Abstract
Background/Purpose:
Cirrhosis – clinically overt, advanced liver disease – is associated with an increased risk of hemorrhagic stroke and poor stroke outcomes. We sought to investigate whether subclinical liver disease, specifically liver fibrosis, is associated with clinical and radiological outcomes in patients with primary intracerebral hemorrhage.
Methods:
We performed a retrospective cohort study using data from the Virtual International Stroke Trials Archive-ICH. We included adult patients with primary ICH presenting within 6 hours of symptom onset. We calculated three validated fibrosis indices—Aspartate aminotransferase Platelet Ratio Index (APRI), Fibrosis-4 (FIB-4) score, and Nonalcoholic Fatty Liver Disease Fibrosis Score (NFS)—and modeled them as continuous exposure variables. Primary outcomes were admission hematoma volume and hematoma expansion. Secondary outcomes were mortality, and the composite of major disability or death, at 90 days. We used linear and logistic regression models adjusted for previously established risk factors.
Results
Among 432 patients with ICH, the mean APRI, FIB-4, and NFS values on admission reflected intermediate probabilities of fibrosis, while standard hepatic assays and coagulation parameters were largely normal. After adjusting for potential confounders, APRI was associated with hematoma volume (β, 0.20; 95% CI, 0.04–0.36), hematoma expansion (OR, 1.6; 95% CI, 1.1–2.3), and mortality (OR, 1.8; 95% CI, 1.1–2.7). FIB-4 was also associated with hematoma volume (β, 0.27; 95% CI, 0.07–0.47), hematoma expansion (OR, 1.9; 95% CI, 1.2–3.0), and mortality (OR, 2.0; 95% CI, 1.1–3.6). NFS was not associated with any outcome. Indices were not associated with the composite of major disability or death.
Conclusions:
In patients with largely normal liver chemistries, two liver fibrosis indices were associated with admission hematoma volume, hematoma expansion, and mortality after ICH.
Keywords: Intracerebral hemorrhage, epidemiology, liver fibrosis, cohort studies
Subject Terms: Fibrosis, Epidemiology, Risk Factors, Intracranial Hemorrhage
Introduction
Liver cirrhosis is associated with an increased risk of stroke, particularly hemorrhagic stroke.1, 2 Furthermore, we previously demonstrated that cirrhosis is associated with in-hospital mortality and unfavorable discharge disposition after intracerebral hemorrhage (ICH).3 While these data highlight a possible link between advanced liver disease and poor stroke outcomes, it is unclear if these findings also apply to subclinical liver disease.
Liver fibrosis, an often clinically silent manifestation of chronic liver disease and a histological precursor to cirrhosis, is present in up to 9% of individuals without known liver disease.4–6 Liver fibrosis is a key predictor of cardiovascular mortality in patients with chronic liver disease7–9 and is also associated with ischemic stroke risk10 and cerebral microbleeds,11 but data are lacking regarding the implications of liver fibrosis for patients with ICH. We therefore investigated the association between subclinical liver disease, defined using liver fibrosis indices, and ICH outcomes using a large, international cohort of patients without overt liver disease enrolled in clinical trials. We hypothesized that serum-based indices of liver fibrosis are associated with larger baseline hematoma volume, greater hematoma expansion, and worse clinical outcomes in patients with ICH.
Methods
Data Source and Study Design
We performed a retrospective cohort study using data from the Virtual International Stroke Trials Archive ICH (VISTA-ICH).12 The VISTA database (www.vistacollaboration.org) houses anonymized, individual patient-level data from completed trials. Trials are eligible for inclusion in VISTA-ICH if they meet the following requirements: 1) documented entry criteria into a trial with a minimum of 50 randomized patients with ICH; 2) documented consent or waiver of consent from a local ethics board; 3) baseline assessment within 24 hours of ICH; 4) baseline assessment of neurologic deficit at the time of admission; 5) confirmation of ICH by cerebral imaging within 7 days; 6) outcome assessment between 1 and 6 months with a validated stroke scale; and 7) data validation through monitoring.13 The VISTA cohort used in this study consisted of only patients in the placebo arm of all trials and intervention arms of negative non-surgical trials. Individual trials that contributed to the dataset used for this analysis were performed with institutional review board and/or regulatory approval. Individual trials obtained informed consent. Our analysis was approved by the Weill Cornell Medicine institutional review board. The data used in this analysis are restricted per the terms of VISTA-ICH’s data use agreement and therefore cannot be shared directly with other investigators. However, investigators can obtain access to these data by submitting a formal application to VISTA-ICH.
Population
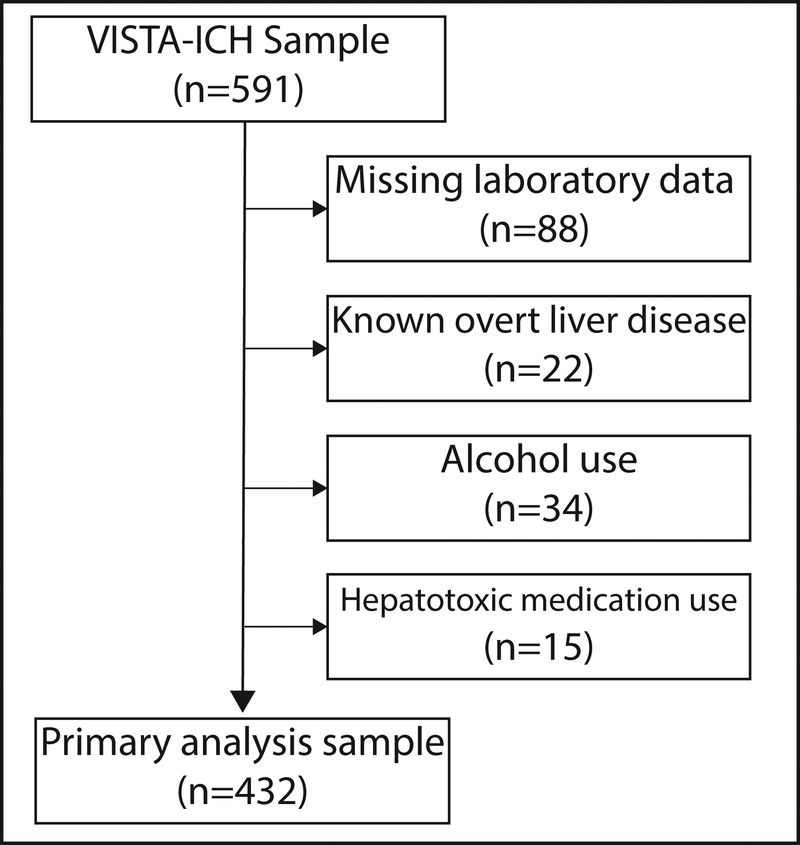
We included only adult patients with primary ICH who presented within 6 hours of symptom onset. We excluded patients with missing data on liver chemistries, baseline imaging, or important clinical covariates such as ICH severity (Figure 1). All patients had follow-up computed tomography (CT) scan at 72 hours and 90-day modified Rankin Scale (mRS) assessments. In order to minimize the contribution of potential confounding by alcohol use and competing causes of liver test abnormalities, we excluded patients with self-reported alcohol use and use of select medications: valproic acid, amiodarone, methotrexate, and tamoxifen.14
Figure 1. Patient flow diagram.
Patients with missing laboratory data, known overt liver disease, alcohol use, and those using potentially hepatotoxic medications were excluded.
Exposures
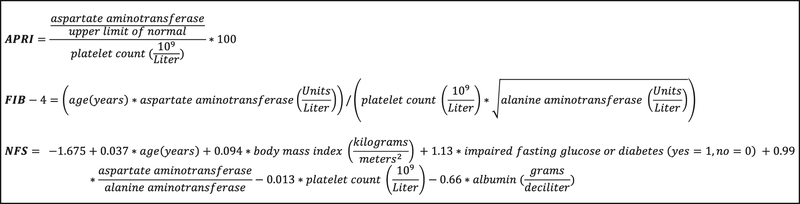
We calculated three non-invasive liver fibrosis indices for each participant at the time of admission: the Aspartate aminotransferase-Platelet Ratio Index (APRI), the Fibrosis-4 (FIB-4) score, and the Nonalcoholic Fatty Liver Disease Fibrosis Score (NFS).15–17 These indices are calculated from demographic variables, medical comorbidities, and laboratory data (Figure 2). Most individuals with covert liver fibrosis, especially after excluding those with alcohol abuse, have non-alcoholic fatty liver disease as the underlying etiology of liver fibrosis.4 Liver imaging data are not available in VISTA-ICH; however all three indices have been validated to have good accuracy for the non-invasive detection of liver fibrosis in patients with non-alcoholic fatty liver disease, with receiver-operating-characteristic curve values ranging from 0.76 to 0.88.18 Studies have validated the predictive accuracy of the indices at different thresholds; thresholds reflective of a high probability of advanced fibrosis are APRI > 1, FIB-4 score > 3.25, and NFS > 0.676.18 The NFS range includes negative values. These indices have also been validated in the setting of hepatitis B (FIB-4), hepatitis C (APRI, FIB-4), and alcoholic liver disease (FIB-4).19–22
Figure 2. Formulas for liver fibrosis indices.
Fibrosis indices were the aspartate aminotransferase – platelet ratio index (APRI), Fibrosis-4 score (FIB-4), and nonalcoholic fatty liver disease – fibrosis score (NFS).
Demographics and ICH Characteristics
Demographic and clinical data included age, sex, hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, smoking, and use of antiplatelet and anticoagulant medication. Admission Glasgow Coma Scale (GCS) score, National Institutes of Health Stroke Scale (NIHSS) score, and systolic blood pressure were also obtained at pre-specified time points. Laboratory data obtained include platelet count, coagulation parameters (International Normalized Ratio and partial thromboplastin time), and several liver chemistries (alanine aminotransferase, aspartate aminotransferase, albumin, and total bilirubin). Baseline radiological data were admission hematoma volume, location (lobar, deep, and infratentorial), and intraventricular hemorrhage (present vs. absent).
Outcomes
Our primary outcomes were admission hematoma volume and hematoma expansion. Our secondary outcomes were all-cause mortality at 90 days, and death or major disability at 90 days. Major disability was defined as a modified Rankin Scale (mRS) score of 4 or greater. Hematoma volumes were calculated using semi-automated planimetry by individual trial neuroradiologists. Hematoma expansion was defined as an increase in the absolute hematoma volume by either 33% or ≥ 6 mL at 72 hours compared to the admission acquisition.23
Statistical Analyses
Patient characteristics were described using standard descriptive statistics. Discrete variables are presented as counts (percentages [%]) and continuous variables as means (standard deviation [SD]) or medians (interquartile range [IQR]), as appropriate. Pearson Chi-squared test was used for categorical variables as appropriate; the Student’s t test or Wilcoxon rank sum test were used for continuous variables depending on the normality of distribution. Fibrosis indices were log-transformed to minimize skewness and treated as continuous variables.
We used multiple logistic regression models to assess the relationships between each fibrosis index and binary outcomes. We a priori selected known predictors of hematoma expansion and clinical outcomes as covariates, which were age, sex, hematoma location, presence of intraventricular hemorrhage, GCS score, anticoagulant use, and time from symptom onset to baseline CT scan.24, 25 The models did not include admission hematoma volume since we expected it to be a mediator in the causal pathway given our hypothesis.
We used multiple linear regression to model associations between fibrosis indices and admission hematoma volume, both of which were log-transformed. Covariates were age, sex, hematoma location, antiplatelet use, and International Normalized Ratio, since these have previously been shown to be independent predictors of baseline hematoma volume in a large cohort of ICH patients.26 Collinear covariates, defined by a variance inflation factor > 4, were subsequently identified and removed from the model. We also performed sensitivity analyses excluding patients with thrombocytopenia (defined as platelet count <150,000 per microliter). Statistical analyses were performed using Stata (version 14.0, College Station, TX). All analyses were two-tailed with the threshold of statistical significance allowing for an alpha error of 0.05.
Results
Characteristics of Study Population
The VISTA-ICH dataset consisted of 591 patients of whom 432 were eligible for our study (Figure 1). The mean age was 66 years (SD, 12). There were 30 patients (7%) taking anticoagulant medications, and 37 patients (9%) had thrombocytopenia. Standard liver chemistry tests and coagulation indices were generally in the normal range in the study sample; 12% had an aspartate aminotransferase > 40 IU/L, 11% had an alanine aminotransferase > 40 IU/L, and 29% had an INR ≥ 1.4 (Table 1). Baseline characteristics were stratified by Fibrosis-4 score (quartile 4 versus quartiles 1–3) to demonstrate how baseline characteristics may differ with fibrosis indices. Mean aspartate aminotransferase values and the proportion with thrombocytopenia were greater among those with a FIB-4 score in the highest quartile (Table 1). The mean APRI, FIB-4, and NFS values were 0.4 (SD, 0.4), 1.9 (SD 1.4), and 0.3 (SD, 1.3), respectively; these means reflect intermediate or indeterminate probabilities of fibrosis. When validated thresholds were used, there were 32 patients (7%) with a high probability of fibrosis by the APRI, 41 patients (9%) by the FIB-4, and 142 patients (33%) by the NFS.
Table 1.
Characteristics of Patients with Intracerebral Hemorrhage, Stratified by Fibrosis-4 Score Quartiles
| Characteristic* | Study Sample (N=432) | FIB-4 Score Quartile 4 (N=108) | FIB-4 Score Quartiles 1–3 (N=324) |
|---|---|---|---|
| Patient Characteristics | |||
| Age, mean (SD), years | 65.5 (11.9) | 73.6 (10.6) | 62.8 (11.0) |
| Female sex | 147 (34) | 33 (31) | 114 (35) |
| Race | |||
| White | 357 (83) | 97 (91) | 260 (83) |
| Black | 19 (4) | 2 (2) | 17 (5) |
| Other | 46 (11) | 8 (8) | 38 (12) |
| Hypertension | 350 (81) | 86 (80) | 264 (82) |
| Hyperlipidemia | 69 (16) | 15 (14) | 54 (17) |
| Diabetes mellitus | 78 (18) | 14 (13) | 64 (20) |
| Atrial fibrillation | 37 (9) | 19 (18) | 18 (6) |
| Anticoagulant use | 30 (7) | 11 (11) | 19 (6) |
| Antiplatelet use | 93 (22) | 35 (32) | 58 (18) |
| Body mass index, mean (SD), kg/m2 | 27.4 (5.7) | 30.3 (6.1) | 27.4 (5.7) |
| Admission Laboratory Data | |||
| Platelet count†, x103 per microliter | 214 (179–258) | 166 (144–194) | 232 (198–272) |
| International Normalized Ratio† | 1.0 (0.9–1.1) | 1.0 (0.9–1.2) | 1.0 (0.9–1.1) |
| International Normalized Ratio ≥ 1.4 | 124 (29) | 35 (32) | 89 (27) |
| aPTT†, seconds | 24.6 (21.5–29.2) | 25.2(19.3–31.1) | 24.6 (21.8–28.8) |
| AST†, units/liter | 23 (19–29) | 30 (23–51) | 22 (18–26) |
| AST > 40 units/liter | 51 (12) | 34 (31) | 17 (5) |
| ALT†, units/liter | 20 (15–29) | 20 (14–34) | 20 (15–28) |
| ALT > 40 units/liter | 46 (11) | 22 (23) | 24 (7) |
| Albumin†, gram/dL | 4.1 (3.9–4.3) | 4.1 (3.8–4.2) | 4.1 (3.9–4.4) |
| Total bilirubin†, milligram/dL | 0.5 (0.7) | 0.6 (0.4–0.8) | 0.4 (0.3–0.5) |
| Thrombocytopenia (<150,000/μL) | 37 (8.6) | 32 (29.6) | 5 (1.5) |
| Admission ICH Data | |||
| Glasgow Coma Scale† | 15 (13–15) | 14 (14–15) | 15 (13–15) |
| National Institutes of Health Stroke Scale† | 13 (9–17) | 14 (10–18) | 13 (9–17) |
| Time to admission CT scan†, hours | 3.3 (1.3–5.5) | 3.4 (1.4–5.0) | 3.2 (1.3–5.6) |
| Hematoma volume at admission†, mL | 16.1 (8.1–29.6) | 21.1 (8.9–35.3) | 14.9 (7.9–27.2) |
| Intraventricular hemorrhage | 138 (32) | 44 (41) | 94 (29) |
| Hematoma location | |||
| Lobar | 90 (21) | 28 (26) | 62 (19) |
| Deep | 333 (77) | 79 (73) | 254 (78) |
| Infratentorial | 9 (2) | 1 (1) | 8 (3) |
| 72-hour ICH Imaging Data | |||
| Hematoma volume at 72 hours†, mL | 16.9 (8.1–33.8) | 25.3 (9.9–43.2) | 16.6 (8.1–31.5) |
| Time to 72-hour CT scan†, hours | 69.9 (64.1–77.1) | 70.5 (65.3–76.4) | 69.6 (64.0–78.2) |
Abbreviations; SD, standard deviation; aPPT, activated partial thromboplastin time; AST, aspartate aminotransferase; ALT, alanine aminotransferase; dl, deciliter; mL, milliliters; μL, microliter.
Data reported as n (%) unless otherwise specified.
Data reported as median (interquartile range).
Fibrosis Indices and Hematoma Admission Hematoma Volume
The median admission hematoma volume was 16.1 mL (IQR, 8.1–29.6). Multiple linear regression models adjusted for age, sex, hematoma location, antiplatelet use, International Normalized Ratio, and time from symptom onset to baseline CT scan showed associations with hematoma volume for APRI (β, 0.20; 95% CI, 0.04–0.36) and FIB-4 (β, 0.27; 95% CI, 0.07–0.47) but not NFS (β, 0.03; 95% CI, −0.07–0.13) (Table 2).
Table 2.
Associations between Liver Fibrosis Indices and Admission Hematoma Volume, Hematoma Expansion, and Outcomes after ICH
| Outcome | APRI* | FIB-4* | NFS* | |||
|---|---|---|---|---|---|---|
| p value | p value | p value | ||||
| Hematoma expansion | 0.01 | 0.01 | 0.22 | |||
| All-cause mortality | 0.01 | 0.02 | 0.22 | |||
| Death or major disability | 0.19 | 0.31 | 0.71 | |||
| p value | p value | p value | ||||
| Admission hematoma volume*,† | 0.01 | 0.01 | 0.53 | |||
Abbreviations: mRS, modified Rankin Scale; APRI, aspartate aminotransferase-platelet ratio index; FIB-4, fibrosis-4 score; NFS, Nonalcoholic Fatty Liver Disease Fibrosis score, SE, Standard Error.
Logarithmic transformation was performed to minimize skewness.
Linear regression was used.
Fibrosis Indices and Hematoma Expansion
One hundred and forty-five patients (34%) had hematoma expansion. Multiple logistic regression models adjusted for age, sex, admission GCS, hematoma location, intraventricular hemorrhage, anticoagulant use, and time from symptom onset to baseline CT scan showed associations with hematoma expansion for APRI (OR per each 10-fold increase, 1.6; 95% CI, 1.1–2.3) and FIB-4 (OR per each 10-fold increase, 1.9; 95% CI, 1.2–3.0), but not for NFS (OR per each 10-fold increase, 1.2; 95% CI, 0.9–1.5) (Table 2).
Fibrosis Indices and Clinical Outcomes
At 90 days, there were 75 (17%) deaths, and 220 (51%) patients had a modified Rankin Scale score of 4 or greater. Multiple logistic regression models adjusted for age, sex, hematoma location, intraventricular hemorrhage, admission GCS, and anticoagulant use showed associations with 90-day mortality for APRI (OR per each 10-fold increase, 1.8; 95% CI, 1.1–2.7) and FIB-4 (OR per each 10-fold increase, 2.0; 95% CI, 1.1–3.6), but not for NFS (OR per each 10-fold increase, 1.2; 95% CI, 0.9–1.8) (Table 2). None of the indices were associated with the composite of major disability or death at 90 days. The direction and magnitude of effect were generally consistent with the primary analysis for all outcomes in post-hoc sex-stratified analyses (please see https://www.ahajournals.org/journal/str).
Sensitivity Analyses
In a sensitivity analysis excluding patients with thrombocytopenia (n=37, 9%), the APRI and FIB-4 fibrosis indices remained associated with admission hematoma volume and hematoma expansion (Table 3). While the direction of effect remained consistent with the primary analyses for the association between APRI and FIB-4 and all-cause mortality, the effect size and statistical significance were attenuated (Table 3). No associations were noted with the composite of 90-day death or major disability, and NFS was not associated with any outcomes of interest, consistent with the results of the primary analysis.
Table 3.
Associations between Liver Fibrosis Indices and Admission Hematoma Volume, Hematoma Expansion, and Outcomes after ICH after Excluding Patients with Thrombocytopenia
| Outcome | APRI* | FIB-4* | NFS* | |||
|---|---|---|---|---|---|---|
| p value | p value | p value | ||||
| Hematoma expansion | 0.02 | 0.02 | 0.36 | |||
| All-cause mortality | 0.08 | 0.15 | 0.44 | |||
| Death or major disability | 0.29 | 0.49 | 0.98 | |||
| p value | p value | p value | ||||
| Admission hematoma volume† | 0.003 | 0.002 | 0.28 | |||
Abbreviations: mRS, modified Rankin Scale; APRI, aspartate aminotransferase-platelet ratio index; FIB-4, fibrosis-4 score; NFS, Nonalcoholic Fatty Liver Disease Fibrosis score, SE, Standard Error.
Logarithmic transformation was performed to minimize skewness.
Linear regression was used.
Discussion
In this international, multicenter cohort of patients with ICH, two serum-based liver fibrosis indices were independently associated with admission hematoma volume, hematoma expansion, and 90-day mortality, but not the composite of death or major disability. Notably, these associations were observed in a population with standard liver chemistries generally in the normal range.
To our knowledge, the association between validated liver fibrosis indices and ICH characteristics and outcomes has not been studied previously. Prior studies have inconclusively suggested that severe derangements in individual liver enzyme tests are associated with higher hematoma volumes and hematoma expansion.27–31 However, these studies evaluated individual, non-specific liver enzyme tests in populations with heavy alcohol use. In contrast, we present a novel association between validated liver fibrosis indices and admission hematoma volume and hematoma expansion. In this context, our study presents novel findings that liver fibrosis may reflect a propensity for increased bleeding as evidenced by higher admission ICH volumes and hematoma expansion, in the absence of obvious clinical manifestations or laboratory derangements otherwise typically expected with overt liver disease. This is consistent with the observation that standard liver chemistries are commonly normal in patients with chronic liver disease; transaminase levels are normal in up to 75% of individuals with imaging evidence of significant liver fibrosis.4, 32 Taken together, these data raise the possibility that subclinical liver fibrosis – liver fibrosis not consistently identifiable with standard liver chemistries – is associated with higher admission ICH volumes and hematoma expansion. Possible mechanisms may include subclinical coagulopathy, endothelial dysfunction, and vascular inflammation.33–36 These mechanisms were described in patients with clinically diagnosed liver disease; whether these mechanisms can be implicated to explain our findings with regards to subclinical liver fibrosis is a hypothesis that requires testing in future studies. Further investigation with more direct measures of liver fibrosis, such as biopsy results or advanced imaging data, detailed liver-specific comorbidity data, and mechanistic data, is warranted to better understand the pathophysiology of our findings and better support any explicit causal inferences regarding liver fibrosis itself.
Liver fibrosis indices were also associated with mortality in our study. Prior studies evaluating the relationship between liver disease and ICH outcomes have considered either isolated abnormalities in individual liver enzyme levels or included patients with clinically overt liver disease. One such study found that subclinical derangements in individual hepatic enzymes, such aspartate aminotransferase and alkaline phosphatase, were associated with worse clinical outcomes in ICH in univariable analysis, but these associations were not present after adjustment for confounders.37 Similarly, isolated hypoalbuminemia has been associated with increased 90-day mortality in ICH.38 With regards to clinically overt liver disease, we have previously demonstrated using administrative claims data that patients with liver disease have less favorable hospital discharge disposition and greater in-hospital mortality compared to those without.3 Additionally, among a small cohort of patients with known chronic liver disease primarily due to alcohol or viral hepatitis, liver cirrhosis was associated with greater in-hospital mortality in ICH.39 Our results build upon these data by demonstrating an association between liver fibrosis indices and 90-day mortality in ICH among individuals with largely normal standard liver chemistries and coagulation indices. Although increased hematoma growth offers one possible explanation, increased susceptibility to infections and liver-related complications, as shown in other studies, may provide alternative reasons for higher mortality after ICH.38–40 The discrepancy between 90-day mortality and the composite of 90-day death or major disability in our study suggests that non-neurological or otherwise non-disabling complications such as those directly related to liver disease, may account for our findings.
Strengths of our study include the use of a well-adjudicated ICH cohort with strict inclusion criteria, pre-specified time points for radiological measurements, the availability of standardized outcome assessments, and the analysis of relatively homogenous clinical trial populations with exclusion of patients with overt liver disease and coagulopathy. Our results should be interpreted while considering an important caveat: our study investigated the associations between serum-based liver fibrosis indices and ICH outcomes. These indices are surrogate markers and screening tools for liver fibrosis, and we did not have advanced liver imaging or liver biopsy data to corroborate the ascertainment of liver fibrosis in our study population. While our use of liver fibrosis indices is consistent with other efforts to understand the impact of liver fibrosis on other disease processes,41, 42 our results should be interpreted while considering the performance of these liver fibrosis indices. Though the prevalence of fibrosis by the APRI and FIB-4 in our cohort was reassuringly consistent with population-based estimates (5–9%),4–6 our data suggest that the NFS performed poorly in the VISTA-ICH cohort. Specifically, the proportion of patients with an NFS above its high-probability cut-off in our cohort was 33%. This suggests that, compared to the APRI and FIB-4, the NFS was non-specific in our sample. The NFS formula includes body mass index, diabetes, and impaired fasting glucose, which reflect this score’s validation primarily for the detection of advanced fibrosis among patients with non-alcoholic fatty liver disease. In contrast, the simpler APRI and FIB-4 have been validated in more liver diseases19–22 and may be more appropriate for the general population, in which liver diseases may occur concurrently. Additionally, some components of the NFS, such as the body mass index, may in fact have a paradoxically protective role after ICH.43, 44 These factors may account for discrepancies in associations across liver fibrosis indices.
Our study has additional noteworthy limitations. First, inclusion of patients with low ICH severity who were enrolled in clinical trials may limit generalizability of our results. Second, our study population had a high mean body mass index; the extent to which liver fibrosis itself is responsible for our findings, as opposed to being an epiphenomenon related to obesity, requires further investigation. Third, viral hepatitis serologies were unavailable and self-reported alcohol use may have been imprecise; however, given that most clinical trials exclude patients with advanced liver disease or known alcohol abuse, we believe our study had few such patients. Some liver fibrosis indices have also been validated in patients with viral hepatitis and alcohol use.19–22
Conclusions
Among patients with ICH, two liver fibrosis indices were associated with admission hematoma volume, hematoma expansion, and 90-day mortality despite largely normal standard liver chemistries and coagulation parameters. Further study to examine the impact of subclinical liver disease on ICH outcomes is warranted. Such work may reveal novel targets for hemostasis and help identify patients at risk of hematoma expansion and poor outcomes who may benefit from targeted hemostatic therapies.
Supplementary Material
Sources of Funding:
NSP: NIH (T32NS07153, PI: MSVE). HK: NIH (R01NS097443, U01NS095869), Michael Goldberg Research Fund. BBN: NIH (K23NS091395), Florence Gould Endowment for Discovery in Stroke. CI: NIH (R01NS34179, R37-NS089323, R01-NS100447, 1R01-NS095441, R01-NS/HL37853). AEM: American Heart Association (AHA) (18CDA34110419), Leon Levy Foundation. AJ: Society of Interventional Radiology Foundation. GJF: NIH (K76AG059992), AHA (18IDDG34280056), Yale Pepper Scholar Award (P30AG021342), Neurocritical Care Society Research Fellowship. KNS: NIH (U01NS106513, U24NS107215, U24NS107136, R01NR018335), AHA (17CSA33550004). MSVE: NIH (R01NS029993, U01NS095869). DH: NIH (1U01NS080824, U24TR001609). WZ: NIH (1U01NS080824). SBM: NIH (K23NS105948), Leon Levy Foundation.
APPENDIX
VISTA-ICH Steering Committee
VISTA-ICH Steering Committee Collaborators: D.F. Hanley (Chair), K. Butcher, S. Davis, B. Gregson, K.R. Lees, P. Lyden, S. Mayer, K. Muir, and T. Steiner.
| Name | Affiliation |
|---|---|
| Daniel F. Hanley (Chair) | Brain Injury Outcomes Center, Johns Hopkins University School of Medicine, Baltimore, MD, USA |
| Kenneth Butcher | Division of Neurology, University of Alberta, Edmonton, AB, Canada |
| Stephen M. Davis | Department of Translational Neuroscience, University of Melbourne, Victoria, Australia |
| Barbara Gregson | Institute of Neuroscience, Newcastle University, Tyne, United Kingdom. |
| Kennedy R. Lees | Institute of Cardiovascular & Medical Sciences, University of Glasgow, Glasgow, United Kingdom |
| Patrick D. Lyden | Department of Neurology, Cedars-Sinai Heart Institute, Los Angeles, CA, USA |
| Stephan A. Mayer | Department of Neurology, Henry Ford Health System, Detroit, MI, USA. |
| Keith Muir | Institute of Neuroscience & Psychology, University of Glasgow, University of Glasgow, Glasgow, Scotland, UK |
| Thorsten Steiner | Department of Neurology, Klinikum Frankfurt Höchst, Frankfurt, Germany. |
Footnotes
Conflicts-of-Interest/Disclosures: HK serves as co-PI for the NIH-funded ARCADIA trial which receives in-kind study drug from the BMS-Pfizer Alliance and in-kind study assays from Roche Diagnostics, serves as Deputy Editor for JAMA Neurology, serves as a steering committee member of Medtronic’s Stroke AF trial (uncompensated), serves on an endpoint adjudication committee for a trial of empagliflozin for Boehringer-Ingelheim, and has served on an advisory board for Roivant Sciences related to Factor XI inhibition. BBN serves as a member of the data and safety monitoring board for the PCORI-funded TRAVERSE trial and has received personal fees for medicolegal consulting on stroke. CI has received fees from Broadview Ventures. KNS serves as the PI for the Novartis-sponsored S1P ICH trial, the co-PI in the Bard-sponsored INTREPID trial, and the co-PI in the Biogen-sponsored CHARM trial, and receives funding from Hyperfine Imaging. MSVE serves as the Chairman of the Advisory Committee to the American Stroke Association and on the National, Founders Affiliate, and New York City boards of the American Heart Association. He receives royalties for chapters on stroke from UpToDate. DFH reports personal fees from Op2Lysis, personal fees from BrainScope, personal fees from Neurotrope, personal fees from Portola Pharmaceuticals, fees for medicolegal consulting, and non-financial support from Genentech outside the submitted work. WCZ reports personal fees and board membership for Portola Pharmaceutical and consulting fees from C.R. Bard, Inc. outside of the area of work commented on here. All other authors report no conflict of interest for this study.
References
- 1.Wu HY, Lin CS, Yeh CC, Hu CJ, Shih CC, Cherng YG, et al. Cirrhosis patients’ stroke risks and adverse outcomes: Two nationwide studies. Atherosclerosis. 2017;263:29–35 [DOI] [PubMed] [Google Scholar]
- 2.Parikh NS, Navi BB, Schneider Y, Jesudian A, Kamel H. Association between cirrhosis and stroke in a nationally representative cohort. JAMA Neurol. 2017;74:927–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parikh NS, Merkler AE, Schneider Y, Navi BB, Kamel H. Discharge disposition after stroke in patients with liver disease. Stroke. 2017;48:476–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caballería L, Pera G, Arteaga I, Rodríguez L, Alumà A, Morillas RM, et al. High prevalence of liver fibrosis among european adults with unknown liver disease: A population-based study. Clin Gastroenterol Hepatol. 2018;16:1138–1145.e1135 [DOI] [PubMed] [Google Scholar]
- 5.Roulot D, Costes JL, Buyck JF, Warzocha U, Gambier N, Czernichow S, et al. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut. 2011;60:977–984 [DOI] [PubMed] [Google Scholar]
- 6.You SC, Kim KJ, Kim SU, Kim BK, Park JY, Kim DY, et al. Factors associated with significant liver fibrosis assessed using transient elastography in general population. World J Gastroenterol. 2015;21:1158–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397.e310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the united states. Hepatology. 2013;57:1357–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le MH, Devaki P, Ha NB, Jun DW, Te HS, Cheung RC, et al. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the united states. PLoS One. 2017;12:e0173499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SU, Song D, Heo JH, Yoo J, Kim BK, Park JY, et al. Liver fibrosis assessed with transient elastography is an independent risk factor for ischemic stroke. Atherosclerosis. 2017;260:156–162 [DOI] [PubMed] [Google Scholar]
- 11.Kim YD, Song D, Heo JH, Kim SU, Kim BK, Park JY, et al. Relationship between cerebral microbleeds and liver stiffness determined by transient elastography. PLoS One. 2015;10:e0139227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali M, Bath P, Brady M, Davis S, Diener HC, Donnan G, et al. Development, expansion, and use of a stroke clinical trials resource for novel exploratory analyses. Int J Stroke. 2012;7:133–138 [DOI] [PubMed] [Google Scholar]
- 13.Murthy SB, Moradiya Y, Dawson J, Lees KR, Hanley DF, Ziai WC, et al. Perihematomal edema and functional outcomes in intracerebral hemorrhage: Influence of hematoma volume and location. Stroke. 2015;46:3088–3092 [DOI] [PubMed] [Google Scholar]
- 14.Kabbany MN, Conjeevaram Selvakumar PK, Watt K, Lopez R, Akras Z, Zein N, et al. Prevalence of nonalcoholic steatohepatitis-associated cirrhosis in the united states: An analysis of national health and nutrition examination survey data. Am J Gastroenterol. 2017;112:581–587 [DOI] [PubMed] [Google Scholar]
- 15.Peleg N, Issachar A, Sneh-Arbib O, Shlomai A. Ast to platelet ratio index and fibrosis 4 calculator scores for non-invasive assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease. Dig Liver Dis. 2017;49:1133–1138 [DOI] [PubMed] [Google Scholar]
- 16.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The nafld fibrosis score: A noninvasive system that identifies liver fibrosis in patients with nafld. Hepatology. 2007;45:846–854 [DOI] [PubMed] [Google Scholar]
- 18.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology. 2017;66(5):1486–1501. [DOI] [PubMed] [Google Scholar]
- 19.Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis c-related fibrosis: An updated meta-analysis. Hepatology. 2011;53:726–736 [DOI] [PubMed] [Google Scholar]
- 20.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. Fib-4: An inexpensive and accurate marker of fibrosis in hcv infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36 [DOI] [PubMed] [Google Scholar]
- 21.Kim BK, Kim DY, Park JY, Ahn SH, Chon CY, Kim JK, et al. Validation of fib-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis b virus-infected patients. Liver Int. 2010;30:546–553 [DOI] [PubMed] [Google Scholar]
- 22.Naveau S, Gaudé G, Asnacios A, Agostini H, Abella A, Barri-Ova N, et al. Diagnostic and prognostic values of noninvasive biomarkers of fibrosis in patients with alcoholic liver disease. Hepatology. 2009;49:97–105 [DOI] [PubMed] [Google Scholar]
- 23.Mayer SA, Brun NC, Broderick J, Davis S, Diringer MN, Skolnick BE, et al. Safety and feasibility of recombinant factor viia for acute intracerebral hemorrhage. Stroke. 2005;36:74–79 [DOI] [PubMed] [Google Scholar]
- 24.Brouwers HB, Chang Y, Falcone GJ, Cai X, Ayres AM, Battey TW, et al. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol. 2014;71:158–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ich score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897 [DOI] [PubMed] [Google Scholar]
- 26.Falcone GJ, Biffi A, Brouwers HB, Anderson CD, Battey TW, Ayres AM, et al. Predictors of hematoma volume in deep and lobar supratentorial intracerebral hemorrhage. JAMA Neurol. 2013;70:988–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujii Y, Tanaka R, Takeuchi S, Koike T, Minakawa T, Sasaki O. Hematoma enlargement in spontaneous intracerebral hemorrhage. J Neurosurg. 1994;80:51–57 [DOI] [PubMed] [Google Scholar]
- 28.Fujii Y, Takeuchi S, Tanaka R, Koike T, Sasaki O, Minakawa T. Liver dysfunction in spontaneous intracerebral hemorrhage. Neurosurgery. 1994;35:592–596 [DOI] [PubMed] [Google Scholar]
- 29.Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Tanaka R. Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke. 1998;29:1160–1166 [DOI] [PubMed] [Google Scholar]
- 30.Kazui S, Minematsu K, Yamamoto H, Sawada T, Yamaguchi T. Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke. 1997;28:2370–2375 [DOI] [PubMed] [Google Scholar]
- 31.Niizuma H, Suzuki J, Yonemitsu T, Otsuki T. Spontaneous intracerebral hemorrhage and liver dysfunction. Stroke. 1988;19:852–856 [DOI] [PubMed] [Google Scholar]
- 32.Calvaruso V, Craxì A. Implication of normal liver enzymes in liver disease. J Viral Hepat. 2009;16:529–536 [DOI] [PubMed] [Google Scholar]
- 33.Lee HJ, Lee CH, Kim S, Hwang SY, Hong HC, Choi HY, et al. Association between vascular inflammation and non-alcoholic fatty liver disease: Analysis by 18f-fluorodeoxyglucose positron emission tomography. Metabolism. 2017;67:72–79 [DOI] [PubMed] [Google Scholar]
- 34.Targher G, Bertolini L, Scala L, Zoppini G, Zenari L, Falezza G. Non-alcoholic hepatic steatosis and its relation to increased plasma biomarkers of inflammation and endothelial dysfunction in non-diabetic men. Role of visceral adipose tissue. Diabet Med. 2005;22:1354–1358 [DOI] [PubMed] [Google Scholar]
- 35.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. The New England journal of medicine. 2011;365:147–156 [DOI] [PubMed] [Google Scholar]
- 36.Shin KH, Kim IS, Lee HJ, Kim HH, Chang CL, Hong YM, et al. Thromboelastographic evaluation of coagulation in patients with liver disease. Ann Lab Med. 2017;37:204–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan G, Hao Z, Lei C, Chen Y, Yuan R, Xu M, et al. Subclinical change of liver function could also provide a clue on prognosis for patients with spontaneous intracerebral hemorrhage. Neurol Sci. 2016;37:1693–1700 [DOI] [PubMed] [Google Scholar]
- 38.Morotti A, Marini S, Lena UK, Crawford K, Schwab K, Kourkoulis C, et al. Significance of admission hypoalbuminemia in acute intracerebral hemorrhage. Journal of neurology. 2017;264:905–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoya K, Tanaka Y, Uchida T, Takano I, Nagaishi M, Kowata K, et al. Intracerebral hemorrhage in patients with chronic liver disease. Neurol Med Chir (Tokyo). 2012;52:181–185 [DOI] [PubMed] [Google Scholar]
- 40.Di Napoli M, Behrouz R, Topel CH, Misra V, Pomero F, Giraudo A, et al. Hypoalbuminemia, systemic inflammatory response syndrome, and functional outcome in intracerebral hemorrhage. J Crit Care. 2017;41:247–253 [DOI] [PubMed] [Google Scholar]
- 41.Yoshihisa A, Sato Y, Yokokawa T, Sato T, Suzuki S, Oikawa M, et al. Liver fibrosis score predicts mortality in heart failure patients with preserved ejection fraction. ESC Heart Fail. 2018;5:262–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pastori D, Lip GYH, Farcomeni A, Del Sole F, Sciacqua A, Perticone F, et al. Incidence of bleeding in patients with atrial fibrillation and advanced liver fibrosis on treatment with vitamin k or non-vitamin k antagonist oral anticoagulants. Int J Cardiol. 2018;264:58–63 [DOI] [PubMed] [Google Scholar]
- 43.Dangayach NS, Grewal HS, De Marchis GM, Sefcik RK, Bruce R, Chhatlani A, et al. Does the obesity paradox predict functional outcome in intracerebral hemorrhage? J Neurosurg. 2018;129:1125–1129 [DOI] [PubMed] [Google Scholar]
- 44.Sun W, Xian Y, Huang Y, Liu R, Li F, Wei JW, et al. Obesity is associated with better survival and functional outcome after acute intracerebral hemorrhage. Journal of the neurological sciences. 2016;370:140–144 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.