Abstract
Haemobilia describes blood loss from the biliary tract and classically presents as Quincke’s triad: upper gastrointestinal bleeding (UGIB), jaundice and right upper quadrant abdominal pain. We discuss the case of a 70-year-old male with a previously stented Bismuth 1 hilar cholangiocarcinoma who presented with haematemesis. He had a similar presentation a month ago where a forward viewing gastroscope identified fresh and altered blood in the distal stomach but no clear source of bleeding. During this admission, a side-viewing duodenoscope identified bleeding from the periampullary region, which was managed by inserting a fully covered self-expanding metal stent (fcSEMS) within his pre-existing uncovered SEMS to tamponade the haemorrhage. This case highlights the importance of using a side-viewing duodenoscope for patients with UGIB on a background of a stented cholangiocarcinoma and inserting a fcSEMS within an uncovered SEMS is feasible and effective in managing these patients.
Keywords: SEMS, cholangiocarcinoma, haemobilia
INTRODUCTION
Haemobilia describes blood loss from the biliary tract, a term first used by Philip Sandblom [1]. Whereas haemobilia classically presents as Quincke’s triad (the association of upper gastrointestinal haemorrhage with jaundice and right upper quadrant abdominal pain), all three components of this triad are only present in 22–35% of patients with haemobilia [2]. Transhepatic percutaneous intervention is the most common cause of haemobilia with rarer causes including trauma, endoscopic intervention, malignancy and inflammatory states. Malignancy itself is a risk factor for haemobilia due to the increased vascularity and friability of the tissues involved. We discuss the case of a patient who developed haemobilia as a result of disease progression of a previously stented hilar cholangiocarcinoma, his subsequent management and a literature review.
CASE REPORT
A 70-year-old male presented to the emergency department after collapsing twice. Paramedics observed him vomiting dark red blood in the ambulance. In the emergency department, he was witnessed having another episode of haematemesis (200 ml fresh blood) and an episode of melaena.
Nine months previously, the patient presented with obstructive jaundice and had computed tomography imaging which revealed an unresectable Bismuth type 1 hilar cholangiocarcinoma. His jaundice resolved after he underwent endoscopic retrograde cholangiopancreatography (ERCP) during which an uncovered SEMS (10 mm × 80 mm uncovered WallFlex biliary Rx stent, Boston Scientific) was deployed across his hilar stricture in an infra-papillary fashion with the proximal end of the stent placed just below the liver hilum and the distal end below the papilla (Fig. 1).
Figure 1.

(A) Perihilar stricture identified at index ERCP due to cholangiocarcinoma; (B) Uncovered SEMS inserted across the perihilar stricture to decompress the biliary tree.
The patient subsequently re-presented to us 8 months post uncovered SEMS insertion with haematemesis and melaena. Whilst an oesophagastroduodenoscopy (OGD), performed with a conventional forward viewing endoscope identified both fresh and altered blood in the distal stomach and duodenum, a source of his bleeding could not be identified. He was transfused and discharged after a period of observation.
He had a past medical history of chronic kidney disease, insulin dependent type 2 diabetes mellitus, hypertension, hypercholesterolaemia, vitamin B12 and folate deficiency. His drug history included folic acid, doxazosin, spironolactone, atorvastatin, lansoprazole and Novomix 30. He had no known drug allergies and did not require any assistance with his activities of daily living.
The patient was transfused with packed red cells and was appropriately resuscitated. An OGD was performed which identified both fresh and altered blood in the duodenum but once again the source of bleeding could not be identified. Given his previous biliary history, we proceeded with repeat endoscopic assessment using a side-viewing duodenoscope to visualise the periampullary area. This examination revealed active bleeding originating from the lumen of the metal stent thereby confirming ongoing haemobilia; this presumably had arisen from his stented cholangiocarcinoma. A repeat ERCP was then performed during which a fully covered self-expanding metal stent (fcSEMS) (10 mm × 80 mm fully covered WallFlex biliary Rx stent, Boston Scientific) was inserted within the uncovered SEMS to tamponade the bleeding area (Fig. 2). Following this intervention further bleeding was not observed.
Figure 2.
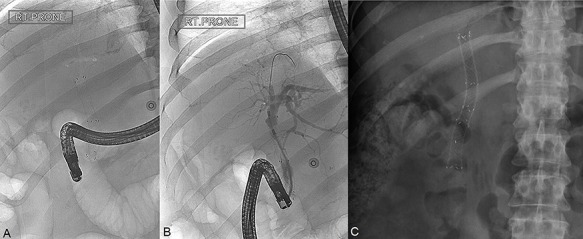
(A) ERCP image taken at the time of presentation with haemobilia identifying a single uncovered SEMS in situ; (B) ERCP image during fcSEMS deployment within the uncovered SEMS; (C) Subsequent abdominal radiograph showing both uncovered SEMS and fcSEMS in place.
On this admission, his baseline observations were heart rate 100/min, blood pressure 132/73 mmHg, respiratory rate 18/min, oxygen saturations 97% on room air and he was afebrile. On examination, there was evidence of mild peripheral oedema. Examination of his cardiovascular and respiratory systems was unremarkable. His abdomen was distended but soft and non-tender with normal bowel sounds.
DISCUSSION
Surgical, endoscopic and radiological intervention can be used to treat haemobilia with the choice of intervention dependent on the cause of haemobilia and patient status. At our patient’s index ERCP, we elected to insert a SEMS instead of a plastic stent as metal stents offer a longer duration of patency due to their greater diameter. Whereas metal stents are used in diameters of up to 10 mm for biliary tract disease, plastic stents used in this context are usually no wider than 10 French (~3.18 mm). This is certainly an important consideration as one wishes to avoid frequent stent changes in a patient population who will undoubtedly become increasingly frail as a result of their advancing malignancy [3]. Two recent meta-analyses and systematic reviews comparing SEMS and plastic stents (11 retrospective and prospective studies, n = 947 and 20 randomised controlled trials, n = 1713, respectively) found SEMS to be associated with longer stent patency, lower re-intervention rates and lower rates of cholangitis [4].
Moreover, at our patient’s index ERCP we elected to insert an uncovered SEMS and not a fcSEMS. As compared to fcSEMS, uncovered SEMS are less likely to migrate or occlude the cystic duct orifice, pancreatic duct or the contralateral biliary tree [5, 6]. These findings may explain why uncovered SEMS have been associated with a higher overall survival than fcSEMS in a subgroup analysis [4].
In this particular case, haemobilia could have arisen from delayed complication of uncovered SEMS insertion or from tumour ingrowth with subsequent bleeding, the first being a complication of stenting and the latter being a natural progression of the disease. However, by considering he presented 9 months post uncovered SEMS insertion, the bleeding would have likely been from the tumour ingrowth. Moreover, Quincke’s triad was not present in our case: we hypothesise that the larger SEMS diameter of 10 mm prevented the development of biliary obstruction. In our case, a fcSEMS was inserted within the patient’s original uncovered SEMS to tamponade his bleeding tumour. In contrast to uncovered SEMs, the stent interstices in fcSEMS are covered by an impermeable plastic covering thereby tamponading any active bleeding from the stricture itself [2–5].
Our literature review highlights that fcSEMS insertion has been used to treat haemobilia arising immediately after endoscopic intervention but never as a delayed manifestation as in our case [7–10].
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING
No funding was obtained for the publication of this case report.
ETHICAL APPROVAL
Not required.
CONSENT
During the write up of the case report, as the patient was deceased, consent was sought from his next of kin(sister). During his in-patient stay, the team had obtained verbal consent from the patient in regards to publishing a case report and would include radiological images, which was obtained during his stay as in in-patient.
GUARANTOR
Tan Yi Chuen.
References
- 1. Sandblom P. Hemorrhage into the biliary tract following trauma; traumatic hemobilia. Surgery 1948;24:571–86. [PubMed] [Google Scholar]
- 2. Murugesan SD, Sathyanesan J, Lakshmanan A, Ramaswami S, Perumal S, Perumal SU, et al. Massive hemobilia: a diagnostic and therapeutic challenge. World J Surg 2014;38:1755–62. [DOI] [PubMed] [Google Scholar]
- 3. Moole H, Jaeger A, Cashman M, Volmar FH, Dhillon S, Bechtold ML, et al. Are self-expandable metal stents superior to plastic stents in palliating malignant distal biliary strictures? A meta-analysis and systematic review. Med J Armed Forces India 2017;73:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Almadi MA, Barkun A, Martel M. Plastic vs. self-expandable metal stents for palliation in malignant biliary obstruction: a series of meta-analyses. Am J Gastroenterol 2017;112:260–73. [DOI] [PubMed] [Google Scholar]
- 5. Kahaleh M, Tokar J, Conaway MR, Brock A, Le T, Adams RB, et al. Efficacy and complications of covered Wallstents in malignant distal biliary obstruction. Gastrointest Endosc 2005;61:528–33. [DOI] [PubMed] [Google Scholar]
- 6. Isayama H, Komatsu Y, Tsujino T, Sasahira N, Hirano K, Toda N, et al. A prospective randomised study of ``covered'' versus ``uncovered'' diamond stents for the management of distal malignant biliary obstruction. Gut 2004;53:729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goenka MK, Harwani Y, Rai V, Goenka U. Fully covered self-expandable metal biliary stent for hemobilia caused by portal biliopathy. Gastrointest Endosc 2014;80:1175. [DOI] [PubMed] [Google Scholar]
- 8. Shinjo K, Matsubayashi H, Matsui T, Kawata N, Uemura S, Yamamoto Y, et al. Biliary hemostasis using an endoscopic plastic stent placement for uncontrolled hemobilia caused by transpapillary forceps biopsy (with video). Clin J Gastroenterol 2016;9:86–8. [DOI] [PubMed] [Google Scholar]
- 9. Bagla P, Erim T, Berzin TM, Chuttani R. Massive hemobilia during endoscopic retrograde cholangiopancreatography in a patient with cholangiocarcinoma: a case report. Endoscopy 2012;44:E1. [DOI] [PubMed] [Google Scholar]
- 10. Barresi L, Tarantino I, Ligresti D, Curcio G, Granata A, Traina M. Fully covered self-expandable metal stent treatment of spurting bleeding into the biliary tract after endoscopic ultrasound-guided fine-needle aspiration of a solid lesion of the pancreatic head. Endoscopy 2015;47:E87–8. [DOI] [PubMed] [Google Scholar]


