucleus, targeting capsid assembly and disassembly is an attractive approach (165). Thus, inhibitors of either encapsidation or compounds that result in capsid disassembly hinder the entry of rcDNA into the nucleus, which impedes the transformation of rcDNA to cccDNA. The capsid assembly modulators (CpAMs) could have multiple effects on the HBV life cycle. Interruption of the assembly of HBcAg dimers into capsids will directly inhibit HBV replication. CpAMs could also prevent cccDNA formation (through the interruption of capsid disassembly) and cccDNA replenishment within the nucleus (165).
CpAMs.
Finally, CpAMs could also restart the host innate immune response through the induction of interferon-stimulated gene (ISG) expression (166, 167). In preclinical studies, CpAMs were found to diminish HBsAg and HBV DNA levels. The initial CpAM study demonstrated that over 28 days of NVR3-778 dosing was associated with 1- to 2-log reductions in HBV DNA levels, although there was no change in HBsAg levels (166, 167). Studies of CpAMs in combination with pegylated interferon or entecavir are in progress. Several CpAMs in current preclinical studies show a significantly higher in vitro potency than NVR3-778 (GS4JHS, AL-034, ARB-423, AT-130, HAP12, HAP_R01, SBA_R01, JNJ-379, ABI-H0731, ABI-H2158, ABI-Nx). However, polymorphisms in the pocket region of the capsid significantly reduce susceptibility to CpAMs. Known signature mutations include T128I and T33N, which confer 20-fold and 80-fold reductions in susceptibility, respectively. CpAM resistance is likely to be against all agents in that class. Although the prevalence of these polymorphisms is low (0.03% for both mutations in untreated patients), they are likely to be rapidly selected by CpAM monotherapy. The clinical significance will be determined by their fitness. Strains with these polymorphisms are fully susceptible to oral antiviral therapy, so their selection would be prevented by combining CpAMs with oral antiviral therapy.
siRNA therapy.
siRNA therapy takes advantage of a natural process in which the body quiets unneeded genes to prevent the translation of unneeded proteins or enzymes. Researchers can use an intracellular RNA-induced silencing complex (RISC) to quiet any unneeded host, bacterial, or viral gene using synthetic siRNAs that complement the invasive viral mRNAs (168, 169). The greatest problem barring therapeutic application of this methodology has been the type of administration and unneeded effects on other organs, such as the kidney. siRNA is digested rapidly in the gut, so it is necessary to administer the therapy parenterally. Infusion reactions can occur with intravenous doses, so dosing often requires medication with corticosteroids or antihistamines in advance of treatment. Subcutaneous injections that target the liver can now be administered. These injections have fewer side effects and require less frequent dosing, which improve patient tolerance. The host immune system is not activated by the metabolically stable siRNAs, so the reduction of off-target effects has been achieved through several techniques, including pairing of siRNA with N-acetylgalactosamine (GalNAc), which enhances liver cell uptake via the asialoglycoprotein receptor (ASGPR) (168, 169).
As HBV is a compact genome with regional overlaps, a single siRNA can quiet the transcription of many genes, blocking the production of the core, surface, polymerase, and X proteins. By directly blocking HBV replication and indirectly facilitating HBV immunity, the expression of HBsAg and HBeAg (tolerogenic antigens) is reduced. Arrowhead’s siRNA ARC520 was the initial siRNA to be developed clinically (168, 169). Different doses of ARC520 lowered HBsAg, HBeAg, HBcAg, and HBV DNA levels in both primates and CHB patients. However, the lowering of HBsAg occurred more often in HBeAg-negative chimpanzees and patients than in HBeAg-positive chimpanzees and patients. This happened because ARC520 targets cccDNA-derived pgRNA, the major source for HBsAg in young HBeAg-positive patients, and not integrated HBs (the major source of HBsAg in older HBeAg-negative patients). ARC250 had no effect on HBs transcriptions in HBV genomes, because the target sequence of ARC520 was upstream from the core promoter site and next to the site of insertion for integrated HBs sequences. To correct this problem, Arrowhead produced a second-generation siRNA that targets this region, which covers transcripts from both integrated and cccDNA-derived HBs. This effectively knocks down HBsAg in both HBeAg-positive and HBsAg-negative patients with chronic hepatitis B (35). Currently, multiple developers, such as Arrowhead and Alnylam, have active siRNA development programs.
LNA technology.
An alternative approach also being actively pursued to silence HBV transcripts and prevent HBV protein synthesis is the locked nucleic acid (LNA) technology, which uses liver-targeted single-strand oligodeoxyribonucleotides complementary to mRNAs derived from cccDNA. These more recent delivery systems for hepatic targeting should eliminate the problem of off-target toxicities due to antisense oligonucleotides (170).
CRISPR/Cas9.
The goal of siRNAs and core inhibitors is a functional cure, which is defined as the clearance of HBsAg with sustained suppression when the patient has discontinued treatment. However, if a patient is immunocompromised, these therapies do not address the lifelong risk of HBV reactivation in this circumstance. The deactivation or elimination of cccDNA is the only way to decrease this risk (171). Therapy could be produced via epigenetic modification of cccDNA using demethylation or deacetylase activation, but this approach is offset by the risk of off-target effects on host genes. Specific cccDNA cleavage via various nucleases and CRISPR/Cas9 would seem to be more attainable.
CRISPR/Cas9 occurs within bacteria, in which the Cas9 nuclease combines with a specific guide RNA (gRNA) that complements plasmid and phage DNA, causing destruction of the genetic material and ending in the acquisition of immunity to the bacteria. In many human diseases of heredity, gene editing via CRISPR/Cas9 has been studied. Most recently, through gene editing of the bone marrow, sickle cell disease was corrected (171). In vitro CRISPR/Cas9 experiments show deep and rapid reductions in the levels of cccDNA and HBV proteins encoded by genes carried by hepatitis B virus cccDNA (172). Combining CRISPR/Cas9 with either gene silencing (siRNA) or another gene editing tool (APOBEC3B) further enhances the disappearance of cccDNA (173, 174).
However, several challenges need to be overcome before this technology can be safely applied to patients with CHB. A lack of cross-reactivity with human genetic material and the ability to specifically deliver the gene editing tool to the hepatocyte nucleus are required to ensure minimum off-target toxicities to host DNA. The lack of any standardized test for cccDNA limits the measurement of endpoints (changes in the levels of cccDNA transcripts and their proteins) (174). If researchers overcome these barriers, then such gene editing technology has the potential to eradicate cccDNA and achieve a complete HBV cure, thereby removing any long-term risk of reactivation. This will necessitate an efficient delivery system with a 100% hit rate. Even one residual cccDNA minichromosome could result in persistent infection. Finally, cccDNA elimination will not completely abrogate the lifelong risk of HCC attributed to integration. Although CRISPR could also be designed to specifically excise integrated HBV sequences from human DNA, any subsequent chromosomal translocation would have disastrous consequences (174).
Summary of new therapeutic targets.
The goal of HBV therapy will always remain complete cure. Functional cure may be attained within the next 5 to 10 years through the use of newly developed antivirals and immunomodulatory agents that will reduce the HBV viral load and build the host’s own immune response against disease. Complete cure will require gene editing approaches which are some years away from human trials. However, safe, well-tolerated, and cost-effective therapies must be the goal of the new therapies so that HBV elimination can be obtained in low-income countries that have the greatest HBV burden. To assist in meeting these goals, an international working group of professionals has come together to help coordinate, promote, and establish public-private partnerships to expedite the cure of CHB. This group, the International Coalition to Eliminate Hepatitis B (ICE-HBV), thus far has developed a global scientific strategy to cure HBV and is actively collaborating on a number of research protocols to cure HBV (https://ice-hbv.org/about/about-ice-hbv/).
COINFECTION: EPIDEMIOLOGY, DIAGNOSIS, AND MANAGEMENT
Hepatitis D and B Virus Coinfection
Introduction to natural history and epidemiology.
Hepatitis D virus (HDV), a unique liver-tropic human virusoid, is the causative agent of chronic hepatitis D but one that can survive only in persons infected with HBV (175). HDV is transmitted either at the same time as HBV or as a superinfection by an established HBV carrier as a result of it being a defective RNA virus.
The HDV virion contains the hepatitis delta antigen (HDAg) and HDV RNA. The virion contains a circular single-strand negative-sense genome. The viroid-like self-complementary genome encodes two varieties of a single protein (large and small), hepatitis D, or delta, antigen. HDV needs a concomitant HBV infection to organize and grow within the host, as the HDV complex requires an envelope made of the three HBV (small, middle, and large HBsAg) proteins during HDV assembly to assemble into an infectious HDV virion and to enter hepatocytes. HBsAg may also be provided by the HBsAg protein encoded by defective integrants of HBV (175).
HDV virions attach to cellular heparin sulfate proteoglycans, in which the penetration of liver cells is regulated by the irreversible attachment of the large HBsAg to the N-terminal pre-S1 region of the hepatocyte-specific human bile salt transporter sodium taurocholate cotransporting polypeptide (NTCP), mediating the liver tropism of both HBV and HDV. Subsequently, HDV RNA moves to the nucleus, where replication occurs by host DNA-dependent RNA polymerases. Replication via a rolling-circle mechanism generates multimeric linear transcripts of the genome and antigenome. These are subsequently cleaved and circularized into the infectious form by the HDV ribozyme.
The antigenomic RNA is edited by adenosine deaminase to catalyze a change in the small HDV RNA stop codon, thus leading to a 3′ elongation of the open reading frame. The subsequent mRNA produces large HDAg. The extension encompasses a binding motif for HBsAg through a prenylated location at Cys211 that is acted on by cellular farnesyltransferase. The identification of the NTCP receptor has enabled the adaptation of hepatically derived HCC cells that express the receptor, such as HepG2-NTCP and HuH7-NTCP cells, rendering these lines susceptible to both HBV and HDV (175).
Of the 240 million people with HBV infection, HDV is thought to affect about 15 million to 20 million. There are regional differences in the epidemiology of HDV. High prevalences have been reported in the Amazon region, central and eastern Africa, Turkey, Mongolia, Iran, and Pakistan. A prevalence of 25% has been reported in Romania. Several genotypes have been described: genotype 1 is prevalent worldwide; in Japan and Taiwan, genotype 2 is the most prevalent; the Amazon Basin harbors genotype 3; genotype 4 is found in Taiwan and Japan; and genotypes 5 to 8 are found in Africa. The disease has declined in Italy after universal HBV vaccination. The current residual reservoir of HDV in European comprises two principal cohorts: aging patients with advanced liver disease and young immigrants with active chronic hepatitis D from areas where HDV infection remains endemic. The increased HDV prevalence in the United Kingdom, France, and Germany reflects the prevalence in young individuals who have migrated from regions of high prevalence. Rates of 1 to 9% have been reported from China, and rates of 3 to 8% have been reported in the United States. The disease is predominantly acquired by horizontal rather than vertical transmission. The transmission of HDV occurs through the parental route through tainted blood and/or body fluids. There is some evidence of sexual transmission. Intrafamilial spread can occur, but perinatal transmission is rare.
Three to 4% of those coinfected contract fulminant hepatitis. The course of HDV infection is affected by the host immune response to HBV, but HDV with HBV causes a high rate of chronicity and the most serious and advancing form of chronic hepatitis, including a high rate of cirrhosis.
Diagnosis and testing.
The diagnosis of HDV infection requires testing of HBsAg-positive individuals for anti-HDV antibodies, and if these are found, the individuals should be tested for HDV RNA to confirm HDV viremia. Testing for HDV antigen in serum has poor sensitivity, and liver tissue staining is reserved for use for testing for HDV antigen (176). All HBsAg-positive individuals should be tested. Testing rates remain low in many countries, including in immigrants and intravenous drug users. Tests for HDV antigen and RNA require standardization.
Treatment.
The treatment of chronic HDV infection remains difficult. HDV may suppress HBV replication but cause progressive disease with a course more severe than that of HBV monoinfection (177). A new endpoint for the treatment of chronic HDV infection has recently been proposed: normal ALT levels and a decline of HDV RNA levels of at least 2 logs at the conclusion of treatment. The sole proven treatment is PEG-IFN (177, 178). PEG-IFN administration is usually weekly for 12 to 18 months, even though HDV RNA may be undetectable in serum after 6 months of therapy in nearly 25% of patients (179). HDV is likely to relapse in patients who remain HBsAg positive. Unfortunately, treatment seldom achieves the endpoint of HBsAg and HDV RNA clearance. However, HDV RNA loss during the follow-up period, along with fewer liver-related complications and disease progression, is more frequent in those treated with IFN. If achieved, the lasting undetectability of HDV RNA is a solid stopping point for treatment with IFN (180).
A nucleoside analogue, in particular, tenofovir or entecavir, can be administered to patients with advancing disease and detectable HBV DNA. However, chain terminators do not directly inhibit HDV RNA replication. There are unconfirmed reports of declines in HDV RNA levels in HIV-positive patients treated with TDF (181). The combination of peginterferon and nucleoside analogues has been assessed. Peginterferon and adefovir for 48 weeks versus peginterferon or adefovir monotherapy was evaluated in the HIDIT-1 trial. HDV RNA became negative in 51%, 26%, and 0% of patients, respectively (182). A greater decline in the HBsAg concentration was observed in the combination arm. However, 56% of the patients relapsed after longer-term follow-up (183). Treatment with tenofovir and PEG-IFN together for 96 weeks offered no incremental benefit in the HIDIT-2 study (184).
There are a number of novel therapies for HDV infection (185). siRNA-mediated silencing of HDV RNA intermediates, in particular, mRNA of HDAg, is a theoretical possibility. Novel therapeutic agents are currently in clinical trials. Lonafarnib is a tricyclic derivative of carboxamide that prevents the prenylation of HDAg by reining in farnesyltransferase, which theoretically prevents the assembly and release of the ribonucleoprotein complex (prenylation is a posttranslational modification whereby prenyl lipids are added to proteins to render them lipophilic). The drug is administered orally (186). In an early-phase study in 12 patients, HDV RNA levels declined after administration of lonafarnib at 200 to 400 mg daily for 28 days. HBsAg concentrations did not change. Lonafarnib influences cell signaling pathways, and its efficacy may be limited by dose and tolerability. Dose-dependent reductions in HDV RNA concentrations were reported, but weight loss and gastrointestinal symptoms, including vomiting, occurred. The results may be improved by ritonavir boosting to effect higher concentrations of the drug in the liver (187). Studies with 25 mg, 50 mg, and 75 mg in combination with ritonavir and PEG-IFN are ongoing.
Myrcludex B (a 47-mer myristoylated pre-S1 lipopeptide) is a synthesized, acylated pre-S peptide derivative of the large HBsAg protein and is a subcutaneously administered allosteric inhibitor of NTCP. In experimental cells, the peptide irreversibly blocks the receptor and blocks the transport of bile salts and the entry of HBV. Currently, the use of myrcludex B in patients with chronic HDV and HBV infection is being explored (163). Myrcludex B in combination with nucleosides and interferon is being tested in phase 1 and 2 studies. In an open-label phase 2b multicenter clinical trial, interim results assessing the safety and efficacy of myrcludex B (bulevirtide) in combination with tenofovir as therapy for chronic HBV and HDV infection have recently been reported. Myrcludex led to reductions in HDV RNA levels and improvements to ALT levels, but after 24 weeks, despite a dose-related suppression of HDV RNA, no change in HBsAg levels was noted (188). In April 2019, the final results of a multicenter open-label phase 2 study of myrcludex with PEG-IFN-α2a were reported. Myrcludex monotherapy (2 mg once daily) led to a continuous HDV RNA decline and ALT reduction over 48 weeks. An HDV RNA response (to undetectable levels or a ≥2-log decline) was observed in 26.7% of patients at week 72. Myrcludex B and PEG-IFN-α in combination therapy resulted in HDV RNA becoming undetectable in 12/30 patients (40%) at week 72. An unexpected result was that for the 15 patients who received the drug combination of 2 mg myrcludex B plus PEG-IFN-α, HBsAg became undetectable in 4/15 patients (27%), and 3 of these 4 patients experienced HBsAg seroconversion. A long duration of therapy will be needed to clear HDV RNA, and thus, long-term studies are being planned (189). The compound inhibits the bile acid transporter function of NTCP to increase conjugated bile acid levels in treated subjects. These drugs have received fast track and prime eligibility status by the U.S. FDA after the need to improve the outlook for HDV infection was recognized.
Phosphorothioated oligonucleotides, such as nucleic acid polymers (NAPS), may prevent viral entry with heparin sulfate proteoglycans (190). In the duck model, the compound REP2055 apparently inhibited HBsAg secretion by inhibiting HBsAg assembly and egress (191). In a phase 2 study, the compound NAP 2139-Ca was studied in patients with HDV infection. Twelve patients were administered REP2139 at 500 mg intravenously once a week for 15 weeks, then received 250 mg intravenous REP2139 combined with PEG-IFN alfa-2a once a week for another 15 weeks, and then underwent monotherapy with PEG-IFN weekly for 8 months. By the conclusion of therapy, six patients had HBsAg levels of less than 50 IU/ml, which were maintained in five patients. Eleven became HDV RNA negative on treatment; seven remained HDV RNA negative after the first year of follow-up. Normalization of serum aminotransferase levels occurred in nine patients (192). Reports of longer follow-up add to the data, but further evidence from controlled trials is required.
Randomized, open-label studies with IFN-λ at 120 or 180 μg, administered by subcutaneous injections every week for 48 weeks, are being conducted in Pakistan, Israel, and New Zealand. Thirty-three patients have been randomized to receive IFN-λ at 180 μg (n = 14) or 120 μg (n = 19). IFN-λ at 180 μg showed antiviral activity comparable to that of IFN-α, suppressing HDV RNA by 2.3 log10 units with better tolerability than historical data for IFN-α (193). An increase in bilirubin levels has been noted with IFN-λ. A study combining IFN-λ with lonafarnib is ongoing (194, 195).
Thus, partial efficacy has been reported for these new strategies, which may complement IFN therapy. Generally, the higher response rates of treatment for chronic HDV can be found in younger and noncirrhotic patients, though there is a risk of relapse. The clearance of HDV RNA may not be sufficient, in the absence of the clearance of HBsAg, as the former does not guarantee that HDV has been eliminated and does not portend a cure. Integration of HBV may provide a significant proportion of the HBsAg that allows HDV to persist. Other strategies may be needed, including quieting of RNAs or other immunomodulatory therapies. The only treatment for those proceeding to end-stage liver disease, HCC, or fulminant hepatitis caused by coinfection or superinfection with HDV and HBV is liver transplantation.
Hepatitis B Virus and HIV Coinfection
Epidemiology and natural history.
Approximately 8% of HIV-infected persons are coinfected with HBV. In regions where HBV is highly endemic, such as sub-Saharan Africa, HBV infections occur during childhood, but HIV infection is usually a sexually transmitted disease acquired in adulthood (196). It is believed that in sub-Saharan Africa, there are 2.6 million people living with HIV and HBV coinfections. Both HBV and HIV can be passed from mother to baby, but infection may also occur via unsafe injections or inadequate blood safety programs.
HIV coinfection may exacerbate liver disease (197–200). Liver-related deaths occur 3.73 times as frequently among those with hepatitis B as among those without; some subjects in some regions may have chronic hepatitis D. The added morbidity of HBV-HIV coinfection may be the result of increased replication, HBV reactivation, a greater likelihood of the chronicity of HBV, and accelerated progression to fibrosis or cirrhosis, which carries the risk of hepatocellular carcinoma at an age younger than that at which it would have occurred without HBV and HIV infections (201). There may also be a risk of hepatotoxicity from antiretroviral therapy (ART), particularly in resource-poor regions. HIV-induced immune deficiency may be aggravated in those with active HBV replication (202, 203).
Diagnosis.
The diagnosis of coinfection requires assessment of risk factors and repeated testing. HIV-infected patients must undergo a thorough assessment and staging for any hepatic condition. The scores obtained by noninvasive tests, including the fibrosis 4 index (FIB-4) and the scores obtained by transient elastography, are useful in this group. All persons with HIV or HBV infection must be assessed for coinfection (204). Innovations to expand testing are required. These include innovations suggested by the WHO, which include strategies to improve the already existing infrastructure to identify high-risk patients while also providing to limited-resource areas and hard-to-reach populations near-patient or point-of-care assays for virological markers (nucleic acid testing). These include different serological and nucleic acid assays with dried blood spot specimens, multiplex and multidisease platforms to enable testing for multiple analytes/pathogens, as well as potential self-testing for viral hepatitis (205, 206).
Treatment.
The first-line therapies used for both HBV and HIV are tenofovir and emtricitabine. These drugs, together with appropriate ART, are usually recommended for the treatment of HIV-HBV coinfection. Renal insufficiency may require a modification of treatment with tenofovir. Entecavir can be added to an appropriately potent suppressive ART regimen. Drug resistance testing is required where lamivudine has been utilized (207). Tenofovir alafenamide is an ART that offers effective activity with less renal or bone toxicity than other therapies (208). Its safety in pregnancy has not yet been established (209). The effective suppression of both viruses can limit the progression of hepatic fibrosis (210, 211). Liver transplantation affords the opportunity of efficacious salvage treatment for those with HBV and HIV coinfection that have progressed to end-stage hepatic disease. The most effective tool in the prevention of HBV infection is vaccination; however, the responsiveness to vaccination is lower in HIV-infected children (212).
Several challenges to the elimination of HBV-HIV coinfection remain, particularly regarding diagnosis and access to treatment of HBV infection in regions where HIV treatment is selectively supported. In many regions, pregnant women are routinely screened for HIV but not HBV (213). Systematic surveillance is required in regions of endemicity (214). Low-cost generic medications have been crucial in expanding programs for the treatment of HIV infection, but their use requires extension to the treatment of HBV infection.
The commonalities between HIV and HBV infection and coinfection lend these diseases to a common strategic policy for elimination (215, 216). Public awareness to increase the public’s understanding of HBV infection as well as HIV infection is growing but is still behind the curve necessary for elimination (217).
Hepatitis B and C Virus Coinfection
Epidemiology.
Because of potentially shared modes of transmission, coinfection with HBV and HCV is common in areas of high endemicity and for individuals with high-risk behaviors and exposure to parenteral methods of transmission. HBV-HCV coinfection is not carefully documented throughout the world and may be understated if occult HBV infection is included (218, 219). The role of each virus in the progression of liver disease is not fully understood at this point.
There is, however, a considerable burden of coinfection in certain groups; coinfection is encountered among people living with AIDS (220–222). As the main routes for the spread of HIV, HBV, and HCV are similar, HBV and HCV infections are prevalent in cohorts positive for HIV (223). In a pooled prevalence study in India, the prevalence of coinfection with HBV and HCV ranged from 0.02% to 3.2%, especially among patients with chronic liver disease, HIV-positive patients, and subjects who injected drugs or had chronic renal disease (224).
Anti-HBc-positive individuals and several high-risk groups (prisoners, intravenous drug users, and people living in regions where HBV is endemic) were reported to have a heightened prevalence of HCV coinfection (225, 226). The prevalence differs in different regions and among HIV-positive cohorts. The TREAT Asia HIV Observational Database (TAHOD), a multicenter cohort of patients with HIV in the Asia-Pacific region, found an approximately 10% prevalence of HBV and HCV coinfection. Elevated alanine aminotransferase levels were found in individuals infected with both HBV and HCV. HIV-positive subjects in Rwanda were very commonly found to be infected with HBV and HCV (227). Coinfections with HIV-HBV, HIV-HCV, and HIV-HBV-HCV were noted in a reference center in southern Brazil at rates of 3.10%, 3.10%, and 0.16%, respectively (228).
Viral interference.
In the interaction between HCV and HBV, HCV is considered the more dominant infection, while HBV replication is inhibited, but this is not always the case. Some studies have shown that HCV is the major cause of active hepatitis in HBV- and HCV-coinfected patients (229). A U.S. study with matched analysis showed that HBV is usually the dominant virus in Asians, while HCV is dominant among non-Asians (230).
Past analyses of long-term outcomes confirm that patterns with viral coinfection may have dynamic profiles over time. Cells with replicating HBV may be infected with HCV, which is evidence against exclusion in superinfection. Also, replicating HBV does not interfere with the production of infectious HCV in cells (231). Genotype 1 HCV strains may have greater inhibitory activity on HBV than genotype 2 HCV strains (232). Chronic inflammatory activity is usually driven by HCV in patients with HCV-HBV coinfection, and the HBV DNA level may be either low or fluctuating. Despite an apparent reciprocal inhibition in plasma, HBV DNA and HCV RNA can be found to coexist in liver tissue, a condition which may explain reactivation in coinfected patients (233, 234).
Natural history and comorbidity.
Acute hepatitis B and C virus coinfection is relatively rare; the kinetics of viremia may be affected. Superinfection and coinfection have been reported and may affect the outcome (235). Coinfection has been observed in patients with acute liver failure, and there are reports of nosocomial transmission.
Chen et al. reported coexisting acute HBV and HCV infections, showing a simultaneous elevation of ALT and bilirubin levels and subsequent continuing HCV infection with HBsAg clearance (236).
It has been suggested that the disease progression is enhanced in coinfected patients. Patients coinfected with HBV and HCV tend to have more serious liver injury and to have a greater likelihood of becoming cirrhotic and developing decompensated cirrhosis and HCC than monoinfected patients (237). Sagnelli et al. documented liver biopsy findings in 142 consecutive subjects with viral chronic hepatitis; those coinfected with HBV and HCV demonstrated a significantly higher fibrosis score. The 27 patients with detectable HBsAg and anti-HCV, despite having undetectable serum HCV RNA, showed histological activity indexes and fibrosis scores significantly higher than those found in the 17 HCV RNA-positive patients (238). Coinfection with any combination of HBV, HCV, or HDV has similarly been associated with a higher total Scheuer score, histological portal and lobular inflammation, and fibrosis (239). The level of regeneration of hepatocytes and the degree of inflammatory cell infiltration were significantly greater in HBV DNA-positive patients than in HBV DNA-negative patients.
A multivariate cause-specific proportional hazards model in Scotland indicated the risk of decompensated cirrhosis was increased in those who had previously been infected by HBV (HBsAg-negative but anti-HBc-positive individuals) (240). Patients identified with HBV coinfection (positive for HBsAg, HBV DNA, or HBeAg) were identified by the National Veterans Affairs HCV Clinical Case Registry. Of 99,548 HCV-infected patients, 1.4% had coinfection with HBV. Patients with HBV coinfection and detectable HBV DNA had a significantly greater chance of cirrhosis, HCC, and death (36.8, 6.9, and 41.7 per 1,000 person-years, respectively) than those infected only with HCV (17.4, 3.6, and 31.4 per 1,000 person-years, respectively) (241).
There may be a risk of undetected HBV infection, especially in HIV- and HCV-coinfected patients, although the clinical impact is uncertain (242). The chance of developing HCC was found to be greater in HBV DNA-positive patients than in HBV DNA-negative patients (243). The clinical outcomes of HIV coinfection with HCV and HBV may differ (244). In both Chinese and African populations and Western populations, HBV infection, HCV infection, or HBV-HCV coinfection may significantly increase the risk of developing HCC (245–249). There are no hard data on the exact way in which tumors develop, although researchers have posited both direct and indirect roles for HBV and HCV.
Antiviral treatment of HCV and reactivation of HBV.
There have been instances of HBV reactivation in patients coinfected with HCV and HBV during both interferon-based and interferon-free DAA therapies, indicating the necessity of close monitoring of HBV coinfection, including infection that is either chronic, occult, or resolved (250). The presence of coinfection with HBV does not, however, appear to impact sustained virological response rates with DAA therapy but may play a role in reactivation (251). There appears to be a very slight risk of reactivation in anti-HBc-seropositive patients, which probably does not warrant prophylactic treatment (252).
Several systematic reviews have been conducted. Chen et al. compared the rate of reactivation of HBV in HCV-infected patients with HBsAg with that in patients with occult HBV infection (HBsAg-negative and HBV DNA-positive individuals) (253). The pooled rate of reactivation of HBV reactivation with obvious HBV infection rather than occult HBV infection was similar among patients who had IFN-based therapy, but reactivation occurred much earlier in those treated with DAAs. CHC patients with occult HBV infection had HBV reactivation, but much less frequently (253).
A study of 62,290 hepatitis C virus-infected veterans identified and characterized HBV reactivation among those who completed DAA treatment. HBV reactivation was characterized by a >1,000-IU/ml increase in the levels of HBV DNA or HBsAg detected in a person who was previously negative. Since this was a retrospective study, the investigators chose only patients who were considered to be at high risk for HBV reactivation (defined as baseline results for HBV infection showing HBsAg positivity and anti-HBc positivity prior to DAA initiation) to be evaluated to better control for normal fluctuations in HBV levels. In total, 9 of the 62,290 veterans demonstrated signs of HBV reactivation during treatment with DAAs. Eight of the cases occurred in HBsAg-positive patients (254).
Little is understood about the specific viral characteristics that facilitate reactivation, as the functional characterization of the reactivated HBV has been conducted only rarely (255). The U.S. FDA reviewed cases that also suggested that further investigation would possibly determine triggers for reactivation and refine recommendations for monitoring but concluded that DAAs continue to be safe and effective therapies for infection with HCV (256).
In one such study, among 14,695 HCV patients, 10,551 were tested for HBsAg and/or HBV DNA. One-hundred fifteen (1.1%) had a positive test result, while 3,836 (27.2%) had received DAA therapy. Only 4.0% of the quarter of the cohort of patients who received DAA therapy were identified as having a single raised ALT level during the 4 to 52 weeks after the start of therapy; no cases of DAA-associated reactivation were detected, but in this analysis, posttreatment HBsAg or HBV DNA testing was rarely performed (257).
A study pooling data from 17 observational investigations involving 1,621 patients with chronic (n = 242) or resolved (n = 1,379) HCV infection treated with different DAAs reported a reactivation rate of 24% in patients with chronic hepatitis B (HBsAg positive) versus a reactivation rate of 1.4% in those who had recovered from HBV infection (HBsAg-negative, anti-HBc-positive patients). HBV reactivation may also be more common in HCV-infected patients undergoing DAA therapy than in patients undergoing IFN-based therapy (258). As a result, nucleoside analogue prophylaxis is warranted in patients who are HBsAg positive, particularly patients with HBV DNA that is quantifiable, and persons who have recovered from HBV infection should be monitored (259–262). An updated and comprehensive review by Loomba et al. summarizes HBV reactivation and nucleoside analogue prophylaxis in other settings of immunosuppression for HBsAg-positive patients and indicated that nucleoside analogue prophylaxis is generally indicated for isolated anti-HBc-positive patients mainly in the setting of stem cell transplantation or anti-CD20 therapies (263).
The long-term effects of hepatitis coinfection (HBV, HCV, or both) on HIV infection or deaths from hepatitis require monitoring, but fortunately, coinfection is readily treatable (264). Anti-HCV-positive individuals should receive vaccinations against hepatitis A and B.
CONCLUSIONS
In summary, an effective and safe vaccine is available to prevent HBV transmission. For those already infected, effective and safe antiviral therapies are also available. Persons infected with HBV as well as HDV, HCV, and/or HIV have a higher risk for progression of disease and complications and must have rigorous surveillance and treatment. Finally, to realize the goal of HBV elimination, improved linkage to care is needed, at-risk populations need to be vaccinated, infected populations need to be diagnosed and treated, and curative therapy with a finite treatment duration is needed. We suggest an algorithm for screening, diagnosis, and linkage to care that starts with universal screening of persons residing or coming from areas with a prevalence of HBV infection of 2% or higher; evaluation of infected persons by at least an assessment of liver enzyme, HBeAg, and HBV DNA levels; and, according to local guidelines and resources, the administration of antiviral therapies for persons at risk for disease progression (Fig. 2) (3).
ACKNOWLEDGMENTS
This work received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
We acknowledge the following members of the Mindie H. Nguyen lab at Stanford University: An Le for her assistance with our EndNote reference management and Karen Ely and Linda Henry for their editorial assistance. We thank Patrick Lane of ScEYEnce Studios for his assistance with the preparation of our figures.
We have the following conflicts of interest. Mindie H. Nguyen receives research support from Pfizer, Gilead Sciences, Janssen, the National Cancer Institute, and the B. K. Kee Foundation and is a consultant for or on the advisory boards of Anylam, Janssen, Intercept, Spring Banks, Bayer, Gilead, Dynavax, Eisai, Exact Sciences, and Novartis. Grace Wong receives research support from AbbVie and Gilead Sciences, is an advisory board member or consultant for Gilead Sciences and Janssen, and is a speaker for Abbott, Bristol-Myers Squibb, Echosens, Furui, Gilead Sciences, Janssen, and Roche. Edward Gane is a consultant for or on the advisory board of Abbvie, Arrowhead, Assembly, Gilead Sciences, Janssen, Roche, and VIR and is a speaker for Abbvie and Gilead Sciences. Jia-Horng Kao is a consultant for or on the advisory board of Abbvie, BMS, Gilead Sciences, and MSD and a speaker for Abbvie, Ascletis, BMS, Gilead Sciences, and MSD. Geoffrey Dusheiko is a consultant for or on the advisory board of Gilead Sciences, AbbVie, Abbott, BMS, Janssen, Merck, and Shionogi.
Biographies

Mindie H. Nguyen is a Professor of Medicine in the Division of Gastroenterology and Hepatology, Stanford Global Health Faculty, and a Fellow and Director for the Hepatology Fellowship and Clerkship at Stanford University, Stanford, CA, USA. After gaining her medical degree at the University of California, San Diego (UCSD), Dr. Nguyen trained in internal medicine at the UCSD Medical Center, where she was Chief Resident. Subsequently, she became Fellow and Chief Fellow in the Division of Gastroenterology and Hepatology at Stanford University before joining the faculty there in 2002. She obtained her master’s in Advanced Studies in Clinical Research at the University of California, San Francisco. Her main fields of scientific and clinical interest have been the epidemiology and management of chronic viral hepatitis, nonalcoholic fatty liver disease, and liver cancer for over 15 years. She is Chair of the American Association for the Study of Liver Diseases (AASLD) Hepatitis B Special Interest Group and a member of the Governing Council of the International Association for the Study of Liver Diseases (IASL). She has also served as an editorial board member for several journals, including The Lancet Gastroenterology and Hepatology, Gastroenterology, and Hepatology.

Grace Wong is a Professor at The Chinese University of Hong Kong. She has been in the field of hepatology research for 15 years. Her main research interest includes noninvasive assessment of liver fibrosis and chronic viral hepatitis and risk prediction for hepatocellular carcinoma. She has published over 250 articles in peer-reviewed journals, including Gastroenterology, Hepatology, and Gut. She is currently the Editor-in-Chief of Hepatology (Hong Kong edition) and the Associate Editor of Alimentary Pharmacology & Therapeutics. She was awarded the Young Investigator Award of the Asian Pacific Association for the Study of the Liver in 2009; the Distinguished Research Paper Award for Young Investigators of the Hong Kong College of Physicians in 2010, 2013, 2014, and 2015; the Ten Outstanding Young Persons (TOYP) of Hong Kong in 2014; the Sir David Todd Lectureship of the Hong Kong College of Physicians in 2016; and the JGHF Emerging Leader Lectureship in 2017.

Edward Gane is Professor of Medicine at the University of Auckland, Auckland, New Zealand, and Hepatologist and Deputy Director of the New Zealand Liver Unit at Auckland City Hospital. Dr. Gane trained in hepatology at the Institute of Liver Studies, King’s College School of Medicine, London, United Kingdom, where he completed his M.D. on the pathogenesis of hepatitis C-related liver injury. In 1998, Dr. Gane was appointed as Chief Physician for the first New Zealand Liver Unit at Auckland City Hospital, which provides a national transplant and HCC program. Dr. Gane helped set up the community-based national HBV Surveillance Programme, which is the largest in the world. He now chairs the Ministry of Health committee responsible for HCV elimination. Dr. Gane is an investigator for many international clinical trials, with particular interest in early-phase development of new direct-acting antiviral therapies against chronic hepatitis C and hepatitis B. He has published almost 300 papers in peer-reviewed journals, including The Lancet and The New England Journal of Medicine. In 2011, Dr. Gane was awarded Member of the Order of New Zealand for Services to Medicine and in 2017 was New Zealand Innovator of the Year for his work toward HCV elimination in New Zealand.

Jia-Horng Kao is the National Chair Professor of the Ministry of Education and Distinguished Professor at the Graduate Institute of Clinical Medicine, National Taiwan University College of Medicine. He serves as the President of the Taiwan Association for the Study of the Liver (TASL) and Advancing Clinical Treatment—Liver Disease (ACT-LD) and Chief of the Division of Hepatology/Gastroenterology at the National Taiwan University Hospital. Professor Kao’s main research interests include the prevention, natural history, molecular virology, pathogenesis, and treatment of chronic viral hepatitis and HCC. He has published more than 520 articles, with the sum of times that his articles have been cited being 18,296 and with an h-index of 63 by the Web of Science. He also contributed to 9 book chapters and edited a monograph on hepatitis B. Professor Kao is the Editor-in-Chief of the Journal of the Formosan Medical Association (JFMA) and sits on the editorial board of Gut, Alimentary Pharmacology & Therapeutics (APT), and the Journal of Infectious Diseases (JID). He also serves as the trustee of the JGH Foundation and is the founding member of AASLD Asia Pacific Regional Advisory Council.

Geoffrey Dusheiko is an Emeritus Professor of Medicine at the Royal Free Hospital and University College London School of Medicine as well as a Consultant Hepatologist at King’s College Hospital, London, United Kingdom. After graduating from the University of the Witwatersrand, he completed his residency at Johannesburg Hospital. His fellowships were conducted at the Johannesburg Hospital Liver Unit, the National Institutes of Health (MD, USA), and the University of Minnesota (MN, USA). Dr. Dusheiko’s research interests include the management and treatment of HCV, HBV, and small hepatocellular carcinoma. He has a special interest in research on viral hepatitis, particularly viral genotyping, applied molecular virology, the natural history of chronic viral hepatitis, and antiviral therapies. He served as educational councilor on the Governing Board of EASL for 4 years and was the recipient of the EASL Recognition Award in 2014. In addition to being on the editorial board of the Journal of Viral Eradication, he is Coeditor of Alimentary Pharmacology & Therapeutics, and he has previously served on the editorial boards for the Journal of Viral Hepatitis, Hepatology, Best Practice & Research: Clinical Gastroenterology, and Gut, among others. Professor Dusheiko has authored or coauthored more than 400 articles published in international peer-reviewed journals or books.
REFERENCES
- 1.Kao JH, Chen DS. 2002. Global control of hepatitis B virus infection. Lancet Infect Dis 2:395–403. doi: 10.1016/s1473-3099(02)00315-8. [DOI] [PubMed] [Google Scholar]
- 2.Polaris Observatory Collaborators. 2018. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 3:383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 3.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. 2015. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 4.Le MH, Yeo YH, Cheung R, Henry L, Lok AS, Nguyen MH. 22 June 2019. Chronic hepatitis B prevalence among foreign-born and US-born adults in the United States, 1999-2019. Hepatology doi: 10.1002/hep.30831. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell T, Armstrong GL, Hu DJ, Wasley A, Painter JA. 2011. The increasing burden of imported chronic hepatitis B—United States, 1974–2008. PLoS One 6:e27717. doi: 10.1371/journal.pone.0027717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen MH, Lim JK, Burak Ozbay A, Fraysse J, Liou I, Meyer N, Dusheiko G, Gordon SC. 2019. Advancing age and comorbidity in a US insured population-based cohort of patients with chronic hepatitis B. Hepatology 69:959–973. doi: 10.1002/hep.30246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu A, Le A, Zhang J, Wong C, Wong C, Henry L, Nguyen MH. 2018. Increasing co-morbidities in chronic hepatitis B patients: experience in primary care and referral practices during 2000–2015. Clin Transl Gastroenterol 9:141. doi: 10.1038/s41424-018-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen MH, Burak Ozbay A, Liou I, Meyer N, Gordon SC, Dusheiko G, Lim JK. 2019. Healthcare resource utilization and costs by disease severity in an insured national sample of US patients with chronic hepatitis B. J Hepatol 70:24–32. doi: 10.1016/j.jhep.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Lin CL, Kao JH. 2017. Natural history of acute and chronic hepatitis B: the role of HBV genotypes and mutants. Best Pract Res Clin Gastroenterol 31:249–255. doi: 10.1016/j.bpg.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. 2016. Global health sector strategy on viral hepatitis, 2016-2021, p 56 World Health Organization, Geneva, Switzerland. [Google Scholar]
- 11.Kao JH. 2015. Hepatitis B vaccination and prevention of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol 29:907–917. doi: 10.1016/j.bpg.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Chang MH, You SL, Chen CJ, Liu CJ, Lai MW, Wu TC, Wu SF, Lee CM, Yang SS, Chu HC, Wang TE, Chen BW, Chuang WL, Soon MS, Lin CY, Chiou ST, Kuo HS, Chen DS, Taiwan Hepatoma Study Group. 2016. Long-term effects of hepatitis B immunization of infants in preventing liver cancer. Gastroenterology 151:472–480.e1. doi: 10.1053/j.gastro.2016.05.048. [DOI] [PubMed] [Google Scholar]
- 13.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J, Cirrhosis Asian Lamivudine Multicentre Study Group. 2004. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 14.Wen WH, Lai MW, Chang MH. 2016. A review of strategies to prevent mother-to-infant transmission of hepatitis B virus infection. Expert Rev Gastroenterol Hepatol 10:317–330. doi: 10.1586/17474124.2016.1120667. [DOI] [PubMed] [Google Scholar]
- 15.Pan CQ, Duan Z, Dai E, Zhang S, Han G, Wang Y, Zhang H, Zou H, Zhu B, Zhao W, Jiang H, China Study Group for the Mother-to-Child Transmission of Hepatitis B. 2016. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med 374:2324–2334. doi: 10.1056/NEJMoa1508660. [DOI] [PubMed] [Google Scholar]
- 16.Zou H, Chen Y, Duan Z, Zhang H, Pan C. 2012. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat 19:e18–e25. doi: 10.1111/j.1365-2893.2011.01492.x. [DOI] [PubMed] [Google Scholar]
- 17.European Association for the Study of the Liver. 2017. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 67:370–398. doi: 10.1016/j.jhep.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell AE, Colvin HM, Palmer Beasley R. 2010. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology 51:729–733. doi: 10.1002/hep.23561. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Department of Health and Human Services. 2011. Combating the silent epidemic of viral hepatitis: action plan for the prevention, care, and treatment of viral hepatitis services. U.S. Department of Health and Human Services, Washington, DC. [Google Scholar]
- 20.Kallman JB, Arsalla A, Park V, Dhungel S, Bhatia P, Haddad D, Wheeler A, Younossi ZM. 2009. Screening for hepatitis B, C and non-alcoholic fatty liver disease: a survey of community-based physicians. Aliment Pharmacol Ther 29:1019–1024. doi: 10.1111/j.1365-2036.2009.03961.x. [DOI] [PubMed] [Google Scholar]
- 21.Foster T, Hon H, Kanwal F, Han S, Spiegel B. 2011. Screening high risk individuals for hepatitis B: physician knowledge, attitudes, and beliefs. Dig Dis Sci 56:3471–3487. doi: 10.1007/s10620-011-1928-z. [DOI] [PubMed] [Google Scholar]
- 22.Ku KC, Li J, Ha NB, Martin M, Nguyen VG, Nguyen MH. 2013. Chronic hepatitis B management based on standard guidelines in community primary care and specialty clinics. Dig Dis Sci 58:3626–3633. doi: 10.1007/s10620-013-2889-1. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen NH, Nguyen V, Trinh HN, Lin B, Nguyen MH. 2013. Treatment eligibility of patients with chronic hepatitis B initially ineligible for therapy. Clin Gastroenterol Hepatol 11:565–571. doi: 10.1016/j.cgh.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 24.Uribe LA, Nguyen N, Kim L, Trinh HN, Wong C, Wong C, Nguyen LH, Nguyen MH. 2016. Rates of treatment eligibility in follow-up of patients with chronic hepatitis B (CHB) across various clinical settings who were initially ineligible at presentation. Dig Dis Sci 61:618–625. doi: 10.1007/s10620-015-3982-4. [DOI] [PubMed] [Google Scholar]
- 25.Shankar H, Blanas D, Bichoupan K, Ndiaye D, Carmody E, Martel-Laferriere V, Culpepper-Morgan J, Dieterich DT, Branch AD, Bekele M, Nichols K, Perumalswami PV. 2016. A novel collaborative community-based hepatitis B screening and linkage-to-care program for African immigrants. Clin Infect Dis 62(Suppl 4):S289–S297. doi: 10.1093/cid/ciw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu JJ, Tien C, Chang M, Rhee J, Tien A, Bae HS, Ho FC, Chan LS, Fong TL. 2013. Demographic and serological characteristics of Asian Americans with hepatitis B infection diagnosed at community screenings. J Viral Hepat 20:575–581. doi: 10.1111/jvh.12073. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Ristau JT, Trinh HN, Garcia RT, Nguyen HA, Nguyen MH. 2012. Undertreatment of Asian chronic hepatitis B patients on the basis of standard guidelines: a community-based study. Dig Dis Sci 57:1373–1383. doi: 10.1007/s10620-012-2137-0. [DOI] [PubMed] [Google Scholar]
- 28.Kim LH, Nguyen VG, Trinh HN, Li J, Zhang JQ, Nguyen MH. 2014. Low treatment rates in patients meeting guideline criteria in diverse practice settings. Dig Dis Sci 59:2091–2099. doi: 10.1007/s10620-014-3283-3. [DOI] [PubMed] [Google Scholar]
- 29.Ha NB, Ha NB, Garcia RT, Trinh HN, Chaung KT, Nguyen HA, Nguyen KK, Levitt BS, Nguyen MH. 2011. Medication nonadherence with long-term management of patients with hepatitis B e antigen-negative chronic hepatitis B. Dig Dis Sci 56:2423–2431. doi: 10.1007/s10620-011-1610-5. [DOI] [PubMed] [Google Scholar]
- 30.Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH, American Association for the Study of Liver Diseases. 2016. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 63:261–283. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. 2016. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan HL, Wong VW, Wong GL, Tse CH, Chan HY, Sung JJ. 2010. A longitudinal study on the natural history of serum hepatitis B surface antigen changes in chronic hepatitis B. Hepatology 52:1232–1241. doi: 10.1002/hep.23803. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen T, Thompson AJ, Bowden S, Croagh C, Bell S, Desmond PV, Levy M, Locarnini SA. 2010. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J Hepatol 52:508–513. doi: 10.1016/j.jhep.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Jaroszewicz J, Calle Serrano B, Wursthorn K, Deterding K, Schlue J, Raupach R, Flisiak R, Bock CT, Manns MP, Wedemeyer H, Cornberg M. 2010. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol 52:514–522. doi: 10.1016/j.jhep.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Wooddell CI, Yuen MF, Chan HL, Gish RG, Locarnini SA, Chavez D, Ferrari C, Given BD, Hamilton J, Kanner SB, Lai CL, Lau JYN, Schluep T, Xu Z, Lanford RE, Lewis DL. 2017. RNAi-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci Transl Med 9:eaan0241. doi: 10.1126/scitranslmed.aan0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan HL, Wong GL, Tse CH, Chan HY, Wong VW. 2011. Viral determinants of hepatitis B surface antigen seroclearance in hepatitis B e antigen-negative chronic hepatitis B patients. J Infect Dis 204:408–414. doi: 10.1093/infdis/jir283. [DOI] [PubMed] [Google Scholar]
- 37.Lee MH, Yang HI, Liu J, Batrla-Utermann R, Jen CL, Iloeje UH, Lu SN, You SL, Wang LY, Chen CJ, R.E.V.E.A.L.-HBV Study Group. 2013. Prediction models of long-term cirrhosis, and hepatocellular carcinoma risk in chronic hepatitis B patients: risk scores integrating host and virus profiles. Hepatology 58:546–554. doi: 10.1002/hep.26385. [DOI] [PubMed] [Google Scholar]
- 38.Chan HL, Wong VW, Tse AM, Tse CH, Chim AM, Chan HY, Wong GL, Sung JJ. 2007. Serum hepatitis B surface antigen quantitation can reflect hepatitis B virus in the liver and predict treatment response. Clin Gastroenterol Hepatol 5:1462–1468. doi: 10.1016/j.cgh.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Sonneveld MJ, Hansen BE, Piratvisuth T, Jia JD, Zeuzem S, Gane E, Liaw YF, Xie Q, Heathcote EJ, Chan HL, Janssen HL. 2013. Response-guided peginterferon therapy in hepatitis B e antigen-positive chronic hepatitis B using serum hepatitis B surface antigen levels. Hepatology 58:872–880. doi: 10.1002/hep.26436. [DOI] [PubMed] [Google Scholar]
- 40.Rijckborst V, Hansen BE, Ferenci P, Brunetto MR, Tabak F, Cakaloglu Y, Lanza AG, Messina V, Iannacone C, Massetto B, Regep L, Colombo M, Janssen HL, Lampertico P. 2012. Validation of a stopping rule at week 12 using HBsAg and HBV DNA for HBeAg-negative patients treated with peginterferon alfa-2a. J Hepatol 56:1006–1011. doi: 10.1016/j.jhep.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Chan HL, Chan CK, Hui AJ, Chan S, Poordad F, Chang TT, Mathurin P, Flaherty JF, Lin L, Corsa A, Gaggar A, Subramanian GM, McHutchison JG, Lau G, Lee S, Gane EJ. 2014. Effects of tenofovir disoproxil fumarate in hepatitis B e antigen-positive patients with normal levels of alanine aminotransferase and high levels of hepatitis B virus DNA. Gastroenterology 146:1240–1248. doi: 10.1053/j.gastro.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 42.Chan HL, Wong GL, Chim AM, Chan HY, Chu SH, Wong VW. 2011. Prediction of off-treatment response to lamivudine by serum hepatitis B surface antigen quantification in hepatitis B e antigen-negative patients. Antivir Ther 16:1249–1257. doi: 10.3851/IMP1921. [DOI] [PubMed] [Google Scholar]
- 43.Chen CH, Lu SN, Hung CH, Wang JH, Hu TH, Changchien CS, Lee CM. 2014. The role of hepatitis B surface antigen quantification in predicting HBsAg loss and HBV relapse after discontinuation of lamivudine treatment. J Hepatol 61:515–522. doi: 10.1016/j.jhep.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 44.Trepo C, Chan HL, Lok A. 2014. Hepatitis B virus infection. Lancet 384:2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 45.Funk ML, Rosenberg DM, Lok AS. 2002. World-wide epidemiology of HBeAg-negative chronic hepatitis B and associated precore and core promoter variants. J Viral Hepat 9:52–61. doi: 10.1046/j.1365-2893.2002.00304.x. [DOI] [PubMed] [Google Scholar]
- 46.Fried MW, Piratvisuth T, Lau GK, Marcellin P, Chow WC, Cooksley G, Luo KX, Paik SW, Liaw YF, Button P, Popescu M. 2008. HBeAg and hepatitis B virus DNA as outcome predictors during therapy with peginterferon alfa-2a for HBeAg-positive chronic hepatitis B. Hepatology 47:428–434. doi: 10.1002/hep.22065. [DOI] [PubMed] [Google Scholar]
- 47.Wong VW, Chan HL. 2009. Severe acute exacerbation of chronic hepatitis B: a unique presentation of a common disease. J Gastroenterol Hepatol 24:1179–1186. doi: 10.1111/j.1440-1746.2009.05924.x. [DOI] [PubMed] [Google Scholar]
- 48.Huang X, Hollinger FB. 2014. Occult hepatitis B virus infection and hepatocellular carcinoma: a systematic review. J Viral Hepat 21:153–162. doi: 10.1111/jvh.12222. [DOI] [PubMed] [Google Scholar]
- 49.Yeo W, Chan TC, Leung NW, Lam WY, Mo FK, Chu MT, Chan HL, Hui EP, Lei KI, Mok TS, Chan PK. 2009. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol 27:605–611. doi: 10.1200/JCO.2008.18.0182. [DOI] [PubMed] [Google Scholar]
- 50.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH, REVEAL-HBV Study Group. 2006. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 51.Wong VW, Chan SL, Mo F, Chan TC, Loong HH, Wong GL, Lui YY, Chan AT, Sung JJ, Yeo W, Chan HL, Mok TS. 2010. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol 28:1660–1665. doi: 10.1200/JCO.2009.26.2675. [DOI] [PubMed] [Google Scholar]
- 52.Wong GL, Chan HL, Mak CW, Lee SK, Ip ZM, Lam AT, Iu HW, Leung JM, Lai JW, Lo AO, Chan HY, Wong VW. 2013. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology 58:1537–1547. doi: 10.1002/hep.26301. [DOI] [PubMed] [Google Scholar]
- 53.Wong GL, Chan HL, Chan HY, Tse PC, Tse YK, Mak CW, Lee SK, Ip ZM, Lam AT, Iu HW, Leung JM, Wong VW. 2013. Accuracy of risk scores for patients with chronic hepatitis B receiving entecavir treatment. Gastroenterology 144:933–944. doi: 10.1053/j.gastro.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 54.Buster EH, Hansen BE, Lau GK, Piratvisuth T, Zeuzem S, Steyerberg EW, Janssen HL. 2009. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology 137:2002–2009. doi: 10.1053/j.gastro.2009.08.061. [DOI] [PubMed] [Google Scholar]
- 55.Honda M, Shirasaki T, Terashima T, Kawaguchi K, Nakamura M, Oishi N, Wang X, Shimakami T, Okada H, Arai K, Yamashita T, Sakai Y, Yamashita T, Mizukoshi E, Kaneko S. 2016. Hepatitis B virus (HBV) core-related antigen during nucleos(t)ide analog therapy is related to intra-hepatic HBV replication and development of hepatocellular carcinoma. J Infect Dis 213:1096–1106. doi: 10.1093/infdis/jiv572. [DOI] [PubMed] [Google Scholar]
- 56.Tada T, Kumada T, Toyoda H, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Kitabatake S, Yama T, Tanaka J. 2016. HBcrAg predicts hepatocellular carcinoma development: an analysis using time-dependent receiver operating characteristics. J Hepatol 65:48–56. doi: 10.1016/j.jhep.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 57.Caviglia GP, Abate ML, Noviello D, Olivero A, Rosso C, Troshina G, Ciancio A, Rizzetto M, Saracco GM, Smedile A. 2017. Hepatitis B core-related antigen kinetics in chronic hepatitis B virus genotype D-infected patients treated with nucleos(t)ide analogues or pegylated-interferon-alpha. Hepatol Res 47:747–754. doi: 10.1111/hepr.12811. [DOI] [PubMed] [Google Scholar]
- 58.Matsumoto A, Tanaka E, Minami M, Okanoue T, Yatsuhashi H, Nagaoka S, Suzuki F, Kobayashi M, Chayama K, Imamura M, Yotsuyanagi H, Nakaoka S, Maki N, Kawata S, Kumada H, Iino S, Kiyosawa K. 2007. Low serum level of hepatitis B core-related antigen indicates unlikely reactivation of hepatitis after cessation of lamivudine therapy. Hepatol Res 37:661–666. doi: 10.1111/j.1872-034X.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 59.Jung KS, Park JY, Chon YE, Kim HS, Kang W, Kim BK, Kim SU, Kim DY, Han KH, Ahn SH. 2016. Clinical outcomes and predictors for relapse after cessation of oral antiviral treatment in chronic hepatitis B patients. J Gastroenterol 51:830–839. doi: 10.1007/s00535-015-1153-1. [DOI] [PubMed] [Google Scholar]
- 60.Khuroo MS, Khuroo NS, Khuroo MS. 2014. Accuracy of rapid point-of-care diagnostic tests for hepatitis B surface antigen—a systematic review and meta-analysis. J Clin Exp Hepatol 4:226–240. doi: 10.1016/j.jceh.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Njai HF, Shimakawa Y, Sanneh B, Ferguson L, Ndow G, Mendy M, Sow A, Lo G, Toure-Kane C, Tanaka J, Taal M, D'alessandro U, Njie R, Thursz M, Lemoine M. 2015. Validation of rapid point-of-care (POC) tests for detection of hepatitis B surface antigen in field and laboratory settings in the Gambia, western Africa. J Clin Microbiol 53:1156–1163. doi: 10.1128/JCM.02980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin CL, Kao JH. 2015. Hepatitis B virus genotypes and variants. Cold Spring Harb Perspect Med 5:a021436. doi: 10.1101/cshperspect.a021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin CL, Kao JH. 2011. The clinical implications of hepatitis B virus genotype: recent advances. J Gastroenterol Hepatol 26(Suppl 1):123–130. doi: 10.1111/j.1440-1746.2010.06541.x. [DOI] [PubMed] [Google Scholar]
- 64.Wong GL, Chan HL. 2005. Molecular virology in chronic hepatitis B: genotypes. Hosp Med 66:13–16. doi: 10.12968/hmed.2005.66.1.17529. [DOI] [PubMed] [Google Scholar]
- 65.Kao JH, Chen PJ, Lai MY, Chen DS. 2000. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology 118:554–559. doi: 10.1016/s0016-5085(00)70261-7. [DOI] [PubMed] [Google Scholar]
- 66.Orito E, Ichida T, Sakugawa H, Sata M, Horiike N, Hino K, Okita K, Okanoue T, Iino S, Tanaka E, Suzuki K, Watanabe H, Hige S, Mizokami M. 2001. Geographic distribution of hepatitis B virus (HBV) genotype in patients with chronic HBV infection in Japan. Hepatology 34:590–594. doi: 10.1053/jhep.2001.27221. [DOI] [PubMed] [Google Scholar]
- 67.Chan HL. 2011. JGH Foundation emerging leadership lecture. Significance of hepatitis B virus genotypes and mutations in the development of hepatocellular carcinoma in Asia. J Gastroenterol Hepatol 26:8–12. doi: 10.1111/j.1440-1746.2010.06514.x. [DOI] [PubMed] [Google Scholar]
- 68.Lin CL, Kao JH. 2013. Hepatitis B viral factors and treatment responses in chronic hepatitis B. J Formos Med Assoc 112:302–311. doi: 10.1016/j.jfma.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 69.Chan HL, Wong GL, Tse CH, Chim AM, Yiu KK, Chan HY, Sung JJ, Wong VW. 2009. Hepatitis B virus genotype C is associated with more severe liver fibrosis than genotype B. Clin Gastroenterol Hepatol 7:1361–1366. doi: 10.1016/j.cgh.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 70.Wong GL, Chan HL, Yiu KK, Lai JW, Chan VK, Cheung KK, Wong EW, Wong VW. 2013. Meta-analysis: the association of hepatitis B virus genotypes and hepatocellular carcinoma. Aliment Pharmacol Ther 37:517–526. doi: 10.1111/apt.12207. [DOI] [PubMed] [Google Scholar]
- 71.Kao JH, Chen PJ, Lai MY, Chen DS. 2004. Hepatitis B virus genotypes and spontaneous hepatitis B e antigen seroconversion in Taiwanese hepatitis B carriers. J Med Virol 72:363–369. doi: 10.1002/jmv.10534. [DOI] [PubMed] [Google Scholar]
- 72.Chan HL, Hui AY, Wong ML, Tse AM, Hung LC, Wong VW, Sung JJ. 2004. Genotype C hepatitis B virus infection is associated with an increased risk of hepatocellular carcinoma. Gut 53:1494–1498. doi: 10.1136/gut.2003.033324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kao JH. 2003. Hepatitis B virus genotypes and hepatocellular carcinoma in Taiwan. Intervirology 46:400–407. doi: 10.1159/000074999. [DOI] [PubMed] [Google Scholar]
- 74.Kao JH, Chen PJ, Chen DS. 2010. Recent advances in the research of hepatitis B virus-related hepatocellular carcinoma: epidemiologic and molecular biological aspects. Adv Cancer Res 108:21–72. doi: 10.1016/B978-0-12-380888-2.00002-9. [DOI] [PubMed] [Google Scholar]
- 75.Gehring A, Bertoletti A, Tavis JE. 2014. Host factor-targeted hepatitis B virus therapies. Intervirology 57:158–162. doi: 10.1159/000360938. [DOI] [PubMed] [Google Scholar]
- 76.Sonneveld MJ, Wong VW, Woltman AM, Wong GL, Cakaloglu Y, Zeuzem S, Buster EH, Uitterlinden AG, Hansen BE, Chan HL, Janssen HL. 2012. Polymorphisms near IL28B and serologic response to peginterferon in HBeAg-positive patients with chronic hepatitis B. Gastroenterology 142:513–520.e1. doi: 10.1053/j.gastro.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 77.Tseng TC, Yu ML, Liu CJ, Lin CL, Huang YW, Hsu CS, Liu CH, Kuo SF, Pan CJ, Yang SS, Su CW, Chen PJ, Chen DS, Kao JH. 2011. Effect of host and viral factors on hepatitis B e antigen-positive chronic hepatitis B patients receiving pegylated interferon-alpha-2a therapy. Antivir Ther 16:629–637. doi: 10.3851/IMP1841. [DOI] [PubMed] [Google Scholar]
- 78.Xu Z, Liu Y, Liu L, Li X, Bai S, Rong Y, Wang H, Mao Y, Xin S, Xu D. 2013. Association of interferon-gamma induced protein 10 promoter polymorphisms with the disease progression of hepatitis B virus infection in Chinese Han population. PLoS One 8:e72799. doi: 10.1371/journal.pone.0072799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laras A, Koskinas J, Dimou E, Kostamena A, Hadziyannis SJ. 2006. Intrahepatic levels and replicative activity of covalently closed circular hepatitis B virus DNA in chronically infected patients. Hepatology 44:694–702. doi: 10.1002/hep.21299. [DOI] [PubMed] [Google Scholar]
- 80.van Bommel F, Bartens A, Mysickova A, Hofmann J, Kruger DH, Berg T, Edelmann A. 2015. Serum hepatitis B virus RNA levels as an early predictor of hepatitis B envelope antigen seroconversion during treatment with polymerase inhibitors. Hepatology 61:66–76. doi: 10.1002/hep.27381. [DOI] [PubMed] [Google Scholar]
- 81.Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, Vianello L, Zanuso F, Mozzi F, Milani S, Conte D, Colombo M, Sirchia G. 2002. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 82.Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, Chan HY, Chan FK, Sung JJ, Chan HL. 2008. Evaluation of alanine transaminase and hepatitis B virus DNA to predict liver cirrhosis in hepatitis B e antigen-negative chronic hepatitis B using transient elastography. Am J Gastroenterol 103:3071–3081. doi: 10.1111/j.1572-0241.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- 83.Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. 2004. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ 328:983. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. 2018. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goodman ZD. 2007. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol 47:598–607. doi: 10.1016/j.jhep.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 86.Amarapurkar D, Amarapurkar A. 2015. Indications of liver biopsy in the era of noninvasive assessment of liver fibrosis. J Clin Exp Hepatol 5:314–319. doi: 10.1016/j.jceh.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wong GL. 2013. Update of liver fibrosis and steatosis with transient elastography (Fibroscan). Gastroenterol Rep (Oxf) 1:19–26. doi: 10.1093/gastro/got007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barr RG, Ferraioli G, Palmeri ML, Goodman ZD, Garcia-Tsao G, Rubin J, Garra B, Myers RP, Wilson SR, Rubens D, Levine D. 2015. Elastography assessment of liver fibrosis: Society of Radiologists in Ultrasound Consensus Conference statement. Radiology 276:845–861. doi: 10.1148/radiol.2015150619. [DOI] [PubMed] [Google Scholar]
- 89.Wong GL, Wong VW, Chim AM, Yiu KK, Chu SH, Li MK, Chan HL. 2011. Factors associated with unreliable liver stiffness measurement and its failure with transient elastography in the Chinese population. J Gastroenterol Hepatol 26:300–305. doi: 10.1111/j.1440-1746.2010.06510.x. [DOI] [PubMed] [Google Scholar]
- 90.Wong VW-S, Vergniol J, Wong GL-H, Foucher J, Chan AW-H, Chermak F, Choi PC-L, Merrouche W, Chu SH-T, Pesque S, Chan HL-Y, de Lédinghen V. 2012. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol 107:1862–1871. doi: 10.1038/ajg.2012.331. [DOI] [PubMed] [Google Scholar]
- 91.Wagner M, Besa C, Bou Ayache J, Yasar TK, Bane O, Fung M, Ehman RL, Taouli B. 2016. Magnetic Resonance elastography of the liver: qualitative and quantitative comparison of gradient echo and spin echo echoplanar imaging sequences. Invest Radiol 51:575–581. doi: 10.1097/RLI.0000000000000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huwart L, van Beers BE. 2008. MR elastography. Gastroenterol Clin Biol 32:68–72. doi: 10.1016/S0399-8320(08)73995-2. [DOI] [PubMed] [Google Scholar]
- 93.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. 2003. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 94.Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. 2002. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 36:986–992. doi: 10.1053/jhep.2002.36128. [DOI] [PubMed] [Google Scholar]
- 95.Bonnard P, Sombie R, Lescure FX, Bougouma A, Guiard-Schmid JB, Poynard T, Cales P, Housset C, Callard P, Le Pendeven C, Drabo J, Carrat F, Pialoux G. 2010. Comparison of elastography, serum marker scores, and histology for the assessment of liver fibrosis in hepatitis B virus (HBV)-infected patients in Burkina Faso. Am J Trop Med Hyg 82:454–458. doi: 10.4269/ajtmh.2010.09-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T, MULTIVIRC Group. 2001. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 357:1069–1075. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 97.European Association for Study of Liver, Asociacion Latinoamericana para el Estudio del Higado. 2015. EASL-ALEH clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 63:237–264. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 98.AASLD HCV Guidance Panel. 2015. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 62:932–954. doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 99.Cales P, Boursier J, Oberti F, Hubert I, Gallois Y, Rousselet MC, Dib N, Moal V, Macchi L, Chevailler A, Michalak S, Hunault G, Chaigneau J, Sawadogo A, Lunel F. 2008. FibroMeters: a family of blood tests for liver fibrosis. Gastroenterol Clin Biol 32:40–51. doi: 10.1016/S0399-8320(08)73992-7. [DOI] [PubMed] [Google Scholar]
- 100.Wong GL, Chan HL, Choi PC, Chan AW, Yu Z, Lai JW, Chan HY, Wong VW. 2014. Non-invasive algorithm of enhanced liver fibrosis and liver stiffness measurement with transient elastography for advanced liver fibrosis in chronic hepatitis B. Aliment Pharmacol Ther 39:197–208. doi: 10.1111/apt.12559. [DOI] [PubMed] [Google Scholar]
- 101.Parkes J, Roderick P, Harris S, Day C, Mutimer D, Collier J, Lombard M, Alexander G, Ramage J, Dusheiko G, Wheatley M, Gough C, Burt A, Rosenberg W. 2010. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut 59:1245–1251. doi: 10.1136/gut.2009.203166. [DOI] [PubMed] [Google Scholar]
- 102.Wong GL, Wong VW, Choi PC, Chan AW, Chan HL. 2010. Development of a non-invasive algorithm with transient elastography (Fibroscan) and serum test formula for advanced liver fibrosis in chronic hepatitis B. Aliment Pharmacol Ther 31:1095–1103. doi: 10.1111/j.1365-2036.2010.04276.x. [DOI] [PubMed] [Google Scholar]
- 103.Marion PL, Salazar FH, Alexander JJ, Robinson WS. 1980. State of hepatitis B viral DNA in a human hepatoma cell line. J Virol 33:795–806. doi: 10.1128/JVI.33.2.795-806.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zlotnick A, Venkatakrishnan B, Tan Z, Lewellyn E, Turner W, Francis S. 2015. Core protein: a pleiotropic keystone in the HBV lifecycle. Antiviral Res 121:82–93. doi: 10.1016/j.antiviral.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bock CT, Schranz P, Schroder CH, Zentgraf H. 1994. Hepatitis B virus genome is organized into nucleosomes in the nucleus of the infected cell. Virus Genes 8:215–229. doi: 10.1007/bf01703079. [DOI] [PubMed] [Google Scholar]
- 106.Bock CT, Schwinn S, Locarnini S, Fyfe J, Manns MP, Trautwein C, Zentgraf H. 2001. Structural organization of the hepatitis B virus minichromosome. J Mol Biol 307:183–196. doi: 10.1006/jmbi.2000.4481. [DOI] [PubMed] [Google Scholar]
- 107.Belloni L, Pollicino T, De Nicola F, Guerrieri F, Raffa G, Fanciulli M, Raimondo G, Levrero M. 2009. Nuclear HBx binds the HBV minichromosome and modifies the epigenetic regulation of cccDNA function. Proc Natl Acad Sci U S A 106:19975–19979. doi: 10.1073/pnas.0908365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lucifora J, Arzberger S, Durantel D, Belloni L, Strubin M, Levrero M, Zoulim F, Hantz O, Protzer U. 2011. Hepatitis B virus X protein is essential to initiate and maintain virus replication after infection. J Hepatol 55:996–1003. doi: 10.1016/j.jhep.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 109.Le Duff Y, Blanchet M, Sureau C. 2009. The pre-S1 and antigenic loop infectivity determinants of the hepatitis B virus envelope proteins are functionally independent. J Virol 83:12443–12451. doi: 10.1128/JVI.01594-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schulze A, Gripon P, Urban S. 2007. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology 46:1759–1768. doi: 10.1002/hep.21896. [DOI] [PubMed] [Google Scholar]
- 111.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. 2012. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 1:e00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ni Y, Lempp FA, Mehrle S, Nkongolo S, Kaufman C, Falth M, Stindt J, Koniger C, Nassal M, Kubitz R, Sultmann H, Urban S. 2014. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology 146:1070–1083. doi: 10.1053/j.gastro.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 113.Urban S, Bartenschlager R, Kubitz R, Zoulim F. 2014. Strategies to inhibit entry of HBV and HDV into hepatocytes. Gastroenterology 147:48–64. doi: 10.1053/j.gastro.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 114.Beasley RP, Hwang LY, Lee GC, Lan CC, Roan CH, Huang FY, Chen CL. 1983. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet ii:1099–1102. doi: 10.1016/s0140-6736(83)90624-4. [DOI] [PubMed] [Google Scholar]
- 115.Beasley RP, Hwang LY, Lin CC, Leu ML, Stevens CE, Szmuness W, Chen KP. 1982. Incidence of hepatitis B virus infections in preschool children in Taiwan. J Infect Dis 146:198–204. doi: 10.1093/infdis/146.2.198. [DOI] [PubMed] [Google Scholar]
- 116.McMahon BJ, Alward WL, Hall DB, Heyward WL, Bender TR, Francis DP, Maynard JE. 1985. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis 151:599–603. doi: 10.1093/infdis/151.4.599. [DOI] [PubMed] [Google Scholar]
- 117.Fattovich G. 2003. Natural history and prognosis of hepatitis B. Semin Liver Dis 23:47–58. doi: 10.1055/s-2003-37590. [DOI] [PubMed] [Google Scholar]
- 118.Fattovich G, Giustina G, Realdi G, Corrocher R, Schalm SW. 1997. Long-term outcome of hepatitis B e antigen-positive patients with compensated cirrhosis treated with interferon alfa. European Concerted Action on Viral Hepatitis (EUROHEP). Hepatology 26:1338–1342. doi: 10.1002/hep.510260536. [DOI] [PubMed] [Google Scholar]
- 119.Fattovich G, Giustina G, Schalm SW, Hadziyannis S, Sanchez-Tapias J, Almasio P, Christensen E, Krogsgaard K, Degos F, Carneiro de Moura M. 1995. Occurrence of hepatocellular carcinoma and decompensation in western European patients with cirrhosis type B. Hepatology 21:77–82. doi: 10.1002/hep.1840210114. [DOI] [PubMed] [Google Scholar]
- 120.Fattovich G, Olivari N, Pasino M, D'Onofrio M, Martone E, Donato F. 2008. Long-term outcome of chronic hepatitis B in Caucasian patients: mortality after 25 years. Gut 57:84–90. doi: 10.1136/gut.2007.128496. [DOI] [PubMed] [Google Scholar]
- 121.Chen CF, Lee WC, Yang HI, Chang HC, Jen CL, Iloeje UH, Su J, Hsiao CK, Wang LY, You SL, Lu SN, Chen CJ, Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer in HBV (REVEAL–HBV) Study Group. 2011. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology 141:1240–1248.e2. doi: 10.1053/j.gastro.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 122.Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R, Bornstein JD, Kitrinos KM, Subramanian GM, McHutchison JG, Heathcote EJ. 2013. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 123.Martin P, Lau DT, Nguyen MH, Janssen HL, Dieterich DT, Peters MG, Jacobson IM. 2015. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2015 update. Clin Gastroenterol Hepatol 13:2071–2087.e16. doi: 10.1016/j.cgh.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 124.Dusheiko G, Lemoine M. 2019. An appraisal of the WHO hepatitis B treatment guidelines applicability to Africans. J Hepatol 70:1046–1048. doi: 10.1016/j.jhep.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 125.Aberra H, Desalegn H, Berhe N, Mekasha B, Medhin G, Gundersen SG, Johannessen A. 2019. The WHO guidelines for chronic hepatitis B fail to detect half of the patients in need of treatment in Ethiopia. J Hepatol 70:1065–1071. doi: 10.1016/j.jhep.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 126.Papatheodoridis G, Vlachogiannakos I, Cholongitas E, Wursthorn K, Thomadakis C, Touloumi G, Petersen J. 2016. Discontinuation of oral antivirals in chronic hepatitis B: a systematic review. Hepatology 63:1481–1492. doi: 10.1002/hep.28438. [DOI] [PubMed] [Google Scholar]
- 127.Jeng WJ, Sheen IS, Chen YC, Hsu CW, Chien RN, Chu CM, Liaw YF. 2013. Off-therapy durability of response to entecavir therapy in hepatitis B e antigen-negative chronic hepatitis B patients. Hepatology 58:1888–1896. doi: 10.1002/hep.26549. [DOI] [PubMed] [Google Scholar]
- 128.Liaw YF, Jia JD, Chan HL, Han KH, Tanwandee T, Chuang WL, Tan DM, Chen XY, Gane E, Piratvisuth T, Chen L, Xie Q, Sung JJ, Wat C, Bernaards C, Cui Y, Marcellin P. 2011. Shorter durations and lower doses of peginterferon alfa-2a are associated with inferior hepatitis B e antigen seroconversion rates in hepatitis B virus genotypes B or C. Hepatology 54:1591–1599. doi: 10.1002/hep.24555. [DOI] [PubMed] [Google Scholar]
- 129.Piratvisuth T, Marcellin P, Popescu M, Kapprell HP, Rothe V, Lu ZM. 2013. Hepatitis B surface antigen: association with sustained response to peginterferon alfa-2a in hepatitis B e antigen-positive patients. Hepatol Int 7:429–436. doi: 10.1007/s12072-011-9280-0. [DOI] [PubMed] [Google Scholar]
- 130.Chan HL, Ahn SH, Chuang WL, Hui AJ, Tabak F, Mehta R, Petersen J, Lee C-M, Ma X, Caruntu FA, Tak WY, Elkhashab M, Lin L, Dinh P, Martins EB, Charuworn P, McHutchinson JG, Subramanian GM, Lim SG, Foster GR, Fung S, Morano L, Samuel D, Agarwal K, Idilman R, Strasser S, Buti M, Gaeta GB, Papatheodoridis G, Flisiak R, Marcellin P. 2015. Predictors of clinical response: results from a large, randomized controlled study with tenofovir disoproxil fumarate (TDF) plus peginterferon alfa-2A (PEG) combination for chronic hepatitis B (CHB). J Hepatol 62:S251–S252. doi: 10.1016/S0168-8278(15)30136-7. [DOI] [Google Scholar]
- 131.Marcellin P, Ahn SH, Ma X, Caruntu FA, Tak WY, Elkashab M, Chuang WL, Lim SG, Tabak F, Mehta R, Petersen J, Foster GR, Lou L, Martins EB, Dinh P, Lin L, Corsa A, Charuworn P, Subramanian GM, Reiser H, Reesink HW, Fung S, Strasser SI, Trinh H, Buti M, Gaeta GB, Hui AJ, Papatheodoridis G, Flisiak R, Chan HL, Study 149 Investigators. 2016. Combination of tenofovir disoproxil fumarate and peginterferon alpha-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology 150:134–144.e10. doi: 10.1053/j.gastro.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 132.Okada M, Enomoto M, Kawada N, Nguyen MH. 2017. Effects of antiviral therapy in patients with chronic hepatitis B and cirrhosis. Expert Rev Gastroenterol Hepatol 11:1095–1104. doi: 10.1080/17474124.2017.1361822. [DOI] [PubMed] [Google Scholar]
- 133.Yeo YH, Ho HJ, Yang HI, Tseng TC, Hosaka T, Trinh HN, Kwak MS, Park YM, Fung JYY, Buti M, Rodriguez M, Treeprasertsuk S, Preda CM, Ungtrakul T, Charatcharoenwitthaya P, Li X, Li J, Zhang J, Le MH, Wei B, Zou B, Le A, Jeong D, Chien N, Kam L, Lee CC, Riveiro-Barciela M, Istratescu D, Sriprayoon T, Chong Y, Tanwandee T, Kobayashi M, Suzuki F, Yuen MF, Lee HS, Kao JH, Lok AS, Wu CY, Nguyen MH. 2019. Factors associated with rates of HBsAg seroclearance in adults with chronic HBV infection: a systematic review and meta-analysis. Gastroenterology 156:635–646.e9. doi: 10.1053/j.gastro.2018.10.027. [DOI] [PubMed] [Google Scholar]
- 134.Nguyen MH, Yang HI, Le A, Henry L, Nguyen N, Lee MH, Zhang J, Wong C, Wong C, Trinh H. 2019. Reduced incidence of hepatocellular carcinoma in cirrhotic and noncirrhotic patients with chronic hepatitis B treated with tenofovir—a propensity score-matched study. J Infect Dis 219:10–18. doi: 10.1093/infdis/jiy391. [DOI] [PubMed] [Google Scholar]
- 135.Dusheiko G. 2013. Treatment of HBeAg positive chronic hepatitis B: interferon or nucleoside analogues. Liver Int 33(Suppl 1):137–150. doi: 10.1111/liv.12078. [DOI] [PubMed] [Google Scholar]
- 136.Burdette D, Cathcart A, Shauf A, Win R, Zaboli S, Hedskog C, Svarovskaia ES, Barry V, Inzunza D, Flaherty JF, Gaggar A, Fletcher S, Chan H, Mo H, Lazerwith S, Feierbach B, Delaney B. 2019. PS-150—evidence for the presence of infectious virus in the serum from chronic hepatitis B patients suppressed on nucleos (t)ide therapy with detectable but not quantifiable HBV DNA. J Hepatol 70:e95. doi: 10.1016/S0618-8278(19)30168-9. [DOI] [Google Scholar]
- 137.Warner N, Locarnini S. 2008. The antiviral drug selected hepatitis B virus rtA181T/sW172* mutant has a dominant negative secretion defect and alters the typical profile of viral rebound. Hepatology 48:88–98. doi: 10.1002/hep.22295. [DOI] [PubMed] [Google Scholar]
- 138.Rehermann B, Nascimbeni M. 2005. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol 5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 139.Pol S, Nalpas B, Driss F, Michel ML, Tiollais P, Denis J, Brecho C, Multicenter Study Group. 2001. Efficacy and limitations of a specific immunotherapy in chronic hepatitis B. J Hepatol 34:917–921. doi: 10.1016/S0168-8278(01)00028-9. [DOI] [PubMed] [Google Scholar]
- 140.Vandepapelière P, Lau GKK, Leroux-Roels G, Horsmans Y, Gane E, Tawandee T, Merican MI, Win KM, Trepo C, Cooksley G, Wettendorff M, Ferrari C, Therapeutic HBV Vaccine Group of Investigators. 2007. Therapeutic vaccination of chronic hepatitis B patients with virus suppression by antiviral therapy: a randomized, controlled study of co-administration of HBsAg/AS02 candidate vaccine and lamivudine. Vaccine 25:8585–8597. doi: 10.1016/j.vaccine.2007.09.072. [DOI] [PubMed] [Google Scholar]
- 141.Gaggar A, Coeshott C, Apelian D, Rodell T, Armstrong BR, Shen G, Subramanian GM, McHutchison JG. 2014. Safety, tolerability and immunogenicity of GS-4774, a hepatitis B virus-specific therapeutic vaccine, in healthy subjects: a randomized study. Vaccine 32:4925–4931. doi: 10.1016/j.vaccine.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 142.Lok AS, Pan CQ, Han SH, Trinh HN, Fessel WJ, Rodell T, Massetto B, Lin L, Gaggar A, Subramanian GM, McHutchison JG, Ferrari C, Lee H, Gordon SC, Gane EJ. 2016. Randomized phase II study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. J Hepatol 65:509–516. doi: 10.1016/j.jhep.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 143.Niu C, Daffis S, Yu M, Cheng G, Delaney WE, Fletcher SP. 2014. Cytokines induced by a Toll-like receptor 7 agonist potently inhibit HBV RNA, DNA and antigen levels in primary human hepatocytes. Hepatology 60:1104A.24753069 [Google Scholar]
- 144.Menne S, Tennant BC, Liu KH, Ascenzi MA, Baldwin BH, Bellezza CA, Cote PJ, Zheng X, Wolfgang G, Tumas D. 2011. Anti-viral efficacy and induction of an antibody response against surface antigen with the TLR7 agonist GS-9620 in the woodchuck model of chronic HBV infection. J Hepatol 54:S363–S534. [Google Scholar]
- 145.Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, Fosdick A, Frey CR, Zheng J, Wolfgang G, Halcomb RL, Tumas DB. 2013. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology 144:1508–1517.e10. doi: 10.1053/j.gastro.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gane EJ, Lim YS, Gordon SC, Visvanathan K, Sicard E, Fedorak RN, Roberts S, Massetto B, Ye Z, Pflanz S, Garrison KL, Gaggar A, Subramanian GM, McHutchison JG, Kottilil S, Freilich B, Coffin CS, Cheng W, Kim YJ. 2015. The oral Toll-like receptor-7 agonist GS-9620 in patients with chronic hepatitis B virus infection. J Hepatol 63:320–328. doi: 10.1016/j.jhep.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 147.Agarwal K, Ahn SH, Elkashab M, Kim K, Lau AH, Gaggar A, Subramanian M, McHutchison JG, Andreone P, Kim HJ, Chuang WL, Nguyen MH. 2017. Safety and efficacy of vesatolimod (GS-9620) in patients with chronic hepatitis B (CHB) who are not currently on antiviral treatment. Hepatology 66:497A–498A. [DOI] [PubMed] [Google Scholar]
- 148.Schinazi RF, Ehteshami M, Bassit L, Asselah T. 2018. Towards HBV curative therapies. Liver Int 38(Suppl 1):102–114. doi: 10.1111/liv.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kumada T, Toyoda H, Tada T, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Niinomi T, Yasuda S, Andou Y, Yamamoto K, Tanaka J. 2013. Effect of nucleos(t)ide analogue therapy on hepatocarcinogenesis in chronic hepatitis B patients: a propensity score analysis. J Hepatol 58:427–433. doi: 10.1016/j.jhep.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 150.Thomsen MK, Nandakumar R, Stadler D, Malo A, Valls RM, Wang F, Reinert LS, Dagnaes-Hansen F, Hollensen AK, Mikkelsen JG, Protzer U, Paludan SR. 2016. Lack of immunological DNA sensing in hepatocytes facilitates hepatitis B virus infection. Hepatology 64:746–759. doi: 10.1002/hep.28685. [DOI] [PubMed] [Google Scholar]
- 151.He J, Hao R, Liu D, Liu X, Wu S, Guo S, Wang Y, Tien P, Guo D. 2016. Inhibition of hepatitis B virus replication by activation of the cGAS-STING pathway. J Gen Virol 97:3368–3378. doi: 10.1099/jgv.0.000647. [DOI] [PubMed] [Google Scholar]
- 152.Guo F, Tang L, Shu S, Sehgal M, Sheraz M, Liu B, Zhao Q, Cheng J, Zhao X, Zhou T, Chang J, Guo JT. 2017. Activation of STING in hepatocytes suppresses the replication of hepatitis B virus. Antimicrob Agents Chemother 61:e00771-17. doi: 10.1128/aac.00771-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guo F, Han Y, Zhao X, Wang J, Liu F, Xu C, Wei L, Jiang JD, Block TM, Guo JT, Chang J. 2015. STING agonists induce an innate antiviral immune response against hepatitis B virus. Antimicrob Agents Chemother 59:1273–1281. doi: 10.1128/AAC.04321-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chang J, Guo JT. 2015. Treatment of chronic hepatitis B with pattern recognition receptor agonists: current status and potential for a cure. Antiviral Res 121:152–159. doi: 10.1016/j.antiviral.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yu S, Chen J, Wu M, Chen H, Kato N, Yuan Z. 2010. Hepatitis B virus polymerase inhibits RIG-I- and Toll-like receptor 3-mediated beta interferon induction in human hepatocytes through interference with interferon regulatory factor 3 activation and dampening of the interaction between TBK1/IKKepsilon and DDX3. J Gen Virol 91:2080–2090. doi: 10.1099/vir.0.020552-0. [DOI] [PubMed] [Google Scholar]
- 156.Sato S, Li K, Kameyama T, Hayashi T, Ishida Y, Murakami S, Watanabe T, Iijima S, Sakurai Y, Watashi K, Tsutsumi S, Sato Y, Akita H, Wakita T, Rice CM, Harashima H, Kohara M, Tanaka Y, Takaoka A. 2015. The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity 42:123–132. doi: 10.1016/j.immuni.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 157.Korolowicz KE, Iyer RP, Czerwinski S, Suresh M, Yang J, Padmanabhan S, Sheri A, Pandey RK, Skell J, Marquis JK, Kallakury BV, Tucker RD, Menne S. 2016. Antiviral efficacy and host innate immunity associated with SB 9200 treatment in the woodchuck model of chronic hepatitis B. PLoS One 11:e0161313. doi: 10.1371/journal.pone.0161313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Crawford A, Wherry EJ. 2009. The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Curr Opin Immunol 21:179–186. doi: 10.1016/j.coi.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen J, Wang XM, Wu XJ, Wang Y, Zhao H, Shen B, Wang GQ. 2011. Intrahepatic levels of PD-1/PD-L correlate with liver inflammation in chronic hepatitis B. Inflamm Res 60:47–53. doi: 10.1007/s00011-010-0233-1. [DOI] [PubMed] [Google Scholar]
- 160.Liu J, Zhang E, Ma Z, Wu W, Kosinska A, Zhang X, Moller I, Seiz P, Glebe D, Wang B, Yang D, Lu M, Roggendorf M. 2014. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathog 10:e1003856. doi: 10.1371/journal.ppat.1003856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gane E, Gaggar A, Nguyen AH, Subramanian GM, McHutchison JG, Schwabe C, Dunbar R. 2017. A phase1 study evaluating anti-PD-1 treatment with or without GS-4774 in HBeAg negative chronic hepatitis B patients. J Hepatol 66:S26–S27. doi: 10.1016/S0168-8278(17)30315-X. [DOI] [Google Scholar]
- 162.Quinet J, Jamard C, Vaillant A, Cova L. 2016. Achievement of surface antigen clearance in the liver by combination therapy with REP 2139-CA and nucleoside analogues against chronic hepatitis B. J Hepatol 64:S385. doi: 10.1016/S0168-8278(16)00590-0. [DOI] [Google Scholar]
- 163.Yan H, Liu Y, Sui J, Li W. 2015. NTCP opens the door for hepatitis B virus infection. Antiviral Res 121:24–30. doi: 10.1016/j.antiviral.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 164.Klumpp K, Lam AM, Lukacs C, Vogel R, Ren S, Espiritu C, Baydo R, Atkins K, Abendroth J, Liao G, Efimov A, Hartman G, Flores OA. 2015. High-resolution crystal structure of a hepatitis B virus replication inhibitor bound to the viral core protein. Proc Natl Acad Sci U S A 112:15196–15201. doi: 10.1073/pnas.1513803112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Berke JM, Dehertogh P, Vergauwen K, Van Damme E, Mostmans W, Vandyck K, Pauwels F. 2017. Capsid assembly modulators have a dual mechanism of action in primary human hepatocytes infected with hepatitis B virus. Antimicrob Agents Chemother 61:e00560-17. doi: 10.1128/AAC.00560-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gane EJ, Schwabe C, Walker K, Flores L, Hartman GD, Klumpp K, Liaw S, Brown NA. 2014. Phase 1a safety and pharmacokinetics of NVR 3-778, a potential first. in-class HBV core inhibitor. Hepatology 60:LB-1C9. [Google Scholar]
- 167.Yuen MF, Kim DJ, Weilert F, Chan H-Y, Lalezari JP, Hwang SG, Nguyen T, Liaw S, Brown N, Klumpp K, Flores O, Hartman G, Gane E. 2016. NVR 3-778, a first-in-class HBV core inhibitor, alone and in combination with peg-interferon (PEGIFN), in treatment-naive HBeAg-positive patients: early reductions in HBV DNA and HBeAg. J Hepatol 64:S210–S211. doi: 10.1016/S0168-8278(16)00175-6. [DOI] [Google Scholar]
- 168.Xu Z, Chavez D, Guerra B, Littlejohn M, Peterson R, Locarnini S, Gish R, Anzalone C, Kanner S, Goetzmann J, Lanford R, Lewis D, Wooddell C. 2016. Treatment of chronically HBV-infected chimpanzees with RNA interference therapeutic ARC-520 led to potent reduction of viral mRNA, DNA and proteins without observed drug resistance. J Hepatol 64:S398. doi: 10.1016/S0168-8278(16)00626-7. [DOI] [Google Scholar]
- 169.Yuen MF, Chan H-Y, Liu K, Given BD, Schluep T, Hamilton J, Lai CL, Locarnini SA, Lau JYN, Ferrari C, Gish RG. 2016. Differential reductions in viral antigens expressed from cccDNA vs integrated DNA in treatment naive HBeAg positive and negative patients with chronic HBV after RNA interference therapy with ARC-520. J Hepatol 64:S390–S391. doi: 10.1016/S0168-8278(16)00606-1. [DOI] [Google Scholar]
- 170.Nakazono K, Ito Y, Wu CH, Wu GY. 1996. Inhibition of hepatitis B virus replication by targeted pretreatment of complexed antisense DNA in vitro. Hepatology 23:1297–1303. doi: 10.1002/hep.510230601. [DOI] [PubMed] [Google Scholar]
- 171.DeWitt MA, Magis W, Bray NL, Wang T, Berman JR, Urbinati F, Heo SJ, Mitros T, Munoz DP, Boffelli D, Kohn DB, Walters MC, Carroll D, Martin DI, Corn JE. 2016. Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells. Sci Transl Med 8:360ra134. doi: 10.1126/scitranslmed.aaf9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ramanan V, Shlomai A, Cox DB, Schwartz RE, Michailidis E, Bhatta A, Scott DA, Zhang F, Rice CM, Bhatia SN. 2015. CRISPR/Cas9 cleavage of viral DNA efficiently suppresses hepatitis B virus. Sci Rep 5:10833. doi: 10.1038/srep10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang J, Lu F. 2015. Dual-gRNAs and gRNA-microRNA (miRNA)-gRNA ternary cassette combined CRISPR/Cas9 system and RNAi approach promotes the clearance of HBV cccDNA. Hepatology 62:223A. [Google Scholar]
- 174.Shi Y, Tu Z, Duan X, Li W, Liu X, Holmes JA, Salloum S, Schaefer EA, Yu ML, Altinbas A, Lin W, Chung RT. 2017. The combination effects of CRISPR/Cas9 and APOBEC3B editing on HBV cccDNA formation. Hepatology 66:782A. [Google Scholar]
- 175.Lempp FA, Urban S. 2017. Hepatitis delta virus: replication strategy and upcoming therapeutic options for a neglected human pathogen. Viruses 9:172. doi: 10.3390/v9070172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shattock AG, Morris MC. 1991. Evaluation of commercial enzyme immunoassays for detection of hepatitis delta antigen and anti-hepatitis delta virus (HDV) and immunoglobulin M anti-HDV antibodies. J Clin Microbiol 29:1873–1876. doi: 10.1128/JCM.29.9.1873-1876.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Manesis EK, Vourli G, Dalekos G, Vasiliadis T, Manolaki N, Hounta A, Koutsounas S, Vafiadis I, Nikolopoulou G, Giannoulis G, Germanidis G, Papatheodoridis G, Touloumi G. 2013. Prevalence and clinical course of hepatitis delta infection in Greece: a 13-year prospective study. J Hepatol 59:949–956. doi: 10.1016/j.jhep.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 178.Yurdaydin C, Abbas Z, Buti M, Cornberg M, Esteban R, Etzion O, Gane EJ, Gish RG, Glenn JS, Hamid S, Heller T, Koh C, Lampertico P, Lurie Y, Manns M, Parana R, Rizzetto M, Urban S, Wedemeyer H, Hepatitis Delta International Network (HDIN). 2019. Treating chronic hepatitis delta: the need for surrogate markers of treatment efficacy. J Hepatol 70:1008–1015. doi: 10.1016/j.jhep.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 179.Hughes SA, Wedemeyer H, Harrison PM. 2011. Hepatitis delta virus. Lancet 378:73–85. doi: 10.1016/S0140-6736(10)61931-9. [DOI] [PubMed] [Google Scholar]
- 180.Wranke A, Serrano BC, Heidrich B, Kirschner J, Bremer B, Lehmann P, Hardtke S, Deterding K, Port K, Westphal M, Manns MP, Cornberg M, Wedemeyer H. 2017. Antiviral treatment and liver-related complications in hepatitis delta. Hepatology 65:414–425. doi: 10.1002/hep.28876. [DOI] [PubMed] [Google Scholar]
- 181.Soriano V, Vispo E, Sierra-Enguita R, Mendoza C, Fernandez-Montero JV, Labarga P, Barreiro P. 2014. Efficacy of prolonged tenofovir therapy on hepatitis delta in HIV-infected patients. AIDS 28:2389–2394. doi: 10.1097/QAD.0000000000000417. [DOI] [PubMed] [Google Scholar]
- 182.Wedemeyer H, Yurdaydìn C, Dalekos GN, Erhardt A, Çakaloğlu Y, Değertekin H, Gürel S, Zeuzem S, Zachou K, Bozkaya H, Koch A, Bock T, Dienes HP, Manns MP, HIDIT Study Group. 2011. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med 364:322–331. doi: 10.1056/NEJMoa0912696. [DOI] [PubMed] [Google Scholar]
- 183.Heidrich B, Yurdaydin C, Kabacam G, Ratsch BA, Zachou K, Bremer B, Dalekos GN, Erhardt A, Tabak F, Yalcin K, Gurel S, Zeuzem S, Cornberg M, Bock CT, Manns MP, Wedemeyer H, HIDIT-1 Study Group. 2014. Late HDV RNA relapse after peginterferon alpha-based therapy of chronic hepatitis delta. Hepatology 60:87–97. doi: 10.1002/hep.27102. [DOI] [PubMed] [Google Scholar]
- 184.Wedemeyer H, Yurdaydin C, Ernst S, Caruntu FA, Curescu MG, Yalcin K, Akarca US, Gürel S, Zeuzem S, Erhardt A, Lüth S, Papatheodoridis GV, Keskin O, Port K, Radu M, Celen MK, Ildeman R, Stift J, Heidrich B, Mederacke I, Hardtke S, Koch A, Dienes HP, Manns MP. 2014. Prolonged therapy of hepatitis delta for 96 weeks with pegylated interferon alpha 2a plus tenofovir or placebo does not prevent HDV RNA relapse after treatment: the HIDIT-2 study. J Hepatol 60:S2–S3. doi: 10.1016/S0168-8278(14)60006-4. [DOI] [Google Scholar]
- 185.Rizzetto M. 2017. Investigational drugs in development for hepatitis D. Expert Opin Invest Drugs 26:999–1005. doi: 10.1080/13543784.2017.1357695. [DOI] [PubMed] [Google Scholar]
- 186.Koh C, Canini L, Dahari H, Zhao X, Uprichard SL, Haynes-Williams V, Winters MA, Subramanya G, Cooper SL, Pinto P, Wolff EF, Bishop R, Han MAT, Cotler SJ, Kleiner DE, Keskin O, Idilman R, Yurdaydin C, Glenn JS, Heller T. 2015. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. Lancet Infect Dis 15:1167–1174. doi: 10.1016/S1473-3099(15)00074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rizzetto M, Ciancio A. 2015. The prenylation inhibitor, lonafarnib: a new therapeutic strategy against hepatitis delta. Lancet Infect Dis 15:1119–1120. doi: 10.1016/S1473-3099(15)00155-3. [DOI] [PubMed] [Google Scholar]
- 188.Wedemeyer H, Alexandrov A, Bogomaolov P, Blank A, Bremer B, Voronkova N , Schöneweis K, Raupach R, Lehmann P, Darnedde M, Pathil A, Burhenne J, Haag M, Schwab M, Haefeli WE, Urban S. 2017. Interim results of a multicenter, open-label phase 2b clinical trial to assess safety and efficacy of Myrcludex B in combination with Tenofovir in patients with chronic HBV/HDV coinfection. Hepatology 66(S1):20A–21A. doi: 10.1002/hep.29500. [DOI] [Google Scholar]
- 189.Wedemeyer H, Schöneweis K, Bogomolov PO, Voronkova N, Chulanov V, Stepanova T, Bremer B, Allweiss L, Dandri M, Burhenne J, Haefeli W-E, Ciesek S, Dittmer U, Alexandrov A, Urban S. 2019. GS-13—final results of a multicenter, open-label phase 2 clinical trial (MYR203) to assess safety and efficacy of myrcludex B in cwith PEG-interferon alpha 2a in patients with chronic HBV/HDV co-infection. J Hepatol 70:e81. doi: 10.1016/S0618-8278(19)30141-0. [DOI] [Google Scholar]
- 190.Vaillant A. 2016. Nucleic acid polymers: broad spectrum antiviral activity, antiviral mechanisms and optimization for the treatment of hepatitis B and hepatitis D infection. Antiviral Res 133:32–40. doi: 10.1016/j.antiviral.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 191.Beilstein F, Blanchet M, Vaillant A, Sureau C. 2018. Nucleic acid polymers are active against hepatitis delta virus infection in vitro. J Virol 92:e01416-17. doi: 10.1128/JVI.01416-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bazinet M, Pantea V, Cebotarescu V, Cojuhari L, Jimbei P, Albrecht J, Schmid P, Le Gal F, Gordien E, Krawczyk A, Mijocevic H, Karimzadeh H, Roggendorf M, Vaillant A. 2017. Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus co-infection (REP 301 and REP 301-LTF): a non-randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol 2:877–889. doi: 10.1016/S2468-1253(17)30288-1. [DOI] [PubMed] [Google Scholar]
- 193.Etzion O, Hamid SS, Lurie Y, Gane E, Bader N, Yardeni D, Nevo-Shor A, Channa S, Mawani M, Parkash O, Yang K, Longo D, Gish RG, Glenn J, Apelian D. 2019. PS-052—end of study results from LIMT HDV study: 36% durable virologic response at 24 weeks post-treatment with pegylated interferon lambda monotherapy in patients with chronic hepatitis delta virus infection. J Hepatol 70:e32. doi: 10.1016/S0618-8278(19)30058-1. [DOI] [Google Scholar]
- 194.Koh C, Da BL, Glenn JS. 2019. HBV/HDV coinfection: a challenge for therapeutics. Clin Liver Dis 23:557–572. doi: 10.1016/j.cld.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yurdaydin C. 2019. New treatment options for delta virus: is a cure in sight? J Viral Hepat 26:618–626. doi: 10.1111/jvh.13081. [DOI] [PubMed] [Google Scholar]
- 196.Inoue T, Tanaka Y. 2016. Hepatitis B virus and its sexually transmitted infection—an update. Microb Cell 3:420–437. doi: 10.15698/mic2016.09.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Thornton AC, Jose S, Bhagani S, Chadwick D, Dunn D, Gilson R, Main J, Nelson M, Rodger A, Taylor C, Youssef E, Leen C, Gompels M, Kegg S, Schwenk A, Sabin C, UK Collaborative HIV Cohort (UK CHIC) Steering Committee. 2017. Hepatitis B, hepatitis C, and mortality among HIV-positive individuals. AIDS 31:2525–2532. doi: 10.1097/QAD.0000000000001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Singh KP, Crane M, Audsley J, Avihingsanon A, Sasadeusz J, Lewin SR. 2017. HIV-hepatitis B virus coinfection: epidemiology, pathogenesis, and treatment. AIDS 31:2035–2052. doi: 10.1097/QAD.0000000000001574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hawkins C, Christian B, Fabian E, Macha I, Gawile C, Mpangala S, Ulenga N, Thio CL, Ammerman LR, Mugusi F, Fawzi W, Green R, Murphy R. 2017. Brief report: HIV/HBV coinfection is a significant risk factor for liver fibrosis in Tanzanian HIV-infected adults. J Acquir Immune Defic Syndr 76:298–302. doi: 10.1097/QAI.0000000000001491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kilonzo SB, Gunda DW, Kashasha F, Mpondo BC. 2017. Liver fibrosis and hepatitis B coinfection among ART naive HIV-infected patients at a tertiary level hospital in northwestern Tanzania: a cross-sectional study. J Trop Med 2017:5629130. doi: 10.1155/2017/5629130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vinikoor MJ, Sinkala E, Chilengi R, Mulenga LB, Chi BH, Zyambo Z, Hoffmann CJ, Saag MS, Davies MA, Egger M, Wandeler G, IeDEA-Southern Africa. 2017. Impact of antiretroviral therapy on liver fibrosis among human immunodeficiency virus-infected adults with and without HBV coinfection in Zambia. Clin Infect Dis 64:1343–1349. doi: 10.1093/cid/cix122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Boyd A, Houghtaling L, Moh R, Chekaraou MA, Gabillard D, Eholie SP, Anglaret X, Zoulim F, Danel C, Lacombe K, for the ANRS 1269 Trivacan and ANRS 12240 VarBVA Studies. 2017. Clinical outcomes during treatment interruptions in human immunodeficiency virus-hepatitis B virus co-infected patients from sub-Saharan Africa. Am J Trop Med Hyg 97:1936–1942. doi: 10.4269/ajtmh.16-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kouame GM, Boyd A, Moh R, Badje A, Gabillard D, Ouattara E, Ntakpe JB, Emieme A, Maylin S, Chekaraou MA, Eholie SP, Zoulim F, Lacombe K, Anglaret X, Danel C, French National Agency for Research on AIDS and Viral Hepatitis (ANRS) 12136 Temprano and ANRS 12240 VarBVA Study Groups. 2018. Higher mortality despite early ART in HIV and hepatitis B virus (HBV)-coinfected patients with high HBV replication. Clin Infect Dis 66:112–120. doi: 10.1093/cid/cix747. [DOI] [PubMed] [Google Scholar]
- 204.Chambal LM, Gudo ES, Carimo A, Corte Real R, Mabunda N, Maueia C, Vubil A, Zicai AF, Bhatt N, Antunes F. 2017. HBV infection in untreated HIV-infected adults in Maputo, Mozambique. PLoS One 12:e0181836. doi: 10.1371/journal.pone.0181836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Easterbrook PJ, Roberts T, Sands A, Peeling R. 2017. Diagnosis of viral hepatitis. Curr Opin HIV AIDS 12:302–314. doi: 10.1097/COH.0000000000000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lange B, Roberts T, Cohn J, Greenman J, Camp J, Ishizaki A, Messac L, Tuaillon E, van de Perre P, Pichler C, Denkinger CM, Easterbrook P. 2017. Diagnostic accuracy of detection and quantification of HBV-DNA and HCV-RNA using dried blood spot (DBS) samples—a systematic review and meta-analysis. BMC Infect Dis 17(Suppl 1):693. doi: 10.1186/s12879-017-2776-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Deressa T, Damtie D, Fonseca K, Gao S, Abate E, Alemu S, Aleka Y, Swain MG, van Marle G, Coffin CS. 2017. The burden of hepatitis B virus (HBV) infection, genotypes and drug resistance mutations in human immunodeficiency virus-positive patients in northwest Ethiopia. PLoS One 12:e0190149. doi: 10.1371/journal.pone.0190149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chan L, Asriel B, Eaton EF, Wyatt CM. 2018. Potential kidney toxicity from the antiviral drug tenofovir: new indications, new formulations, and a new prodrug. Curr Opin Nephrol Hypertens 27:102–112. doi: 10.1097/mnh.0000000000000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.De Clercq E. 2018. Role of tenofovir alafenamide (TAF) in the treatment and prophylaxis of HIV and HBV infections. Biochem Pharmacol 153:2–11. doi: 10.1016/j.bcp.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 210.Boyd A, Bottero J, Miailhes P, Lascoux-Combe C, Rougier H, Girard PM, Serfaty L, Lacombe K. 2017. Liver fibrosis regression and progression during controlled hepatitis B virus infection among HIV-HBV patients treated with tenofovir disoproxil fumarate in France: a prospective cohort study. J Int AIDS Soc 20:21426. doi: 10.7448/IAS.20.1.21426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Grant J, Agbaji O, Kramvis A, Yousif M, Auwal M, Penugonda S, Ugoagwu P, Murphy R, Hawkins C. 2017. Hepatitis B virus sequencing and liver fibrosis evaluation in HIV/HBV co-infected Nigerians. Trop Med Int Health 22:744–754. doi: 10.1111/tmi.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Haban H, Benchekroun S, Sadeq M, Benjouad A, Amzazi S, Oumzil H, Elharti E. 2017. Assessment of the HBV vaccine response in a group of HIV-infected children in Morocco. BMC Public Health 17:752. doi: 10.1186/s12889-017-4776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Manyahi J, Msigwa Y, Mhimbira F, Majigo M. 2017. High sero-prevalence of hepatitis B virus and human immunodeficiency virus infections among pregnant women attending antenatal clinic at Temeke municipal health facilities, Dar es Salaam, Tanzania: a cross sectional study. BMC Pregnancy Childbirth 17:109. doi: 10.1186/s12884-017-1299-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Forbi JC, Dillon M, Purdy MA, Drammeh BS, Tejada-Strop A, McGovern D, Xia GL, Lin Y, Ganova-Raeva LM, Campo DS, Thai H, Vaughan G, Haule D, Kutaga RP, Basavaraju SV, Kamili S, Khudyakov YE. 2017. Molecular epidemiology of hepatitis B virus infection in Tanzania. J Gen Virol 98:1048–1057. doi: 10.1099/jgv.0.000776. [DOI] [PubMed] [Google Scholar]
- 215.Hutin Y, Low-Beer D, Bergeri I, Hess S, Garcia-Calleja JM, Hayashi C, Mozalevskis A, Rinder Stengaard A, Sabin K, Harmanci H, Bulterys M. 2017. Viral hepatitis strategic information to achieve elimination by 2030: key elements for HIV program managers. JMIR Public Health Surveill 3:e91. doi: 10.2196/publichealth.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ndow G, Gore ML, Shimakawa Y, Suso P, Jatta A, Tamba S, Sow A, Toure-Kane C, Sadiq F, Sabally S, Njie R, Thursz MR, Lemoine M. 2017. Hepatitis B testing and treatment in HIV patients in the Gambia—compliance with international guidelines and clinical outcomes. PLoS One 12:e0179025. doi: 10.1371/journal.pone.0179025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Omatola CA, Onoja BA, Thomas T. 2017. High rate of hepatitis B virus surface antigenemia among people living with HIV/AIDS in Kakuri, Kaduna State, north west Nigeria. Viral Immunol 30:516–521. doi: 10.1089/vim.2016.0163. [DOI] [PubMed] [Google Scholar]
- 218.Chu CJ, Lee SD. 2008. Hepatitis B virus/hepatitis C virus coinfection: epidemiology, clinical features, viral interactions and treatment. J Gastroenterol Hepatol 23:512–520. doi: 10.1111/j.1440-1746.2008.05384.x. [DOI] [PubMed] [Google Scholar]
- 219.Perumalswami PV, Bini EJ. 2006. Epidemiology, natural history, and treatment of hepatitis B virus and hepatitis C virus coinfection. Minerva Gastroenterol Dietol 52:145–155. [PubMed] [Google Scholar]
- 220.Yachimski P, Chung RT. 2005. Update on hepatitis B and C coinfection in HIV. Curr Infect Dis Rep 7:299–308. doi: 10.1007/s11908-005-0063-4. [DOI] [PubMed] [Google Scholar]
- 221.Zhang C, Li X, Liu Y, Qiao S, Chen Y, Zhou Y, Shen Z. 2017. Co-infections of tuberculosis, hepatitis B or C viruses in a cohort of people living with HIV/AIDS in China: predictors and sequelae. AIDS Care 29:974–977. doi: 10.1080/09540121.2016.1271388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yang R, Gui X, Xiong Y, Gao S. 2017. Long-term follow-up of patients triply infected with HIV and hepatitis B and C viruses in a comprehensive hospital in central China. J Viral Hepat 24:1192–1193. doi: 10.1111/jvh.12739. [DOI] [PubMed] [Google Scholar]
- 223.Saravanan S, Velu V, Kumarasamy N, Nandakumar S, Murugavel KG, Balakrishnan P, Suniti S, Thyagarajan SP. 2007. Coinfection of hepatitis B and hepatitis C virus in HIV-infected patients in south India. World J Gastroenterol 13:5015–5020. doi: 10.3748/wjg.v13.i37.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Desikan P, Khan Z. 2017. Prevalence of hepatitis B and hepatitis C virus co-infection in India: a systematic review and meta-analysis. Indian J Med Microbiol 35:332–339. doi: 10.4103/ijmm.IJMM_17_257. [DOI] [PubMed] [Google Scholar]
- 225.Mohammadi M, Talei G, Sheikhian A, Ebrahimzade F, Pournia Y, Ghasemi E, Boroun H. 2009. Survey of both hepatitis B virus (HBsAg) and hepatitis C virus (HCV-Ab) coinfection among HIV positive patients. Virol J 6:202. doi: 10.1186/1743-422X-6-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.KhS D, Singh NB, Singh HL, Singh YM. 2009. Coinfection by human immunodeficiency virus, hepatitis B virus and hepatitis C virus in injecting drug users. J Indian Med Assoc 107:144, 146–147. [PubMed] [Google Scholar]
- 227.Umutesi J, Simmons B, Makuza JD, Dushimiyimana D, Mbituyumuremyi A, Uwimana JM, Ford N, Mills EJ, Nsanzimana S. 2017. Prevalence of hepatitis B and C infection in persons living with HIV enrolled in care in Rwanda. BMC Infect Dis 17:315. doi: 10.1186/s12879-017-2422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Silva CMD, Peder LD, Guelere AM, Horvath JD, Silva ES, Teixeira JJV, Bertolini DA. 2018. Seroprevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) among human immunodeficiency virus (HIV)-infected patients in an HBV endemic area in Brazil. PLoS One 13:e0203272. doi: 10.1371/journal.pone.0203272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sato S, Fujiyama S, Tanaka M, Yamasaki K, Kuramoto I, Kawano S, Sato T, Mizuno K, Nonaka S. 1994. Coinfection of hepatitis C virus in patients with chronic hepatitis B infection. J Hepatol 21:159–166. doi: 10.1016/s0168-8278(05)80389-7. [DOI] [PubMed] [Google Scholar]
- 230.Nguyen LH, Ko S, Wong SS, Tran PS, Trinh HN, Garcia RT, Ahmed A, Lutchman GA, Keeffe EB, Nguyen MH. 2011. Ethnic differences in viral dominance patterns in patients with hepatitis B virus and hepatitis C virus dual infection. Hepatology 53:1839–1845. doi: 10.1002/hep.24308. [DOI] [PubMed] [Google Scholar]
- 231.Bellecave P, Gouttenoire J, Gajer M, Brass V, Koutsoudakis G, Blum HE, Bartenschlager R, Nassal M, Moradpour D. 2009. Hepatitis B and C virus coinfection: a novel model system reveals the absence of direct viral interference. Hepatology 50:46–55. doi: 10.1002/hep.22951. [DOI] [PubMed] [Google Scholar]
- 232.Pontisso P, Gerotto M, Benvegnu L, Chemello L, Alberti A. 1998. Coinfection by hepatitis B virus and hepatitis C virus. Antivir Ther 3:137–142. [PubMed] [Google Scholar]
- 233.Coppola N, Pisapia R, Tonziello G, Martini S, Imparato M, Piai G, Stanzione M, Sagnelli C, Filippini P, Piccinino F, Sagnelli E. 2008. Virological pattern in plasma, peripheral blood mononuclear cells and liver tissue and clinical outcome in chronic hepatitis B and C virus coinfection. Antivir Ther 13:307–318. [PubMed] [Google Scholar]
- 234.Cheruvu S, Marks K, Talal AH. 2007. Understanding the pathogenesis and management of hepatitis B/HIV and hepatitis B/hepatitis C virus coinfection. Clin Liver Dis 11:917–943. ix-x. doi: 10.1016/j.cld.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 235.Yan BM, Lee SS. 2005. Acute coinfection with hepatitis B and hepatitis C viruses. Can J Gastroenterol 19:729–730. doi: 10.1155/2005/514813. [DOI] [PubMed] [Google Scholar]
- 236.Chen SW, Lee TS, Hu CC, Chang LC, Chien RN. 2007. Simultaneously acute hepatitis B virus and C virus coinfection and subsequent chronic hepatitis C. Scand J Infect Dis 39:351–354. doi: 10.1080/00365540600951358. [DOI] [PubMed] [Google Scholar]
- 237.Crockett SD, Keeffe EB. 2005. Natural history and treatment of hepatitis B virus and hepatitis C virus coinfection. Ann Clin Microbiol Antimicrob 4:13. doi: 10.1186/1476-0711-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sagnelli E, Pasquale G, Coppola N, Scarano F, Marrocco C, Scolastico C, Santantonio T, Gentile A, Piccinino F. 2004. Influence of chronic coinfection with hepatitis B and C virus on liver histology. Infection 32:144–148. doi: 10.1007/s15010-004-3080-6. [DOI] [PubMed] [Google Scholar]
- 239.Weltman MD, Brotodihardjo A, Crewe EB, Farrell GC, Bilous M, Grierson JM, Liddle C. 1995. Coinfection with hepatitis B and C or B, C and delta viruses results in severe chronic liver disease and responds poorly to interferon-alpha treatment. J Viral Hepat 2:39–45. doi: 10.1111/j.1365-2893.1995.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 240.Wang H, Swann R, Thomas E, Innes HA, Valerio H, Hayes PC, Allen S, Barclay ST, Wilks D, Fox R, Bhattacharyya D, Kennedy N, Morris J, Fraser A, Stanley AJ, Gunson R, McLntyre PG, Hunt A, Hutchinson SJ, Mills PR, Dillon JF. 2018. Impact of previous hepatitis B infection on the clinical outcomes from chronic hepatitis C? A population-level analysis. J Viral Hepat 25:930–938. doi: 10.1111/jvh.12897. [DOI] [PubMed] [Google Scholar]
- 241.Kruse RL, Kramer JR, Tyson GL, Duan Z, Chen L, El-Serag HB, Kanwal F. 2014. Clinical outcomes of hepatitis B virus coinfection in a United States cohort of hepatitis C virus-infected patients. Hepatology 60:1871–1878. doi: 10.1002/hep.27337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Morsica G, Ancarani F, Bagaglio S, Maracci M, Cicconi P, Cozzi Lepri A, Antonucci G, Bruno R, Santantonio T, Tacconi L, Baldelli F, Piscopo R, Santoro D, Lazzarin A, D'Arminio Monforte A, HepaICONA amd the ICONA Study Group. 2009. Occult hepatitis B virus infection in a cohort of HIV-positive patients: correlation with hepatitis C virus coinfection, virological and immunological features. Infection 37:445–449. doi: 10.1007/s15010-008-8194-9. [DOI] [PubMed] [Google Scholar]
- 243.Matsuoka S, Nirei K, Tamura A, Nakamura H, Matsumura H, Oshiro S, Arakawa Y, Yamagami H, Tanaka N, Moriyama M. 2008. Influence of occult hepatitis B virus coinfection on the incidence of fibrosis and hepatocellular carcinoma in chronic hepatitis C. Intervirology 51:352–361. doi: 10.1159/000187720. [DOI] [PubMed] [Google Scholar]
- 244.He Y, Zhao QX, Ren YJ, Ding LM. 2006. Coinfection with HBV and HCV in 128 AIDS patients infected through blood transmission. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 28:662–664. (In Chinese.) [PubMed] [Google Scholar]
- 245.Li L, Lan X. 2016. Association between hepatitis B virus/hepatitis C virus infection and primary hepatocellular carcinoma risk: a meta-analysis based on Chinese population. J Cancer Res Ther 12:C284–C287. doi: 10.4103/0973-1482.200763. [DOI] [PubMed] [Google Scholar]
- 246.Kew MC, Yu MC, Kedda MA, Coppin A, Sarkin A, Hodkinson J. 1997. The relative roles of hepatitis B and C viruses in the etiology of hepatocellular carcinoma in southern African blacks. Gastroenterology 112:184–187. doi: 10.1016/S0016-5085(97)70233-6. [DOI] [PubMed] [Google Scholar]
- 247.Sagnelli E, Coppola N, Scolastico C, Mogavero AR, Filippini P, Piccinino F. 2001. HCV genotype and “silent” HBV coinfection: two main risk factors for a more severe liver disease. J Med Virol 64:350–355. doi: 10.1002/jmv.1057. [DOI] [PubMed] [Google Scholar]
- 248.Stransky J, Malina L, Cieslarova B, Stritesky J, Putova I, Horak J. 2000. Overt and hidden coinfection with hepatitis B and C viruses in chronic liver disease and porphyria cutanea tarda. Acta Virol 44:23–28. [PubMed] [Google Scholar]
- 249.Benvegnu L, Noventa F, Bernardinello E, Pontisso P, Gatta A, Alberti A. 2001. Evidence for an association between the aetiology of cirrhosis and pattern of hepatocellular carcinoma development. Gut 48:110–115. doi: 10.1136/gut.48.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.De Monte A, Courjon J, Anty R, Cua E, Naqvi A, Mondain V, Cottalorda J, Ollier L, Giordanengo V. 2016. Direct-acting antiviral treatment in adults infected with hepatitis C virus: reactivation of hepatitis B virus coinfection as a further challenge. J Clin Virol 78:27–30. doi: 10.1016/j.jcv.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 251.Fukuda R, Ishimura N, Niigaki M, Hamamoto S, Satoh S, Tanaka S, Kushiyama Y, Uchida Y, Ihihara S, Akagi S, Watanabe M, Kinoshita Y. 1999. Serologically silent hepatitis B virus coinfection in patients with hepatitis C virus-associated chronic liver disease: clinical and virological significance. J Med Virol 58:201–207. doi:. [DOI] [PubMed] [Google Scholar]
- 252.Yeh ML, Huang CF, Hsieh MH, Ko YM, Chen KY, Liu TW, Lin YH, Liang PC, Hsieh MY, Lin ZY, Chen SC, Huang CI, Huang JF, Kuo PL, Dai CY, Yu ML, Chuang WL. 2017. Reactivation of hepatitis B in patients of chronic hepatitis C with hepatitis B virus infection treated with direct acting antivirals. J Gastroenterol Hepatol 32:1754–1762. doi: 10.1111/jgh.13771. [DOI] [PubMed] [Google Scholar]
- 253.Chen G, Wang C, Chen J, Ji D, Wang Y, Wu V, Karlberg J, Lau G. 2017. Hepatitis B reactivation in hepatitis B and C coinfected patients treated with antiviral agents: a systematic review and meta-analysis. Hepatology 66:13–26. doi: 10.1002/hep.29109. [DOI] [PubMed] [Google Scholar]
- 254.Belperio PS, Shahoumian TA, Mole LA, Backus LI. 2017. Evaluation of hepatitis B reactivation among 62,920 veterans treated with oral hepatitis C antivirals. Hepatology 66:27–36. doi: 10.1002/hep.29135. [DOI] [PubMed] [Google Scholar]
- 255.Blackard JT, Sherman KE. 2018. Hepatitis B virus (HBV) reactivation—the potential role of direct-acting agents for hepatitis C virus (HCV). Rev Med Virol 28:e1984. doi: 10.1002/rmv.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Bersoff-Matcha SJ, Cao K, Jason M, Ajao A, Jones SC, Meyer T, Brinker A. 2017. Hepatitis B virus reactivation associated with direct-acting antiviral therapy for chronic hepatitis C virus: a review of cases reported to the U.S. Food and Drug Administration Adverse Event Reporting System. Ann Intern Med 166:792–798. doi: 10.7326/M17-0377. [DOI] [PubMed] [Google Scholar]
- 257.Moorman AC, Xing J, Rupp LB, Gordon SC, Spradling PR, Boscarino JA, Schmidt MA, Daida YG, Teshale EH, Holmberg SD, Chronic Hepatitis Cohort Study Investigators. 2018. Hepatitis B virus infection and hepatitis C virus treatment in a large cohort of hepatitis C-infected patients in the United States. Gastroenterology 154:754–758. doi: 10.1053/j.gastro.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Jiang XW, Ye JZ, Li YT, Li LJ. 2018. Hepatitis B reactivation in patients receiving direct-acting antiviral therapy or interferon-based therapy for hepatitis C: a systematic review and meta-analysis. World J Gastroenterol 24:3181–3191. doi: 10.3748/wjg.v24.i28.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Potthoff A, Berg T, Wedemeyer H, HEP-NET B/C Coinfection Study Group. 2009. Late hepatitis B virus relapse in patients co-infected with hepatitis B virus and hepatitis C virus after antiviral treatment with pegylated interferon-a2b and ribavirin. Scand J Gastroenterol 44:1487–1490. doi: 10.3109/00365520903329585. [DOI] [PubMed] [Google Scholar]
- 260.Wang C, Ji D, Chen J, Shao Q, Li B, Liu J, Wu V, Wong A, Wang Y, Zhang X, Lu L, Wong C, Tsang S, Zhang Z, Sun J, Hou J, Chen G, Lau G. 2017. Hepatitis due to reactivation of hepatitis B virus in endemic areas among patients with hepatitis C treated with direct-acting antiviral agents. Clin Gastroenterol Hepatol 15:132–136. doi: 10.1016/j.cgh.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 261.Liu CJ, Chuang WL, Sheen IS, Wang HY, Chen CY, Tseng KC, Chang TT, Massetto B, Yang JC, Yun C, Knox SJ, Osinusi A, Camus G, Jiang D, Brainard DM, McHutchison JG, Hu TH, Hsu YC, Lo GH, Chu CJ, Chen JJ, Peng CY, Chien RN, Chen PJ. 2018. Efficacy of ledipasvir and sofosbuvir treatment of HCV infection in patients coinfected with HBV. Gastroenterology 154:989–997. doi: 10.1053/j.gastro.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 262.Mucke MM, Backus LI, Mucke VT, Coppola N, Preda CM, Yeh ML, Tang LSY, Belperio PS, Wilson EM, Yu ML, Zeuzem S, Herrmann E, Vermehren J. 2018. Hepatitis B virus reactivation during direct-acting antiviral therapy for hepatitis C: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 3:172–180. doi: 10.1016/S2468-1253(18)30002-5. [DOI] [PubMed] [Google Scholar]
- 263.Loomba R, Liang TJ. 2017. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology 152:1297–1309. doi: 10.1053/j.gastro.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zhou J, Dore GJ, Zhang F, Lim PL, Chen YM, TREAT Asia HIV Observational Database. 2007. Hepatitis B and C virus coinfection in The TREAT Asia HIV Observational Database. J Gastroenterol Hepatol 22:1510–1518. doi: 10.1111/j.1440-1746.2007.05062.x. [DOI] [PubMed] [Google Scholar]
- 265.Xiao H, Shi M, Xie Y, Chi X. 2017. Comparison of diagnostic accuracy of magnetic resonance elastography and Fibroscan for detecting liver fibrosis in chronic hepatitis B patients: a systematic review and meta-analysis. PLoS One 12:e0186660. doi: 10.1371/journal.pone.0186660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Hu X, Liu Y, Qian L. 2017. Diagnostic potential of real-time elastography (RTE) and shear wave elastography (SWE) to differentiate benign and malignant thyroid nodules: a systematic review and meta-analysis. Medicine 96:e8282. doi: 10.1097/MD.0000000000008282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Xiao G, Yang J, Yan L. 2015. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology 61:292–302. doi: 10.1002/hep.27382. [DOI] [PubMed] [Google Scholar]
- 268.Salkic N, Jovanovic P, Hauser G, Brcic M. 2014. FibroTest/Fibrosure for significant liver fibrosis and cirrhosis in chronic hepatitis B: a meta-analysis. Am J Gastroenterol 109:796–809. doi: 10.1038/ajg.2014.21. [DOI] [PubMed] [Google Scholar]
- 269.Wong GL-H, Chan HL-Y, Choi PC-L, Chan AW-H, Yu Z, Lai JW-Y, Chan HY, Wong VW-S. 2014. Non-invasive algorithm of enhanced liver fibrosis and liver stiffness measurement with transient elastography for advanced liver fibrosis in chronic hepatitis B. Aliment Pharmacol Ther 39:197–208. doi: 10.1111/apt.12559. [DOI] [PubMed] [Google Scholar]
- 270.Tong MJ, Pan CQ, Hann HW, Kowdley KV, Han SH, Min AD, Leduc TS. 2011. The management of chronic hepatitis B in Asian Americans. Dig Dis Sci 56:3143–3162. doi: 10.1007/s10620-011-1841-5. [DOI] [PubMed] [Google Scholar]
- 271.Yim HJ, Lok AS. 2006. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology 43(2 Suppl 1):S173–S181. [DOI] [PubMed] [Google Scholar]