Abstract
Natural killer (NK) cells are non-T, non-B lymphocytes are part of the innate immune system and function without prior activation. The human NK cell surface determinant, CD94, plays a critical role in regulation of NK cell activity as a heterodimer with NKG2 subclasses. Canine NK cells are not as well defined as the human and murine equivalents, due in part to the paucity of reagents specific to cell surface markers. Canines possess NK/NKT cells that have similar morphological characteristics to those found in humans, yet little is known about their functional characteristics nor of cell surface expression of CD94. Here, we describe the development and function of a monoclonal antibody (mAb) to canine (ca) CD94. Freshly isolated canine CD94+ cells were CD3+/–, CD8+/–, CD4–, CD21–, CD5low, NKp46+, and were cytotoxic against a canine target cell line. Anti-caCD94 mAb proved useful in enriching NK/NKT cells from PBMC for expansion on CTAC feeder cells in the presence of IL-2 and IL-15. The cultured cells were highly cytolytic with co-expression of NKp46 and reduced expression of CD3. Transmission electron microscopy revealed expanded CD94+ lymphocytes were morphologically large granular lymphocytes with large electron dense granules. Anti-caCD94 (mAb) can serve to enrich NK/NKT cells from dog peripheral blood for ex vivo expansion for HCT and is a potentially valuable reagent for studying NK/NKT regulation in the dog.
Keywords: CD94, NK/NKT cell, monoclonal antibody, canine, expansion, CTAC
1. INTRODUCTION
Natural killer (NK) cells are classified as part of the mammalian innate immune system. They provide the host with a rapid response against virus-infected cells and tumor cells that lack or have defective major histocompatibility complex (MHC) class I expression. To date, the precise regulation of NK cells is not fully understood; however, mounting evidence indicates that the activation or inhibition of NK cells is the result of a network of several activating and inhibitory receptors that interact mainly with MHC molecules (Backstrom et al., 2004; Iwaszko and Bogunia-Kubik, 2011). The best understood regulator of NK function is the killer immunoglobulin-like receptor (KIR), which interacts with host MHC-class I epitopes to prevent NK cells from damaging host tissue (Vilches and Parham, 2002). A second well-studied regulator is the killer cell lectin like receptor, subfamily D, member 1, (KLRD-1), also known as CD94, which is a MHC-class I specific NK-cell receptor belonging to the C-type lectin family of proteins. In humans, CD94 appears on the surface of nearly all NK-cells as a heterodimer with 5 of the 7 members of the NKG2 family (Backstrom et al., 2004; Iwaszko and Bogunia-Kubik, 2011). CD94/NKG2 heterodimers either activate (CD94/NKG2C, E, or H) or inhibit NK function (CD94/NKG2A, B). Regulation of NK cell activity in species other than humans and mice is poorly understood (Saether et al., 2011).
In canines, NK cells may play an important role in anti-viral activity (Park et al., 2015) and cancer immunotherapy (Addissie and Klingemann, 2018) and function in the outcome of dog leukocyte antigen (DLA)-nonidentical hematopoietic cell transplantation (HCT) (Raff et al., 1986; Raff et al., 1994). Studies have shown a rise in host peripheral blood NK cells in dogs that rejected DLA-nonidentical marrow grafts within one week after conditioning with high-dose total body irradiation (TBI) (Raff et al., 1994). These NK cells also inhibited growth of marrow donor granulocyte macrophage colony forming units (GM-CFU) in a MHC-unrestricted manner, suggesting that host NK cells inhibited proliferation of donor cells. In addition to dogs, studies have also shown that host NK cells play an important role in graft rejection in mice (Cudkowicz and Bennett, 1971), non-human primates (Kawai et al., 2000), and humans (Mattsson et al., 2008).
Although the morphological characteristics of canine NK cells appear similar to those of humans and mice, canine NK cell function and surface markers remain poorly characterized (Shin et al., 2013). To date, only a few monoclonal antibodies (mAb) that recognize canine NK cells specifically have been described. One is to the cell surface marker CD44 (Tan et al., 1993), a second is to CD5 (Huang et al., 2008), and a third recognizes a NK cell-specific receptor, NCR1 (Foltz et al., 2016). CD94 was first described in dogs as a pseudogene (Hao et al., 2006). However, subsequent investigations showed that anti-human CD94 antibodies cross reacted with canine cells, indicating that the antigen is expressed on the cell surface (Schuberth et al., 2007). To further investigate the expression profile of CD94, we produced a canine-specific mAb against CD94 with the aim to establish mAb specificity for dog NK cells, modulate NK activity in vivo and/or to select NK cells for ex vivo expansion and use in adoptive immunotherapy. Additionally, an anti-canine CD94 mAb may prove useful in future mechanistic studies investigating dog NK regulation. Here, we describe the immunophenotypic properties of an anti-canine (ca)CD94mAb, clone 8H10, and demonstrate the potential application of the antibody for selecting and expanding cytolytically active canine NK and NKT cells with a large granular lymphocyte (LGL) phenotype.
2. MATERIALS AND METHODS
2.1. Experimental animals and blood cell preparations
Peripheral blood mononuclear cells (PBMC) were obtained from healthy male and female beagles, mini-mongrels, basenjis, and golden retriever crossbreeds. The dogs were raised at the Fred Hutchinson Cancer Research Center (“Fred Hutch,” Seattle, WA) or purchased from commercial kennels. The animals were housed in Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited facilities and the study was approved by the Fred Hutch Institutional Animal Care and Use Committee. Blood was collected in heparin (10%), and PBMC isolated by Ficoll-Hypaque density gradient centrifugation (density, 1.074 g/ml).
2.2. Cloning of canine CD94
Canine CD94 was originally cloned from dog PBMC RNA (Genbank Accession No. DQ228356) by RT-PCR using primers based on the predicted DNA sequence (Forward: ATGGCTGTTTCTCAGACCACTATATGGAATTTTG; Reverse: CTACATAAGCTCTTGCTTACATATTAAAACGACT). The cDNA of the extracellular domain of CD94 was inserted into the pcDNA3.1 expression vector as a fusion with murine IgG2a (pcDNA3.1-CD94-muIgG2a) or canine IgG1 (pcDNA3.1-CD94-caIgG1) using previously reported methods (Graves et al., 2011). Comparison of the experimentally obtained sequence with the dog genome revealed localization of the canine CD94 gene on chromosome 27.
2.3. Generation of mouse anti-caCD94 mAb
Production of anti-caCD94 was done using previously reported methods (Graves et al., 2011). Briefly, NS0 cell were stably transfected with pcDNA3.1-CD94-muIgG2a or pcDNA3.1-CD94-caIgG1 and the resulting fusion proteins were purified by immuno-affinity chromatography. BALB/cJ mice were immunized with purified canine CD94-muIg2a fusion protein, and spleen cells were harvested and hybridomas generated using the ClonaCell-Hy Hybridoma Cloning Kit (STEMCELL Technologies, Vancouver, BC, Canada). Culture supernatants from individual hybridoma clones were screened for canine CD94 reactivity by ELISA using CD94-canine-IgG1 fusion protein to capture and an HRP-labeled F(ab’)2 donkey anti-mouse antibody for detection (Southern Biotech, Birmingham, AL). Immuno-reactivity of selected supernatants to CD94 on the cell surface was confirmed by flow cytometry analysis of canine PBMC using a FITC-labeled donkey anti-mouse F(ab’)2 secondary antibody (Jackson ImmunoResearch, West Grove, PA). Clone 8H10 was expanded in culture in serum-free medium and the antibody was purified by HiTrap MAbSelect SuRe immunoaffinity chromatography (GE Healthcare, Pittsburg, PA).
2.4. Flow cytometry
PBMC, CD94+-selected or CD94+-cultured cells were collected, resuspended in flow cytometry buffer (DPBS + 2% horse serum), and phenotyped using the following antibodies: anti-CD3 (CA17.6F9 or CA17.6B3), anti-CD4 (CA13.1E4), anti-CD8 (CA9.JD3), anti-CD21 (1D6), anti-CD45 (10C12), antiCD11b (16.ED1) (all gifted from Dr. Peter Moore, UCD, Davis, CA), anti-CD5 (RPE-labeled; YKIX322.3, Serotech, (Biorad, Hercules, CA) or PerCP700-labeled eBiosciences (ThermoFisher, Grand Island, NY), Live/Dead fixable Viability Dye eFluor 780 (cat# 65–0865, ThermoFischer, eBiosciences) and anti-human CD94 (clone HP3D9, Becton Dickinson, Franklin Lakes, NJ). Anti-CD3, -CD4, and -CD8 were FITC-labeled using NHS-Fluorescein at a 15:1 molar ratio of fluorescein to antibody (ThermoFisher Scientific, Waltham, MA). Anti-caCD94 mAb used for flow cytometry was conjugated to Alexa Fluor 647 or Pacific Blue according to the manufacturer’s instructions (Thermo Fisher Scientific). Flow cytometry data was analyzed using FlowJo software (version 10). NCR-1 or NKp46 (a generous gift from Drs. J. Foltz and D. Lee, Nationwide Children’s Hospital, Ohio State University, Columbus, Ohio) was conjugated with anti-mouse IgG2a PE secondary (SouthernBiotech, Birmingham AL),
2.5. RT-PCR
Total RNA was isolated from ex vivo expanded canine CD94+ cells, ConA-activated canine PBMC, and primary canine myoblasts, and used as a template to generate cDNA (Applied Biosystems High Capacity cDNA Reverse Transcription Kit, Waltham, MA). End-point PCR was performed using 1/20th of the cDNA reaction as template and Platinum Taq, for 30 cycles (ThermoFisher Scientific, Waltham, MA). Primers were designed to cross exon-exon boundaries to generate cDNA-specific amplicons: CD94-F: ATTGCGTTTTGTGAGCTCCA; CD94-R: GAGAGCCAAGCCATTTTCCC; CD16-F: TCTTCGGAGGCTGTGAACAT; CD16-R: AGGGAGAAAGGGATTTGGGG; CD56-F: GCGATGACAGTTCTGAGCTG; CD56-R: GAGGCTTCACAGGTCAGAGT; NCR1/NKp46-F: GGAGCTCTGGTCAAAGCCTA; NCR1/NKp46-R: TATCTGTGTTGTGGGTGGCT; GranzymeB-F: AAGTGTGGTGGGTTCCTGAT; GranzymeB-R: CCCAGGGTGACTTTGATGGA; Eomes-F: GACTGCCTACCAAAACACCG; Eomes-R: GAAGGATTGAACGCCGTACC; TBP-F: GAA ATG CTG AAT ATA ATC CCA AGC; TBP-R: CAG CTC CCC ACC ATG TTC T.
Original 2,6 section on CTAC/41BBL cells has been deleted
2.6. 51Cr-release assay
NK-cell mediated cytotoxicity was tested using a standard 51Cr-release assay with canine thyroid adenocarcinoma (CTAC) cell line serving as target cells (Knapp et al., 1993). Briefly, fresh or 9–11 day cultured CD94-selected canine PBMC were incubated with 51CR-labeled CTAC for approximately 15 hours at 37°C, 5% CO2 humidified atmosphere. Effector-to-target cell ratios (E:T) were assessed in quadruplicate. Supernatants were measured for 51Cr release using a gamma counter (Packard, Meridian, CT). The percent cytotoxicity was determined by the following equation:
2.7. Anti-caCD94-mediated NK cell enrichment and culture
Canine NK cells were selected from PBMCs with anti-caCD94 mAb (45 minutes at 4°C) and anti-mouse IgG microbeads (Miltenyi Biotec, Auburn, CA). The cells were positively selected using the Miltenyi magnetic column method. The cells were cultured in complete medium (45% Waymouth’s medium, 45% Iscove’s medium, 10% heat-inactivated dog serum, 10 ng/ml recombinant canine IL-2 (R&D Systems, Minneapolis, MN) 15 ng/ml recombinant human IL-15 (R&D Systems), penicillin/streptomycin, sodium pyruvate, glutamine) on a feeder layer of 5 × 105 lethally irradiated CTAC Cells were transferred to fresh wells containing irradiated CTAC cells and complete medium every other day.
2.8. Transmission electron microscopy
Ex vivo expanded CD94+ cells were harvested after 9 days and suspended in EM fixative (1:1 Karnovsky’s and cells in Iscove’s medium) for 10 minutes at room temperature. After centrifugation, the cells were respended in Karnovsky’s fixative for 1 hour, followed by treatment with 1% osmium tetroxide in 0.1M cacodylate buffer. The cells were dehydrated through an ethanol gradient followed by incubation in propylene oxide. The cells were embedded in 1:1 PO/Epon resin and examined using a a 120 KV JEOL JEM-1400 transmission electron microscope (JEOL USA, Peabody, MA) with a Gatan 2K × 2K pixel Ultrascan 1000XP bottom mount camera designed for high resolution (Gatan, Inc., Pleasanton, CA).
3. RESULTS
3.1. Sequencing and mapping of canine CD94
The complete open reading frame of the 540-nucleotide sequence of canine CD94 (KLRD-1) cDNA was obtained from GenBank (Accession No. DQ228356.1). Sequence comparison of canine CD94 with its human (Genbank Accession No. U30610.1), murine (Genbank Accession No. AF039025.1), rat (Genbank Accession No. AF009133), bovine (Genbank Accession No. NM001002890), and chimpanzee (Genbank Accession No AF259054) homologs revealed that these proteins range from 68% to 77% identity at the nucleotide level and from 50% to 59% identity at the amino acid level (Table 1).
Table 1.
Sequence comparison of canine CD94 with chimpanzee, human, murine, rat, and bovine CD94 at cDNA and amino acid levels
| Sequence identity at nucleotide/amino acid level [%] | |||||
|---|---|---|---|---|---|
| C. familiaris | P. troglodytes | H. sapiens | M. musculus | R. norvegicus | |
| P. troglodytes | 77 / 59 | ||||
| H. sapiens | 77 / 59 | 99 / 99 | |||
| M. musculus | 68 / 50 | 73 / 55 | 72 / 54 | ||
| R. norvegicus | 68 / 50 | 71 / 55 | 71 / 55 | 89 / 78 | |
| B. taurus | 70 / 59 | 72 / 57 | 71 / 59 | 92 / 54 | 92 / 55 |
3.2. Production and specificity of anti-caCD94
Using our validated immunogen production strategy (Graves et al., 2011) of linking the extracellular domain of the antigen (CD94) to the Fc domain of mouse Ig, we produced three functional anti-caCD94 clones. Clones of anti-caCD94 mAb were first selected for antibody binding to canine CD94-canine Ig1 fusion protein by ELISA. Additional selection was based on clone growth rate and stability, antibody expression level, and antibody immunoreactivity with dog PBMC. Based on these parameters, clone 8H10 was deemed superior to other clones tested. Antigen specificity of anti-caCD94 (8H10) mAB was also indicated using competitive binding to the CD94 antigen with and without an anti-human CD94 mAb, shown to be cross reactive with dog lymphocytes (Schuberth et al., 2007). Increasing concentrations of the anti-caCD94 mAb resulted in reduced staining with the anti-human CD94 mAb (Figure 1). Double antibody staining using an anti-human anti-CD94 and anti-caCD94 mAbs revealed a “rocket” double-positive scatter pattern, suggesting staining of a single cell population but recognition by the two antibodies. In one experiment, anti-caCD94 bound to 7.7% of freshly isolated canine PBMC. In several similar experiments, fresh CD94+ PBMC were also CD3+, CD4–, CD8+/–, CD5low, CD21–, and CD45+ (Figure 2). These results are consistent with the conclusion that anti-caCD94 recognizes canine NK and NKT cells. As shown in Figure 3, live mononuclear lymphocytes were gated according to CD5 and NKp46 expression profiles and further phenotyped according to CD94 and CD3 expression. NKp46 (NCR1) is present on dog NK cells and has been characterized using a murine anti-NKp46 mAb (Foltz et al., 2016). CD5bright NKp46– cells are CD3+ with a minor CD94+ lymphocyte population. A CD5+ intermediate intensity, NKp46+ population can be separated into a CD94+ CD3– and a CD94+ and CD3+; this latter population we consider as NKT cells. In the CD5dim and NKp46+ population we observe a strong population of CD94+CD3– cells which we classify as NK cells. Thus, the CD94+ and CD3– lymphocyte population is enriched for NKp46 and CD5low double positive lymphocytes. We conclude from these data that anti-caCD94 mAb recognizes both NK and NKT cells.
Figure 1.
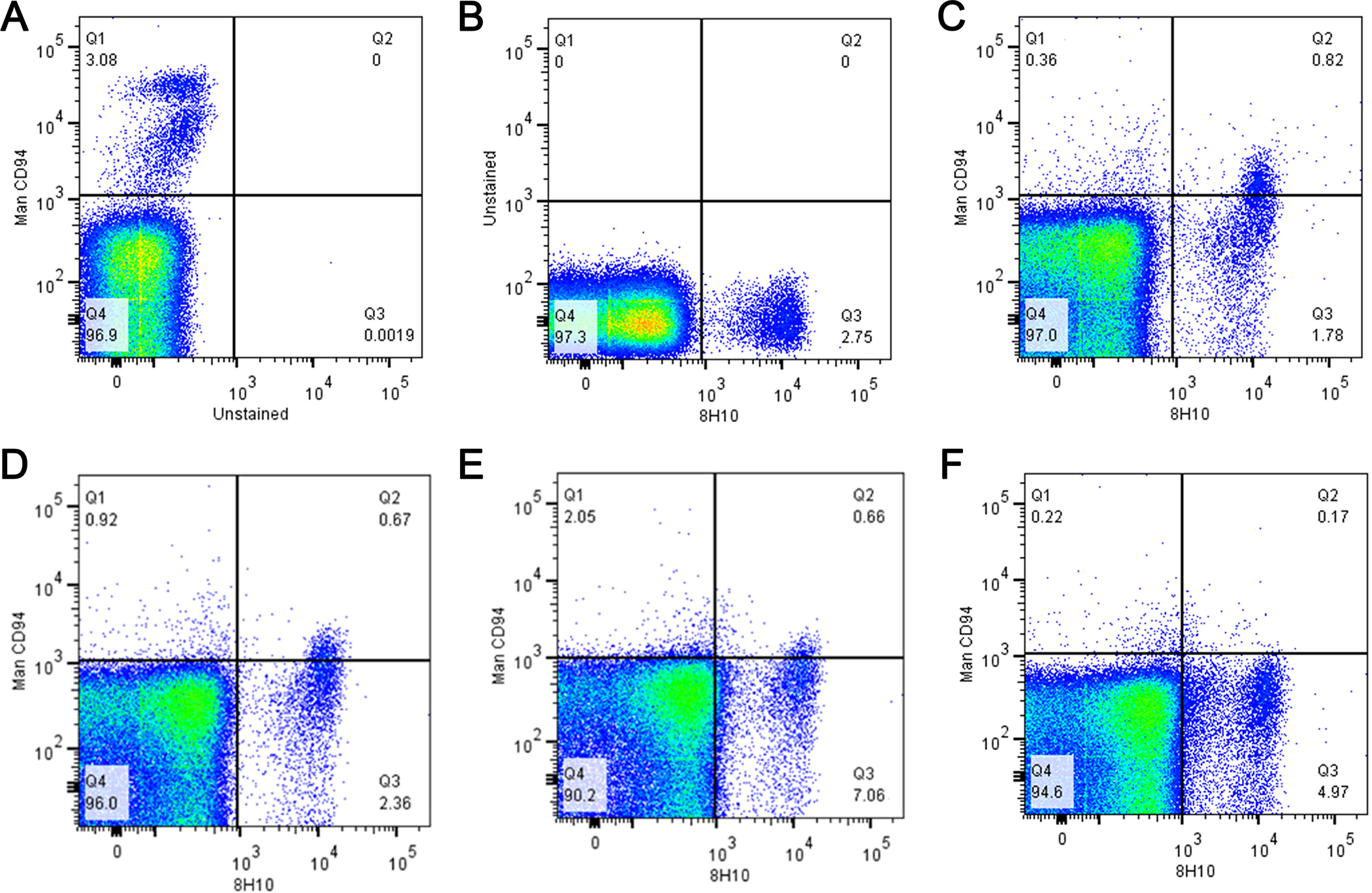
Competition between anti-human CD94 and anti-canine clone 8H10 mAb on canine PBMC. Upper row: (A) isotype control, (B) middle, 8H10 alone 5 μg/mL, and (C) anti- human CD94 costaining with anti-caCD94 (10 μg/mL). Bottom row: anti-human CD94 (10 μg/mL) with increasing concentrations of anti-caCD94 at (D) 10 μg, (E) 20 μg and (F) 40 μg/mL.
Figure 2.
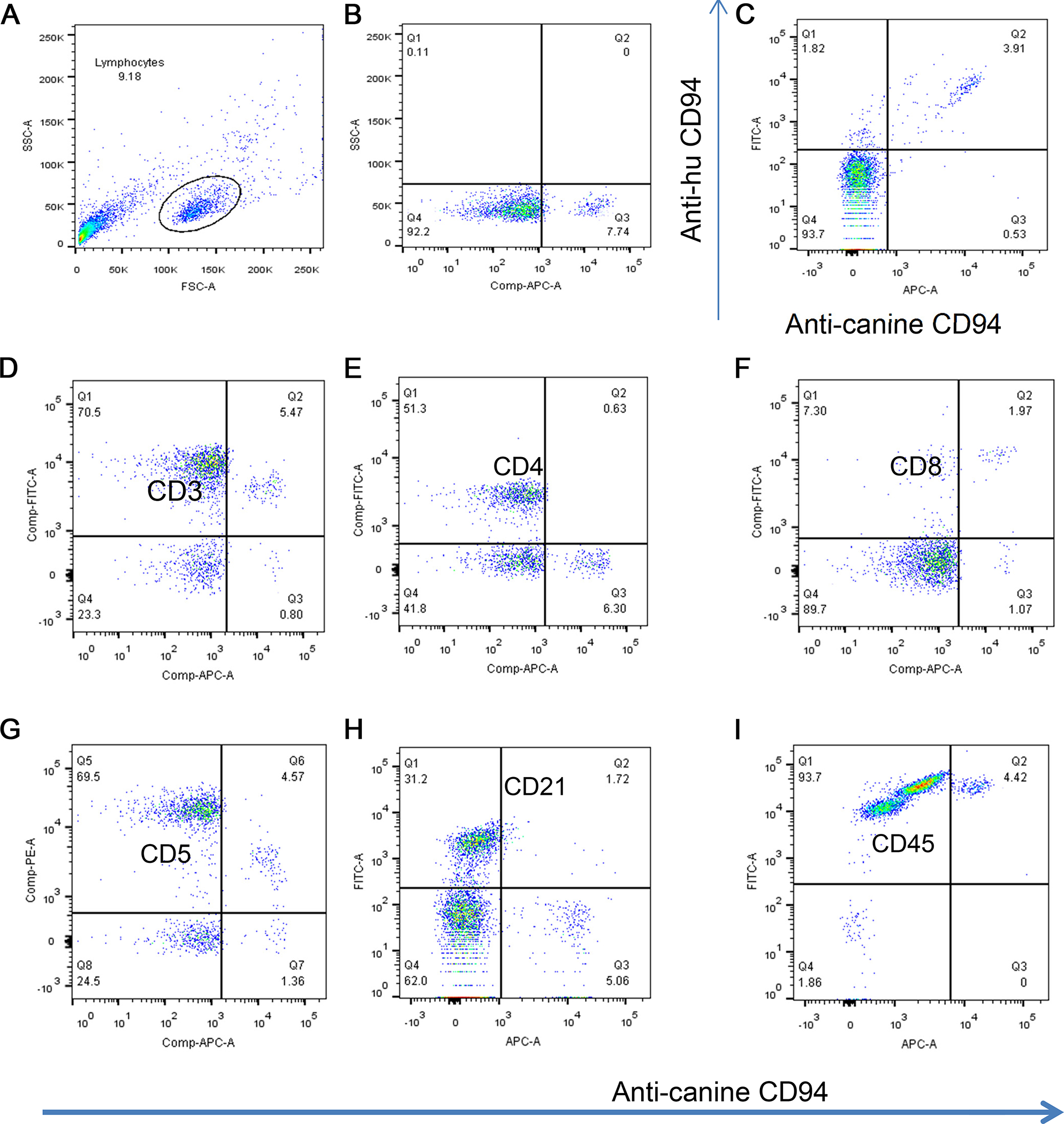
Phenotype of CD94+ resting canine lymphocytes. Moving left to right and from top to bottom, dot plots show the gated canine lymphocyte subset of PBMC (A), gated expression of anti-caCD94 on resting PBMC (B), and double positive staining of PBMC using anti-human CD94 and anti-ca CD94 (C). Subsequent figures indicate double staining of lymphocytes for expression of CD94 and CD3 (D), CE4 (E), CD8 (F), CD5 (G), CD21 (H), and CD45 (I). All gates were set using isotype-matched controls. The data is collectively representative of 3 or more dogs.
Figure 3. Immunophenotype of normal dog PBMC with caCD94, CD3, CD5 and NKp46.
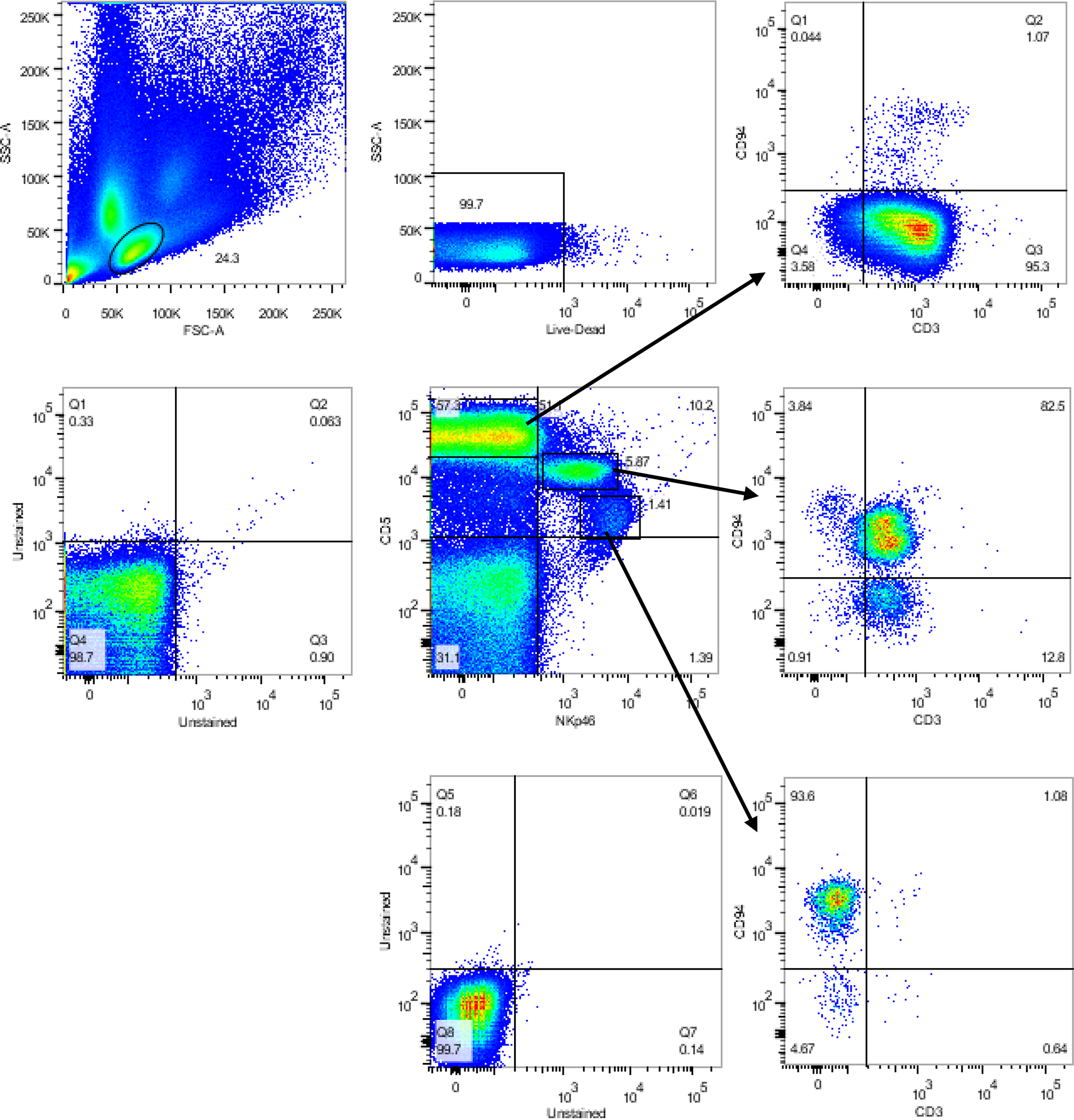
Live mononuclear cells (FSCloSSClo) were placed into and NKp46 by CD5 gate (center scatter-gram). CD5hiNKp46– are almost entirely CD3+, (top right scatter-gram). CD5+intermediate NKp46+ are mostly almost entirely CD3+, but with a high CD94+ population (ostensibly NKT cells) and a small portion CD94– (center right scatter-gram). In the CD5 dim NKp46hi population almost all cells are CD3–CD94hi putative NK cells, (lower right scatter-gram).
3.3. Anti-caCD94 fails to affect NK cell-mediated cytotoxicity
Human anti-CD94 mAb has been shown to increase, decrease, or have no effect on human NK redirected cytotoxicity against P815 cells (Perez-Villar et al., 1995). To assess the presence or absence of effector function of anti-caCD94, PBMC from three dogs were isolated and incubated for 16 hours with or without recombinant canine IL-2 and tested for cytolytic activity against 51Cr-labeled CTAC cells. As shown in Figure 4, anti-caCD94 mAb had no effect on resting or IL-2-activated cultured NK-mediated cytotoxicity.
Figure 4. Effect of anti-caCD94 mAb on cytotoxic activity of canine PBMC.
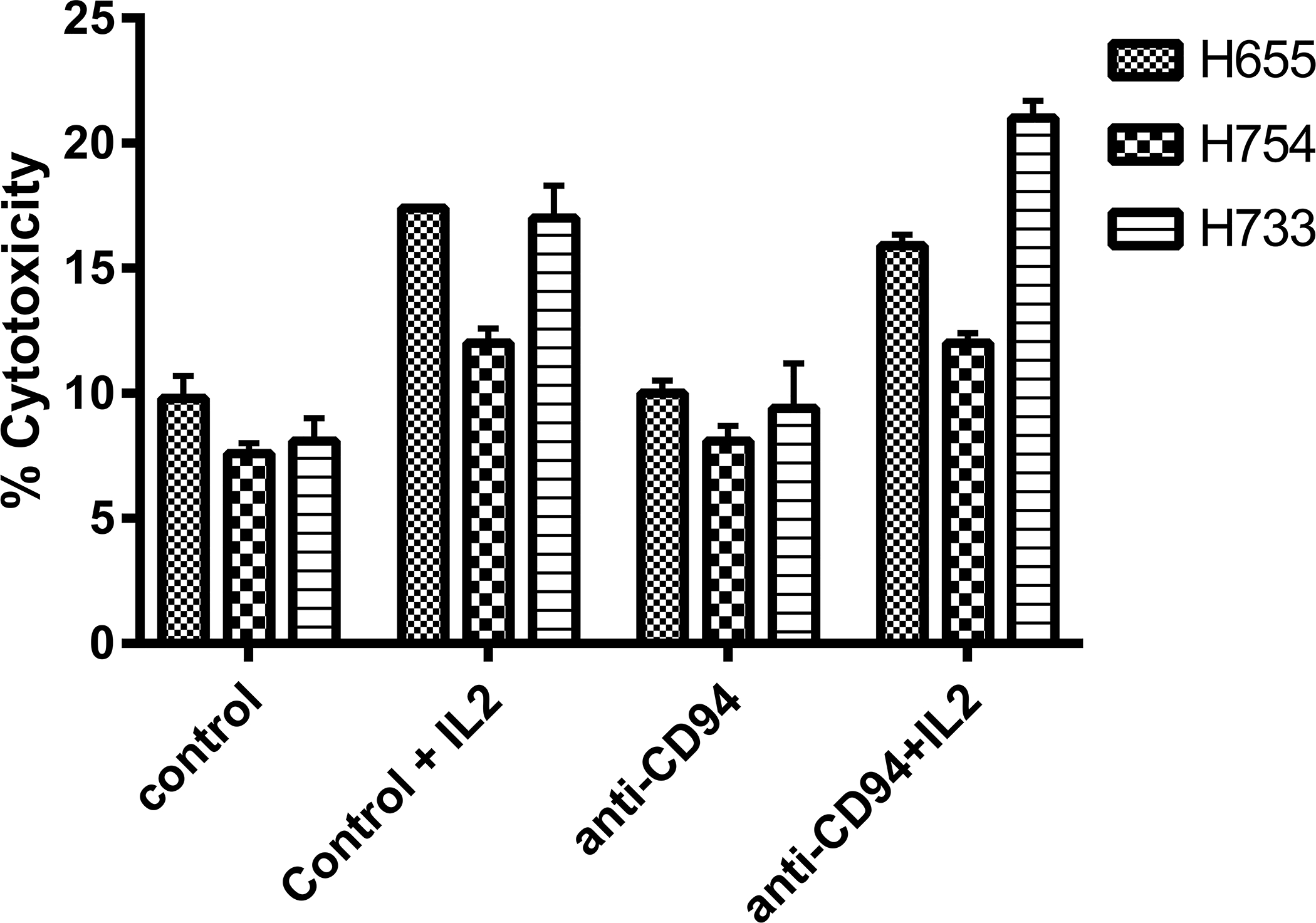
PBMC (5 × 105 cells per well) were incubated with and without anti-CD94 at 10 μg/m and recombinant canine IL-2 at 20 ng/ml. CTAC were labeled with 51Cr and added at 1 × 104 per well. Cells were incubated for 16 hours before harvesting supernatants. Data is presented as mean cytotoxicity at an E:T ratio of 25:1 (n=4).
3.4. Enrichment of NK cells using anti-ca CD94 mAb
Because anti-caCD94 mAb did not positively or negatively impact NK/NKT-mediated cytotoxicity either in the presence or absence of IL-2 in preliminary studies, we reasoned that the antibody could be used to enrich NK/NKT cells from PBMC without inhibiting cell function. To this end, 100–200 mL of peripheral blood were collected from healthy dogs and CD94+ cells were isolated using the anti-caCD94 mAb and magnetic bead sorting methodology. Unsorted PBMC, CD94– and CD94+ cells were tested directly in a CTAC cytotoxicity assay at effector-to-target (E:T) ratios ranging from 50:1 to 3:1. In two separate studies, CD94+ cells were more cytolytically active than unsorted PBMC (Figure 5). Compared to unsorted PBMC, CD94+ cells showed approximately a 4-fold greater cytotoxic activity at all E:T ratios.
Figure 5. NK cytotoxicity of naïve PBMC and CD94 sorted cells against 51Cr-labeled CTAC.
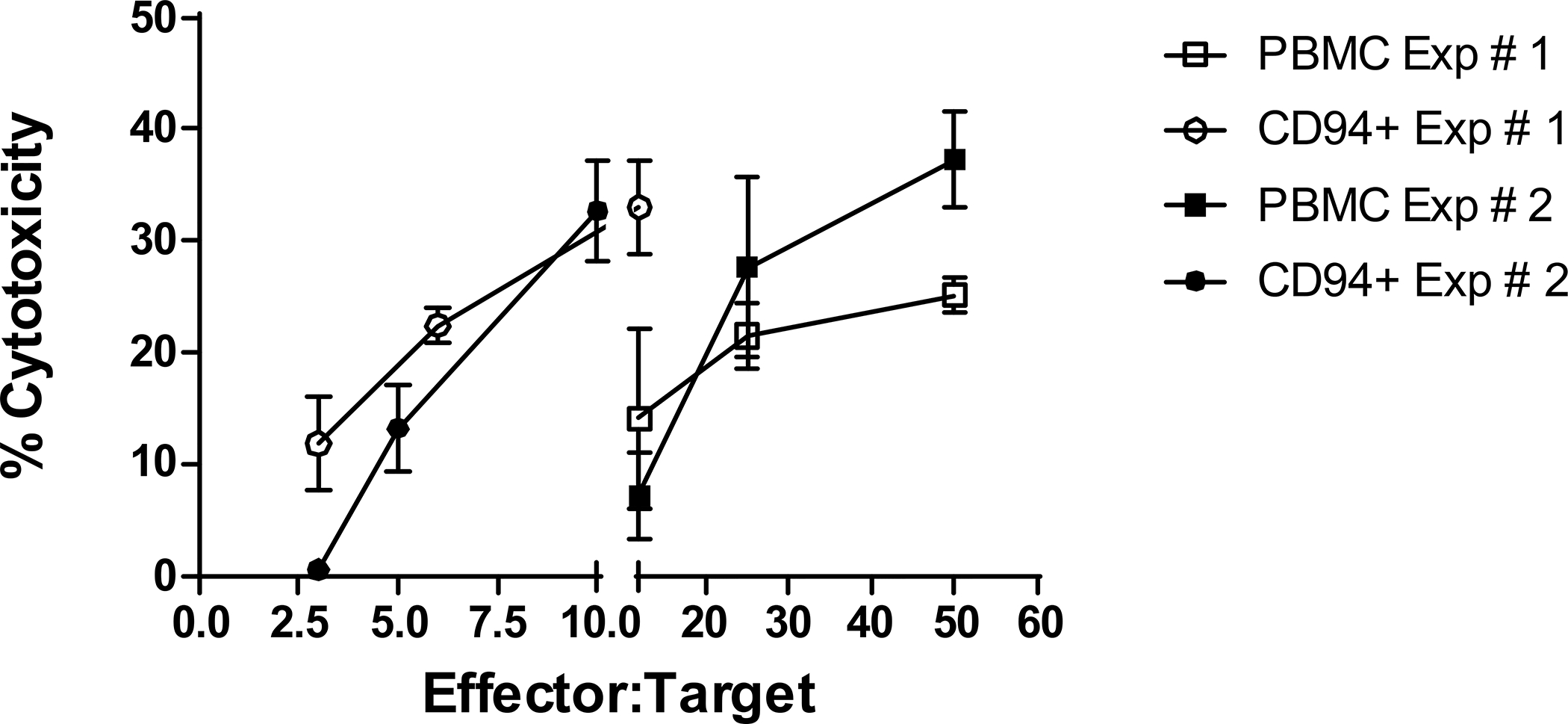
(A) Unsorted PBMC, CD94+ sorted cells were tested for cytotoxicity against 51Cr-labeled CTAC. Effector and target cells were added to microtiter wells at the E:T ratios indicated in the figures. Data are representative of duplicated experiments and presented as means for each E:T tested (n=4).
3.5. In vitro culture and expansion of CD94+ cells
Previous studies showed that canine NK/NKT cells with a CD3+ and CD8+ phenotype can be expanded from PBMC in culture medium containing IL-2 and IL-15 and using K562 cells genetically modified to express membrane-bound IL-15 and the 41BB ligand (Shin et al., 2013). We hypothesized that anti-caCD94 mAb plus magnetic bead selection and using CTAC feeder cells would improve selective expansion of NK/NKT cells. Flow cytometry studies on CTAC cells labeled with FITC-labeled 41BB-IgG2a revealed innate expression of 41BB ligand. Starting with 100–200 mL of anti-coagulated dog blood, 0.5–2.5 × 106 anti-CD94-selected cells were cultured in complete medium containing IL-2 and IL-15 on a feeder layer of irradiated CTAC cells. In one 10-day expansion experiment, CD94+ cells could be expanded to 79% of the total recovered population (data not shown). As shown in Figure 6, Day 11 expanded cells labeled with antibodies to additional lymphocyte surface markers revealed that the CD94+ population was primarily CD3– and NKp46± with the NKP46+ population being CD3± as well. Using this expansion method, we were successful in expanding our original CD94+ selected cells with low levels of CD3+ cells up to approximately 100-fold.
Figure 6.
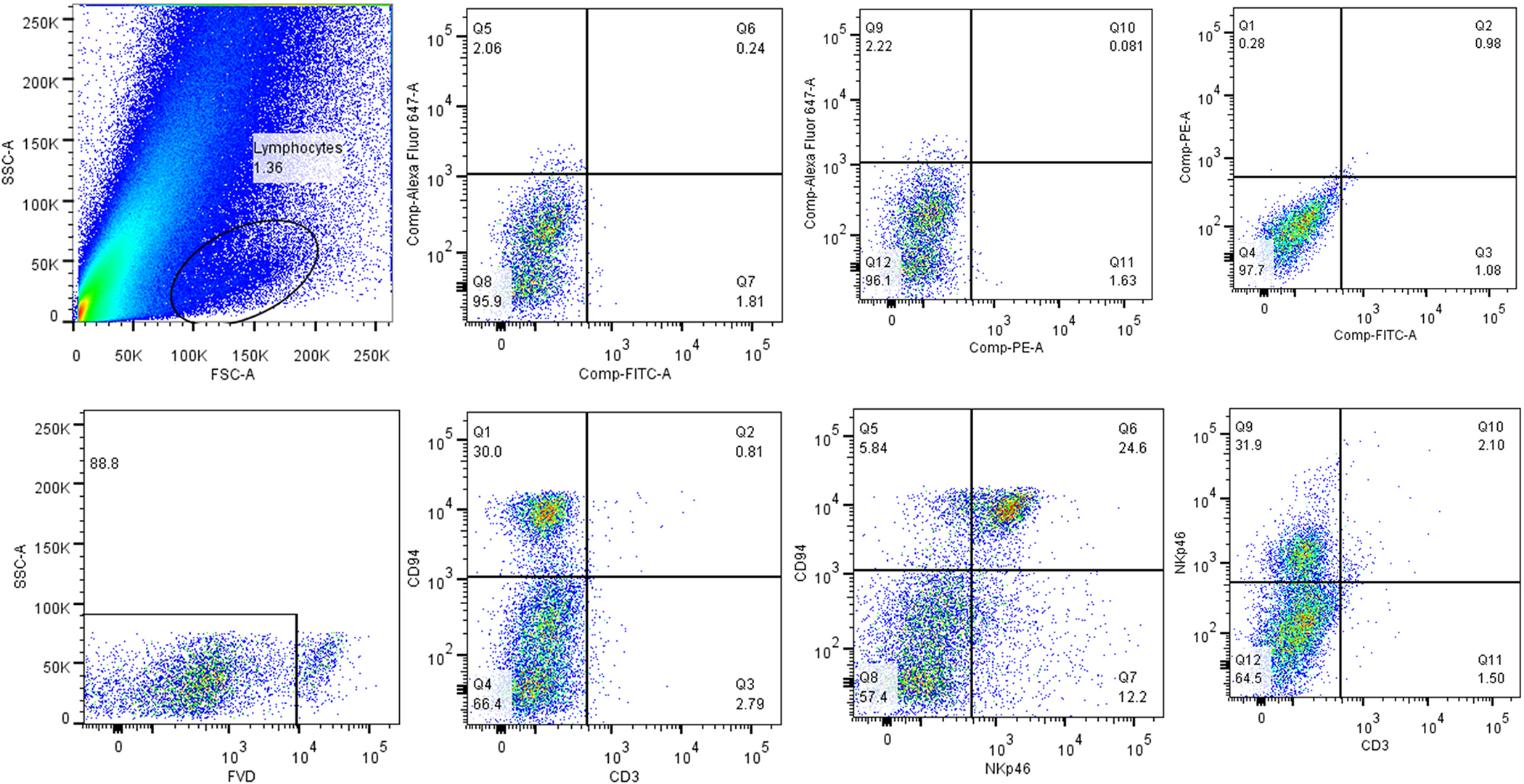
Phenotype of CD94 selected and expanded lymphocytes. Day 11 cultured cells were gated in FSC/SSC then placed into a viability gate (FVD/SSC). Immunophenotypes were compared to unstained controls or to anti-mouse IgG2a PE secondary (NKp46). CD94 cells at the end of culture were composed of CD3–, NKp46+/–.
Due to the scarcity of antibodies specific for canine NK cells, we used RT-PCR to further confirm the phenotype of ex vivo expanded CD94+ cells. To this end, we evaluated expression of NK/NKT cell-associated transcripts (CD16, CD56, CD94, Eomes, NCR1/NKp46, granzyme B, and perforin) in expanded cells (days 6 and 10 or 11) from three different dogs. Clear expression of CD16, CD56, and Eomes was observed in cell preparations in 3 of 3 dogs while NCRI, granzyme and perforin expression was seen in the cell preparations of 2 of 3 dogs (data not shown). Possible differences in expression between dogs, and different concentrations of NK cells in the expanded cultures may bear upon the expression levels of the NK cell- specific transcripts.
3.6. Cytolytic activity of expanded CD94+ cells
Visual inspection of the tissue culture plates of CD94+-selected cells after expansion on CTAC feeder cells suggested the presence of NK/NKT cells exhibiting high cytolytic activity by days 8–10 after initiation of culture. The cultured CD94+-selected lymphocytes effectively eliminated nearly all CTAC feeder cells overnight (data not shown). Cytolytic activity was tested empirically using a CTAC 51Cr release assay. Cytotoxicity of CD94+ cultured cells exceeded that of fresh PBMC and IL-2-stimulated PBMC at all E:T ratios Specifically, ex vivo expanded CD94+ cells mediated 60% cytotoxicity at an E:T ratio of 6:1, while freshly isolated PBMC mediated only 20% cytotoxicity at an E:T ratio of 100:1 (Figure 7).
Figure 7. Cytotoxicity and phenotype of CD94+-selected and expanded canine lymphocytes.
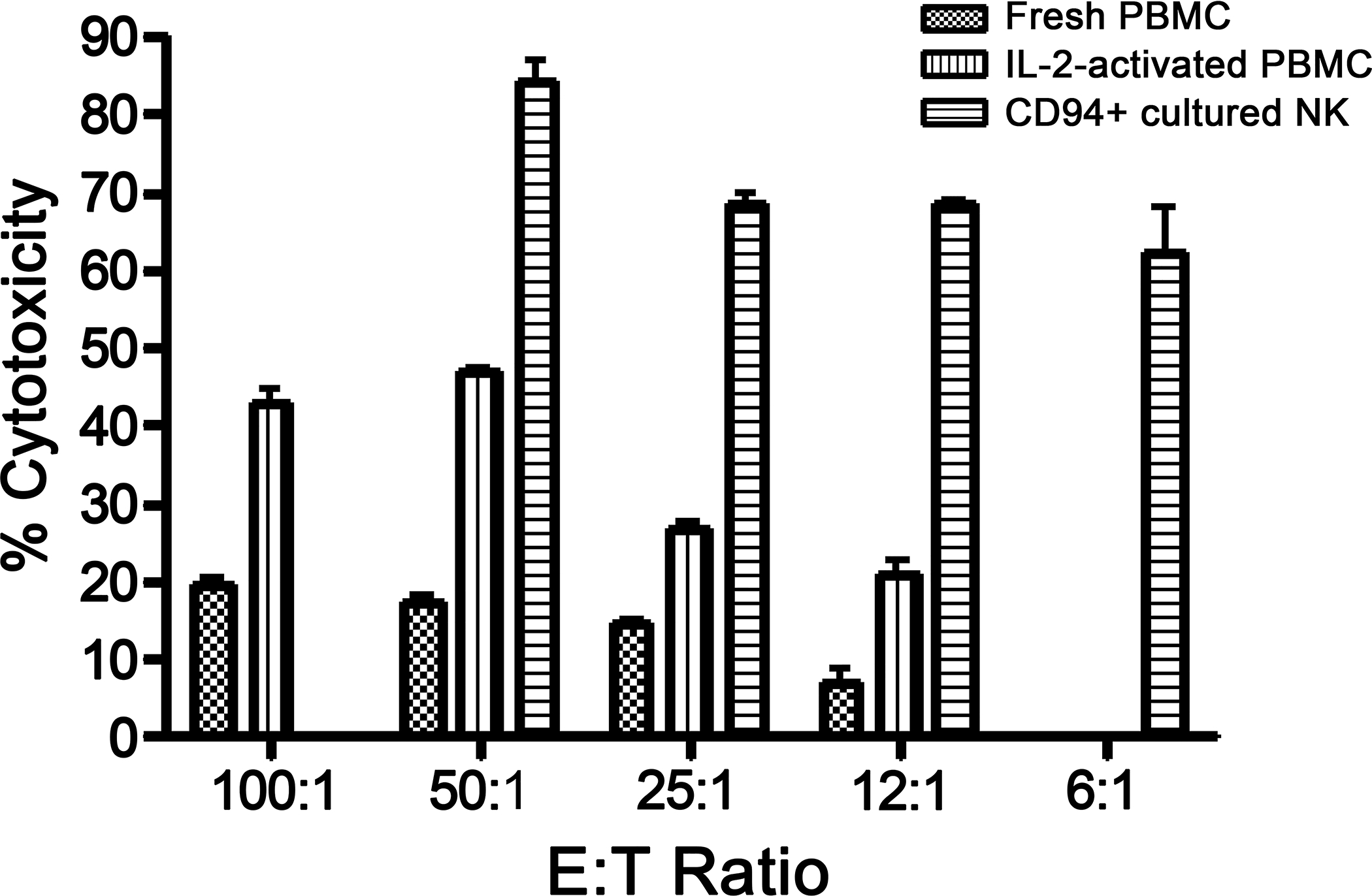
(A) Canine fresh PBMC, IL-2 activated PBMC, or CD94+-selected lymphocytes were tested for cytotoxicity against 51CR-labeled CTAC cells in an 18-hour assay. Data are presented as mean values of quadruplicate samples at each E:T ratio. CD94+ cultured cell-mediated cytotoxicity is significantly greater at each E:T ratio than fresh PBMC or IL-2 activated PBMC (p≤0001, Student’s T test). Representative data of three experiments with different dogs.
3.7. Morphology of CD94+-selected and cultured cells
Human and murine NK/NKT cells are characterized as large granular lymphocytes (LGL). Canine NK cells have been similarly classified (Michael et al., 2013). To verify that CD94 selected and cultured lymphocytes were of LGL morphology, we collected CD94+ cells after a 9-day culture period and examined them by transmission electron microscopy for the presence of cytoplasmic large electron dense granules and reniform nuclei. As shown in Figure 8, anti-CD94 mAb-selected and cultured lymphocytes present typical LGL morphology.
Figure 8. Transmission electron micrographs of CD94 selected cells.
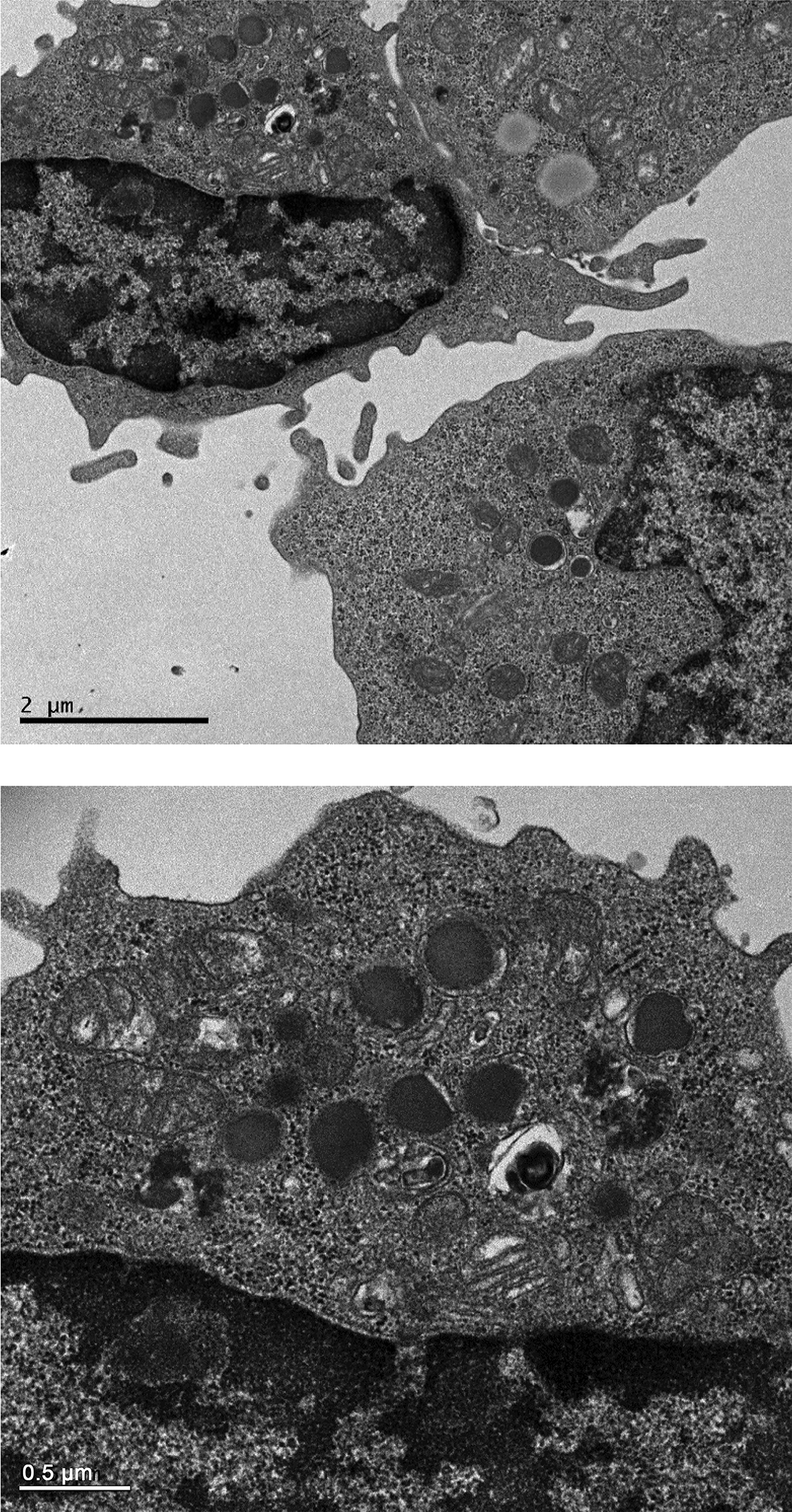
Electron-dense granules and reniform nuclei, typical of LGL, are shown in both figures. Top photo is 2000X and the lower is 5000X magnification with the bars indicating 2.0 and 0.5 microns, respectively.
4. DISCUSSION
The present study was undertaken to generate and describe a new mouse anti-canine specific mAb against CD94, a cell surface marker known to be present on human NK/NKT cells (Hromadnikova et al., 2016). Here, we ascertain the specificity of anti-caCD94 mAb based on the following criteria. A recombinant version of the extracellular domain of canine CD94 was used to produce anti-CD94 mAb. Hybridoma screening was done by ELISA using recombinant canine CD94-Ig1 Fc fusion protein for capture. Anti-caCD94 competed for binding to canine lymphocytes with an anti-human CD94 shown to be cross-reactive with canine lymphocytes (Schuberth et al., 2007). Anti-caCD94 mAb bound to CD5low+ canine lymphocytes; CD5low expression reported to be characteristic for canine NK/NKT cells (Huang et al., 2008). Anti-ca CD94 mAb bound lymphocytes that also tested positive for NKp46 expression (Foltz et al., 2016). Collectively, these studies are consistent with the notion that anti-caCD94 was specific for canine NK/NKT cells and is applicable for ELISA, flow cytometry, cell binding studies, and CD94 enrichment studies.
In humans, the function of CD94 has been well-defined, including its dimerization with NKG2 molecules that serve as receptors for either NK cell activation or inhibition (Iwaszko and Bogunia-Kubik, 2011). Anti-human CD94 has been shown to augment proliferation and IFN-γ production by human NK cells in the presence of IL-2 or IL-15 (Perez-Villar et al., 1995). Antibodies directed against CD94 can either augment, inhibit, or have a null effect on human NK cell proliferation. However, in canines, the function of CD94 has been inadequately described. It is currently unknown whether CD94 is expressed as a monomer or a heterodimer with an NKG2-like molecule on the surface of canine NK cells, what is the nature of the cognitive ligand for canine CD94, and whether an antibody directed against CD94 affects cell function. As to the latter point, the understanding of the regulation of canine NK cells is to date essentially limited to characterization of single Ly49 gene. The protein contains an immune tyrosine-based inhibition motif (ITIM) suggesting that the receptor functions as an inhibitory molecule. (Gagnier et al., 2003).
Previous reports suggest that based on morphology and phenotype, canine NK/NKT cells are of a T-cell lineage (Foltz et al., 2016; Loughran et al., 1985; Ringler and Krakowka, 1985). Our studies showed that anti-caCD94 bound to CD3+ as well as CD3– lymphocytes but not to B cells or monocytes obtained from freshly isolated PBMC. Freshly isolated CD94+ cells were CD5low, and NCR-1/NKp46+ which has been previously shown to be a characteristic of canine NK/NKT cells (Foltz et al., 2016; Huang et al., 2008). Anti-caCD94 mAb recognized an epitope on canine NK/NKT cells that upon ligation failed to either augment or inhibit NK cell-mediated cytotoxicity against CTAC target cells when the antibody was added during a cytotoxicity assay with resting PBMC. Also, anti-caCD94 enriched lymphocytes were more cytolytic than resting PBMC suggesting cytotoxicity of anti-caCD94 mAb purified cells was not affected by antibody binding to CD94. However, it is possible that binding of anti-caCD94 to the NK/NKT cell fraction of PBMC initiated expansion of the NK/NKT cell population cultured in medium containing IL-2 and IL-15. Clearly, additional studies are warranted for better understanding of the effect of anti-CD94 binding to NK/NKT cells.
CD94 has been shown to identify distinct subsets within the mouse NK cell population (Yu et al., 2009). One population, CD94high, delineates, a population of NK cells showing a greater proliferative and cytolytic capacity than the CD94low population while the CD94low population showed a lower expression of Ly49D (activating) and LY49G2 (inhibitory) markers. In these studies, purified mouse CD94low cells became CD94high when injected into mice (Yu et al., 2009). In humans, CD94 expression has been applied to NK cell differentiation. Studies conducted by Yu et al (Yu et al., 2009) showed that CD94high, CD56dim NK cells produce less IFNγ but have higher granzyme B and perforin expression than CD56bright cells. The high levels of canine CD94 expression correlate with the NK phenotypic profile of NKp46+ CD5dim and therefore may be of use in further definition of canine NK cells. Our studies also indicate canine CD94 cells can also be divided into different subsets with respect to CD3, CD5, and NKp46 expression profiles. Anti-caCD94 may provide insight into the similar functional distinctions of NK/NKT cells as has been reported for mouse NK cells.
Anti-caCD94 mAb was effective in selecting NK/NKT cells for ex vivo expansion. Expansion of CD94+ cells obtained from freshly isolated PBMC resulted in changes in cell surface markers compared to freshly isolated CD94+ cells. CD5low was expressed on freshly isolated CD94+ cells but was not observed on highly cytotoxic cultured CD94+ selected cells, suggesting that maturation or other phenotypic changes occurred following CD94+ cell expansion using the culture methods outlined in this study. CD94+ cell enrichment for in vitro NK cell expansion resulted at times in a reduction in the number of CD3+ or CD8+ cells that was not observed by others using cell culture to expand canine NK cells (Shin et al., 2013). CD94+-selected and cultured cells contained cytoplasmic azurophilic granules and reniform nuclei characteristic of canine NK cells when examined by electron microscopy (Loughran et al., 1985). Enrichment of CD94+CD3– cells was variable and possibly due to differences in the efficacy of the CD94 selection process and the amount of contamination by T cells during enrichment. T cell depletion using anti-CD5 prior to CD94 enrichment is a logical solution to this problem (Michael et al., 2013). Depletion of CD3+ followed by CD94+ cell selection may also be an alternative. A clinical study using CD3+ cell depletion CD56+ cell selection regimen followed by IL-15/41BBL-activated NK cell was performed on patients with ultra-high-risk tumors (Shah et al., 2015). Unfortunately, this protocol failed to prevent occurrence of acute GVHD, leading to the conclusion that the activated NK cells contributed to the disease. However, such approaches beg the question as to what is the outcome or role of the CD3low population of which NK cells are comprised?
Because NK cells represent a small fraction of the total PBMC (<15%), ex vivo expansion methods provide a means to enhance NK numbers sufficiently for in vivo transfer (Cheng et al., 2013). Ex vivo culture techniques resulting in specific expansion of cytolytically active NK cells for adoptive immunotherapy offers a promising approach for administration of sufficiently high doses of donor NK cells to mediate anti-tumor effects. Various strategies for NK cell expansion prior to infusion have been described including the use of cytokines (Siegler et al., 2010; Spanholtz et al., 2011), feeder cells (Miller et al., 1994), CD137 ligand (Lapteva et al., 2012; Voskens et al., 2010), and Epstein-Barr virus-infected feeder cells (Berg et al., 2009). Foltz et al. (Foltz et al., 2016) reported that efficient expansion of canine NK cells could be obtained using irradiated K562 feeder cells transfected with 4–1BBL and recombinant IL-21. Adoptive immunotherapy of in vitro expanded NK cells using this approach proved therapeutically active against a canine sarcoma model (Canter et al., 2017). Expansion methods resulting in the generation of NK memory-like NK cells is a particularly important area of study due to the limited in vivo survival period of allogeneic NK cells.
In summary, we describe the development and immunoreactivity of an anti-caCD94 mAb that recognizes dog NK / NKT cells. Furthermore, anti-ca CD94 was effective in expanding cells with natural killer cytotoxicity. We anticipate future studies will enable exploration of effective NK cell immunotherapy protocols for the treatment of malignancies in both canines and humans. Establishing the appropriate mAb to analogs of human NK cell surface molecules in the dog is an important step in this direction.
HIGHLIGHTS.
We describe a new anti-canine CD94 monoclonal antibody
The antibody specifically binds to dog NK and NKT cells
Antibody and magnetic bead selected CD94+ cells expand on canine thyroid adenocarcinoma cells.
CD94 selected lymphocytes are highly cytolytic with large granular lymphocyte morphology
ACKNOWLEDGEMENTS
The authors thank Alix McPhearson and the Fred Hutchinson Cancer Research Center’s animal research technicians and veterinarians for their assistance with animal care, and Helen Crawford for her assistance with manuscript preparation.
ROLE OF THE FUNDING SOURCE
Research reported in this manuscript was supported by the National Cancer Institute of the National Institutes of Health under award number P01CA078902 and P30CA015704. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, which had no involvement in the in study design; the collection, analysis and interpretation of data; the writing of the report; nor in the decision to submit the article for publication.
Footnotes
DECLARATION OF INTEREST: There are no conflicts of interest to report.
SUBMISSION DECLARATION AND VERIFICATION: We affirm that this work is original and has not been submitted elsewhere for publication in any form.
REFERENCES
- Addissie S, Klingemann H, 2018. Cellular immunotherapy of canine cancer. Veterinary sciences 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom E, Kristensson K, Ljunggren HG, 2004. Activation of natural killer cells: underlying molecular mechanisms revealed (Review). Scandinavian Journal of Immunology 60, 14–22. [DOI] [PubMed] [Google Scholar]
- Berg M, Lundqvist A, McCoy P Jr., Samsel L, Fan Y, Tawab A, Childs R, 2009. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy 11, 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canter RJ, Grossenbacher SK, Foltz JA, Sturgill IR, Park JS, Luna JI, Kent MS, Culp WTN, Chen M, Modiano JF, Monjazeb AM, Lee DA, Murphy WJ, 2017. Radiotherapy enhances natural killer cell cytotoxicity and localization in pre-clinical canine sarcomas and first-in-dog clinical trial. Journal for immunotherapy of cancer 5, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M, Chen Y, Xiao W, Sun R, Tian Z, 2013. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol 10, 230–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudkowicz G, Bennett M, 1971. Peculiar immunobiology of bone marrow allografts. I. Graft rejection by irradiated responder mice. Journal of Experimental Medicine 134, 83–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz JA, Somanchi SS, Yang Y, Aquino-Lopez A, Bishop EE, Lee DA, 2016. NCR1 Expression Identifies Canine Natural Killer Cell Subsets with Phenotypic Similarity to Human Natural Killer Cells. Front Immunol 7, 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnier L, Wilhelm BT, Mager DL, 2003. Ly49 genes in non-rodent mammals. Immunogenetics 55, 109–115. [DOI] [PubMed] [Google Scholar]
- Graves SS, Stone DM, Loretz C, Peterson LJ, Lesnikova M, Hwang B, Georges GE, Nash R, Storb R, 2011. Antagonistic and agonistic anti-canine CD28 monoclonal antibodies: tools for allogeneic transplantation. Transplantation 91, 833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Klein J, Nei M, 2006. Heterogeneous but conserved natural killer receptor gene complexes in four major orders of mammals. Proc Natl Acad Sci U S A 103, 3192–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromadnikova I, Li S, Kotlabova K, Dickinson AM, 2016. Influence of in vitro IL-2 or IL-15 alone or in combination with Hsp 70 derived 14-Mer peptide (TKD) on the expression of NK cell activatory and inhibitory receptors on peripheral blood T cells, B cells and NKT cells. PLoS One 11, e0151535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YC, Hung SW, Jan TR, Liao KW, Cheng CH, Wang YS, Chu RM, 2008. CD5-low expression lymphocytes in canine peripheral blood show characteristics of natural killer cells. Journal of Leukocyte Biology 84, 1501–1510. [DOI] [PubMed] [Google Scholar]
- Iwaszko M, Bogunia-Kubik K, 2011. Clinical significance of the HLA-E and CD94/NKG2 interaction. Arch Immunol Ther Exp (Warsz) 59, 353–367. [DOI] [PubMed] [Google Scholar]
- Kawai T, Wee SL, Bazin H, Latinne D, Phelan J, Boskovic S, Ko DS, Hong HZ, Mauiyyedi S, Nadazdin O, Abrahamian G, Preffer F, Colvin RB, Sachs DH, Cosimi AB, 2000. Association of natural killer cell depletion with induction of mixed chimerism and allograft tolerance in non-human primates. Transplantation 70, 368–374. [DOI] [PubMed] [Google Scholar]
- Knapp DW, Leibnitz RR, DeNicola DB, Turek JJ, Teclaw R, Shaffer L, Chan TC, 1993. Measurement of NK activity in effector cells purified from canine peripheral lymphocytes. Vet Immunol Immunopathol 35, 239–251. [DOI] [PubMed] [Google Scholar]
- Lapteva N, Durett AG, Sun J, Rollins LA, Huye LL, Fang J, Dandekar V, Mei Z, Jackson K, Vera J, Ando J, Ngo MC, Coustan-Smith E, Campana D, Szmania S, Garg T, Moreno-Bost A, Vanrhee F, Gee AP, Rooney CM, 2012. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy 14, 1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughran TP Jr., Deeg HJ, Storb R, 1985. Morphologic and phenotypic analysis of canine natural killer cells: Evidence for T-cell lineage. Cellular Immunology 95, 207–217. [DOI] [PubMed] [Google Scholar]
- Mattsson J, Ringdén O, Storb R, 2008. Graft failure after allogeneic hematopoietic cell transplantation. Biology of Blood and Marrow Transplantation 14 (Suppl. 1), 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael HT, Ito D, McCullar V, Zhang B, Miller JS, Modiano JF, 2013. Isolation and characterization of canine natural killer cells. Vet Immunol Immunopathol 155, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Alley KA, McGlave P, 1994. Differentiation of natural killer (NK) cells from human primitive marrow progenitors in a stroma-based long-term culture system: identification of a CD34+7+ NK progenitor. Blood 83, 2594–2601. [PubMed] [Google Scholar]
- Park JY, Shin DJ, Lee SH, Lee JJ, Suh GH, Cho D, Kim SK, 2015. The anti-canine distemper virus activities of ex vivo-expanded canine natural killer cells. Vet Microbiol 176, 239–249. [DOI] [PubMed] [Google Scholar]
- Perez-Villar JJ, Melero I, Rodriguez A, Carretero M, Aramburu J, Sivori S, Orengo AM, Moretta A, Lopez-Botet M, 1995. Functional ambivalence of the Kp43 (CD94) NK cell-associated surface antigen. J Immunol 154, 5779–5788. [PubMed] [Google Scholar]
- Raff RF, Deeg HJ, Loughran TP Jr., Graham TC, Aprile JA, Sale GE, Storb R, 1986. Characterization of host cells involved in resistance to marrow grafts in dogs transplanted from unrelated DLA-nonidentical donors. Blood 68, 861–868. [PubMed] [Google Scholar]
- Raff RF, Sandmaier BM, Graham T, Loughran TP Jr., Pettinger M, Storb R, 1994. Resistance to DLA-nonidentical canine unrelated marrow grafts is unrestricted by the major histocompatibility complex. Experimental Hematology 22, 893–897. [PubMed] [Google Scholar]
- Ringler SS, Krakowka S, 1985. Cell surface markers of the canine natural killer (NK) cell. Vet Immunol Immunopathol 9, 1–11. [DOI] [PubMed] [Google Scholar]
- Saether PC, Hoelsbrekken SE, Fossum S, Dissen E, 2011. Rat and mouse CD94 associate directly with the activating transmembrane adaptor proteins DAP12 and DAP10 and activate NK cell cytotoxicity. J Immunol 187, 6365–6373. [DOI] [PubMed] [Google Scholar]
- Schuberth HJ, Kucinskiene G, Chu RM, Faldyna M, 2007. Reactivity of cross-reacting monoclonal antibodies with canine leukocytes, platelets and erythrocytes. Vet Immunol Immunopathol 119, 47–55. [DOI] [PubMed] [Google Scholar]
- Shah NN, Baird K, Delbrook CP, Fleisher TA, Kohler ME, Rampertaap S, Lemberg K, Hurley CK, Kleiner DE, Merchant MS, Pittaluga S, Sabatino M, Stroncek DF, Wayne AS, Zhang H, Fry TJ, Mackall CL, 2015. Acute GVHD in patients receiving IL-15/4–1BBL activated NK cells following T-cell-depleted stem cell transplantation. Blood 125, 784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DJ, Park JY, Jang YY, Lee JJ, Lee YK, Shin MG, Jung JY, Carson WE 3rd, Cho D, Kim SK, 2013. Ex vivo expansion of canine cytotoxic large granular lymphocytes exhibiting characteristics of natural killer cells. Vet Immunol Immunopathol 153, 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegler U, Meyer-Monard S, Jorger S, Stern M, Tichelli A, Gratwohl A, Wodnar-Filipowicz A, Kalberer CP, 2010. Good manufacturing practice-compliant cell sorting and large-scale expansion of single KIR-positive alloreactive human natural killer cells for multiple infusions to leukemia patients. Cytotherapy 12, 750–763. [DOI] [PubMed] [Google Scholar]
- Spanholtz J, Preijers F, Tordoir M, Trilsbeek C, Paardekooper J, de Witte T, Schaap N, Dolstra H, 2011. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PLoS One 6, e20740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan PHS, Santos EB, Rossbach HC, Sandmaier BM, 1993. Enhancement of natural killer activity by an antibody to CD44. Journal of Immunology 150, 812–820. [PubMed] [Google Scholar]
- Vilches C, Parham P, 2002. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity (Review). Annual Review of Immunology 20, 217–251. [DOI] [PubMed] [Google Scholar]
- Voskens CJ, Watanabe R, Rollins S, Campana D, Hasumi K, Mann DL, 2010. Ex-vivo expanded human NK cells express activating receptors that mediate cytotoxicity of allogeneic and autologous cancer cell lines by direct recognition and antibody directed cellular cytotoxicity. J Exp Clin Cancer Res 29, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Wei M, Mao H, Zhang J, Hughes T, Mitsui T, Park IK, Hwang C, Liu S, Marcucci G, Trotta R, Benson DM Jr., Caligiuri MA, 2009. CD94 defines phenotypically and functionally distinct mouse NK cell subsets. J Immunol 183, 4968–4974. [DOI] [PMC free article] [PubMed] [Google Scholar]


