Abstract
BACKGROUND:
Fermented foods were favourable because of its properties in enhancing the shelf life, safety, function, sensory and nutrition. There are many fermented foods tested in vitro as an α-glucosidase enzyme inhibitor. Dengke naniura is one of Indonesia’s traditional food made using fermentation.
AIM:
To identify lactic acid bacteria (LAB) strains in dengke naniura and its properties in inhibiting the α-glucosidase enzyme.
METHODS:
The carp were sacrificed, and soaked with rough lemon for 6 hours then spices added to it for another 1 hour. Then the isolation of LAB conducted using a serial dilution of the samples. The selected isolates of the LAB were then characterised by its morphology under the microscope, gram staining, growth at 15°C and 45°C and biochemical identification. The isolates were then tested for its inhibiting properties against the α-glucosidase enzyme.
RESULTS:
The isolates (DL-109 and DL-107) were a gram-positive, nonspore-forming and non-motile rod. The Physiological and biochemical properties of the isolates confirm its LAB properties. On the test against α-glucosidase enzyme activity inhibition, isolate DL-109 LAB (4) showed dominant activity with very low IC50 compared to Acarbose (IC50 = 128.06 ppm) and DL-107 (46.32 ppm) while at the lowest dosage of 25 µg/ml DL-109 showed activity as much as 54.76%.
CONCLUSION:
These findings concluded that the isolates were LAB by its properties and can be used for lowering blood glucose in term of inhibition of the α-glucosidase enzyme.
Keywords: Lactic acid bacteria, Traditional food products, Dengke naniura
Introduction
The research of LAB (probiotics) in food is blooming, not only because of the health it served, but also its properties which obstruct the growth of pathogen [1] and food spoiling bacteria, so that the LAB improve the quality of the food [2]. Nowadays, the traditional way of serving food products has been chosen over the modern way because of long-proven safety while being served for many centuries [3]. The Fermentation is playing an important role in serving the safety of traditional food [4]. The basic technique, using maintaining the low pH using organic acids will serve not only as protein hydrolytic but also preventing the growth of spoiling bacteria [5] while promoting acid-resistant bacteria like the LAB to grow [6]. The famous dengke naniura among Batak people served without additional heat along the process and undergo the fermentation process, while the fish used is common carp (Cyprinus carpio). The common carp was acidified with an organic acid (Citrus jambhiri extract) and then added to it spices, and it stands for 3-9 hours without cooking process [7]. Not only the spices but the carp also possess Bacilli that may serve as the source of the LAB in dengke naniura [8]. The LAB especially Lactobacillus as the living microorganism being unknown by the ancient people, but after much exhaustive research, had brought an intention when it was proved to be improving the immune system [9], intestine microflora modification and antipathogenic effect [10] and particularly help the diabetes type 2 by inhibiting the α-glucosidase in intestine [11].
This work aimed to characterise the strains of LAB isolated from dengke naniura a traditional food from Batak tribe in Indonesia; we embarked on an effort to isolate and identify candidate probiotic lactobacilli from it; along with its properties in inhibiting the α-glucosidase enzyme.
Material and Methods
The carp fish (Cyprinus carpio) was purchased in the traditional market of Parapat, Simalungun regency, Sumatera Utara, Indonesia. All the standard spices for naniura (Citrus jambhiri, andaliman, cayenne, red onion, white onion, turmeric, rias, candlenut and table salt) were purchased in the traditional market of Balige, Tobasa regency, Sumatera Utara, Indonesia. The de Man, Rogosa and Sharpe (MRS agar medium and MRS broth) were obtained from Himedia lab, India. Acarbose, yeast α-glucosidase, p-nitrophenyl-α-D-glucopyranoside were obtained from Sigma (MO, USA). Other chemical and reagents used were of the pure and analytical grade.
The live carp fish with 1 kg weighted were sacrificed, washed with tap water followed by removing the gut, then were cutted from the centre axis of the fish into two identic part. The flesh then washed with sterilised water to remove extra blood. Then it was soaked with the juice of 300 g rough lemon (Citrus jambhiri) and an additional of 3% of salt weight per weight of the samples, allowed to stand for 6 hours. Then all the spices were added into it (45 g red onion, 12 g garlic, 45 g powdered candlenut, 56 g turmeric, 20 g Sichuan pepper, 50 g cayenne, 162 g powdered torch ginger) and allowed to stand for 1 hour covered along with the soaking [7].
Dengke Naniura of common carp (Cyprinus carpio) were used for the isolation of lactic acid bacteria. Ten grams of sample was homogenised with 90 mL sterile NaCl solution (0.85%, w/v) to a homogenous suspension and then a tenfold serial dilution in NaCl solution (0.85%, was carried out, plated on MRS agar containing 1.0% CaCO3 and incubated at 37°C for two days. Isolated colonies of different appearances and surrounded by a halo were picked up and tested for catalase and Gram staining. Pure isolated colonies were tested for cell morphology (based on colour, shape, size) by phase-contrast microscopy, rough or smooth surface, Gram and catalase reaction. The strains with Gram-positive and catalase-negative were selected. The pure colonies were kept as stocks in MRS broth supplemented with 10% (v/v) glycerol at-18°C [12]. Before the test, all frozen stocks were recultivated in MRS broth and were incubated at 37°C. Growth of the isolates was observed in MRS broth after incubation at 15°C and 45°C [13].
This study was used 20 µl α-glucosidase (0.5 unit/ml) and 120 µl 0.1 M phosphate buffer pH 6.8. As the substrate were used p-nitrophenyl-α-D-glucopyranoside (p-NPG) 5 mM in the same buffer. As much as 10 µl of the samples dissolved in DMSO in various concentration, were mixed with enzymes solution in 96-well microplate and incubated at 37°C for 15 minutes. During the preparation and test, the enzyme must be taken care of under 2-8°C. Then were added 20 µl substrate solution and were incubated again at 37°C for 15 minutes. Enzymatic reaction halted with the addition of 80 µl Sodium carbonate 0.2 M. The test was done in triplicate. Samples were measured using a microplate reader at 405 nm [14]. Enzyme inhibitory effect was calculated by the formula:
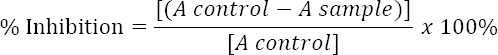
Linear regression equation between the samples and inhibition activity were used in determining IC50 number. The activity of inhibiting α-glucosidase enzyme by inhibitor concentration as much as 50% were called IC50 [15].
Results
The two isolates (DL-109 and DL-107) were a gram-positive, nonspore-forming and non-motile rod. Its physiological and biochemical characteristics, as shown in Figure 1, Figure 2 and Table 1.
Figure 1.
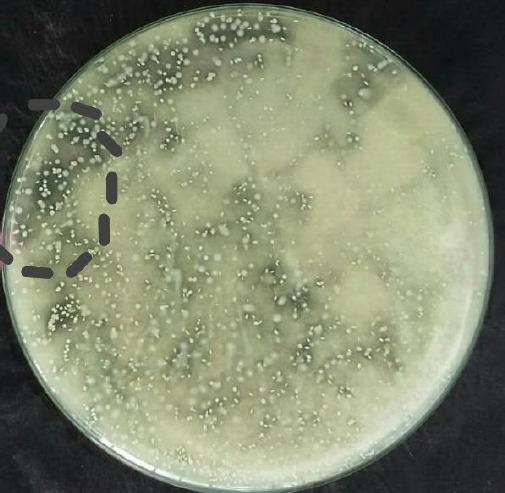
Lactic acid bacteria in MRSA growth media
Figure 2.
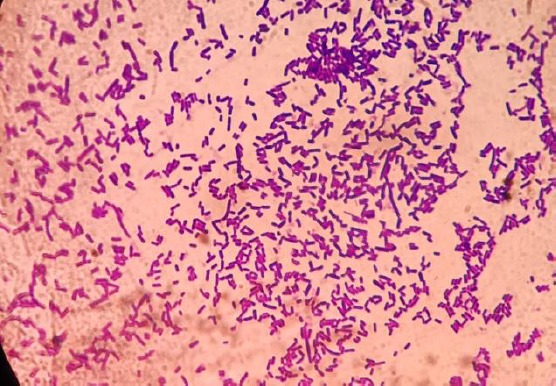
Cell form in gram staining from an isolate of dengke naniura
Table 1.
Morphology characteristic and biochemistry isolate from dengke naniura
| Characteristic | DL-109 | DL-107 |
|---|---|---|
| Colony Morphology | ||
| Colony Form | Small oval | Moderate spheric |
| Periphery colony form | Flat | Flat |
| Height of surface | Flat | Flat |
| Colony colour | White | White |
| Cell Morphology | ||
| Gram | + | + |
| Cell Form | Basil | Basil |
| Biochemistry | ||
| Catalase | - | - |
| TSIA | + | + |
| Gas | - | - |
| H2S | - | - |
| Fermentation Type | Homo | Homo |
| Growth on Temperature | ||
| 15°C | - | - |
| 37°C | ++ | ++ |
| 45°C | +++ | + |
| Growth on PH | ||
| PH 2 | + | + |
| PH 3 | ++ | +++ |
Characterisation of bacterial isolates was carried out morphologically by observing the shape of the colonies on the media visually and Misco-microscopically after gram staining. The biochemical characterisation was carried out by catalase test, Triple Sugar Iron Agar (TSIA), fermentation type test, growth at low pH and growth of isolates at low and high temperatures. Observation results can be seen in Table 1. On the microscope of bacterial isolates from dengke naniura showed that the bacteria were gram-positive, which was characterised by purple bacterial cells with round and rod shapes as shown in Figure 1 and Figure 2 [16].
Lactic Acid Bacteria have limited biosynthetic abilities. The acquisition of energy is solely dependent on fermentative metabolism that is carried out in its place. The catalytic test results on the four bacterial isolates from dengke naniura showed negative results, as shown in Table 1. In the review of bacterial isolates, there was no visible gas bubble when dripping with hydrogen peroxide (H2O2). Lactic Acid Bacteria can produce a variety of other metabolite compounds in addition to lactic acid, including hydrogen peroxide (H2O2) [17].
Catalase test is a test to identify microbes that are capable of producing catalase enzyme which can cleavage the hydrogen peroxide that is formed from aerobic respiration and is toxic to bacteria, to dihydrogen oxide (H2O) and oxygen (O2) which are no longer toxic [16] Triple Sugar Iron Agar (TSIA) test results in table 1 show positive results for all isolates. All isolates were able to ferment glucose, sucrose, and lactose contained in TSIA medium and produce acid as indicated by the colour changes in the slant and butt parts to yellow. Heterofermentative lactic acid, besides producing lactic acid is also ethanol and other acids such as acetic acid and CO2 gas [16]. TSIA test is a biochemical test to determine the ability of microbes to ferment glucose, sucrose and lactose contained in the medium.
From the test results, it was shown that all isolates did not produce gas and did not produce H2S, according to Freeman (1979) which stated that the genus Lactobacillus did not produce H2S. The fermentation type testing results show that four isolates are homofermentative because they do not produce gas. Homofermentative Lactic Acid Bacteria only produce lactic acid as the main product of fermentation. Growth test at 15°C, 37°C and 45°C for the four bacterial isolates from dengke naniura showed different growth at each temperature variation. At a temperature of 15°C, there is no visible growth of bacteria in the test tube. At a temperature of 37°, C DL109 and DL107 grew by producing moderate amounts of sediment. At a temperature of 45°C, DL109 can grow best with many deposits below the tube, DL107 grow not optimally with the fewest deposits. Based on the optimum temperature of growth, Lactic Acid Bacteria were grouped into two groups, namely mesophilic (optimal temperature of 25°C growth and maximum temperature of 40°C) and thermophilic (optimal temperature of 37°C growth and maximum temperature of 52°C) [16].
The activity of the isolates in inhibiting the α-glucosidase enzyme can be seen in Table 2.
Table 2.
Inhibition Activity and IC50 of the LAB
| Test solution concentration (µg/mL) | Absorbance (A) | S1-S0 (Asample) | % Inhibition | IC50 (ppm) | |
|---|---|---|---|---|---|
| S1 | S0 | ||||
| Blank | 0.016 (Ablank) | - | - | - | |
| Control | 0.294 (Acontrol) | - | - | - | |
| Acarbose | |||||
| 100 | 0.240 | 0.080 | 0.160 | 45.58 | 128.06 |
| 75 | 0.258 | 0.085 | 0.173 | 41.16 | |
| 50 | 0.277 | 0.092 | 0.185 | 37.07 | |
| 25 | 0.290 | 0.094 | 0.196 | 33.33 | |
| Isolate DL-109 | |||||
| 100 | 0.146 | 0.019 | 0.127 | 56.80 | (-) |
| 75 | 0.139 | 0.010 | 0.129 | 56.12 | |
| 50 | 0.136 | 0.005 | 0.131 | 55.44 | |
| 25 | 0.135 | 0.002 | 0.133 | 54.76 | |
| Isolate DL-107 | |||||
| 100 | 0.205 | 0.064 | 0.141 | 52.04 | 46.32 |
| 75 | 0.164 | 0.020 | 0.144 | 51.02 | |
| 50 | 0.154 | 0.007 | 0.147 | 50.00 | |
| 25 | 0.152 | 0.003 | 0.149 | 49.32 | |
Note: (-) for DL-109 means that the inhibition under 0 ppm based on calculation.
The results showed that the LAB isolated from dengke naniura possessed inhibitory activity better than the positive control (acarbose). The isolate DL109 have an unmeasured IC50 (below 0) and showed dominant inhibition compared to acarbose (128.06 ppm) and isolate (L107 (46,32 ppm). While the lowest concentration of DL109 (25 µg/ml) showed inhibition as much as 54.76%, which is higher than the highest concentration (100 µg/ml) of acarbose and DL107.
Discussion
The results shown above revealed the fermentation conducted by the LAB in dengke naniura. From the results, we have four isolates that have morphology and biochemical properties of lactic acid bacteria. Lactic acid bacteria have the ability to grow under low pH and by addition of 3% of salt favour the growth of lactic acid bacteria in this spontaneous fermentation [18]. The sources of LAB could be from the fish as it was reported having Bacilli (belonging to the phylum Firmicutes) by using pyrosequencing of 16S rRNA gene amplicons method revealed the bacteria including Lactobacillus and Leuconostoc bacteria [8]. Dengke naniura in its preparation also included many fresh spices which could also be the sources of the LAB, as it was reported in many vegetable fermentations that contains Lactobacillus and Leuconostoc as dominant species [18].
The viability of the LAB in fermented foods like dengke naniura depend not only by the pH level and salt, but also by the utilisation of bonded sugar creating an aglycon, and the aglycon may become one of the therapeutic ingredients produced by the LAB [19]. Under the stress condition such as low pH, the LAB synthesised a polyphosphate compound to protect itself under that condition. Some of our pure LAB colonies isolate were unable to grow under subsequent cultivation; this growth failure might because of particular nutrient needed by the LAB isolates [20].
All of the LAB isolates were having inhibition activity against the α-glucosidase enzyme, where the enzyme has the role in degrading polysaccharide into monosaccharide [21]. Thus inhibition makes the isolates can be an alternative way of treating type 2 diabetes. The study for LAB α-glycosidic breaking activity was not many, but this ability replaced by its ability to form exopolysaccharide from lignin (β-glycosidic bond compound). That exopolysaccharide produced by the LAB, protect the LAB from a hard condition such as dehydration and acidity even by bile acid [22].
Carp fish is the uncommon raw material for being fermented foods, but in Batak tribe of Indonesia, this food is delicacies to the local people. From the findings mentioned above, it showed that dengke naniura not only serve us the nutrition but also health especially to the grown-up people for the treatment of some health issues like diabetes since the LAB can reduce the sugar bonded or not and synthesising metabolites that benefit human health [19]. The dengke naniura can also benefit children with the lack of protein because dengke naniura have more protein that comes from its raw material. The nutritional value of dengke naniura not only come from the fish itself but also the spices added to it. The herbs and spices may serve special nutrient that can be utilized by the LAB to synthesised compound that brings benefit to us.
Footnotes
Funding: Universitas Sumatera Utara supported this research, Indonesia through “Hibah Penelitian Guru Besar” funding 2018
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Lievin-Le Moal V, Servin AL. Anti-Infective Activities of Lactobacillus Strains in the Human Intestinal Microbiota:from Probiotics to Gastrointestinal Anti-Infectious Biotherapeutic Agents. Clinical Microbiology Reviews. 2014;27(2):167–99. doi: 10.1128/CMR.00080-13. https://doi.org/10.1128/CMR.00080-13 PMid:24696432 PMCid:PMC3993101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wayah SB, Philip K. Pentocin MQ1:A Novel, Broad-Spectrum, Pore-Forming Bacteriocin From Lactobacillus pentosus CS2 With Quorum Sensing Regulatory Mechanism and Biopreservative Potential. Frontiers in Microbiology [Internet]. Frontiers Media SA; 2018:9. doi: 10.3389/fmicb.2018.00564. https://doi.org/10.3389/fmicb.2018.00564 PMid:29636737 PMCid:PMC5880951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mokoena MP, Mutanda T, Olaniran AO. Perspectives on the probiotic potential of lactic acid bacteria from African traditional fermented foods and beverages. Food & Nutrition Research. 2016;60(1):29630. doi: 10.3402/fnr.v60.29630. https://doi.org/10.3402/fnr.v60.29630 PMid:26960543 PMCid:PMC4785221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gawai KM, Prajapati JB. Safety Aspects of Fermented and Probiotic Foods. International Journal of Fermented Foods. 2017;6(1):45. https://doi.org/10.5958/2321-712X.2017.00005.9. [Google Scholar]
- 5.Baygar T, Alparslan Y, Kaplan M. Determination of changes in chemical and sensory quality of sea bass marinades stored at +4 (±1)°C in marinating solution. CyTA-Journal of Food. 2012;10(3):196–200. https://doi.org/10.1080/19476337.2011.614016. [Google Scholar]
- 6.Kindossi JM, Anihouvi VB, Vieira-Dalodé G, Akissoé NH, Hounhouigan DJ. Optimization of the marinating conditions of cassava fish (Pseudotolithussp.). fillet for Lanhouin production through application of Doehlert experimental design. Food Science & Nutrition. 2015;4(2):261–8. doi: 10.1002/fsn3.285. https://doi.org/10.1002/fsn3.285 PMid:27004115 PMCid:PMC4779494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manik M. Karakterisasi Kimia dan Mikrobiologis Serta Pengujian Potensi Probiotik dari Dengke Naniura Sebagai Makanan Tradisional Hasil Fermentasi Ikan Mas (Cyprinus carpio) Asal Kawasan Danau Toba. Disertasi. Fakultas Farmasi Universitas Sumatera Utara. 2017:51–3. [Google Scholar]
- 8.Van Kessel MA, Dutilh BE, Neveling K, Kwint MP, Veltman JA, Flik G, et al. Pyrosequencing of 16S rRNA gene amplicons to study the microbiota in the gastrointestinal tract of carp (Cyprinus carpio L.) AMB Express. 2011;1(1):41. doi: 10.1186/2191-0855-1-41. https://doi.org/10.1186/2191-0855-1-41 PMid:22093413 PMCid:PMC3226434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He S, Ran C, Qin C, Li S, Zhang H, de Vos WM, et al. Anti-Infective Effect of Adhesive Probiotic Lactobacillus in Fish is Correlated With Their Spatial Distribution in the Intestinal Tissue. Scientific Reports. 2017;7:1. doi: 10.1038/s41598-017-13466-1. https://doi.org/10.1038/s41598-017-13466-1 PMid:29038557 PMCid:PMC5643340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faghfoori Z, Gargari BP, Saber A, Seyyedi M, Khosroushahi AY. The Investigation of The Diversity of Lactobacilli spp. And Assesment Their Some Probiotic Properties in Traditional Dairy Products in East Azerbaijan Province in Iran. Iranian Journal of Pharmaceutical Research. 2017;16(4):1538–1545. [PMC free article] [PubMed] [Google Scholar]
- 11.Bajpai VK, Han J-H, Nam G-J, Majumder R, Park C, Lim J, et al. Characterization and pharmacological potential of Lactobacillus sakei 1I1 isolated from fresh water fish Zacco koreanus. DARU Journal of Pharmaceutical Sciences. 2016;24:1. doi: 10.1186/s40199-016-0147-8. https://doi.org/10.1186/s40199-016-0147-8 PMid:26980217 PMCid:PMC4793658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawalata JH, Satiman U. Identification of Lactic Acid Bacteria Proteolytic Isolated from An Indonesian Traditional Fermented Fish Sauce Bakasang by Amplified Ribosomal DNA Restriction Analysis (ARDRA) International Journal of ChemTech Research. 2015;8(12):630–636. [Google Scholar]
- 13.Nguyen MT. The effect of temperature on the growth of the bacteria Escherichia coli DH5α. Saint Martin's University Biology Journal. 2006;1:87–94. [Google Scholar]
- 14.Salehi P, Asghari B, Esmaeili MA, Dehghan H, Ghazi I. α-Glucosidase and α-amylase inhibitory effect and antioxidant activity of ten plant extracts traditionally used in Iran for diabetes. J. Med. Plants Res. 2013;7:257–266. [Google Scholar]
- 15.Mohan C, Long KD, Mutneja M. An Introduction to Inhibitors and Their Biological Applications. EMD Millipore Corp. 2013:3–13. https://doi.org/10.13070/mm.en.3.158. [Google Scholar]
- 16.Surono IS. Yayasan Pengusaha Makanan dan Minuman Seluruh Indonesia (YAPMMI) Jakarta: TRICK; 2004. Probiotik, Susu Fermentasi dan Kesehatan. [Google Scholar]
- 17.Ouwehand A, Vesterlund S. Antimicrobial Components from Lactic Acid Bacteria. Lactic Acid Bacteria. 2004:389–403. https://doi.org/10.1201/9780824752033.ch11 PMCid:PMC522068. [Google Scholar]
- 18.Reina LD, Pérez-Díaz IM, Breidt F, Azcarate-Peril MA, Medina E, Butz N. International Journal of Food Microbiology. Vol. 203. Elsevier BV; 2015. Characterization of the microbial diversity in yacon spontaneous fermentation at 20°C; pp. 35–40. https://doi.org/10.1016/j.ijfoodmicro.2015.03.007 PMid:25777679 PMCid:PMC4587664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazlan FA, Annuar MSM, Sharifuddin Y. Biotransformation of Momordica charantiafresh juice byLactobacillus plantarumBET003 and its putative anti-diabetic potential. Peer J. 2015;3:e1376. doi: 10.7717/peerj.1376. https://doi.org/10.7717/peerj.1376 PMid:26539336 PMCid:PMC4631465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin H, Sun Q, Pan X, Qiao Z, Yang H. Microbial Diversity and Biochemical Analysis of Suanzhou:A Traditional Chinese Fermented Cereal Gruel. Frontiers in Microbiology. 2016:7. doi: 10.3389/fmicb.2016.01311. https://doi.org/10.3389/fmicb.2016.01311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toeller M. α-Glucosidase inhibitors in diabetes:efficacy in NIDDM subjects. European Journal of Clinical Investigation. 2010;24(S3):31–5. doi: 10.1111/j.1365-2362.1994.tb02253.x. https://doi.org/10.1111/j.1365-2362.1994.tb02253.x PMid:8001625. [DOI] [PubMed] [Google Scholar]
- 22.Owusu-Kwarteng J, Tano-Debrah K, Akabanda F, Jespersen L. Technological properties and probiotic potential of Lactobacillus fermentum strains isolated from West African fermented millet dough. BMC Microbiology. 2015;15:1. doi: 10.1186/s12866-015-0602-6. https://doi.org/10.1186/s12866-015-0602-6 PMid:26560346 PMCid:PMC4642623. [DOI] [PMC free article] [PubMed] [Google Scholar]


