Abstract
Formation of the vertebrate visual system involves complex interplays of cell-extrinsic cues and cell-intrinsic determinants. Studies in several vertebrate species demonstrate that multiple classes of signaling molecules participate in pattern formation of the eye and neurogenesis of the retina. Certain signals, such as hedgehog, BMP, and FGF molecules, are repeatedly deployed at varying concentration thresholds and in different cellular contexts. Accumulating evidence reveals a striking conservation of molecular mechanisms regulating the neurogenic process between Drosophila and vertebrate retinas. The remaining challenge is to understand how these well-characterized signaling pathways are activated and integrated to impact eye morphogenesis and retinal progenitor cell fate determination.
Keywords: Vertebrates, Eye morphogenesis, Retinogenesis, Extrinsic growth factors
1. Introduction
The vertebrate central nervous system (CNS) is comprised of a vast array of neuronal cell types. A major focus of developmental neuroscience is to decipher how neural progenitor cells give rise to distinct neuronal cell types that constitute a given region of the CNS. Accumulating evidence indicates that both cell-extrinsic cues and cell-intrinsic determinants play important roles in progenitor cell fate determination and their subsequent differentiation [1]. Significant progress has been made towards the identification of various cell-intrinsic factors and the characterization of biochemical mechanisms mediating various extrinsic signals involved in neural progenitor fate specification. An outstanding example illuminating the interplay between cell-extrinsic cues and cell-intrinsic determinants is the establishment of distinct neuronal phenotypes along the dorsal-ventral axis of the developing spinal cord, where graded morphogenic signals are translated into spatially restricted expression patterns of nuclear transcription factors that ultimately specify cell fates in a combinatorial fashion [2–5]. One of the remaining challenges is to elucidate regulatory mechanisms by which cell-extrinsic cues impact upon key cell-intrinsic determinants in the context of a specific CNS region to result in the appropriate biological readout at the transcriptional and morphological levels.
During vertebrate development, several major classes of cell–cell signaling pathways, including Notch, Hedgehog (Hh), Wnt, TGFβ/BMP, receptor tyrosine kinase (RTK), Jak/STAT, and nuclear receptor pathways, are repeatedly deployed to control progenitor cell fate choices [6]. Not surprisingly, all of these signaling systems appear to be involved in vertebrate eye morphogenesis and/or retinal neurogenesis. The biochemical mechanisms that mediate the various types of signals are divergent, ranging from the very direct mode of signal transmission found in small ligand triggered activation of nuclear receptors to the fairly complex mode of signal relay found in RTK-induced phosphorylation cascade. Nonetheless, one of the major consequences of extrinsic signaling is the alteration of cellular transcription profiles. This article focuses on recent advancements in understanding cell-extrinsic factors in vertebrate eye development, with a particular emphasis on signaling molecules affecting pattern formation of the eye and early retinogenesis.
2. Intrinsic programs versus cell-extrinsic cues: lessons from cell birthdating, lineage tracing, and transplantation studies
The vertebrate retina is derived from the anterior neural tube, and thus shares a common origin with the rest of the CNS. The mature retina is organized as a laminar neural network composed of specialized sensory neurons, interneurons, and projection neurons, which together accomplish the tasks of image detection, processing, and transmission. The early retinal primordium is a pseudostratified neural epithelium containing proliferating progenitor cells with extending processes contacting both the ventricular and vitreal surfaces of the epithelium. Throughout neurogenesis the nuclei of progenitor cells undergo a cell cycle-dependent movement, with occurrence of the DNA synthesis phase (S-phase) within the ventricular zone and the mitotic phase (M-phase) along the ventricular surface. Following the M-phase, progenitor cells give rise to different postmitotic neurons as well as new progenitor cells [7]. The cell bodies of postmitotic neurons exiting the cell cycle amidst neurogenesis are generally localized to their final laminar positions, which are either outside or embedded within the ventricular zone. Thus, from the onset to the completion of neurogenesis, the vertebrate retina contains a mixture of proliferating progenitors and postmitotic neurons distributed in a polarized manner between the ventricular and vitreal surfaces within a 100–200-μm thick epithelium.
Two common developmental features are shared among vertebrate retinas. First, the seven major retinal cell types are generated in a sequential yet overlapping order that is conserved among diverse vertebrate species, with retinal ganglion cells typically differentiating first and Müller glia last [8–11]. This chronological cell birth sequence may be a reflection of conserved molecular events underlying vertebrate retinogenesis. Second, cell-lineage analyses have demonstrated that vertebrate retinal progenitor cells are multipotent at different developmental stages, as progenies derived from individual progenitor cells can assume a variety of cell fates [12–16]. Furthermore, cell fate decisions can be made during or after the last mitotic division, since two daughter cells of a given progenitor often adopt distinct cell fates. The multipotency of retinal progenitor cells throughout neurogenesis suggests that local environmental factors play important roles in cell fate decisions.
Despite their persisting multipotency and common proliferative behavior, existing evidence indicate that retinal progenitor cells do not remain homogeneous or static throughout development [17]. Heterochronic transplantations have demonstrated that early and late retinal progenitor cells have distinct differentiation capacities when placed in similar environments [18–21]. In addition, molecular markers analyses have shown that proliferating progenitor cells are heterogeneous with regard to their gene expression profiles [22–26]. Thus, the intrinsic components that define properties of retinal progenitor cells, including but not limited to nuclear transcription factors, cell surface receptors, and intracellular signaling components, undergo progressive changes as development proceeds [17,27,28]. A current model of retinal development proposes that retinal progenitor cells progress through a series of “competent states” during development, and each competent state favors specification of one or more cell fates. The “competent states” are presumably defined by cell-intrinsic properties that determine the responsiveness of the progenitor cell to extrinsic cues and the developmental potential of the progenitor. Thus, according to this model extrinsic cues derived from the retinal environment as well as intrinsic properties of progenitors both contribute to the specification of retinal cell fates [29,30].
Consistent with the above model, several classes of transcription factors containing homeobox, bHLH, leucine zipper, or nuclear receptor motifs have been shown to be crucial in generating specific retinal cell types (see other articles in this issue) [27]. Increasing evidence also indicates that at least some of the cell-extrinsic cues impinging upon uncommitted progenitor cells and differentiating neurons are, in fact, derived from postmitotic retinal neurons. Several issues relevant to this model remain unresolved and are worthy of further exploration. First, do retinal progenitor cells progress through a finite number of competent states, which can be defined by gene expression profiles and corresponding developmental potentials, or continuously exist as a heterogeneous population at any given stage? Second, do all progenitor cells traverse a consecutive series of competent states during retinogenesis, or follow distinct paths of progression? Third, is the progression of progenitor cells during development propelled by cell autonomous programs or regulated by the changing retinal environment as a consequence of accumulating postmitotic neurons? In other words, what are the relative contributions of cell-intrinsic programs versus cell-extrinsic cues in determining competent states or cell fate choices of a progenitor cell? Finally, what are mechanisms by which extrinsic signals modulate progenitor cell-intrinsic properties and fate choices? Future analyses of progenitor cells at different developmental stages using powerful molecular tools now available will provide important clues needed o resolve these questions.
3. Hedgehog family of signaling molecules
The vertebrate hedgehog (Hh) proteins are an important family of secreted signaling molecules involved in a variety of developmental processes from embryonic pattern formation, cell fate specification, to cell proliferation [31]. In Hh producing cells, the Hh proteins are synthesized as precursors that undergo an autocleavage reaction to yield the N-terminal portion of the protein (Hh-N) covalently linked to a cholesterol moiety at its C terminus. Fully processed Hh proteins also receive an N-terminal palmitoyl group. Hh-N is active in intercellular signaling and can diffuse from its site of production within the extracellular space to signal to cells resides many cell diameters away, thus acting as a typical morphogen. In cells exposed to Hh, two transmembrane proteins Patched (Ptc) and Smoothened (Smo) are involved in mediating Hh signals. Binding of Hh to Ptc prevents Ptc from inhibiting Smo, thus resulting in the activation of Smo and subsequent transcriptional response through the Gli family of transcription factors.
During vertebrate eye morphogenesis and retinogenesis, Hh molecules are expressed in dynamic patterns and play multiple roles to influence tissue pattern formation and cell type specification. The sources that provide Hh signals include the anterior ventral midline tissues, the retinal pigmented epithelium (RPE), and specific types of differentiated retinal neurons. The cellular responses to Hh signals in the eye are determined by the local concentration of Hh signals as well as the intrinsic properties of cells within the Hh morphogen gradient.
3.1. Shh in eye pattern formation
Emergence of the bilaterally symmetrical vertebrate eye fields requires Hh signals emanating from the midline of the embryo during gastrulation and neurulation. At the anterior axial position where the optic primordium forms, Sonic hedgehog (Shh), a member of the Hh family, is expressed by the axial mesoderm, the prechordal plate, and the overlying ventral anterior neural tube. Germ line disruption of the Shh gene in mouse results in a single centrally positioned primitive optic vesicle [32]. Similarly, mutations in the human SHH gene cause a form of holoprosencephaly (HPE3), which is marked by fusion of the cerebral hemispheres, and in severe cases leads to the formation of cyclopic eyes [33]. Inhibiting Shh signals in chick embryos by the steroidal alkaloid cyclopamine, which binds and blocks the activity of Smo, causes cyclopia in a concentration-dependent manner [34]. These data indicate that Shh signals derived from the anterior ventral midline of the embryo play a critical role in the formation of separate vertebrate eye fields.
Molecular genetic studies in several vertebrate species have further established the function of Hh in the proximodistal (dorsoventral) pattern formation and ocular tissue specification of the eye. The optic vesicle initially forms as an evagination of the anterior neural tube, and then undergoes a base constriction to form the optic stalk and a ventral-lateral invagination to result in the double-layered optic cup. The outer layer of the optic cup differentiates into the retinal pigmented epithelium whereas the inner layer becomes the neural retina. The morphological transition of the optic vesicle to the optic cup coincides with formation of the three primary ocular tissue types, the optic stalk, the RPE, and the retina, and is accompanied by differential gene expression that demarcates these emerging ocular tissues. The portion of the optic primordium proximal to the midline, including the optic stalk and the ventral retina, initially expresses the paired domain gene Pax2 [35] and the Vax family of homeodomain proteins [36–40], whereas the portion distal to the midline, the RPE and the retina, initially express the homeo box containing genes Pax6 [41] and Rx (Rax) [42,43]. Subsequently, Pax6 expression in the presumptive RPE is down regulated and replaced by expression of the bHLH zipper gene Mitf [44] and transient expression of the homeo domain gene Otx2 [45], whereas Pax6 and Rx gene expression persists in the neural retinal primordium.
Overexpression of Shh in early zebrafish embryos causes a reduction of the Pax6 expression domain and an expansion of Pax2 expression domain, and consequently the formation of small eyes with expanded optic stalks [46,47]. Hh signals also promote the expression of Vax genes in zebrafish eye [40]. Similarly, elevating Shh signals using a retrovirus in chick during the optic vesicle to optic cup transition suppresses Pax6 and increases Pax2 and Vax gene expression, resulting in microphthalmia with an expanded optic stalk [48]. Reducing Shh signals during chick eye morphogenesis by a neutralizing antibody also results in microphthalmia with severe disruption of ventral eye structures [48]. In this case, reduced Shh levels caused decreased Pax2 and Vax expression in the ventral eye and optic stalk as well as the loss of Otx2 in the RPE layer. This suppression of proximal markers is accompanied by a compensatory increase of Pax6 expression in the presumptive RPE and optic stalk, resulting in the conversion of both the optic stalk and ventral RPE into the neural retina. Activating Hh signaling pathway by a dominant negative PKA mutant or inhibiting Hh signaling using cyclopamine in Xenopus embryos also results in defects of the eye along the proximodistal axis, especially in the ventral eye, and abnormal RPE differentiation [49]. In mouse, targeted removal of Shh signals from the ventral midline has not been reported. However, ablation of ventral forebrain tissues that express Shh in BF1-deficient mice resulted in dorsoventral patterning defects in the eye similar to those found in chick when Shh signals are reduced, including the conversion of the ventral PRE and optic stalk into the neural retina [50]. Moreover, mutational and molecular analyses in mouse have shown that Pax2 and Pax6 proteins mutually suppress the transcription activation of each other through direct binding to promoter/enhancer sequences, thus establish and refine the boundary between the optic stalk and the optic cup [51].
Manipulations of Hh signaling levels in zebrafish, chick, mouse, and Xenopus, thus lead to several important conclusions regarding the function of Shh in vertebrate eye pattern formation. First, the temporal requirement for proper Shh signal persists throughout the optic vesicle to optic cup transition and after formation of the optic cup [48–50]. Second, Shh signals emanating from the ventral midline play a critical role in pattern formation of the vertebrate optic primordium along the proximodistal axis. These Shh signals are likely forming a medial to lateral concentration gradient and define distinct ocular tissue identities by regulating expression of key transcription factors at distinct concentration thresholds (Fig. 1). In response to graded Shh, Pax2 and Vax genes are expressed in regions adjacent the midline and specify the optic stalk and ventral retinal fates, whereas Pax6 and Rx are expressed in regions further distal to the midline and defines the optic cup. In addition, Shh signal levels also play a role in regulating expression of the RPE determinants Otx2 and Mitf [44,45]. The precise molecular mechanism by which distinct Shh signal thresholds control the expression of various transcription factors along the proximodistal axis remains to be established. Third, the vertebrate optic primordium at the optic cup stage (including the optic stalk, the RPE and retinal layers) may be subdivided into dorsal and ventral compartments reminiscent of the early Drosophila eye imaginal disc [52,53]. This intriguing possibility is supported by observations that perturbing Shh signals results in the emergence of a sharp morphological boundary between the dorsal and ventral optic primordium. Furthermore, the ventral eye compartment is more sensitive to altered levels of Shh signal as reflected by the fate switch of ventral RPE as well as the restricted gene expression pattern changes observed along a D-V division in the ventral retina [37,48,49,54–56]. Further investigations are necessary to determine if the vertebrate eye primordium contains dorsoventral compartments and a D-V boundary, what function these putative compartmentalization may have in visual system development, and by what mechanisms are these compartments established.
Fig. 1.
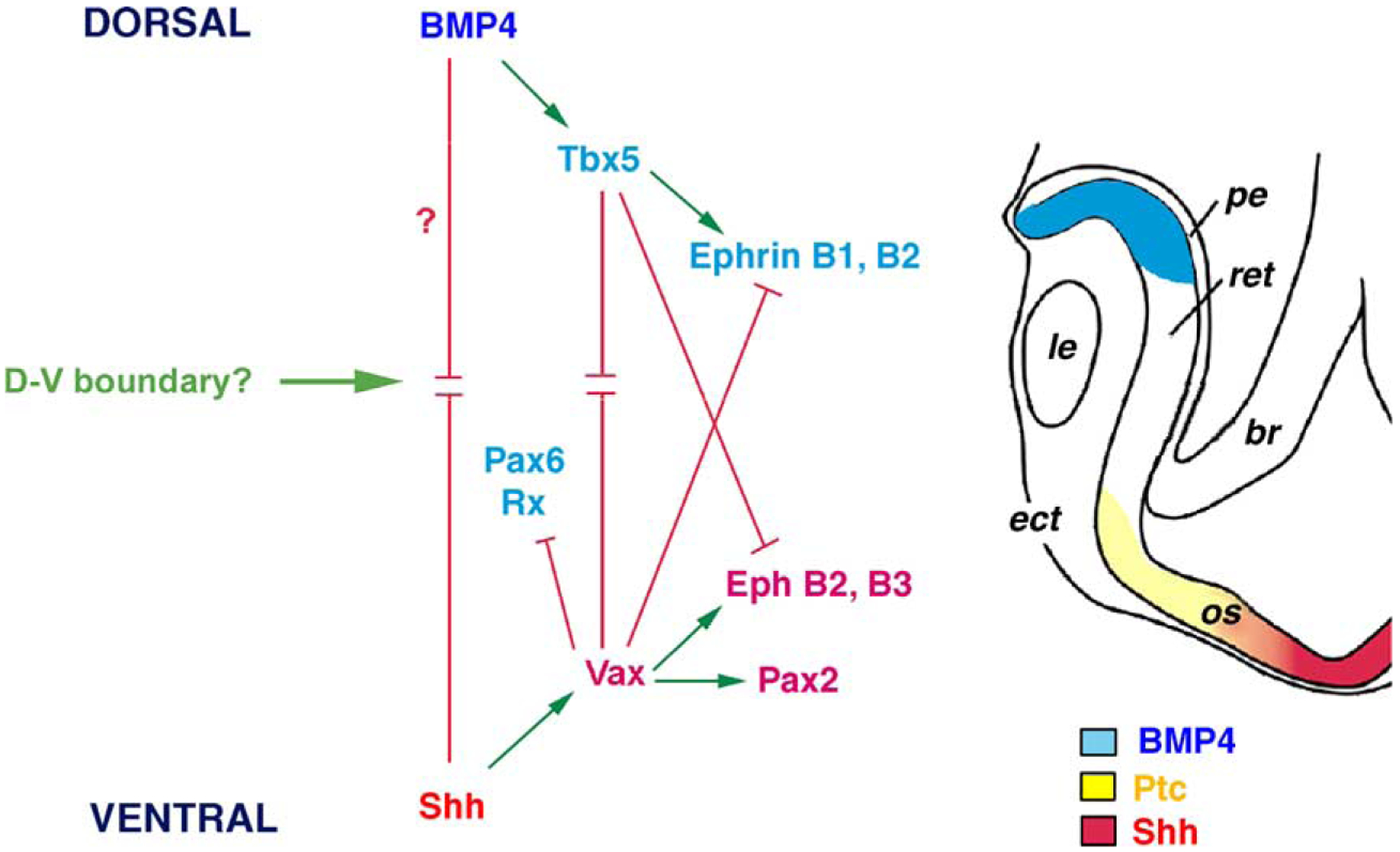
A model for the roles of Shh and BMP4 in D-V pattern formation of the vertebrate eye. The schematic drawing depicts a section plain through the optic fissure of an early optic cup. During the transition from the optic vesicle to the optic cup, Shh signals emanating from the ventral forebrain and BMP4 signals expressed in the dorsal retina act antagonistically to establish D-V properties within this morphogenetic field. The opposing Shh and BMP signaling induces expression of transcription factors Tbx5 and Vax2 at distinct concentration thresholds. Tbx5 and Vax suppress each other and regulate downstream factors including Pax2, Pax6, and Rx. The differential activities of Tbx5 and Vax in the neural retina in turn control expression patterns of axonal guidance molecules Ephs and Ephrins, which are critical for retinotectal mapping. In addition, Pax6 and Pax2 mutually suppress each other to delineate the optic cup and optic stalk boundary. Furthermore, BMP4 and Shh may also influence other factors such as Otx2 and Mitf to specify the RPE identity. Thus, normal D-V patterning of the optic cup is dependent on Shh and BMP signals. It remains to be determined if a D-V boundary exists in the vertebrate eye primordium. Arrows and bars indicate promoting and repressing activities, respectively. le, lens; pe, pigmented epithelium; ret, retina; br, forebrain; os, optic stalk; ect, ectoderm.
3.2. Hh signals and retinal ganglion cell genesis
The early vertebrate retina contains only proliferating progenitor cells. The neurogenic process that gives rise to postmitotic neurons commences near the center of the retina and propagates in a wave like fashion towards the periphery [57,58]. The progression of the vertebrate neurogenic wave is reminiscent of the morphogenetic furrow sweeping through the developing eye imaginal disc of Drosophila. Unlike the morphogenetic furrow, which is created by cell shape changes due to synchronous entry into the cell cycle, no cell cycle synchrony has been detected in the vertebrate retinal neurogenic wave front [58]. Nonetheless, recent evidence has shown that mechanisms controlling progression of the neurogenic waves appear to be evolutionarily conserved.
In the fly eye disc, differentiated photoreceptor cells behind the morphogenetic furrow produce Hh, which is required for the progression of the furrow towards undifferentiated areas across the entire eye disc [59]. In zebrafish, the first postmitotic retinal neurons, the retinal ganglion cells (RGCs), express Shh and another Hh family member, tiggywinkle hedgehog (Twhh) [60]. In the zebrafish Shh mutant (sonic you, syu), neurogenesis is initiated in the retina but fails to spread further. Similarly, treatment of zebrafish embryos with cyclopamine, a general inhibitor of the Hh signaling pathway, also blocks spread of the neurogenic wave. Furthermore, transplanting wild type cells and expressing Shh from a heat shock promoter in the syu mutant retina induce expression of a GFP reporter from the Shh promoter. These results demonstrate convincingly that Shh produced by differentiated neurons behind the neurogenic wave front is necessary and sufficient for the progression of the neurogenic wave and the induction of Shh gene expression in nascent postmitotic neurons. In the fly eye disc, activation of the extracellular signal-regulated kinase (ERK) occurs in a wave-like process that parallels the movement of the morphogenetic furrow. This phospho-ERK wave is dependent upon Hh signals derived from differentiated photoreceptors and is required for retinal neurogenesis [61]. Strikingly, a wave of activated ERK paralleling the neurogenic wave front also propagates across the zebrafish retina, and can be blocked with cyclopamine, suggesting that Hh activity is involved in the generation and/or progression of the ERK wave [60]. These findings in zebrafish demonstrate that during the initial stages of vertebrate retinogenesis, Hh signals produced by RGC play a fundamental role in triggering the entry of the retinal primordium from a proliferative state to a neurogenic state.
The roles of Hh in retinogenesis are not limited to expansion of the neurogenic wave into the undifferentiated retinal territory. At the neurogenic wave front of vertebrate retina, newly postmitotic RGCs emerge in non-random arrays [58], while behind the wave front increasing numbers of RGCs differentiate in the wake of the wave and begin to express Shh [62]. Since differentiated RGCs reside in the inner retina opposing the ventricular zone, RGC derived Shh molecules presumably form a concentration gradient across both the neurogenic wave front and the ventricular zone containing proliferating progenitor cells. Indeed, expression of Ptc, a target gene induced by Hh signaling, is increased in the retina ventricular zone adjacent to the accumulating RGCs [62,63]. Elevating Shh signal levels leads to a reduction of differentiated RGCs, whereas decreasing Shh signals suppresses RGC genesis during the peak period of RGC production in the chick retina [62]. Moreover, by monitoring responses of cohorts of early progenitor cells, it has been established that Shh signaling affects progenitor cell specification towards the ganglion cell fate during or soon after their last mitotic cycle [62]. Thus, secreted Shh molecules derived from differentiated RGCs act as negative feedback signals to modulate the further production of RGC from the early retinal progenitor pool. This finding is consistent with a previous report that differentiated RGCs produce unidentified inhibitory factors to negatively regulate their own production [64].
Negative feedback regulation of the first-born retinal neurons by Hh also appears to be an evolutionarily conserved mechanism. In the Drosophila eye disc, Hh produced by the R8 photoreceptor cells, the founder cell of each ommatidium, controls ommatidial assembly through regulation of the proneural gene atonal, a bHLH transcription factor and a determinant of the R8 cell [65,66]. Genetic manipulation of Hh signals in the fly eye has demonstrated that low levels of Hh signal occurring at a distance from the Hh producing cell act to induce atonal expression; while higher levels of Hh signal found in the vicinity of newly differentiated ommatidial units suppress atonal expression between nascent proneural clusters, and thus critically control the position and number of future R8 cells [67]. Strikingly, vertebrate homologs of the Drosophila atonal gene are essential for the production of the first born retinal neurons. Mice carrying deletions in the Math5 gene show profound defect in RGC genesis [68,69], and in the zebrafish ath5 mutant lakritz, ganglion cell production is eliminated [70]. These data further demonstrate the conserved molecular mechanisms in early retinogenesis between Drosophila and vertebrate species, and support that Hh signals secreted by the first-born neurons play multiple roles at distinct concentration thresholds to instruct the entry of naïve progenitors into a competent state for neurogenesis and to bias the choice of competent progenitors between differentiation and continued proliferation (Fig. 2).
Fig. 2.
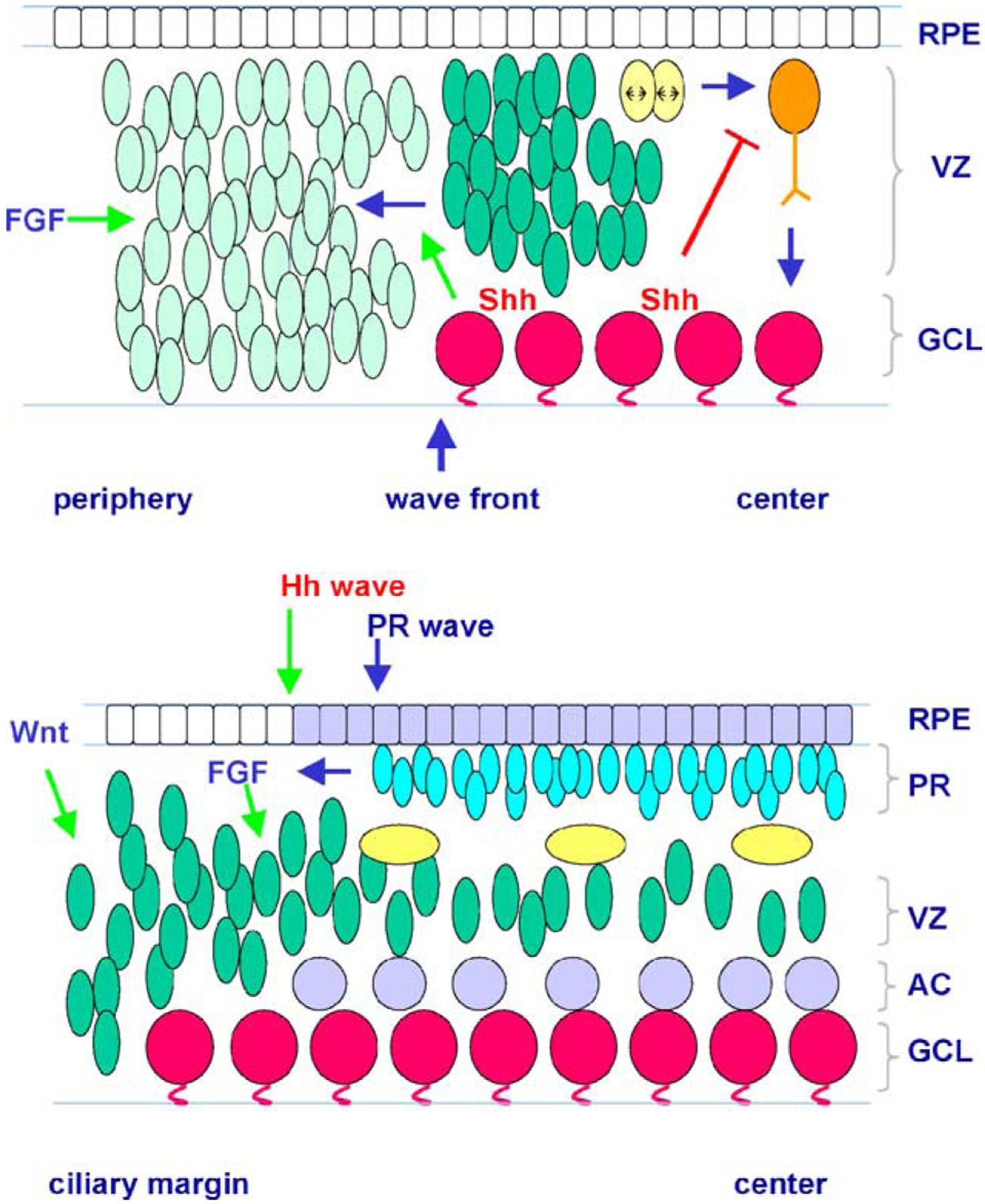
Roles of secreted growth factors in vertebrate retinogenesis. The cartoons depict two distinct stages of retinal development. (A) During early retinal differentiation, a neurogenic wave spreads from the central retina towards the peripheral retina. Ahead of the wave, retinal progenitor cells remain in a naïve incompetent state (light blue), while behind the wave front, progenitor cells (green) have entered into a competent state to produce postmitotic neurons. Shh molecules are secreted by nascent retinal ganglion cells (RGCs) and may diffuse to form a gradient across the neurogenic wave front and the ventricular zone. Shh signaling is required for the acquisition of progenitor competence and for the progression of the neurogenic wave. Shh signaling behind the wave front negatively affects RGC fate specification of competent progenitor cells during or soon after M-phase (yellow) of the cell cycle, or influences further differentiation of nascent RGCs (orange) into mature RGCs (magenta). FGF signals present in the peripheral retina may also facilitate the early retinogenic process. (B) During the period of photoreceptor production, RPE derived Hh signals may promote photoreceptor differentiation. In zebrafish, a Hh wave (gray) in the RPE precedes the wave of photoreceptor (bright blue) genesis sweeping across the retina. In addition, FGF signaling may be involved in photoreceptor cell specification. At the ciliary margin, RPE derived Wnt signals are important for the maintenance of a progenitor pool at the peripheral retina. Blue and green arrows indicate developmental events and promoting activities, respectively; the red bar indicates inhibitory activities. AC, amacrine cells; GCL, ganglion cell layer; PR, photoreceptors; RPE, retinal pigmented epithelium; VZ, ventricular zone.
Besides modulation of RGC cell numbers through a feedback mechanism, Shh signals have also been shown to act as a negative regulator of RGC axon extension in chick [71]. Shh suppresses both the number and the length of neurites from retinal explants but not neural tube or the dorsal root ganglia explants in vitro. In addition, retroviral mediated ectopic expression of Shh along the visual pathway interferes with the growth of RGC axons. Moreover, Shh decreases the intracellular cAMP levels, which is associated with the inhibition and retraction of the growth cone.
The intriguing question of how neurogenesis initiates in the vertebrate retina primordium requires further consideration. In zebrafish, mutations causing defects in the axial mesoderm also prevent the onset and spread of ath5 gene in the retina [72]. Transplanting Pax2 expressing optic stalk tissues induces ectopic expression of ath5 in the zebrafish retina. This suggests that either midline signals that have diffused into the optic stalk or secondary cues elicited by these midline signals can initiate the retinal neurogenic process. A recent study in zebrafish has tested if Hh molecules derived from the midline play a role in the initiation of RGC differentiation [73]. Injecting antisense morpholino oligonucleotides (MOs) targeting both Shh and twhh or treating embryos with cyclopamine at 10 h post-fertilization (hpf) when prechordal plate derived signals are effective caused the reduction of ath5 expression in 20% of the retinas, whereas injecting anti-Hh MOs at 27hpf when RGCs are producing Hh signals showed only slight effects on the progression of RGC wave [73]. In the zebrafish muscle-omitted (smu) mutant, which carries a point mutation in the smoothened gene, ath5 expression is affected in 14% of the embryos whereas Pax2 is affected in 83% of the embryos. In the zebrafish syu mutants, half of the mutant retinas fails to initiate retinogenesis, while the other half of mutant retinas undergoes retinal differentiation but fails to propagate the neurogenic wave [74]. These data show that Hh signals produced outside of the eye prior to the onset of retinogenesis play an important role in the initiation of retinal neurogenesis. However, it remains to be determined if extra-retinal Hh signals act directly or indirectly to control ath5 expression, or if perturbation of Hh signal transduction causes early eye pattern defects, which in turn result in the misplacement or failure of retinogenesis.
3.3. Hh signaling and photoreceptor development
In addition to being expressed by differentiated retinal neurons, Hh family members are also produced by the RPE layer directly opposing the vertebrate retina. For example, Indian hedgehog (Ihh) is expressed in the embryonic and mature rat RPE [75], and Banded hedgehog and Cephalic hedgehog, which are related to the mammalian Ihh and desert hedgehog, respectively, are expressed by the Xenopus central embryonic RPE [49]. In a rat retinal monolayer culture system, Shh causes transient increases in cell proliferation, and a 2–10-fold increase in cells expressing photoreceptor cell markers [75]. In mouse retinal cultures, Shh stimulates late embryonic retinal cell proliferation and enhances production of all postnatally born retinal cell types [63]. In the zebrafish eye, expression of Shh and twhh begins in the ventral RPE at a discrete position, and then sweeps through the rest of the RPE. This wave of Hh expression precedes that of the differentiating photoreceptor cell wave in the retina, suggesting that RPE derived Hh may play a role in promoting photoreceptor cell differentiation [76]. Treatment of embryos with antisense oligonucleotides either slows or arrests the progression of the photoreceptor wave [76]. Injection of anti-Hh MO at a time when the RPE Hh is spreading (51hpf) also leads to the reduction of opsin expression accompanied by the reduced expression of the homeobox gene Rx1 in the outer nuclear layer [73]. Thus, RPE derived Hh signals are likely to be involved in promoting the differentiation of photoreceptor cells in the zebrafish retina. Tissue-specific inactivation of Hh genes will further elucidate the functions of Hh signals produced by the RPE cell layer in other vertebrates.
3.4. Hh and retinal gliogenesis
In the developing mouse retina, Shh is initially expressed by differentiated RGCs, and then also by a subset of postmitotic inner nuclear layer (INL) cells that are likely to be amacrine cells [63]. As the retina becomes more mature, Shh expression is limited to the RGC layer and the inner nuclear layer while the expression of the Ptc receptor is restricted to the Müller glia [63]. Conditional disruption of the Shh gene in RGCs using the Thy1 promoter driving the Cre recombinase results in a smaller eye with lamination defects, as indicated by the disorganized photoreceptor cell layer [77]. This phenotype is reminiscent of the Math5 null mice that show abnormal development of cone photoreceptors, bipolar interneurons, and Müller glia [68,69]. Thus, loss of Shh signals from the RGCs affects subsequent development of the neural retina. It has been suggested that the lamination defects found in the mutant retina lacking RGC derived Shh is due to the malformation of Müller glia [77]. However, the Thy1-Cre conditional disruption of the Shh gene leads to lethality at birth prior to Müller cell differentiation, and the full range of retinal defects in this mutant remain to be further characterized.
In the developing optic nerve, astrocytes and their precursors express high levels of Ptc, indicating that they receive Hh signals. Transection of the optic nerve and injecting periocular tissues with Shh neutralizing antibodies cause reduction of Ptc expression and decreased astrocyte proliferation in the optic nerve, supporting the notion that RGC axons may anterogradely transport Shh signals to influence astrocyte proliferation in the optic nerve. Recent conditional ablation of Shh in RGCs using the Thy1-Cre shows a loss of astrocyte precursors in the optic disc as well as RGC axon guidance defects [78]. Thus, RGC derived Shh is crucial for proper development of the optic nerve especially with regard to expansion of the astroglia. Interestingly, eliminating Shh activity from RGCs also results in a fate switch of the optic stalk. However, instead of becoming neural retina as found in other Hh perturbation studies [36,48–50] removal of RGC derived Shh alone leads to the conversion of the optic stalk into RPE tissue [78]. Therefore, optic stalk specification appears to require Hh signals emanating both from the anterior ventral midline tissues and the differentiated RGCs in mouse.
4. TGFβ/BMP family of signaling molecules
The TGFβ super family of signaling molecules have multiple functions in the development of the nervous system, including neural induction, D-V patterning of the neural tube, apoptotic cell death, and neuronal differentiation. Members of the TGFβ family signal through heteromeric transmembrane protein complexes containing type I and type II receptors. Both types of TGFβ receptors are single membrane spanning serine/threonine protein kinases [79]. Ligand binding to the extracellular domain of the receptors activates the type II receptors which in turn activate the type I receptors by phosphorylation. Activated type I receptors then recruit and phosphorylate specific SMAD transcription factors, which form a complex with co-SMAD and enter the nucleus to activate transcription. Increasing evidence shows that the TGFβ family of molecules plays important roles during vertebrate eye morphogenesis and retinogenesis.
4.1. TGFβ family molecules in ocular tissue specification
Multiple tissue interactions occur during vertebrate eye morphogenesis and members of the TGFβ family are implicated in these cross-tissue signaling events critical for patterning, cell specification, and differentiation. For example, in both chick and mouse, BMP4 mRNA expression is initially detected in the distal optic vesicle, then in the dorsal retina and ventral RPE at the optic cup stage, and finally in the peripheral margins of the differentiating retina [48,55,80]. Targeted deletion of the BMP4 gene causes early embryonic lethality, but explant assays indicate that lens formation is affected in BMP4-deficient mice [80]. BMP7 is expressed in the surface ectoderm opposing the optic vesicle in mouse, and BMP7 null mice frequently show an eyeless phenotype, possibly due to the necessary ectoderm–optic vesicle interaction in eye morphogenesis [81]. Tissue ablation experiments have shown that formation of the double-layered optic cup requires signal(s) from the pre-lens ectoderm as its removal disrupts the invagination of the optic vesicle [82]. Ectopic BMP signals near the optic vesicle similarly prevents formation of the optic cup from the optic vesicle, although lens vesicle formation still occurs. These data suggest that BMPs are involved in signaling events between the surface ectoderm and the optic vesicle, and that proper regulation of BMP signals is crucial for the early stages of eye morphogenesis.
In addition to interactions between the lens placode and the optic vesicle, mesenchymal tissues surrounding the optic vesicle also impact upon ocular tissue specification. Optic vesicle explant culture experiments show that extraocular mesenchyme is required for the induction and formation of the RPE. Extraocular mesenchyme promotes expression of genes normally expressed in RPE such as Mitf and suppresses the neural retina markers Chx10, Pax6, and Six6 [83]. Furthermore, the TGFβ family member Activin can substitute for the extraocular mesenchyme in the induction and maintenance of RPE markers, suggesting an Activin-like signal from the tissues surrounding the optic vesicle may play a role in RPE specification.
BMP signals are also involved in vertebrate ocular tissue differentiation. The mature ciliary body contains components derived from the margin of the optic cup and the periocular mesenchyme. Lens-specific expression of Noggin, a secreted BMP antagonist, blocks formation of the ciliary body at the periphery of the mouse optic cup, whereas co-expression of BMP7 with Noggin restores normal ciliary body formation, thus supporting the role of BMPs in ciliary body differentiation [84]. In chick, phospho-SMAD1 is detected in the equator of the lens vesicle as lens epithelial cells undergo elongation and differentiation. Overexpression of Noggin by a retrovirus in the chick eye delays lens fiber cell differentiation, however, this inhibition is reversible by the addition of BMP molecules. Thus, BMPs appears to play a role in the transition of lens cells from a proliferative state to a postmitotic state.
4.2. BMP signaling in retinal pattern formation
The D-V axial pattern formation of the vertebrate nervous system is largely dependent upon ventrally derived Shh and dorsally derived BMP signals [2]. These two signals also serve as important morphogens in the D-V axial determination of the optic cup and the neural retina. BMP signals are particularly crucial for establishing the topographic axonal projection map of the RGC axons in their target fields.
The initial D-V morphological asymmetry in the chick optic primordium appears prior to the invagination of optic vesicle (stage 11), when Shh expression occurs prominently at the base of the ventral optic vesicle [48]. As soon as the optic cup is formed (stage 14), BMP4 is expressed in the dorsal retina and persists until neurogenesis commences (stage 18). Ectopic expression of Shh at optic vesicle stage abolishes BMP4 expression in the dorsal retina, and expands the ventral expression domains of cVax and Pax2 more dorsally [48]. Conversely, reducing Shh signals causes ventral expansion of BMP4 expression and suppression of cVax and Pax2 in the ventral half of the retina. Ectopic expression of BMP4 in the early chick optic cup also inhibits ventral expression of cVax and Pax2, and promotes ectopic expression in the ventral retina of a T box gene Tbx5, which is normally restricted to the dorsal half of the retina [56]. These results indicate that ventrally derived Shh and dorsally derived BMP4 signals antagonize each other to establish D-V expression territories for key transcription factors in the optic primordium.
Although the precise molecular mechanisms of how Shh and BMP4 mutually oppose each other in the D-V axis of the eye primordium remain unknown, one likely possibility involves the antagonistic activities of transcription factors regulated by these two morphogens. Indeed, forced expression of Tbx5 in the ventral retina suppresses Pax2 and Vax expression [56], and ectopic expression of mVax2 or cVax in the dorsal retina reduces Tbx5 expression territory and expands Pax2 expression dorsally [37]. Thus, the two transcription factors Tbx5 and Vax act to mutually suppress each other. More importantly, the dorsoventral differential expression of Tbx5 and Vax is critical for the graded retinal expression of the Eph family of receptors and Ephrin ligands that play fundamental roles in establishing the retinotectal topographic map. For example, misexpressing mVax2 or cVax completely suppress the dorsal expression of EphrinB1 in the embryonic day 8 (E8) chick retina and induces ectopic dorsal expression of EphB2, which is normally restricted to the ventral retina [37]. Consequently, retinas infected with Vax expressing viruses show retinotectal mapping errors. Targeted disruption of the mouse Vax1 [36,38] and Vax2 [39,85] genes further confirms that these genes play important roles in patterning the ventral eye and retina as loss of either Vax1 or Vax2 results in flattened gradients of EphB receptors and EphrinB ligands as well as axonal guidance defects. Although effects of misexpressing Tbx5 on Eph and Ephrins have not been directly demonstrated, disruption of retinotectal projection is observed in Tbx5 virus infected chick retina, consistent with a role of Tbx5 in regulating Eph and/or Ephrin expression [56]. Thus, the opposing activities of Shh and BMP4 in the early retina are translated into domain-specific expressions of key transcription factors that govern the D-V identity of the retinal projection neurons and the graded guidance cues for the correct connectivity map in the vertebrate visual system (Fig. 1).
Recent findings have indicated that the activities of BMP signals are greatly influenced by extracellular binding proteins [86]. A BMP4 antagonist Ventroptin is expressed in a “ventral high and dorsal low” fashion in the optic cup [55]. Misexpression of Ventroptin in the dorsal retina abolishes dorsal Tbx5 expression, induces dorsal expression of cVax, and disrupts the retinotectal projection. Interestingly, Ventroptin is also normally expressed in an anteroposterior gradient later in retinal development (E6) and regulates axonal properties along the A-P axis [55]. Misexpression of another BMP binding protein Noggin at the optic vesicle and the optic cup stages causes microphthalmia and the conversion of ventral RPE into the neural retina, respectively [87]. In addition, misguided RGC axons are seen in Noggin virus infected eyes. These phenotypes are similar to the effects of reducing Shh signals [48], and are likely due to D-V patterning defects in the optic cup and retina.
A number of BMPs and their receptors are similarly expressed in the developing chick and mouse retinas [54,71,88]. Among the BMP receptors, BMPRIa and BMPRIb can each complex with the BMPRII and activate SMAD1, SMAD5, or SMAD8. In contrast to BMPRIa and BMPRII, which are expressed ubiquitously in the retina, BMPRIb show prominent ventral expression. Targeted deletion of BMPRIb in mice causes retinal RGC axonal projection defects, with a subset of ventral axons making abrupt turns and failing to enter the optic nerve head [88]. Thus, genetic evidence combined with perturbation studies in chick indicate that BMP signaling is crucial in D-V patterning of the vertebrate retina.
4.3. BMP and TGFβ signals in retinal differentiation and cell survival
The potential functions of BMP or TGFβ signaling in retinal proliferation and differentiation are not well understood. During retinogenesis, a number of BMP molecules initially expressed in specific regions adopt layer specific expression patterns later in development [54]. In a rat E18 retinal culture, addition of the TGFβ family member Activin A increases the number of rod photoreceptor cells, but has no apparent effect on other cell types [89]. Correspondingly, in Activin betaA-deficient mice, the expression of recoverin, a marker for photoreceptors, at early postnatal stages is decreased, suggesting the involvement of Activin in photoreceptor differentiation.
Several studies implicate the involvement of BMP or TGFβ in retinal cell survival. In BMPRIb-deficient mice, there is a marked increase of apoptotic cell death in the inner retina in the first postnatal week, suggesting that BMP signaling postnatally maybe necessary for the survival of certain retinal cells [88]. In contrast, TGFβ molecules and BMPs are implicated in promoting cell death in the developing chick retina. TGFβ2 and TGFβ3 expression are detected in the central chick retina near the optic nerve head between E5 and E7, a period of programmed cell death mediated by NGF [90]. Application of TGFβ neutralizing antibodies results in decreased cell death in the retina, suggesting a role of TGFβ in controlling apoptosis. Similarly, a spatially restricted retinal cell death occurring in the dorsal retina at stage 17 in chick and E10 in mouse [91], and local application of Noggin decreased this early retinal cell death, supporting the involvement of TGFβ/BMP signaling in programmed cell death.
5. Wnt family of signaling molecules
The Wnt family of signaling molecules regulates a variety of cell behaviors, including proliferation, differentiation, polarity, and movement [92]. In the canonical Wnt signaling pathway, Wnt ligands interact with two types of transmembrane receptors, the Frizzled (Fz) serpentine receptors and the single membrane spanning proteins LRP5 or LRP6 (LDL receptor-related proteins). In the absence of Wnt signals, β-catenin is phosphorylated sequentially by CKI and GSK3 in a complex containing Axin and APC, and then targeted for degradation. Wnt stimulation results in a Dishevelled (Dvl)-dependent blockade of β-catenin phosphorylation and degradation. The accumulation of β-catenin induces formation of β-catenin and TCF/LEF (T-cell factor/lymphoid enhancer factor) complex that activates transcription. In addition to regulating patterning and cell fate, Wnt signals can influence axonal growth, pathfinding and synaptogenesis. However, this aspect of Wnt function is mediated by the effects of Dvl and GSKs on microtubule stability and is independent of β-catenin and TCF-activated transcription. In addition, Wnt, in conjunction with the Fz receptor, regulates planar cell polarity in both Drosophila and mammals via Dv1-dependent stimulation of RhoA/ROK and RAC/JNK activity. Therefore, the cellular effects of Wnt signaling are mediated by a variety of complex inter-related signaling pathways.
To date, little is known regarding the potential function of Wnt in vertebrate eye patterning and neurogenesis. A recent comprehensive study has described the expression patterns of Wnt signaling components during mouse eye development [93]. RT-PCR assays detected transient expression of Wnt1, Wnt3, Wnt5a, Wnt5b, Wnt7b, and Wnt13 (Wnt2b) in the embryonic retina, and persistent expression of Wnt13 in the peripheral RPE opposing the neural retina in the ciliary margin. Several mouse Fz receptors are also found in the developing retina. For example, both Mfz3 and Mfz7 are transcribed at high levels in prenatal retinal progenitor cells, with Mfz7 transcripts particularly concentrated in the peripheral retinal margin. Interestingly, the Wnt antagonists Sfrp (secreted frizzled related proteins) also show dynamic expression patterns in the retina. For example, Sfrp2 mRNA is highly expressed between E12 and E15 throughout the proliferative zone with the exception of the peripheral margin of the retina. Moreover, a TCF/LEF responsive LacZ reporter has revealed activation of the canonical Wnt signaling pathway in the ciliary margin region. Together, these data suggest that the Wnt family of molecules may play multiple roles in eye development and retinogenesis.
The expression patterns of Wnt13 (Wnt2b) and Mfz receptors in the ciliary margin of the eye imply that Wnt signaling may regulate retinal progenitor proliferation [94]. In the chick retina, the ciliary margin zone retina contains high levels of LEF that colocalizes with progenitor markers. Over expressing Wnt13 in the central retina suppresses neuronal differentiation, and blocking Wnt signaling with a dominant-negative LEF1 protein inhibits cell proliferation at the ciliary margin and causes premature neuronal differentiation [95]. Furthermore, retinal progenitor cells prolong their proliferative period in vitro in the presence of Wnt13. Thus, Wnt13 may function in the ciliary margin to maintain an undifferentiated progenitor pool.
6. Growth factor signals mediated by receptor tyrosine kinases
A large number of growth factors signal through transmembrane proteins that encode an intracellular protein tyrosine kinase domain [96]. These receptor tyrosine kinases (RTKs) are activated through dimerization triggered by ligand binding, and proceed to cross phosphorylate each other on tyrosine residues. RTK phosphorylation consequently provides docking sites for a variety of signaling proteins containing SH2 (Src homology2) or PTB (phosphotyrosine binding) domains and initiates downstream signaling events. The most commonly activated and well studied signal transduction pathway mediated by RTK is the Ras/MAP signaling cascade, which involves the phosphotyrosine-mediated binding of the Grb2/Sos complex to the receptor and the subsequent activation of the small G protein RAS by the guanine nucleotide exchange factor Sos. Ras interacts with the effector protein Raf, and activated Raf stimulates MAP kinase-kinase (MEK), which in turn phosphorylates MAPK (ERK) on Thr and Tyr residues. Activated MAPK is rapidly translocated to the nucleus to phosphorylate and activate transcription factors. Here, roles of RTK-mediated FGF signals in ocular tissue specification and retinogenesis will be discussed.
6.1. FGFs in neural retina versus RPE formation
A potential role for FGF in vertebrate ocular tissue specification was initially suggested by manipulating early chick optic cup tissues. During chick eye morphogenesis, the surface ectoderm expresses FGFs while the adjacent optic vesicle expresses FGFRs [97]. Removal of the ectoderm results in the co-mingling of retina and RPE. Implanting FGF-soaked beads or infection with FGF-expressing retroviruses partially restores the segregated retina and RPE domains with the retina tissue always forming adjacent to the source of FGF [97]. Chick optic vesicles cultured in vitro reproducibly form the lens vesicle and a double layered optic cup with an outside RPE layer. Inclusion of FGF to the culture medium causes the presumptive RPE to undergo neuronal differentiation resulting in two retinal layers [98]. Conversely, addition of neutralizing antibodies to FGF2 blocks neural differentiation in the retinal layer without affecting RPE differentiation. These data indicate that neural retina specification requires FGF function, and FGF signals emanating from the surface ectoderm may help organize the double-layered optic cup.
The function of FGF in retinal versus RPE fate choices in the early optic vesicle is at least in part mediated through suppression of genes required for RPE determination. The bHLH zipper transcription factor Mitf is initially expressed in the entire mouse optic vesicle and later restricted to the RPE [99]. In mice carrying a naturally occurring Mitf mutation, the dorsal half of the RPE transdifferentiates into the neural retina [99]. Furthermore, FGF1 or FGF2 coated beads can down regulate Mitf expression and interfere with pigmentation of cultured mouse eyes. In chick, bFGF also suppresses Mitf, and retroviral-mediated overexpression of Mitf causes hyperpigmentation and inhibition of Pax6 expression in retinal cultures [44]. Therefore, intracellular determinants for RPE may be suppressed by high concentrations of FGF. Since Mitf promotes the RPE fate and suppresses neural retina determinants, down regulation of Mitf in the distal optic vesicle is necessary for induction of the neural retina.
Recent studies have provided insight into how FGF signaling prevents Mitf expression. In melanocytes, RTK-mediated Ras/ERK activation can lead to phosphorylation of Mitf protein on Ser73, which together with a subsequent phosphorylation event, further enhances Mitf’s transcriptional activity while simultaneously targeting Mitf for ubiquitin-dependent degradation [100]. In the chick eye, expression of a constitutively activated MEK1, the upstream activator of ERK, induced the transdifferentiation of RPE into neural retina with the concomitant down regulation of Mitf [101]. In addition to surface ectoderm derived FGFs, FGF9 is normally expressed by the distal optic vesicle in mouse [102]. Misexpression of FGF9 by the alpha crystalline promoter in the lens causes the dorsal RPE to convert into neural retina, which shows decreased Mitf expression, and ectopic expression of the neural retinal markers Rx, Chx10, and Math5. Similar studies have shown that ectopic expression in the RPE of FGF9 or a constitutively active Ras protein induces dramatic transdifferentiation of the presumptive RPE layer into a duplicated neural retina [102,103]. In these studies, however, FGF9 and active RAS protein were expressed by a tyrosinase-related protein 2 (TRP2) promoter, which is only transiently active in the RPE. Thus, transient activation of Ras is sufficient to specify the neural retina fate. Moreover, since retinal development in FGF9-deficient mice is normal except in the ciliary margin, where the RPE extends into the retinal layer, the role of FGF9 may be to define the boundary between the retina and the RPE.
6.2. FGFs in retinal cell fate specification
FGF signals are not only involved in retina versus RPE fate determination, but also in specification of retinal cell types. During the initial stage of chick retinogenesis, FGF1 is expressed at high levels in the peripheral retina. Blocking FGF signaling with a protein kinase inhibitor retards the progression of the RGC wave in retinal explants, while FGF1 but not FGF8 treatment accelerates the RGC wave [58]. This result highlights the proneural activity associated with FGF in vertebrate eye formation. However, in both Drosophila and vertebrate retinas, the ERK inducing signal(s) remain to be identified.
In the developing Xenopus embryo, inhibiting FGF signaling by expressing a dominant negative form of the Xenopus FGFR causes a 50% loss of both photoreceptor and amacrine cells, accompanied by a 3.5-fold increase of Müller glia [104]. Furthermore, overexpressing FGF2 in retinal progenitor cells causes a 35% increase of RGCs and a 50% increase of Müller cells. Despite the unaltered proportion of photoreceptors among total cells, the ratio of rod versus cone photoreceptors is also affected by FGF2 oevrexpression [105]. Interestingly, transgenic tadpoles expressing a dominant-negative FGFR4a under the control of the Xenopus Rx1A (Xrx1A) promoter, which is active among retinal progenitors, show disorganized retinas that either specifically lack photoreceptors or contain a few ectopic photoreceptors [106]. These findings provide evidence that FGF signaling during retinogenesis participates in retinal progenitor cell fate choices.
6.3. FGFs and retinal stem cells
Accumulating evidence also shows that FGF activity is critical for maintaining retinal stem cells, which have been identified in the ciliary margin of the adult mouse retina [107]. Dissociated cells derived from the RPE layer at the ciliary margin, when plated at clonal density, can form neural spheres if treated with FGF2 or EGF. The mouse retinal ciliary margin stem cells can differentiate into various retinal neuronal types including photoreceptors, bipolar neurons, and Müller glia. Thus, the adult mammalian eye may harbor stem cells, which can be induced to re-enter the cell cycle and initiate neuronal differentiation.
In post hatching chick retina, a proliferative zone has been identified in the ciliary margin, as evidenced by the ability of cells to incorporate BrdU [108]. It was later determined that Müller glia re-enter the cell cycle in response to acute damage caused by injections of neurotoxins [109]. The BrdU-labeled cycling cells express Pax6, Chx10, and CASH1, markers normally expressed by chick retinal progenitor cells, and transiently express neurofilament. Later these cells integrate into the inner and out nuclear layer of the retina. However, a majority of these cells remain undifferentiated, and only a minority of these cells has differentiated into neurons and Müller glia. Injecting toxin-treated chick eye with a combination of insulin and FGF2 enhances the number as well as the differentiation of neurons that expressing ganglion cell markers [110]. Furthermore, co-injection of insulin and FGF2 without toxin also causes Müller cells to reenter the cell cycle and express progenitor cell markers [111]. These results demonstrate that in the mature chick retina, Müller glia are plastic and can respond to environmental cues, especially certain growth factor signals, to proliferate and differentiate.
7. Concluding remarks: target identification and signal integration
In summary, multiple classes of extracellular signaling molecules influence vertebrate eye patterning and retinal differentiation. Despite the significant achievements in identifying these signaling molecules and characterizing their potential functions, much effort is still needed to elucidate molecular mechanisms by which cell-extrinsic cues impact upon key cell-intrinsic determinants in the developing eye primordium and retina. Many intriguing questions that remain unanswered include: How secreted signals are modulated in the extracellular space? What are the direct target genes regulated by different signaling pathways? How various signals are integrated at the cellular and transcriptional levels? Progress in these areas will greatly enhance our knowledge of the developmental processes by which the vertebrate visual system forms and thus hopefully provide us with a greater ability to manipulate progenitor or stem cells.
Acknowledgements
I thank Xiang-Mei Zhang and Jeffrey Goliger for helping to prepare this manuscript. X.J.Y. is supported by grants from the Research to Prevent Blindness Foundation, the March of Dimes Birth Defect Foundation, the Karl Kirchgessner Foundation, and the National Eye Institute (EY012270, EY014440).
References
- [1].Edlund T, Jessell TM. Progression from extrinsic to intrinsic signaling in cell fate specification: a view from the nervous system. Cell 1999;96:211–24. [DOI] [PubMed] [Google Scholar]
- [2].Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet 2000;1:20–9. [DOI] [PubMed] [Google Scholar]
- [3].Marquardt T, Pfaff SL. Cracking the transcriptional code for cell specification in the neural tube. Cell 2001;106:651–4. [DOI] [PubMed] [Google Scholar]
- [4].Timmer JR, Wang C, Niswander L. BMP signaling patterns the dorsal and intermediate neural tube via regulation of homeobox and helix-loop-helix transcription factors. Development 2002;129:2459–72. [DOI] [PubMed] [Google Scholar]
- [5].Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci 2002;3:517–30. [DOI] [PubMed] [Google Scholar]
- [6].Barolo S, Posakony JW. Three habits of highly effective signaling pathways: principles of transcriptional control by developmental cell signaling. Genes Dev 2002;16:1167–81. [DOI] [PubMed] [Google Scholar]
- [7].Sidman RL. Histogenesis of mouse retina studied with thymidine In: The structure of the eye. New York: Academic Press; 1961. [Google Scholar]
- [8].Young RW. Cell differentiation in the retina of the mouse. Anat Rec 1985;212:199–205. [DOI] [PubMed] [Google Scholar]
- [9].Young RW. Cell proliferation during postnatal development of the retina in the mouse. Brain Res 1985;353:229–39. [DOI] [PubMed] [Google Scholar]
- [10].Spence SG, Robson JA. An autoradiographic analysis of neurogenesis in the chick retina in vitro and in vivo. Neuroscience 1989;32:801–12. [DOI] [PubMed] [Google Scholar]
- [11].Altshuler DM, Turner DL, Cepko DL. Specification of cell type in the vertebrate retina In: Development of the visual system. Cambridge, MA: MIT Press; 1991. [Google Scholar]
- [12].Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature 1987;328:131–6. [DOI] [PubMed] [Google Scholar]
- [13].Holt CE, Bertsch TW, Ellis HM, Harris WA. Cellular determination in the Xenopus retina is independent of lineage and birth date. Neuron 1988;1:15–26. [DOI] [PubMed] [Google Scholar]
- [14].Wetts R, Fraser SE. Multipotent precursors can give rise to all major cell types of the frog retina. Science 1988;239:1142–5. [DOI] [PubMed] [Google Scholar]
- [15].Turner DL, Snyder EY, Cepko CL. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron 1990;4:833–45. [DOI] [PubMed] [Google Scholar]
- [16].Fekete DM, Perez-Miguelsanz J, Ryder EF, Cepko CL. Clonal analysis in the chicken retina reveals tangential dispersion of clonally related cells. Dev Biol 1994;166:666–82. [DOI] [PubMed] [Google Scholar]
- [17].Lillien L Neural progenitors and stem cells: mechanisms of progenitor heterogeneity. Curr Opin Neurobiol 1998;8:37–44. [DOI] [PubMed] [Google Scholar]
- [18].Watanabe T, Raff MC. Rod photoreceptor development in vitro: intrinsic properties of proliferating neuroepithelial cells change as development proceeds in the rat retina. Neuron 1990;4:461–7. [DOI] [PubMed] [Google Scholar]
- [19].Morrow EM, Belliveau MJ, Cepko CL. Two phases of rod photoreceptor differentiation during rat retinal development. J Neurosci 1998;18:3738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Belliveau MJ, Cepko CL. Extrinsic and intrinsic factors control the genesis of amacrine and cone cells in the rat retina. Development 1999;126:555–66. [DOI] [PubMed] [Google Scholar]
- [21].Belliveau MJ, Young TL, Cepko CL. Late retinal progenitor cells show intrinsic limitations in the production of cell types and the kinetics of opsin synthesis. J Neurosci 2000;20:2247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jasoni CL, Reh TA. Temporal and spatial pattern of MASH-1 expression in the developing rat retina demonstrates progenitor cell heterogeneity. J Comp Neurol 1996;369:319–27. [DOI] [PubMed] [Google Scholar]
- [23].Yang XY, Cepko CL. Flk-1, a receptor for vascular endothelial growth factor (VEGF), is expressed by retinal progenitor cells. J Neurosci 1996;16:6089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Alexiades MR, Cepko CL. Subsets of retinal progenitors display temporally regulated and distinct biases in the fates of their progeny. Development 1997;124:1119–31. [DOI] [PubMed] [Google Scholar]
- [25].Perron M, Kanekar S, Vetter ML, Harris WA. The genetic sequence of retinal development in the ciliary margin of the Xenopus eye. Dev Biol 1998;199:185–200. [DOI] [PubMed] [Google Scholar]
- [26].Matter-Sadzinski L, Matter JM, Ong MT, Hernandez J, Ballivet M. Specification of neurotransmitter receptor identity in developing retina: the chick ATH5 promoter integrates the positive and negative effects of several bHLH proteins. Development 2001;128:217–31. [DOI] [PubMed] [Google Scholar]
- [27].Cepko CL. The roles of intrinsic and extrinsic cues and bHLH genes in the determination of retinal cell fates. Curr Opin Neurobiol 1999;9:37–46. [DOI] [PubMed] [Google Scholar]
- [28].Lillien L Changes in retinal cell fate induced by overexpression of EGF receptor. Nature 1995;377:158–62. [DOI] [PubMed] [Google Scholar]
- [29].Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci USA 1996;93:589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci 2001;2:109–18. [DOI] [PubMed] [Google Scholar]
- [31].Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev 2001;15:3059–87. [DOI] [PubMed] [Google Scholar]
- [32].Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 1996;383:407–13. [DOI] [PubMed] [Google Scholar]
- [33].Muenke M, Beachy PA. Genetics of ventral forebrain development and holoprosencephaly. Curr Opin Genet Dev 2000;10:262–9. [DOI] [PubMed] [Google Scholar]
- [34].Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev 2002;16:2743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nornes HO, Dressler GR, Knapik EW, Deutsch U, Gruss P. Spatially and temporally restricted expression of Pax2 during murine neurogenesis. Development 1990;109:797–809. [DOI] [PubMed] [Google Scholar]
- [36].Hallonet M, Hollemann T, Pieler T, Gruss P. Vax1, a novel homeobox-containing gene, directs development of the basal forebrain and visual system. Genes Dev 1999;13:3106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schulte D, Furukawa T, Peters MA, Kozak CA, Cepko CL. Misexpression of the Emx-related homeobox genes cVax and mVax2 ventralizes the retina and perturbs the retinotectal map. Neuron 1999;24:541–53. [DOI] [PubMed] [Google Scholar]
- [38].Bertuzzi S, Hindges R, Mui SH, O’Leary DD, Lemke G. The homeodomain protein vax1 is required for axon guidance and major tract formation in the developing forebrain. Genes Dev 1999;13:3092–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mui SH, Hindges R, O’Leary DD, Lemke G, Bertuzzi S. The homeodomain protein Vax2 patterns the dorsoventral and nasotemporal axes of the eye. Development 2002;129:797–804. [DOI] [PubMed] [Google Scholar]
- [40].Take-uchi M, Clarke JD, Wilson SW. Hedgehog signalling maintains the optic stalk-retinal interface through the regulation of Vax gene activity. Development 2003;130:955–68. [DOI] [PubMed] [Google Scholar]
- [41].Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development 1991;113:1435–49. [DOI] [PubMed] [Google Scholar]
- [42].Mathers PH, Grinberg A, Mahon KA, Jamrich M. The Rx homeobox gene is essential for vertebrate eye development. Nature 1997;387:603–7. [DOI] [PubMed] [Google Scholar]
- [43].Furukawa T, Kozak CA, Cepko CL. Rax, a novel paired-type homeobox gene, shows expression in the anterior neural fold and developing retina. Proc Natl Acad Sci USA 1997;94:3088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mochii M, Mazaki Y, Mizuno N, Hayashi H, Eguchi G. Role of Mitf in differentiation and transdifferentiation of chicken pigmented epithelial cell. Dev Biol 1998;193:47–62. [DOI] [PubMed] [Google Scholar]
- [45].Martinez-Morales JR, Signore M, Acampora D, Simeone A, Bovolenta P. Otx genes are required for tissue specification in the developing eye. Development 2001;128:2019–30. [DOI] [PubMed] [Google Scholar]
- [46].Macdonald R, Barth KA, Xu Q, Holder N, Mikkola I, Wilson SW. Midline signalling is required for Pax gene regulation and patterning of the eyes. Development 1995;121:3267–78. [DOI] [PubMed] [Google Scholar]
- [47].Ekker SC, Ungar AR, Greenstein P, von Kessler DP, Porter JA, Moon KT, Beachy PA. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr Biol 1995;5:944–55. [DOI] [PubMed] [Google Scholar]
- [48].Zhang XM, Yang X- J. Temporal and spatial effects of Sonic hedgehog signaling in chick eye morphogenesis. Dev Biol 2001;233:271–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Perron M, Boy S, Amato MA, Viczian A, Koebernick K, Pieler T, Harris WA. A novel function for Hedgehog signalling in retinal pigment epithelium differentiation. Development 2003;130:1565–77. [DOI] [PubMed] [Google Scholar]
- [50].Huh S, Hatini V, Marcus RC, Li SC, Lai E. Dorsal-ventral patterning defects in the eye of BF-1-deficient mice associated with a restricted loss of shh expression. Dev Biol 1999;211:53–63. [DOI] [PubMed] [Google Scholar]
- [51].Schwarz M, Cecconi F, Bernier G, Andrejewski N, Kammandel B, Wagner M, Gruss P. Spatial specification of mammalian eye territories by reciprocal transcriptional repression of Pax2 and Pax6. Development 2000;127:4325–34. [DOI] [PubMed] [Google Scholar]
- [52].Cho KO, Choi KW. Fringe is essential for mirror symmetry and morphogenesis in the Drosophila eye. Nature 1998;396:272–6. [DOI] [PubMed] [Google Scholar]
- [53].Papayannopoulos V, Tomlinson A, Panin VM, Rauskolb C, Irvine KD. Dorsal-ventral signaling in the Drosophila eye. Science 1998;281:2031–4. [DOI] [PubMed] [Google Scholar]
- [54].Belecky-Adams T, Adler R. Developmental expression patterns of bone morphogenetic proteins, receptors, and binding proteins in the chick retina. J Comp Neurol 2001;430:562–72. [PubMed] [Google Scholar]
- [55].Sakuta H, Suzuki R, Takahashi H, Kato A, Shintani T, Iemura S, Yamamoto TS, Ueno N, Noda M. Ventroptin: a BMP-4 antagonist expressed in a double-gradient pattern in the retina. Science 2001;293:111–5. [DOI] [PubMed] [Google Scholar]
- [56].Koshiba-Takeuchi K, Takeuchi JK, Matsumoto K, Momose T, Uno K, Hoepker V, Ogura K, Takahashi N, Nakamura H, Yasuda K, Ogura T. Tbx5 and the retinotectum projection. Science 2000;287:134–7. [DOI] [PubMed] [Google Scholar]
- [57].Hu M, Easter SS. Retinal neurogenesis: the formation of the initial central patch of postmitotic cells. Dev Biol 1999;207:309–21. [DOI] [PubMed] [Google Scholar]
- [58].McCabe KL, Gunther EC, Reh TA. The development of the pattern of retinal ganglion cells in the chick retina: mechanisms that control differentiation. Development 1999;126:5713–24. [DOI] [PubMed] [Google Scholar]
- [59].Heberlein U, Moses K. Mechanisms of Drosophila retinal morphogenesis: the virtues of being progressive. Cell 1995;81:987–90. [DOI] [PubMed] [Google Scholar]
- [60].Neumann CJ, Nuesslein-Volhard C. Patterning of the zebrafish retina by a wave of sonic hedgehog activity. Science 2000;289: 2137–9. [DOI] [PubMed] [Google Scholar]
- [61].Greenwood S, Struhl G. Progression of the morphogenetic furrow in the Drosophila eye: the roles of Hedgehog, Decapentaplegic and the Raf pathway. Development 1999;126:5795–808. [DOI] [PubMed] [Google Scholar]
- [62].Zhang XM, Yang X- J. Regulation of retinal ganglion cell production by Sonic hedgehog. Development 2001;128:943–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Jensen AM, Wallace VA. Expression of Sonic hedgehog and its putative role as a precursor cell mitogen in the developing mouse retina. Development 1997;124:363–71. [DOI] [PubMed] [Google Scholar]
- [64].Waid DK, McLoon SC. Ganglion cells influence the fate of dividing retinal cells in culture. Development 1998;125:1059–66. [DOI] [PubMed] [Google Scholar]
- [65].Jarman AP, Grell EH, Ackerman L, Jan LY, Jan YN. Atonal is the proneural gene for Drosophila photoreceptors. Nature 1994;369:398–400. [DOI] [PubMed] [Google Scholar]
- [66].White NM, Jarman AP. Drosophila atonal controls photoreceptor R8-specific properties and modulates both receptor tyrosine kinase and Hedgehog signalling. Development 2000;127:1681–9. [DOI] [PubMed] [Google Scholar]
- [67].Dominguez M Dual role for Hedgehog in the regulation of the proneural gene atonal during ommatidia development. Development 1999;126:2345–53. [DOI] [PubMed] [Google Scholar]
- [68].Wang SW, Kim BS, Ding K, Wang H, Sun D, Johnson RL, Klein WH, Gan L. Requirement for math5 in the development of retinal ganglion cells. Genes Dev 2001;15:24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Brown NL, Patel S, Brzezinski J, Glaser T. Math5 is required for retinal ganglion cell and optic nerve formation. Development 2001;128:2497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kay JN, Finger-Baier KC, Roeser T, Staub W, Baier H. Retinal ganglion cell genesis requires lakritz, a Zebrafish atonal Homolog. Neuron 2001;30:725–36. [DOI] [PubMed] [Google Scholar]
- [71].Trousse F, Marti E, Gruss P, Torres M, Bovolenta P. Control of retinal ganglion cell axon growth: a new role for Sonic hedgehog. Development 2001;128:3927–36. [DOI] [PubMed] [Google Scholar]
- [72].Masai I, Stemple DL, Okamoto H, Wilson SW. Midline signals regulate retinal neurogenesis in zebrafish. Neuron 2000;27:251–63. [DOI] [PubMed] [Google Scholar]
- [73].Stenkamp DL, Frey RA. Extraretinal and retinal hedgehog signaling sequentially regulate retinal differentiation in zebrafish. Dev Biol 2003;258:349–63. [DOI] [PubMed] [Google Scholar]
- [74].Stenkamp DL, Frey RA, Mallory DE, Shupe EE. Embryonic retinal gene expression in sonic-you mutant zebrafish. Dev Dyn 2002;225:344–50. [DOI] [PubMed] [Google Scholar]
- [75].Levine EM, Roelink H, Turner J, Reh TA. Sonic hedgehog promotes rod photoreceptor differentiation in mammalian retinal cells in vitro. J Neurosci 1997;17:6277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Stenkamp DL, Frey RA, Prabhudesai SN, Raymond PA. Function for Hedgehog genes in zebrafish retinal development. Dev Biol 2000;220:238–52. [DOI] [PubMed] [Google Scholar]
- [77].Wang YP, Dakubo G, Howley P, Campsall KD, Mazarolle CJ, Shiga SA, Lewis PM, McMahon AP, Wallace VA. Development of normal retinal organization depends on Sonic hedgehog signaling from ganglion cells. Nat Neurosci 2002;5:831–2. [DOI] [PubMed] [Google Scholar]
- [78].Dakubo GD, Wang YP, Mazerolle C, Campsall K, McMahon AP, Wallace VA. Retinal ganglion cell-derived sonic hedgehog signaling is required for optic disc and stalk neuroepithelial cell development. Development 2003;130:2967–80. [DOI] [PubMed] [Google Scholar]
- [79].Attisano L, Wrana JL. Signal transduction by the TGF-beta super-family. Science 2002;296:1646–7. [DOI] [PubMed] [Google Scholar]
- [80].Furuta Y, Hogan BL. BMP4 is essential for lens induction in the mouse embryo. Genes Dev 1998;12:3764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Dudley AT, Robertson EJ. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev Dyn 1997;208:349–62. [DOI] [PubMed] [Google Scholar]
- [82].Hyer J, Kuhlman J, Afif E, Mikawa T. Optic cup morphogenesis requires pre-lens ectoderm but not lens differentiation. Dev Biol 2003;259:351–63. [DOI] [PubMed] [Google Scholar]
- [83].Fuhrmann S, Levine EM, Reh TA. Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Development 2000;127:4599–609. [DOI] [PubMed] [Google Scholar]
- [84].Zhao S, Chen Q, Hung FC, Overbeek PA. BMP signaling is required for development of the ciliary body. Development 2002;129:4435–42. [DOI] [PubMed] [Google Scholar]
- [85].Barbieri AM, Broccoli V, Bovolenta P, Alfano G, Marchitiello A, Mocchetti C, Crippa L, Bn lfone A, Marigo V, Ballabio A, Banki S. Vax2 inactivation in mouse determines alteration of the eye dorsal-ventral axis, misrouting of the optic fibres and eye coloboma. Development 2002;129:805–13. [DOI] [PubMed] [Google Scholar]
- [86].Larrain J, Oelgeschlager M, Ketpura NI, Reversade B, Zakin L, De Robertis EM. Proteolytic cleavage of chordin as a switch for the dual activities of twisted gastrulation in BMP signaling. Development 2001;128:4439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Adler R, Belecky-Adams TL. The role of bone morphogenetic proteins in the differentiation of the ventral optic cup. Development 2002;129:3161–71. [DOI] [PubMed] [Google Scholar]
- [88].Liu J, Wilson S, Reh T. BMP receptor 1b is required for axon guidance and cell survival in the developing retina. Dev Biol 2003;256:34–48. [DOI] [PubMed] [Google Scholar]
- [89].Davis AA, Matzuk MM, Reh TA. Activin A promotes progenitor differentiation into photoreceptors in rodent retina. Mol Cell Neurosci 2000;15:11–21. [DOI] [PubMed] [Google Scholar]
- [90].Dunker N, Schuster N, Krieglstein K. TGF-beta modulates programmed cell death in the retina of the developing chick embryo. Development 2001;128:1933–42. [DOI] [PubMed] [Google Scholar]
- [91].Trousse F, Esteve P, Bovolenta P. BMP4 mediates apoptotic cell death in the developing chick retina. J Neurosci 2001;21: 1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].He X A Wnt–Wnt situation. Dev Cell 2003;4:791–7. [DOI] [PubMed] [Google Scholar]
- [93].Liu H, Mohamed O, Dufort D, Wallace VA. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn 2003;227:323–34. [DOI] [PubMed] [Google Scholar]
- [94].Jasoni C, Hendrickson A, Roelink H. Analysis of chicken Wnt-13 expression demonstrates coincidence with cell division in the developing eye and is consistent with a role in induction. Dev Dyn 1999;215:215–24. [DOI] [PubMed] [Google Scholar]
- [95].Kubo F, Takeichi M, Nakagawa S. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development 2003;130:587–98. [DOI] [PubMed] [Google Scholar]
- [96].Schlessinger J Cell signaling by receptor tyrosine kinases. Cell 2000;103:211–25. [DOI] [PubMed] [Google Scholar]
- [97].Hyer J, Mima T, Mikawa T. FGF1 patterns the optic vesicle by directing the placement of the neural retina domain. Development 1998;125:869–77. [DOI] [PubMed] [Google Scholar]
- [98].Pittack C, Grunwald GB, Reh TA. Fibroblast growth factors are necessary for neural retina but not pigmented epithelium differentiation in chick embryos. Development 1997;124:805–16. [DOI] [PubMed] [Google Scholar]
- [99].Nguyen M, Arnheiter H. Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Development 2000;127:3581–91. [DOI] [PubMed] [Google Scholar]
- [100].Wu M, Hemesath TJ, Takemoto CM, Horstmann MA, Wells AG, Price ER, Fisher DZ, Fisher DE. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev 2000;14:301–12. [PMC free article] [PubMed] [Google Scholar]
- [101].Galy A, Neron B, Planque N, Saule S, Eychene A. Activated MAPK/ERK kinase (MEK-1) induces transdifferentiation of pigmented epithelium into neural retina. Dev Biol 2002;248:251–64. [DOI] [PubMed] [Google Scholar]
- [102].Zhao S, Hung FC, Colvin JS, White A, Dai W, Lovicu FJ, et al. Patterning the optic neuroepithelium by FGF signaling and Ras activation. Development 2001;128:5051–60. [DOI] [PubMed] [Google Scholar]
- [103].Zhao S, Overbeek PA. Tyrosinase-related protein 2 promoter targets transgene expression to ocular and neural crest-derived tissues. Dev Biol 1999;216:154–63. [DOI] [PubMed] [Google Scholar]
- [104].McFarlane S, Zuber ME, Holt CE. A role for the fibroblast growth factor receptor in cell fate decisions in the developing vertebrate retina. Development 1998;125:3967–75. [DOI] [PubMed] [Google Scholar]
- [105].Patel A, McFarlane S. Overexpression of FGF-2 alters cell fate specification in the developing retina of Xenopus laevis. Dev Biol 2000;222:170–80. [DOI] [PubMed] [Google Scholar]
- [106].Zhang L, El-Hodiri HM, Ma HF, Zhang X, Servetnick M, Wensel TG, Jamrich M. Targeted expression of the dominant-negative FGFR4a in the eye using Xrx1A regulatory sequences interferes with normal retinal development. Development 2003;130:4177–86. [DOI] [PubMed] [Google Scholar]
- [107].Tropepe V, Coles BL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, van der Kooy D. Retinal stem cells in the adult mammalian eye. Science 2000;287:2032–6. [DOI] [PubMed] [Google Scholar]
- [108].Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev Biol 2000;220:197–210. [DOI] [PubMed] [Google Scholar]
- [109].Fischer AJ, Reh TA. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci 2001;4:247–52. [DOI] [PubMed] [Google Scholar]
- [110].Fischer AJ, Dierks BD, Reh TA. Exogenous growth factors induce the production of ganglion cells at the retinal margin. Development 2002;129:2283–91. [DOI] [PubMed] [Google Scholar]
- [111].Fischer AJ, McGuire CR, Dierks BD, Reh TA. Insulin and fibroblast growth factor 2 activate a neurogenic program in Müller glia of the chicken retina. J Neurosci 2002;22:9387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]


