Abstract
Malaria is an infectious disease that affects over 216 million people worldwide, killing over 445,000 patients annually. Due to the constant emergence of parasitic resistance to the current antimalarial drugs, the discovery of new drug candidates is a major global health priority. Aiming to make the drug discovery processes faster and less expensive, we developed binary and continuous Quantitative Structure-Activity Relationships (QSAR) models implementing deep learning for predicting antiplasmodial activity and cytotoxicity of untested compounds. Then, we applied the best models for a virtual screening of a large database of chemical compounds. The top computational predictions were evaluated experimentally against asexual blood stages of both sensitive and multi-drug-resistant Plasmodium falciparum strains. Among them, two compounds, LabMol-149 and LabMol-152, showed potent antiplasmodial activity at low nanomolar concentrations (EC50 <500 nM) and low cytotoxicity in mammalian cells. Therefore, the computational approach employing deep learning developed here allowed us to discover two new families of potential next generation antimalarial agents, which are in compliance with the guidelines and criteria for antimalarial target candidates.
Author summary
Malaria is a serious infectious disease caused by parasites of the genus Plasmodium. The recommended treatment is a combination of antimalarial drugs. However, the rise of parasites resistant to the current antimalarial drugs means that new therapeutics are continually required. To meet this challenge, we developed and applied models using deep learning, a powerful artificial intelligence method, supported by experimental validation to identify new drug candidates against malaria. We used the developed computational models to prioritize novel, active, and nontoxic compounds from virtual chemical libraries for experimental evaluation. Then, the predicted antimalarial compounds were experimentally validated in assays on Plasmodium falciparum culture. This allowed us to discover two new potential antimalarial candidates. The use of computational approaches is an attractive route to expedite the discovery of new therapeutics, especially to infectious tropical diseases, as it can reduce time and development costs. Future directions include in vivo studies on animal models.
Introduction
Malaria is a serious worldwide health problem that affects 216 million people, killing over 445,000 patients annually, especially children younger than five-years-old and pregnant women in Sub-Saharan Africa [1]. The disease is transmitted to humans through the bites of infected female Anopheles mosquitoes and caused by parasites of the genus Plasmodium [2,3]. Among them, P. falciparum is the most prevalent and dangerous species, causing the severe form of the disease, i.e., cerebral malaria [3,4].
Current control and eradication of malaria demands a multifaceted approach. The World Health Organization recommends a combination of at least two drugs with different mechanism of action. However, the efficacy of antimalarial drugs is threatened by the emergence and spread of resistant strains to all major antimalarial drugs, such as chloroquine [5], atovaquone [6], pyrimethamine [7], and sulfadoxine [8]. More recently, drug resistance has also been reported to front-line artemisinin-based combination therapies (ACTs) in the Greater Mekong Subregion and southeast Asia [9–11]. All these aspects highlight the compelling need for the development of new therapies to solve the challenges of drug resistance and treatment adherence by identifying molecules with novel mechanisms of action and activity against all known resistant parasite strains [12].
In this context, computational approaches, especially quantitative structure-activity relationships (QSAR) modeling, have had a profound impact in drug discovery [13]. Methodologically, QSAR modeling can be presented as a three-part process. Initially, a set of chemicals with experimentally-determined biological properties is converted into molecular descriptors (independent variables). Then, statistical methods are employed to establish relationships between descriptors and the biological properties (dependent variable) [14,15]. Early statistical methods used in QSAR applications were linear regression models [16–18], but these were quickly supplanted by Bayesian neural networks [19,20], followed by Support Vector Machines [21] and Random Forests [22–24]. Once statistically validated using appropriate metrics, the generated model represents a helpful tool for the virtual screening (VS) of new chemicals with desired biological properties.
The availability of large datasets of chemical compounds with at least one biological property measured [25,26], associated with thousands of molecular descriptors paired with the popularization of in silico approaches resulted in the widespread use of QSAR for a diverse array of biological properties relevant to drug discovery [27–32]. However, dealing with big datasets has posed a challenge to model biological properties using classical machine learning algorithms [33,34]. To address this issue, deep learning methods (deep neural networks) have been presented as a practical solution [35,36]. Deep learning is particularly well-suited for QSAR modeling because it possesses multiple hidden layers capable of computing adaptive non-linear features that increasingly capture complex data patterns with each iterative additional layer, which makes this approach useful for tackling more complex chemical data [37,38].
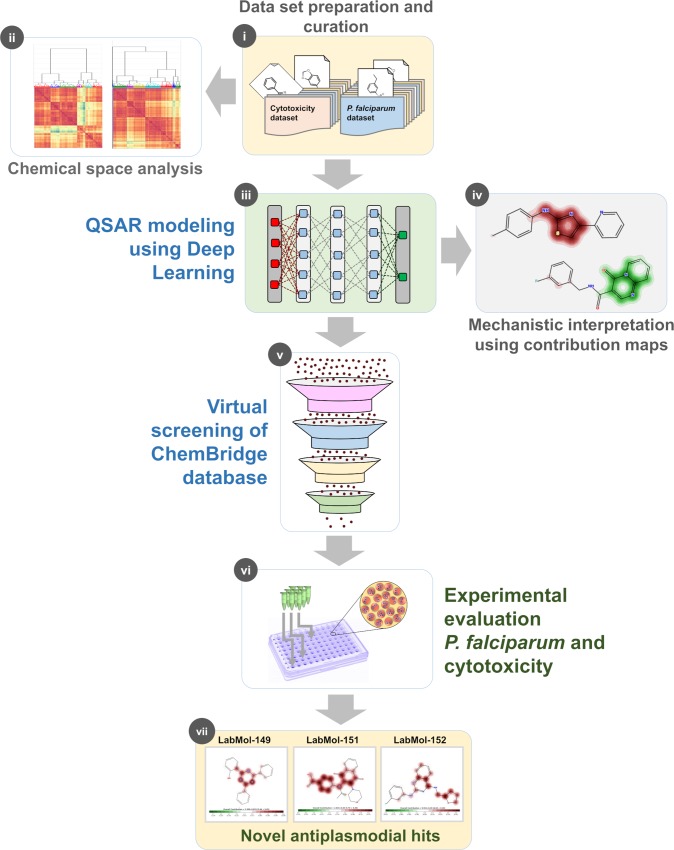
Recently, there have been some exciting studies implementing deep learning for de novo design of molecules [39–41] compounds with desired activity. Here, we developed a modeling protocol employing deep learning to build binary and continuous QSAR models based on large datasets and applied them for predicting the antiplasmodial activity and cytotoxicity of untested compounds. Then, the prioritized compounds were experimentally evaluated against asexual blood stages P. falciparum and mammalian cells. The general study design is presented in Fig 1. Briefly, we followed the following successive steps: (i) dataset collection, curation, and integration of molecules with activity against P. falciparum and cytotoxicity in fibroblasts; (ii) chemical space analysis of curated datasets; (iii) development of both binary and continuous QSAR models using deep learning; (iv) mechanistic interpretation of continuous models to provide structural and biological insights useful for design of new antiplasmodial compounds; (v) VS of ChemBridge chemical database (∼500,000 compounds); (vi) experimental validation of prioritized compounds on asexual blood stages of P. falciparum (sensitive and multi-drug resistant strains) and mammalian cells; and (vii) identification of novel antiplasmodial compounds.
Fig 1. Study design.
(i) dataset collection, curation, and integration of molecules with activity against P. falciparum and cytotoxicity in fibroblasts; (ii) chemical space analysis; (iii) development of QSAR models using deep learning; (iv) mechanistic interpretation of models; (v) VS of ChemBridge chemical database; (vi) experimental validation of prioritized compounds; and (vii) identification of novel antiplasmodial compounds.
Results
Chemical space analysis
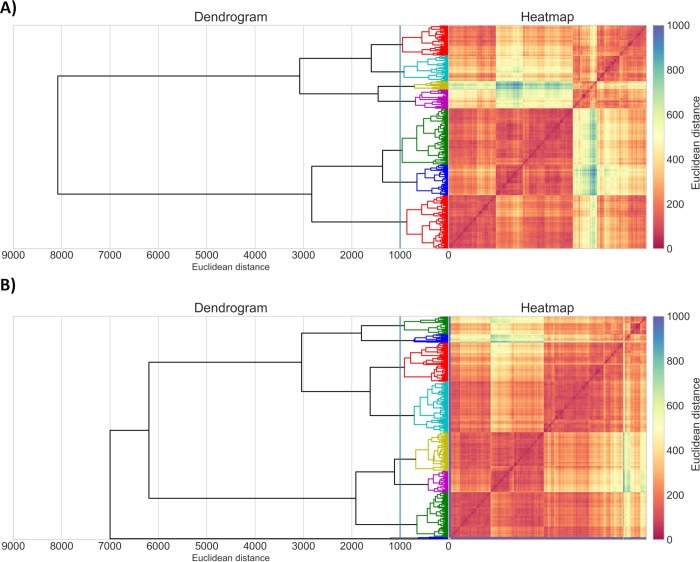
Initially, an activity threshold of 1 μM based on half maximal effective concentration (EC50) against P. falciparum was defined for discrimination between active and inactive compounds previously tested against asexual blood stages of P. falciparum. In addition, a threshold of 10 μM based on half-maximal cytotoxic concentration (CC50) for the NIH/3T3 cells was defined for discrimination between toxic and nontoxic compounds [42]. The analysis of chemical space was performed by using the curated datasets (see Materials and Methods) for erythrocytic stages of P. falciparum 3D7 strain (chloroquine sensitive) dataset containing 1,162 compounds (P. falciparum dataset) and cytotoxicity dataset tested against mouse embryonic fibroblasts (NIH/3T3 cell line) containing 1,270 compounds (cytotoxicity dataset). This analysis has been performed by clustering both datasets separately, which revealed that both are very structurally dissimilar, containing smaller clusters of similar compounds (Fig 2).
Fig 2.
Cluster analysis of A) 1,162 compounds from P. falciparum dataset and B) 1,270 compounds from cytotoxicity dataset. Dendrogram and heatmap of the distance matrix are both colored according to structural similarity (orange/red = similar; blue/violet = dissimilar). The x- and y-axis labels of the heatmap represent compounds.
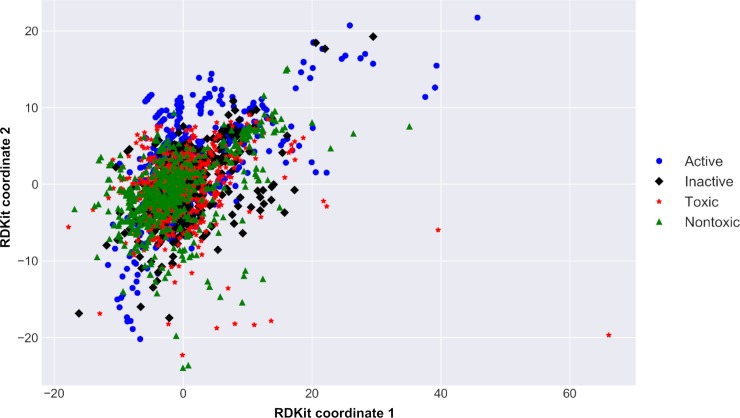
Although the datasets are structurally diverse, they share the same regions of chemical space. When analyzing the chemical space of both datasets together, by protting the two-dimensional barcentric coordinates [43] (Fig 3, see Materials and Methods for details), one can see that most of the active and inactive compounds from P. falciparum dataset overlap within the same regions of chemical space of toxic and nontoxic compounds from the cytotoxicity dataset. This analysis reveals that multiple compounds active against P. falciparum in the erythrocytic stage are potentially toxic in mouse embryonic fibroblasts. For this reason, we developed predictive computational models for both biological properties in order to select only compounds predicted as active for P. falciparum and nontoxic for mammalian cells.
Fig 3. Chemical space of investigated compounds.
The plot was obtained using barycentric coordinates from 2D RDKit descriptors showing active (blue dots) and inactive (black diamonds) compounds of P. falciparum dataset and toxic (red stars) and nontoxic (green triangles) from cytotoxicity dataset.
Performance of binary QSAR models
Binary QSAR models were built to distinguish active vs. inactive compounds for P. falciparum and toxic vs. nontoxic compounds for NIH/3T3 cells. According to the statistical results of a 5-fold external cross-validation procedure (see Materials and Methods), the combination of Morgan and FeatMorgan fingerprints (radius 2: FeatMorgan_2, Morgan_2; radius 4: FeatMorgan_4, Morgan_4) with deep learning (see Materials and Methods for details) led to predictive binary QSAR models. Statistical characteristics of developed QSAR models estimated by 5-fold external cross-validation are reported in Table 1. Briefly, correct classification rate (CCR) values were ranging between 0.82–0.87; sensitivity (SE)– 0.82–0.87; specificity (SP)– 0.82–0.87, and a coverage– 0.77–0.87. Table 1 shows the detailed performances of the binary QSAR models. The model built using Morgan_2 demonstrated the best performance among all other models developed for P. falciparum (CCR = 0.84; SE = 0.82; SP = 0.86; and PPV = 0.86). On the other hand, the best model developed for prediction of cytotoxicity for mammalian fibroblasts was built using FeatMorgan_4 (CCR = 0.87; SE = 0.87; and SP = 0.87).
Table 1. Summarized statistical characteristics of binary QSAR models.
| Model | CCR | SE | SP | PPV | NPV | Coverage |
|---|---|---|---|---|---|---|
| P. falciparum | ||||||
| FeatMorgan_2 | 0.84 | 0.82 | 0.86 | 0.85 | 0.83 | 0.80 |
| FeatMorgan_4 | 0.82 | 0.82 | 0.83 | 0.83 | 0.83 | 0.78 |
| Morgan_2 | 0.84 | 0.82 | 0.86 | 0.86 | 0.83 | 0.80 |
| Morgan_4 | 0.84 | 0.83 | 0.85 | 0.85 | 0.83 | 0.77 |
| Cytotoxicity | ||||||
| FeatMorgan_2 | 0.84 | 0.85 | 0.84 | 0.84 | 0.85 | 0.80 |
| FeatMorgan_4 | 0.87 | 0.87 | 0.87 | 0.87 | 0.87 | 0.85 |
| Morgan_2 | 0.84 | 0.86 | 0.83 | 0.83 | 0.85 | 0.79 |
| Morgan_4 | 0.84 | 0.84 | 0.85 | 0.85 | 0.84 | 0.87 |
CCR: correct classification rate; SE: sensitivity; SP: specificity; PPV: positive predictive value; and NPV: negative predictive value; Coverage: percentage of test set compounds within the applicability domain.
Performance of continuous QSAR models
We have developed continuous QSAR models aiming to predict negative logarithmic units of EC50 values (pEC50) against P. falciparum and CC50 values (pCC50) against NIH/3T3 cell line. According to the statistical results of a 5-fold external cross-validation procedure, the combination of Morgan and FeatMorgan fingerprints (radius 2: FeatMorgan_2, Morgan_2) with deep learning led to statistically predictive models (Table 2), with predictive squared correlation coefficient for the test set () values ranging between 0.70–0.88, root mean square error of cross-validation (RMSECV) of 0.44–0.55, mean absolute error (MAE) of 0.31–0.43, and coverage of 0.79–0.81. The model built using Morgan_2 demonstrated the best performance among all other models developed for P. falciparum ( = 0.88, RMSECV = 0.49, and MAE = 0.43). On the other hand, the best model developed for prediction of cytotoxicity for mammalian fibroblasts was built using FeatMorgan_2 ( = 0.74, RMSECV = 0.44, and MAE = 0.31).
Table 2. Statistical characteristics of developed continuous QSAR models.
| Model | RMSECV | MAE | Coverage | ||
|---|---|---|---|---|---|
| P. falciparum | |||||
| Morgan_2 | 0.88 | 0.49 | 0.43 | 0.88 | 0.79 |
| FeatMorgan_2 | 0.71 | 0.55 | 0.40 | 0.72 | 0.81 |
| Cytotoxicity | |||||
| Morgan_2 | 0.70 | 0.51 | 0.38 | 0.70 | 0.79 |
| FeatMorgan_2 | 0.73 | 0.44 | 0.31 | 0.74 | 0.79 |
R2: correlation coefficient; RMSECV: root mean square error of cross-validation; MAE: mean absolute error; : predictive squared correlation coefficient for the test set; Coverage: percentage of test set compounds within the applicability domain (AD).
Model interpretation
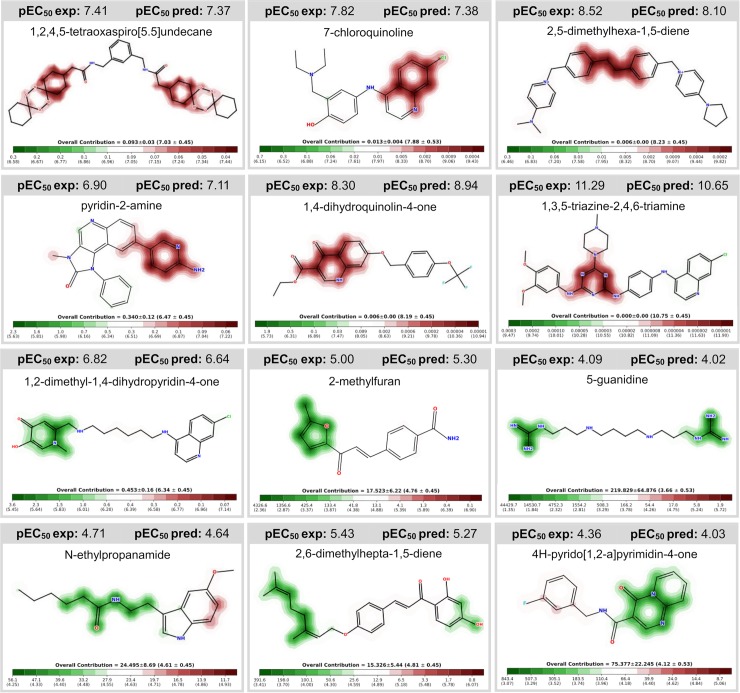
To provide a mechanistic interpretation and shed some light from the structural and biological data used to build the continuous QSAR models, we plotted the predicted feature (fingerprint) importance to visualize how the fragments contributed for the antiplasmodial activity and the cytotoxicity (Fig 4 and S1 Fig). According to our results, atoms or fragments promoting positive contribution for the antiplasmodial activity are highlighted in red, while structural moieties decreasing the activity are highlighted in green.
Fig 4. Predicted influence of structural fragments on the antiplasmodial activity.
Compounds experimentally tested in P. falciparum assay, extracted from the literature and used to build/validate our models. Fragments increasing the activity are colored in red; structural moieties decreasing the activity are highlighted in green; indifferent fragments are not highlighted. pEC50 exp = pEC50 experimental; pEC50 pred = pEC50 predicted.
By analyzing the contribution maps generated for the P. falciparum dataset, we identified six major fragments with favorable contribution for antiplasmodial activity. Examples of favorable fragments are as follows: 1,2,4,5-tetraoxaspiro[5.5]undecane; 7-chloroquinoline; 2,5-dimethylhexa-1,5-diene; pyridin-2-amine; 1,4-dihydroquinolin-4-one; and 1,3,5-triazine-2,4,6-triamine. We also identified six fragments with unfavorable contribution for antiplasmodial activity, such as: 1,2-dimethyl-1,4-dihydropyridin-4-one; 2-methylfuran; 5-guanidine; N-ethylpropanamide; 2,6-dimethylhepta-1,5-diene; and 4H-pyrido[1,2-a]pyrimidin-4-one. Moreover, we also calculated the predicted influence of structural fragments on the cytotoxicity. A summarized list of atoms or fragments with favorable and unfavorable contribution for cytotoxicity on mammalian fibroblasts is available in S1 Fig. The structural and biological information provided by the QSAR models developed using deep learning could be useful for designing or optimizing potent and selective antiplasmodial compounds by replacing unfavorable fragments by favorable fragments, assuming true independence of physicochemical effects.
Virtual screening
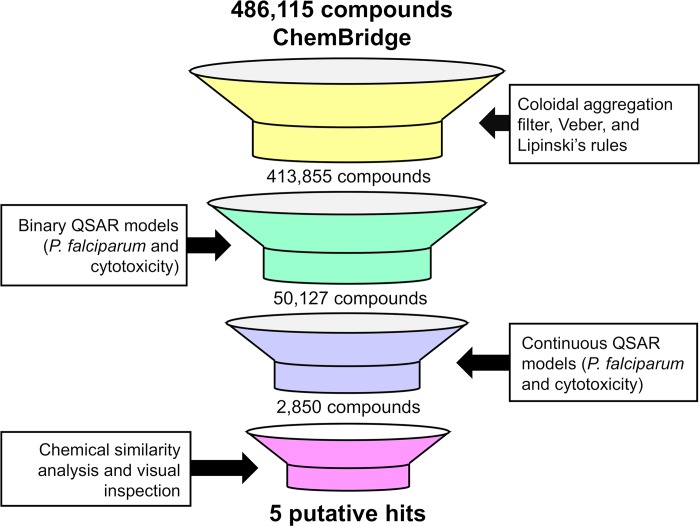
The virtual screening (VS) was carried out following the workflow presented in Fig 5. Initially, 486,115 compounds available on EXPRESS-Pick collection of ChemBridge were downloaded and standardized for VS. Then, drug-likeness filters (Veber [44] and Lipinski’s rules [45]) were applied to prioritize molecules with good oral bioavailability, to ensure that the compound has basic properties of active drugs. In parallel, colloidal aggregation tool was used to filter out molecules that are known to aggregate in experimental assays [46,47] After these steps, 72,260 compounds were excluded. Afterwards, the remaining compounds were submitted to conservative binary and continuous QSAR models for prediction of the activity against blood stages of P. falciparum and cytotoxicity against mammalian cells. The final selection of candidate compounds can be summarized as follows: (i) the compounds predicted as active and non-cytotoxic by the binary QSAR models; (ii) compounds with pEC50 ≥6.00 (i.e., EC50 ≤1 μM for P. falciparum) and pCC50 <5.00 (i.e., CC50 >10 μM for mammalian cells) predicted by the continuous QSAR models; (iii) and compounds inside the applicability domain (AD) of the QSAR models. The combination of binary and continuous QSAR models was implemented to increase success rates in virtual screening campaign. In addition, the AD was determined in order to set “reliable” and “unreliable” predictions [48,49]. The predictions were considered reliable when they were within the chemical space used for training the models. Finally, we performed a chemical similarity analysis to select a subset of structurally diverse compounds. At the end of this process, five candidate compounds were selected for biological evaluation (Fig 6).
Fig 5. Virtual screening workflow used for identifying new compounds active against P. falciparum and non-toxic to mammalian cells.
Veber and Lipinski rules were used to prioritize candidate compounds with good oral bioavailability, to ensure that the compound has basic properties of active drugs; colloidal aggregation tool was used to filter out molecules that are known to aggregate in experimental assays; chemical similarity analysis and visual inspection were performed to select a subset of structurally diverse candidate compounds.
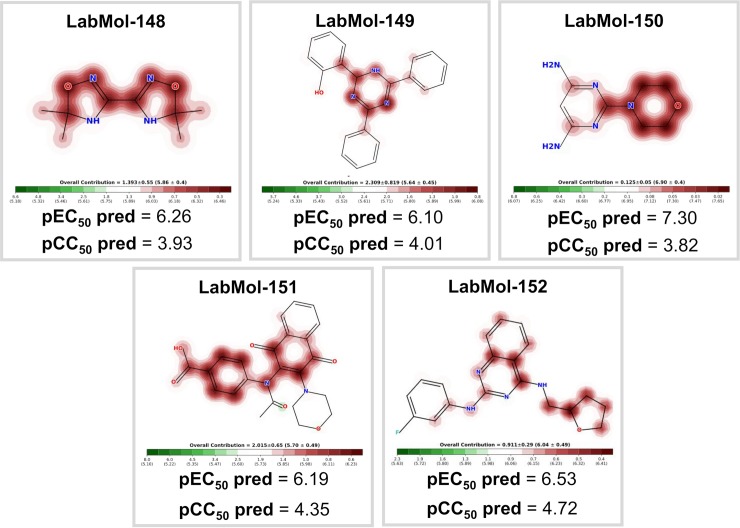
Fig 6. Five computationally-determined candidate compounds prioritized for further experimental evaluation.
Atoms or fragments promoting positive contribution for the antiplasmodial activity are highlighted in red.
Experimental validation
The five candidate compounds were evaluated in vitro against asexual blood stages of P. falciparum sensitive (3D7), and multi-drug-resistant (W2) strains. The EC50 for each compound (Table 3) indicate that two compounds, 2-(4,6-diphenyl-1,2-dihydro-1,3,5-triazin-2-yl)phenol (LabMol-149), 4-{N-[3-(morpholin-4-yl)-1,4-dioxo-1,4-dihydronaphthalen-2-yl]acetamido}benzoic acid (LabMol-151) and N2-(3-fluorophenyl)-N4-[(oxolan-2-yl)methyl]quinazoline-2,4-diamine (LabMol-152), were potent at inhibiting the parasite growth, showing activities in submicromolar and low nanomolar range against both 3D7 and W2 strains. Moreover, the compound LabMol-152 (EC50 = 0.049 μM and 0.078 μM for 3D7 and W2, respectively) showed efficacy in the same range of activity of the reference drugs, chloroquine (EC50 = 0.011 μM) and pyrimethamine (EC50 = 0.037 μM). The candidate compounds were also evaluated for their cytotoxicity against fibroblast-like cell lines derived from monkey kidney (COS-7 cells). With respect to selectivity, LabMol-149 and LabMol-152 showed the most promising results (selectivity index, SI, ranging between 71.4–340.8, Table 3).
Table 3. In vitro evaluation of selected compounds against asexual blood stage of P. falciparum 3D7 and W2 strains, cytotoxicity on mammalian cells (COS7), and selectivity indices.
| Compound | EC50 (Pf3D7) (μM) |
EC50 (PfW2) (μM) |
CC50 (COS-7) (μM) | SIa | SIb |
|---|---|---|---|---|---|
| LabMol-148 | >40 | >40 | >200 | − | − |
| LabMol-149 | 1.450 ± 0.524 | 0.509 ± 0.177 | >100 | >71.4 | >196 |
| LabMol-150 | >40 | >40 | >200 | − | − |
| LabMol-151 | 1.911 ± 0.292 | 1.616 ± 0.321 | 140.8 ± 11.2 | 73.7 | 87.1 |
| LabMol-152 | 0.049 ± 0.029 | 0.078 ± 0.014 | 16.7 ± 10.3 | 340.8 | 214.1 |
| Chloroquine | 0.011 ± 0.001 | 0.173 ± 0.020 | >50 | 4,545 | 289 |
| Pyrimethamine | 0.037 ± 0.007 | 18.240 ± 4.537 | >100 | 2,702 | 5.5 |
EC50: half maximal effective concentration on 3D7 and W2 Plasmodium falciparum strains; CC50: half maximal cytotoxic concentration on COS7 cells; COS7: fibroblast-like cells derived from monkey kidney tissue; SIa: Selectivity index calculated by COS7 CC50/3D7 EC50; and SIb: Selectivity index calculated by COS7 CC50/W2 EC50; The data are expressed as mean ± SD of three independent assays. Dashed SI values means that SI could be calculated because compounds did not show activity even at highest concentrations used in the assay.
Discussion
In this work, we used a deep learning technique to obtain both binary and continuous QSAR models to predict the antiplasmodial activity and cytotoxicity of untested compounds. Models were developed following the best practices of QSAR modeling [14,15], which are fully compliant to Organization for Economic Co-operation and Development (OECD) guidance [50], such as (i) a defined endpoint (biological properties in our case), (ii) an unambiguous algorithm, (iii) a defined applicability domain (AD), (iv) appropriate measures of goodness-of-fit, robustness, and predictivity, and (v) mechanistic interpretation, if possible [50].
Our study follows the most recent tendencies in the usage of deep learning to probe chemical space of drug-like molecules [38,51]. As a result, we have obtained predictive QSAR models, with CCR values ranging between 0.82–0.87 (binary models) and values ranging between 0.70–0.88 (continuous models). These results suggest that deep learning can be efficiently used to rationalize the identification of potent and selective antiplasmodial compounds in early stages of drug discovery. In addition, analysis of chemical space of P. falciparum and cytotoxicity dataset revealed that the compounds active against P. falciparum share the same chemical space of many compounds that are toxic in mouse embryonic fibroblasts.
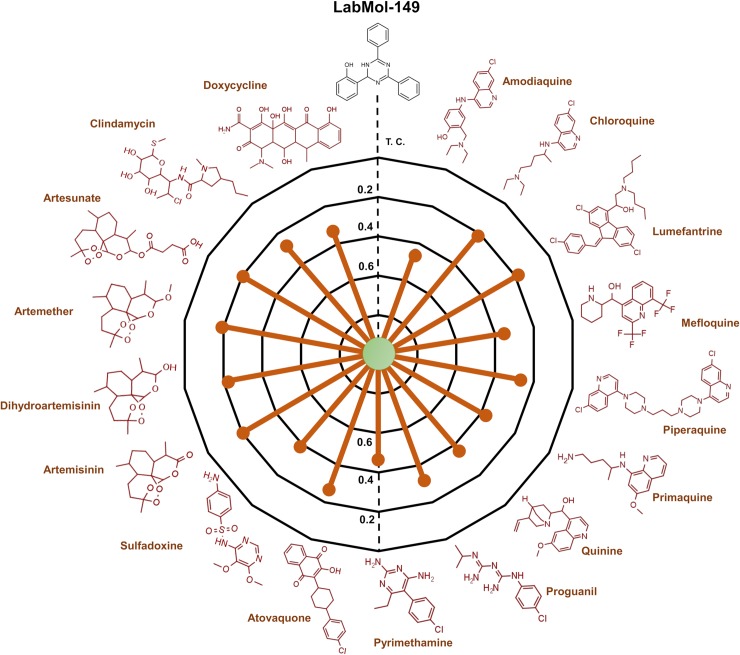
By applying the workflow presented on Fig 5, we were able prioritize five new compounds for further experimental testing in vitro against sensitive (3D7) and multidrug-resistant (W2) strains of P. falciparum. Two compounds (LabMol-149 and LabMol-151) showed activity at submicromolar concentrations against asexual blood stages and low cytotoxicity in mammalian cells. More remarkable, the compound LabMol-152 showed efficacy in the same range of activity as the reference drug pyrimethamine against 3D7 strain (EC50 = 0.049 and 0.037 μM, respectively). Drug resistance in Plasmodium spp. is a complex daunting issue, and there is an opinion that resistant parasite strains will always emerge [12]. Although not fully understood, clinical resistance is probably due to high parasite genetic diversity and the misuse of therapeutics. The compounds tested here present chemical scaffolds dissimilar from current antimalarial drugs, according to the Tanimoto coefficient calculated using MACCS structural keys descriptors (Fig 7, S2 and S3 Figs, Supporting Information). Furthermore, no compound showed cross-resistance with a P. falciparum multidrug-resistant strain, thus indicating new mechanisms of action and potentially representing new weapons in our arsenal of antimalarials. However, parasite resistance to new compounds seems more likely to be a problem by de novo acquisition than pre-existing resistance [52]. Thus, how quickly resistance against our compounds would occur is an interesting question and needs to be better elucidated in future studies.
Fig 7. Radial plot showing the similarities of LabMol-149 to known anti-malarial drugs (red).
The similarity was accessed by the Tanimoto coefficient (Tc) and MACCS structural key fingerprints.
To summarize, the approach developed in this study allowed us to discover three new chemicals belonging to three different structural families (triazines, naphtoquinones, and quinazolines), which are promising starting points for developing of potential next generation antimalarial agents. Moreover, two of these compounds (LabMol-149 and LabMol-152) completely satisfy the guidelines and criteria for discovery of new antimalarial drugs, i.e., activity at low nanomolar concentrations (EC50 <500nM) against sensitive and multiple resistant strains of Plasmodium spp. and SI greater than 10 folds [12,42]. Future directions include structural optimization of potency and selectivity, determination of the stage in the asexual life cycle of P. falciparum where these compounds seem to act, as well as in vivo assays.
Materials and methods
Computational
Datasets
In this study, deep learning algorithms were explored to build binary and continuous QSAR models using two datasets extracted from the ChEMBL database (https://www.ebi.ac.uk/chembl/) [25]. A brief description of the datasets is presented below.
P. falciparum (Target ID: CHEMBL2366922): 1,757 compounds with EC50 data for asexual blood stages of P. falciparum 3D7 strain (chloroquine sensitive). Based on a threshold of 1 μM, it consisted of 1,058 active compounds with EC50 ≤ 1 μM, and 699 inactive compounds (EC50 > 1 μM).
Cytotoxicity (Target ID: CHEMBL614822): 2,061 compounds with CC50 data for mouse embryonic fibroblasts (NIH/3T3 cell line). Based on a threshold of 10 μM, it consisted of 773 toxic compounds with CC50 ≤ 10 μM, and 1,288 nontoxic compounds (CC50 > 10 μM).
Data curation
All chemical structures and correspondent biological information were carefully standardized using Standardizer v.16.9.5.0 (ChemAxon, Budapest, Hungary, http://www.chemaxon.com) according to the protocols proposed by Fourches and colleagues [53–55]. Briefly, explicit hydrogens were added, whereas polymers, salts, metals, organometallic compounds, and mixtures were removed. In addition, specific chemotypes such as aromatic rings and nitro groups were normalized. Furthermore, we performed the analysis and exclusion of duplicates. Different criteria were adopted, as follows:
Binary QSAR models: (i) if duplicates presented discordance in biological activity, both entries would be excluded; and (ii) if the reported outcomes of the duplicates were the same, one entry would be retained in the dataset and the other excluded. This analysis showed high concordance between duplicate records for P. falciparum dataset (92%), and cytotoxicity dataset (100%), revealing the high quality of these datasets. Considering the different size of classes in P. falciparum dataset (789 actives and 581 inactives), and cytotoxicity dataset (635 toxic compounds and 933 nontoxic compounds), the curated datasets were balanced using a linear under-sampling approach (i.e., reducing the size of the majority class) [29]. The under-sampling strategy used here retains most of the representative molecules of the majority class in balanced dataset, ensuring the structural diversity of original chemical space [29]. Initially, the Euclidean distances between each compound in majority class and whole set of minority class are measured using k-Nearest Neighbor (k-NN) algorithm [56]. Then, the samples on majority classes were linearly extracted over the whole set by using k-distances and used to generate balanced datasets. Finally, we obtained two under-sampled datasets with 1,162 compounds (see full P. falciparum dataset in S1 File, Support Information), and 1,270 compounds (see full cytotoxicity dataset in S2 File, Support Information).
Continuous QSAR models: (i) duplicates were inspected visually, (ii) if duplicates presented discordant potencies, both entries would be excluded; and (iii) if the reported potencies were similar, an average of the values was calculated, and one entry would be retained in the dataset. Subsequently, the EC50 (P. falciparum) and CC50 (cytotoxicity) values were converted to negative logarithmic (−log) units, pEC50 and pCC50, respectively. At the end of this process, the P. falciparum dataset had 1,246 compounds (full dataset available on S3 File, Support Information) while the cytotoxicity dataset had 1,144 compounds (full dataset available on S4 File).
Chemical space analysis
Chemical space formed by P. falciparum and cytotoxicity datasets was analyzed by plotting the barycentric coordinates of all the structures encountered in both datasets, which were defined by the 2D RDKit descriptors. Barycentric coordinates correspond to the location of the points of a simplex (a triangle, tetrahedron, etc.) in the space, defined by the vertices [43]. In other words, the location of a chemical in a multidimensional space of 2D RDKit descriptors has been scaled to two dimensions. In this case, a simplex is defined by all the RDKit descriptors of a particular chemical substance. Barycentric coordinates were determined using Methods of Data Analysis module of HiT QSAR software [57]. In addition, both datasets were independently clustered using the Sequential Agglomerative Hierarchical Non-overlapping method [58] implemented in Python v.3.6. Briefly, a dendrogram of the parent-child relationships between clusters and a heatmap of the proximity matrix colored according to the pairwise chemical similarity between compounds. To better visualize the clusters, the distance matrix of the compounds from the two datasets were independently calculated and the compounds were clustered.
Molecular fingerprints
Morgan and FeatMorgan fingerprints were calculated in the open-source cheminformatics software RDKit (http://www.rdkit.org, [59]) executed on Python v.3.6 (https://www.python.org). Both fingerprints were generated with radius 2−4 and bit vector of 2,048 bits. Morgan is a type circular fingerprint built by applying the Morgan algorithm to a set of user-supplied 2D chemical structures [60,61]. The fingerprint generation process systematically records the neighborhood of each non-hydrogen atom into multiple circular layers up to a stablished radius. The radius is a dominant parameter which controls the number and the maximum size of considered atom neighborhoods, thus it controls the complexity of fragment representation. These atom-centered substructural features are interpreted as indexes of bits in a huge virtual bit string. Each position in this bit string accounts for the presence or absence of a specific fragment feature [60,61]. The Morgan captures highly specific atomic information enabling the representation of a large set of precisely defined structural features [60]. Additionally, invariants of Morgan called as FeatMorgan fingerprints can also be calculated by including functional features (i.e., hydrogen-bond donor and acceptors, aromatic, halogen, basic and acid groups) [62].
Deep learning
The binary and continuous QSAR models were developed using Keras (https://keras.io/), a deep learning library, and Tensorflow (www.tensorflow.org), a GPU training and CPU for prediction), as backend. Binary models were trained using previously established activity/toxicity thresholds, while continuous models were developed using pEC50 (P. falciparum) and pCC50 (cytotoxicity). The following parameters of the deep learning method were optimized prior to model training: layer type (dense), hidden layers (8), activation function (ReLU), output layer function (sigmoid), model optimizer (Adam). The “binary cross-entropy” and “mean squared error” were used as loss functions in binary and continuous QSAR modeling, respectively. The “accuracy” and “mean absolute error” were used as parameters to judge the performance of binary and continuous models, respectively. The following hyperparameters were used for further deep learning training: epochs (5, 10, 50, 100), and batch size (10, 20, 40, 60, 80, 100).
5-fold external cross-validation (5FECV)
According to the best practices of QSAR modeling [15], we chose five-fold external cross-validation for the estimation of predictivity of developed models. The procedure can be described as follows: the entire dataset of compounds was randomly divided into five subsets of equal size; then one of these subsets (20% of all compounds) is set aside as an external validation set and the remaining four sets together form the modeling set (80% of the full set). This procedure was repeated five times, allowing each of the five subsets to be used as an external validation set. Models were built using the modeling set while the compounds in momentary external set (fold) were employed to evaluation of predictive performance.
Performance of QSAR models
The predictive performance of binary QSAR models was evaluated using sensitivity (SE), specificity (SP), correct classification rate (CCR), positive predictive value (PPV), and negative predictive value (NPV). These metrics were calculated as follows:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
Here, TP and TN represent the number of true positives and true negatives, respectively, while FP and FN represent the number of false positives and false negatives, respectively.
The predictive performance of continuous QSAR models was evaluated using correlation coefficient (R2), root mean square error of cross validation (RMSECV), mean absolute error (MAE), and predictive squared correlation coefficient for the test set () [63]. These metrics were calculated as follows:
| (6) |
| (7) |
| (8) |
| (9) |
In the above equations, Yobs represents experimental pEC50 or pCC50 value, Ypred represents the predicted pEC50 or pCC50 value, ntrain and ntest are the number of compounds in training and test set, respectively, and is the average of experimental values of the training set.
Applicability domain
The AD was estimated based on the Euclidean distances among the training set of each QSAR model generated in the 5-fold external cross-validation procedure. The distance of a test set compound to its nearest neighbor in the training set was compared to the predefined AD threshold level. The prediction was considered to be less reliable if the distance was greater than the threshold level. In our study, the AD was defined as a distance threshold (DT) between a compound under prediction and the closest nearest neighbors in training set. The following equation was used for calculation of distance threshold [64]:
| (10) |
In which ӯ is the average Euclidean distance of the k nearest neighbors within the modeling set, σ is the standard deviation of these Euclidean distances, and Z is an arbitrary parameter to control the significance level. We set the default value of this parameter Z at 0.5. If the compound distance exceeded the threshold, the prediction was considered to be less trustworthy [65].
Mechanistic interpretation
Contribution maps were generated from continuous QSAR models to visualize the atomic and fragment contributions for antimalarial activity and cytotoxicity. Here, the "weight" of an atom was considered as predicted-potency difference obtained when the bits in the fingerprint corresponding to the atom are removed. Then, the normalized weights were used to color the atoms in a topography-like map in which green indicating a negative difference (i.e., potency increases when the bits are removed), and red indicating a positive difference in biological property.
Virtual screening (VS)
Developed QSAR models were used for VS of EXPRESS-Pick collection of ChemBridge Corporation (http://www.chembridge.com/) aiming to identify new potential antiplasmodial compounds, which could be potentially selective against the parasite (i.e. non-toxic to mammalian cells). Prior to screening, the database was filtered using a aggregator advisor tool to identify molecules that are known-to aggregate in experimental assays [46,47]. Subsequently, Veber [44] and Lipinski’s rules [45] were employed in screening to prioritize drug-like compounds. Then, the remaining compounds had their antiplasmodial activity and cytotoxicity against mammalian cells predicted by binary and continuous QSAR models. In addition, the structural diversity of candidate compounds was investigated using pairwise Tanimoto coefficients between compounds. Finally, the selected candidate compounds were purchased and submitted to in vitro experimental evaluation.
Experimental
Materials
Candidate compounds were purchased from ChemBridge (San Diego-CA, USA) and resuspended in 100% DMSO. It is important to mention that all compounds had a minimum purity of 95%. The DMEM and RPMI 1640 media were purchased from Vitrocell Embriolife (Campinas-SP, Brazil). All other reagents were purchased from Sigma-Aldrich (St. Louis-MO, USA).
Parasite culture
The 3D7 and W2 strains were cultured in RPMI 1640 medium supplemented with 0.05 mg/mL gentamycin, 38.4 mM HEPES, 0.2% sodium bicarbonate, and 10% O+ human serum, as previously described in standardized protocol [66]. Then, erythrocytes were added to the culture to obtain a 5% of hematocrit, and incubated at 37°C under 5% CO2 atmosphere, with daily exchange of medium. The parasitemia was monitored daily in smears, stained with Giemsa. Synchronic cultures in ring stage were obtained by two consecutive treatments, at 48h intervals with a 5% solution of D-sorbitol [67].
Antiplasmodial assay
Parasites synchronized at the ring stage, with 0.5% parasitemia and 2% hematocrit, were distributed in the wells of a 96-well plate. The compounds were tested in triplicates using 12-point dilution series (0.019 μM– 40 μM) over 72h. Chloroquine and pyrimethamine were used as positive controls. The in vitro susceptibility of parasite to tested drugs was measured by SYBR Green according to Hartwig and colleagues [68]. Briefly, 100 μL of lysis buffer (20 mM Tris, 5 mM EDTA, 0,008% wt/vol saponin, 0,08% vol/vol Triton X-100 and 0.4 μL/mL of SYBR Green) were added in each well of a new black 96-well plate and 100 μL of parasite culture incubated with drugs were transferred. After homogenization, the plates were incubated for 1h in the dark. Fluorescence was measured at 490 nm excitation and 540 nm emission (CLARIOstar, Labtech BMG).
Cytotoxicity assay
Cytotoxicity assays used fibroblast-like cell lines derived from monkey kidney tissue (COS7 cells), grown in DMEM medium supplemented with 10% fetal bovine serum and 0.05 mg/mL gentamicin in atmosphere containing 5% CO2 at 37°C. Drug cytotoxicity in COS-7 cells was determined in duplicate, using 12 dilution series (0.097 μM– 200 μM). After an incubation period (72 hours), cell viability analysis was performed via the MMT reduction method (3- [4,5- dimethyl-thiazol-2-yl] -2,5-diphenyltetrazolium chloride) [69]. The optical density was determined at 570 nm (CLARIOstar, Labtech BMG) and the 50% cytotoxicity concentrations (CC50) was expressed as the percent viability relative to the control.
Statistics
The EC50 and CC50 values were calculated by plotting the Log doses vs. inhibition (expressed as a percentage relative to the control) in GraphPad Prism v.6 (GraphPad Software, La Jolla California USA, www.graphpad.com).
Supporting information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Fragments contributing for the cytotoxicity are colored in red, atoms or fragments decreasing the cytotoxicity are highlighted in green, and no highlighting means no influence to cytotoxicity.
(TIF)
The similarity was calculated using Tanimoto coefficient (Tc) and MACCS structural keys descriptors.
(TIF)
The similarity was calculated using Tanimoto coefficient (Tc) and MACCS structural keys descriptors.
(TIF)
Acknowledgments
We are thankful for Kirsten Overdahl for her kind help with editing the manuscript. We gratefully acknowledge the support of NVIDIA Corporation with the donation of the Titan Xp GPU used for this research and ChemAxon for providing us with academic license for their software.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors would like to thank Brazilian funding agencies, CNPq, FAPEG, FAPESP, and CAPES for financial support and fellowships. FTMC, GCC, and CHA were supported by FAPESP (grants #2012/16525-2, #2015/20774-6, 2017/18611-7 and #2017/02353-9). ENM appreciate financial support of NIH (grant 1U01CA207160). CHA and FTMC are CNPq research fellows. CHA also thanks the “L'Oréal-UNESCO-ABC Para Mulheres na Ciência” and “L’Oréal-UNESCO International Rising Talents” for the awards and fellowships received, which partially funded this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization, WHO, Phillips MA, Burrows JN, Manyando C, van Huijsduijnen RH, et al. Malaria. In: Nature Reviews Disease Primers [Internet]. Aug 2017 [cited 3 Nov 2017] pp. 1–24. 10.1038/nrdp.2017.50 [DOI] [PubMed]
- 2.Ashley EA, Pyae Phyo A, Woodrow CJ. Malaria. Lancet. 2018;391: 1608–1621. 10.1016/S0140-6736(18)30324-6 [DOI] [PubMed] [Google Scholar]
- 3.Phillips MA, Burrows JN, Manyando C, van Huijsduijnen RH, Van Voorhis WC, Wells TNC. Malaria. Nat Rev Dis Prim. 2017;3: 17050 10.1038/nrdp.2017.50 [DOI] [PubMed] [Google Scholar]
- 4.Wassmer SC, Grau GER. Severe malaria: what’s new on the pathogenesis front? Int J Parasitol. 2017;47: 145–152. 10.1016/j.ijpara.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wellems TE, Plowe C V. Chloroquine‐Resistant Malaria. J Infect Dis. 2001;184: 770–776. 10.1086/322858 [DOI] [PubMed] [Google Scholar]
- 6.Srivastava IK, Morrisey JM, Darrouzet E, Daldal F, Vaidya AB. Resistance mutations reveal the atovaquone-binding domain of cytochrome b in malaria parasites. Mol Microbiol. 1999;33: 704–711. 10.1046/j.1365-2958.1999.01515.x [DOI] [PubMed] [Google Scholar]
- 7.Wu Y, Kirkman LA, Wellems TE. Transformation of Plasmodium falciparum malaria parasites by homologous integration of plasmids that confer resistance to pyrimethamine. Proc Natl Acad Sci U S A. 1996;93: 1130–1134. 10.1073/pnas.93.3.1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Triglia T, Wang P, Sims PFG, Hyde JE, Cowman AF. Allelic exchange at the endogenous genomic locus in Plasmodium falciparum proves the role of dihydropteroate synthase in sulfadoxine-resistant malaria. EMBO J. 1998;17: 3807–3815. 10.1093/emboj/17.14.3807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers WO, Sem R, Tero T, Chim P, Lim P, Muth S, et al. Failure of artesunate-mefloquine combination therapy for uncomplicated Plasmodium falciparum malaria in southern Cambodia. Malar J. 2009;8: 10 10.1186/1475-2875-8-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of Artemisinin Resistance in Plasmodium falciparum Malaria. N Engl J Med. 2014;371: 411–423. 10.1056/NEJMoa1314981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witkowski B, Khim N, Chim P, Kim S, Ke S, Kloeung N, et al. Reduced Artemisinin Susceptibility of Plasmodium falciparum Ring Stages in Western Cambodia. Antimicrob Agents Chemother. 2013;57: 914–923. 10.1128/AAC.01868-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burrows JN, Duparc S, Gutteridge WE, Hooft van Huijsduijnen R, Kaszubska W, Macintyre F, et al. New developments in anti-malarial target candidate and product profiles. Malar J. 2017;16: 26 10.1186/s12936-016-1675-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider G. Automating drug discovery. Nat Rev Drug Discov. Nature Publishing Group; 2017;17: 97–113. 10.1038/nrd.2017.232 [DOI] [PubMed] [Google Scholar]
- 14.Tropsha A. Best Practices for QSAR Model Development, Validation, and Exploitation. Mol Inform. 2010;29: 476–488. 10.1002/minf.201000061 [DOI] [PubMed] [Google Scholar]
- 15.Cherkasov A, Muratov EN, Fourches D, Varnek A, Baskin II, Cronin M, et al. QSAR modeling: where have you been? Where are you going to? J Med Chem. 2014;57: 4977–5010. 10.1021/jm4004285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansch C, Fujita T. p -σ-π Analysis. A Method for the Correlation of Biological Activity and Chemical Structure. J Am Chem Soc. 1964;86: 1616–1626. 10.1021/ja01062a035 [DOI] [Google Scholar]
- 17.Cramer RD, Patterson DE, Bunce JD. Comparative Molecular Field Analysis (CoMFA). 1. Effect of Shape on Binding of Steroids to Carrier Proteins. J Am Chem Soc. 1988;110: 5959–5967. 10.1021/ja00226a005 [DOI] [PubMed] [Google Scholar]
- 18.Kubinyi H, Kehrhahn OH. Quantitative structure-activity relationships. VI. Non-linear dependence of biological activity on hydrophobic character: calculation procedures for bilinear model. Arzneimittelforschung. 1978;28: 598–601. [PubMed] [Google Scholar]
- 19.Ajay Walters WP, Murcko MA. Can We Learn To Distinguish between “Drug-like” and “Nondrug-like” Molecules? J Med Chem. 1998;41: 3314–3324. 10.1021/jm970666c [DOI] [PubMed] [Google Scholar]
- 20.Burden FR, Winkler DA. Robust QSAR models using bayesian regularized neural networks. J Med Chem. 1999;42: 3183–3187. 10.1021/jm980697n [DOI] [PubMed] [Google Scholar]
- 21.Du H, Wang J, Hu Z, Yao X, Zhang X. Prediction of fungicidal activities of rice blast disease based on least-squares support vector machines and project pursuit regression. J Agric Food Chem. 2008;56: 10785–10792. 10.1021/jf8022194 [DOI] [PubMed] [Google Scholar]
- 22.Svetnik V, Liaw A, Tong C, Christopher Culberson J, Sheridan RP, Feuston BP. Random Forest: A Classification and Regression Tool for Compound Classification and QSAR Modeling. J Chem Inf Comput Sci. 2003;43: 1947–1958. 10.1021/ci034160g [DOI] [PubMed] [Google Scholar]
- 23.Alves VM, Muratov E, Fourches D, Strickland J, Kleinstreuer N, Andrade CH, et al. Predicting chemically-induced skin reactions. Part I: QSAR models of skin sensitization and their application to identify potentially hazardous compounds. Toxicol Appl Pharmacol. 2015;284: 262–272. 10.1016/j.taap.2014.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alves VM, Muratov E, Fourches D, Strickland J, Kleinstreuer N, Andrade CH, et al. Predicting chemically-induced skin reactions. Part II: QSAR models of skin permeability and the relationships between skin permeability and skin sensitization. Toxicol Appl Pharmacol. Elsevier Inc.; 2015;284: 273–280. 10.1016/j.taap.2014.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, et al. ChEMBL: a large-scale bioactivity database for drug discovery. Nucleic Acids Res. 2012;40: D1100–D1107. 10.1093/nar/gkr777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Xiao J, Suzek TO, Zhang J, Wang J, Zhou Z, et al. PubChem’s BioAssay Database. Nucleic Acids Res. 2012;40: D400–D412. 10.1093/nar/gkr1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braga RC, Alves VM, Silva AC, Nascimento MN, Silva FC, Liao LM, et al. Virtual screening strategies in medicinal chemistry: the state of the art and current challenges. Curr Top Med Chem. 2014;14: 1899–1912. 10.2174/1568026614666140929120749 [DOI] [PubMed] [Google Scholar]
- 28.Braga RC, Alves VM, Silva MFB, Muratov E, Fourches D, Tropsha A, et al. Tuning HERG out: antitarget QSAR models for drug development. Curr Top Med Chem. 2014;14: 1399–1415. 10.2174/1568026614666140506124442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neves BJ, Dantas RF, Senger MR, Melo-Filho CC, Valente WCG, de Almeida ACM, et al. Discovery of New Anti-Schistosomal Hits by Integration of QSAR-Based Virtual Screening and High Content Screening. J Med Chem. 2016;59: 7075–7088. 10.1021/acs.jmedchem.5b02038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melo-Filho CCCC, Dantas RF, Braga RCRCRC, Neves BJBJBJ, Senger MR, Valente WCGWCG, et al. QSAR-Driven Discovery of Novel Chemical Scaffolds Active against Schistosoma mansoni. J Chem Inf Model. 2016;56: 1357–1372. 10.1021/acs.jcim.6b00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lima MNN, Melo-Filho CC, Cassiano GC, Neves BJ, Alves VM, Braga RC, et al. QSAR-Driven Design and Discovery of Novel Compounds With Antiplasmodial and Transmission Blocking Activities. Front Pharmacol. 2018;9: 146 10.3389/fphar.2018.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomes MN, Braga RC, Grzelak EM, Neves BJ, Muratov E, Ma R, et al. QSAR-driven design, synthesis and discovery of potent chalcone derivatives with antitubercular activity. Eur J Med Chem. 2017;137: 126–138. 10.1016/j.ejmech.2017.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu H, Zhang J, Kim MT, Boison A, Sedykh A, Moran K. Big Data in Chemical Toxicity Research: The Use of High-Throughput Screening Assays To Identify Potential Toxicants. Chem Res Toxicol. 2014;27: 1643–1651. 10.1021/tx500145h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartung T. Making big sense from big data in toxicology by read-across. ALTEX. 2016;33: 83–93. 10.14573/altex.1603091 [DOI] [PubMed] [Google Scholar]
- 35.Ramsundar B, Liu B, Wu Z, Verras A, Tudor M, Sheridan RP, et al. Is Multitask Deep Learning Practical for Pharma? J Chem Inf Model. 2017;57: 2068–2076. 10.1021/acs.jcim.7b00146 [DOI] [PubMed] [Google Scholar]
- 36.Ekins S. The Next Era: Deep Learning in Pharmaceutical Research. Pharm Res. Pharmaceutical Research; 2016; 2594–2603. 10.1007/s11095-016-2029-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goh GB, Hodas NO, Vishnu A. Deep learning for computational chemistry. J Comput Chem. 2017;38: 1291–1307. 10.1002/jcc.24764 [DOI] [PubMed] [Google Scholar]
- 38.Ma J, Sheridan RP, Liaw A, Dahl GE, Svetnik V. Deep Neural Nets as a Method for Quantitative Structure–Activity Relationships. J Chem Inf Model. 2015;55: 263–274. 10.1021/ci500747n [DOI] [PubMed] [Google Scholar]
- 39.Popova M, Isayev O, Tropsha A. Deep reinforcement learning for de novo drug design. Sci Adv. 2018;4: eaap7885 10.1126/sciadv.aap7885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gómez-Bombarelli R, Wei JN, Duvenaud D, Hernández-Lobato JM, Sánchez-Lengeling B, Sheberla D, et al. Automatic Chemical Design Using a Data-Driven Continuous Representation of Molecules. ACS Cent Sci. 2018;4: 268–276. 10.1021/acscentsci.7b00572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bjerrum E, Sattarov B. Improving Chemical Autoencoder Latent Space and Molecular De Novo Generation Diversity with Heteroencoders. Biomolecules. 2018;8: 131 10.3390/biom8040131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katsuno K, Burrows JN, Duncan K, van Huijsduijnen RH, Kaneko T, Kita K, et al. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat Rev Drug Discov. 2015;14: 751–758. 10.1038/nrd4683 [DOI] [PubMed] [Google Scholar]
- 43.Vityuk N, Voskresenskaja E, Kuz’min V. The Synergism of Methods Barycentric Coordinates and Trend-vector for Solution ―Structure-Property Tasks. Pattern Recognit Image Anal. 1999;3: 521–528. [Google Scholar]
- 44.Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45: 2615–2623. 10.1021/jm020017n [DOI] [PubMed] [Google Scholar]
- 45.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23: 3–25. 10.1016/S0169-409X(96)00423-1 [DOI] [PubMed] [Google Scholar]
- 46.Irwin JJ, Duan D, Torosyan H, Doak AK, Ziebart KT, Sterling T, et al. An Aggregation Advisor for Ligand Discovery. J Med Chem. 2015;58: 7076–87. 10.1021/acs.jmedchem.5b01105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Owen SC, Doak AK, Wassam P, Shoichet MS, Shoichet BK. Colloidal Aggregation Affects the Efficacy of Anticancer Drugs in Cell Culture. ACS Chem Biol. 2012;7: 1429–1435. 10.1021/cb300189b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gadaleta D, Mangiatordi GF, Catto M, Carotti A, Nicolotti O. Applicability Domain for QSAR Models. Int J Quant Struct Relationships. 2016;1: 45–63. 10.4018/IJQSPR.2016010102 [DOI] [Google Scholar]
- 49.Netzeva TI, Worth A, Aldenberg T, Benigni R, Cronin MTD, Gramatica P, et al. Current status of methods for defining the applicability domain of (quantitative) structure-activity relationships. The report and recommendations of ECVAM Workshop 52. Altern Lab Anim. 2005;33: 155–73. 10.1177/026119290503300209 [DOI] [PubMed] [Google Scholar]
- 50.OECD principles for the validation, for regulatory purposes, of (Quantitative) Structure-Activity Relationship models. In: Organisation for Economic Cooperation and Development [Internet]. 2004 [cited 1 Oct 2015] pp. 1–2. Available: http://www.oecd.org/chemicalsafety/risk-assessment/37849783.pdf
- 51.Ghasemi F, Mehridehnavi A, Pérez-Garrido A, Pérez-Sánchez H. Neural network and deep-learning algorithms used in QSAR studies: merits and drawbacks. Drug Discov Today. 2018;23: 1784–1790. 10.1016/j.drudis.2018.06.016 [DOI] [PubMed] [Google Scholar]
- 52.Corey VC, Lukens AK, Istvan ES, Lee MCS, Franco V, Magistrado P, et al. A broad analysis of resistance development in the malaria parasite. Nat Commun. 2016;7: 11901 10.1038/ncomms11901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fourches D, Muratov E, Tropsha A. Trust, but verify: on the importance of chemical structure curation in cheminformatics and QSAR modeling research. J Chem Inf Model. 2010;50: 1189–204. 10.1021/ci100176x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fourches D, Muratov E, Tropsha A. Curation of chemogenomics data. Nat Chem Biol. Nature Publishing Group; 2015;11: 535–535. 10.1038/nchembio.1881 [DOI] [PubMed] [Google Scholar]
- 55.Fourches D, Muratov E, Tropsha A. Trust, but Verify II: A Practical Guide to Chemogenomics Data Curation. J Chem Inf Model. 2016;56: 1243–1252. 10.1021/acs.jcim.6b00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Altman N. An introduction to kernel and nearest-neighbor nonparametric regression. Am Stat. 1992;46: 175–185. 10.1080/00031305.1992.10475879 [DOI] [Google Scholar]
- 57.Kuz’min VE, Artemenko AG, Muratov EN. Hierarchical QSAR technology based on the Simplex representation of molecular structure. J Comput Aided Mol Des. ELSEVIER SCIENCE BV; 2008;22: 403–21. 10.1007/s10822-008-9179-6 [DOI] [PubMed] [Google Scholar]
- 58.Varnek A, Fourches D, Horvath D, Klimchuk O, Gaudin C, Vayer P, et al. ISIDA—Platform for Virtual Screening Based on Fragment and Pharmacophoric Descriptors. Curr Comput Aided-Drug Des. 2008;4: 191–198. 10.2174/157340908785747465 [DOI] [Google Scholar]
- 59.Riniker S, Landrum G a. Open-source platform to benchmark fingerprints for ligand-based virtual screening. J Cheminform. 2013;5: 26 10.1186/1758-2946-5-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rogers D, Hahn M. Extended-Connectivity Fingerprints. J Chem Inf Model. 2010;50: 742–754. 10.1021/ci100050t [DOI] [PubMed] [Google Scholar]
- 61.Morgan HL. The Generation of a Unique Machine Description for Chemical Structures-A Technique Developed at Chemical Abstracts Service. J Chem Doc. 1965;5: 107–113. 10.1021/c160017a018 [DOI] [Google Scholar]
- 62.Gobbi A, Poppinger D. Genetic optimization of combinatorial libraries. Biotechnol Bioeng. 1998;61: 47–54. [DOI] [PubMed] [Google Scholar]
- 63.Consonni V, Ballabio D, Todeschini R. Comments on the Definition of the Q 2 Parameter for QSAR Validation. J Chem Inf Model. 2009;49: 1669–1678. 10.1021/ci900115y [DOI] [PubMed] [Google Scholar]
- 64.Golbraikh A, Shen M, Xiao Z, Xiao Y-D, Lee K-H, Tropsha A. Rational selection of training and test sets for the development of validated QSAR models. J Comput Aided Mol Des. 2003;17: 241–253. 10.1023/a:1025386326946 [DOI] [PubMed] [Google Scholar]
- 65.Tropsha A, Golbraikh A. P redictive QSAR Modeling Workflow, Model Applicability Domains, and Virtual Screening. Curr Pharm Des. 2007;13: 3494–3504. 10.2174/138161207782794257 [DOI] [PubMed] [Google Scholar]
- 66.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193: 673–5. 10.1126/science.781840 [DOI] [PubMed] [Google Scholar]
- 67.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum Erythrocytic Stages in Culture. J Parasitol. 1979;65: 418 10.2307/3280287 [DOI] [PubMed] [Google Scholar]
- 68.Hartwig CL, Ahmed AOA, Cooper RA, Stedman TT. SYBR Green I®-Based Parasite Growth Inhibition Assay for Measurement of Antimalarial Drug Susceptibility in Plasmodium falciparum In: Moll K, Kaneko A, Scherf A, Wahlgren M, editors. Methods in Malaria Research. 6th ed Glasgow: EVIMalaR; 2013. pp. 122–129. [Google Scholar]
- 69.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65: 55–63. 10.1016/0022-1759(83)90303-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Fragments contributing for the cytotoxicity are colored in red, atoms or fragments decreasing the cytotoxicity are highlighted in green, and no highlighting means no influence to cytotoxicity.
(TIF)
The similarity was calculated using Tanimoto coefficient (Tc) and MACCS structural keys descriptors.
(TIF)
The similarity was calculated using Tanimoto coefficient (Tc) and MACCS structural keys descriptors.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.