Abstract
Background:
Improvements in disease free survival for epithelial ovarian, peritoneal or fallopian tube cancer (EOC) will only come with improved primary therapy. Incorporation of poly-ADP-ribose inhibitors (PARPi) in the frontline setting may represent one strategy. This study sought to determine the maximum tolerated and feasible doses of the PARPi veliparib in combination with chemotherapy for EOC.
Methods:
A phase I, 3+3 dose escalation evaluated dose-limiting toxicities (DLTs) in cycles 1–2. Once <2/6 patients experienced a DLT, that dose level expanded to evaluate feasibility over 4 cycles. This study opened 10/2009 and closed 8/2016. Eligible patients had untreated, stage II-IV EOC. Veliparib was added either continuous (day 1–21) or intermittent (day - 2 to 5) during 6 cycles of chemotherapy. Three chemotherapy backbones were evaluated (2 intravenous (q3week and weekly) and 1 intraperitoneal (IP)) all inclusive of bevacizumab with and as maintenance to 22 cycles.
Findings:
Dose evaluations for 424 treated patients were available. Regimen 1 (q3 week), continuous (Reg1c) the maximum tolerated dose (MTD) was 250mg veliparib BID and feasible dose was 150mg BID. For regimen 1, intermittent (Reg1i) the MTD and feasible dose were 400 and 250mg BID. For Reg2c (weekly paclitaxel) the MTD and feasible dose were 150mg BID. For Reg2i the MTD and feasible dose were 250 and 150mg BID. For Reg3c (IP) the MTD and feasible dose were 150mg BID and for Reg3i (IP), the MTD and feasible dose were 400mg and 300mg BID.
Interpretation:
The feasible dose for Reg1c, 2c, 2i and 3c was 150mg po BID. For Reg1i and 3i the dose was pushed to 250 and 300mg po BID respectively. There is no apparent difference in efficacy between continuous and intermittent dosing indicating that the higher doses achieved in intermittent dosing may not be needed. ()
Funding:
National Cancer Institute
Introduction:
Epithelial ovarian cancer (EOC) remains the most lethal of gynecologic cancers with 22,530 new cases and 13,980 deaths estimated in the United States in 2019.1 Recent estimates place the prevalence of long term, disease free survival (defined as 10 years or greater from time of diagnosis) at approximately 16%.2 There are a myriad of efforts attempting to shift the proportion of patients who present with advanced stage disease to long term, disease free survival including improved selection for and execution of high quality primary cytoreduction3–5, tailored delivery of platinum/taxane based chemotherapy6–9, inclusive of intraperitoneal (IP) and weekly (dd) delivery, and combination of novel therapeutics with and/or to follow front line chemotherapy10, 11.
The emergence of poly [ADP ribose] polymerase inhibitors (PARPi) as treatment for EOC has dramatically challenged the established treatment paradigms for recurrent and recently diagnosed, treatment naïve EOC.12 PARPi would be predicted to be most efficacious in patients who harbor either germline (g) or somatic/tumor (t) mutations in BRCA. However, the Cancer Genome Atlas (TCGA) reports that up to 50% of patients with high grade serous EOC harbor molecular alterations in other homologous recombination genes and epigenetic changes to BRCA in addition to the 20% with BRCA mutations making EOC an ideal target for use of PARPi. The treatment paradigm for recurrent EOC shifted with a series of new approvals. First, approval of both olaparib13 and rucaparib14 for treatment of recurrent EOC with either g or tBRCA mutations respectively made PARPi an available alternative to chemotherapy. Second, the approval of olaparib15, niraparib16 and rucaparib17 for use in EOC as a switch maintenance agent to follow response to platinum based induction treatment in the recurrent setting opened up use of PARPi to BRCAwt.
Most recently, the results of SOLO-1() which used olaparib as switch maintenance following response to front line therapy among patients with g or tBRCA showed a HR for PFS of 0.3 with a median PFS that has not been reached in favor of olaparib (95% CI for HR 0.23, 0.41; p<0.0001).12
The clinical questions at present are whether incorporation of PARPi into front line therapy will result in a clinically meaningful impact among all patients with EOC and how PARPi should be incorporated: concomitant with chemotherapy and continued as maintenance following completion of chemotherapy, or started as switch maintenance among patients with response following platinum based chemotherapy.
Veliparib (ABT-888) is an orally bioavailable PARP 1 and 2 inhibitor which has single agent activity in recurrent, gBRCA EOC.18 Veliparib has also been successfully combined with chemotherapy in EOC, breast, pancreas and other solid tumors.19–21 Given the ability to combine veliparib with standard dose chemotherapy and interest in exploiting the high prevalence of HRD in high grade EOC, this multi-cohort, phase I trial was initiated to determine the maximum tolerated dose (MTD) of veliparib, given both continuously and intermittently in combination with standard intravenous (IV) every 21 day paclitaxel and carboplatin, IV weekly (dd) paclitaxel and carboplatin and intraperitoneal (IP) cisplatin, IV paclitaxel and IP paclitaxel day 8. All regimens were given with bevacizumab and with bevacizumab maintenance given the benefit demonstrated in prior phase 3 trials10, 11.
Methods:
This open label, multi-cohort, phase I study was open through the Gynecologic Oncology Group (GOG) phase I sites. The trial was registered with ClinicalTrials.gov (). All patients gave written informed consent before study entry in compliance with institutional, state and federal regulations. The study’s primary objectives were (i) to determine the MTD and dose-limiting toxicities (DLTs) of veliparib when administered using continuous versus intermittent dosing schedules with IV carboplatin, paclitaxel and bevacizumab using two different treatment regimens; or with IP cisplatin and IV and IP paclitaxel and IV bevacizumab in women with newly diagnosed, EOC, (ii) to determine the feasibility of these treatment regimens over four cycles in a 2-stage group sequential design once the MTD was established, and (iii) to assess the toxicity of these regimens using the CTEP NCI Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. The primary endpoints were: (i) first or second-cycle dose-limiting toxicities (DLTs) in the dose escalation phase, and (ii) DLTs occurring in the first four cycles in the feasibility phase.
Patients:
Eligible patients were those with FIGO stage II-IV EOC. Optimal (≤1cm) or suboptimal residual disease was allowed. However, patients with neoadjuvant chemotherapy or with planned interval cytoreductive surgery were excluded. All EOC histologic subtypes were eligible. Adequate bone marrow, renal, hepatic, neurologic and blood coagulation functions and a GOG performance status of 0–2 were required. A study amendment also required an albumin ≥ 3.0g/dL. Patients with tumors of low malignant potential (aka borderline tumors), a history of other malignancies within 5 years, prior radiation to the abdomen and/or pelvis, prior chemotherapy within 5 years, history of significant cardiovascular disease, bleeding conditions or evidence of active central nervous system involvement were excluded. Patient characteristics are presented for the 424 treated patients. Demographics are shown in table1.
Table 1:
Demographics for study participants
| Characteristic | Regimen I N(%) | Regimen II N(%) | Regimen III N(%) | Total N(%) |
|---|---|---|---|---|
| Age (y) | ||||
| ≤40 | 9 (5.2) | 7 (5.4) | 6 (4.9) | 22 (5.2) |
| 40–49 | 29 (16.8) | 16(12.5) | 26(21.1) | 71 (16.7) |
| 50–59 | 60 (34.7) | 47 (36.7) | 42(34.1) | 149(35.1) |
| 60–69 | 54(31.2) | 41 (32) | 40 (32.5) | 135(31.8) |
| 70–79 | 21 (12.1) | 16(12.5) | 9 (7.3) | 46(10.8) |
| ≥80 | 0 | 1 (0.8) | 0 | 1 (0.2) |
| BRCA 1 or 2 | ||||
| BRCA+ | 18(10.3%) | 16(12.2%) | 25(19.8%) | 59 (13.7%) |
| BRCAwt | 90(51.7%) | 61(46.6%) | 59(46.8%) | 210 (48.7%) |
| Unknown | 66(37.9%) | 54(41.2%) | 42(33.3%) | 162 (37.6%) |
| Race | ||||
| White | 154 (89) | 113(88.3) | 115(93.5) | 382(90.1) |
| Black | 7 (4.0) | 8 (6.3) | 4(3.3) | 19 (4.5) |
| Asian | 8 (4.6) | 3 (2.3) | 3 (2.4) | 14 (3.3) |
| Am Indian | 1 (0.6) | 0 | 0 | 1 (0.2) |
| Ukn | 3(1.7) | 4(3.1) | 1 (0.8) | 8(1.9) |
| Performance Status | ||||
| 0 | ||||
| 1 | 118(68.2) | 83 (64.8) | 87 (70.7) | 288 (67.9) |
| 2 | 54(31.2) | 43 (33.6) | 36 (29.3) | 133(31.4) |
| 1 (0.6) | 2(1.6) | 0 | 3 (.7) | |
| Histology | ||||
| Serous | 128 (74) | 100(78.1) | 101 (82.1) | 329 (77.6) |
| Endometrioid | 15(8.7) | 7 (5.5) | 10(8.1) | 32 (7.5) |
| Clear Cell | 14(8.1) | 7 (5.5) | 4 (3.3) | 25 (5.9) |
| Other | 16 (9.3) | 14(10.9) | 8 (6.5) | 38 (9.0) |
| Stage | ||||
| 2 | 24 (14%) | 19(15%) | 10 (8%) | 53 (12.5%) |
| 3 | 119(69%) | 83 (65%) | 104 (85%) | 306 (72.5%) |
| 4 | 30 (17%) | 26 (20%) | 9 (7%) | 65 (15%) |
| Residual Disease | ||||
| >0 cm | 54(31%) | 47 (35.9%) | 21(16.7%) | 122(28.3%) |
| Microscopic | 120 (68.9%) | 84 (64%) | 105 (83.3%) | 309(71.7%) |
Am Indian = American Indian; Ukn = unknown; NOS = not otherwise specified
Study Design:
This study consisted of 3 regimens (each with 2 veliparib dosing cohorts), each with a dose escalation and dose expansion component. Assignment to each of the 3 regimens was per physician selection and slot availability. (Supplemental Figure 1) Patients must have received >75% of their planned veliparib dose to be evaluable for a DLT for both dose escalation and dose feasibility. Patients failing to meet these criteria were replaced. During dose escalation, DLTs were assessed during the first 2 cycles of treatment.
Dose escalation was run separately for each regimen. Following common 3+3 escalation rules, patients enrolled in dose-level cohorts of 3 until a DLT occurred. If 1 of 3 patients experienced a DLT, up to 3 additional patients were treated at that dose level. If no further DLTs were observed, dose escalation continued. When ≥2 patients at a dose level experienced a DLT, that dose level was discontinued and the dose level was de-escalated. The highest dose with less than 2 DLTs observed in 6 evaluable patients was deemed the MTD.
Starting with regimen’s MTD, the feasibility of the regimen was evaluated by DLT assessments through cycle 4 of treatment. The feasibility component included two stages. For stage 1, an additional 11 patients were added to the MTD dose level (for a total of 17 patients). If ≥7 DLT events occurred in stage I, the regimen was considered not feasible and the dose was deescalated. If ≤2 DLT events were observed, the regimen was considered feasible and no further patients were enrolled. If > 2 but < 7 DLTs were observed, a second stage of 16 feasibility patients was enrolled. If ≥9 events occurred following accrual of up to 33 patients, the regimen would be considered not feasible. If ≤9 events occurred, the regimen could be considered feasible.
DLTs for this study included both hematologic and non-hematologic toxicities. Hematologic toxicities included a dose delay > 3 weeks due to failure to recover counts, febrile neutropenia, grade 4 neutropenia ≥ 7 days and grade 4 thrombocytopenia or bleeding associated with grade 3 thrombocytopenia.
Non-hematologic toxicities included study related grade 3 or 4 non-hematologic toxicity (excluding alopecia, fatigue, hypersensitivity reactions, nausea, vomiting, constipation, diarrhea, hypokalemia, hypomagnesemia, hypocalcemia, hypophosphatemia and grade 3 hypertension), and any drug related death.
Treatment:
The study evaluated 3 regimens, each with two dosing sub-cohorts to evaluate continuous (c) veliparib dosing twice daily PO (BID) days 1–21 or intermittent (i) veliparib dosing twice daily PO BID days −2 to 5. Veliparib was only administered during the 6 cycles of chemotherapy, not as maintenance. Regimen 1 (Reg1) treated patients with paclitaxel 175mg/mg2 IV, carboplatin AUC 6 IV, bevacizumab 15mg/kg IV all given day 1 (starting cycle 2) followed by bevacizumab maintenance cycles 7–22.
Regimen 2 (Reg2) used weekly paclitaxel 80mg/m2, carboplatin AUC 6 IV day 1, bevacizumab 15mg/kg IV day 1 (starting cycle 2) followed by bevacizumab maintenance cycles 7–22.
Regimen 3 (Reg3) used paclitaxel 135mg/m2 IV day 1, cisplatin 75mg/m2 IP day 1 or 2, paclitaxel 60mg/m2 IP day 8, bevacizumab 15mg/kg IV day 1 (starting cycle 2) followed by bevacizumab maintenance cycles 7–22.
All cycles were repeated every 21 days for a total of 6 cycles. Bevacizumab was continued as maintenance at 15mg/kg every 21 days for cycles 7–22. Standard pre-chemotherapy anti-emetics, H1 and H2 blockers and dexamethasone were used.
Ten dose levels (DL) were planned, starting with veliparib dose level 1 (DL1) of 30mg, DL2 50mg, DL3 80mg, DL4 100mg, DL5 150 mg, DL 6 200mg, DL7 250mg, DL8 300mg, DL9 350mg, and DL10 400mg BID. Intermittent dosing was added during initial escalation of the 3 chemotherapy regimens with continuous veliparib dosing in anticipation of lower toxicity. The intermittent dosing cohorts were started at the MTD of the companion continuous regimen, ensuring a dose that had cleared the initial DLT evaluation.
During dose escalation, toxicity and laboratory assessments were done weekly. From cycles 3–6 and during maintenance, toxicity assessments were done prior to each cycle every 21 days. Adverse events were assessed using CTCAE version 4.0. Response and progression were evaluated using the revised Response Evaluation Criteria in Solid Tumors (RECIST) guideline (version 1.1)22.
Statistics
Descriptive statistics and contingency tables were used to summarize baseline patient characteristics, tumor response and adverse events for this study. The Kaplan Meier23 methods were used to estimate the progression free and overall survival distributions and the related medians. 95% confidence intervals were estimated using Greenwood methods24.
This study was sponsored by the National Cancer Institute/National Clinical Trials Network. The corresponding author had access to all data in the study and had final responsibility to submit for publication.
Adverse Events
Table 2 displays adverse events (AEs) that occurred during the study by system organ class and preferred term. Eight patients had grade 5 AEs: Four of these were cases of sepsis: 1 in Regimen 1, 1 in Regimen 2, and 2 in Regimen 3. Two of these, were determined to be study related. The first patient was on Reg1c, DL 2 (50mg veliparib BID). She had hypoalbuminemia at enrollment with an albumin of 2.3g/dl. She was admitted cycle 3 day 12 with pseudomonal sepsis (WBC=0.6k/ mc°L on admission) and died after a brief intensive care stay. Following this event, the protocol was amended to include albumin ≥ 3g/dl as an eligibility criterion. The second patient was on Reg3c, DL 6 (200mg veliparib). She had persistent ascites requiring weekly paracenteses following enrollment on protocol. Her albumin was 3.5g/dl at screening but continued to decline while on study to 2.1. She was admitted with sepsis on cycle 2, day 14 and died one day later in the ICU. Her WBC/ANC at the time of admission was 0.55 and 0.17 k/mc°L respectively.
Table 2:
Adverse events reported during study participation. (Neuro = neurologic, Malig. = malignancy, AML = acute myelogenous leukemia, MDS = myelodysplastic syndrome)
| Regimen 1 | Regimen 2 | Regimen 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Adverse Events | A11G | G3/4 | G5 | A11G | G3/4 | G5 | A11G | G3/4 | G5 |
| Hematologic | |||||||||
| Anemia | 95% | 25% | 0 | 99% | 47% | 0 | 92% | 26% | 0 |
| Neutropenia | 98% | 92% | 0 | 94% | 83% | 0 | 91% | 74% | 0 |
| Febrile Neutropenia | 13% | 13% | 0 | 4% | 4% | 0 | 4% | 4% | 0 |
| Thrombocytopenia | 91% | 33% | 0 | 59% | 27% | 0 | 63% | 15% | 0 |
| Gastrointestinal | |||||||||
| Abdominal Pain | 43% | 5% | 0 | 54% | 6% | 0 | 66% | 7% | 0 |
| Perforation (Colon) | 1% | 1% | 0 | 0 | 0 | 0 | 3% | 3% | 0 |
| Perforation (SI) | 1% | 1% | 0 | 1% | 1% | 0 | 0 | 0 | 0 |
| Constipation | 66% | 2% | 0 | 62% | 1% | 0 | 68% | 1% | 0 |
| Diarrhea | 45% | 1% | 0 | 59% | 6% | 0 | 57% | 6% | 0 |
| Dyspepsia | 15% | 1% | 0 | 13% | 0 | 0 | 14% | 0 | 0 |
| Mucositis | 28% | 0 | 0 | 30% | 0 | 0 | 34% | 2% | 0 |
| Nausea | 84% | 6% | 0 | 77% | 6% | 0 | 90% | 12% | 0 |
| Vomiting | 40% | 4% | 0 | 38% | 6% | 0 | 59% | 10% | 0 |
| Cardiovascular | |||||||||
| Thrombo-embolic | 10% | 5% | 1% | 13% | 5% | 0 | 19% | 9% | 0 |
| Stroke | 1% | 0 | 0 | 0 | 0 | 0 | 2% | 1% | 0 |
| Hypertension | 55% | 27% | 0 | 58% | 30% | 0 | 64% | 29% | 0 |
| Epistaxis | 29% | 1% | 0 | 47% | 1% | 0 | 24% | 0 | 0 |
| Vaginal Bleeding | 5% | 0 | 0 | 8% | 0 | 0 | 2% | 1% | 0 |
| General/Neuro | 0 | ||||||||
| Anorexia | 43% | 1% | 0 | 42% | 3% | 0 | 59% | 1% | 0 |
| Fatigue | 89% | 5% | 0 | 90% | 6% | 0 | 88% | 9% | 0 |
| Headache | 39% | 1% | 0 | 52% | 2% | 0 | 50% | 1% | 0 |
| Insomnia | 29% | 0 | 0 | 33% | 1% | 0 | 23% | 0 | 0 |
| Myalgia | 36% | 0 | 0 | 29% | 1% | 0 | 27% | 0 | 0 |
| Sensory Neuropathy | 67% | 0 | 0 | 65% | 2% | 0 | 61% | 0 | 0 |
| Motor Neuropathy | 8% | 1% | 0 | 6% | 0 | 0 | 4% | 0 | 0 |
| Renal | |||||||||
| Creatinine elevation | 14% | 0 | 0 | 13% | 0 | 0 | 27% | 1% | 0 |
| Proteinuria | 12% | 3% | 0 | 9% | 3% | 0 | 14% | 2% | 0 |
| Respiratory | |||||||||
| Dyspnea | 39% | 0 | 0 | 45% | 5% | 0 | 35% | 2% | 0 |
| Secondary Malig. | |||||||||
| AML | 1% | 1% | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MDS | 1% | 1% | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Infections/Infestation | |||||||||
| Lung infection | 1.8% | 1.2% | 0% | 4.7% | 3.1% | .8% | 2.4% | 1.6% | 0 |
| Sepsis | 1.8% | 1.2% | .6% | 3.1% | 2.3% | .8% | 4.0% | 2.4% | 1.6% |
| Psychiatric | |||||||||
| Suicide (attempt) | 0.6% | 0.6% | 0 | 0 | 0 | 0 | .8% | 0 | .8% |
The other grade 5 events were not deemed study related. There were two grade 5 thromboembolic events, both on Reg1i. One on DL 8 (300 mg BID); the other occurred on DL 9 (350 mg BID). Two additional deaths due to sepsis were study related, 1 in Reg2i DL5 and 1 in Reg3i DL10. A grade 5 lung infection in Reg2i, DL 6 (200 mg BID) and a grade 5 suicide in Reg3i, DL 10 (400 mg BID) were also considered unrelated to study treatment.
Treatment Results
Details of dose escalation for the 6 cohorts is summarized in Table 3. For Reg1c, DL 7 (250mg veliparib BID) was considered the MTD. In order to find a feasible dose through cycle 4, the dose had to be de-escalated to DL 5 (150mg veliparib BID). DLTs were primarily hematologic with 9 reported episodes of febrile neutropenia (FN), 7 episodes of grade 4 thrombocytopenia (or grade 3 with bleeding) (PLT), and 1 reported case of sepsis.
Table 3:
Dose limiting toxicities (DLTs) by dose level and regimen. (n)= number of evaluable patients
| Reg | DL1 (n) | DL2(n) | DL3(n) | DL4(n) | DL5(n) | DL6(n) | DL7(n) | DL8(n) | DL9(n) | DL10(n) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ic | (6) 1 DLT: PE | (6) 1 DLT: FN | (3) 0 | (3) 0 | (3) 0 DLTs Expansion (14) 2 DLTs:G3FN, G3Na | (3) 0 DLTs Expansion (14) 6 DLTs: G3FN (2); G4PLT (2); G3 syncope; >3 week delay r/t PLT | (3) 0 DLTs Expansion (14) 7 DLTs:G4PLTS (4); FN(2); G4Sepsis/G3FN C3 and G4 SBO C4 (1 patient) | (6) 2 DLT: G4FN; G4 PLTS | ||
| Ii | (3) 0 DLTs | (3) 0 DLTs Expansion (14) 4DLTS:G4FN; G4TEE/G3 pain; G4 duodenal ulcer; G4 ANC; G3FN/G3AP | (3) 0 DLTs Expansion (12) 4DLTs:G3FNx 2; G3 PLTS/G4 epistaxis; FN/G4PLTS; G3FN/G3AP C4 | (6) 1DLT: G4 PLTS Expansion (16) 7 DLTs:G4ANC; G3AP/G4PLTs; G3 Afib/G3DH; G4ANC × 2; FN/G3PE; G4 perf/G4Ca; G3FN/G3PE Expansion 2 (9) 3 DLTS: G3Pain/G3 Afib/G3 DH; G3AP/G3NV/G4PLTs; G4ANC | (6) 0 DLTs Expansion (8) 4 DLTs: G3 DH;G3 PNA/FN × 2; G3FN | |||||
| 2c | (6) 1 DLT: G3FN | (6) 1 DLT: G4ANC | (3) 0 DLTs | (6) 1 DLT: G3 QTc | (6) 1 DLT: G3 HA Expansion (11) 0 DLT | (3) 0 DLTs | ||||
| 2i | (4) 0 DLTs | (3) 0 DLTs Expansion (13) 2 DLTs: G3Na; G3AP | (3) 0 DLTs Expansion (14) 6 DLTs: G3 syncope; G4 ANC; G4 neuropathy; G3 liver, G5 cardiac arrest/ G4 DH/G3Na(lpt);FN | (6) 1 DLT: G4PLTs Expansion 1 (10) 3 DLTs; G4PLTs/G3 MW; G3PE; G4 ANC Expansion 2 (14) 6 DLTs: G4PLTs/G3 DH; G3 MW; G3 Na, G4PLTs, G3 syncope, G3 neuropathy; G4ANC | (6) 2 DLTS: G4PLTs; G4ANC | |||||
| 3c | (3) 0 DLTs | (6) 0 DLTs Expansion1 (11) 5 DLTs; G3FN, G4PE, G4M1, G3 PE, G4CVA Expansion2 (14) 4 DLTs: G3 AP (Cl)/G4 pelvic ifx (C4); G3PNA (C2)/ G3FN/PNA/Sepsis (C3); G3 syncope; G3 mouth sores | (5) 2 DLTs: G3HA, G5 Sepsis (1) | |||||||
| 3i | (3) 0 DLTs | (3) 0 DLTs | (4) 0 DLTs | (6)1DLT: G3 IP Infection | (3) 0 DLTs Expansion (14) 3 DLTs: G3Na; G3FN; G3PE | (3) 0 DLTs | (5)0 DLTs Expansion (8) 5 DLTs: G3 syncope/G3 fatigue; G3PE; G3 fatigue/G3Na; G4ANC; G3Na |
Regimens: 1c: paclitaxel 175mg/mg2 IV, carboplatin AUC 6 IV, bevacizumab 15mg/kg IV all given day 1 (starting cycle 2), veliparib po BID days 1–21 followed by bevacizumab maintenance cycles 7–22. 1i: paclitaxel 175mg/mg2 IV, carboplatin AUC 6 IV, bevacizumab 15mg/kg IV all given day 1 (starting cycle 2), veliparib po BID days −2 to 5 followed by bevacizumab maintenance cycles 7–22. 2c: weekly paclitaxel 80mg/m2, carboplatin AUC 6 IV day 1, bevacizumab 15mg/kg IV day 1 (starting cycle 2) and veliparib po BID days 1–21 followed by bevacizumab maintenance cycles 7–22; 2i: weekly paclitaxel 80mg/m2, carboplatin AUC 6 IV day 1, bevacizumab 15mg/kg IV day 1 (starting cycle 2) and veliparib po BID day −2 to 5 followed by bevacizumab maintenance cycles 7–22; 3c weekly paclitaxel 80mg/m2, carboplatin AUC 6 IV day 1, bevacizumab 15mg/kg IV day 1 (starting cycle 2) and veliparib po BID days 1–21 followed by bevacizumab maintenance cycles 7–22; 3i weekly paclitaxel 80mg/m2, carboplatin AUC 6 IV day 1, bevacizumab 15mg/kg IV day 1 (starting cycle 2) and veliparib days −2 to 5 followed by bevacizumab maintenance cycles 7–22. Dose escalation presented first and includes DLTs through cycle 2, patients in dose escalation were included for feasibility assessment if they were evaluable through cycle 4. c = continuous; i= intermittent; G= grade; PE = pulmonary embolus; FN = febrile neutropenia; Na = hyponatremia, PLT = thrombocytopenia; r/t = related to; C= cycle; SBO = small bowel obstruction; TEE= thromboembolic event; AP= abdominal pain; ANC = G4 neutropenia >7 days; DH= dehydration; Afib = atrial fibrillation; perf= colonic perforation; Ca= hypocalcemia; NV= nausea vomiting; PNA= pneumonia; QTc = prolonged QTc interval; HA= headache; liver = elevated transaminases, MW= muscle wasting; MI = myocardial infarction; CVA = cerebral vascular accident; IP= intraperitoneal
(N) = evaluable patients on the cohort
Dose escalation for Reg1i dosing was initiated at DL6 and continued to DL 10 (400mg veliparib BID days −2 to 5) which was the highest planned dose. For DL10, no DLTs were noted in the first 3 patients and, as this was the highest planned DL, it was expanded to 6 patients. No DLTs were observed, and DL 10 was considered the MTD. DL10 was then expanded for feasibility with an additional 8 patients, 4 of whom had DLTs. The protocol required that 17 be evaluable, but due to concerns over tolerability of this DL (2 patients discontinued due to inability to tolerate oral dosing), the feasibility dose was de-escalated to DL 9. Two additional de-escalations were required to find the feasible dose at DL 7 (250mg BID). Although there were 4 DLTs, 2 of these were due exclusively to bevacizumab and were within the expected toxicities for this agent and so the decision was made to not further dose de-escalate. Similar to Reg1c, the DLTs for Reg1i were primarily hematologic with 11 reported episodes of FN, 4 PLT, and 5 episodes of grade 4 neutrophils lasting more than 7 days.
Dose escalation for Reg2c continued to DL 6 (200mg BID) where 3 patients were treated, and no DLTs were observed. However, there were significant early delays and dose modifications in 2 of 3 patients. Therefore, rather than continuing with escalation, the dose was reduced to DL 5. This dose was used as the estimated MTD and was expanded to the feasibility phase. Of twenty patients accrued, 15 were DLT evaluable across four cycles, and only 1 DLT was observed. Therefore, this dose level (150mg BID) was declared feasible.
Dose escalation for Reg2i identified DL 7 (250mg veliparib BID) as the MTD. The feasible dose was identified at DL5 (150mg veliparib BID). DLTs in DLs above DL 5 included 1 FN, 5 PLTs and 4 ANC > 7 days.
Dose escalation for Reg3c identified DL5 as the MTD (150mg veliparib BID) as well as the feasible dose. While feasible, this does level still had one patient with repeated episodes of grade 3 pneumonia as well as FN and sepsis.
Dose escalation for Reg3i proceeded to DL 10 (400mg BID) which was declared the MTD. Because of the need in all other cohorts to de-escalate by at least 2 DL from the feasible dose, dosing was dropped to DL8 which identified the feasible dose of 300mg veliparib BID.
Progression Free and Overall Survival
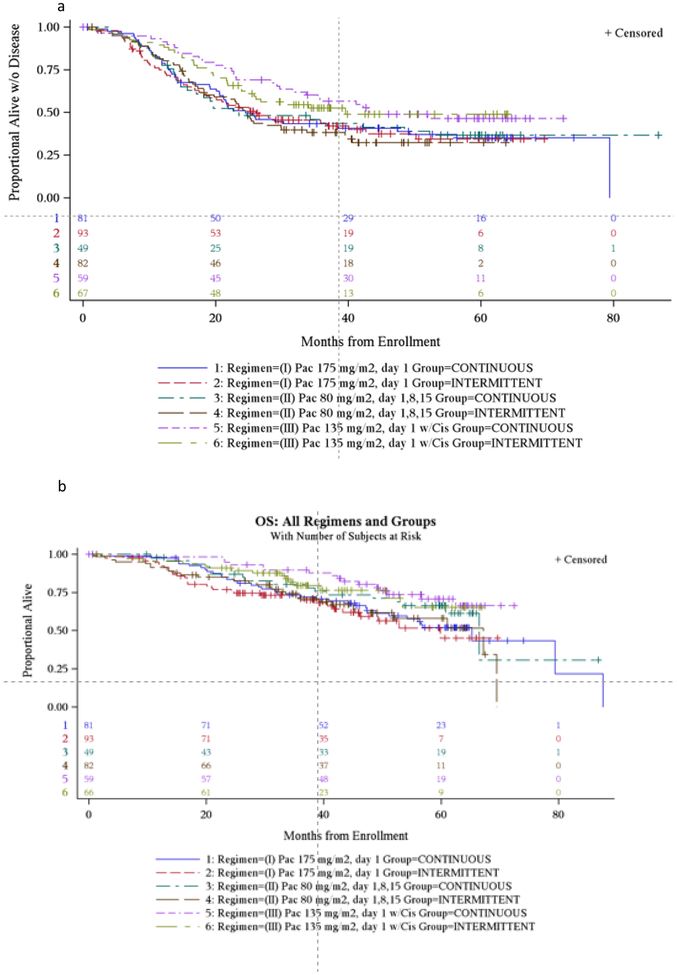
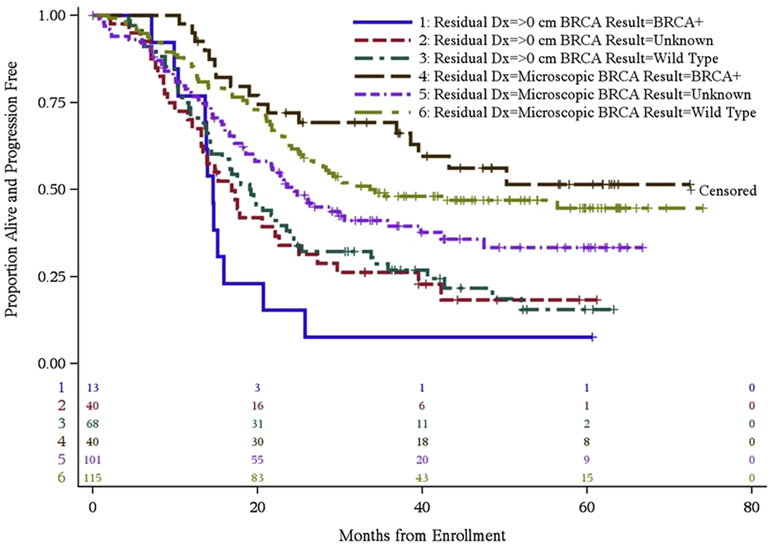
Progression free and overall survival for each regimen is summarized in Figure 1. Figure 2 displays the progression free survival (PFS) for patients by BRCA status and residual disease. There is no treatment effect with continuous versus intermittent dosing and so these categories were collapsed. The median PFS for no gross residual disease was not reached (NR) (36- NR); 34.2 (25.5 – NR) and 24.5 (18.6–35.7) in BRCA+, BRCAwt and BRCA unknown (unk) respectively. Median PFS for residual disease was 14.6 (10.3–15.9), 19.1 (14.3 – 23.5) and 16.9 (13.1–22.6) respectively.
Figure 1:
Progression free and overall survival (PFS and OS) by regimen. (a) Progression free survival for all regimens/cohorts. (b) Overall survival for all regimens/cohorts.
Figure 2:
PFS by residual disease and BRCA status.
Discussion:
Incorporation of PARPi into the treatment paradigm of EOC is a marked step forward in providing patients with EOC another active treatment and hopefully, the chance to live longer. With the approval of PARPi in both front line and recurrent treatment scenario, our data helps answer the important question of how best to use PARPi.
At the time this study was designed, three front line delivery models for chemotherapy were available: 1) every 21 days IV, 2) dose dense paclitaxel (dd) IV and 3) intraperitoneal cisplatin and paclitaxel (IP). This study incorporated veliparib into each of these delivery models.25,8 ICON8 reported that in 1,500 patients, dd paclitaxel and carboplatin was not statistically superior to every 21 day paclitaxel and carboplatin or weekly dosing of both drugs.26 Our study demonstrates that veliparib at a dose of 150mg BID (continuous or intermittent) can be added to dd paclitaxel and carboplatin but the necessity of utilizing this regimen over the more convenient every 21 day dosing is under question. When utilizing the every 21 day regimen for both agents, veliparib was able to be combined at a dose of 150mg BID continuous and 250mg BID intermittent. Of note, this dose was higher than the doses used in the breast (50mg BID) and lung (120mg BID) trials of veliparib with paclitaxel and carboplatin which were negative.27, 28
GOG protocol 172 demonstrated a significant improvement in OS among optimally debulked stage 3 EOC patients receiving triplet vs doublet platinum therapy, even out to 10 years.6, 29 However, GOG 252 which used a lower dose of IP cisplatin and added bevacizumab, failed to show an improvement in any IP regimen over IV.30 Our study demonstrated the feasibility of adding continuous veliparib 150mg BID or 300mg BID with intermittent dosing to an IP regimen. In addition, median PFS outcomes among the population selected to receive IP therapy was impressive at 43.2 and 39.6 months for continuous and intermittent dosing respectively. Median OS was not reached at data cut off in either arm. There is provocative, albeit retrospective data, demonstrating superior survival outcomes with IP therapy among patients with BRCA mutations as well31 prompting the question as to whether IP therapy plus PARPi has a role for selected patients with BRCA mutations. As with dd paclitaxel, the question remains whether patients need to be exposed to this more toxic therapy and whether this finding has relevance moving forward.
Given that every 21-day IV paclitaxel and carboplatin may become the favored regimen for front line EOC, the finding that veliparib combined with this regimen did not compromise the dose intensity of the chemotherapy is important when developing transformative trials moving forward. The PFS for this trial is comparable with historical data sets such as GOG 218 and ICON7 where the median PFS and OS inclusive of maintenance bevacizumab was 14.1/43.8 and 21/58 months respectively10, 11. Considering just the every-21 day IV regimen for GOG 9923 with continuous dosing, the median PFS was 24.5 and median OS 65.2. Further, the BRCA+ patients on GOG 9923 had a median PFS of 14.6 to NR depending on residual disease as compared to 19.6 months on GOG 21832 which was predominantly patients with residual disease. Addition of veliparib to chemotherapy was feasible and did not impact dose intensity but was associated with hematologic toxicity and did not appear to greatly impact the PFS or OS of patients who participated.
This finding raises the question of where PARPi should be positioned in front line therapy. Evidence to date would suggest that the earlier PARPi is incorporated into therapy, the more efficacious the activity is.33 Several trials are exploring use of PARPi following front line chemotherapy as a maintenance compared to placebo. These include SOLO-1 which evaluated olaparib following front line chemotherapy in patients with g or tBRCA mutations. This study reported an unprecedented improvement in PFS for patients randomized to maintenance olaparib with a HR of 0.30 (95% CI of 0.23–0.41; p<0001).12 PRIMA evaluated switch maintenance with niraparib as compared to placebo in high grade serous and endometrioid patients following front line chemotherapy. (). The primary analysis demonstrated superiority for use of niraparib in the intention to treat (ITT) population with a HR 0.62 (95% CI 0.5–0.76) as well as the HRD+ population with a HR of 0.43 (95% CI 0.31–0.59). PAOLA-1 evaluated use of bevacizumab with chemotherapy and as maintenance with added olaparib switch maintenance versus placebo (). The primary analysis is only in the ITT population and was positive for the combination with a HR of 0.59 (95% CI 0.49–0.72).
Only Velia () has evaluated incorporation of a PARPi (veliparib) with and following carboplatin and paclitaxel as continued maintenance in a randomized phase 3, 3-arm study. The primary endpoint for Velia was in the BRCA+ tumors, followed by HRD+ and finally IIT. All 3 primary endpoints were positive with a HR of 0.44 (95% CI), HR of 0.57 (95% CI 0.43–0.76) and HR of 0.68 (95% CI 0.56–0.83) in the 3 groups respectively. Our study, GOG 9923, provided the safety data that recommended the veliparib dose with chemotherapy for Velia and also provides safety data for combination with bevacizumab, which is critical given the recent approval of bevacizumab in front line EOC34. How best to use PARPi in terms of population, timing and as a single agent or in combination is now the challenge for patient management given the positive read out on the above phase 3 trials all performed in different populations and with different primary endpoints.
Supplementary Material
Figure 1 Supplemental: Schema for treatment regimens on GOG 9923
Key Points:
Question: Is it feasible to give veliparib concurrently with platinum based chemotherapy in treatment naïve, advanced ovarian cancer?
Findings: In this large, phase I study of over 400 patients, veliparib was successfully combined with platinum based chemotherapy given every 3 weeks, using weekly paclitaxel or intraperitoneal delivery. The feasible dose of veliparib was 150mg p.o. BID given either continuously or intermittently.
Meaning: This study demonstrates the feasibility of using concurrent poly-ADP ribose polymerase inhibitor with platinum based chemotherapy in untreated epithelial ovarian cancer. An ongoing phase 3 study will demonstrate possible efficacy.
Highlights:
The PARP inhibitor veliparib can be combined with chemotherapy
A feasible combination dose was accomplished without compromised dose intensity
Combination veliparib and chemotherapy may improve responses in front line treatment
ACKNOWLEDGEMENTS
This work was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical and Data Center (CA 37517), the NRG Oncology SDMC (U10 CA180822), NRG Oncology Operations (U10CA 180868), NIH/NCI Support Grant P30 CA008748 (Dr. O’Cearbhaill, Dr. Aghajanian).
The following institutions participated in this study: University of Oklahoma Health Sciences Center, Women and Infants Hospital, University of Virginia, University of Colorado Cancer Center - Anschutz Cancer Pavilion, Memorial Sloan Kettering Cancer Center, Ohio State University Comprehensive Cancer Center, Washington University School of Medicine, Fred Hutchinson Cancer Research Center, Virginia Commonwealth University, Georgia Center for Oncology Research and Education (CORE), Georgia Cares Minority Underserved NCORP, University of Iowa Hospitals and Clinics, University of Chicago, Johns Hopkins University/Sidney Kimmel Cancer Center, Roswell Park Comprehensive Cancer Center, Fox Chase Cancer Center, Cleveland Clinic Foundation, Case Western Reserve University and University of Wisconsin Hospital and Clinics.
Disclosures: Dr. Kathleen Moore discloses reimbursement for advisory board participation for Astra Zeneca, Immunogen, Genentech/Roche, Tesaro, Clovis, OncoMed, Samumed, Aravive, Pfizer, Merck, Janssen and VBL Therapeutics. Dr. Moore also discloses steering committee involvement with Astra Zeneca, Tesaro, Clovis, Aravive and VBL Therapeutics.
Dr. Russell Schilder reports personal fees from Incyte, Flatiron, Celsion and Immunogen.
Dr. Roisin O’Cearbhaill discloses reimbursement for advisory board participation for Tesaro and Clovis and steering committee involvement with Tesaro.
Dr. Bell-McGuinn reports other payments from Lilly Oncology, outside the submitted work
Dr. Duska reports personal fees from Astra Zeneca, grants, personal fees and other from Genentech/Roche, grants from Cerulean/NextGen/(GOG 3008), grants from AbbVie/(GOG 3005), grants from Tesaro, grants from Pfizer, grants and other from GlaxoSmithKlein/Novartis, grants from Morab, grants and personal fees from MorphoTek, grants, personal fees and other from Merck, grants from Aduro BioTech, grants from Syndax, grants from Ludwig, grants from LEAP Therapeutics, grants from Eisai, grants from Lycera, grants and personal fees from Genentech/Roche, grants and personal fees from Inovio, personal fees from Advance Medical, personal fees from UpToDate, personal fees from Cue Biopharma, personal fees from British Journal of OB/GYN, personal fees from Parexel, personal fees from State of California, personal fees from Elsevier, personal fees from ASCO, personal fees from Expert review, personal fees from ClearView Health Care, personal fees from National Cancer Institute, personal fees from JB Learning, grants from Advaxis, outside the submitted work.
Dr. David O’Malley reports personal fees and other from AstraZeneca, personal fees and other from Clovis, personal fees and other from Tesaro, personal fees and other from Immunogen, personal fees from Ambry, personal fees and other from Janssen/J&J, personal fees and other from Abbvie, personal fees and other from Regeneron, personal fees and other from Amgen, personal fees and other from Novocure, personal fees and other from Genentech/Roche, other from VentiRx, other from Array Biopharma, other from EMD Serono, other from Ergomed, other from Ajinomoto Inc., other from Ludwig Cancer Research, other from Stemcentrx, Inc, other from CERULEAN PHARMA, other from GOG Group, other from Bristol-Myers Squibb Co, other from Serono Inc, other from TRACON Pharmaceuticals, other from Yale University, other from New Mexico Cancer Care Alliance, other from INC Research, Inc, other from inVentiv Health Clinical, other from Iovance Biotherapeutics, Inc, other from PRA Intl, other from Agenus, outside the submitted work.
Dr. Carol Aghajanian reports personal fees from Tesaro, personal fees from Immunogen, grants and personal fees from Clovis, personal fees from Mateon Therapeutics, personal fees from Cerulean Pharma, grants from Genentech, grants from AbbVie, and grants from AstraZeneca, outside the submitted work.
Dr. Austin Miller, Dr. Andrea Hagemann, Dr. Alice Chen, Dr. Cara Mathews, Dr. Joan Walker, Dr. Saketh Guntupalli, Dr. Deborah Armstrong, Dr. Heidi Gray and Dr. Sarah Gordon have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.American Cancer Society. Cancer Facts & Figures 2019. Atlanta: American Cancer Society; 2019. [Google Scholar]
- 2.Cress RD, Chen YS, Morris CR, Petersen M, Leiserowitz GS. Characteristics of Long-Term Survivors of Epithelial Ovarian Cancer. Obstetrics and gynecology 2015; 126(3): 491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2002; 20(5): 1248–59. [DOI] [PubMed] [Google Scholar]
- 4.Winter WE 3rd, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2007; 25(24): 3621–7. [DOI] [PubMed] [Google Scholar]
- 5.Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2016; 34(28): 3460–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. The New England journal of medicine 2006; 354(1): 34–43. [DOI] [PubMed] [Google Scholar]
- 7.Jaaback K, Johnson N, Lawrie TA. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. The Cochrane database of systematic reviews 2016; (1): CD005340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan JK, Brady MF, Penson RT, et al. Weekly vs. Every-3-Week Paclitaxel and Carboplatin for Ovarian Cancer. The New England journal of medicine 2016; 374(8): 738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katsumata N, Yasuda M, Takahashi F, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet 2009; 374(9698): 1331–8. [DOI] [PubMed] [Google Scholar]
- 10.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. The New England journal of medicine 2011; 365(26): 2473–83. [DOI] [PubMed] [Google Scholar]
- 11.Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. The New England journal of medicine 2011; 365(26): 2484–96. [DOI] [PubMed] [Google Scholar]
- 12.Moore K, Colombo N, Scambia G, et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. The New England journal of medicine 2018. [DOI] [PubMed] [Google Scholar]
- 13.FDA label for Olaparib. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208558s000lbl.pdf.
- 14.Rucaparib Approved for Ovarian Cancer. Cancer discovery 2017; 7(2): 120–1. [DOI] [PubMed] [Google Scholar]
- 15.Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. The Lancet Oncology 2017; 18(9): 1274–84. [DOI] [PubMed] [Google Scholar]
- 16.Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. The New England journal of medicine 2016; 375(22): 2154–64. [DOI] [PubMed] [Google Scholar]
- 17.Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390(10106): 1949–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman RL, Sill MW, Bell-McGuinn K, et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation - An NRG Oncology/Gynecologic Oncology Group study. Gynecologic oncology 2015; 137(3): 386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Reilly EM, Lee JW, Lowery MA, et al. Phase 1 trial evaluating cisplatin, gemcitabine, and veliparib in 2 patient cohorts: Germline BRCA mutation carriers and wild-type BRCA pancreatic ductal adenocarcinoma. Cancer 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han HS, Dieras V, Robson M, et al. Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/metastatic breast cancer: randomized phase II study. Annals of oncology: official journal of the European Society for Medical Oncology 2018; 29(1): 154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray HJ, Bell-McGuinn K, Fleming GF, et al. Phase I combination study of the PARP inhibitor veliparib plus carboplatin and gemcitabine in patients with advanced ovarian cancer and other solid malignancies. Gynecologic oncology 2018; 148(3): 507–14. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer 2009; 45(2): 228–47. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan EaMP. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association 1958; 53: 457–81. [Google Scholar]
- 24.Greenwood M The natural duration of cancer. Reports on Public Health and Medical Subjects 1926; 33: 1–26. [Google Scholar]
- 25.Katsumata N, Yasuda M, Isonishi S, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. The Lancet Oncology 2013; 14(10): 1020–6. [DOI] [PubMed] [Google Scholar]
- 26.Clamp A, McNeish I, Dean A, Gallardo D, Weon-Kim J, O’Donnell D, Hook J, Coyle C, Blagden SP, Brenton J, Naik R, Perren T, Sundar S, Cook A, James E, Swart AM, Steenning S, Kaplan R, Ledermann J. ICON8: A GCIG phase III randomsed trial evaluating weekly dose dense chemotherpay integration in fist line epithelial ovarian/fallopiantube/primary peritoneal carcinoma (EOC) treatment: Results of primary progression free survival (PFS) analysis. Annals of Oncology 2017; 28(suppl_5). [Google Scholar]
- 27.Ramalingam SS, Blais N, Mazieres J, et al. Randomized, Placebo-Controlled, Phase II Study of Veliparib in Combination with Carboplatin and Paclitaxel for Advanced/Metastatic Non-Small Cell Lung Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research 2017; 23(8): 1937–44. [DOI] [PubMed] [Google Scholar]
- 28.Loibl S, O’Shaughnessy J, Untch M, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. The Lancet Oncology 2018; 19(4): 497–509. [DOI] [PubMed] [Google Scholar]
- 29.Tewari D, Java JJ, Salani R, et al. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2015; 33(13): 1460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker JL BM, DiSilvestro PA, et al. . A phase III trial of bevacizumab with IV versus IP chemotherapy in ovarian, fallopian tube and peritoneal carcinoma NCI- supplied agent(s): A GOG/NRG trial (GOG 252) Gynecologic oncology 2016; Presented at the Society of Gynecologic Oncology; San Diego CA. [Google Scholar]
- 31.Naumann RW, Morris JC, Tait DL, et al. Patients with BRCA mutations have superior outcomes after intraperitoneal chemotherapy in optimally resected high grade ovarian cancer. Gynecologic oncology 2018; 151(3): 477–80. [DOI] [PubMed] [Google Scholar]
- 32.Norquist BM, Brady MF, Harrell MI, et al. Mutations in Homologous Recombination Genes and Outcomes in Ovarian Carcinoma Patients in GOG 218: An NRG Oncology/Gynecologic Oncology Group Study. Clinical cancer research: an official journal of the American Association for Cancer Research 2018; 24(4): 777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matulonis UA, Penson RT, Domchek SM, et al. Olaparib monotherapy in patients with advanced relapsed ovarian cancer and a germline BRCA1/2 mutation: a multistudy analysis of response rates and safety. Annals of oncology: official journal of the European Society for Medical Oncology 2016; 27(6): 1013–9. [DOI] [PubMed] [Google Scholar]
- 34.FDA label for bevacizumab; https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125085s305lbl.pdf accessed August 14, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1 Supplemental: Schema for treatment regimens on GOG 9923