Nrf2 (NF-E2-related-factor 2) is a stress-responsive transcription factor that protects cells against oxidative stresses. To clarify whether Nrf2 prevents Alzheimer’s disease (AD), AD model AppNL-G-F/NL-G-F knock-in (AppNLGF) mice were studied in combination with genetic Nrf2 induction model Keap1FA/FA mice. While AppNLGF mice displayed shorter latency to escape than wild-type mice in the passive-avoidance task, the impairment was improved in AppNLGF::Keap1FA/FA mice.
KEYWORDS: Nrf2, Alzheimer’s disease, oxidative stress, inflammation, glutathione, MALDI-MSI, Alzheimer’s disease, Nrf2
ABSTRACT
Nrf2 (NF-E2-related-factor 2) is a stress-responsive transcription factor that protects cells against oxidative stresses. To clarify whether Nrf2 prevents Alzheimer’s disease (AD), AD model AppNL-G-F/NL-G-F knock-in (AppNLGF) mice were studied in combination with genetic Nrf2 induction model Keap1FA/FA mice. While AppNLGF mice displayed shorter latency to escape than wild-type mice in the passive-avoidance task, the impairment was improved in AppNLGF::Keap1FA/FA mice. Matrix-assisted laser desorption ionization–mass spectrometry imaging revealed that reduced glutathione levels were elevated by Nrf2 induction in AppNLGF::Keap1FA/FA mouse brains compared to AppNLGF mouse brains. Genetic Nrf2 induction in AppNLGF mice markedly suppressed the elevation of the oxidative stress marker 8-OHdG and Iba1-positive microglial cell number. We also determined the plasmalogen-phosphatidylethanolamine (PlsPE) level as an AD biomarker. PlsPE containing polyunsaturated fatty acids was decreased in the AppNLGF mouse brain, but Nrf2 induction attenuated this decline. To evaluate whether pharmacological induction of Nrf2 elicits beneficial effects for AD treatment, we tested the natural compound 6-MSITC [6-(methylsulfinyl)hexyl isothiocyanate]. Administration of 6-MSITC improved the impaired cognition of AppNLGF mice in the passive-avoidance task. These results demonstrate that the induction of Nrf2 ameliorates cognitive impairment in the AD model mouse by suppressing oxidative stress and neuroinflammation, suggesting that Nrf2 is an important therapeutic target of AD.
INTRODUCTION
The global incidence and prevalence of neurocognitive disorders are increasing worldwide (1). Alzheimer’s disease (AD) is one of the most common neurocognitive disorders and is characterized by unique pathological changes, including amyloid β (Aβ) accumulation, plaque formation, hyperphosphorylation of tau protein, and neurofibrillary tangles (NFTs) (2). Oxidative stress and inflammation are increased in the brains of AD patients, and these increases have been widely verified in a number of model animals (3–5). Suppression of the onset and/or progression of AD is an important issue in modern society, and studies have suggested that improvements in these pathological conditions may be beneficial for maintaining or improving the neuronal functions of AD patients.
Our bodies are continuously exposed to various stresses, including reactive oxygen/nitrogen species and electrophiles (6), and Nrf2 (NF-E2-related-factor 2) plays critical roles in protecting cells against these stresses (7, 8). Nrf2 is a basic region-leucine zipper-type transcription factor (9) that belongs to the cap’n’collar family (10). In normal unstressed conditions, Keap1 (Kelch-like ECH-associated protein 1) acts as an adaptor for the Cul3-based ubiquitin E3 ligase complex. The E3 ligase efficiently ubiquitinates Nrf2, which brings about rapid Nrf2 degradation through the ubiquitin-proteasome pathway and constitutively suppresses the transcriptional activity of Nrf2 (8, 11). In contrast, when Keap1 is exposed to oxidative and electrophilic stresses, Keap1 cysteine residues are modified, resulting in impairment of the ubiquitin ligase activity of Keap1. Accordingly, Nrf2 degradation is suppressed, and Nrf2 is stabilized and accumulates in the nucleus (11–15). Nrf2 forms a heterodimer with small Maf (sMaf) proteins and binds to the CNC-sMaf-binding element (CsMBE) (16). This stress-responsive transcriptional regulation is called the Keap1-Nrf2 regulatory system (17).
The Keap1-Nrf2 system concomitantly regulates both oxidative stress responses and anti-inflammatory responses. It has been shown that the Keap1-Nrf2 system acts as a key regulator of protective responses against oxidative stresses (17), and Nrf2 induces the expression of many antioxidant enzyme genes (7, 18). An important recent observation is that, in addition to antioxidant enzyme genes, Nrf2 negatively regulates the expression of proinflammatory cytokine genes (19) and modulates the process of inflammation (20). In fact, activation of Nrf2 signaling ameliorates autoimmune disease in mouse models (19, 21–23).
The Keap1-Nrf2 system has been shown to protect neurons in the hypothalamus against oxidative damage (24). A number of studies have also shown that this system plays important roles in the maintenance of brain function (25–28). Indeed, in the brains of AD patients and AD model AppNL-G-F/NL-G-F knock-in mice (29), the mRNA and protein expression levels of NRF2 have been shown to be altered (30, 31). Similarly, glutathione levels and neuroinflammation are shown to be influenced in the brains of mild cognitive impairment and AD patients (32–35). These lines of evidence support the hypothesis that perturbation of the Nrf2-mediated defense system may lead to the pathogenesis of AD. Indeed, Nrf2 deficiency aggravates the phenotypes of AD model APP/TAU and APP/PS1 mice (36–39), and overexpression of Nrf2 by virus vectors protects hippocampal neurons of APP/PS1 mice and cultured hippocampal cells (40, 41).
Despite these accumulating lines of evidence, however, the roles that Nrf2 plays in AD model animals have not been studied extensively. It remains to be clarified whether Nrf2 induction strongly contributes to protection against AD. To this end, we decided to use Keap1 knockdown mice, which generally express high levels of Nrf2 in various tissues (42). We refer to this Nrf2 induction as “genetic induction of Nrf2” in contrast to drug-mediated “pharmacological induction of Nrf2.” In this study, we exploited Keap1floxA/floxA (Keap1FA/FA) mice as a genetic Nrf2 induction model. We crossed Keap1FA/FA mice with AppNL-G-F/NL-G-F knock-in mice (referred to as AppNLGF mice in this study) as an AD model. The AppNLGF mice harbor the humanized App gene with mutations of familial AD, including mutations of Swedish (KM670/671NL), Beyreuther/Iberian (I716F), and Arctic (E693G) (29).
Through analyses of AppNLGF::Keap1FA/FA mice, we found that genetic Nrf2 induction by Keap1FA/FA elevated the level of reduced glutathione (GSH) and suppressed oxidative stress and neuroinflammation in the brains of AppNLGF::Keap1FA/FA mice. Genetic Nrf2 induction improved the impaired cognition of AppNLGF::Keap1FA/FA compound mice compared to AppNLGF mice. We also found that pharmacological Nrf2 induction by a natural compound with mild efficacy and a nonstressful administration route also ameliorated the cognitive impairment of AppNLGF mice. Thus, this study supports our hypothesis that the induction of Nrf2 in the brain exerts beneficial effects in mice against the development of AD.
RESULTS
Genetic Nrf2 induction in AD model mouse brains.
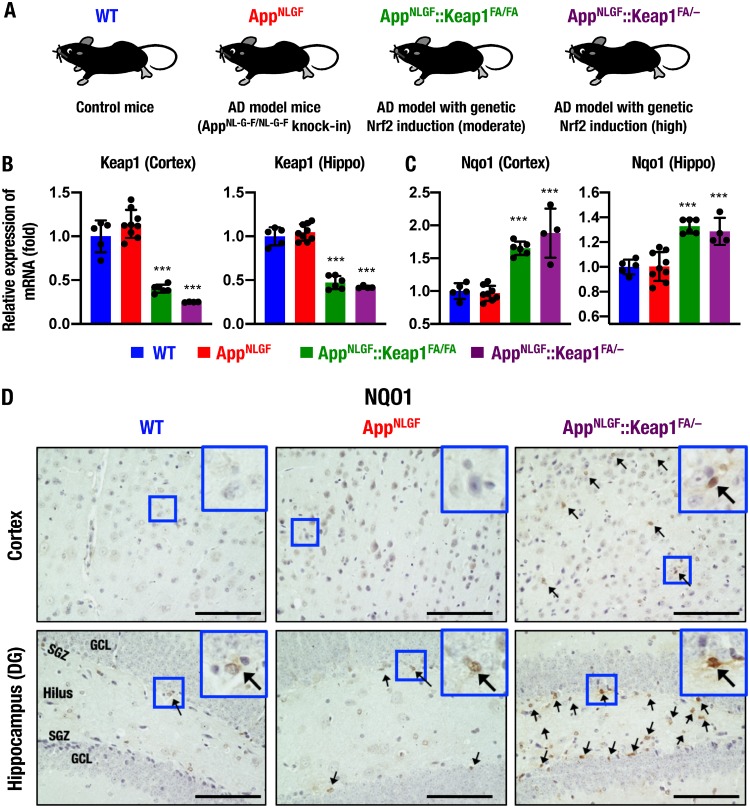
To assess whether Nrf2 induction protects AD model mice against disease progression, we crossed Keap1 knockdown (Keap1FA/FA) or heterozygous Keap1 knockout (Keap1+/–) mice with AppNL-G-F/NL-G-F (abbreviated here as AppNLGF) mice to generate AppNLGF::Keap1FA/FA and AppNLGF::Keap1FA/– mice. We expected that these mutant mice would serve as AD models with moderately and highly activated Nrf2 expression, respectively (Fig. 1A). When we evaluated the expression levels of Keap1 mRNA, we found that the expression levels of Keap1 in the cerebral cortices and hippocampi of 11-month-old male AppNLGF mice were comparable with those of age-matched wild-type (WT) male mice. However, Keap1 expression was decreased to 30 to 50% in the cortices and the hippocampi of AppNLGF::Keap1FA/FA and AppNLGF::Keap1FA/– mice compared with AppNLGF and WT mice (Fig. 1B).
FIG 1.
Genetic induction of Nrf2 in AD model AppNLGF mice. (A) Schematic of WT, AppNLGF, AppNLGF::Keap1FA/FA, and AppNLGF::Keap1FA/– mice. (B and C) Expression levels of Keap1 (B) and Nqo1 (C) gene mRNAs in the cerebral cortex and the hippocampus (Hippo), normalized by Actb gene expression. The expression levels in WT mice were set to 1, and the results are presented as means ± the SD. ANOVA, followed by the Fisher LSD post hoc test, was performed. **, P < 0.01; ***, P < 0.001 versus AppNLGF mice. (D) Immunohistochemistry of NQO1 in the cerebral cortex (upper) and the hippocampal dentate gyrus (DG, lower) in WT, AppNLGF, and AppNLGF::Keap1FA/– mice. Arrows indicate NQO1-positive cells. Scale bars, 100 μm. GCL, granule cell layer; SGZ, subgranular zone.
We also examined expression of the Nqo1 gene, a representative Nrf2 target gene that exerts an antioxidative response and found that Nqo1 expression was significantly augmented in both the cortices and the hippocampi of APPNLGF::Keap1FA/FA and AppNLGF::Keap1FA/– mice (Fig. 1C). These data indicate that the Keap1FA/FA and Keap1FA/– mutants decrease Keap1 expression in the brains of AppNLGF mice, and Nrf2 signaling is indeed activated in AppNLGF::Keap1FA/FA and AppNLGF::Keap1FA/– mouse brains.
To determine cell types in which Nrf2 signaling is activated, immunohistochemical analysis of NQO1 was performed. We could not find NQO1-positive cells in the cortex of WT or AppNLGF mice; in contrast, NQO1 was expressed in glia-like cells but not in neuron-like cells in the AppNLGF::Keap1FA/– mouse cortex (Fig. 1D, upper). In contrast, several weakly NQO1-positive cells were found in the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) in WT and AppNLGF mice, and in AppNLGF::Keap1FA/– mice strongly NQO1-positive cells were detected in the SGZ and the hilus but not in the granule cell layer (GCL) (Fig. 1D, lower). Consistent with previous reports (43, 44), these data suggest that Nrf2 signaling is activated in glial cells but not in neurons in the AppNLGF mouse brain.
Nrf2 opposes cognitive impairment in AppNLGF mice.
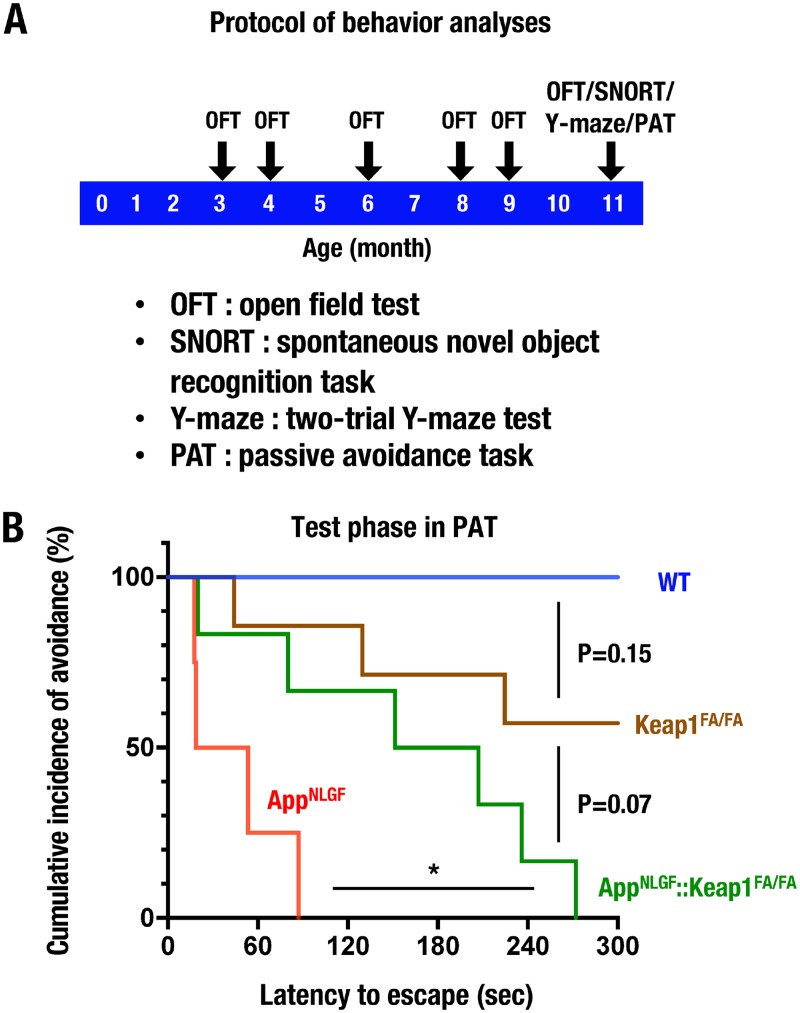
To investigate the roles that Nrf2 plays in the preservation of cognitive functions in AppNLGF mice, we executed a series of behavioral analyses for WT, Keap1FA/FA, AppNLGF, and AppNLGF::Keap1FA/FA mice. As shown in Fig. 2A, mice first performed an open-field test (OFT) at six time points of 3, 4, 6, 8, 9, and 11 months of age. The mice also performed a spontaneous novel object recognition task (SNORT), a two-trial Y-maze test, and a passive-avoidance task (PAT) at 11 months of age.
FIG 2.
Behavior analyses of AppNLGF mice. (A) Schematic of the behavior analysis protocol. (B) PAT of 11-month-old male WT (n = 4), Keap1FA/FA (n = 7), AppNLGF (n = 4), and AppNLGF::Keap1FA/FA (n = 6) mice. The cumulative incidence of avoidance during the test phase in the PAT is presented as a Kaplan-Meier curve. The log rank test was performed. *, P < 0.05.
In 3- to 9-month-old mice, we found no significant differences in the total distance of OFT among the WT, Keap1FA/FA, AppNLGF, and AppNLGF::Keap1FA/FA mouse groups (see Fig. S1A in the supplemental material). At 11 months of age, the total distance traveled in the OFT in AppNLGF mice was slightly higher than that in WT mice, but there were no statistically significant differences among the four mouse groups (Fig. S1B). We also performed SNORT using appetitive behavior to assess the objective learning and memory abilities of mice. However, the discrimination ratios in the test phase of SNORT were comparable among the WT, Keap1FA/FA, AppNLGF, and AppNLGF::Keap1FA/FA mouse groups (data not shown). Similarly, the Y-maze test was used to assess spatial memory, but no obvious change was observed among the four genotype groups (data not shown).
We then conducted PAT to evaluate the associative learning and memory of an aversive condition. Latency to escape of Keap1FA/FA mice was slightly shorter than that of WT mice, although there was no statistically significant difference in cumulative incidence of avoidance between WT and Keap1FA/FA mice (Fig. 2B). Notably, AppNLGF mice displayed shorter latency to escape and lower cumulative incidence of avoidance than WT mice in the PAT analysis (Fig. 2B). In contrast, the latency to escape was significantly prolonged in AppNLGF::Keap1FA/FA mice compared to AppNLGF mice. These results thus demonstrate impaired cognitive functions in AppNLGF mice. However, genetic Nrf2 induction improves the impaired cognition, especially the decline of emotional associative memory, in the AppNLGF mice.
Nrf2 suppresses proinflammatory response and phagocytic cells in the AppNLGF mouse brain.
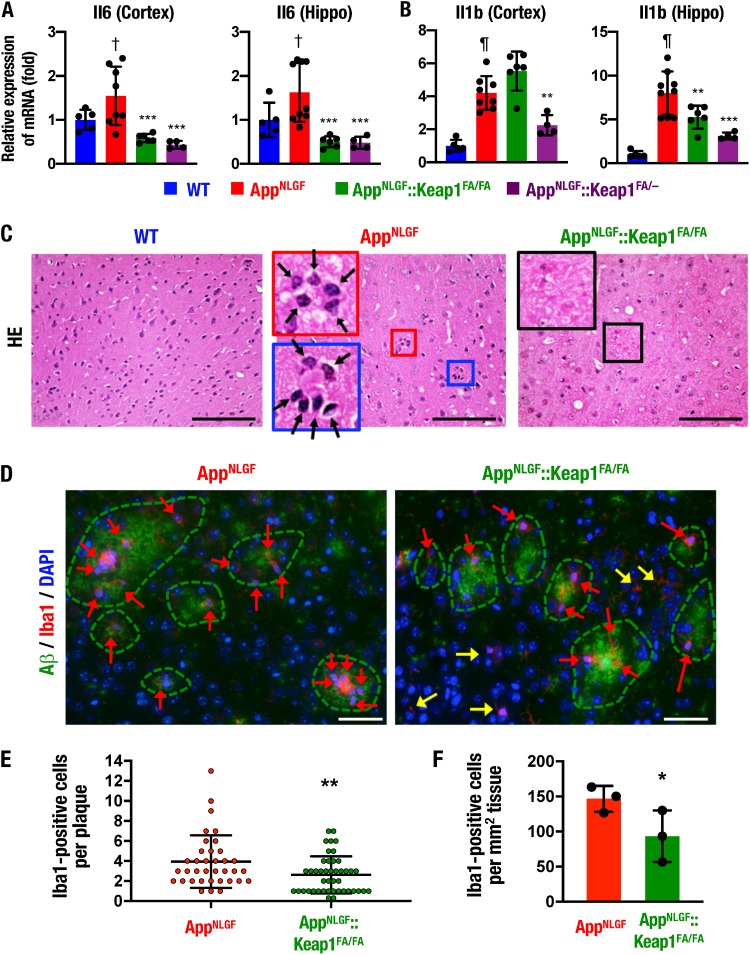
To clarify the molecular basis of how Nrf2 improves the AD phenotype of AppNLGF mice, we examined whether Nrf2 ameliorates proinflammatory response in the AppNLGF mouse brain. To this end, we analyzed the expression of proinflammatory cytokine genes in the mouse brains. Although the mRNA levels of proinflammatory cytokine genes Il6 and Il1b were significantly increased in the cerebral cortex and the hippocampus of AppNLGF mice compared to WT mice (Fig. 3A and B), the expression levels of these genes were reduced in both the cerebral cortex and the hippocampus of AppNLGF::Keap1FA/FA and AppNLGF::Keap1FA/– mice compared to AppNLGF mice, except for Il1b gene expression in the cerebral cortex of AppNLGF::Keap1FA/FA mice. These results suggest that the induction of Nrf2 may ameliorate the AD phenotype of AppNLGF mice by reducing inflammation in the brain.
FIG 3.
Proinflammatory response and phagocytic cells and Nrf2 expression in the AppNLGF mouse brain. (A and B) Expression levels of Il6 (A) and Il1b (B) gene mRNAs in the cerebral cortex and the hippocampus (Hippo), normalized by Actb gene expression. The expression levels of WT mice were set to 1. (C) HE staining in the cerebral cortices of WT, AppNLGF, and AppNLGF::Keap1FA/FA mice. Arrows, phagocytic cells. (D) Immunofluorescence staining of Aβ (green) and Iba1 (red) in the AppNLGF and AppNLGF::Keap1FA/FA mouse cortex. Red and yellow arrows indicate plaque-related and plaque-unrelated Iba1-positive cells, respectively. (E) Numbers of Iba1-positive cells in each amyloid plaque in the cerebral cortex. Thirty-six plaques from three AppNLGF mouse cortices and 44 plaques from three AppNLGF::Keap1FA/FA mouse cortices were counted. (F) Numbers of Iba1-positive cells associated with amyloid plaque per tissue area (square millimeter) in the cerebral cortex. The averages of the numbers in each mouse were calculated in three AppNLGF and three AppNLGF::Keap1FA/FA mice. The results are presented as means ± the SD. Statistical analyses were performed using ANOVA, followed by Fisher LSD post hoc test (A and B) or Student t test (E and F). †, P < 0.05; ¶, P < 0.001 versus WT mice. *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus AppNLGF mice. Scale bars: 100 μm (C) and 50 μm (D).
We next analyzed the distribution of phagocytic cells in hematoxylin-eosin (HE)-stained sections of the AppNLGF mouse cortex. We found clusters of phagocytic cells with small and condensed nuclei accumulated around the Aβ depositions in the AppNLGF mouse cortex (Fig. 3C, middle panel, arrows), which were not observed in the WT mouse cortex (left panel). Notably, the phagocyte-like cells around the Aβ depositions were rarely observed in the brains of AppNLGF::Keap1FA/FA mice (right panel). Although it has been reported that the Nrf2-inducing compounds dimethyl fumarate (DMF) and sulforaphane increase phagocytic activity (45, 46), these data demonstrate that phagocytic cells are suppressed by genetic Nrf2 induction in the brain.
We also performed immunofluorescent staining for the microglial marker ionized calcium-binding adapter molecule 1 (Iba1) (24). Consistent with the results of the HE-stained sections, in the AppNLGF mouse cortex, Iba1-positive microglia (Fig. 3D, left panel, arrows) were clustered around the amyloid plaques, as shown by the dotted lines. In stark contrast, in AppNLGF::Keap1FA/FA mice, Iba1-positive microglia were not clustered near the amyloid plaques, but Iba1-positive microglia were frequently found at locations not typically associated with amyloid plaques (right panel, yellow arrows). The number of amyloid plaque-associated Iba1-positive microglia in each plaque was significantly decreased in the AppNLGF::Keap1FA/FA mouse cortex compared to the AppNLGF mouse brain cortex (Fig. 3E). In addition, the number of amyloid plaque-associated Iba1-positive microglia per square millimeter of tissue was also decreased in the cortices of AppNLGF::Keap1FA/FA mice (Fig. 3F).
To further evaluate inflammation in AppNLGF mouse brain, inflammation mediator genes were examined. Nos2 mRNA expression was increased in the hippocampi, but not in the cerebral cortices, of AppNLGF mice compared to WT mice, but the expression levels in the brains of AppNLGF::Keap1FA/FA and AppNLGF::Keap1FA/– mice were comparable to AppNLGF mice (Fig. S2A). In contrast, Ptgs2 gene mRNA expression levels were comparable among the four mouse groups (Fig. S2B). These data support the notion that genetic Nrf2 induction suppresses the proinflammatory response and phagocytic cells in the AppNLGF mouse brain.
Nrf2 suppresses transition of homeostatic microglia to disease-associated microglia.
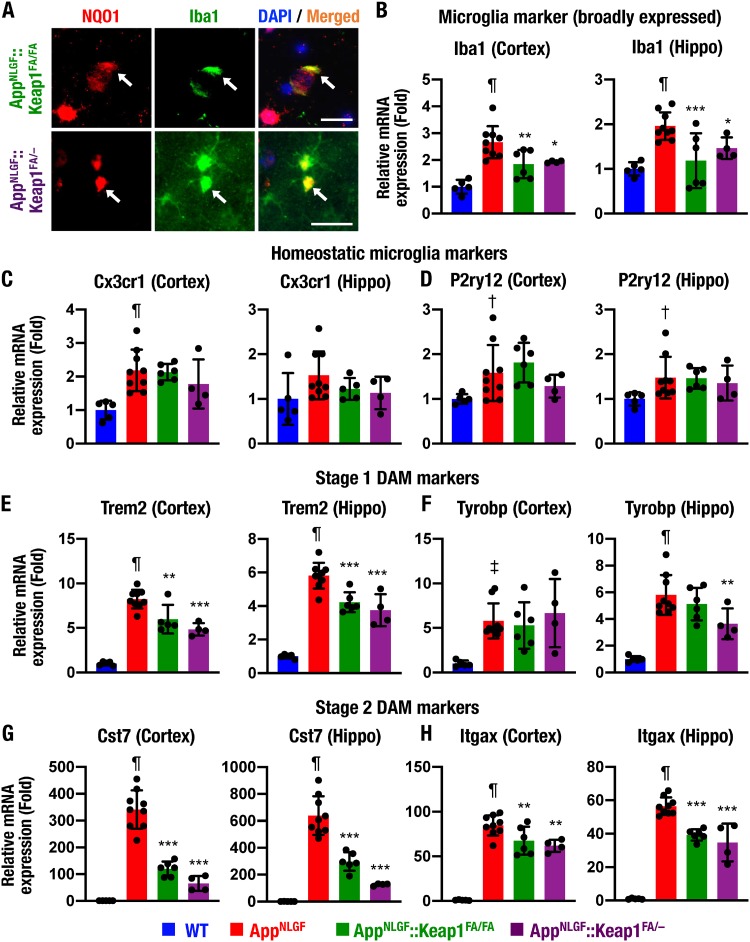
AppNLGF::Keap1FA/FA mice displayed a reduction in Iba1-positive cells associated with amyloid plaques. We then focused on the role of Nrf2 in microglia regulation. We examined NQO1 and Iba1 immunostaining and found that the Iba1-positive cells expressed NQO1 in AppNLGF::Keap1FA/FA and AppNLGF::Keap1FA/– mouse brains (Fig. 4A, arrows), indicating that Nrf2 signaling is activated in microglia.
FIG 4.
Nrf2 suppresses transition of homeostatic microglia to DAM. (A) Immunofluorescence analysis of NQO1 and Iba1 in the hippocampi of AppNLGF::Keap1FA/FA and AppNLGF::Keap1FA/– mice. Arrows indicate NQO1-positive cells. (B to H) Expression levels of microglia subtype marker gene mRNAs, including a broadly expressed microglia marker Iba1 (B), homeostatic microglia markers Cx3cr1 and P2ry12 (C and D), stage 1 DAM markers Trem2 and Tyrobp (E and F), and stage 2 DAM markers Cst7 and Itgax (G and H), in the cerebral cortex and the hippocampus (Hippo), normalized by Actb gene expression. The expression levels in WT mice were set to 1 and are presented as means ± the SD. Statistical analyses were performed using ANOVA, followed by the Fisher LSD post hoc test. †, P < 0.05; ‡, P < 0.01; ¶, P < 0.001 versus WT mice. *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus AppNLGF mice.
We also examined the expression levels of Iba1 mRNA in the cortices and the hippocampi of WT, AppNLGF, AppNLGF::Keap1FA/FA, and AppNLGF::Keap1FA/– mouse brains. The Iba1 mRNA expression levels were markedly increased in both the cortices and the hippocampi of AppNLGF mice compared to WT mice (Fig. 4B). Importantly, showing very good agreement with the results of histological and immunofluorescent analyses, the induction of Iba1 mRNA in the AppNLGF mouse brain was suppressed in both the cortices and the hippocampi of AppNLGF::Keap1FA/FA and AppNLGF::Keap1FA/– mouse brains.
It has been reported that there are subtypes of microglia (47), including phagocytic and activated subtypes, named disease-associated microglia (DAM) (48–50). Since the phagocytic cells surrounding amyloid plaques were suppressed in AppNLGF::Keap1FA/FA mouse brain, we evaluated whether Nrf2 influences the transition of homeostatic microglia to DAM by examining homeostatic and disease-associated microglial markers.
Homeostatic microglial marker Cx3cr1 and P2ry12 gene expression levels were slightly elevated in the cortex and hippocampus of AppNLGF mice (Fig. 4C and D). However, these expression levels were not suppressed in the cortices or hippocampi of AppNLGF::Keap1FA/FA and AppNLGF::Keap1FA/– mice compared to AppNLGF mice.
When microglia are activated to exert phagocytic efficacy, they are initially activated to an intermediate subtype, named stage 1 DAM, which increases Trem2 and Tyrobp expression levels (48). The expression of the Trem2 gene, a stage 1 DAM marker, was markedly increased in AppNLGF mouse cortex and hippocampus compared to WT mice but was suppressed in AppNLGF::Keap1FA/FA and AppNLGF::Keap1FA/– mouse cortices and hippocampi (Fig. 4E). Tyrobp gene expression was also increased in AppNLGF mouse cortices and hippocampi and repressed in the hippocampi of AppNLGF::Keap1FA/– mice (Fig. 4F).
The stage 1 DAM subtype is activated to stage 2 DAM, which induces the expression of phagocytic cell-related Cst7 and Itgax genes (48). The gene expression levels of the stage 2 DAM markers Cst7 and Itgax were strongly induced in the cortices and hippocampi of AppNLGF mice, and their inductions were markedly repressed in AppNLGF::Keap1FA/FA and AppNLGF::Keap1FA/– mouse cortices and hippocampi (Fig. 4G and H). These data support the notion that genetic Nrf2 induction suppresses the transition of homeostatic microglia to DAM in the AppNLGF mouse brain.
Nrf2 attenuates reactive astrocytosis in the AppNLGF mouse brain.
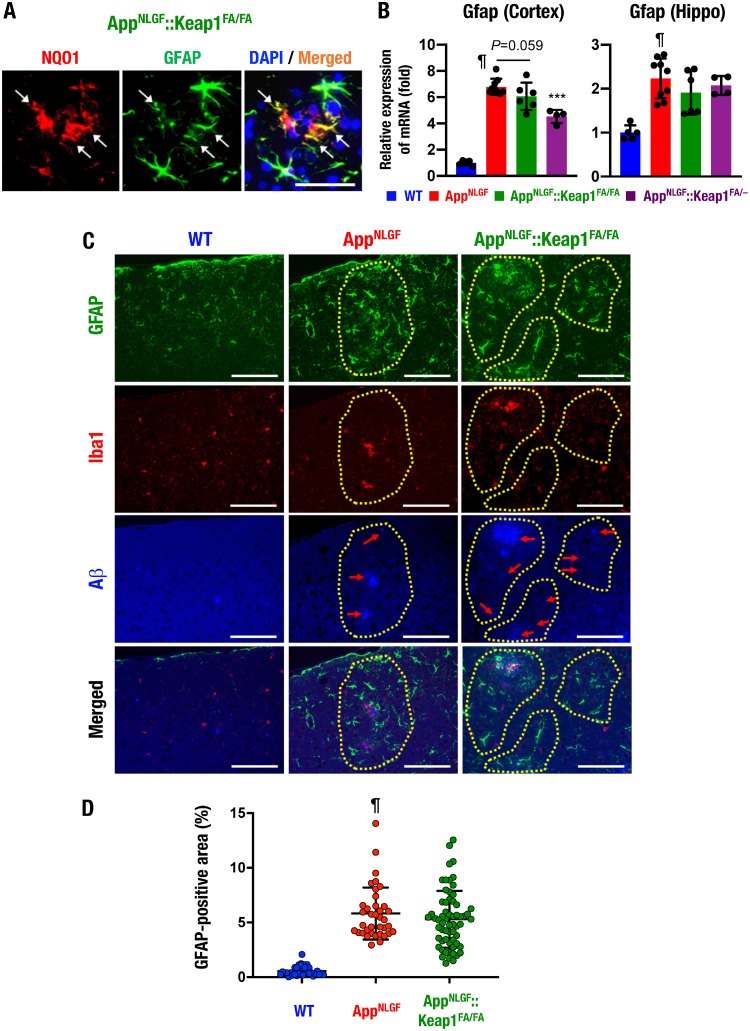
It is known that reactive astrocytosis is a pathological reaction of astrocytes frequently observed in inflammation, and a hallmark of the condition is increased glial fibrillary acidic protein (GFAP)-positive astrocytes (5). NQO1 and GFAP immunostaining revealed that the GFAP-positive cells indeed expressed NQO1 in the AppNLGF::Keap1FA/FA mouse brain (Fig. 5A, arrows).
FIG 5.
Nrf2 induction ameliorates reactive astrocytosis in the AppNLGF mouse brain. (A) Immunofluorescence analysis of NQO1 and GFAP in the cerebral cortices of AppNLGF::Keap1FA/FA mice. Arrows indicate NQO1-positive cells. (B) Expression of Gfap mRNA in the cerebral cortex and the hippocampus (Hippo), normalized by Actb gene expression. The expression levels of WT mice were set to 1. (C) Immunofluorescence analysis of GFAP (green), Iba1 (red), and Aβ (blue) in the cerebral cortices of WT, AppNLGF, and AppNLGF::Keap1FA/FA mice. Dotted lines indicate Aβ deposit-related areas. (D) GFAP-positive area in the brain cortex. We analyzed 33, 37, and 62 images from three WT, three AppNLGF, and two AppNLGF::Keap1FA/FA mouse cortices. The results were calculated as the percentage of tissue area and are presented as means ± the SD. Statistical analyses were performed using ANOVA followed by Fisher LSD post hoc test (B) or the Kruskal-Wallis test (D). ¶, P < 0.001 versus WT mice. ***, P < 0.001 versus AppNLGF mice. Scale bars: 50 μm (A) and 100 μm (C).
It has been reported that GFAP-positive astrocytes are increased in the AppNLGF mouse brain (29). Consistent with this observation, we found that Gfap mRNA expression levels were significantly elevated in both the cortices and the hippocampi of AppNLGF mice compared to WT mice (Fig. 5B). Gfap mRNA induction was significantly and moderately suppressed in the cortex of AppNLGF::Keap1FA/– mice and AppNLGF::Keap1FA/FA mice, respectively (left panel). In the hippocampus, these changes in Gfap mRNA expression were marginal (right panel).
We also conducted immunofluorescence staining for GFAP, Iba1, and Aβ and found that GFAP-positive cells were broadly increased in the cerebral cortices of AppNLGF mice but were highly induced around the Aβ-deposited area (Fig. 5C, middle panels, dotted lines) compared to WT mice (left panels). In contrast, a limited number of Iba1-positive cells were expressed around Aβ depositions in the AppNLGF mouse cortex (middle panels) compared to the WT mouse cortex (left panels). GFAP-positive cells were observed around the Aβ-deposited area, but fewer were found in the cortex of AppNLGF::Keap1FA/FA mice (right panels, dotted lines) than in the cortices of AppNLGF mice. The quantified GFAP-positive area was higher in the cortices of AppNLGF mice than in WT mice (Fig. 5D). Although low-level GFAP-stained areas were observed in the AppNLGF::Keap1FA/FA mouse cortex, there was no statistically significant difference between AppNLGF and AppNLGF::Keap1FA/FA mice. Taken together, these results indicate that genetic Nrf2 induction by Keap1 gene knockdown partially ameliorates reactive astrocytosis in the AppNLGF mouse cortex.
Nrf2 inhibits neuronal damage around amyloid plaques.
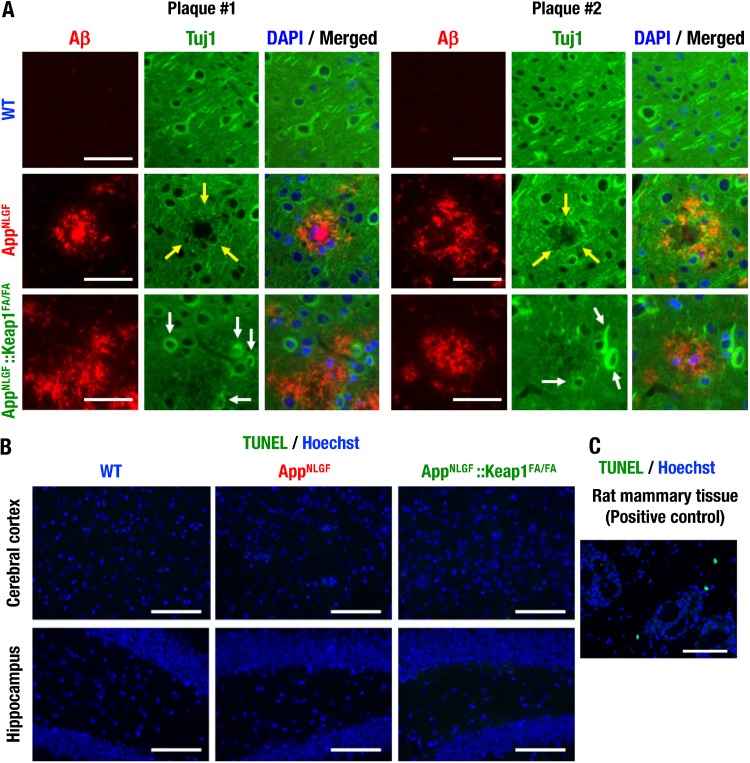
It has been reported that synaptic alterations around amyloid plaques were observed in the brains of AppNLGF mice and AD patients (29). We performed immunostaining of the neuron fiber marker Tuj1 and found that Tuj1-positive staining cells were decreased around amyloid plaques in the cortices of AppNLGF mice compared to WT mice (Fig. 6A, top and middle, arrows). Importantly, in the AppNLGF::Keap1FA/FA mouse brain, Tuj1 strongly positive neurons were found around plaques (Fig. 6A, bottom, arrows).
FIG 6.
Nrf2 inhibits neuron damage around amyloid plaques. (A) Immunofluorescence analysis of Tuj1 and Aβ in the cerebral cortices of 11-month-old mice. Yellow arrows indicate the decreased Tuj1-positive staining around amyloid plaques. White arrows indicate retained neurons. (B and C) TUNEL assay and Hoechst 33342 straining in the cerebral cortices and the hippocampi of 11-month-old mouse brains (B), with rat mammary tissue as a positive control (C). Scale bars: 50 μm (A) and 100 μm (B and C).
To detect neuronal apoptosis in the brains of AppNLGF mice, in situ detection of fragmented DNA by terminal deoxynucleotidyl-transferase dUTP nick-end labeling (TUNEL) analysis was next performed. The TUNEL-positive cells were not found in the cerebral cortices or hippocampi of WT, AppNLGF, and AppNLGF::Keap1FA/FA mice (Fig. 6B). In contrast, TUNEL-positive cells were found in rat mammalian tissue as a positive control (Fig. 6C). These data indicate that Nrf2 induction inhibits the decrease in neuronal damage around amyloid plaques independent of apoptosis.
Oxidative stress accumulation in the AppNLGF mouse brain.
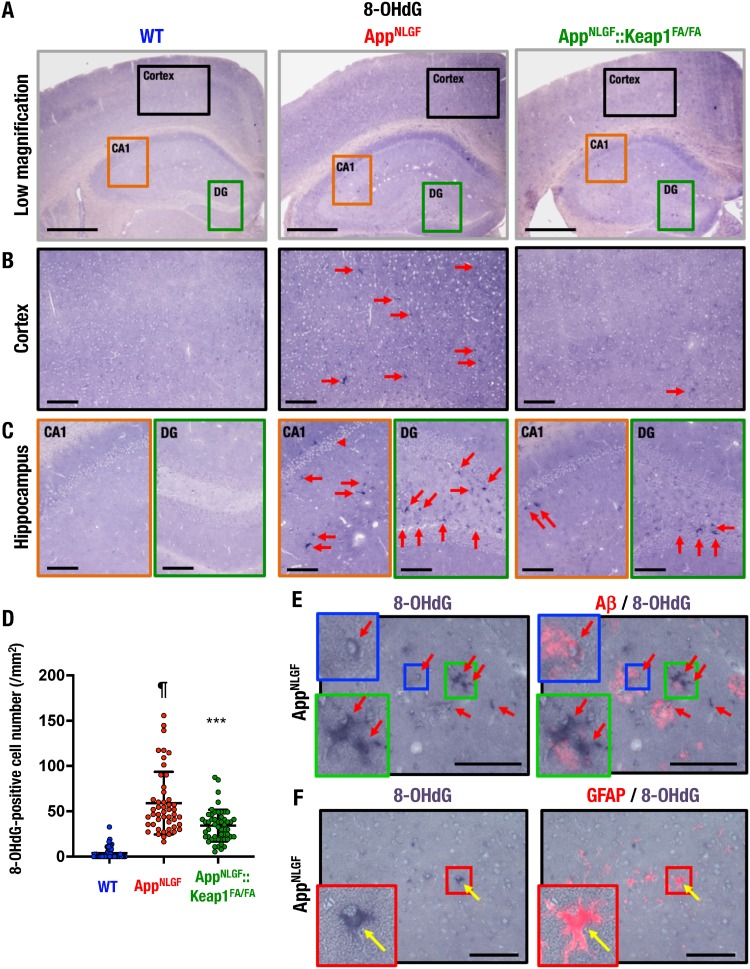
To assess oxidative stress levels in the AppNLGF mouse brain, we conducted an immunohistochemical analysis of the oxidative stress marker 8-hydroxy-2′-deoxyguanosine (8-OHdG) in the brains of WT, AppNLGF, and AppNLGF::Keap1FA/FA mice. We captured high-magnification images of the regions shown in the low-magnification images in Fig. 7A of the cortex (black boxes), the hippocampal CA1 region (orange boxes), and the DG (green boxes).
FIG 7.
Oxidative stress accumulation in the AppNLGF mouse brain. (A to C) Immunohistochemistry of the oxidative stress marker 8-OHdG. Shown are low-magnification images acquired using a 4× objective (A) and high-magnification images acquired using a 20× objective of the cerebral cortex (B), the hippocampal CA1 (C, left), and the DG (C, right). (D) Number of 8-OHdG-positive cells in the cortex. We analyzed 48, 48, and 52 images from three each WT, AppNLGF, and AppNLGF::Keap1FA/FA mouse cortex. The results are presented as the number per square millimeter of tissue and as means ± the SD. Statistical analyses were performed using ANOVA, followed by the Fisher LSD post hoc test. ¶, P < 0.001 versus WT mice. ***, P < 0.001 versus AppNLGF mice. (E and F) Immunostaining of 8-OHdG and Aβ (E) or GFAP (F) in AppNLGF mouse cerebral cortex. Arrows indicate 8-OHdG-positive cells. Scale bars: 500 μm (A) and 100 μm (B, C, E, and F).
Importantly, large numbers of 8-OHdG-positive cells were found in the cortices, the hippocampal CA1 regions, and the DGs of AppNLGF mice (Fig. 7B and C, middle panels), but essentially no such cells were found in the WT mouse brain (left panels). Importantly, 8-OHdG-positive cells were markedly decreased in the respective regions of the AppNLGF::Keap1FA/FA mouse brain (right panels). A quantifiable number of 8-OHdG-positive cells were induced in the cerebral cortices of AppNLGF mice compared to WT mice, and the number was decreased in the cortices of AppNLGF::Keap1FA/FA mice compared to AppNLGF mice (Fig. 7D). Double immunostaining for 8-OHdG and Aβ revealed that the 8-OHdG-positive cells were found around Aβ-stained areas in the AppNLGF mouse cortex (Fig. 7E, arrows). The 8-OHdG staining was detected in astrocyte-like (Fig. 7E, green boxes) and microglia-like (blue boxes) cells. We also found 8-OHdG- and GFAP-double-stained cells in the cerebral cortices of AppNLGF mice (Fig. 7F). These results indicate that genetic Nrf2 induction suppresses oxidative stress and 8-OHdG formation in the AppNLGF mouse brain.
Nrf2 increases GSH levels in the AppNLGF mouse brain.
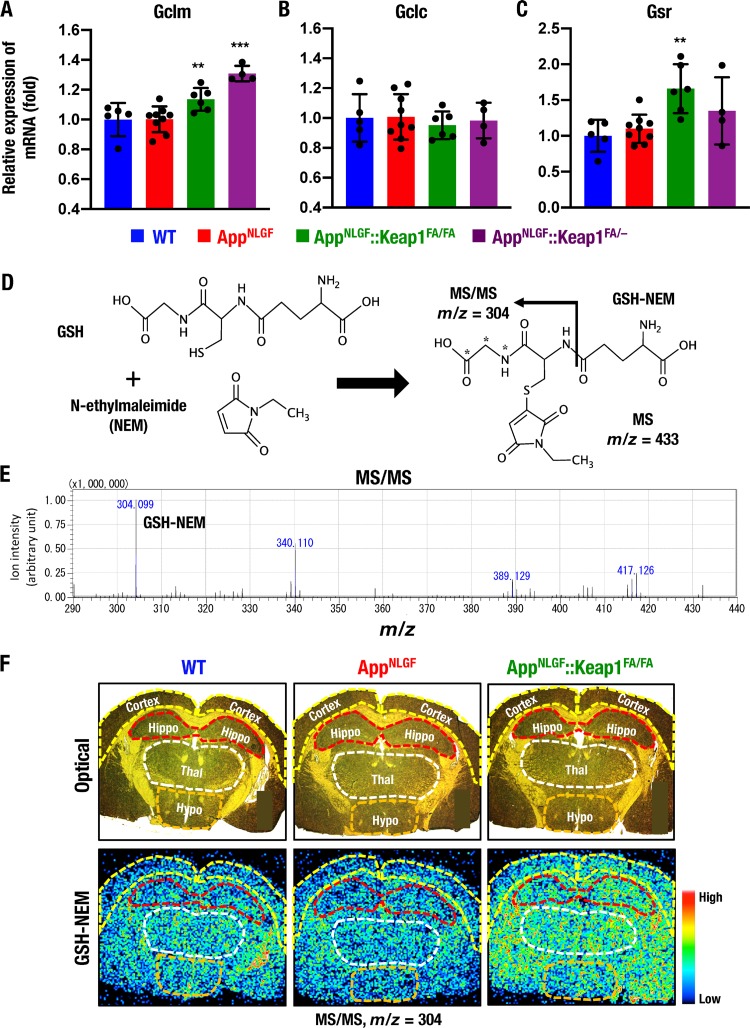
To clarify how Nrf2 protects the AppNLGF mouse brain against oxidative tissue damage, we conducted a series of experiments that assessed changes in the antioxidative stress activity of the brain. Nrf2 has been shown to regulate the expression of glutathione synthesis-related enzyme genes (6); therefore, we analyzed the expression levels of Gclm, Gclc, and Gsr genes encoding glutamate-cysteine ligase modifier and catalytic subunits and glutathione reductase, respectively. The expression of Gclm mRNA in the cerebral cortex was comparable between WT and AppNLGF mice, but the mRNA expression level was increased significantly in AppNLGF::Keap1FA/FA and AppNLGF::Keap1FA/– mice compared to AppNLGF mice (Fig. 8A). In contrast, the expression of Gclc mRNA in the cerebral cortex was comparable among these four mouse groups (Fig. 8B). Gsr mRNA expression in the cerebral cortex was increased in AppNLGF::Keap1FA/FA mice compared to AppNLGF mice (Fig. 8C).
FIG 8.
Nrf2 increases GSH levels in AppNLGF::Keap1FA/FA mouse brains. (A to C) Expression levels of glutathione-related Gclm (A), Gclc (B), and Gsr (C) genes in the cerebral cortex, normalized by Actb gene expression. The expression levels in WT mice were set to 1, and the results are presented as means ± the SD. Note that the Gclm expression levels were significantly elevated in AppNLGF and AppNLGF::Keap1FA/FA mouse brains. Statistical analyses were performed using ANOVA, followed by the Fisher LSD post hoc test. **, P < 0.01; ***, P < 0.001 versus AppNLGF mice. (D to F) MALDI-MSI analysis of GSH in the brain. (D) In the presence of NEM, the thiol residue of GSH conjugates with NEM, and MS/MS signals of GHS-NEM were calculated to be detected as m/z 304. (E) MS/MS signals of GHS-NEM from NEM-treated brain sections were detected as m/z 304.099 by MALDI-MSI analysis. (F) Optical (upper) and MS/MS (lower) images of GHS-NEM signals (m/z 304) in brain sections of 11-month-old male WT, AppNLGF and AppNLGF::Keap1FA/FA mice at positions 8 mm posterior to the bregma. Cortex, cerebral cortex (yellow lines); Hippo, hippocampus (red lines); Thal, thalamus (white line); Hypo, hypothalamus (orange lines).
We next sought to evaluate GSH levels in the mouse brain in situ by means of matrix-assisted laser desorption ionization–mass spectrometry imaging (MALDI-MSI). However, since GSH is highly reactive and easily generates oxidized glutathione (GSSG) (51, 52), we realized the necessity to avoid nonspecific reactions to the thiol residue of GSH. Therefore, we decided to generate GSSG by utilizing N-ethylmaleimide (NEM), since NEM has been used for this purpose in liquid chromatography-mass spectrometry (LC-MS) analysis (53). Thus, we applied a challenge application of NEM in MALDI-MSI in this analysis. In the presence of NEM, the cysteine residue of GSH forms a conjugate with NEM and generates GSH-NEM (Fig. 8D), and the tandem mass spectrometry (MS/MS) signal of GSH-NEM should be detected as m/z 304 by LC-MS (53). In the MALDI-MSI analysis, the MS/MS signal of GSH-NEM was also detected as m/z 304 in brain sections (Fig. 8E), indicating that the NEM method is applicable for MALDI-MSI analysis.
MALDI-MSI analysis coupled with the NEM modification method was used to evaluate the distribution of GSH-NEM signals in brain coronal sections of WT mice at 1.8 mm posterior to the bregma. GSH-NEM signals were detected in the inner cortex, the hippocampus, the hypothalamus, and the thalamus, whereas signals were rarely detected in the outer cortex (Fig. 8F, left panels). GSH-NEM signals were lower in the hippocampi, thalami, and hypothalami of AppNLGF mice than in those regions of WT mice (middle panels), but these signals were significantly and broadly elevated in the AppNLGF::Keap1FA/FA mouse brain (right panels). These results thus demonstrate that GSH levels in various parts of the brain are increased in the AppNLGF::Keap1FA/FA mouse brain, perhaps due to the increased Nrf2 activity.
Nrf2 induction does not significantly change Aβ deposition.
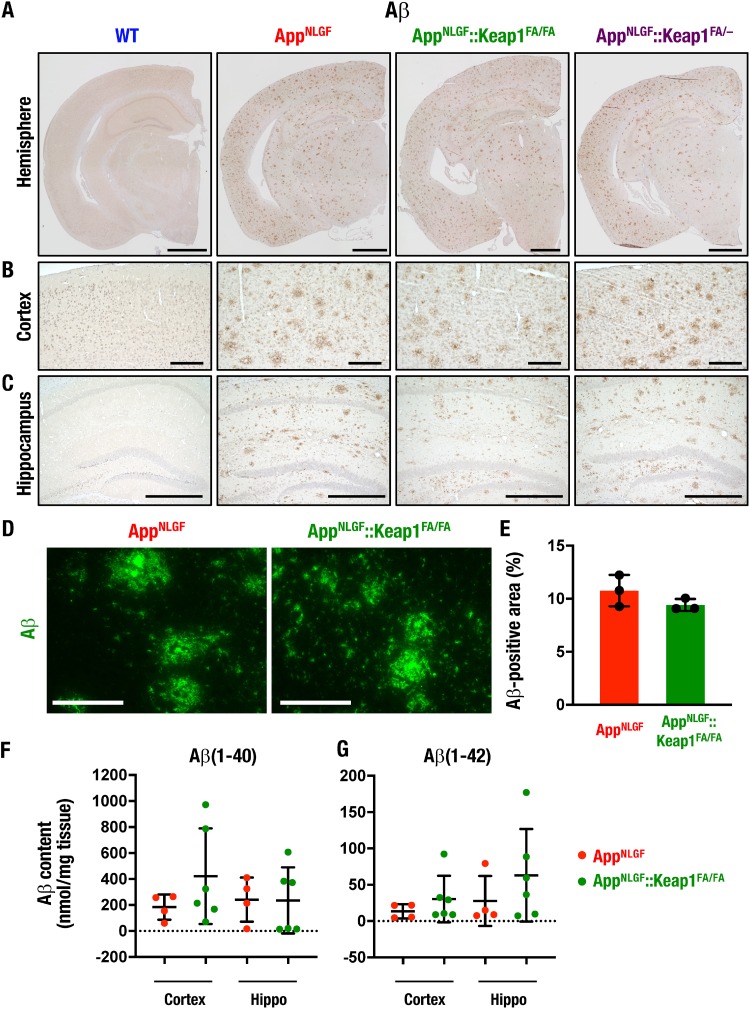
We next examined Aβ accumulation in the brains of WT, AppNLGF, AppNLGF::Keap1FA/FA, and AppNLGF::Keap1FA/– mice by means of immunohistochemistry. Consistent with the previous report that Aβ accumulated in the brains of AppNLGF mice from 2 to 7 months of age (29), we found in this study that Aβ was highly deposited in 11-month-old AppNLGF mouse brains. Aβ depositions in AppNLGF::Keap1FA/FA and AppNLGF::Keap1FA/– mice were comparable with those of AppNLGF mice, as shown by the low-magnification images in Fig. 9A.
FIG 9.
Nrf2 induction does not significantly change Aβ deposition. (A to C) Immunohistochemistry of Aβ in brain sections as shown in a low-magnification image of the hemisphere (A) and high-magnification images of the cerebral cortex (B) and the hippocampus (C) in 11-month-old WT, AppNLGF, AppNLGF::Keap1FA/FA, and AppNLGF::Keap1FA/– mouse brains. (D and E) Quantification of Aβ accumulation. (D) Aβ immunofluorescence in cortex sections of 11-month-old mouse brains. (E) Nine to eleven cortex images per mouse were analyzed, and the percentage of Aβ-positive area in each mouse cortex was calculated (n = 3). The percentages of Aβ-positive areas of tissue are represented. (F and G) Aβ content in the brain. Soluble Aβ(1-40) (F) and Aβ(1-42) (G) in the cerebral cortex and the hippocampus (Hippo) of four AppNLGF and six AppNLGF::Keap1FA/FA 11-month-old mouse brains are shown. Scale bars: 1 mm (A), 200 μm (B), 500 μm (C), and 100 μm (D). The results are presented as means ± the SD. Statistical analyses were performed using the Mann-Whitney U test (E) or ANOVA, followed by the Fisher LSD post hoc test (F and G).
Inspection of higher-magnification images of the cortex (Fig. 9B) and the hippocampus (Fig. 9C) further verified that Aβ deposition levels in AppNLGF::Keap1FA/FA and AppNLGF::Keap1FA/– mice were comparable with that in AppNLGF mice. To determine the Aβ accumulation, we also performed immunofluorescent staining and enzyme-linked immunosorbent assay (ELISA) of Aβ in the AppNLGF mouse brain. The immunofluorescent staining of Aβ revealed that the percentages of the Aβ-positive area in the cortex were comparable between AppNLGF and AppNLGF::Keap1FA/FA mice (Fig. 9D and E). The ELISA showed that the soluble Aβ(1-40) (F) and Aβ(1-42) levels were comparable among cerebral cortices and hippocampi in AppNLGF and AppNLGF::Keap1FA/FA mouse brains (Fig. 9F and G). These results demonstrate that the genetic induction of Nrf2 ameliorates oxidative tissue damage and proinflammatory response in the AppNLGF mouse brain without significantly changing Aβ deposition.
6-MSITC prevents cognitive impairment in AppNLGF mice by inducing Nrf2.
A natural compound, 6-(methylsulfinyl)hexyl isothiocyanate (6-MSITC), which is contained in Japanese horseradish, has been reported to mildly activate Nrf2 signaling (54). As we sought to identify a mild Nrf2 inducer that could be administered safely long-term to mice through a nonstressful route, we decided to test the ability of 6-MSITC to preserve the cognitive functions of AppNLGF mice.
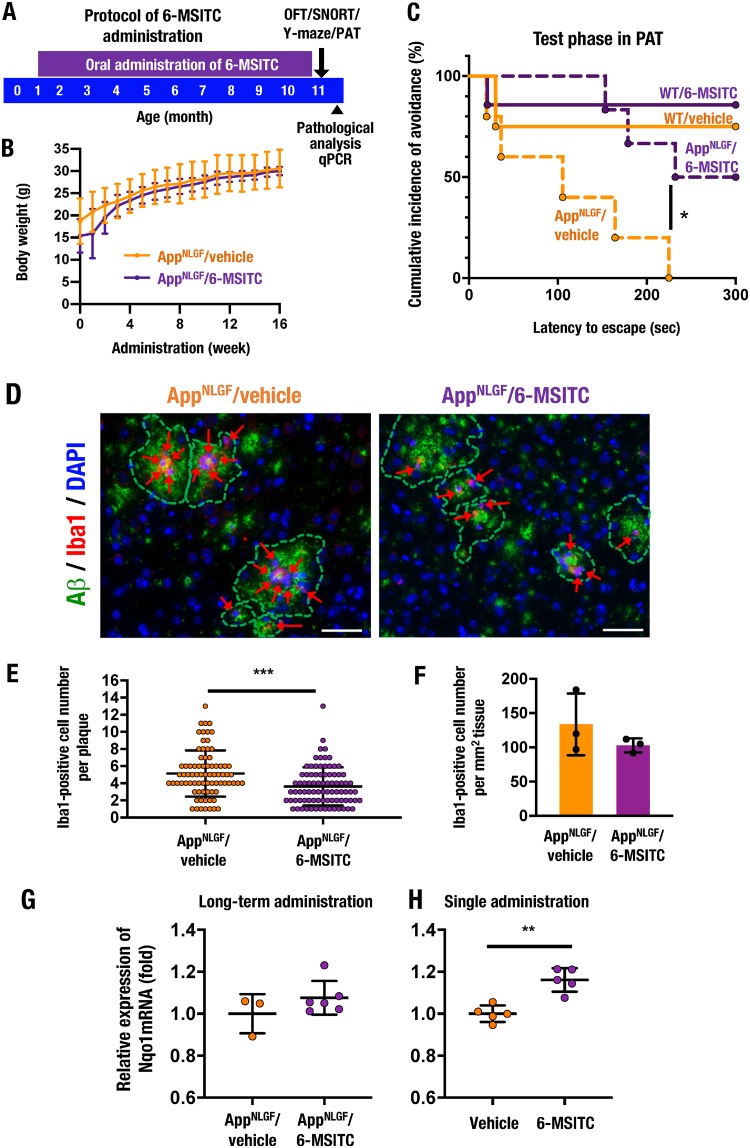
We administered 6-MSITC orally in drinking water during the period from 1 month of age to 11 months of age (Fig. 10A). As we expected, 6-MSITC treatment did not lead to deleterious effects in AppNLGF mice. Although 6-MSITC is known to be a safe compound without affecting general body conditions or body weight, we examined the body weight changes of the AppNLGF mice in this study for 16 weeks after the start of administration at 1 month of age. We found that the body weights of the 6-MSITC-treated group of AppNLGF mice were within a comparable range to those of the water (vehicle)-treated group of mice over the 16 weeks evaluated (Fig. 10B).
FIG 10.
The Nrf2-inducing natural compound 6-MSITC improves cognitive impairment in AppNLGF mice. (A) A schematic of the protocol for long-term 6-MSITC administration. Vehicle (water) or 6-MSITC was administered to male WT and AppNLGF mice from 1 month of age to 11 months of age through drinking water. Behavior tests, including the OFT, the SNORT, the Y-maze test, and the PAT, were performed at 11 months of age, followed by pathological analysis and quantitative PCR (qPCR). (B) Time course analysis of body weight in AppNLGF mice for 16 weeks during long-term administration of vehicle or 6-MSITC. AppNLGF/vehicle (n = 8) and AppNLGF/6-MSITC (n = 9) mice were analyzed. (C) Results of the PAT in 11-month-old male WT/vehicle (n = 4), WT/6-MITC (n = 7), AppNLGF/vehicle (n = 10), and AppNLGF/6-MSITC (n = 10) mice. The cumulative incidence of avoidance during the test phase is presented. (D) Immunofluorescence staining for Aβ (green) and Iba1 (red) in the cerebral cortices of AppNLGF mice. ARrows indicate Iba1-positive cells around amyloid plaques. Scale bars, 50 μm. (E) The number of Iba1-positive cells in each amyloid plaque. A total of 78 plaques in the cortices from three AppNLGF/vehicle mice and 85 plaques in the cortices of three AppNLGF/6-MSITC mice were counted. (F) Amyloid plaque-associated Iba1-positive cell number per tissue area in the cerebral cortex. Ten images per mouse were counted, and the average of numbers in each mouse was calculated in three each of the AppNLGF/vehicle and AppNLGF/6-MSITC mice. (G) Expression levels of Nqo1 mRNA after long-term oral 6-MSITC administration. The cerebral cortices of 11-month-old male AppNLGF/vehicle (n = 3) and AppNLGF/6-MSITC (n = 6) mice were analyzed. (H) Expression levels of Nqo1 mRNA in the cerebral cortex of 5-week-old male WT mice 12 h after single administration of vehicle or 6-MSITC (50 mg/kg [body weight], intraperitoneally, n = 5 each). Nqo1 expression was normalized to Actb expression, and the levels in vehicle-treated mice were set to 1. The results are presented as means ± the SD (B, E to H) or as a Kaplan-Meier curve (C). Statistical analyses were performed using repeated-measures ANOVA (B), the log rank test (C), or the Student t test (E to H). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We also performed behavior and pathological analyses of these 6-MSITC and vehicle-treated mice, as summarized in Fig. 10A. In very good agreement with the analyses shown in Fig. 2, vehicle-treated AppNLGF mice displayed impaired escape latency in the PAT compared to vehicle-treated WT mice. Of note, 6-MSITC-treated AppNLGF mice displayed significant recovery of this impaired escape latency (Fig. 10C). In contrast, the administration of 6-MSITC to WT mice did not change the latency to escape in the PAT. No obvious change was observed in the OFT, the SNORT, or the Y-maze test between vehicle- and 6-MSITC-treated AppNLGF mice (data not shown).
We then performed immunofluorescent staining for Aβ and Iba1 employing vehicle- and 6-MSITC-treated AppNLGF mice at 11 months of age. Long-term treatment with 6-MSITC decreased the number of Iba1-positive microglia associated with each amyloid plaque in the AppNLGF mouse cerebral cortex (Fig. 10D and E). The number of amyloid plaque-associated Iba1-positive microglia per mm2 of tissue was not significantly reduced in the cortices of 6-MSITC-treated AppNLGF mice compared to vehicle-treated AppNLGF mice (Fig. 10F). These results support the notion that long-term treatment with mild chemical Nrf2 inducers can exert partially similar anti-AD activity to that observed by genetic Keap1 knockdown and Nrf2 induction.
To examine whether 6-MSITC has the ability to activate Nrf2 signaling in the brain, we examined the expression levels of the Nqo1 gene after 6-MSITC treatment. Since long-term treatment of 6-MSITC to AppNLGF mice tended to mildly increase Nqo1 gene expression (Fig. 10G), we used acute Nrf2 induction conditions for this purpose. Intraperitoneal administration of high-dose 6-MSITC (50 mg/kg [body weight]) to WT mice increased Nqo1 mRNA expression in the cerebral cortex (Fig. 10H), indicating that 6-MSITC can activate Nrf2 signaling in the mouse brain. Taken together, these results support our hypothesis that long-term treatment with mild Nrf2 inducers prevents the onset of cognitive impairment in AD model mice.
Plasmalogen phosphatidylethanolamines in the AppNLGF mouse brain.
It has been reported that plasmalogen phosphatidylethanolamine (PlsPE) levels decrease in the brain and serum of both AD patients and model animals (55–57). PlsPE is a unique class of glycerophospholipid containing a fatty alcohol with a vinyl-ether bond at the sn-1 position and a fatty acid at the sn-2 position (Fig. 11A). Polyunsaturated fatty acids (PUFAs) are enriched at the sn-2 position (58). To verify whether PlsPE acts as a new biomarker for AD and to determine the relationship of PlsPE with Nrf2 in AD pathology, we considered whether the AD mouse model with genetic Keap1 induction would be an optimal system. Therefore, we assessed PlsPE levels in AppNLGF mice by means of MALDI-MSI analysis.
FIG 11.
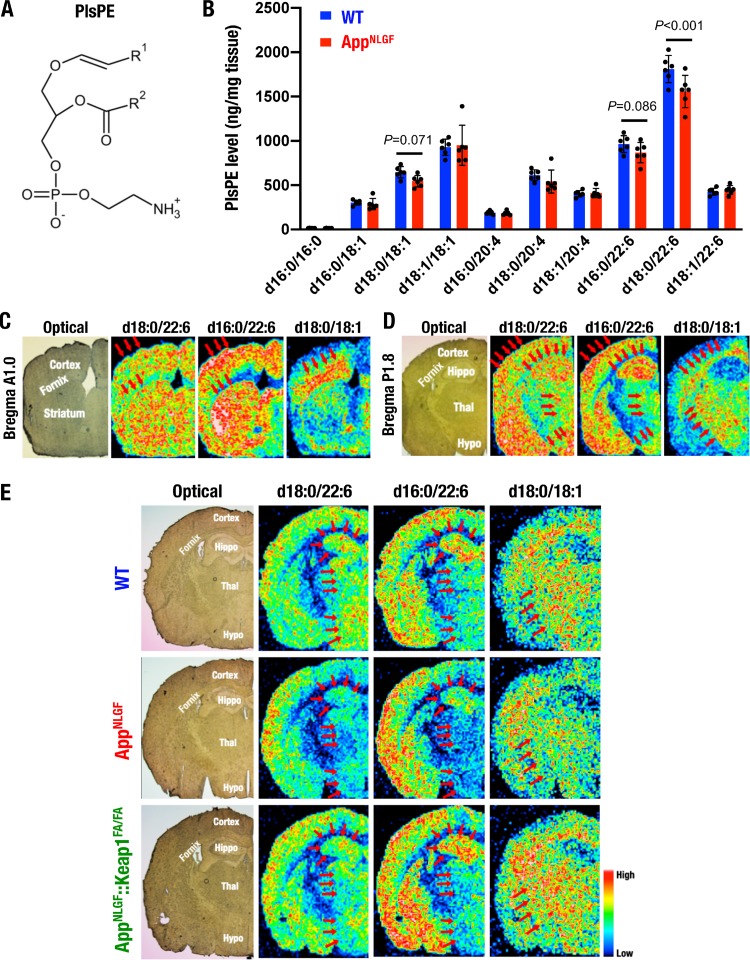
PlsPE as an AD marker in the AppNLGF mouse brain. (A) Chemical structure of PlsPE containing a fatty alcohol with a vinyl-ether bond at the sn-1 position (R1) and a fatty acid at the sn-2 position (R2). (B) LC-MS analysis of PlsPE in the brains of 8-month-old female WT and AppNLGF mice (both n = 6). The results are presented as means ± the SD. Statistical analyses were performed using ANOVA, followed by the Fisher LSD post hoc test. (C to E) MALDI-MSI analyses of PlsPE in the brains of 8-month-old female WT mice (C and D) and 11-month-old male WT, AppNLGF, and AppNLGF::Keap1FA/FA mice (E). Coronal sections at 1.0 mm anterior (A1.0) (C) and 1.8 mm posterior (P1.8) (D and E) to the bregma were analyzed as special references for the distributions of PlsPEs containing d18:0/22:6, d16:0/22:6, and d18:0/18:1. Experiments were performed at least three times with three mouse brains, and the results were fairly reproducible. Hippo, hippocampus; Thal, thalamus; Hypo, hypothalamus.
We first conducted LC-MS analysis and identified nine PlsPE compounds in WT and AppNLGF mouse brains; this group of PlsPE contained 18:1, 20:4, and 22:6 fatty acids (Fig. 11B). Notably, PlsPE d18:0/22:6 levels were significantly decreased (P < 0.001), and PlsPE d18:0/18:1 and d16:0/22:6 levels were mildly decreased (P = 0.071 and 0.086, respectively) in AppNLGF mouse brains compared to WT mouse brains.
To confirm this finding in light of the distributions in the mouse brain, we performed MALDI-MSI analysis with coronal sections from two distinct sites of WT mouse brains, i.e., 1.0 mm anterior and 1.8 mm posterior to the bregma. We focused on three PlsPE compounds, d18:0/22:6, d18:0/18:1, and d16:0/22:6, which were downregulated in AppNLGF mice compared to WT mice in whole-brain LC-MS analysis (Fig. 11B). We found that the expression profiles of these three PlsPEs were distinct and unique. PlsPE d18:0/22:6 and d16:0/22:6 were both distributed in the cortex, the striatum, the hippocampus, and the center region of the thalamus, whereas PlsPE d18:0/18:1 was highly expressed in the fornix and the thalamus (Fig. 11C and D).
We also compared the levels of these PlsPEs between AppNLGF and AppNLGF::Keap1FA/FA mice. The levels of PlsPE d16:0/22:6 and d18:0/22:6 were decreased in the hippocampi, thalami, and hypothalami of AppNLGF mice compared to WT mice; in addition, PlsPE d18:0/18:1 levels were also decreased in the thalami of AppNLGF mice (Fig. 11E, top and middle panels, arrows). Importantly, the decreases in these PlsPEs were mitigated in AppNLGF::Keap1FA/FA mice (bottom panels, arrows). These data indicate that PlsPE seems to be a useful biomarker for predicting AD conditions and may also be available for evaluating the improvement of AD by Nrf2 inducers.
DISCUSSION
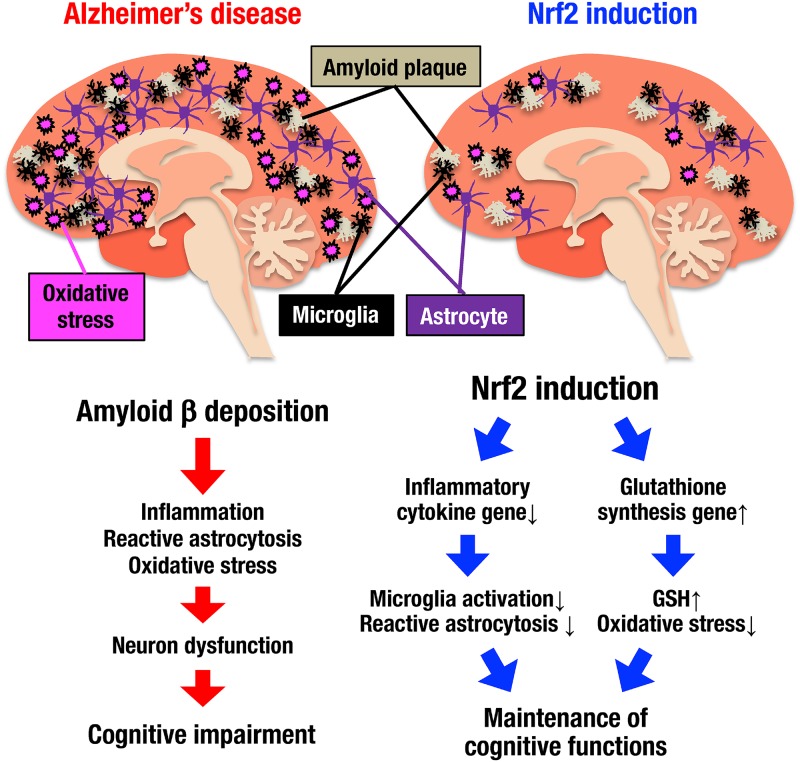
In this study, we addressed the question of how Nrf2 activation prevents the progression of the AD phenotype utilizing AD model mice crossed with Keap1 knockdown mice. Although several preceding reports have implied an Nrf2 contribution to reducing AD phenotypes, these studies heavily depended on loss-of-function analyses relying on the use of Nrf2 knockout mice (36–39). In contrast, we found in this study that genetic Nrf2 induction by Keap1 gene knockdown in mice provokes the induction of glutathione synthesis and the repression of inflammatory cytokine gene expression. As summarized in Fig. 12, these changes in gene expression profiles bring about the suppression of amyloid deposition-induced oxidative stress, inflammation, and reactive astrocytosis in AppNLGF model mouse brains. Existing lines of evidence further support the idea that Nrf2 induction ameliorates the impaired cognitive functions in AppNLGF mice. In addition to these gene-modified mouse studies, in this study, we also provide evidence that mild, long-term pharmacological induction of Nrf2 by 6-MSITC is able to suppress AD-like pathology in model mice. Based on these findings, we propose that the activation of Nrf2 signaling prevents cognitive impairment in AD.
FIG 12.
Nrf2 contributes to the maintenance of cognitive functions in AD. Amyloid β deposition (white threads) in the AppNLGF mouse brain induces inflammation (microglia; black cells), reactive astrocytosis (violet cells), and oxidative stress (pink bombs) and induces the development of neuron dysfunction and cognitive impairment. In contrast, when Nrf2 is induced in the AppNLGF mouse brain, the expression levels of inflammatory cytokine genes are reduced, and the expression levels of glutathione synthesis-related genes are increased. The number of microglia is decreased, and reactive astrocytosis and oxidative stress are suppressed, while amyloid β deposition remains constant. Nrf2 suppresses inflammation, reactive astrocytosis and oxidative stress in the AppNLGF mouse brain and contributes to the maintenance of cognitive functions in AD.
Whereas the antioxidant system operating in the central nervous system remains to be clarified, Nrf2 has been reported to play critical roles in the regulation of GSH metabolism genes in the brain (7). It has been demonstrated that Nrf2 is strongly induced in astrocytes and microglia but poorly activated in neurons (24, 44, 59). Nrf2 elevates the expression levels of glutathione synthesis-related genes, including the gene for glutamate-cysteine ligase, and enhances the synthesis of GSH in astrocytes (26). Importantly, GSH produced in astrocytes is transported from astrocytes to neurons and exerts beneficial effects in protecting neurons from oxidative damage (24, 60). In this study, we demonstrated that Nrf2 enhanced GSH levels by MALDI-MSI analysis. The elevation of GSH will play important roles in the protection of neurons against various stresses in the AppNLGF mouse brain.
We believe that GSH-mediated suppression of oxidative stress in the brain is a promising strategy for the prevention and/or early intervention of AD (61). In this regard, however, it has been reported that supplements intended to repress oxidative stresses do not improve the symptoms of AD patients (62–64). In this study, since Nrf2 suppressed both inflammation and oxidative stress in the AD mouse brain, we also focused on inflammation. Importantly, immunoglobulins and complement factors have been reported to be deposited around amyloid plaques in AD patient brains (65). We also found that Nrf2 inhibited DAM marker expressions in AppNLGF mouse brain, indicating that Nrf2 suppresses the transition of homeostatic microglia to DAM. It has been reported that TREM2 is expressed in DAM and needed for activation of microglia (48), and Trem2 depletion decrease Iba1-positive cells and improves pathological changes in AD model mouse brain (66). These observations suggest that the suppression of inflammation is important for controlling the pathogenesis of AD (67, 68). In contrast, anti-inflammatory drugs failed to improve AD symptoms in a previous clinical study (64), and the surveillance in AD patients demonstrated that the loss-of-function TREM2 variant R47H increases the risk of AD (69, 70). Nonetheless, Nrf2 inducers are expected to exert beneficial effects by suppressing the onset and development of AD by simultaneously suppressing oxidative stress and inflammation in the brain.
It has been reported that AppNLGF mice display amyloid depositions but lack tauopathy or neurofibrillary tangles in the brain (29). Importantly, the PAT analysis in the present study revealed the presence of significant cognitive impairment in AppNLGF mice. Consistent with this observation, analyses of the other AD mouse models, including Tg2576, APP23, APP/PS1, and 3×Tg-AD mice, also showed the presence of cognitive impairment (71). In this regard, it is interesting to note that AD profiles differ from model to model. For instance, neurofibrillary tangles (NFTs) are observed in 3×Tg-AD mouse brains (72), but NFTs are not observed in the brains of Tg2576, APP/PS1, APP23, or AppNLGF mice (29, 73–75). Both genetic and pharmacological induction of Nrf2 improved the abnormalities of AppNLGF mice in the PAT, supporting our belief that the induction of Nrf2 prevents cognitive impairment in the early stage of neurocognitive disorders.
In this study, we found through MALDI-MSI analyses that PUFA-containing PlsPEs are decreased in the hippocampus and the thalamus of the AppNLGF mouse brain, but genetic Nrf2 induction rescued the suppression of PUFA-containing PlsPEs in the mouse brain. Although the physiological significance of PlsPE changes has not been fully clarified, we posit that PlsPEs may play important roles in the biological membrane, including the maintenance of curved lipid membrane structures, specialized membrane microdomains, and ether-linked glycosylphosphatidylinositols. As it has been reported that the double bond in PUFA contributes to decreasing reactive oxygen species levels (76), the PUFA-containing PlsPEs may contribute to the protection of an AD brain against oxidative stress in collaboration with GSH. While PlsPEs are under evaluation as serum biomarkers of AD in humans (56, 57), our present findings further suggest that PlsPEs may act as useful antioxidants in the AD brain.
In this study, we employed 6-MSITC via a stress-free administration route expecting a mild therapeutic efficacy, as we planned to treat AppNLGF mice for a long period of time. We found that 6-MSITC improved the pathogenic conditions of AppNLGF mice in several aspects. Consistent with this finding, it has been reported that 6-MSITC protects neuronal functions in Parkinson’s disease model mice (77) and improves memory functions in Aβ1-42 injection-induced cognitive impairment model mice (78). In addition to 6-MSITC, the Nrf2-inducing chemical CDDO-methyl-amide and DMF have been shown to improve cognitive function in other AD model mice (38, 79). These wide-ranging observations provide evidence that Nrf2 inducers are useful drugs for the suppression of AD onset and development.
In conclusion, this study demonstrates that Nrf2 induction improves the antioxidative functions in the brain and ameliorates pathological neuroinflammation in AppNLGF model mice. This study further provides important lines of evidence supporting the notion that Nrf2 activation suppresses the onset and/or progression of AD, indicating that the Keap1-Nrf2 system is a promising target for the development of drugs for neurocognitive disorders, including AD.
MATERIALS AND METHODS
Animals.
AppNLGF, Keap1+/–, and Keap1FA/FA mice were previously described (29, 42, 80, 81), and these mice were backcrossed to the C57BL/6J strain for at least 10 generations. For pathological and behavioral experiments, we exploited Nrf2-inducing natural compound 6-MSITC (Abcam). 6-MSITC was dissolved in water (0.4 mg/ml) and orally administered ad libitum in drinking water to AppNLGF mice and C57BL/6J strain WT mice for 10 months. To evaluate the expression of the Nrf2 target Nqo1 gene, 6-MSITC was intraperitoneally administered at 15 mg/kg (body weight), and the brain was collected 12 h after administration. All of the animal experiments were approved by the Animal Committee at Tohoku University.
RNA isolation and real-time quantitative PCR.
Total RNA was extracted from the cerebral cortex and the hippocampus with Sepasol-RNA I Super G reagent (Nacalai Tesque). Extracted RNA was used for reverse transcription with ReverTra Ace (Toyobo) according to the manufacturer’s instructions. The resulting templates were used for qPCR with Thunderbird qPCR Mix (Toyobo). The primer sets used are listed in Table S1 in the supplemental material. Relative RNA equivalents were obtained by normalization with the expression of Actb (encoding β-actin) mRNA levels.
Immunostaining.
Immunostaining was performed using mouse monoclonal anti-Aβ (1:300, clone 82E1; IBL), anti-GFAP (clone GA5, 1:300; Chemicon), rabbit polyclonal anti-Iba1 (1:300; Wako), anti-8-OHdG (1:200; Bioss), anti-Tuj1 (1:2,000, ab18207; Abcam) and goat polyclonal anti-NQO1 (1:200, ab2346; Abcam). Secondary antibodies conjugated with horseradish peroxidase, alkaline phosphatase or a fluorescent marker were utilized and visualized according to standard protocols (24). A TUNEL assay for detecting cell death was performed with an in situ apoptosis detection kit (TaKaRa). Rat mammary tissue included in the kit was used as a positive control for TUNEL.
The Aβ-positive and GFAP-positive areas were quantified by thresholding the fluorescence intensity in these fluorescent images using ImageJ software. The amyloid plaque-associated Iba-positive cells were manually counted in each Aβ-stained area in the Iba1- and Aβ-double-staining images.
OFT.
Mice were videotaped in an open-field test system (O’Hara & Co., Ltd.) to evaluate locomotor, anxiety-like, and exploratory behaviors. A chamber with an open top box (width 50 cm by height 50 cm by depth 30 cm) made of gray acrylic that had photobeam sensors placed 5 cm above the bottom was used to detect vertical activities. Mice were placed in the same chamber for 10 min again to assess habituation behavior in the chamber after 24 h. The behavior of mice in the chamber was monitored for 10 min and recorded by a charge-coupled device camera mounted above the chamber. Videos were analyzed with TimeOFCR4 software (O’Hara & Co., Ltd.). Increased time spent in the central area has been shown to be an index of lower anxiety. The OFT was conducted at 3, 4, 6, 8, 9, and 11 months of age.
PAT.
The PAT is a behavioral task assessing learning and memory of aversive spatial information using electric shocks (82). A step-through chamber was prepared consisting of an illuminated acrylic transparent compartment (width 15 cm by height 8.5 cm by depth 25 cm) and a black opaque acrylic chamber (width 25 cm by height 25 cm by depth 25 cm). Two compartments were connected with a hole (5 cm by 5 cm) and a guillotine door. The chamber was placed in a sound-attenuated chamber (width 40 cm by height 60 cm by depth 55 cm; Muromachi Co.), which had a movable and bright-adjustable LED light. The behavior of mice in the chamber was monitored and recorded by a camera mounted above the illuminated compartment.
First, mice were allowed to acclimate to the chamber for 2 min, during which time mice could freely explore the chamber. In the training phase, a mouse was placed in the illuminated chamber with a closed door. The guillotine door was opened 30 s after exposure. A scrambled foot shock (0.35 mA, 2 s) was delivered 3 s after the four paws of the mouse completely entered the dark compartment (LE10026; Panlab). The mouse was moved into a waiting cage 30 s after the foot shock. After 24 h, the mouse was exposed to the chamber again. The latency to enter the dark chamber was calculated in the retention phase.
MALDI-MSI.
Mouse brain samples were frozen in liquid nitrogen and dissected for cryosectioning at 8-μm thickness using a cryostat (CM 3050S; Leica Microsystems). Sections were thaw mounted on indium-tin oxide slides (100 Ω/square; Matsunami Co.). For detection of GSH, NEM (Tokyo Chemical Industry) was used to generate GSH-NEM. NEM was dissolved in 15% methanol solution at 100 mmol/liter, and the solution was splayed by using a Mr. Hobby Procon Boy FWA Platinum 0.2 double-action apparatus (GSI Creos). After spraying with NEM solution, specimens were incubated for 60 min at room temperature, after which α-cyano-4-hydroxycinnamic acid (CHCA; Sigma-Aldrich) was applied to the specimens as a matrix at a thickness of 1.5 μm using an iMLayer (Shimadzu).
MALDI-MSI analysis was performed with iMScope (Shimadzu). MS/MS spectra were acquired with 100 laser shots per data point in positive-ion mode. The laser was irradiated at a 25-μm diameter and a 70-μm spatial interval of each data point. Regions of the tissue samples exposed to laser irradiation were determined by light microscopic observations. Metabolites were identified by the MS/MS spectrum using chemical standards. The data were processed using Imaging MS solution v1.30 analysis software (Shimadzu).
For PlsPE detection, CHCA was applied to 0.7-μm-thick specimens, and MS spectra were acquired by 100 laser shots per data point in positive-ion mode. The diameter of laser irradiation was 25 μm, and the spatial interval of each data point was 70 μm.
LC-MS.
Frozen mouse brains were dissected for cryosectioning at 8-μm thickness (approximately 0.3 mg) using a cryostat. Sections were placed in 2-ml plastic tubes, and 500 μl of internal standard (sulfide d18:1/17:0, 100 nmol/liter in methanol containing 0.1% formic acid) was added. Samples were vigorously mixed for 15 sec and homogenized in an ultrasonic bath for 10 min and then centrifuged at 16,000 × g for 20 min. The supernatant was then injected into the UPLC-MS/MS system. UHPLC-MS/MS analysis was performed on an Acquity Ultra Performance LC I-class system equipped with a binary solvent manager, a sample manager, and a column heater (Waters) interfaced with a Waters Xevo TQ-S MS/MS system equipped with electrospray ionization operated in positive-ion mode (83).
MS/MS was performed using multiple reaction monitoring mode; the transitions of the precursor ion to the product ion, cone voltage (V), and collision energy (eV) are listed in Table S2 in the supplemental material. The capillary voltage was 2.5 kV, and the cone voltage was 100 V. The source offset and temperature were set at 50 V and 150°C, respectively, with a cone gas flow rate of 150 liters/h. The desolvation temperature was set to 500°C, and the desolvation gas flow, collision gas flow, and nebulization gas flow were set to 1,000 liters/h, 0.15 ml/min, and 7.00 × 105 Pa, respectively. Both the cone and the nebulization gases were nitrogen. LC separation was performed using a reversed-phase column (Acquity UPLC BEH C8; 150 mm by 2.1 mm [inner diameter], 1.7-μm particle size; Waters Corp.) with a gradient elution of solvent A (5 mmol/liter ammonium formate in water, pH 4) and solvent B (5 mmol/liter ammonium formate in 95% acetonitrile, pH 4) at 0.4 ml/min. The initial condition was set to 40% solvent B and maintained for 1 min, and solvent B was increased linearly to 80% over 4 min. The gradient continued from 80 to 95% solvent B in the next 3 min and from 95 to 100% in 2 min. Subsequently, solvent B was immediately set to 100% and maintained for 8 min. Finally, the mobile phase was returned to the initiated conditions and maintained for 7 min until the end of the run (84). The oven temperature was 45°C. Data were collected using MassLynx v4.1 software (Waters) and analyzed using Traverse MS v1.2.7 software (Reifycs).
Aβ ELISA.
Cerebral cortices and hippocampi were added with 5× volume Tris-buffered saline (pH 7.4) with protease inhibitor cocktail Complete (Roche) and sonicated with a Sonifier 250 sonicator (Branson) for 45 s (85), followed by centrifugation at 17,400 × g for 60 min. To quantitate the levels of Aβ(1-40) and Aβ(1-42), the supernatant was analyzed by a Human β Amyloid(1-40)ELISA kit Wako II and a Human β Amyloid(1-42) ELISA kit Wako, High Sensitive (Fujifilm Wako Pure Chemical) according to the manufacturer’s instructions.
Statistical analyses.
Data are presented as the means ± the standard deviations (SD) or as a Kaplan-Meier survival curve. Statistical analyses were performed using Student's t test and the Mann-Whitney U test for two groups. Analyses of variance (ANOVA), followed by the Fisher least-significant-difference (LSD) post hoc test and the Kruskal-Wallis test, were performed for multiple comparisons. A log rank test was performed for the Kaplan-Meier survival curve.
Supplementary Material
ACKNOWLEDGMENTS
We thank Nao Ota (Tohoku University) and the Tohoku University Graduate School of Medicine Biomedical Research Core for technical support.
This research was supported by the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research [BINDS]) from the Japan Agency for Medical Research and Development (AMED; grant JP19am0101001 [M.Y.]), by the Tohoku Medical Megabank Project from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), by AMED (JP18km0105001 and JP18km0105002 [M.Y.]), by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS; grants 24249015 and 19H01019 [M.Y.], grants 17K01837 and 16KK0195 [A.U.], and grant 19K07361 [D.M.]), by the Takeda Science Foundation (M.Y.), and by the Naito Foundation (M.Y.).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M, Alzheimer’s Disease International. 2005. Global prevalence of dementia: a Delphi consensus study. Lancet 366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. 2011. Alzheimer’s disease. Lancet 377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 3.Chauhan V, Chauhan A. 2006. Oxidative stress in Alzheimer’s disease. Pathophysiology 13:195–208. doi: 10.1016/j.pathophys.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Henstridge CM, Hyman BT, Spires-Jones TL. 2019. Beyond the neuron-cellular interactions early in Alzheimer disease pathogenesis. Nat Rev Neurosci 20:94–108. doi: 10.1038/s41583-018-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito T, Saido TC. 2018. Neuroinflammation in mouse models of Alzheimer’s disease. Clin Exp Neuroimmunol 9:211–218. doi: 10.1111/cen3.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uruno A, Yagishita Y, Yamamoto M. 2015. The Keap1-Nrf2 system and diabetes mellitus. Arch Biochem Biophys 566:76–84. doi: 10.1016/j.abb.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. 1997. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 8.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. 1999. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itoh K, Igarashi K, Hayashi N, Nishizawa M, Yamamoto M. 1995. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol Cell Biol 15:4184–4193. doi: 10.1128/mcb.15.8.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sykiotis GP, Bohmann D. 2010. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci Signal 3:re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, Uchida K, Yamamoto M. 2006. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol 26:221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong KI, Padmanabhan B, Kobayashi A, Shang C, Hirotsu Y, Yokoyama S, Yamamoto M. 2007. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol Cell Biol 27:7511–7521. doi: 10.1128/MCB.00753-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukutomi T, Takagi K, Mizushima T, Ohuchi N, Yamamoto M. 2014. Kinetic, thermodynamic, and structural characterizations of the association between Nrf2-DLGex degron and Keap1. Mol Cell Biol 34:832–846. doi: 10.1128/MCB.01191-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takaya K, Suzuki T, Motohashi H, Onodera K, Satomi S, Kensler TW, Yamamoto M. 2012. Validation of the multiple sensor mechanism of the Keap1-Nrf2 system. Free Radic Biol Med 53:817–827. doi: 10.1016/j.freeradbiomed.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saito R, Suzuki T, Hiramoto K, Asami S, Naganuma E, Suda H, Iso T, Yamamoto H, Morita M, Baird L, Furusawa Y, Negishi T, Ichinose M, Yamamoto M. 2016. Characterizations of three major cysteine sensors of Keap1 in stress response. Mol Cell Biol 36:271–284. doi: 10.1128/MCB.00868-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamazaki H, Katsuoka F, Motohashi H, Engel JD, Yamamoto M. 2012. Embryonic lethality and fetal liver apoptosis in mice lacking all three small Maf proteins. Mol Cell Biol 32:808–816. doi: 10.1128/MCB.06543-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto M, Kensler TW, Motohashi H. 2018. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev 98:1169–1203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. 2000. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem 275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, Tanaka N, Moriguchi T, Motohashi H, Nakayama K, Yamamoto M. 2016. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun 7:11624. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itoh K, Mochizuki M, Ishii Y, Ishii T, Shibata T, Kawamoto Y, Kelly V, Sekizawa K, Uchida K, Yamamoto M. 2004. Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Δ12,14-prostaglandin J2. Mol Cell Biol 24:36–45. doi: 10.1128/mcb.24.1.36-45.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yagishita Y, Uruno A, Chartoumpekis DV, Kensler TW, Yamamoto M. 2019. Nrf2 represses the onset of type 1 diabetes in non-obese diabetic mice. J Endocrinol doi: 10.1530/JOE-18-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higashi C, Kawaji A, Tsuda N, Hayashi M, Saito R, Yagishita Y, Suzuki T, Uruno A, Nakamura M, Nakao K, Furusako S, Yamamoto M. 2017. The novel Nrf2 inducer TFM-735 ameliorates experimental autoimmune encephalomyelitis in mice. Eur J Pharmacol 802:76–84. doi: 10.1016/j.ejphar.2017.02.044. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki T, Murakami S, Biswal SS, Sakaguchi S, Harigae H, Yamamoto M, Motohashi H. 2017. Systemic activation of NRF2 alleviates lethal autoimmune inflammation in scurfy mice. Mol Cell Biol 37:e00063-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yagishita Y, Uruno A, Fukutomi T, Saito R, Saigusa D, Pi J, Fukamizu A, Sugiyama F, Takahashi S, Yamamoto M. 2017. Nrf2 improves leptin and insulin resistance provoked by hypothalamic oxidative stress. Cell Rep 18:2030–2044. doi: 10.1016/j.celrep.2017.01.064. [DOI] [PubMed] [Google Scholar]
- 25.Muramatsu H, Katsuoka F, Toide K, Shimizu Y, Furusako S, Yamamoto M. 2013. Nrf2 deficiency leads to behavioral, neurochemical, and transcriptional changes in mice. Genes Cells 18:899–908. doi: 10.1111/gtc.12083. [DOI] [PubMed] [Google Scholar]
- 26.Kraft AD, Johnson DA, Johnson JA. 2004. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci 24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida H, Mimura J, Imaizumi T, Matsumiya T, Ishikawa A, Metoki N, Tanji K, Ota K, Hayakari R, Kosaka K, Itoh K, Satoh K. 2011. Edaravone and carnosic acid synergistically enhance the expression of nerve growth factor in human astrocytes under hypoxia/reoxygenation. Neurosci Res 69:291–298. doi: 10.1016/j.neures.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Yang L, Calingasan NY, Thomas B, Chaturvedi RK, Kiaei M, Wille EJ, Liby KT, Williams C, Royce D, Risingsong R, Musiek ES, Morrow JD, Sporn M, Beal MF. 2009. Neuroprotective effects of the triterpenoid, CDDO methyl amide, a potent inducer of Nrf2-mediated transcription. PLoS One 4:e5757. doi: 10.1371/journal.pone.0005757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito T, Matsuba Y, Mihira N, Takano J, Nilsson P, Itohara S, Iwata N, Saido TC. 2014. Single App knock-in mouse models of Alzheimer’s disease. Nat Neurosci 17:661–663. doi: 10.1038/nn.3697. [DOI] [PubMed] [Google Scholar]
- 30.Youssef P, Chami B, Lim J, Middleton T, Sutherland GT, Witting PK. 2018. Evidence supporting oxidative stress in a moderately affected area of the brain in Alzheimer’s disease. Sci Rep 8:11553. doi: 10.1038/s41598-018-29770-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castillo E, Leon J, Mazzei G, Abolhassani N, Haruyama N, Saito T, Saido T, Hokama M, Iwaki T, Ohara T, Ninomiya T, Kiyohara Y, Sakumi K, LaFerla FM, Nakabeppu Y. 2017. Comparative profiling of cortical gene expression in Alzheimer’s disease patients and mouse models demonstrates a link between amyloidosis and neuroinflammation. Sci Rep 7:17762. doi: 10.1038/s41598-017-17999-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ansari MA, Scheff SW. 2010. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol 69:155–167. doi: 10.1097/NEN.0b013e3181cb5af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karelson E, Bogdanovic N, Garlind A, Winblad B, Zilmer K, Kullisaar T, Vihalemm T, Kairane C, Zilmer M. 2001. The cerebrocortical areas in normal brain aging and in Alzheimer’s disease: noticeable differences in the lipid peroxidation level and in antioxidant defense. Neurochem Res 26:353–361. doi: 10.1023/a:1010942929678. [DOI] [PubMed] [Google Scholar]
- 34.Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, Araoz C. 1989. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A 86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers J, Luber-Narod J, Styren SD, Civin WH. 1988. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer’s disease. Neurobiol Aging 9:339–349. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- 36.Rojo AI, Pajares M, Rada P, Nuñez A, Nevado-Holgado AJ, Killik R, Van Leuven F, Ribe E, Lovestone S, Yamamoto M, Cuadrado A. 2017. NRF2 deficiency replicates transcriptomic changes in Alzheimer’s patients and worsens APP and TAU pathology. Redox Biol 13:444–451. doi: 10.1016/j.redox.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Branca C, Ferreira E, Nguyen TV, Doyle K, Caccamo A, Oddo S. 2017. Genetic reduction of Nrf2 exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. Hum Mol Genet 26:4823–4835. doi: 10.1093/hmg/ddx361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rojo AI, Pajares M, García-Yagüe AJ, Buendia I, Van Leuven F, Yamamoto M, López MG, Cuadrado A. 2018. Deficiency in the transcription factor NRF2 worsens inflammatory parameters in a mouse model with combined tauopathy and amyloidopathy. Redox Biol 18:173–180. doi: 10.1016/j.redox.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joshi G, Gan KA, Johnson DA, Johnson JA. 2015. Increased Alzheimer’s disease-like pathology in the APP/PS1ΔE9 mouse model lacking Nrf2 through modulation of autophagy. Neurobiol Aging 36:664–679. doi: 10.1016/j.neurobiolaging.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanninen K, Malm TM, Jyrkkänen HK, Goldsteins G, Keksa-Goldsteine V, Tanila H, Yamamoto M, Ylä-Herttuala S, Levonen AL, Koistinaho J. 2008. Nuclear factor erythroid 2-related factor 2 protects against beta amyloid. Mol Cell Neurosci 39:302–313. doi: 10.1016/j.mcn.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Kanninen K, Heikkinen R, Malm T, Rolova T, Kuhmonen S, Leinonen H, Ylä-Herttuala S, Tanila H, Levonen AL, Koistinaho M, Koistinaho J. 2009. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 106:16505–16510. doi: 10.1073/pnas.0908397106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taguchi K, Maher JM, Suzuki T, Kawatani Y, Motohashi H, Yamamoto M. 2010. Genetic analysis of cytoprotective functions supported by graded expression of Keap1. Mol Cell Biol 30:3016–3026. doi: 10.1128/MCB.01591-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Habas A, Hahn J, Wang X, Margeta M. 2013. Neuronal activity regulates astrocytic Nrf2 signaling. Proc Natl Acad Sci U S A 110:18291–18296. doi: 10.1073/pnas.1208764110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bell KF, Al-Mubarak B, Martel MA, McKay S, Wheelan N, Hasel P, Márkus NM, Baxter P, Deighton RF, Serio A, Bilican B, Chowdhry S, Meakin PJ, Ashford ML, Wyllie DJ, Scannevin RH, Chandran S, Hayes JD, Hardingham GE. 2015. Neuronal development is promoted by weakened intrinsic antioxidant defences due to epigenetic repression of Nrf2. Nat Commun 6:7066. doi: 10.1038/ncomms8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lastres-Becker I, García-Yagüe AJ, Scannevin RH, Casarejos MJ, Kügler S, Rábano A, Cuadrado A. 2016. Repurposing the NRF2 activator dimethyl fumarate as therapy against synucleinopathy in Parkinson’s disease. Antioxid Redox Signal 25:61–77. doi: 10.1089/ars.2015.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suganuma H, Fahey JW, Bryan KE, Healy ZR, Talalay P. 2011. Stimulation of phagocytosis by sulforaphane. Biochem Biophys Res Commun 405:146–151. doi: 10.1016/j.bbrc.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stratoulias V, Venero JL, Tremblay M, Joseph B. 2019. Microglial subtypes: diversity within the microglial community. EMBO J 38:e101997. doi: 10.15252/embj.2019101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I. 2017. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169:1276–1290.e1217. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 49.Deczkowska A, Keren-Shaul H, Weiner A, Colonna M, Schwartz M, Amit I. 2018. Disease-associated microglia: a universal immune sensor of neurodegeneration. Cell 173:1073–1081. doi: 10.1016/j.cell.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O’Loughlin E, Xu Y, Fanek Z, Greco DJ, Smith ST, Tweet G, Humulock Z, Zrzavy T, Conde-Sanroman P, Gacias M, Weng Z, Chen H, Tjon E, Mazaheri F, Hartmann K, Madi A, Ulrich JD, Glatzel M, Worthmann A, Heeren J, Budnik B, Lemere C, Ikezu T, Heppner FL, Litvak V, Holtzman DM, Lassmann H, Weiner HL, Ochando J, Haass C, Butovsky O. 2017. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47:566–581.e569. doi: 10.1016/j.immuni.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossi R, Milzani A, Dalle-Donne I, Giustarini D, Lusini L, Colombo R, Di Simplicio P. 2002. Blood glutathione disulfide: in vivo factor or in vitro artifact? Clin Chem 48:742–753. [PubMed] [Google Scholar]
- 52.Giustarini D, Tsikas D, Colombo G, Milzani A, Dalle-Donne I, Fanti P, Rossi R. 2016. Pitfalls in the analysis of the physiological antioxidant glutathione (GSH) and its disulfide (GSSG) in biological samples: an elephant in the room. J Chromatogr B Analyt Technol Biomed Life Sci 1019:21–28. doi: 10.1016/j.jchromb.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moore T, Le A, Niemi AK, Kwan T, Cusmano-Ozog K, Enns GM, Cowan TM. 2013. A new LC-MS/MS method for the clinical determination of reduced and oxidized glutathione from whole blood. J Chromatogr B Analyt Technol Biomed Life Sci 929:51–55. doi: 10.1016/j.jchromb.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 54.Hou DX, Korenori Y, Tanigawa S, Yamada-Kato T, Nagai M, He X, He J. 2011. Dynamics of Nrf2 and Keap1 in ARE-mediated NQO1 expression by wasabi 6-(methylsulfinyl)hexyl isothiocyanate. J Agric Food Chem 59:11975–11982. doi: 10.1021/jf2032439. [DOI] [PubMed] [Google Scholar]
- 55.Han X, Holtzman DM, McKeel DW. 2001. Plasmalogen deficiency in early Alzheimer’s disease subjects and in animal models: molecular characterization using electrospray ionization mass spectrometry. J Neurochem 77:1168–1180. doi: 10.1046/j.1471-4159.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- 56.Wood PL, Locke VA, Herling P, Passaro A, Vigna GB, Volpato S, Valacchi G, Cervellati C, Zuliani G. 2016. Targeted lipidomics distinguishes patient subgroups in mild cognitive impairment (MCI) and late onset Alzheimer’s disease (LOAD). BBA Clin 5:25–28. doi: 10.1016/j.bbacli.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goodenowe DB, Cook LL, Liu J, Lu Y, Jayasinghe DA, Ahiahonu PW, Heath D, Yamazaki Y, Flax J, Krenitsky KF, Sparks DL, Lerner A, Friedland RP, Kudo T, Kamino K, Morihara T, Takeda M, Wood PL. 2007. Peripheral ethanolamine plasmalogen deficiency: a logical causative factor in Alzheimer’s disease and dementia. J Lipid Res 48:2485–2498. doi: 10.1194/jlr.P700023-JLR200. [DOI] [PubMed] [Google Scholar]
- 58.Braverman NE, Moser AB. 2012. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta 1822:1442–1452. doi: 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 59.Soriano FX, Léveillé F, Papadia S, Higgins LG, Varley J, Baxter P, Hayes JD, Hardingham GE. 2008. Induction of sulfiredoxin expression and reduction of peroxiredoxin hyperoxidation by the neuroprotective Nrf2 activator 3H-1,2-dithiole-3-thione. J Neurochem 107:533–543. doi: 10.1111/j.1471-4159.2008.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dringen R, Pfeiffer B, Hamprecht B. 1999. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci 19:562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pocernich CB, Butterfield DA. 2012. Elevation of glutathione as a therapeutic strategy in Alzheimer disease. Biochim Biophys Acta 1822:625–630. doi: 10.1016/j.bbadis.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Persson T, Popescu BO, Cedazo-Minguez A. 2014. Oxidative stress in Alzheimer’s disease: why did antioxidant therapy fail? Oxid Med Cell Longev 2014:427318. doi: 10.1155/2014/427318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim GH, Kim JE, Rhie SJ, Yoon S. 2015. The role of oxidative stress in neurodegenerative diseases. Exp Neurobiol 24:325–340. doi: 10.5607/en.2015.24.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nichols MR, St-Pierre MK, Wendeln AC, Makoni NJ, Gouwens LK, Garrad EC, Sohrabi M, Neher JJ, Tremblay ME, Combs CK. 2019. Inflammatory mechanisms in neurodegeneration. J Neurochem 149:562–581. doi: 10.1111/jnc.14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eikelenboom P, Stam FC. 1982. Immunoglobulins and complement factors in senile plaques: an immunoperoxidase study. Acta Neuropathol 57:239–242. doi: 10.1007/bf00685397. [DOI] [PubMed] [Google Scholar]
- 66.Jay TR, Miller CM, Cheng PJ, Graham LC, Bemiller S, Broihier ML, Xu G, Margevicius D, Karlo JC, Sousa GL, Cotleur AC, Butovsky O, Bekris L, Staugaitis SM, Leverenz JB, Pimplikar SW, Landreth GE, Howell GR, Ransohoff RM, Lamb BT. 2015. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J Exp Med 212:287–295. doi: 10.1084/jem.20142322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arranz AM, De Strooper B. 2019. The role of astroglia in Alzheimer’s disease: pathophysiology and clinical implications. Lancet Neurol 18:406–414. doi: 10.1016/S1474-4422(18)30490-3. [DOI] [PubMed] [Google Scholar]
- 68.Edwards FA. 2019. A unifying hypothesis for Alzheimer’s disease: from plaques to neurodegeneration. Trends Neurosci 42:310–322. doi: 10.1016/j.tins.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 69.Guerreiro R, Alzheimer Genetic Analysis Group, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J. 2013. TREM2 variants in Alzheimer’s disease. N Engl J Med 368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K. 2013. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med 368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Webster SJ, Bachstetter AD, Nelson PT, Schmitt FA, Van Eldik LJ. 2014. Using mice to model Alzheimer’s dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front Genet 5:88. doi: 10.3389/fgene.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. 2003. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron 39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 73.Radde R, Bolmont T, Kaeser SA, Coomaraswamy J, Lindau D, Stoltze L, Calhoun ME, Jäggi F, Wolburg H, Gengler S, Haass C, Ghetti B, Czech C, Hölscher C, Mathews PM, Jucker M. 2006. Aβ42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep 7:940–946. doi: 10.1038/sj.embor.7400784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Bürki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B. 1997. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci U S A 94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. 1996. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 76.Markesbery WR. 1997. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med 23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 77.Morroni F, Sita G, Tarozzi A, Cantelli-Forti G, Hrelia P. 2014. Neuroprotection by 6-(methylsulfinyl)hexyl isothiocyanate in a 6-hydroxydopamine mouse model of Parkinson’s disease. Brain Res 1589:93–104. doi: 10.1016/j.brainres.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 78.Morroni F, Sita G, Graziosi A, Turrini E, Fimognari C, Tarozzi A, Hrelia P. 2018. Protective effects of 6-(methylsulfinyl)hexyl isothiocyanate on Aβ. Int J Mol Sci 19:E2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dumont M, Wille E, Calingasan NY, Tampellini D, Williams C, Gouras GK, Liby K, Sporn M, Nathan C, Flint Beal M, Lin MT. 2009. Triterpenoid CDDO-methylamide improves memory and decreases amyloid plaques in a transgenic mouse model of Alzheimer’s disease. J Neurochem 109:502–512. doi: 10.1111/j.1471-4159.2009.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, Harada T, Engel JD, Yamamoto M. 2003. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet 35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- 81.Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. 2006. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun 339:79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- 82.Ader R. 1973. Effects of early experiences on shock-and illness-induced passive avoidance behaviors. Dev Psychobiol 6:547–555. doi: 10.1002/dev.420060611. [DOI] [PubMed] [Google Scholar]
- 83.Saigusa D, Okudaira M, Wang J, Kano K, Kurano M, Uranbileg B, Ikeda H, Yatomi Y, Motohashi H, Aoki J. 2014. Simultaneous quantification of sphingolipids in small quantities of liver by LC-MS/MS. Mass Spectrom (Tokyo) 3:S0046. doi: 10.5702/massspectrometry.S0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sato K, Saigusa D, Saito R, Fujioka A, Nakagawa Y, Nishiguchi KM, Kokubun T, Motoike IN, Maruyama K, Omodaka K, Shiga Y, Uruno A, Koshiba S, Yamamoto M, Nakazawa T. 2018. Metabolomic changes in the mouse retina after optic nerve injury. Sci Rep 8:11930. doi: 10.1038/s41598-018-30464-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang J, Dickson DW, Trojanowski JQ, Lee VM. 1999. The levels of soluble versus insoluble brain Aβ distinguish Alzheimer’s disease from normal and pathologic aging. Exp Neurol 158:328–337. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.