Abstract
Background:
Abdominal pain is one of the major symptoms of inflammatory Bowel Disease (IBD). The inflammatory mediators released by colon inflammation are known to sensitize the afferent neurons, which is one of the contributors to abdominal pain. However, not all IBD patients have abdominal pain, and some patients report abdominal pain during remission, suggesting contributions of other pathological factors to abdominal pain in IBD. Epidemiological studies found early-life gastrointestinal infections a risk factor for IBD symptoms and adult-life gastrointestinal infections may trigger the onset of IBD. We investigated the hypothesis that neonatal colon immune challenge followed by an adult colon immune challenge upregulates spinal cord BDNF that aggravates visceral sensitivity over and above that induced by adult colon immune challenge alone.
Methods:
We induced neonatal and adult colon immune challenges by intraluminal administration of trinitrobenzene sulfonic acid to the rat colon.
Results:
We found that neonatal immune challenge triggers epigenetic programming that upregulates tyrosine hydroxylase in the locus ceruleus when these rats are subjected to an adult colon immune challenge. The upregulation of locus ceruleus tyrosine hydroxylase, upregulates norepinephrine in the cerebrospinal fluid that acts on adrenergic receptors to enhance pCREB binding to the cAMP response element, which recruits histone acetylene transferase (HAT) to the BDNF gene to enhance its transcription resulting in aggravated visceromotor response to colorectal distension. HAT and adrenergic receptor antagonists block the aggravation of visceral sensitivity.
Conclusion:
HAT and adrenergic receptor inhibitors may serve as alternates to opioids and NSAIDS in suppressing abdominal pain in IBD.
Keywords: visceral sensitivity, Sympathetic activity, IBD abdominal pain, BDNF
Introduction:
Abdominal pain/cramping is one of the major presenting symptoms of inflammatory bowel disease (IBD) patients experiencing onset or relapse of the disease (1–5). Abdominal pain or fear of it is a significant burden in these patients that diminishes their quality of life. (5, 6). Visceral sensitivity to rectal distension, one of the factors contributing to the sensation of visceral pain, is increased in IBD patients (7, 8). Visceral hypersensitivity (VHS) in response to colon inflammation is thought to be caused by the actions of multiple inflammatory mediators acting on afferent neurons (9–14). However, not all IBD patients with active disease present with abdominal pain (1, 15). On the other hand, it is not uncommon for some IBD patients to continue to have the symptom of abdominal pain during clinical and endoscopic remission (15), which suggests that pathologic factors, such as sensitization of the spinal cord neurons and altered processing of the afferent signals in the CNS, may also contribute to abdominal pain in IBD patients independent of colon inflammation.
Little is known about potential contribution of spinal cord neurons in inducing visceral hypersensitivity in IBD patients. However, epidemiological studies suggest that exposure to postnatal and childhood infections are risk factors for the onset of IBD in later years (16–20), and adult immune challenges by infectious gastroenteritis may trigger the onset of IBD, exacerbate the disease activity or relapse IBD during remission (21–24). These epidemiological findings have not been validated, mainly because of lack of availability of human visceral tissues and ethical and safety considerations that preclude interventional experiments in humans. Taking the epidemiological findings of early-life immune challenges as risk factors and adult-life immune challenges as triggers of IBD, we hypothesized that neonatal immune challenge triggers epigenetic programming that targets vulnerable genes in the CNS and the spinal cord to upregulate nociceptive proteins, such as neurotrophins, in the dorsal root ganglia and the spinal cord to aggravate VHS over and above that caused by colon inflammation alone.
We found that epigenetic programming triggered by neonatal colon immune challenge upregulates tyrosine hydroxylase in the locus ceruleus; the resulting increase of the central norepinephrine epigenetically upregulates the transcription of brain-derived neurotrophic factor (BDNF) in the spinal cord and the dorsal root ganglia to aggravate visceral sensitivity to colorectal distension, when the neonatal immune challenged rats are exposed to an adult colon immune challenge.
Methods:
Animals:
All experimentation utilized male Sprague-Dawley rats. The Institutional Animal Care and Use Committee at the University of Texas Medical Branch at Galveston approved all procedures performed in this study. We did not include female rats due to lack of clear evidence for female bias in abdominal pain in IBD.
Neonatal colon immune challenge:
Ten-days old neonatal rats received intraluminal colon infusion of trinitrobenzene sulfonic (TNBS) acid (130 mg/kg, dissolved in 0.2 ml saline containing 10% ethanol) to trigger immune challenge. The leakage of TNBS was prevented by holding the animals in trendelenburg position and holding the anus closed for one minute. The control rats received colonic infusion of saline only.
Adult colonic inflammation:
Eight to twelve weeks-old rats received intraluminal colonic infusion of TNBS (68 mg/kg, dissolved in 0.25 ml of 40% ethanol in water). These animals received inhaled isolflourane anesthesia to receive TNBS infusion with anus held closed for 5 minutes. All experiments were performed 7-days after the infusion of TNBS.
Spinal cord tissues:
The spinal cord was expelled using hydrostatic pressure and the attached roots were removed. The spinal cord was cut at the center of the lumbar enlargement and segments caudal to this cut to the end of the cord constituted lumba-sacral spinal cord.
Western Blot:
Western blot was performed as previously described (25). Antibodies were used as follows: primary BDNF antibody (Santa Cruz Biotechnologies, Santa Cruz, CA) at 1/200 dilution, β-actin antibody (Sigma Aldrich, St Louis, MO) at 1/5000 dilution, beta-III tubulin antibody (Promega, Madison, WI) at 1/1000. Secondary antibodies utilized were as follows: donkey anti-rabbit alexa fluor 680 (Invitrogen, Carlsbad, CA) at 1/5000 dilution; goat anti-mouse IRDye 800 (Rockland, Pottstown, PA) at 1/5000 dilution in PBS. Li-cor Odyssey system (Li-Cor, Lincoln, NE) was utilized to acquire images and measure band intensity.
Real-time RT-PCR:
Total RNA was extracted by using RNeasy Mini Kit (QIAGEN, Valencia, CA). One microgram of total RNA was reverse-transcribed using SuperScript™ III First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA). BDNF and tyrosine hydroxylase (TH) mRNA levels were measured by TAQMAN RT-PCR using a StepOnePlus thermal cycler with 18S as the normalizer and Applied Biosystems primer/probe set Rn02531967_s1 directed against the translated exon IX of Bdnf and Rn00562500_m1 for TH. Fold-change relative to control was calculated using the ΔΔCt method (Applied Biosystems, Foster City, CA).
ChIP Assays:
ChIP assays utilized the ChIP-IT Express Enzymatic kit from Active Motif, (Carlsbad, CA). Antibodies used were as follows: pCREB (17–10131, Millipore, Billerica, MA); CBP (sc-369, Santa Cruz Biotechnologies, Santa Cruz, CA); RNA pol II (102660), Histone 3K9 (39917) and Histone 4K12 (39927) from Active Motif.
Visceromotor response to colorectal distention:
Bipolar electrodes were sutured into the external oblique abdominal wall muscles of adult rats under inhaled 2% isoflurane anesthesia. Electrode wires were routed subcutaneously and externalized in the subscapular region. Following at least one week of recovery from surgery, a 5 cm long balloon attached to a tygon tube was placed into the distal colon under 2% isoflurane anesthesia. The animals were confined in Lucite cubicles and allowed to adapt for at least 30 minutes prior to pneumatic colonic distention for 20 seconds followed by 2 minutes of rest at the following constant pressures: 20, 30, 40, 50, 60, and 80 mm Hg performed in duplicate, as previously described (25).
Electromyographic (EMG) activity was detected from the surgically implanted abdominal wall electrodes using Biopac MP100A and EMG100C amplifier (Biopac Systems, Inc, Santa Barbara, CA). The EMG signal was amplified, filtered at 300 Hz and digitized. The area under the curve (AUC) for the EMG signal during each 20 seconds of distention was calculated using Acknowledge software (Biopac Systems Inc.). The net value for each distension period was calculated by subtracting the baseline value derived from the average AUC for 20 seconds before and 20 seconds after the distention period (25). This AUC (VxS) was plotted as a function of distention pressure. The area under this curve was calculated for each rat and used for statistical analyses.
Intrathecal infusions:
Thirty-two gauge catheters were inserted through the atlanto-occipital membrane and extended to LS spinal cord segment. The location of each catheter was confirmed following euthanasia. Rats received either BDNF antagonist trkB-Fc (R&D Systems, Minneapolis, MN), 5 μg in 10 μl sterile saline or saline twice/day for seven days following adult TNBS application. Propranolol+phentolamine (50 μg each in 10 μl sterile saline) were administered in each rat daily for seven days following adult TNBS application. In separate groups of adult rats, peripheral propranolol+phentolamine cocktail was administered i.p. at 2 mg/kg each in saline once daily for seven days starting with the day of TNBS administration. Garcinol (Tocris Minneapolis, MN) was dissolved in DMSO and diluted to 4 nmol per μl in 50% DMSO/water. Garcinol was administered intrathecally via an osmotic pump (model 2001, Durect Corporation, Cupertino, CA) at a flow rate of 4 nmol per hour over a period of 7 days.
Plasma and cerebrospinal fluid norepinephrine measurements:
Plasma and cerebrospinal fluid norepinephrine was measured by ELISA (Rocky Mountain Diagnostics, Colorado Springs, CO).
Locus ceruleus (LC) plugs:
A 14 gauge tissue corer was used to remove plugs bilaterally from a 1.0 mm thick coronal section at Bregma −9.5 according to Rat Brain Atlas by Watson and Paxinos.
Laser Capture Micro-Dissection:
We injected CTB-488 (Invitrogen, Calsbad, CA), 4 mg/ml in PBS, into the distal colon wall (6 injections of 2 μl each/rat); ACI was induced three days later. S1 DRG were collected 7 days later and frozen in OCT on dry ice. Twelve micron sections, prepared from both S1 DRGs, were fixed and dehydrated. We identified CTB-488 labeled neuronal profiles and captured them with a Pixel IIe LCM microscope (Applied Biosystems, Foster City, CA). RNA was prepared with a Qiagen microRNA kit. SYBR green RT-PCR was performed with Applied Biosystems reagents and Step One Plus real-time PCR apparatus. We used β-III-Tub as a normalizer and compared fold-change to control by using the ΔΔCt procedure. Primers were designed using Primer Express Software (Applied Biosystems) and validated through control experiments: a single amplimer was observed by melting curve analysis; no amplimer was produced without reverse transcription or template; amplification efficiency was 100%. Primer sequences: BDNF, For- GGACATATCCATGACCAGAAAGAAA, Rev- GCAACAAACCAAACATTATCGAG; β-III-Tub, For- GGGAGATCGTGCACATCCA, Rev- CTATGCCATGCTCGTCACTGA.
Statistics:
We utilized two-way ANOVA and subsequently compared individual means with Tukey post-hoc analysis to determine the presence of significant differences among treatment groups of rats; p<0.05 was considered statistically significant. Analyses were performed on Sigmaplot 12.0 (Systat software).
Results:
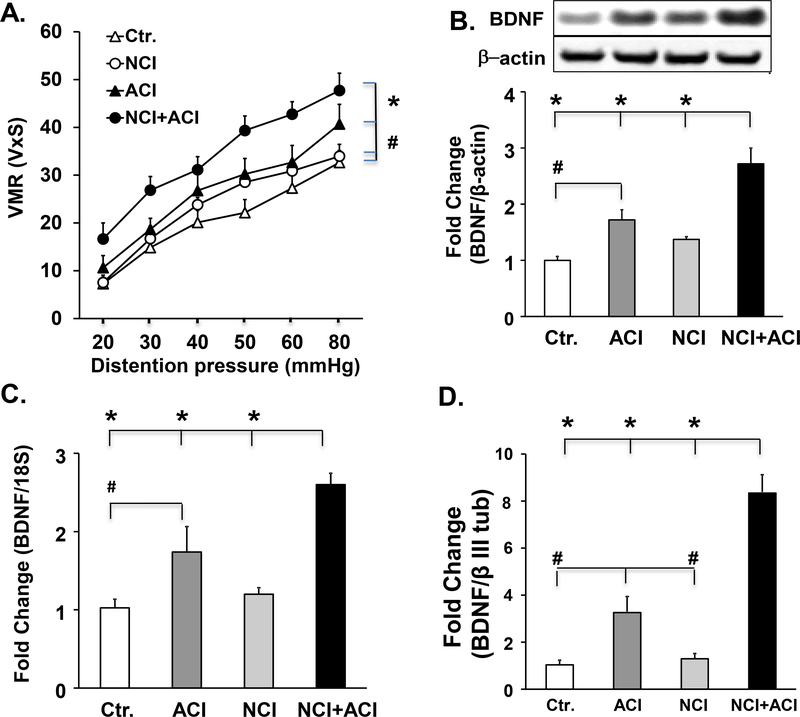
Neonatal colon inflammation is a risk factor for aggravating visceral hypersensitivity (VHS), when followed by adult colon inflammation: We investigated whether the upregulation of the visceromotor response to colorectal distention in response to adult colon inflammation is enhanced further in rats exposed previously to neonatal colon inflammation. We used four groups of rats (n=6 each group). Naïve adult control rats subjected to intraluminal colonic administration of saline (control rats); adult rats subjected previously to neonatal colon inflammation; adult naive rats subjected only to adult colon inflammation and rats subjected to neonatal colon inflammation followed by adult colon inflammation (neonatal/adult inflammation). We found that the visceromotor response to colorectal distention in neonatal/adult inflammation rats was significantly greater than in adult colon inflammation rats (27%, p=0.013), neonatal colon inflammation rats (46%, p=0.001) and ctr. rats (65%, p<0.001) (Fig. 1A). Adult colon inflammation alone produced a significant increase in the visceromotor response to colorectal distention vs. control rats (30%, p=0.03). There was no significant difference in the visceromotor response to colorectal distention in neonatal colon inflammation vs. control rats (p=0.33).
Fig. 1:
Enhanced visceromotor response to colorectal distention was observed in response to adult colon inflammation in adult rats subjected previously to neonatal colon inflammation. A. Graph showing the responses of control, neonatal colon inflammation, adult colon inflammation and neonatal/adult inflammation rats to graded colorectal distention, 7 days after initiation of adult colitis, n=6. We found significant effects of both neonatal colon inflammation (F1,27=6.84, p=0.017) and adult colon inflammation (F1,27=19.4, p<0.001) on visceral sensitivity. The visceromotor response to colorectal distention was significantly greater in neonatal/adult inflammation rats vs. adult colon inflammation (*p=0.013), neonatal colon inflammation alone (*p=0.001) and control (*p<0.001) rats. The visceromotor response to colorectal distention in adult colon inflammation rats was significantly greater in adult colon inflammation rats vs. the control (#p=0.03) rats. There was no significant difference in the visceromotor response to colorectal distention between neonatal colon inflammation and control rats. B. Western blot panels and bar graphs showing that BDNF protein expression in lumbar-sacral spinal cord segments was significantly higher in neonatal/adult inflammation rats vs. adult colon inflammation (*p<0.001), control (*p<0.001) and neonatal colon inflammation (*p<0.001) rats. Spinal cord BDNF was also significantly greater in adult colon inflammation rats vs. control rats (#p<0.001, n=6). C. Graph showing BDNF mRNA expression in lumbar/sacral spinal cord segments normalized to 18S rRNA and expressed as fold change with respect to control BDNF mRNA was significantly upregulated in neonatal/adult inflammation vs. adult colon inflammation *p=0.003), control (*p<0.001) and neonatal colon inflammation (*p<0.001) rats, and it was significantly upregulated in adult colon inflammation vs. control (#p=0.013) rats; significant effect of adult colon inflammation: F1,27=32.1, p<0.001 and of neonatal colon inflammation: F1,27=7.63, p=0.012. n=6). D. Bar graph showing BDNF mRNA expression in colon projecting neurons from the S1 dorsal root ganglia that were isolated by laser capture microdissection. We detected a significant neonatal/adult inflammation interaction: F1,23= 9.15, p=0.008, n=5. BDNF mRNA was significantly upregulated in neonatal/adult inflammation vs. adult colon inflammation (*p<0.001), control (*p<0.001) and neonatal colon inflammation rats (*p<0.001), and it was significantly upregulated in adult colon inflammation vs. control (#p=0.035) and neonatal colon inflammation (#p=0.03) rats. There were no significant differences between control and neonatal colon inflammation (p=0.49). Separate groups of animals contributed to visceromotor response data and to tissue for molecular analyses.
Upregulation of spinal cord and dorsal root ganglia BDNF in neonatal/adult inflammation rats:
We investigated whether upregulation of BDNF in the lumbar/sacral spinal cord underlies the aggravation of visceromotor response to colorectal distention in neonatal/adult inflammation rats. BDNF protein expression was significantly greater in neonatal/adult inflammation rats vs. the adult colon inflammation rats (51%, p<0.001), control rats (160%, p<0.001) and neonatal colon inflammation rats (100%, p<0.001) (Fig. 1B), n=6. BDNF expression in the lumbar/sacral spinal cord of adult colon inflammation rats was also significantly greater than that in control rats (73%, p<0.001) (Fig. 1B). There was no significant difference in BDNF expression between the neonatal colon inflammation and control rats (Fig. 1B). BDNF mRNA was significantly upregulated in neonatal/adult inflammation vs. adult colon inflammation rats (50%, p=0.003), control rats (160%, p<0.001) and neonatal colon inflammation rats (120%, p<0.001), n=6. BDNF mRNA was also significantly upregulated in adult colon inflammation vs. control rats (75%, p=0.013), but not vs. neonatal colon inflammation rats (p=0.21) (Fig. 1C), There was no significant difference in BDNF mRNA expression between the control and neonatal colon inflammation rats (p=0.54) (Fig. 1C).
In colon projecting neurons from S1 dorsal root ganglia isolated by laser capture micro-dissection, BDNF mRNA was significantly upregulated in neonatal/adult inflammation vs. adult colon inflammation rats (2.4 fold, p<0.001), control rats (7.8 fold, p<0.001) and neonatal colon inflammation rats (4.5 fold, p<0.001), and it was significantly upregulated in adult colon inflammation vs. control rats (3.2 fold, p=0.035) and neonatal colon inflammation rats (3-fold, p=0.03) (Fig. 1D), n=5 rats in each group. There were no significant differences between control and neonatal colon inflammation rats (p=0.49) (Fig. 1D). By contrast, there was no significant difference in the expression of spinal cord nerve growth factor (NGF) between the neonatal/adult inflammation and adult colon inflammation rats (data not shown).
Upregulation of spinal cord BDNF underlies the aggravation of visceromotor response to colorectal distention in neonatal/adult inflammation rats:
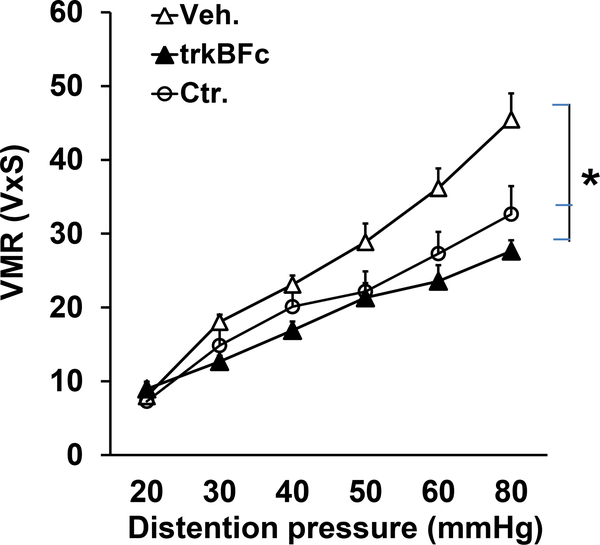
Daily intrathecal injections of BDNF antagonist TrkB-Fc (5 μg in 10 μl sterile saline or vehicle twice/day) into the lumbar/sacral spinal cord for seven days starting with the day of intraluminal administration of TNBS significantly suppressed the visceromotor response to colorectal distention to the level observed in control rats (p <0.05, Fig. 2).
Fig. 2:
BDNF inhibitor reverses the enhanced visceral hypersensitivity in neonatal/adult inflammation rats. Graph showing that intrathecal treatment with BDNF antagonist trkB-Fc significantly reduced the visceral sensitivity in neonatal/adult inflammation rats compared to intrathecal vehicle treatment to a level that was not statistically different from the visceral sensitivity in control rats (*p<0.05, n=6).
Upregulation of sympathetic activity contributes to aggravated VHS and BDNF upregulation in neonatal/adult inflammation rats:
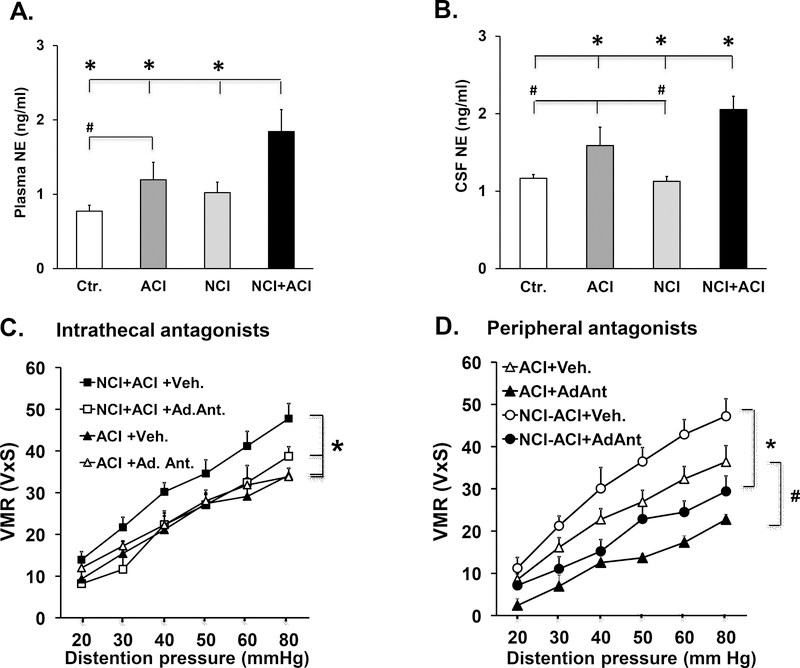
Clinical findings show an increase in sympathetic tone as measured by heart rate variability test in IBD patients (26). We found that the Plasma norepinephrine levels were significantly increased in neonatal/adult inflammation vs. adult colon inflammation rats (54%, p=0.02), control rats (140%, p<0.001) and neonatal colon inflammation rats (80%, p=0.003) (Fig. 3A). The plasma norepinephrine levels were also significantly greater in adult colon inflammation rats vs. control rats (66%, p=0.04) (Fig. 3A, n=10).
Fig. 3:
Increased sympathetic activity contributes to enhanced visceral sensitivity in neonatal/adult inflammation rats. A. Graph showing plasma levels of norepinephrine one week after induction of adult colitis. We found significant main effects of neonatal colon inflammation F1,40=5.36, p=0.026 and adult colonic inflammation F1,40=10.3, p=0.003, n=10. The plasma norepinephrine levels were significantly elevated in neonatal/adult inflammation vs. adult colonic inflammation (*p=0.02), control (*p<0.001) and neonatal colon inflammation (*p=0.003) rats. The plasma norepinephrine levels were significantly elevated in adult colonic inflammation vs. control rats ( p=0.04). B. Graph showing norepinephrine levels in the cerebrospinal fluid. We found a significant neonatal colon inflammation x adult colonic inflammation interaction F1,40=4.57, p=0.039, n=10. Cerebrospinal fluid norepinephrine was significantly upregulated in neonatal/adult inflammation vs. adult colonic inflammation (*p=0.019), control (*p<0.001) and neonatal colon inflammation (*p<0.001) rats. The cerebrospinal fluid norepinephrine levels in the adult colonic inflammation rats were significantly greater than in control (#p=0.046) and in neonatal colon inflammation rats (#p=0.04). C. Graph showing the visceromotor response to colorectal distention in adult colonic inflammation and neonatal/adult inflammation rats that were treated intrathecally with either propranolol+phentolamine cocktail or vehicle. We found a significant interaction between neonatal colon inflammation and drug treatment: F1,28=9.31, p=0.006, n=6–7. Drug treatment significantly reduced the enhanced visceral hypersensitivity in neonatal/adult inflammation rats *p<0.001 compared to vehicle treatment. D. Graph showing the effects of systemic daily treatment with adrenergic antagonists propranolol and phentolamine on the visceromotor response to colorectal distention. We found significant main effects of neonatal colon inflammation F1,27=14.8, p=0.001 and adrenergic antagonists F1,27=48.2, p<0.001, n=6. Systemic adrenergic antagonist treatment significantly decreased visceral hypersensitivity in both in neonatal/adult inflammation and adult colonic inflammation groups of rats (neonatal/adult inflammation, *p<0.001 and adult colonic inflammation, #p<0.001). Separate groups of animals contributed to visceromotor response data and to tissue for molecular analyses.
The norepinephrine levels in the cerebrospinal fluid were significantly greater in neonatal/adult inflammation vs. adult colon inflammation rats (36%, p=0.019), control rats (58%, p<0.001) and neonatal colon inflammation rats (63%, p<0.001) (Fig. 3B). There was no significant difference in cerebrospinal fluid norepinephrine levels between the control and neonatal colon inflammation rats (p=0.3) (Fig. 3B). The cerebrospinal fluid norepinephrine levels in the adult colon inflammation rats were significantly greater than in control (36%, p=0.046 and neonatal colon inflammation rats (29%, p=0.04) (Fig. 3B)
We investigated the functional role of increase in the central and peripheral sympathetic activity by measuring the visceromotor response to colorectal distention in neonatal/adult inflammation and adult colon inflammation rats treated with intrathecal or systemic administration of a combination of β1/2-adrenergic receptor antagonist propranolol and α1-adrenergic receptor antagonist phentolamine during the 7 days of adult colon inflammation. Intrathecal administration of adrenergic receptor antagonists (Ad. Ant.) significantly reduced the enhanced visceral hypersensitivity in neonatal/adult inflammation rats 36% p<0.001, n=6 compared to vehicle but was without significant effect on visceral sensitivity in adult colon inflammation rats (Fig. 3C). Systemic adrenergic antagonist treatment significantly decreased visceral hypersensitivity in both groups of rats (neonatal/adult inflammation, 41% decrease, p<0.001 and adult colon inflammation, 48% decrease, p<0.001) n=6–7(Fig. 3D).
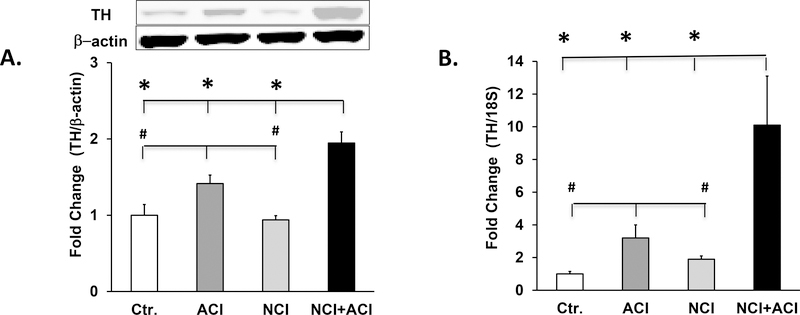
Locus ceruleus is the primary source of norepinephrine in the brain (27). The expression of tyrosine hydroxylase was significantly upregulated in the locus ceruleus of the neonatal/adult inflammation rats vs. adult colon inflammation rats (38%, p=0.005), control rats (94%, p<0.001) and neonatal colon inflammation rats (100%, p<0.001); and in adult colon inflammation rats vs. control rats (42%, p=0.017) and neonatal colon inflammation rats (47%, p=0.011), n=6–8, (Fig. 4A). No significant differences were detected between control and neonatal colon inflammation rats. The expression of tyrosine hydroxylase mRNA was significantly upregulated in the locus ceruleus in neonatal/adult inflammation vs. adult colon inflammation rats (3 fold, p=0.001), control rats (10-fold, p<0.001) and neonatal colon inflammation rats (5.6 fold, p<0.001) and in adult colon inflammation rats vs. control rats (3.2 fold, p=0.001) and neonatal colon inflammation rats (0.7 fold, p=0.043), n=5 or 6, (Fig. 4B).
Fig.4.
Tyrosine hydroxylase expression was elevated in the locus ceruleus of neonatal/adult inflammation rats. A. Graph and western blot panel showing tyrosine hydroxylase expression in central nervous system plugs containing the locus ceruleus. We found a significant neonatal colon inflammation x adult colonic inflammation interaction: F1,28=6.36, p=0.019, n=6–8. Expression of tyrosine hydroxylase protein was significantly elevated in neonatal/adult inflammation rats vs. adult colonic inflammation (*p=0.005), control (*p<0.001) and neonatal colon inflammation (*p<0.001) rats; and in adult colonic inflammation rats vs. control (#p=0.017) and neonatal colon inflammation (#p=0.011) rats. B. Graph showing tyrosine hydroxylase mRNA levels in locus ceruleus containing plugs normalized to 18S rRNA and expressed relative to control. We found a significant neonatal colon inflammation x adult colonic inflammation interaction: F1,28=5.60, p=0.029, n=5 or 6. Tyrosine hydroxylase mRNA was significantly upregulated in neonatal/adult inflammation rats vs. adult colonic inflammation (*p=0.001), control (*p<0.001) and neonatal colon inflammation (*p<0.001) rats; and in adult colonic inflammation rats vs. ctr. (#p=0.001) and neonatal colon inflammation (#p=0.043) rats.
Epigenetic upregulation of spinal cord BDNF by elevated cerebrospinal fluid norepinephrine in neonatal/adult inflammation rats:
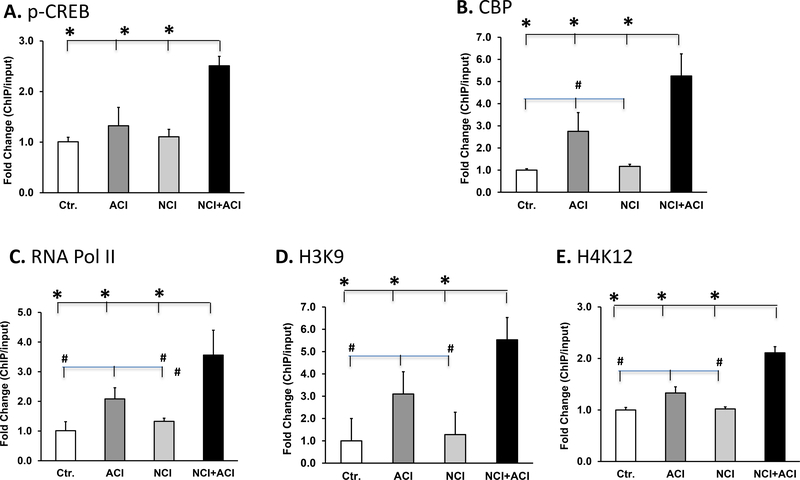
We investigated whether the elevation of norepinephrine in the cerebrospinal fluid epigenetically upregulates the expression of spinal cord BDNF in neonatal/adult inflammation rats. Norepinephrine, acting on adrenergic receptors, elevates intracellular cAMP, which activates protein kinase A (PKA). The catalytic subunit of PKA translocates to the nucleus to phosphorylate the nuclear transcription factor cAMP response element-binding (CREB) protein, which recruits CREB binding protein (CBP), a histone acetylene transferase (HAT), resulting in relaxation of chromatin and enhanced gene transcription. Promoter IX of the BDNF gene has two cAMP response elements CRE1 (TGACGTCA) and CRE2 (TGACGTAA). ChIP assay showed significantly greater binding of pCREB at CRE2 in neonatal/adult inflammation rats vs. adult colon inflammation rats (2-fold, p<0.001), control rats (2.5 fold, p<0.001) and neonatal colon inflammation rats (2.5 fold, p<0.001, n=5 each, (Fig. 5A). There was no significant difference in pCREB binding at CRE1 between the neonatal/adult inflammation and the other groups of rats (data not shown). The CBP binding at CRE2 in neonatal/adult inflammation rats was also significantly greater than in adult colon inflammation rats (2-fold, p=0.003), control rats (5-fold, p<0.001) and neonatal colon inflammation rats (5-fold, p<0.001). In addition, the CBP binding to CRE2 in adult colon inflammation rats was significantly greater than in control rats (2.5 fold, p=0.021) and neonatal colon inflammation rats (2.5 fold, p=0.025), n=5 each group, (Fig. 5B).
Fig. 5.
Bar graphs displaying the results of ChIP assay using chromatin prepared from lumbar/sacral spinal cords. A. Average pCREB binding at the BDNF promoter CRE2 in neonatal/adult inflammation lumbar/sacral spinal cords was significantly greater than in adult colon inflammation (*p<0.001), control (*p<0.001) and neonatal colon inflammation (*p<0.001) rats. We found a significant neonatal colon inflammation x adult colon inflammation interaction F 1,23=30.0, p<0.001, n=5). B. CBP binding at the BDNF promoter CRE2 in neonatal/adult inflammation lumbar/sacral spinal cords was significantly greater than in adult colon inflammation (*p=0.003), control (*p<0.001) and neonatal colon inflammation (*p<0.001) rats; and in adult colon inflammation rats, it was significantly greater than in control (#p=0.021) and neonatal colon inflammation (#p=0.025) rats. (We found a significant neonatal colon inflammation x adult colon inflammation interaction F1,23=6.24, p=0.028, n=5). C. RNA Pol II binding at the BDNF core promoter in neonatal/adult inflammation lumbar/sacral spinal cords was significantly greater vs. that in adult colon inflammation (p=0.03), control (p=0.003) and neonatal colon inflammation (p=0.004); and this binding in adult colon inflammation rats was significantly greater vs. control (#p=0.009) and neonatal colon inflammation (#p=0.012) rats. We found a significant effect of adult colon inflammation (F1,23=13.8, p=0.003} on Pol II binding, n=5). D and E. Both, H3K9 (D) and H4K12 acetylations at the BDNF core promoter in neonatal/adult inflammation lumbar/sacral spinal cords were significantly greater vs. those in adult colon inflammation (*p<0.001), control (*p<0.001) and neonatal colon inflammation (*p<0.001) spinal cord tissues; and both, H3K9 (D) and (E) H4K12 acetylations in adult colon inflammation spinal cord tissues were significantly greater vs. those in control (#p=0.002) and neonatal colon inflammation (#p=0.002) spinal cord tissues. We found a significant interaction between neonatal colon inflammation and adult colon inflammation, F1,23=9.54, p=0.009, n=5).
We investigated whether the above epigenetic modulations underlie pro-transcriptional epigenetic changes at the BDNF core promoter. We found that RNA polymerase II (RNAP II) binding to the BDNF core promoter was significantly greater in neonatal/adult inflammation rats vs. adult colon inflammation rats (57%, p<0.001), control rats (3.4 fold, p<0.001) and neonatal colon inflammation rats (3 fold, p<0.001) (Fig. 5C). RNAP II binding to the core BDNF reporter in adult colon inflammation rats was significantly greater than in control rats (2-fold, p=0.009) and neonatal colon inflammation rats (2-fold, p=0.012), n=6, (Fig. 5C). H3K9 acetylation at the BDNF core promoter in neonatal/adult inflammation lumbar/sacral spinal cord was significantly greater than in adult colon inflammation spinal cord (0.73 fold, p<0.001), control spinal cord (5.2 fold, p<0.001) and neonatal colon inflammation spinal cord (5 fold, p<0.001) (Fig. 5D); The H3K9 acetylation in adult colon inflammation rats was significantly greater than in control (3 fold, p=0.002) and neonatal colon inflammation rats (3 fold, p=0.002), n=5, Fig. 5D. H4K12 acetylation at the BDNF core promoter in neonatal/adult inflammation lumbar/sacral spinal cord was significantly greater than in adult colon inflammation (0.1.65-fold, p<0.001), control (2.2-fold, p<0.001) and neonatal colon inflammation rats (2.2 fold, p<0.001). H3K12 acetylation in adult colon inflammation rats was significantly greater than in control (0.1.65-fold, p=0.003) and neonatal colon inflammation rats (1.65-fold, p=0.004), n=5 each (Fig. 5E).
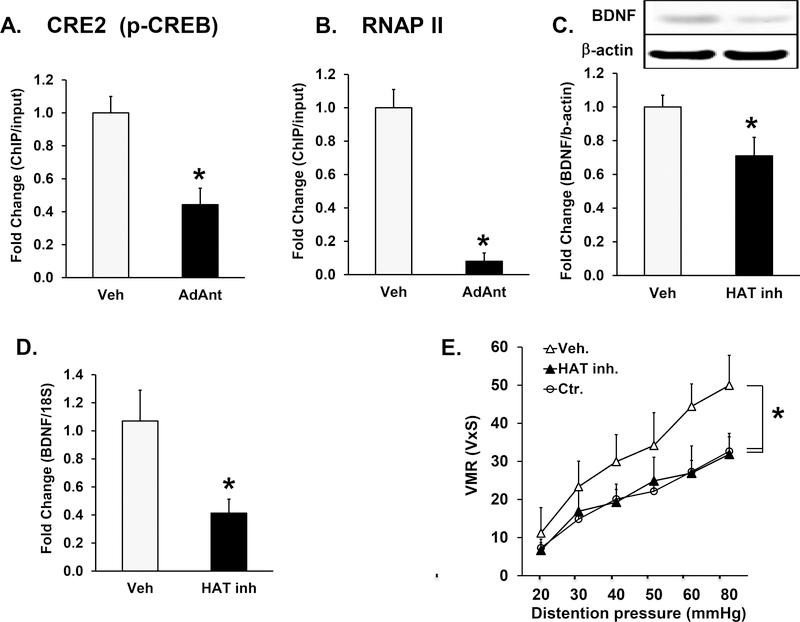
We investigated whether the intrathecal adrenergic antagonist treatment-induced suppression of visceromotor response to colorectal distention in neonatal/adult inflammation rats (Fig. 3C) was mediated by reversing the epigenetic modifications of the BDNF promoter lX in neonatal/adult inflammation rats. ChIP assay revealed that intrathecal treatment with adrenergic antagonists propranolol and phentolamine (Ad. Ant.) significantly decreased pCREB binding to CRE2 (p<0.05), n=5) (Fig. 6A) and RNAP II binding to the core BDNF IX promoter (p<0.05), n=5, (Fig. 6B).
Fig. 6.
Epigenetic regulation of BDNF expression in neonatal/adult inflammation rats. A, B. Bar graphs showing the results of ChIP experiments using chromatin isolated from rats that received intrathecal treatment with either adrenergic antagonists, propranolol and phentolamine, (Ad. Ant.) or vehicle. A. pCREB binding to the CRE2 in BDNF promoter IX was significantly decreased by antagonist treatment (*p<0.05, n=5). B. RNA polymerase II (pol II) binding to the core promoter was significantly decreased by antagonist treatment (*p<0.05, n=5). C, D, E. Intrathecal infusion of the HAT inhibitor garcinol via continuous infusion from an implanted osmotic pump during the seven day inflammation period significantly reduced: C. BDNF protein (*p<0.05, n=5) and D. mRNA expression (*p<0.05, n=5) in lumbar-sacral spinal cord segments were significantly decreased by garcinol treatments. E. HAT inhibitor treatment significantly decreased visceral sensitivity in neonatal/adult inflammation rats (*p<0.05, n=5). The tissue for molecular experiments was obtained from same animals that underwent visceromotor response testing.
Finally, we investigated whether intrathecal administration of HAT inhibitor, garcinol, by an osmotic pump at 4 nmol per hour over a period of 7 days starting with the onset of adult colon inflammation would block the epigenetic upregulation of spinal cord BDNF and hence the aggravation of visceromotor response to colorectal distention in neonatal/adult inflammation rats. We found that the HAT inhibitor blocked the up-regulation of spinal cord BDNF protein (p<0.05) n=5; (Fig. 6C) and the upregulation of spinal cord mRNA (p<0.05) n=5 (Fig. 6D). Garcinol treatment significantly suppressed the visceromotor response to colorectal distention in neonatal/adult inflammation rats to the level seen in ctr. rats (p<0.05, n=7 each; Fig. 6E).
Discussion:
Our findings show that robust neonatal colon immune challenge triggers epigenetic programming that upregulates tyrosine hydroxylase expression in the locus ceruleus, when these rats were subjected to a follow up adult colon immune challenge. The upregulation of tyrosine hydroxylase elevates norepinephrine levels in the cerebrospinal fluid. Norepinephrine, acting on its adrenergic receptors is known to upregulate intracellular cAMP that activates protein kinase A (PKA). The catalytic subunit of PKA translocates to the nucleus to phosphorylate the transcription factor cAMP response element binding protein (CREB), which recruits CREB binding protein (CBP), a histone acetyl transferase (HAT) to the promoter to increase gene transcription (28). We found that the increase of cerebrospinal fluid norepinephrine as well as the binding pCREB and CBP to the cAMP response element 2 (CRE 2) on the promoter IX of the BDNF gene was greater in neonatal/adult inflammation than in adult colon inflammation rats. The greater HAT recruitment in neonatal/adult inflammation than in adult colon inflammation rats recruited greater RNAP II to the BDNF core promoter to enhance BDNF transcription in neonatal/adult inflammation vs. the adult colon inflammation rats. The higher levels of acH3K9 and acH4K12 at the BDNF core promoter in neonatal/adult inflammation vs. adult colon inflammation rats indicates increased chromatin relaxation resulting in greater recruitment of RNA pol II and greater transcription of BDNF in neonatal/adult inflammation vs. the adult colon inflammation rats. The end result of stronger activation of the spinal cord adrenergic pathway in neonatal/adult inflammation rats than in adult colon inflammation rats was greater expression of spinal cord BDNF and elevation of visceromotor response to colorectal distention in neonatal/adult inflammation vs. adult colon inflammation rats. Overall, these findings suggest epigenetic-susceptibility to developing aggravated visceral sensitivity that contributes to abdominal pain (7, 8).
It is notable that neonatal colon inflammation by itself, had minimal effect on spinal cord BDNF expression or the visceromotor response to colorectal distention in later life, suggesting that the induction of intense visceral hypersensitivity is a two-step process; early-life colon immune challenge followed by a follow up adult-life immune challenge. Our findings in a preclinical animal model consolidate the observations in two separate groups of epidemiological studies; one that in a subset of IBD patients, childhood immune challenge may be a risk factor for the development of IBD symptoms (16–20); and the other that adult immune challenge triggers the onset or relapse of IBD symptoms, including abdominal pain (21–24). Note that the epigenetic marks can be modified or erased by life styles and other environmental factors during adolescence and adult-life (29), suggesting that not all subjects exposed to childhood immune challenges may develop epigenetic-susceptibility to developing IBD symptoms.
It is noteworthy that the specific type of epigenetic programming by adverse early-life experiences (AELE) and its target cells in organs depend on the type of stress, such as immune challenge, psychological stress and nutritional deficiency, severity and frequency of stress as well as on the timing of the stress during development, such as fetal, neonatal and childhood (25, 30). In this regard, our findings suggest that the future epidemiological studies on the early-life risk factors for the development of IBD and its symptoms may be strengthened in their outcomes and uniformity among different studies by identifying the age range of early-life infections and their severity, if such data are available.
The targets of childhood immune challenges to induce epigenetic-susceptibility-to aggravated immune response, when challenged with adult-life immune challenges remain unknown. However, findings in an animal model show that neonatal immune challenge by lipopolysaccharide (LPS) followed by adult immune challenge by TNBS exacerbated colitis compared with that induced by TNBS alone in naive adult rats (31). It appears, therefore, that the same risk factors, early-life infection followed by adult-life infection may underlie the aggravated immune response as well the symptom of abdominal pain.
Attempts to identify a specific infectious agent as the risk factor for IBD have not been successful (32). Multiple types of infectious bacteria and viruses causing childhood gastrointestinal enteritis have been identified as risk factors for inducing susceptibility to IBD as well as trigger factors for IBD (16–24). It is thought that an invasive pathogen may breakdown the epithelial barrier and the resulting exposure to the commensal microbes may trigger the immune response (33). Each species of pathogens may trigger the release of a different profile of inflammatory mediators in the gut wall. However, each profile of inflammatory mediators triggers the hypothalamic-pituitary-adrenal axis, whose products, including glucocorticoids and catecholamines, trigger epigenetic programming (34). It appears that the epithelial barrier breakdown by a generic chemical, such as TNBS, or bacterial breakdown products, such as LPS, in animals works the same way to trigger epigenetic programming.
The current therapeutic approaches for the treatment of abdominal pain in IBD by opioids (35–37) and non-steroidal anti-inflammatory drugs (38) remain problematic and unsatisfactory. We show that intrathecal administration of a HAT inhibitor blocked the upregulation of spinal cord BDNF and visceromotor response to colorectal distention in neonatal/adult inflammation rats, suggesting the potential of epigenetic modulators as candidates to suppress abdominal pain in IBD patients. In a previous study, we reported that orally administered Hat inhibitors cross the blood-brain barrier to block the epigenetic upregulation of spinal cord BDNF in response to chronic prenatal stress (25).
Both, the cerebrospinal and plasma levels of norepinephrine, were upregulated in neonatal/adult inflammation rats vs. the adult colonic inflammation rats. However, the peripheral norepinephrine does not cross the blood-brain barrier (39). Therefore, it is unlikely that the increase of plasma norepinephrine contributed to the upregulation of spinal cord BDNF or the visceromotor response to colorectal distention, as evidenced by blockade of the above effects by intrathecal administration of β1/2 and α1 adrenergic receptor antagonists. It is notable that peripheral administration of β1/2 and α1 adrenergic receptor antagonists also blocked the upregulation of spinal cord BDNF and increase of visceromotor response to colorectal distention; these antagonists cross the blood-brain barrier (40). The profiles of increase of cerebrospinal fluid norepinephrine and peripheral norepinephrine in neonatal/adult inflammation rats were similar. Even though the peripheral norepinephrine does not contribute to the upregulation of visceral sensitivity, it may serve as a biomarker of visceral hypersensitivity in neonatal/adult inflammation rats. It is noteworthy that IBD patients show a higher sympathetic tone than healthy controls as measured by heart rate variability (26).
IBD is a complex multi-organ and heterogeneous disease. There is no preclinical model that can completely simulate a complex human disease such as IBD. However, the preclinical models are used widely to mimic specific aspects of complex diseases, such as visceral hypersensitivity in our case, which is one of the prominent biomarkers of abdominal pain. The findings of these models can then be tested in human subjects by clinical studies as well as clinical trials. In our case, TNBS-induced colon inflammation is an established and widely used preclinical model of IBD and specifically of Crohn’s disease because TNBS induces transmural inflammation (41,42).
In conclusion, neonatal immune challenge triggers epigenetic programming that upregulates tyrosine hydroxylase in the locus ceruleus, when these rats are exposed to a second immune challenge as adults. The resulting upregulation of cerebrospinal fluid norepinephrine epigenetically upregulates spinal cord BDNF, which aggravates visceral sensitivity to colorectal distension. Epidemiological studies show that childhood immune challenges are a risk tor factor for IBD and adult immune challenges are trigger factors for the onset of IBD. Our findings consolidate the epidemiological observations into a unified hypothesis that the severity of abdominal pain in IBD may be determined not only by the severity of colitis but also by the upregulation of BDNF in the spinal cord. HAT inhibitors as well as adrenergic receptor antagonists may be used to mitigate the severity of abdominal pain in IBD patients as an alternate to the use of opioids or non-steroidal anti-inflammatory drugs. Manipulation of the spinal cord BDNF expression has been proposed also to treating nerve injury-induced neuropathic pain (41) In support of our hypothesis, several types of epigenetic modulations, including DNA methylation, histone modifications and changes in microRNA expressions have been noted in the inflamed tissues of IBD patients (42–45), but these modifications have not yet been related to a specific environmental factor or to the symptoms they may contribute to in IBD patients.
Key Points:
Treatment of visceral inflammatory pain remains unsatisfactory. The current focus is on identifying inflammatory mediators that induce visceral hypersensitivity.
We found that epigenetic programming triggered by early-life colon inflammation upregulates spinal cord BDNF that aggravates visceral sensitivity when exposed to adult-life colon inflammation.
Our findings suggest alternate approaches to manage visceral pain in IBD patients.
Acknowledgement
X performed the research JHW, JEA
X designed the research study SKS, JHW
X contributed essential reagents or tools JHW SKS
X analyzed the data JHW, JEA, X wrote the paper. SKS, JHW JEA
Supported in part by NIDDK Grants 5R01DK088796 (SKS) and DK 32346 (SKS)
References:
- 1.Morrison G, Van Langenberg DR, Gibson SJ, Gibson PR. Chronic pain in inflammatory bowel disease: characteristics and associations of a hospital-based cohort. Inflamm Bowel Dis 2013; 19: 1210–1217. [DOI] [PubMed] [Google Scholar]
- 2.Bielefeldt K, Davis B, Binion DG. Pain and inflammatory bowel disease. Inflamm Bowel Dis 2009; 15: 778–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cross RK, Wilson KT, Binion DG. Narcotic use in patients with Crohn’s disease. Am J Gastroenterol 2005; 100: 2225–2229. [DOI] [PubMed] [Google Scholar]
- 4.Edwards JT, Radford-Smith GL, Florin TH. Chronic narcotic use in inflammatory bowel disease patients: prevalence and clinical characteristics. J Gastroenterol Hepatol 2001; 16: 1235–1238. [DOI] [PubMed] [Google Scholar]
- 5.Zeitz J, Ak M, Muller-Mottet S, et al. Pain in IBD Patients: Very Frequent and Frequently Insufficiently Taken into Account. PLoS One 2016; 11: e0156666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lix LM, Graff LA, Walker JR, et al. Longitudinal study of quality of life and psychological functioning for active, fluctuating, and inactive disease patterns in inflammatory bowel disease. Inflamm Bowel Dis 2008; 14: 1575–1584. [DOI] [PubMed] [Google Scholar]
- 7.Chang L, Munakata J, Mayer EA, et al. Perceptual responses in patients with inflammatory and functional bowel disease. Gut 2000; 47: 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faure C, Giguere L. Functional gastrointestinal disorders and visceral hypersensitivity in children and adolescents suffering from Crohn’s disease. Inflamm Bowel Dis 2008; 14: 1569–1574. [DOI] [PubMed] [Google Scholar]
- 9.Beyak MJ, Vanner S. Inflammation-induced hyperexcitability of nociceptive gastrointestinal DRG neurones: the role of voltage-gated ion channels. Neurogastroenterol Motil 2005; 17: 175–186. [DOI] [PubMed] [Google Scholar]
- 10.Zhao P, Lieu T, Barlow N, et al. Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4. J Biol Chem 2014; 289: 27215–27234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes PA, Brierley SM, Martin CM, Brookes SJ, Linden DR, Blackshaw LA. Post-inflammatory colonic afferent sensitisation: different subtypes, different pathways and different time courses. Gut 2009; 58: 1333–1341. [DOI] [PubMed] [Google Scholar]
- 12.O’Malley D, Julio-Pieper M, O’Mahony SM, Dinan TG, Cryan JF. Differential visceral pain sensitivity and colonic morphology in four common laboratory rat strains. Exp Physiol 2014; 99: 359–367. [DOI] [PubMed] [Google Scholar]
- 13.Qiao LY, Grider JR. Colitis elicits differential changes in the expression levels of receptor tyrosine kinase TrkA and TrkB in colonic afferent neurons: a possible involvement of axonal transport. Pain 2010; 151: 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji Y, Tang B, Cao DY, Wang G, Traub RJ. Sex differences in spinal processing of transient and inflammatory colorectal stimuli in the rat. Pain 2012; 153: 1965–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long MD, Drossman DA. Inflammatory bowel disease, irritable bowel syndrome, or what?: A challenge to the functional-organic dichotomy. Am J Gastroenterol 2010; 105: 1796–1798. [DOI] [PubMed] [Google Scholar]
- 16.Montgomery SM, Morris DL, Pounder RE, Wakefield AJ. Paramyxovirus infections in childhood and subsequent inflammatory bowel disease. Gastroenterology 1999; 116: 796–803. [DOI] [PubMed] [Google Scholar]
- 17.Wurzelmann JI, Lyles CM, Sandler RS. Childhood infections and the risk of inflammatory bowel disease. Dig Dis Sci 1994; 39: 555–560. [DOI] [PubMed] [Google Scholar]
- 18.Gradel KO, Nielsen HL, Schonheyder HC, Ejlertsen T, Kristensen B, Nielsen H. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology 2009; 137: 495–501. [DOI] [PubMed] [Google Scholar]
- 19.Aspberg S, Dahlquist G, Kahan T, Kallen B. Fetal and perinatal risk factors for inflammatory bowel disease. Acta Paediatr 2006; 95: 1001–1004. [DOI] [PubMed] [Google Scholar]
- 20.Whorwell PJ, Holdstock G, Whorwell GM, Wright R. Bottle feeding, early gastroenteritis, and inflammatory bowel disease. Br Med J 1979; 1: 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banaszkiewicz A, Kowalska-Duplaga K, Pytrus T, Pituch H, Radzikowski A. Clostridium difficile infection in newly diagnosed pediatric patients with inflammatory bowel disease: prevalence and risk factors. Inflamm Bowel Dis 2012; 18: 844–848. [DOI] [PubMed] [Google Scholar]
- 22.Mann EA, Saeed SA. Gastrointestinal infection as a trigger for inflammatory bowel disease. Curr Opin Gastroenterol 2012; 28: 24–29. [DOI] [PubMed] [Google Scholar]
- 23.Hansen R, Thomson JM, El-Omar EM, Hold GL. The role of infection in the aetiology of inflammatory bowel disease. J Gastroenterol 2010; 45: 266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia Rodriguez LA, Ruigomez A, Panes J. Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastroenterology 2006; 130: 1588–1594. [DOI] [PubMed] [Google Scholar]
- 25.Winston JH, Li Q, Sarna SK. Chronic prenatal stress epigenetically modifies spinal cord BDNF expression to induce sex-specific visceral hypersensitivity in offspring. Neurogastroenterol Motil 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maule S, Pierangeli G, Cevoli S, et al. Sympathetic hyperactivity in patients with ulcerative colitis. Clin Auton Res 2007; 17: 217–220. [DOI] [PubMed] [Google Scholar]
- 27.Aston-Jones G, Waterhouse B. Locus coeruleus: From global projection system to adaptive regulation of behavior. Brain Res 2016; 1645: 75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2001; 2: 599–609. [DOI] [PubMed] [Google Scholar]
- 29.Laird PW. Cancer epigenetics. Hum Mol Genet 2005; 14 Spec No 1: R65–76. [DOI] [PubMed] [Google Scholar]
- 30.Faulk C, Dolinoy DC. Timing is everything: the when and how of environmentally induced changes in the epigenome of animals. Epigenetics 2011; 6: 791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ardizzone S, Puttini PS, Cassinotti A, Porro GB. Extraintestinal manifestations of inflammatory bowel disease. Dig Liver Dis 2008; 40 Suppl 2: S253–259. [DOI] [PubMed] [Google Scholar]
- 32.Rogler G, Zeitz J, Biedermann L. The Search for Causative Environmental Factors in Inflammatory Bowel Disease. Dig Dis 2016; 34 Suppl 1: 48–55. [DOI] [PubMed] [Google Scholar]
- 33.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008; 134: 577–594. [DOI] [PubMed] [Google Scholar]
- 34.Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal ‘programming’ of adult pathophysiology. Nat Clin Pract Endocrinol Metab 2007; 3: 479–488. [DOI] [PubMed] [Google Scholar]
- 35.Tuteja AK, Biskupiak J, Stoddard GJ, Lipman AG. Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol Motil 2010; 22: 424–430, e496. [DOI] [PubMed] [Google Scholar]
- 36.Hanson KA, Loftus EV Jr., Harmsen WS, Diehl NN, Zinsmeister AR, Sandborn WJ. Clinical features and outcome of patients with inflammatory bowel disease who use narcotics: a case-control study. Inflamm Bowel Dis 2009; 15: 772–777. [DOI] [PubMed] [Google Scholar]
- 37.Jones JL, Loftus EV Jr. Avoiding the vicious cycle of prolonged opioid use in Crohn’s disease. Am J Gastroenterol 2005; 100: 2230–2232. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi K, Smale S, Premchand P, et al. Prevalence and mechanism of nonsteroidal anti-inflammatory drug-induced clinical relapse in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2006; 4: 196–202. [DOI] [PubMed] [Google Scholar]
- 39.MacKenzie ET, McCulloch J, O’Kean M, Pickard JD, Harper AM. Cerebral circulation and norepinephrine: relevance of the blood-brain barrier. Am J Physiol 1976; 231: 483–488. [DOI] [PubMed] [Google Scholar]
- 40.Neil-Dwyer G, Bartlett J, McAinsh J, Cruickshank JM. Beta-adrenoceptor blockers and the blood-brian barrier. Br J Clin Pharmacol 1981; 11: 549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renn CL, Leitch CC, Lessans S, et al. Brain-derived neurotrophic factor modulates antiretroviral-induced mechanical allodynia in the mouse. J Neurosci Res 2011; 89: 1551–1565. [DOI] [PubMed] [Google Scholar]
- 42.Nimmo ER, Prendergast JG, Aldhous MC, et al. Genome-wide methylation profiling in Crohn’s disease identifies altered epigenetic regulation of key host defense mechanisms including the Th17 pathway. Inflamm Bowel Dis 2012; 18: 889–899. [DOI] [PubMed] [Google Scholar]
- 43.Tsaprouni LG, Ito K, Powell JJ, Adcock IM, Punchard N. Differential patterns of histone acetylation in inflammatory bowel diseases. J Inflamm (Lond) 2011; 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stylianou E Epigenetics: the fine-tuner in inflammatory bowel disease? Curr Opin Gastroenterol 2013; 29: 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu F, Zikusoka M, Trindade A, et al. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology 2008; 135: 1624–1635 e1624. [DOI] [PubMed] [Google Scholar]