Abstract
PURPOSE
The 21-gene recurrence score (RS) assay is prognostic among women with early-stage estrogen receptor–positive (ER+) and human epidermal growth factor receptor 2–negative (HER2−) breast cancer and is used to inform recommendations for chemotherapy. Women ≤ 40 years of age represent a minority of patients studied using gene expression profiles.
METHODS
The Young Women's Breast Cancer Study is a prospective cohort of women diagnosed with breast cancer at age ≤ 40 years and enrolled patients between 2006 and 2016 (N = 1,302). We identified patients with stage I-III ER+/HER2− breast cancer. The RS assay was performed on banked specimens for patients who had not been tested clinically. Distant recurrence-free survival (DRFS) was assessed by TAILORx and traditional RS risk groups among patients with axillary node–negative (N0) and limited node–positive (N1) breast cancer.
RESULTS
Among eligible women (N = 577), 189 (33%) had undergone RS testing, and 320 (56%) had banked specimens sufficient for testing. Median follow-up was 6.0 years. Median age at diagnosis was 37.2 years; 300 of 509 patients (59%) had N0 breast cancer, of whom 195 (65%) had an RS of 11-25 and fewer than half (86 of 195; 44%) received chemotherapy. Six-year DRFS rates were 94.4% and 92.3% (RS < 11), 96.9% and 85.2% (RS 11-25), and 85.1% and 71.3% (RS ≥ 26) among women with N0 and N1 disease, respectively.
CONCLUSION
The RS assay is prognostic among young women with node-negative and limited node-positive breast cancer, representing a valuable tool for risk stratification. Disease outcomes with a median follow-up of 6 years among young women with N0 disease and an RS of 0-25, a minority of whom received chemotherapy, and node-positive disease with an RS < 11 were very good, whereas those with N0 disease and an RS ≥ 26 or N1 disease with an RS ≥ 11 experienced substantial risk of early distant recurrence.
INTRODUCTION
Young women, traditionally defined as age ≤ 40 years at diagnosis,1 account for 4% of women with breast cancer in the United States2 and are more likely than older women to be diagnosed at later stages and with more aggressive subtypes, including poorly differentiated, estrogen receptor (ER)-negative, and human epidermal growth factor receptor 2–positive (HER2+) disease.3-7 Young age has also been considered an independent risk factor for recurrence, particularly with ER-positive (ER+) disease.5-10 In multiple studies, over 85% of young women with breast cancer have received adjuvant chemotherapy.3,11,12 Yet, the majority of breast cancers diagnosed in young women are ER+/HER2-negative (HER2−),9 a subgroup with lesser chemotherapy benefit than the HER2+ and triple-negative subgroups.13
Correspondingly, efforts have been made in recent years to improve risk stratification in ER+/HER2− disease, particularly with gene expression profiles (GEP), with the aim of reserving chemotherapy for those likely to benefit and sparing those who are not. The 21-gene recurrence score (RS; Oncotype Dx; Genomic Health, Redwood City, CA) assay was initially evaluated among axillary lymph node–negative (N0) patients and later for postmenopausal women with node-positive breast cancer.14-18 Recently, results of the phase III Trial Assigning Individualized Options for Treatment (TAILORx; ClinicalTrials.gov identifier: NCT00310180) demonstrated very low distant recurrence risk (DRR) among women with N0 breast cancer and RS 0-10, and no benefit from chemotherapy among women with RS 11-25, with a suggestion of benefit among women ≤ 50 years of age with RS 16-25 in a subset analysis.19,20 In contrast, DRR was higher among women with RS ≥ 26.20 In previous studies, chemotherapy reduced 10-year DRR among women with RS ≥ 31 by 28%, although only 14% of patients undergoing RS testing have RS ≥ 31.15,21 These and other data have led to the widespread use of the RS assay and an associated decline in chemotherapy use for ER+/HER2− breast cancer.22,23
However, concerns about avoiding chemotherapy have arisen for young women with ER+/HER2− breast cancer, given their higher risk of recurrence, and use of the RS assay has not been adopted to the same extent as in older women.22 The small proportion (5%-10%) of women < 40 years of age in validation studies contributes to this apprehension.14-17,19 In an effort to address this gap, we sought to evaluate the prognostic impact of the RS assay in young women with stage I-III ER+/HER2− breast cancer unselected for RS testing enrolled in a prospective cohort study.
METHODS
Study Design
The Young Women’s Breast Cancer Study is a multicenter, prospective cohort of women diagnosed with breast cancer at ≤ 40 years of age, enrolled between 2006 and 2016 from 13 sites in the United States and Canada. Women with newly diagnosed breast cancer (< 6 months from diagnosis to enrollment) and able to respond to questionnaires in English were eligible. Potential participants at the Dana-Farber/Harvard Cancer Center (DF/HCC) sites were identified by the Rapid Case Identification Core through pathology review and at other sites through systematic review of clinic lists. Participants provided written informed consent. Institutional review board approval was obtained through DF/HCC and other participating centers.
Patient, disease, and treatment information was obtained through medical record review. ER and progesterone receptor positivity were defined as immunohistochemistry ≥ 1% and HER2 status using American Society of Clinical Oncology (ASCO) guidelines in use at diagnosis. Amenorrhea was evaluated by patient report on surveys completed at baseline, every 6 months for 3 years, then annually, and defined as absence of menses in the 6 months prior to the survey completed 1 year following diagnosis, to reflect that chemotherapy-related amenorrhea (CRA) has commonly been evaluated at the 1 year time point but that menses may continue beyond initiation of treatment.24 If data were unavailable at 1 year, amenorrhea was defined as absence of menses in the 12 months before the 18-month survey.
Selection for RS testing and RS results were abstracted from medical records when records indicated that testing was performed. For those who did not undergo RS testing as part of clinical care, RS testing was performed by Genomic Health (Redwood City, CA) on banked tissue obtained before initiation of systemic therapy. Recurrences reported by patients on surveys were confirmed (or refuted) through medical record review and characterized as distant recurrence versus other (ie, local/regional). Date of death was obtained through medical record review, obituaries, and family report. Analysis of selection for RS testing included all women with stage I-III breast cancer. Analyses of distant recurrence-free survival (DRFS) were limited to women with N0 or N1 (1-3 positive nodes, including micrometastases) breast cancer and an available RS result.
Statistical Analysis
The χ2 test was used to compare chemotherapy use by nodal status and receipt of RS testing during clinical care. Logistic regression models were used to assess factors associated with RS testing during clinical care. DRFS (primary outcome) was defined as the interval from diagnosis to distant recurrence or (if the latter was unknown), breast cancer–specific death. Patients were censored at last follow-up. DRFS was assessed using Kaplan-Meier survival estimates through 6 years, the median follow-up when this analysis was performed. DRFS was analyzed both by TAILORx risk groups (< 11, 11-25, and ≥ 26) and traditional risk groups (RS < 18, 18-30, ≥ 31) to reflect that TAILORx risk groups have been widely adopted in clinical practice since spring 2018, when these data became public, but traditional risk groups were used while patients in this study made chemotherapy decisions and to evaluate whether the different groups better stratified risk in N0 versus N1 breast cancer. A 2-degrees-of-freedom log-rank test was used to compare outcomes across RS groups. Multivariable Cox proportional hazards models were used to assess factors associated with DRFS. Factors significant at a level of P < .20 in univariable analysis were tested in the multivariable model.25 Outcomes by receipt of chemotherapy were evaluated in the RS 11-25 group using Kaplan-Meier survival as an exploratory analysis because administration of chemotherapy was not randomly assigned. All analyses were performed using SAS, Version 9.4 (SAS Institute, Cary, NC). All statistical tests were 2-sided, with a .05 level of significance.
RESULTS
Disease and Treatment Information
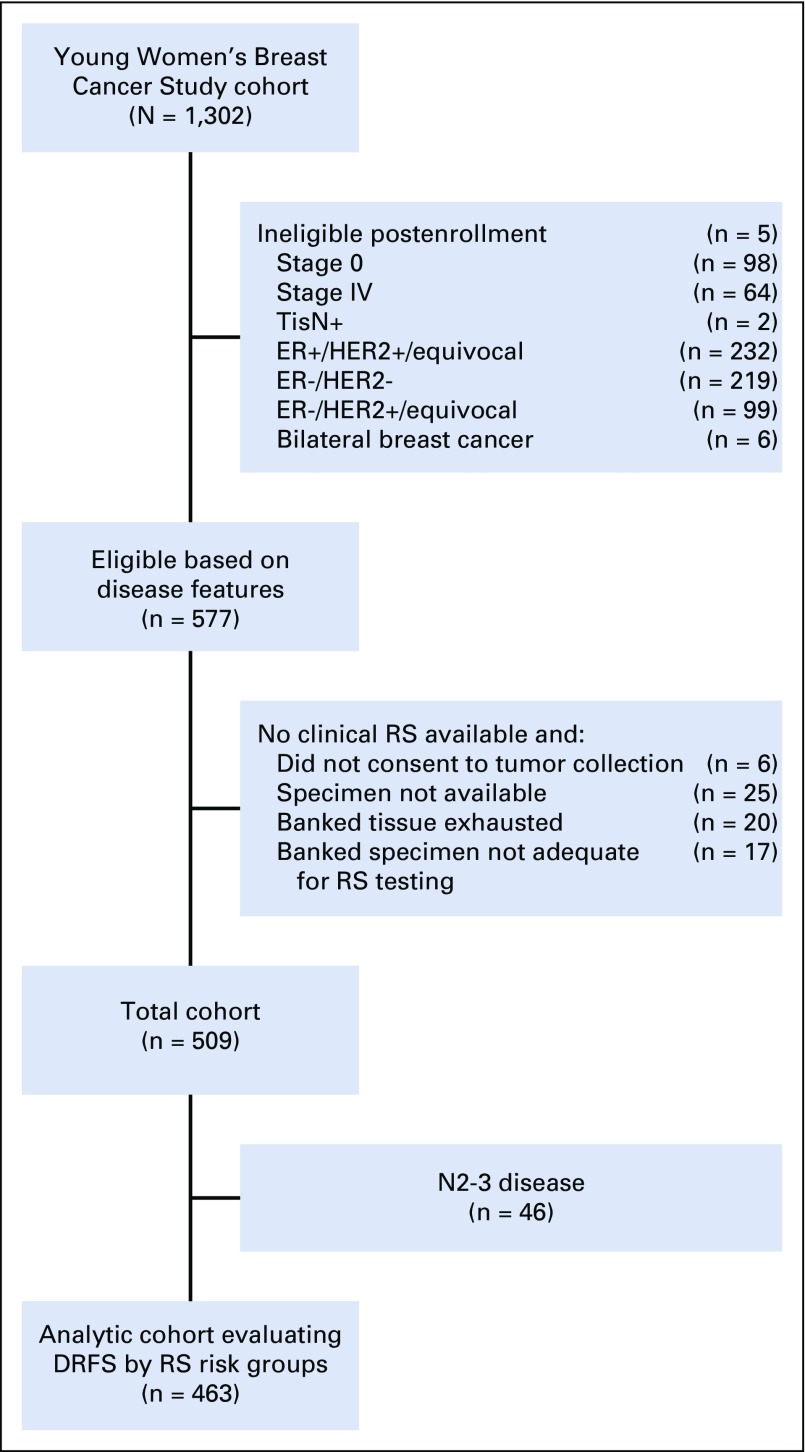
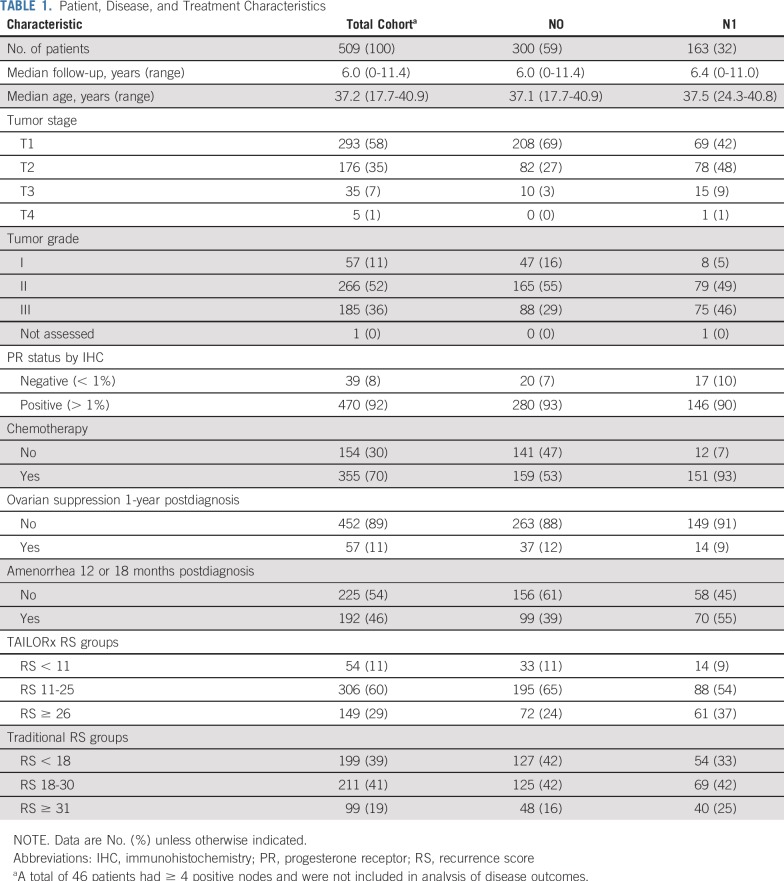
Of 577 eligible women with stage I-III ER+/HER2− breast cancer, 509 had an RS performed during clinical care (n = 189) or on banked tissue (n = 320; Fig 1). Disease, demographic, and treatment data are presented in Table 1. Median age at diagnosis was 37.2 years (17.7-40.9 years). Most women (n = 300; 58.9%) had N0 disease; 163 women (32.0%) had N1 disease. Three hundred fifty-five (69.7%) received chemotherapy, although use varied by nodal status (N0 disease, 53.0% v N1 disease, 92.6%; P < .0001). Fifty-seven patients (11.2%) received ovarian suppression, either with a gonadotropin-releasing hormone agonist or bilateral oophorectomy. Of 417 evaluable women, including the 57 who received ovarian suppression, 192 (46.0%) reported amenorrhea. RS ranged from 3-77. Among N0 patients (n = 300), 11.0% had RS < 11, 65.0% had RS 11-25, and 24.0% had RS ≥ 26, among whom 21.2%, 44.1%, and 91.7% received chemotherapy, respectively.
FIG 1.
Study flow diagram. DRFS, distant recurrence-free survival; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; RS, recurrence score; TisN+, in situ disease in breast, axillary node-positive.
TABLE 1.
Patient, Disease, and Treatment Characteristics
Selection for RS Testing During Clinical Care
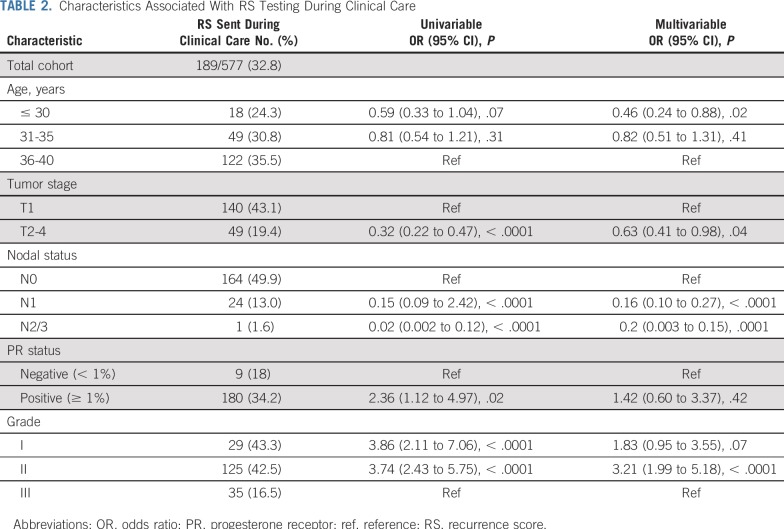
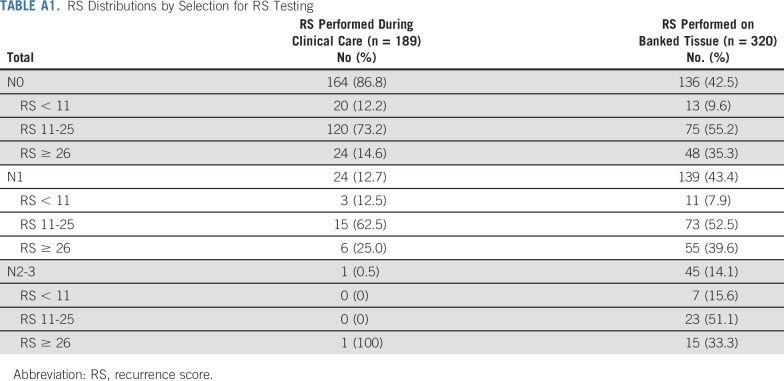
Selection for RS testing during clinical care among women with stage I-III ER+/HER2− breast cancer varied by disease and demographic features (Table 2). In multivariable analysis, women who were younger (ORage < 30 v 36-40 years = 0.46; 95% CI, 0.24 to 0.88), had larger tumors (ORT2-4 v T1 = 0.61; 95% CI, 0.39 to 0.95), or had node-positive disease (ORN1 v N0 = 0.16, 95% CI, 0.10 to 0.27; ORN2/3 v N0 = 0.02, 95% CI, 0.003 to 0.15) were less likely to have an RS, and patients with grade II tumors were more likely to have an RS (ORgrade II v III = 3.23; 95% CI, 2.00 to 5.20). Among the 189 women who had an RS assay during clinical care, 164 (86.8%) were N0, 24 (12.7%) were N1, and 1 (0.5%) had ≥ 4 positive nodes. RS distributions appeared to vary by selection for RS testing (Appendix Table A1, online only). Patients who had an RS performed during clinical care were less likely to receive chemotherapy for N0 (65/164 [39.6%] v 94/135 [69.1%]; P < .0001) and N1 disease (18/24 [75.0%] v 133/139 [95.7%]; P = .0003).
TABLE 2.
Characteristics Associated With RS Testing During Clinical Care
Disease Outcomes by RS Risk Groups
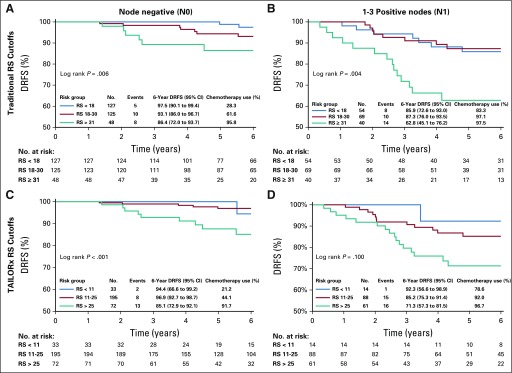
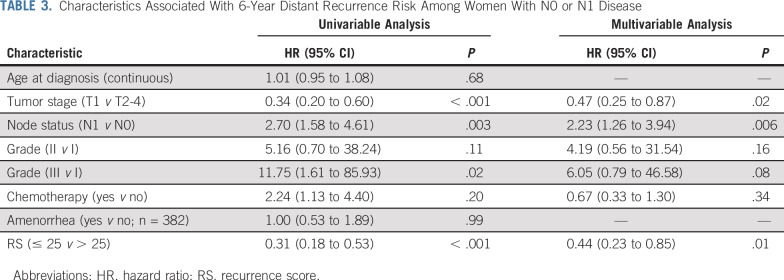
Median follow-up for the 509 women with an RS was 6.0 years (range, 0-11.4 years). All DRFS events (n = 55) were distant recurrence, and no breast cancer deaths without distant recurrence occurred. Among women with N0 disease, 6-year DRFS rates were 94.4% (RS, < 11), 96.9% (RS, 11-25), and 85.1% (RS, ≥ 26; Fig 2). Among women with N1 disease, 6-year DRFS rates were 92.3% (RS < 11), 85.2% (RS, 11-25), and 71.3% (RS, ≥ 26). In univariable analysis, features associated with 6-year DRR were tumor stage, node status, grade, chemotherapy use, and RS. Young age (≤ 30 or 31-35 v 36-40 years) and amenorrhea were not associated with 6-year DRR. In the multivariable model, tumor size (hazard ratio [HR]T1 v T2-4 = 0.45; 95% CI, 0.26 to 0.80), node status (HRN1 v N0 = 2.23; 95% CI, 1.29 to 3.85), and RS (HR< 25 v > 25 = 0.48; 95% CI, 0.27 to 0.85) remained associated with 6-year DRR, whereas grade (HRII v I = 2.58, 95% CI, 0.61 to 10.97; HRIII v I = 2.92, 95% CI, 0.67 to 12.83) and chemotherapy (HRyes v no = 0.74, 95% CI, 0.33 to 1.64) did not (Table 3).
FIG 2.
Distant recurrence-free survival (DRFS) by (A and B) traditional and (C and D) TAILORx recurrence score (RS) risk groups.
TABLE 3.
Characteristics Associated With 6-Year Distant Recurrence Risk Among Women With N0 or N1 Disease
DRFS varied significantly using TAILORx cutoffs for those with N0 (log-rank P < .001) but not N1 disease (log-rank P = .10; Fig 2). DRFS varied significantly using traditional cutoffs both in N0 (log-rank P = .006) and N1 disease (log-rank P = .004).
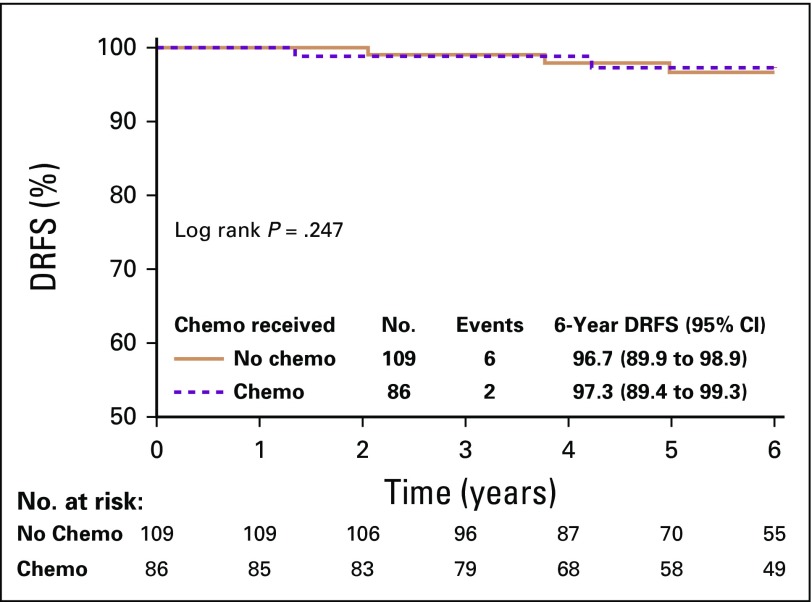
Among women with N0 disease and RS 11-25 (n = 195), 86 (44.1%) received chemotherapy and 109 (55.9%) did not. Two women (2.3%) who received chemotherapy and 6 (5.5%) who did not experienced a distant recurrence. In an exploratory analysis, chemotherapy use was not associated with DRFS (log-rank P = .25) in this subgroup (Fig 3).
FIG 3.
Distant recurrence-free survival (DRFS) by chemotherapy (chemo) use in women with N0 breast cancer and recurrence score 11-25.
DISCUSSION
Consensus guidelines specific to young women with breast cancer support use of GEP to assist in chemotherapy decision making, but comment that only a small minority of women in validation studies were ≤ 40 years of age at diagnosis.26 This population has experienced inferior disease outcomes, including survival, relative to women age 40-49 years.6,27 For this reason and others, adoption of GEP in the care of young women with ER+/HER− breast cancer has lagged behind older women, and data regarding GEP specifically in women age < 40 years, rather than grouping all women age < 50 years, are needed to inform chemotherapy decision making. Recently, TAILORx demonstrated that the RS is prognostic of outcomes and predictive of chemotherapy benefit among women age ≤ 50 years, but raised questions about how to incorporate assay results into chemotherapy decision making.20 Correspondingly, the ASCO Clinical Practice Guideline was updated to incorporate the TAILORx data and supports chemoendocrine therapy or endocrine therapy for women age ≤ 50 years with RS 16-25.28 However, only 5% of women with RS 11-25 in TAILORx were ≤ 40 years of age (n = 203), and the analysis did not adjust for CRA. Our study demonstrates that, in a large prospective cohort of women ≤ 40 years of age at diagnosis, RS is independently prognostic of DRR among women with N0 and N1 disease, further supporting that both traditional and genomic risk factors are important in selecting treatment of women ≤ 40 years of age.
Although young age has not been an independent risk factor in studies associating RS with outcomes,14 concerns have remained that host or tumor differences that impart independent risk to young women with ER+/HER2− breast cancer could alter the performance of GEP. Supported by data demonstrating greater chemotherapy benefit,13 some have suggested that the threshold for chemotherapy in young women might be lower to address risk due to young age. Furthermore, an unplanned, post hoc subset analysis of TAILORx found that chemotherapy was associated with a reduction in DRR among women < 50 years of age with RS 21-25 and, to a lesser extent, RS 16-20.20 Many have wondered whether the observed chemotherapy benefit could be attributable at least in part to CRA,29 particularly because ovarian function suppression (OFS) was used in a small minority and because the benefit became evident only after 5 years postdiagnosis. In our study, amenorrhea 1 year after diagnosis was not associated with DRR, raising the question of whether amenorrhea at a later time point may be a more valuable biomarker, given that many women ≤ 40 years of age later resume menstrual function.30 A subsequent analysis of TAILORx found that chemotherapy benefit among women ≤ 50 years of age with RS 11-25 was limited to premenopausal women 46-50 years of age, supporting the hypothesis that chemotherapy benefit may have been derived through CRA, given that women older than premenopausal women are significantly more likely to undergo CRA than younger women.31
Our data show that DRFS was high for women with N0 disease and RS < 11 or RS < 18, among whom only a minority received chemotherapy. Importantly, our population includes those for whom the RS was not performed during clinical care, a group enriched for higher-risk clinical features. Low DRR through 6 years further demonstrates that young women with RS < 11 and RS 11-15 have little risk within the first 5 years to improve on, supporting the application of TAILORx findings regarding lack of chemotherapy benefit for women ≤ 50 years of age with RS < 11 and RS 11-15 to women ≤ 40 years of age. In this cohort, 10% of node-negative patients who did not have an RS assay performed during clinical care were found to have an RS < 11, suggesting that broader RS assay use among young patients may reduce chemotherapy use without compromising outcomes.
Whether there is benefit from chemotherapy for patients ≤ 40 years of age with RS 16-20 or 21-25 is not directly addressed by our analysis. We observed no difference in DRFS in the intermediate risk group (RS 11-25) by receipt of chemotherapy, and the event rate was low in both subgroups, suggesting that there may be no or limited benefit. The recent TAILORx analysis showing no chemotherapy benefit among women ≤ 50 years of age with RS 16-20 and low clinical risk raises additional questions about the benefit of chemotherapy in this setting.31
On the other end of the spectrum, our data demonstrate that young women with node-negative disease and RS ≥ 26 or RS ≥ 31, the majority of whom had T1 tumors, experience moderate rates of early distant recurrence despite high rates of chemotherapy use. Most striking is the relatively poor 6-year DRFS among those with N1 disease and high risk scores despite near universal chemotherapy use, underscoring the need for improved therapies. The vast majority of patients in this study did not receive OFS, which substantially improved distant recurrence rates and survival in the Suppression of Ovarian Function (SOFT; ClinicalTrials.gov identifier: NCT00066690) and Tamoxifen and Exemstane Trial (TEXT; ClinicalTrials.gov identifier: NCT00066703) trials, with particularly large benefits for women < 35 years of age, highlighting the importance of considering OFS use for those with higher-risk disease.32-35
Whether and to what degree young women with node-positive disease but low or low-intermediate RS benefit from chemotherapy remain unanswered questions. In 2010, Albain et al17 published the first validation study evaluating the RS assay in node-positive breast cancer. The analysis was limited to postmenopausal women, and data evaluating whether RS is predictive of chemotherapy benefit for node-positive premenopausal women remain limited. The Microarray in Node-Negative and 1 to 3 Positive Lymph Node Disease May Avoid Chemotherapy (MINDACT; ClinicalTrials.gov identifier: NCT00433589) trial randomly assigned 1,550 women with high clinical and low genomic risk using the MammaPrint assay (Agendia, Irvine, CA), 48% of whom had N1 disease, to endocrine therapy with or without chemotherapy and showed no difference in 5-year DRFS (95.9 v 94.9%; P = .27), with no difference in outcomes by age, although fewer than 10% were < 40 years of age.36,37 The West German Study Group Plan B trial (ClinicalTrials.gov identifier: NCT01049425) included 348 women with RS ≤ 11 treated with endocrine therapy alone (median age of 56 years; 31% with N1 disease), among whom 5-year DRFS was 98% in both N0 and N1 patients.38,39 The most recent TAILORx analysis also found no association between clinical risk, albeit in the node-negative setting, and distant recurrence among women ≤ 50 years of age with RS 0-10.31 These studies provide reasonably strong data suggesting that endocrine therapy alone may be appropriate for select premenopausal women with N1 disease and low GEP risk. Premenopausal women are included in RxPONDER (ClinicalTrials.gov identifier: NCT 01272037), in which patients with node-positive ER+/HER2− breast cancer with RS 0-25 are randomly assigned to endocrine therapy with or without chemotherapy, but will represent a minority, particularly those ≤ 40 years of age. In our study, although the majority of women with N1 disease and RS < 11 had received chemotherapy, their fairly good outcomes, coupled with prior data showing no benefit from adjuvant chemotherapy in N1 patients with low-risk GEP, suggest that GEP are likely to be valuable tools in chemotherapy decision making for premenopausal women with N1 breast cancer in the future. In addition, RS cutpoints (traditional v TAILORx) may stratify disease outcomes differently in N0 versus N1 breast cancer. Given the increased anatomic risk, it is conceivable that lower-risk cutoffs will better differentiate risk among node-positive patients, although our data do not clearly demonstrate this. Additional data (eg, RxPONDER) regarding an optimal clinical cutpoint in node-positive breast cancer are awaited, and we encourage additional analyses from other datasets of young women, even in which chemotherapy was not randomly assigned, such as SOFT/TEXT and the Austrian Breast and Colorectal Cancer Study Group trial 12, to further explore GEP in premenopausal women.
Our findings should be interpreted in light of their limitations. First, we are not at this time able to address risk of later recurrences because our median follow-up was 6 years. Future analyses evaluating longer-term disease outcomes in this cohort are planned, although chemotherapy benefit, which has generally been limited to the first 5 years after diagnosis, seems unlikely to be affected substantially. Second, we were unable to formally evaluate whether RS is predictive of chemotherapy benefit because receipt of chemotherapy was not randomly assigned in this prospective observational cohort. An exploratory analysis among those with N0 disease and RS 11-25 showed no difference in 6-year DRFS by receipt of chemotherapy. We attempted to perform an additional exploratory analysis in this subgroup using propensity score analysis, but were unable to develop a balanced model because of limitations on measured covariables associated with treatment decisions. Third, our ability to evaluate the association between CRA and DRR may have been limited by missing information and by resumption of menses after CRA evaluation on the 1-year survey.
In conclusion, in a cohort of young women unselected for RS testing during clinical care, RS is prognostic of DRR among those with N0 and N1 breast cancer and is a valuable tool for risk stratification. Future analyses should seek to determine whether GEP are predictive of chemotherapy benefit among young women, with the goal of optimizing breast cancer outcomes and minimizing short- and long-term toxicities.
Appendix
TABLE A1.
RS Distributions by Selection for RS Testing
PRIOR PRESENTATION
Presented as a poster at the 40th annual San Antonio Breast Cancer Symposium, San Antonio, TX, December 4-8, 2018.
SUPPORT
Supported by the Conquer Cancer Foundation, Susan G. Komen, and the Breast Cancer Research Foundation.
AUTHOR CONTRIBUTIONS
Conception and design: Philip D. Poorvu, Shari I. Gelber, Shoshana M. Rosenberg, Rulla M. Tamimi, Eric P. Winer, Ann H. Partridge
Provision of study materials or patients: Kathryn J. Ruddy, Rulla M. Tamimi, Jeffrey Peppercorn, Lidia Schapira, Virginia F. Borges, Steven E. Come, Ellen Warner, Eric P. Winer, Ann H. Partridge
Collection and assembly of data: Philip D. Poorvu, Shari I. Gelber, Shoshana M. Rosenberg, Laura C. Collins, Debbie M. Jakubowski, Ann H. Partridge
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prognostic Impact of the 21-Gene Recurrence Score Assay Among Young Women With Node-Negative and Node-Positive ER-Positive/HER2-Negative Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Philip D. Poorvu
Other Relationship: Medscape
Shoshana M. Rosenberg
Stock and Other Ownership Interests: Edwards Lifesciences, Cantel Medical
Kathryn J. Ruddy
Stock and Other Ownership Interests: Merck, Pfizer
Patents, Royalties, Other Intellectual Property: My husband is a co-inventor of technology licensed by Mayo Clinic to AliveCor (MountainView, CA), which makes a smartphone-enabled remote ECG monitoring system (I)
Jeffrey Peppercorn
Employment: GlaxoSmithKline (I)
Stock and Other Ownership Interests: GlaxoSmithKline (I)
Consulting or Advisory Role: Athenex
Research Funding: Pfizer
Lidia Schapira
Consulting or Advisory Role: Rubedo, Bluenote Therapeutics
Virginia F. Borges
Research Funding: Abbott/AbbVie (Inst), Seattle Genetics (Inst)
Steven E. Come
Research Funding: AstraZeneca
Employment: Genomic Health
Stock and Other Ownership Interests: Genomic Health
Christy Russell
Employment: Genomic Health International
Stock and Other Ownership Interests: Genomic Health International
Honoraria: Novartis, Pfizer, Genentech, Genomic Health
Speakers' Bureau: Novartis, Pfizer, Genentech, Genomic Health
Travel, Accommodations, Expenses: Novartis, Pfizer, Genentech, Genomic Health
Eric P. Winer
Stock and Other Ownership Interests: Verastem
Honoraria: Genentech, Tesaro, Genomic Health
Consulting or Advisory Role: Leap Therapeutics, Seattle Genetics, Jounce Therapeutics, GlaxoSmithKline, Carrick Therapeutics, Lilly, Genomic Health
Research Funding: Genentech (Inst), Novartis (Inst), Merck (Inst)
Ann H. Partridge
Patents, Royalties, Other Intellectual Property: I receive small royalty payments for co-authoring the breast cancer survivorship section of UpToDate
No other potential conflicts of interest were reported.
REFERENCES
- 1.Adami HO, Malker B, Rutqvist LE, et al. Temporal trends in breast cancer survival in Sweden: Significant improvement in 20 years. J Natl Cancer Inst. 1986;76:653–659. doi: 10.1093/jnci/76.4.653. [DOI] [PubMed] [Google Scholar]
- 2. American Cancer Society: Breast cancer facts & figures 2017-2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2017-2018.pdf. [Google Scholar]
- 3.Han W, Kang SY. Relationship between age at diagnosis and outcome of premenopausal breast cancer: Age less than 35 years is a reasonable cut-off for defining young age-onset breast cancer. Breast Cancer Res Treat. 2010;119:193–200. doi: 10.1007/s10549-009-0388-z. [DOI] [PubMed] [Google Scholar]
- 4.Keegan TH, DeRouen MC, Press DJ, et al. Occurrence of breast cancer subtypes in adolescent and young adult women. Breast Cancer Res. 2012;14:R55. doi: 10.1186/bcr3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han W, Kim SW, Park IA, et al. Young age: An independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer. 2004;4:82. doi: 10.1186/1471-2407-4-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gnerlich JL, Deshpande AD, Jeffe DB, et al. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg. 2009;208:341–347. doi: 10.1016/j.jamcollsurg.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azim HA, Jr, Michiels S, Bedard PL, et al. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res. 2012;18:1341–1351. doi: 10.1158/1078-0432.CCR-11-2599. [DOI] [PubMed] [Google Scholar]
- 8.Fredholm H, Eaker S, Frisell J, et al. Breast cancer in young women: Poor survival despite intensive treatment. PLoS One. 2009;4:e7695. doi: 10.1371/journal.pone.0007695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Partridge AH, Hughes ME, Warner ET, et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol. 2016;34:3308–3314. doi: 10.1200/JCO.2015.65.8013. [DOI] [PubMed] [Google Scholar]
- 10.Johansson ALV, Trewin CB, Hjerkind KV, et al. Breast cancer-specific survival by clinical subtype after 7 years follow-up of young and elderly women in a nationwide cohort. Int J Cancer. 2019;144:1251–1261. doi: 10.1002/ijc.31950. [DOI] [PubMed] [Google Scholar]
- 11.Copson E, Eccles B, Maishman T, et al. Prospective observational study of breast cancer treatment outcomes for UK women aged 18-40 years at diagnosis: The POSH study. J Natl Cancer Inst. 2013;105:978–988. doi: 10.1093/jnci/djt134. [DOI] [PubMed] [Google Scholar]
- 12.Elkum N, Dermime S, Ajarim D, et al. Being 40 or younger is an independent risk factor for relapse in operable breast cancer patients: The Saudi Arabia experience. BMC Cancer. 2007;7:222. doi: 10.1186/1471-2407-7-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 14.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 15.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 16.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: A TransATAC study. J Clin Oncol. 2010;28:1829–1834. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 17.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts MC, Dusetzina SB. Use and costs for tumor gene expression profiling panels in the management of breast cancer from 2006 to 2012: Implications for genomic test adoption among private payers. J Oncol Pract. 2015;11:273–277. doi: 10.1200/JOP.2015.003624. [DOI] [PubMed] [Google Scholar]
- 19.Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373:2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379:111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kizy S, Huang JL, Marmor S, et al. Distribution of 21-gene recurrence scores among breast cancer histologic subtypes. Arch Pathol Lab Med. 2018;142:735–741. doi: 10.5858/arpa.2017-0169-OA. [DOI] [PubMed] [Google Scholar]
- 22.Hassett MJ, Silver SM, Hughes ME, et al. Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol. 2012;30:2218–2226. doi: 10.1200/JCO.2011.38.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. doi: 10.1093/jnci/djx239. Kurian AW, Bondarenko I, Jagsi R, et al: Recent trends in chemotherapy use and oncologists’ treatment recommendations for early-stage breast cancer. J Natl Cancer Inst110:493-400, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrek JA, Naughton MJ, Case LD, et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: A prospective study. J Clin Oncol. 2006;24:1045–1051. doi: 10.1200/JCO.2005.03.3969. [DOI] [PubMed] [Google Scholar]
- 25.Bursac Z, Gauss CH, Williams DK, et al. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paluch-Shimon S, Pagani O, Partridge AH, et al. ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3) Breast. 2017;35:203–217. doi: 10.1016/j.breast.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 27. Thomas A, Rhoads A, Pinkerton E, et al: Incidence and survival among young women with stage I-III breast cancer: SEER 2000-2015. JNCI Cancer Spectr 3:pkz040, 2019. [DOI] [PMC free article] [PubMed]
- 28.Andre F, Ismaila N, Henry NL, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: ASCO clinical practice guideline update summary. J Clin Oncol. 2019;37:1956–1964. doi: 10.1200/JCO.19.00945. [DOI] [PubMed] [Google Scholar]
- 29.Stearns V. TAILORing adjuvant systemic therapy for breast cancer. N Engl J Med. 2018;379:191–192. doi: 10.1056/NEJMe1806329. [DOI] [PubMed] [Google Scholar]
- 30.Sukumvanich P, Case LD, Van Zee K, et al. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: A prospective study. Cancer. 2010;116:3102–3111. doi: 10.1002/cncr.25106. [DOI] [PubMed] [Google Scholar]
- 31.Sparano JA, Gray RJ, Ravdin PM, et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med. 2019;380:2395–2405. doi: 10.1056/NEJMoa1904819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371:107–118. doi: 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436–446. doi: 10.1056/NEJMoa1412379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Francis PA, Pagani O, Fleming GF, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379:122–137. doi: 10.1056/NEJMoa1803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saha P, Regan MM, Pagani O, et al. Treatment efficacy, adherence and quality of life among women younger than 35 years of age in the International Breast Cancer Study Group TEXT and SOFT adjuvant endocrine therapy trials. J Clin Oncol. 2017;35:3113–3122. doi: 10.1200/JCO.2016.72.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cardoso F, van’t Veer LJ, Bogaerts J, et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375:717–729. doi: 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- 37. Aalders K, Genbrugge E, Poncet C, et al: Young age and the risk of disease recurrence as assessed by the 70-gene signature – an analysis from the EORTC 10041/BIG 03-04 MINDACT trial. Cancer Research 78, 2018 (abstr P1-07-08)
- 38.Gluz O, Nitz UA, Christgen M, et al. West German Study Group phase III PlanB trial: First prospective outcome data for the 21-gene Recurrence Score Assay and concordance of prognostic markers by central and local pathology assessment. J Clin Oncol. 2016;34:2341–2349. doi: 10.1200/JCO.2015.63.5383. [DOI] [PubMed] [Google Scholar]
- 39.Nitz U, Gluz O, Clemens M, et al. West German Study PlanB trial: Adjuvant four cycles of epirubicin and cyclophosphamide plus docetaxel versus six cycles of docetaxel and cyclophosphamide in HER2-negative early breast cancer. J Clin Oncol. 2019;37:799–808. doi: 10.1200/JCO.18.00028. [DOI] [PubMed] [Google Scholar]