Abstract
AIM:
The objective of this study was to evaluate the inhibitory activity of Picria fel-terrae Lour on Nitric Oxide production toward RAW 264.7 cells.
METHODS:
The extraction was obtained by maceration method using n-hexane, ethyl acetate and ethanol solvents and then nitric oxide (NO) production was obtained using Griess reagent.
RESULTS:
Extract of Picria fel-terrae Lour herbs can reduce the NO production toward RAW 264.7 cells with induced by lipopolysaccharide has obtained nitric concentrations 12.5 and 25 μg/mL from n-hexane extract (72.50 ± 4.51 and 10.42 ± 1.82), ethyl acetate extract: (88.33 ± 6.51 and 30.83 ± 6.86), ethanol extract: (75.00 ± 1.91 and 22.08 ± 2.53).
CONCLUSION:
n-hexane extract of Picria fel-terrae Lour Herbs has a high potential to reduce the NO production in LPS-stimulated RAW 264.7 cells compared to ethyl acetate and ethanol extracts of Picria fel-terrae Lour Herbs.
Keywords: Picria fel-terrae Lour Herbs Extract, Nitric Oxide production, Immunosuppressive effects
Introduction
Nitric oxide (NO) is a necessary molecule to protect against various pathogens such as bacteria, viruses, fungi, and parasites [1], [2]. Under normal physiological conditions, NO plays a notable role in the regulation of various pathophysiological processes such as neuronal communication, vasodilatation, and neurotoxicity. However, overproduction of NO induces tissue damage associated with acute and chronic inflammations. Therefore, many researchers developed new drug as a potential inhibition on NO production related to the treatment of chronic inflammatory diseases [2]. Macrophages are significant components of the mammalian immune system, and they play a key role by providing an immediate defence against foreign agents before leukocyte migration and production of various pro-inflammatory mediators including the short-lived free radical NO. Lipopolysaccharide (LPS), a component from the cell walls of gram-negative bacteria is one of the most efficacious activators of macrophages and involves the production of pro-inflammatory cytokines. Therefore, inhibition of NO production in LPS-stimulated RAW 264.7 cells is one of the possible ways to screen various anti-inflammatory drugs [2].
Poguntano (Picria fel-terrae Lour.) have been various modern pharmacological investigations indicated that the extract of Picria fel-terrae Lour exerts diuretic, antioxidant, antipyretic, anti-diabetic, anthelmintic, anti-inflammatory, hepatoprotective, cardioprotective, analgesic activities and have inhibits activity of hepatitis B virus [3], [4], [5], [6], [7], [8], [9], [10], [11]. It can be developed a co-chemotherapeutic regimen for breast cancer, and it has antioxidant and antiproliferative activities of ethyl acetate fraction [12], [13]. Therefore, the present study was aimed to evaluate reduced of Nitric oxide production in LPS-induced on Picria fel-terrae Lour herbs extract toward RAW 264.7 murine macrophage cell line.
Material and Methods
Fresh Picria fel-terrae Lour herbs were collected from Tiga Lingga village, Dairi regency, Sumatera Utara province, Indonesia. Lipopolysaccharide is obtained Escherichia coli bacteria O111.B4 (Sigma), Dexamethasone (Harsen), Griess reagent and Nitrite Standard Solution (Biotium), n-hexane, ethyl acetate, ethanol 96% were procured from Smart lab.
RAW 264.7 cells were obtained from Parasitology Laboratory, Faculty of Medicine, Gadjah Mada University. The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% Fetal bovine serum and 100 units/mL each of penicillin and streptomycin was grown at 37°C and 5% CO2 in humidified air [14].
The extracts were prepared by Yuandani et al., 2017. The dried material was sequently macerated, briefly an amount of 500 g of P. fel-terrae Lour herbs. The following extracts were obtained after removal of solvents under reduced pressure [14], [15].
The NO production assay was conducted according to a previous paper by Yuandani et al. [14]. Briefly, RAW 264.7 cells (3 × 103 cells/mL) were seeded in 96-well plates for 24 h. Then, cells were incubated with test samples (12.5 and 25 μg/mL) and dexamethasone (1.25 and 2.5 μg/mL) for another 24h, then stimulated with LPS (1 μg/mL). After incubation for 24h at 37°C, 5% CO2, the production of nitric oxide was determined by measuring the quantity of nitrite in the medium using Griess reagent (0.1% naphthyl ethylenediamine dihydrochloride in 2.5% phosphoric acid and 1% sulfanilamide). One hundred μL of Griess reagent was added to culture supernatant, then incubated for 10 min in a dark room. A microplate reader was used to measure absorbance at 595 nm, and a standard solution of sodium nitrite was used to calculate nitrite concentrations. The concentration of nitrite in the samples was determined concerning a sodium nitrite standard curve (Biotium catalogue #30100).
Data were expressed as means ± standard error minimum (SEM) of the mean from three independent experiments. Statistical analysis was conducted using SPSS software (version 22.0). Statistical comparisons were performed by one-way analysis of variance (ANOVA), followed by a Tukey HSD multiple comparison test. Differences were considered statistically significant at P < 0.05.
Results
The inhibitory activity of n-hexane, ethyl acetate and ethanol extracts from P. fel-terrae Lour on NO production toward RAW 264.7 cells.
All the samples tested revealed significant inhibition with inhibition value at concentration 12.5 and 25 μg/mL had decreased compared to LPS. As shown in Table 1, n-hexane extracts of P. fel-terrae Lour depicted the strongest NO inhibitory activity with a concentration value of 25 μg/mL (10.42 ± 1.82). However, its value was higher than that of dexamethasone as a positive control (5.83 ± 3.33). The negative control (LPS) shows the highest value of nitrite inhibitory too because the normal cell (CC) was used the normal cell did not release much nitrite like LPS-stimulated cells, so the inhibitory activity becomes high.
Table 1.
Mean of nitric oxide production and Tukey HSD post hoc test of nitrite concentration over the various concentration extract and dexamethasone measured in triplicate
| Samples | Mean of nitric oxide production ± SEM |
|---|---|
| CC | 2.67 ± 1.10b |
| LPS | 100.83 ± 2.20ac |
| NEPFH 12.5 | 72.50 ± 4.51abc |
| NEPFH 25 | 10.42 ± 1.82b |
| EAEPFH 12.5 | 88.33 ± 6.51ac |
| EAEPFH 25 | 30.83 ± 6.86abc |
| EEPFH 12.5 | 75.00 ± 1.91abc |
| EEPFH 25 | 22.08 ± 2.53abc |
| Dexamethasone 1.25 | 7.42 ± 4.53b |
| Dexamethasone 2.5 | 5.83 ± 3.33b |
Values are mean of three replicated determinations (n = 3) ± Standard error of the mean.a P < 0.05 vs Cells Control,bP < 0.05 vs LPS,c P < 0.05 vs Dexamethasone. NEPFH: n-Hexane Extract of Picria fel-terrae Lour Herbs, EAEPFH: Ethylacetate Extract of Picria fel-terrae Lour Herbs, EEPFH: Ethanol Extract of Picria fel-terrae Lour Herbs. CC: Cells Control, LPS: Lipopolysaccharides.
In this study, n-hexane, ethyl acetate and ethanol extracts of Picria fel-terrae Lour in reduced the NO production in RAW 264.7 cells with induced by LPS. NO production was measured as nitrite concentration in culture media and compared with normal cell (control) release lower NO production than compared with negative control (LPS) as shown in Figure 1.
Figure 1.
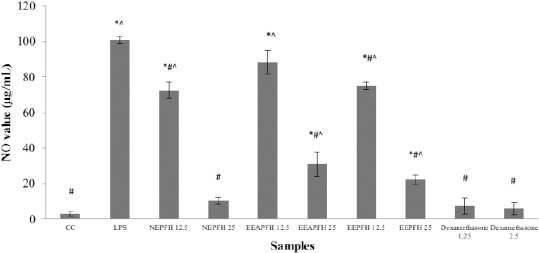
Inhibitory activity extracts of P.fel-terrae herbs on NO production by LPS-stimulated RAW 264.7 cells; NEPFH: n-Hexane Extract of Picria fel-terrae Lour Herbs; EAEPFH: Ethylacetate Extract of Picria fel-terrae Lour Herbs; EEPFH: Ethanol Extract of Picria fel-terrae Lour Herbs; CC: Cells Control; LPS: Lipopolysaccharides; Values are mean of three replicated determinations (n = 3) ± Standard error of the mean;*P < 0.05 vs Cells Control;# P < 0.05 vs LPS;^ P < 0.05 vs Dexamethasone.
Discussion
NO is a multifunctional signalling molecule. Thus the impact of the extract or compound on NO production likely has further effects on signalling pathways in many cell types [2], [16]. RAW 264.7 cell a murine macrophage cell line had been often used for the screening of anti-inflammatory drugs and immunomodulatory [2]. The extracts showed the reduced of NO production in cells indicating that the presence of antioxidant molecules would be responsible for the inhibitory action [13]. The results study demonstrated that the n-hexane extract significantly decreased the nitrite accumulation in LPS-stimulated RAW 264.7 cells. This is caused the secondary metabolite. The n-hexane extract of P. fel-terrae Lour herb contained steroids [13] likely dexamethasone. Dexamethasone is a steroid agent which can reduce NO production. It used as positive control. While ethyl acetate and ethanol extracts contained flavonoids, saponins, tannin, glycoside reduce NO production too [13], [18], [19], [20], [21], [22], [23].
The results of this study indicate that the n-hexane extract from Picria fel-terrae Lour Herbs has a high potential to reduce the production of NO in LPS-stimulated RAW 264.7 cells compared ethyl acetate and ethanol extract Picria fel-terrae Lour Herbs. The findings of this study provided evidence that supports the traditional use of Picria fel-terrae Lour Herbs in the treatment of inflammatory diseases and immunomodulatory agents.
Footnotes
Funding: This research was funding by PDUPT 2018 ministry of research technology and higher education
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Mu MM, Chakravortty D, Sugiyama T, Koide N, Takahashi K, Mori I, et al. Journal of Endotoxin Research [Internet] 6. Vol. 7. SAGE Publications; 2001. The inhibitory action of quercetin on lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophage cells; pp. 431–8. https://doi.org/10.1177/09680519010070060601. [DOI] [PubMed] [Google Scholar]
- 2.Joo T, Sowndhararajan K, Hong S, Lee J, Park S-Y, Kim S, et al. Saudi Journal of Biological Sciences [Internet] 5. Vol. 21. Elsevier BV; 2014. Inhibition of nitric oxide production in LPS-stimulated RAW 264.7 cells by stem bark of Ulmus pumila L; pp. 427–35. https://doi.org/10.1016/j.sjbs.2014.04.003 PMid:25313277 PMCid:PMC4191610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalimunthe A, Urip H, Rosidah G, Pandapotan NM. Evaluation of diuretic activity of Picria fel-terrae (Lour.). leaves extracts. Asian J Pharm Clin Resc. 2015;8:204–5. [Google Scholar]
- 4.Huang Y, Cimanga K, Lasure A, Poel VB. Biological activities of Picria fel-terrae Lour. Pharm World Sci. 1994;16:18. [Google Scholar]
- 5.Thuan ND, Ha DT, Thuong PT, Na MK, Bae K, Lee JP, et al. Archives of Pharmacal Research. 9. Vol. 30. Springer Nature; 2007. A phenylpropanoid glycoside with antioxidant activity from picria tel-ferae; pp. 1062–6. https://doi.org/10.1007/BF02980238 PMid:17958321. [DOI] [PubMed] [Google Scholar]
- 6.Zou J-M, Wang L-S, Niu X-M, Sun H-D, Guo Y-J. Journal of Integrative Plant Biology. 5. Vol. 47. Wiley; 2005. Phenylethanoid Glycosides from Picria felterrae Lour; pp. 632–6. https://doi.org/10.1111/j.1744-7909.2005.00082.x. [Google Scholar]
- 7.Harfina F, Bahri S, Saragih A. Pengaruh serbuk daun puguntano (Curanga fel-terrae Merr.) pada pasien diabetes mellitus. Journal of Pharmaceutics and Pharmacology. 2012;1(2):112–8. [Google Scholar]
- 8.Sitorus P, Harahap U, Barus T. Isolation of β-sitosterol from n-hexane extract of Picria fel-terrae Lour. leave and study of its antidiabetic effect in alloxan induced diabetic mice. 2014 [Google Scholar]
- 9.Sihotang Y, Silalahi J, Hadisahputra S, Hasibuan PA, Satria D. Cardioprotective effect of ethylacetate extract of poguntano (Picria fel-terrae Lour.). against doxorubicin-induced cardiotoxicity in rats. Cardioprotective Effect of Ethylacetate Extract of Poguntano (Picria fel-terrae Lour.) Against Doxorubicin-Induced Cardiotoxicity in Rats. 2016 [Google Scholar]
- 10.Patilaya P, Husori DI. Preliminary study on the anthelmintic activity of the leaf ethanolic extract of Indonesian Curanga fel-terrae (Lour.) Merr. Int J Pharmtech Res. 2015;8(3):347–51. [Google Scholar]
- 11.Zeng J, Pan X, Yang K, Wei Z, Chen C. Experimental study on the inhibitory effect on HBeAg and HBsAg excreted by 2215 cells of different extracts of Picria fel-tarrae Lour. China Medical Herald. 2010;7(16):27. [Google Scholar]
- 12.Satria D, Furqan M, Hadisahputra S. Rosidah. Combinational Effects Of Ethylacetate Extract Of Picria Fel-Terrae Lour and Doxorubicin On T47d Breast Cancer Cells. International Journal of Pharmacy and Pharmaceutical Sciences. 2015;7(7):73. [Google Scholar]
- 13.Satria D, Silalahi J, Haro G, Ilyas S, Hsb PA. Antioxidant and Antiproliferative Activities of an Ethylacetate Fraction of Picria Fel-Terrae Lour. Herbs. Asian Pacific journal of cancer prevention: APJCP. 2017;18(2):399. doi: 10.22034/APJCP.2017.18.2.399. https://doi.org/10.5220/0008359701900193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuandani Jantan I, Husain K. 4,5,4′-Trihydroxychalcone, 8,8′-(ethene-1,2-diyl)-dinaphtalene-1,4,5-triol and rutin from Gynura segetum inhibit phagocytosis, lymphocyte proliferation, cytokine release and nitric oxide production from phagocytic cells. BMC Complementary and Alternative Medicine. 2017;17(1) doi: 10.1186/s12906-017-1726-z. https://doi.org/10.1186/s12906-017-1726-z PMid:28399868 PMCid:PMC5387197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutagaol SH, Rosidah M, Satria D. Combination effect of ethylacetate extract leaves of Moringa oleifera L. and Doxorubicin against MCF-7 cell lines [Google Scholar]
- 16.Lander HM, Jacovina AT, Davis RJ, Tauras JM. Differential Activation of Mitogen-activated Protein Kinases by Nitric Oxide-related Species. Journal of Biological Chemistry [Internet]. American Society for Biochemistry &Molecular Biology (ASBMB); 1996;271(33):19705–9. doi: 10.1074/jbc.271.33.19705. https://doi.org/10.1074/jbc.271.33.19705 PMid:8702674. [DOI] [PubMed] [Google Scholar]
- 17.Venkatesha SH, Dudics S, Astry B, Moudgil KD. Control of autoimmune inflammation by celastrol, a natural triterpenoid. In: Flajnik M, editor. Pathogens and Disease [Internet] 6. Vol. 74. Oxford University Press (OUP); 2016. p. ftw059. https://doi.org/10.1093/femspd/ftw059 PMid:27405485 PMCid:PMC5985506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durga M, Nathiya S, Devasena T. Immunomodulatory and antioxidant actions of dietary flavonoids. Int J Pharm Pharm Sci. 2014;6(2):50–6. [Google Scholar]
- 19.Liu X, Jia L, Gao Y, Li B, Tu Y. Anti-inflammatory activity of total flavonoids from seeds of Camellia oleifera Abel. Acta Biochimica et Biophysica Sinica. 2014;46(10):920–2. doi: 10.1093/abbs/gmu071. https://doi.org/10.1093/abbs/gmu071 PMid:25189429. [DOI] [PubMed] [Google Scholar]
- 20.Bondonno CP, Croft KD, Ward N, Considine MJ, Hodgson JM. Nutrition Reviews [Internet] 4. Vol. 73. Oxford University Press (OUP); 2015. Dietary flavonoids and nitrate:effects on nitric oxide and vascular function; pp. 216–35. https://doi.org/10.1093/nutrit/nuu014 PMid:26024545. [DOI] [PubMed] [Google Scholar]
- 21.Gyeong-JIN Y, Il-Whan C, Gi-Young K, Byung-Woo K, Cheol P, Su-Hyun H, et al. International Journal of Molecular Medicine. 6. Vol. 35. Spandidos Publications; 2015. Anti-inflammatory potential of saponins derived from cultured wild ginseng roots in lipopolysaccharide-stimulated RAW 264.7 macrophages; pp. 1690–8. https://doi.org/10.3892/ijmm.2015.2165 PMid:25847675. [DOI] [PubMed] [Google Scholar]
- 22.Ahn S, Siddiqi MH, Noh H-Y, Kim Y-J, Kim Y-J, Jin C-G, et al. Science Bulletin [Internet] 8. Vol. 60. Elsevier BV; 2015. Anti-inflammatory activity of ginsenosides in LPS-stimulated RAW 264.7 cells; pp. 773–84. https://doi.org/10.1007/s11434-015-0773-4. [Google Scholar]
- 23.Jang K-J, Choi SH, Yu GJ, Hong SH, Chung YH, Kim C-H, et al. Experimental and Therapeutic Medicine. 3. Vol. 11. Spandidos Publications; 2015. Anti-inflammatory potential of total saponins derived from the roots of Panax ginseng in lipopolysaccharide-activated RAW 264.7 macrophages; pp. 1109–15. https://doi.org/10.3892/etm.2015.2965 PMid:26998045 PMCid:PMC4774435. [DOI] [PMC free article] [PubMed] [Google Scholar]


