Abstract
Background
Unresectable or metastatic cutaneous squamous cell cancers (cSCCs) are rare but potentially life-threatening diseases. In this setting, systemic therapy has a palliative intent with limited benefit, but there is no established consensus regarding the proper management of this tumour. This retrospective study aimed to review outcomes in patients with non-curable cSCC treated with platinum-based chemotherapy and cetuximab.
Methods
We considered 12 consecutive patients treated between June 2010 and March 2016. All patients had received previous treatment for the local disease.
Results
The overall response rate was 50%, and the disease control rate was 67%. Median progression-free survival and overall survival were 6.6 (95% confidence interval [CI]: 1.9–8.4) and 14.6 (95% CI: 9.4–20.1) months, respectively. The median duration of response was 4.8 months (95% CI: 1.2–5.9). The most frequent toxicities were skin reactions (58%; grade 3: 25%) and anaemia (10%). No grade 4 toxicities were observed.
Conclusions
Cetuximab and platinum-based chemotherapy were shown to be feasible and active in cSCC, with an acceptable toxicity profile, even if with a limited duration of response.
Keywords: cetuximab, combination, cSCC, platinum-based chemotherapy
Introduction
Cutaneous squamous cell cancers (cSCCs) are frequent in the Caucasian population, accounting for approximately 20% of non-melanoma skin cancers.1 In the last few decades, population-based studies have highlighted a rapid global increase in cSCC incidence.2,3 Typically, cSCC can be cured with surgical excision or radiotherapy. More options for early lesions are Mohs surgery, curettage, cryosurgery, laser, and photodynamic therapy. Nonetheless, it can recur and rarely metastasize (1.9–2.6%).4 cSCC not amenable to curative treatments can lead to physical disfigurement with a major impact on the patient’s quality of life, resulting in a life-threatening disease. At present, in addition or as an alternative to radiotherapy, chemotherapy (CT) is reserved in case of recurrent or metastatic (R/M) disease, but data supporting efficacy are still limited without established treatment standards.5–7
The most common chemotherapeutic agent used in the non-curable setting is cisplatin, which has been used as a single agent or combined with other drugs (e.g. 5-fluorouracil, doxorubicin, or bleomycin).8–10
The epidermal growth factor receptor (EGFR) is highly expressed in many epithelial tumours including cSCC11; this glycoprotein plays a crucial role in signal transduction pathways that regulate key cellular functions.12 Therefore, anti-EGFR agents have been investigated in unresectable/metastatic cSCC. In this setting, monotherapy with both anti-EGFR antibodies, cetuximab and panitumumab, and tyrosine kinase inhibitors, such as gefitinib, erlotinib, and dacomitinib, showed some activity, with a range of response between 10% and 31%.12–20 The combination of cisplatin-based chemotherapy and cetuximab is widely employed in head and neck squamous cell carcinoma, where it provided a survival benefit over chemotherapy alone.
In a French retrospective study, the authors evaluated patients with locally advanced, non-metastatic cSCC who were non-operable because no complete resection was possible or due to the risk of critical cosmetic or functional outcomes. Patients received neoadjuvant CT with cetuximab, platinum salt, and 5-fluorouracil, to increase the indication for surgery. This combination led to the resection of the majority (92%) of previously unresectable cSCC.21 The potential efficacy of cetuximab in this setting has also been shown in other small reports, investigating the use of cetuximab in association with radiotherapy (RT) or in patients who are refractory to platinum-based regimens.5,18,22
However, at present, there are no data, to our knowledge, regarding the concurrent use of platinum and cetuximab in R/M cSCC patients. Therefore, we report herein a retrospective series of patients treated with this combination, to review its safety profile and feasibility, and to be considered as a historical comparator with immunotherapeutic agents, which have been recently reported or are in study in the same setting of disease.23
Patients and methods
After approval by the Institutional Review Board (Fondazione IRCCS Istituto Nazionale dei Tumori, Milano, Italy), we analysed patient data recorded on an electronic cancer database, thus identifying all the patients treated at our Institution between June 2010 and March 2016 with a combination of platinum-based CT and cetuximab. All information was de-identified for the analysis of data and manuscript drafting. All patients who were not amenable to curative treatment due to rapid progressing disease or unfeasibility of curative approaches and fit for CT received this protocol. In total, we identified 12 patients, whose characteristics are listed in Table 1. Response to systemic therapy was assessed according to RECIST 1.1.
Table 1.
Patients’ characteristics.
| n | % | |
|---|---|---|
| Gender: | ||
| • Male | 11 | 92 |
| • Female | 1 | 8 |
| Median age (range); years | 74 (46–82) | |
| ECOG performance status: | ||
| • 0 | 2 | 17 |
| • 1 | 9 | 75 |
| • 2 | 1 | 8 |
| Primary localization: | ||
| • H&N | 10 | 83 |
| • Gluteal region | 2 | 17 |
| Local disease: | ||
| • No local recurrence | 1 | 8 |
| • T1 | 0 | 0 |
| • T2 | 3 | 25 |
| • T3 | 6 | 50 |
| • T4 | 1 | 8 |
| • NA | 1 | 8 |
| Node involvement: | ||
| • N0 | 5 | 42 |
| • N1 | 3 | 25 |
| • N2 | 3 | 25 |
| • NA | 1 | 8 |
| Metastatic at treatment start | 4 | 33 |
| Histotype: | ||
| • Histological subtypes of SCC | 12 | 100 |
| ○ Invasive SCC | 9 | 75 |
| ○ Poorly differentiated SCC | 1 | 8 |
| ○ SCC with pseudosarcomatous traits | 2 | 17 |
| Previous therapies: | ||
| • Surgery | 12 | 100 |
| • Radiotherapy | 6 | 50 |
| • Chemotherapy | 1 | 8 |
| • TKI | 1 | 8 |
| • Sirolimus | 1 | 8 |
| • Metastasectomy | 1 | 8 |
| • CTRT | 1 | 8 |
| Chemotherapy associated with cetuximab: | ||
| • Cisplatin | 4 | 33 |
| • Carboplatin | 7 | 58 |
| • Cisplatin + 5-fluoruracil | 1 | 8 |
Cetuximab was administered weekly in association with 3-weekly cisplatin (five patients, one patient also received 5-fluorouracil) or carboplatin (seven patients) when cisplatin was contraindicated (e.g. patient frailty and impaired renal function).
Males were predominant (11 males/1 female), and the median age was 73 years (range: 46–82). ECOG performance status (PS) was 0 in two patients, 1 in nine patients, and 2 in one patient. Most patients (83%) had a primary disease localized in the head and neck area and the remaining ones in the gluteal region. Most patients had T3–T4 local disease (60%), whilst node metastases were present in half of them. Four patients had metastatic disease at the start of treatment. Histological characteristics showed poorly differentiated aspects in one case and pseudosarcomatoid traits in two cases. All patients received previous surgery, whilst RT was performed in 7 out of 12 patients. Amongst patients who had not received radiotherapy, three had rapid disease progression after surgery, which contraindicated postoperative radiation; in the other two cases, there was no clinical indication to radiotherapy. Other previous treatments consisted of metastasectomy (8%), CT with cisplatin and 5-fluorouracil (8%), concurrent chemoradiation (8%), gefitinib (8%), and sirolimus (8%). Median disease-free interval from the first curative treatment was 4.5 months (95% confidence interval [CI]: 2.0–18.9). One-third of patients were metastatic at the start of the treatment.
Results
In the period of enrolment, 12 patients were recruited, and only 15% of patients presenting with recurrent and/or metastatic disease were judged unfit for CT and received best supportive care. All patients experienced at least one treatment-related adverse event. Skin reactions (58%) and anaemia (50%) were the most frequent toxicities; neutropenia, thrombocytopenia, and nausea accounted for less than 20%. Grade 3 adverse events occurred in 25% of patients (skin rash 25% and neutropenia 8%), and no grade 4 or grade 5 toxicities were observed.
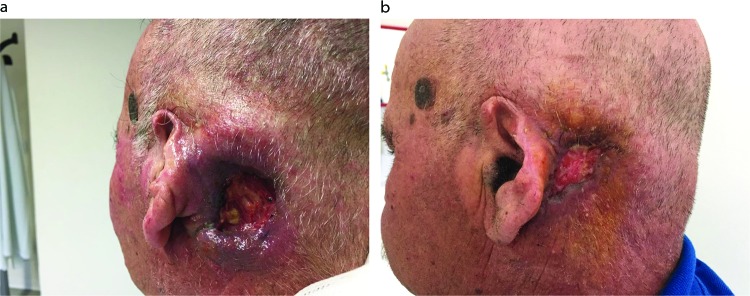
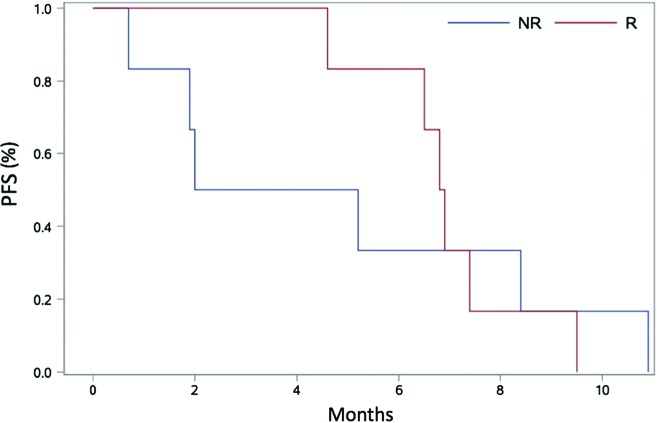
Amongst 12 patients, the overall response rate (RR) was 50%: complete response 1/12 (8%) and partial response 5/12 (42%). Two patients had stable disease leading to a disease control rate of 67% in the entire study population. Moreover, 4 out of 12 patients (33.3%) progressed during treatment without any clinical benefit, including 1 patient with a pseudosarcomatous tumour who had a rapid disease progression after the first cycle of treatment. One frail patient (PS 2) received carboplatin obtaining a partial response, and the treatment was tolerated though complicated by G3 myelotoxicity. Figure 1 shows a case of partial response to treatment (three-weekly carboplatin + cetuximab). This exemplary patient received five cycles of carboplatin + cetuximab achieving a PR and had a clinical benefit in terms of pain control. He received radiotherapy afterwards, and progression-free survival (PFS) was 9.5 months. After systemic treatment, 6 patients (50%) underwent further local treatments: 3 patients (25% of the total) received surgery and 4 patients (33%) received RT; of these 6 patients, 4 had obtained a response (CR + PR) to drug combination. Median duration of response was 4.8 months (95% CI: 1.2–5.9). Median PFS and overall survival (OS) were 6.6 (95% CI: 1.9–8.4) and 14.6 (95% CI: 9.4–20.1) months, respectively. The longest OS was observed in a patient who underwent surgery despite of disease progression (25.3 months) and in 3 responding patients (1 complete response – 84.7 months and 2 partial responses – 20.1 and 17.6 months), 2 of the latter were consolidated with a local treatment after CT (surgery and RT with cetuximab). No differences in PFS (Figure 2) or OS between responders and not responders were reported (PFS: 6.9 months in responders [95% CI 4.6–9.5] versus 3.6 months in non-responders [0.7–10.9]; p=0.88. OS: 17.6 [9.4–NR] versus 14.5 months [2.6–25.3]; p=0.2) (Figure 2).
Figure 1.
A case of partial response, obtained 2 months after treatment initiation. (A) Baseline and (B) 2 months.
Figure 2.
Progression-free survival curves of responder and non-responder patients.
Patients with pseudosarcomatoid differentiation had a dismal prognosis (PFS <2 months).
Discussion
Consensus on the treatment strategy for the management of advanced cSCC is still to be reached. Recently, EGFR inhibition by cetuximab has shown some efficacy in the non-operable setting.5,6,18,22 However, information on the feasibility of cetuximab in combination with platinum-based CT is lacking.
The combination of cetuximab and platinum-based CT showed to be feasible and active in advanced cSCC. The toxicity profile was acceptable, considering the overall fragile patient population. In fact, the series was mainly composed of heavily pretreated patients with advanced age and recurrent tumours, all conditions potentially exposing the patients to a higher toxicity risk. Notwithstanding, the adverse events were common but of limited severity; this could be explained by the choice to combine a mono-CT with cetuximab in almost all the cases and the use of carboplatin instead of cisplatin in more than half of the patients, therefore tailoring the treatment to the frailty of the patients. Because of this tailored approach, therapy was heterogeneous in our small number of patients (i.e. patients were in different stages and had received several types of previous treatment) and this limited reliability and interpretation of results. The possible underestimation of adverse events collection in a retrospective series should be also considered as a limitation of the study. Another limitation is the lack of data on quality of life and a systematic evaluation of the treatment benefit.
In comparison to the previous series of R/M cSCC patients treated with single anti-EGFR agents (RR: 10–31%),12–16 the combination of cetuximab and CT showed higher responses (50%). Conversely, CT achieved higher responses rates when doublet or triplet regimens were used (RR: 37.5–85.7%),8,10,24 whilst results with mono-CT are dismal (RR: 14.3%).25
Median PFS was comparable to those of patients treated with anti-EGFR monotherapies (range: 3.8–8 months).12–16 Therefore, the benefit from the use of this drug combination appears to be mainly an increased response rate. However, achieving responses in these aggressive and destructive tumours cannot be underestimated, as it could reflect in symptom improvement and thus a better quality of life for patients. Moreover, most patients (83%) showing a response to CT and anti-EGFR agent subsequently received surgery or RT. This emphasizes the benefit of systemic treatments in increasing the rate of patients initially not amenable to curative therapies who become candidates to these approaches, although we cannot rule out that these patients presented a less aggressive disease than the others. In our experience, two patients had pseudosarcomatous tumours, and they had either no response or a very short response. This could be expected, as this type of tumour presents with a lower expression of EGFR, and is less responsive to chemotherapy.26,27 Findings reported in a retrospective series of cases by a French group20 were similar to our results, identifying more than 90% of the patients treated with chemotherapy and targeted treatment able to undergo curative surgery. In this French series, patients had no distant metastases and more than 40% of the cases were naïve to any therapy.
The same authors reported median PFS of 8.5 months in operated patients after the use of neo-adjuvant cetuximab combined with a platinum salt and 5-fluorouracil. Keeping in mind the different populations of the two studies, the PFS is not so different when compared to the one obtained in our series of R/M patients (6.6 months). This fact reinforces the concept of a short-lived duration of response with the currently available systemic treatments.
Therefore, locally advanced or R/McSCCs not amenable to curative approaches remains an unmet need for clinical oncology.
Recent data showed a promising activity of immune checkpoint inhibitors in locally advanced or metastatic cSCC. Approximately half of the patients treated with cemiplimab (anti-PD-1 agent) within a phase I–II trial obtained partial or complete responses. Responses were rapid (median time to response: 1.9 months) and durable in most patients; in fact, in more than half of the patients duration of response exceeded 6 months and the estimated probability of PFS at 12 months was 53% (95% CI: 37–66), whilst the same figure for OS was 81%.28 Median duration of response and median OS were not reached.
Our results may help to evaluate the position of immunotherapy in the course of the disease. Other trials employing checkpoint inhibitors in the recurrent/metastatic setting, as well as in adjuvant setting, are ongoing. New knowledge about immunotherapy makes it necessary to reconsider the role and timing of all available systemic treatments. Treatment combinations or sequences should be exploited to maximize the response to immunotherapy and to tackle primary and acquired resistance.
Considering the limitations of our retrospective analysis conducted in a limited number of cases and on a quite heterogeneous population, we presented the outcome of a series of patients with RM cSCCs not amenable to salvage approaches and treated with a combination of cetuximab and platinum-based chemotherapy. Toxicity profile, response rate, and rescue of salvage local treatments are the main strengths of this therapy, whilst short-lived responses and PFS are the corresponding drawbacks.
Acknowledgements
Editorial assistance was provided by Laura Brogelli, PhD, and Aashni Shah (Polistudium SRL, Milan, Italy); this editorial assistance was paid for by Fondazione IRCCS Istituto Nazionale dei Tumori Milan, with funds assigned to P Bossi.
Footnotes
Contributions: Data Collection: D.G., S.A., F.P. Data interpretation: P.B., C.B., L.L., L.G., L.F.L. Statistical analysis: S.C., C.R., E.O. Manuscript writing: P.B., D.G. Manuscript editing: all authors. Review and approval of the final draft: all authors. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: P.B.: Advisory board: Merck, Sanofi, Merck Sharp & Dohme, Sun Pharma, Angelini, AstraZeneca. Conference honoraria: Bristol-Myers Squibb, Kyowa Hakko Kirin, Angelini, Roche. L.G.: Consultancy: Eisai, LeoPharma, Grunenthal, Pierre-Fabre, Indena, Abbvie, CSL Behring, Santhera, Recordati. L.D.L.: Research funding: EISAI. Honoraria: EISAI; IPSEN; BMS; MSD; Merck Serono; Biogen; Eli Lilly. L.F.L.: Honoraria or consultation fees: (for public speaking/teaching in medical meetings and/or for expert opinion in advisory boards): Astrazeneca, Bayer, BMS, Eisai, MSD, Merck–Serono, Boehringer Ingelheim, Novartis, Roche, Debiopharm, Sobi, Incyte Biosciences Italy srl, Doxa Pharma srl, Amgen, Nanobiotics Sa, GSK. (For public speaking/teaching from research companies & commercial education providers): AccMed, Medical Science Fundation, G. Lorenzini, Associazione Sinapsi, Think 2 IT, Aiom Servizi, Prime Oncology, WMA Congress Education, Fasi, DueCi promotion Srl, MI&T, Net Congress & Education, PRMA Consulting, Kura Oncology, Health & Life srl, Ipsen Innovation, Immuno-Oncology Hub. Funding (to her institution) for clinical studies and research: Astrazeneca, BMS, Boehringer Ingelheim, Celgene International, Eisai, Exelixis inc, Hoffmann-La roche ltd, IRX Therapeutics inc, Medpace inc, Merck–Serono, MSD, Novartis, Pfizer, Roche. Travel coverage for medical meetings: Merck–Serono, BMS, MSD, Debiopharm, Sobi, Bayer, Stilema, AccMed, Aiocc, Aiom. D.G., S.C., S.A., C.R., E.O., C.B., F.P. declare no conflict of interest. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at https://www.drugsincontext.com/wp-content/uploads/2019/11/dic.212611-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article. No funding was received for this study.
Correct attribution: Copyright © 2019 Galbiati D, Cavalieri S, Alfieri S, Resteghini C, Bergamini C, Orlandi E, Platini F, Locati LD, Giacomelli L, Licitra LF, Bossi P. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: submitted; externally peer reviewed.
Peer review comments to author: 17 September 2019
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2010;344(13):975–983. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 2.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166(5):1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 3.Perera E, Gnaneswaran N, Staines C, et al. Incidence and prevalence of non-melanoma skin cancer in Australia: a systematic review. Australas J Dermatol. 2015;56(4):258–267. doi: 10.1111/ajd.12282. [DOI] [PubMed] [Google Scholar]
- 4.Brougham NDLS, Dennett ER, Cameron R, et al. The incidence of metastasis from cutaneous squamous cell carcinoma and the impact of its risk factors. J Surg Oncol. 2012;106(7):811–815. doi: 10.1002/jso.23155. [DOI] [PubMed] [Google Scholar]
- 5.Trodello C, Higgins S, Ahadiat O, et al. Cetuximab as a component of multimodality treatment of high-risk cutaneous squamous cell carcinoma: a retrospective analysis from a single tertiary academic medical center. Dermatol Surg. 2019;45(2):254–267. doi: 10.1097/DSS.0000000000001755. [DOI] [PubMed] [Google Scholar]
- 6.Dereure O, Missan H, Girard C, et al. Efficacy and tolerance of cetuximab alone or combined with chemotherapy in locally advanced or metastatic cutaneous squamous cell carcinoma: an open study of 14 patients. Dermatology. 2016;232(6):721–730. doi: 10.1159/000461578. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald K, Tsai KK. Systemic therapy for advanced cutaneous squamous cell carcinoma. Semin Cutan Med Surg. 2019;38(1):E67–E74. doi: 10.12788/j.sder.2019.010. [DOI] [PubMed] [Google Scholar]
- 8.Khansur T, Kennedy A. Cisplatin and 5-fluoruracil for advanced locoregional and metastatic squamous cell carcinoma of the skin. Cancer. 1991;67:2030–2032. doi: 10.1016/B978-0-12-800206-3.00023-9. [DOI] [PubMed] [Google Scholar]
- 9.DeConti RC. Chemotherapy of squamous cell carcinoma of the skin. Semin Oncol. 2012;39(2):145–149. doi: 10.1053/j.seminoncol.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura K, Okuyama R, Saida T, et al. Platinum and anthracycline therapy for advanced cutaneous squamous cell carcinoma. Int J Clin Oncol. 2013;18(3):506–509. doi: 10.1007/s10147-012-0411-y. [DOI] [PubMed] [Google Scholar]
- 11.Maubec E, Duvillard P, Velasco V, et al. Immunohistochemical analysis of EGFR and HER-2 patients with metastatic squamous cell carcinoma of the skin. Anticancer Res. 2005;25:1205–1210. [PubMed] [Google Scholar]
- 12.Maubec E, Petrow P, Scheer-Senyarich I, et al. Phase II study of cetuximab as first-line single-drug therapy in patients with unresectable squamous cell carcinoma of the skin. J Clin Oncol. 2011;29(25):3419–3426. doi: 10.1200/JCO.2010.34.1735. [DOI] [PubMed] [Google Scholar]
- 13.Foote MC, McGrath M, Guminski A, et al. Phase II study of single-agent panitumumab in patients with incurable cutaneous squamous cell carcinoma. Ann Oncol. 2014;25(10):2047–2052. doi: 10.1093/annonc/mdu368. [DOI] [PubMed] [Google Scholar]
- 14.William WN, Feng L, Ferrarotto R, et al. Gefitinib for patients with incurable cutaneous squamous cell carcinoma: a single-arm phase II clinical trial. J Am Acad Dermatol. 2017;77(6):1110–1113.e2. doi: 10.1016/j.jaad.2017.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gold KA, Kies MS, William WN, et al. Erlotinib in the treatment of recurrent or metastatic cutaneous squamous cell carcinoma: a single-arm phase 2 clinical trial. Cancer. 2018:2169–2173. doi: 10.1002/cncr.31346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavalieri S, Perrone F, Miceli R, et al. Efficacy and safety of single-agent pan-human epidermal growth factor receptor (HER) inhibitor dacomitinib in locally advanced unresectable or metastatic skin squamous cell cancer. Eur J Cancer. 2018;97:7–15. doi: 10.1016/j.ejca.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Chapalain M, Baroudjian B, Dupont A, et al. Stage IV cutaneous squamous cell carcinoma (cSCC): treatment outcomes in a series of 42 patients. J Eur Acad Dermatol Venereol. 2019 Oct 6; doi: 10.1111/jdv.16007. [DOI] [PubMed] [Google Scholar]
- 18.Joseph K, Alkaabi K, Warkentin H, et al. Cetuximab-radiotherapy combination in the management of locally advanced cutaneous squamous cell carcinoma. J Med Imaging Radiat Oncol. 2019;63(2):257–263. doi: 10.1111/1754-9485.12842. [DOI] [PubMed] [Google Scholar]
- 19.Berliner JG, Schulman JM, Lazarova Z, et al. Response of cutaneous squamous cell carcinoma to treatment with cetuximab. Dermatol Surg. 2019;45(2):313–316. doi: 10.1097/DSS.0000000000001583. [DOI] [PubMed] [Google Scholar]
- 20.Lu SM, Lien WW. Concurrent radiotherapy with cetuximab or platinum-based chemotherapy for locally advanced cutaneous squamous cell carcinoma of the head and neck. Am J Clin Oncol. 2018;41(1):95–99. doi: 10.1097/COC.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 21.Reigneau M, Robert C, Routier E, et al. Efficacy of neoadjuvant cetuximab alone or with platinum salt for the treatment of unresectable advanced nonmetastatic cutaneous squamous cell carcinomas. Br J Dermatol. 2015;173(2):527–534. doi: 10.1111/bjd.13741. [DOI] [PubMed] [Google Scholar]
- 22.Capalbo C, Belardinilli F, Filetti M, et al. Effective treatment of a platinum-resistant cutaneous squamous cell carcinoma case by EGFR pathway inhibition. Mol Clin Oncol. 2018;9(1):30–34. doi: 10.3892/mco.2018.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi FD, Kraus CN, Elsensohn AN, et al. D-1 and PD-L1 inhibitors in the treatment of non-melanoma skin cancer: a systematic review. J Am Acad Dermatol. 2019 doi: 10.1016/j.jaad.2019.05.077. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Sadek H, Azli N, Wendling JL, et al. Treatment of advanced squamous cell carcinoma of the skin with cisplatin, 5-fluorouracil, and bleomycin. Cancer. 1990;66(8):1692–1696. doi: 10.1002/1097-0142(19901015)66:8<1692::AID-CNCR2820660807>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 25.Cartei G, Cartei F, Interlandi G, et al. Oral 5-fluorouracil in squamous cell carcinoma of the skin in the aged. Am J Clin Oncol Cancer Clin Trials. 2000;23(2):181–184. doi: 10.1097/00000421-200004000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Battifora H. Diagnosis of human tumors. Case 10: pseudosarcomatous squamous cell carcinoma. Ultrastruct Pathol. 1983;5(4):335–339. doi: 10.3109/01913128309141460. [DOI] [PubMed] [Google Scholar]
- 27.Yozu M, Glengarry J, Ahmed SS. Cutaneous squamous cell carcinoma associated with proliferation of osteoclast-like giant cells. J Pak Med Assoc. 2011;61(9):922–925. [PubMed] [Google Scholar]
- 28.Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379(4):341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]