Abstract
BACKGROUND:
Combination of betamethasone valerate and neomycin sulfate in cream is used to treat the itching, redness, dryness, scaling, inflammation and discomfort of various skin conditions caused by infection. The combination of active ingredients has side effects which can cause dry skin, thinning of the skin, hypertrichosis, and stretch marks.
AIM:
The purpose of this study was to make a formula containing vitamin E and quantitative analysis of betamethasone valerate and neomycin sulfate in creams using High Performance Liquid Chromatography and Spectrophotometry Area Under Curve methods.
METHODS:
Cream preparation includes smelting and emulsification processes, with oil phases namely stearic acid and vitamin E as well as water phases are glycerin, sodium bi-borate, tri-ethanolamine. Physical tests for the cream were organoleptic, homogeneity, pH, evaluation of dispersion, and viscosity. HPLC analysis for cream was carried out using C18 column, and the mobile phase of methanol: water with comparison optimization beforehand. Spectrophotometry analysis for cream was carried out using application of Area Under Curves methods.
RESULTS:
The formula used was betamethasone valerate 5 mg, neomycin sulfate 25 mg, stearic acid, glycerin, sodium bi-borate, tri-ethanolamine, vitamin E and distilled water. The obtained cream was in the form of semi-solid, odorless, white (colorless), homogeneous, pH 7, the dispersion power of 500 mg cream is 4.0-4.3 cm in diameter and viscosity is 7500 Cps. Analysis of the determination of the levels of the two components was carried out by the HPLC method C-18 column with the mobile phase of methanol: water (90: 10). Betamethasone valerate and neomycin sulfate levels in formulas made HPLC methods were 94.15%, and 136.56%, respectively and using AUC spectrophotometry methods were 107.98% and 94.81%.
CONCLUSION:
Cream that made by new formula with vitamin E shows good result in physical evaluation. HPLC methods with a mobile phase of methanol: water (90:10) was not recommended, while the AUC spectrophotometry method shows the valid result of quantitative analysis of betamethasone valerate and neomycin sulfate in cream.
Keywords: Betamethasone valerate, Neomycin sulfate, Cream, Spectrophotometry, Area under curve
Introduction
The UV spectrophotometry is drug analysis method that able to determine the levels of multi-component compounds which have overlapping problems in UV spectra [1], [2]. Multi-component drug products are increasingly distributed to patients, and the determination of each substance level in the mixture of drug components is need to find simple, fast, validated and safe method for researchers. Various methods for determining multi-component levels in tablet preparations have been developed [3].
The spectrophotometric method had been modified so that it can be used for mixed drug analysts that have more than two maximum wavelengths. The spectrophotometric technique applied in the manufacture of quantitative calibration curves in adjacent spectral analysis is very important in the quality control. The multi-component product consisting of 2 or more drug components usually had an adjacent wavelength when the spectrum overlaps. In addition, the spectrophotometric technique can be applied to analyze multi-component drug [4].
The High Performance Liquid Chromatography (HPLC) method can be used to determine the concentration of drug combinations in preparations or product [5]. In the implementation of this method, optimization must be done by using columns that correspond to the mobile phase that can separate the mixture. By using the mobile phase with a variety of mixtures and comparisons of the mobile phase mixture of water methanol it is expected that the HPLC method can produce a separation system with high speed and efficiency while being supported by advances in column technology, high pressure pump systems, and highly sensitive and diverse detectors, analyze various samples qualitatively and quantitatively, both in single and mixed components [6].
The analysis method for determining the mixture content of betamethasone valerate and neomycin sulfate with the HPLC and spectrophotometric methods has never been done. To be able to determine the content of the mixture by the application method of spectrophotometry and HPLC is influenced by the success of simultaneous analysis and type of solvent, absorption wavelength, optimization and flow rate to obtain results that meet the validation requirements with levels that meet the requirements of Indonesian Pharmacopoeia.
Betamethasone valerate is white powder, difficult to dissolve in water, rather difficult to dissolve in ethanol, and is an active semi-synthetic pharmaceutical raw material which is glucocorticoid. Betamethasone can be determined using spectrophotometry methods, betamethasone valerate in ethanol has a maximum wavelength of 240 nm (A11 = 390a) [7]. Neomycin sulfate is a sulfate salt from neomycin sulfate, which has usually use as antimicrobial agent. Based on Indonesian pharmacopeia, the analysis on neomycin sulfate is using microbial testing [5]. Another study shown that absorption ration and chemometric spectrophotometry methods was valid for determination betamethasone valerate and neomycin sulfate in cream [8], [9].
Thus, the present study aimed to make a formula containing vitamin E and make a comparison of determine levels of betamethasone valerate and neomycin sulfate in creams using High Performance Liquid Chromatography and Spectrophotometry Area Under Curve methods.
Material and Methods
The present study was conducted at Research laboratory in Faculty Pharmacy Universitas Sumaera Utara, Medan, Indonesia. The tools used in this study included a set of Ultra Violet Spectrophotometer instruments, complete High-Performance Liquid Chromatography (Shimadzu Prominence series) Whatman Cellulose Nitrate membrane filter 0.45 μm, Cellulose Nitrate 0.2 μm and PTFE 0.5 μm; analytical balance (Boeco BBL31); pH meter (Hanna) and glassware.
The material used were analytical grade from Merck, namely ethanol, methanol, phosphate buffer pH 7.2, sodium hydroxide, distilled water (PT Ikapharmindo Putramas), stearic acid, vitamin E, glycerin, sodium bi-borate, triethanolamine, betamethasone valerate and neomycin sulfate standard obtain from PPOM Jakarta.
The preparation of cream includes the smelting process and the emulsification process. Immiscible components with water are thawed together in a water bath at a temperature of 70-75°C, while all aqueous solutions that are heat-resistant and components that dissolve in water were heated at the same temperature as the fat component. Then the aqueous solution is slowly added to the liquid fat mixture and stirred constantly, the temperature is maintained for 5-10 minutes to prevent crystallization from the material. Next the mixture is slowly cooled with continuous stirring until the mixture thickens. After thickening, betamethasone valerate and neomycin sulfate, the active compounds were added to be mashed little by little and slowly so that the cream did not separate until homogeneous. The evaluation of cream preparations carried out was organoleptic, including odor, color, texture of preparation, and consistency using respondents. Then an evaluation of pH, evaluation of dispersion, and viscosity conducted to the cream.
Analysis of the active compound by using spectrophotometry method procedure started by making of standard spectrum. The standard of betamethasone valerate was weighed 50 mg, into a 100-ml flask, and dissolve using 70% ethanol so that a solution with concentration 500 µg/ml was obtained. This solution then diluted to obtain a concentration of 11 µg/ml. While for neomycin sulfate, the standard was weighed 100 mg into a 100 ml flask, and dissolve using 70% ethanol so that a solution with concentration 1000 µg/ml was obtained. This solution then diluted to obtain a concentration of 170 µg/ml. The calibration curve was made by measuring absorption from various concentrations of standard by diluting the standard solution to a concentration of 5.5; 11.5; 15.5; 20.5 µg/ml for betamethasone valerate and 85; 170; 255; 340 µg/ml for neomycin sulfate. Then the determination of the maximal absorption spectrum of betamethasone valerate and neomycin sulfate was carried out by making a mixture of betamethasone valerate and neomycin sulfate standard mixtures. The area under curve (AUC) absorption spectrum was carried out using the UV probe 2.42 software to obtain the spectrum. This software operated calculation of the area value at the maximum length of the two active compounds. The absorption spectrum produced was then made into a spectrum of ratios for each betamethasone valerate and neomycin sulfate, then the making of the AUC spectrum and carried out with the level determination as in the sample.
The method is validated with validation parameter accuracy, precision, linearity, limit of detection (LOD) and limit of quantization (LOQ). Accuracy test is done by standard addition method [10]. The precision determination was done based on relative standard deviation (RSD) values with relative standard deviation requirements is worth less than 2% [11]. Linearity was the ability of the analytical method to obtain the results that are in accordance with the range of concentrations of certain analytes. The regression used in the calibration curve is obtained from the equation y = ax + b. This equation will produce a relation coefficient (r). This coefficient of relations is used to determine the linearity, LOD and LOQ of an analytical method used [10], [11].
The HPLC method for cream analysis was done by using C18 column, and the mobile phase of methanol: water with comparison optimization first. The HPLC analysis procedure includes conditioning the HPLC, determining the mobile phase composition between methanol and water, determining the optimum flow rate of methanol and water, determining the levels of betamethasone valerate and neomycin sulfate and calculating the respective levels.
Results
The formula of cream was made based on the Table 1. It shows good result of physical evaluation of cream.
Table 1.
Formula of cream
| No. | Formula | |
|---|---|---|
| 1 | Betamethasone valerate | 5 mg |
| 2 | Neomycin sulfate | 25 mg |
| 3 | Stearic acid | 0.7061 g |
| 4 | Glycerin | 0.4972 g |
| 5 | Na biborate | 0.0124 g |
| 6 | TEA | 0.0497 g |
| 7 | Vitamin E | 0.0049 g |
| 8 | Water | 3.7294 g |
| Total | 5 g | |
| Oil phase | Stearic acid and vitamin E | |
| Water base | Glycerin, Na bi-borate, TEA, water | |
The organoleptic test was intended to see the physical appearance of the cream, which included shape, color and smell where the results obtained were in the form of semi-solid, odorless, white (unchanged), homogeneous, pH 7 with spread power 4.0-4.3 cm and viscosity 7500 Cps.
The maximum absorption spectrum of neomycin sulfate and betamethasone valerate can be seen in Figure 1.
Figure 1.

Maximum absorption spectrum of neomycin sulfate (170 µg/ml) and betamethasone valerate (11 µg/ml)
The absorption spectrum of neomycin sulfate and betamethasone valerate in different concentrations were shown in Figure 2 and Figure 3.
Figure 2.

Absorption spectrum of Neomycin sulfate at concentrations 85 µg/ml-340 µg/ml
Figure 3.

Absorption spectrum of Betamethasone valerate at concentration 5.5 µg/ml-20.5 µg/ml
Measurement of the absorption spectrum of betamethasone valerate and neomycin sulfate standard mixture was carried out based on a comparison of sample concentration (drug). Where the concentration of neomycin sulfate and betamethasone valerate was in ratio 1 to 5. Neomycin sulfate standard reach maximum absorption that meets the law of Lambert-beer, in the concentration of 170 µg/ml.
The standard mixture of neomycin sulfate and betamethasone valerate concentration were 170 µg/mL and 11 µg/mL produces a spectrum similar to the betamethasone valerate spectrum because neomycin sulfate has insignificant absorption so that the mixture does not affect the shape of the standard absorption spectrum. The standard and cream mixture spectrum can be seen in Figure 4.
Figure 4.

The Absorption Spectrum of Neomycin sulfate and Betamethasone valerate standard mixture in cream
The sample solution preparation was done by the standard addition method until it reaches the maximum concentration. Addition of neomycin sulfate carried out with a ratio of 1: 5, where the measured Neomycin sulfate uptake did not meet the Lambert-beer law (absorption is not in the range 0.2-0.6), so that it is added until it reaches maximum concentration. A number of samples were analyzed by addition of the analytes mixed, and analyzed, again. The difference between the two results is compared with the actual level.
The prepared sample was then measured at a wavelength of 200-400 nm. The absorption spectrum of the samples obtained was a mixture of the spectrum of neomycin sulfate and betamethasone valerate to obtain the area under curve by the wavelength 244-254 nm for neomycin sulfate and 240-250 nm for betamethasone valerate, then the concentration is calculated using equations.
Spectrum of AUC neomycin sulfate and spectrum of AUC Betamethasone valerate in cream is seen in Figure 5.
Figure 5.

AUC Spectrum of Neomycin sulfate and Betamethasone valerate in Cream
The value of the area under the neomycin sulphate and betamethasone valerate curves in cream can be seen in Table 2 and the levels of the active compounds in cream preparations can be seen in Table 3.
Table 2.
Area under curves of neomycin sulfate and betamethasone valerate
| No. | Area under curves of neomycin sulfate | Area under curves of betamethasone valerate | ||
|---|---|---|---|---|
| 244 nm | 240 nm | 250 nm | 254 nm | |
| 1 | 0.235 | 0.189 | 0.300 | 0.327 |
| 2 | 0.236 | 0.189 | 0.299 | 0.327 |
| 3 | 0.236 | 0.189 | 0.299 | 0.328 |
| 4 | 0.236 | 0.189 | 0.299 | 0.327 |
| 5 | 0.236 | 0.189 | 0.299 | 0.327 |
| 6 | 0.236 | 0.189 | 0.300 | 0.328 |
Table 3.
Level of neomycin sulfate and betamethasone valerate in cream
| No. | Active compounds | Level of active compounds | Composition of active compounds | Requirements |
|---|---|---|---|---|
| 1 | Neomycin sulfate | (94.81 ± 2.6958)% | 25 mg | (90-135)% |
| 2 | Betamethasone valerate | (107.98 ± 1.6491)% | 5 mg | (90-110)% |
Determination of the maximum wavelength that has been done shows that each compound has a different maximum wavelength. The maximum wavelength of the research results for betamethasone valerate and neomycin sulfate is 240 nm.
Chromatogram of each raw compound with the mobile phase of methanol: water with a ratio of 90: 10 for optimal results after optimization.
Chromatogram samples of cream preparations can be seen in Figures 6 and 7.
Figure 6.

The chromatogram of betamethasone valerate at concentration 11 μg/ml and retention time 5.3 minutes
Figure 7.

The chromatogram of neomycin sulfate at concentration 50 μg/ml and retention time 2.7 minute
Figure 8 is the chromatogram of a cream. The retention time used for testing was 5.3 minutes for betamethasone valerate and 2.7 minutes for neomycin sulfate.
Figure 8.
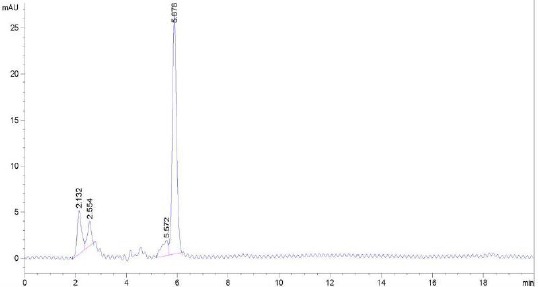
The chromatogram of cream
The results of betamethasone valerate and neomycin sulfate levels in cream were describe in Table 4.
Table 4.
The betamethasone valerate and neomycin sulfate levels in cream
| No | Name Sample | Betamethasone valerate | Neomycin Sulfate | ||
|---|---|---|---|---|---|
| 1 | Standard | 100 μg/ml | 46,71983 | 11 μg/ml | 411,6 |
| 2 | Cream | 109.46 % | 91.96 % | ||
| 119.70 % | 181.37 % | ||||
| 79.92 % | 117.32 % | ||||
| 83.98 % | 149.41 % | ||||
| 83.70 % | 148.93 % | ||||
| 88.12 % | 130.37 % | ||||
| 3 | Level of active compound | 0.9415 ± 0.1338 mg/g | 6.8279 ± 11.8105 | ||
| 94.15 % | 136.56 % | ||||
The levels of neomycin sulfate and betamethasone valerate in cream were 90-135% and 90-110%, respectively. The relative standard deviation (RSD) for neomycin sulfate and betamethasone valerate were 1.73% and 0.92%. Neomycin sulfate and betamethasone valerate have good precision because both RSD values are less than 2%. The validation parameters tested were accuracy, precision, linearity, limit of detection (LOD), and limit of quantization (LOQ). Accuracy tests are expressed in percent recovery which is determined by the standard addition method. In this study validation tests were carried out with a standard addition method in cream samples.
Accuracy tests were carried out by making three concentrations of samples with a specific range of 80%, 100%, and 120% calculated from the equality of weighing on sample concentration, each specific range consisted of three repetitions containing 70% analyte and 30% standard. The spectrum absorption of neomycin sulfate and betamethasone valerate for the recovery test can be seen in Figure 9.
Figure 9.

The spectrum absorption of neomycin sulfate and betamethasone valerate for the recovery test
The percent recovery was eligible for method validation with an average of 98%-102% for neomycin sulfate was 99.44% and betamethasone valerate was 100.07%. The relative standard deviation (RSD) meets the precision requirements because each was less than 2% with 1.54% for neomycin sulfate and 0.87% for betamethasone valerate. The limit of detection and limit of quantization was illustrated in table 5.
Table 5.
The validation methods parameters
| No | Active Compounds | % Recovery | RSD | LOD | LOQ |
|---|---|---|---|---|---|
| 1 | Neomycin sulfate | 99.44% | 1.54% | 11.2184 µg/ml | 37.3949 µg/ ml |
| 2 | Betamethasone valerate | 100.07% | 0.87%. | 0.6481 µg/ ml | 2.1604 µg/ ml |
Discussion
Area under curve spectrophotometry is a method that performs the determination of levels by measuring and calculating the absorbance of the area under the curve of 2 wavelengths around the maximum absorption wavelength of one component of the drug, and this method is called the measurement method in the area under curve and abbreviated by the name of the AUC method. This method is one of the prevailing spectrophotometric methods which have no valid curve where no sharp peaks or spectra are a parabolic or broad spectrum. This method has a calculation of the integrated absorbance value between the two selected wavelengths λ1 and λ2.
Single form determination of betamethasone valerate and neomycin sulfate levels can be determined by ultra-violet spectrophotometry method. Betamethasone valerate has maximum absorption at 240 nm wavelength (A11 = 390a) in ethanol while neomycin sulfate has the maximum absorption at 285 nm (A11 = 39b) in water [7]. In other literature state that determination of levels of neomycin sulfate by ultraviolet spectrophotometric method has insignificant absorbance, with wavelengths of 230-360 nm [12].
Because the conditions for measuring multi-component levels are using the same solvent, the orientation of the solvent done to find the solvent that can dissolved the active compounds. The orientation was carried out by trying 2 treatments, namely treatment one dissolved the two-active compound with 50% ethanol and the second treatment dissolved the two-active compound with 70% ethanol. Based on the orientation results showed that 70% ethanol can dissolve the two active substances of the drug. Determination of the maximum absorption spectrum of neomycin sulfate and betamethasone valerate was made in a wavelength range of 200-400 nm. Based on the results of the qualitative absorption spectrum, the maximum wavelength of neomycin sulfate (concentration 170 µg/ml) was obtained at 244 nm and for betamethasone valerate (concentration 11 µg/ml) at 240 nm.
The second absorbance point of the wavelength was not the wavelength point which has maximum absorptions, but the point that was bound to the curve is the wavelength point on the left and right side of the peak area. The calculation of area was by calculates the area bound by the curve and horizontal axis. The axis is chosen by entering the wavelength range where the area must be calculated. This wavelength range is chosen based on repeated observations so that it gets linearity between the area under the curve and concentration. The spectrum mentioned was used to calculate AUC. Thus, a calibration curve can be constructed by plotting concentration versus AUC.
High Performance Liquid Chromatography (HPLC) is a separation system with high speed and efficiency, because it is supported by advancements in column technology, high pressure pump systems and very sensitive and diverse detectors so as to be able to analyze various samples qualitatively and quantitatively, both in single components or mixture. HPLC is a method often used to analyze drug compounds. HPLC can be used to check the purity of active compounds, supervise the process of synthesis and quality control [5], [13]. Among the ratio of mobile phase that had identified, (90: 10) is the best mobile phase for betamethasone valerate and neomycin sulfate.
In conclusion, cream that made by new formula with vitamin E shows good result in physical evaluation. HPLC methods with various ratio of methanol: water was conducted to obtain the best mobile phase for betamethasone valerate and neomycin sulfate analysis. The best mobile phase of methanol: water (90: 10), but this method not recommended, while the AUC spectrophotometry method shows the valid result for quantitative analysis of betamethasone valerate and neomycin sulfate in cream.
Acknowledgment
We are gratefully Rector of Universitas Sumatera Utara and Chairman of the USU Research Institutes for financial support in order to carried out this research.
Footnotes
Funding: This work was supported by the Universitas Sumatera Utara grant numbers 447/UN5.2.3.1/PPM/ KP-TALENTA USU 2018
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Asra R, Rivai H, Astuty W. Pengembangan dan Validasi Metode Analisis Betametason Tablet dengan Metode Absorbansi dan Luas Daerah di Bawah Kurva Secara Spektrofotometri Ultraviolet Pengembangan dan Validasi Metode Analisis Betametason Tablet dengan Metode semi sintetis aktif yang me. J Farm Higea. 2017;9(2):118–26. [Google Scholar]
- 2.Rivai H, Astuty W, Asra R. Pengembangan dan Validasi Metode Analisis Betametason dalam Tablet dengan Metode Absorbansi dan Luas Daerah di Bawah Kurva Secara Spektrofotometri Ultraviolet. J Sains dan Teknol Farmasu. 2017;19(1):s52–7. [Google Scholar]
- 3.Bendre SD, Ghule PJ. Analytical Method Development, Validation, and Assay of Betamethasone Dipropionate Craem by HPLC Method. Int Res J Pharm. 2016;7(12):74–83. https://doi.org/10.7897/2230-8407.0712151. [Google Scholar]
- 4.Muchlisyam Pardede TR. Spectrofotometri dan Analisis Multikomponen Obat. Medan: USU Press; 2017. [Google Scholar]
- 5.Ditjen POM RI. Farmakope Indonesia [Internet] 4th ed. Jakarta: Departemen Kesehatan Republik Indonesia, Departemen Kesehatan RI; 1995. Available from: https://doi.org/10.6066/jtip.2013.24.2.121 . [Google Scholar]
- 6.Harahap Y, Amalia GA, Maggadani BP. Analysis of Rifampicin in Dried Blood Spots Using High Performance Liquid Chromatography. Asian J Sci Res. 2018;11(2):232–9. https://doi.org/10.3923/ajsr.2018.232.239. [Google Scholar]
- 7.Moffat AC, Osselton MD, Widdop B. Clarke's Analysis of Drug and Poisons. 4th ed. London: Pharmaceutical Press; 2011. [Google Scholar]
- 8.Bachri M, Reveny J, Permata YM, Situmorang CEA. Validation of Intersection Absorption Spectrum For Simultaneous Determination of Betamethasone Valerate and Neomycin Sulfate in Cream. Rasayan J Chem. 2019;12(1):232–9. https://doi.org/10.31788/RJC.2019.1215013. [Google Scholar]
- 9.Bachri M, Permata YM, Permadi R. Validation and Simultaneous Estimation of Betamethasone Valerate and Neomycin Sulfate Cream By Absorbance Ratio Spectrophotometry Methods. Int Res J Pharm. 2019;10(2):48–52. https://doi.org/10.7897/2230-8407.100240. [Google Scholar]
- 10.Harmita Petunjuk pelaksanaan validasi. Maj Ilmu Kefarmasian. 2004;1(3):117–35. https://doi.org/10.7454/psr.v1i3.3375. [Google Scholar]
- 11.Satiadarma K, Mulja M, Tjahyono DH, Kartasasmita RE. Asas Pengembangan Prosedur Analisis. 1st ed. Surabaya: Airlangga University Press; 2004. [Google Scholar]
- 12.Sweetman SC. Martindale The Complete Drug Reference. 36th ed. New York: Pharmaceutical Press; 2009. [Google Scholar]
- 13.Jasprica I, Mršić N, Dragić T, Cetina-Čižmek B. Determination of meglumine in pharmaceutical formulations using high performance liquid chromatography. Die Pharmazie-An International Journal of Pharmaceutical Sciences. 2011;66(12):916–9. [PubMed] [Google Scholar]


