Abstract
Experience-dependent neuronal plasticity is a fundamental substrate of learning and memory. Intrinsic excitability is a form of neuronal plasticity that can be altered by learning and indicates the pattern of neuronal responding to external stimuli (e.g. a learning or synaptic event). Associative fear conditioning is one form of learning that alters intrinsic excitability, reflecting an experience-dependent change in neuronal function. After fear conditioning, intrinsic excitability changes are evident in brain regions that are a critical part of the fear circuit, including the amygdala, hippocampus, retrosplenial cortex, and prefrontal cortex. Some of these changes are transient and/or reversed by extinction as well as learning-specific (i.e. they are not observed in neurons from control animals). This review will explore how intrinsic neuronal excitability changes within brain structures that are critical for fear learning, and it will also discuss evidence promoting intrinsic excitability as a vital mechanism of associative fear memories. This work has raised interesting questions regarding the role of fear learning in changes of intrinsic excitability within specific subpopulations of neurons, including those that express immediate early genes and thus demonstrate experience-dependent activity, as well as in neurons classified as having a specific firing type (e.g. burst-spiking vs. regular-spiking). These findings have interesting implications for how intrinsic excitability can serve as a neural substrate of learning and memory, and suggest that intrinsic plasticity within specific subpopulations of neurons may promote consolidation of the memory trace in a flexible and efficient manner.
Keywords: learning, memory, intrinsic plasticity, fear memories, extinction, memory modulation
1. Introduction
Experience-driven cellular changes are a critical component of learning and memory and are necessary for learning-related plasticity. Intrinsic excitability is one example of a learning-related change in neuronal plasticity and reflects alterations in the way a neuron responds to incoming information (e.g. from a learning event or synaptic stimulation). One learning paradigm that has received considerable attention for its role in learning-related changes of intrinsic excitability is classical fear conditioning. This learning paradigm has been shown to lead to distinct changes of intrinsic excitability in brain regions integral to the fear circuit. Intrinsic excitability is often learning-specific (i.e. it does not occur in animals that do not learn), transient (i.e. it lasts for a brief period of time after the learning event), and can be observed in specific subpopulations of neurons that likely reflect the memory trace. Thus, intrinsic plasticity is thought to be a substrate of learning that is independent of synaptic changes. In this review, we will briefly highlight the mechanisms of intrinsic plasticity, followed by a discussion of the role of several prominent brain regions in the fear circuit, as well as the role of fear conditioning in intrinsic plasticity within these regions. Evidence from these studies will support the idea that intrinsic excitability is an experience-dependent form of plasticity, establishing it as a critical substrate of fear learning and memory.
1.1. Intrinsic plasticity versus synaptic plasticity
The substrates for learning can be revealed by examining experience-dependent changes in neuronal function, i.e. plasticity. Such plasticity can be observed as synaptic changes, including long-term potentiation (LTP) or long-term depression (LTD), as well as non-synaptic changes, including intrinsic excitability. Since both synaptic and intrinsic plasticity are closely linked to learning and memory (Zhang & Linden, 2003; Lynch, 2004; Mayford, Siegelbaum, & Kandel, 2012; Sehgal, Song, Ehlers, & Moyer, 2013), they are invaluable for uncovering the neural substrates of memory.
Synaptic plasticity is a well-known cellular mechanism of learning and memory and has been extensively studied in a variety of preparations (for review see Lynch, 2004). The observation of enhanced synaptic transmission in the dentate gyrus following high frequency stimulation of the perforant path by Bliss and Lomo (1973) spurred similar observations shortly thereafter that supported the idea that learning and LTP depend upon similar changes of synaptic efficacy. Indeed, learning and LTP are linked in diverse ways. While protein synthesis is vital for long-term memory, short-term memory is unaffected when protein synthesis is blocked (Davis & Squire, 1984; Emptage & Carew, 1993; Izquierdo & Medina, 1998; McGaugh, 2000). Similarly, long-lasting LTP (L-LTP) critically depends on de novo protein synthesis, but a shorter, earlier phase of LTP (E-LTP) does not (Korte et al., 1995; Kang et al., 1997; Poo, 2001; Lynch, 2004; Pang et al., 2004). This suggests that learning-induced biochemical changes parallel those associated with LTP. Additionally, mechanisms that block LTP also block learning. For example, blockade of NDMA receptors (NMDARs) using APV disrupts spatial memory as well as LTP induction (Morris, Anderson, Lynch, & Baudry, 1986). Although a critical component of learning and memory, synaptic plasticity is not an exclusive form of experience-dependent plasticity, but is coupled with other forms of plasticity, including intrinsic excitability.
Intrinsic neuronal excitability is a non-synaptic form of cellular plasticity that supports learning and memory. The pattern of neuronal responding to learning-related stimuli can be observed by measuring spike frequency adaptation (the number of action potentials (APs) fired in response to sustained excitation), and post-burst afterhyperpolarization (AHP; hyperpolarizing current following a burst of APs). An experience like associative fear learning can affect either or both of these measures, often in the form of reduced spike frequency adaptation, as well as reduced post-burst AHP (Kaczorowski & Disterhoft, 2009; McKay, Matthews, Oliveira, & Disterhoft, 2009; Song, Detert, Sehgal, & Moyer, 2012; Sehgal, Ehlers, & Moyer, 2014; Oh & Disterhoft, 2015). Additionally, intrinsic excitability is thought to be a form of metaplasticity, acting as a catalyst for future synaptic changes, and influencing future learning (Abraham, 2008; Sehgal et al., 2013). It is clear that intrinsic and synaptic plasticity are independent mechanisms but are directly linked, such that enhanced intrinsic excitability promotes synaptic strength (for review see Sehgal et al., 2013). Historically, compared to synaptic plasticity, intrinsic plasticity has received far less attention as a mechanism of learning and memory. Therefore, this review will focus on mechanisms of intrinsic plasticity as well as recent developments in understanding how intrinsic plasticity changes as a consequence of fear learning.
1.1. Mechanisms of intrinsic plasticity
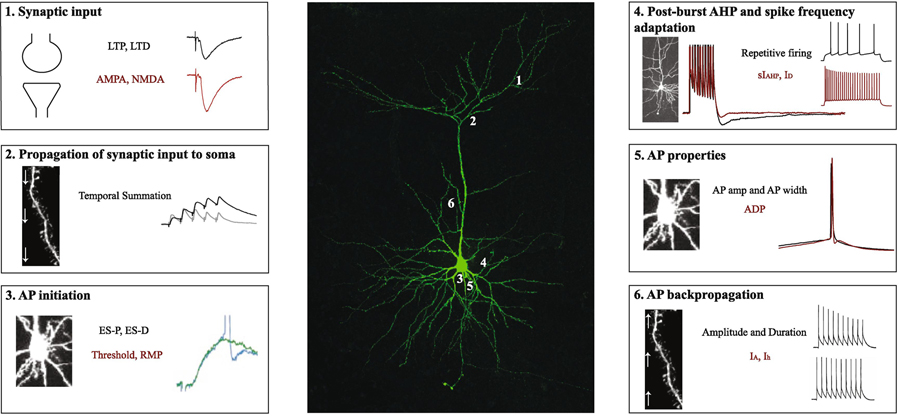
Following synaptic transmission (Figure 1, Panel 1), an AP will be initiated if excitatory postsynaptic potentials (EPSP) exceed inhibitory postsynaptic potentials (IPSP). Moreover, EPSPs and IPSPs are required to propagate from their site of generation to the AP zone in the soma (Figure 1, Panel 2). Propagation of synaptic potentials are influenced by 1) the complex dendritic morphology, 2) basic dendritic cable properties and, 3) voltage-gated conductances (for reviews see Spruston, 2008; Spruston, Stuart, & Häusser, 2016). Thus, changes in dendritic cable properties and/or changes in active dendritic conductances can influence the magnitude of local EPSPs, their integration, and their propagation to the soma (Papoutsi, Sidiropoulou, & Poirazi, 2012). The slow afterhyperpolarization current (sIAHP) has been shown to modulate synaptic input propagating to the soma (Hotson & Prince, 1980; Lancaster & Adams, 1986; Storm, 1989; Lancaster, Hu, Ramakers, & Storm, 2001). For example, activation of sIAHP reduces the amplitude of EPSPs (Sah & Bekkers, 1996), which leads to a reduction in intrinsic plasticity. Conversely, inhibition of sIAHP enhances intrinsic excitability by slowing the decay of summated EPSPs (Lancaster et al., 2001) in hippocampal pyramidal neurons. Furthermore, blockers of sIAHP promote LTP induction in hippocampal neurons (Sah & Bekkers, 1996; Cohen, Coussens, Raymond, & Abraham, 1999). The role of sIAHP in synaptic propagation and integration has also been observed in other fear-related brain structures including the amygdala and prefrontal cortex (Faber, Delaney, & Sah, 2005; Power, Bocklisch, Curby, & Sah, 2011; Zaitsev & Anwyl, 2011). Thus, sIAHP regulates synaptic efficacy by propagating and integrating synaptic potentials from the dendrites to soma.
Figure 1. Synaptic and intrinsic properties modulate the flow of information within a neuron.
Middle panel shows a confocal image of a retrosplenial cortical neuron that was filled with biocytin during whole-cell patch-clamp recording. Numbers 1, 2, 3, 4, 5 and 6 refer to the boxes in the left and right panels. (1) Most neuronal inputs originate via synapses on the dendrites and dendritic spines, which can undergo bidirectional plasticity in the form of LTP and LTD. Such plasticity is modulated by AMPA and NMDA receptor-mediated transmission as well as intrinsic membrane properties. (2) Following synaptic transmission, EPSPs are propagated from their site of generation towards the soma and AP zone. Propagation of EPSPs is influenced by dendritic cable as well as active membrane properties. (3) Once the signal reaches the soma, the increased likelihood for an EPSP to fire an AP is termed E-S potentiation. Factors including AP threshold and resting membrane potential determine AP initiation. (4) Increased neuronal excitability (e.g., reduction in the postburst AHP and/or spike frequency adaptation) promotes an output signal. (5) Other intrinsic factors including AP amplitude, AP duration, and the presence or absence of an afterdepolarization also influence neuronal excitability, which modulates neuronal processing and synaptic throughput. (6) Synaptic efficacy is also influenced by backpropagating APs, which are mediated by complex dendritic morphology, and dendritic ionic conductances such as IA currents. Abbreviations: long-term potentiation (LTP); long-term depression (LTD); excitatory postsynaptic potential (EPSP); EPSP-spike (E-S). Electrophysiological traces in boxes 3 and 6 were adapted from Daoudal et al., 2002 (Copyright (2002) National Academy of Sciences, USA) and Tsubokawa et al., 2000 (Copyright (2000) Society for Neuroscience).
When synaptic inputs reach the AP initiation zone, the increased propensity for an EPSP to fire an AP is a phenomenon termed EPSP-spike (E-S) potentiation (Bliss & Lomo, 1973; Figure 1, Panel 3). LTP induction (Noguchi, Saito, & Abe, 1998), activation of NMDARs, and elevated intracellular calcium (Ca2+) concentration (Aizenman & Linden, 2000) and/or enhanced intrinsic excitability (Pugliese, Ballerini, Passani, & Corradetti, 1994) can promote E-S potentiation. Furthermore, E-S coupling can be bidirectional as LTD induction in CA1 hippocampal neurons results in E-S depression (Daoudal, Hanada, & Debanne, 2002). Other intrinsic factors that may influence AP initiation include AP threshold and resting membrane potential, which depend on ion channels in the soma (Papoutsi et al., 2012). Taken together, intrinsic plasticity can regulate dendritic integration of synaptic input and impact E-S coupling.
In addition to the all-or-none firing nature of an AP, enhanced excitability (e.g. increased input resistance, reduced current required to elicit an AP, reduction in spike frequency adaptation) promotes a final neuronal output signal (Figure 1, Panel 4). Moreover, enhanced intrinsic excitability is correlated with enhanced learning (Disterhoft, Coulter, & Alkon, 1986; Disterhoft, Golden, Read, Coulter, & Alkon, 1988). Spike frequency adaptation is mediated by AHP current, and increased AHP reduces AP firing frequency. The AHP acts as a negative feedback mechanism and has three components: fast afterhyperpolarization (fAHP; within 2–5 ms of an AP), medium afterhyperpolarization (mAHP; 50–150 ms following one or more APs), and slow afterhyperpolarization (sAHP; 1 s following a burst of APs; Storm, 1987; Storm, 1989; Sah & Bekkers, 1996; Kaczorowski, Disterhoft, & Spruston, 2007; Song & Moyer, 2017). The fAHP and mAHP are mediated by Ca2+-activated potassium (SK) channels (Faber & Sah, 2002; McKay et al., 2012). Furthermore, mAHP is also modulated by M-type K+ channels or hyperpolarization-activated cyclic nucleotide-gated (HCN) cation channels (Gu, Vervaeke, Hu, & Storm, 2005). The sAHP component is regulated by the apamin-insensitive sIAHP (Sah, 1996; Storm, 1989; Gasparini & DiFrancesco, 1999; Stocker, Krause, & Pedarzani, 1999). Overall, enhanced neuronal excitability or reduced spike frequency adaptation is mediated by the AHP, which may promote synaptic throughput (Moyer, Thompson, & Disterhoft, 1996).
Single AP characteristics including AP amplitude, AP half-width, and the afterdepolarization (ADP) following an AP influence neuronal excitability (Figure 1, Panel 5). AP amplitude and AP half-width influence the duration and extent of Ca2+ influx at the presynaptic terminal (Deng et al., 2013). The ADP property is mediated by metabotropic glutamate receptors (mGluRs; Greene, Schwindt, & Crill, 1994; Young, Chuang, & Wong, 2004; Park et al., 2010) and muscarinic receptors (Haj-Dahmane & Andrade, 1998; Yan, Villalobos, & Andrade, 2009). The ADP has been shown to trigger burst firing in hippocampal neurons (Schwartzkroin, 1975; Azouz, Jensen, & Yaari, 1996; Jensen, Azouz, & Yaari, 1996; Sanabria, Su, & Yaari, 2001; Su, Alroy, Kirson, & Yaari, 2001) and bursting is a requirement for synaptic plasticity at the Schaffer collateral to CA1 synapse (Thomas, Watabe, Moody, Makhinson, & O’dell, 1998; Pike, Meredith, Olding, & Paulsen, 1999).
Following initiation in the axon, APs can backpropagate (bAPs) to the soma and dendritic trees (Figure 1, Panel 6). Backpropagation of APs are influenced by dendritic morphology (Goldstein & Rall, 1974) and various ionic conductances in the dendrites including voltage-gated Na+ channels (Häusser, Stuart, Racca, & Sakmann, 1995), A-type K+ channels (Hoffman, Magee, Colbert, & Johnston, 1997; Frick, Magee, & Johnston, 2004), and Ca2+ influx into the dendritic compartments (Larkum, Kaiser, & Sakmann, 1999). Specifically, induction of LTP increases dendritic excitability and bAP amplitude, which is modulated by Ca2+ influx and A-type K+ channels (Frick et al., 2004). Moreover, excitatory and inhibitory synaptic transmission influence bAPs (Spruston, 2016). For example, synaptic depolarization facilitates bAPs in the apical dendrites (Hoffman et al., 1997; Stuart & Häusser, 2001; Watanabe, Hoffman, Migliore, & Johnston, 2002) whereas GABAergic inhibitory conductances attenuate bAPs (Tsubokawa & Ross, 1996; Pérez-Garci, Gassmann, Bettler, & Larkum, 2006). Therefore, by influencing intrinsic excitability, bAPs promote synaptic plasticity in the brain.
2. Fear learning
Different forms of learning can induce local and global changes by modulating various intrinsic properties including resting and voltage-dependent channels, thereby leading to changes in neuronal excitability. Understanding plasticity of intrinsic excitability is a critical component in the analysis of learning and memory mechanisms. Although both operant (Saar, Grossman, & Barkai, 1998, 1999; Zelcer et al., 2005; Motanis, Maroun, & Barkai, 2012) and classical (Disterhoft et al., 1986; Moyer et al., 1996; Moyer, Power, Thompson, & Disterhoft, 2000; Kaczorowski & Disterhoft, 2009; Oh & Disterhoft, 2015) conditioning paradigms modulate intrinsic excitability, this article focuses on intrinsic changes associated with classical fear conditioning.
2.1. Fear conditioning paradigms
The first laboratory study of fear conditioning was conducted in infants and is famously known as the “Little Albert” study (Watson & Rayner, 1920). In this study, a 9 month old infant (Albert) was conditioned to associate a white rat (conditioned stimulus; CS) with a loud noise (unconditioned stimulus; US). Several decades later, Ingram & Fitzgerald, 1974 demonstrated that infants as young as 3 months showed greater skin conductance responses to a CS associated with aversive stimuli (CS+) compared to the CS that was presented alone (CS-), suggesting that fear conditioning can be acquired during the early stages of development. Numerous studies on fear conditioning have been carried out in many other species. Further, there has been an exponential growth in different types of fear conditioning studies (Fanselow & Sterlace, 2014) as it not only serves as a model for anxiety disorders but is also useful for studying basic cellular mechanisms of learning and memory. In general, classical fear conditioning involves pairing a neutral cue such as a light or tone (CS) with an aversive cue such as a mild footshock (US) that naturally elicits a stereospecific freezing or crouching response as the unconditioned response (UR). A learned association between CS and US occurs over multiple pairings of the two stimuli, resulting in freezing behaviors (conditioned response; CR) to the CS alone. Thus, classical fear conditioning is invaluable for studying the neurobiology of learning and memory (Kim & Jung, 2006).
Animals can acquire a conditioned fear response to a surrounding environment or context as well as to discrete stimuli, such as an auditory CS. In the absence of discrete cues, unsignaled presentations of a US leads to acquisition of fear to the foreground context. When discrete cues are present, such as an auditory CS, presentations of the US can lead to acquisition of fear to both the CS and the background context (Phillips & LeDoux, 1994; Fanselow, 2000; Gould & Bevins, 2012). There are two basic fear conditioning paradigms involving discrete cues: delay and trace. In delay fear conditioning, paired CS-US presentations are contiguous (i.e. there is temporal overlap between onset of the CS and onset of the US – they often co-terminate), and acquisition depends predominantly on the amygdala (Phillips & LeDoux, 1992). In contrast, trace fear conditioning involves paired CS-US presentations that are not contiguous, but rather are separated by a brief temporal gap between offset of the CS and onset of the US called the trace interval. The presence of the trace interval necessitates explicit awareness of the CS-US relationship, and requires the interaction of subcortical and cortical brain regions, including the amygdala, hippocampus, prefrontal, rhinal, and retrosplenial cortices (McEchron, Bouwmeester, Tseng, Weiss, & Disterhoft, 1998; Detert, Kampa, & Moyer, 2008; Kholodar-Smith, Boguszewski, & Brown, 2008; Esclassan, Coutureau, Di Scala, & Marchand, 2009a, 2009b; Gilmartin & Helmstetter, 2010; Kwapis, Jarome, Schiff, & Helmstetter, 2011; Gilmartin, Kwapis, & Helmstetter, 2012; Kwapis, Jarome, Lee, & Helmstetter, 2015). Since auditory delay or trace fear paradigms also produce background fear to the training context, later exposure to the original training context after a conditioning session results in increased freezing. These contextual fear memories are dependent on dorsal hippocampus (Phillips & LeDoux, 1992, 1994). Classical fear conditioning paradigms provide critical insight into how learning affects intrinsic neuronal plasticity of many cortical and subcortical neurons that are part of the fear circuit (for review see Johansen, Wolff, Luthi, & LeDoux, 2012; Tovote, Fadok, & Luthi, 2015), and while there are many brain regions involved in fear conditioning, this section will focus on the role of the amygdala, hippocampus, retrosplenial cortex (RSC) and medial prefrontal cortex (mPFC; see Figure 2 for basic schematic).
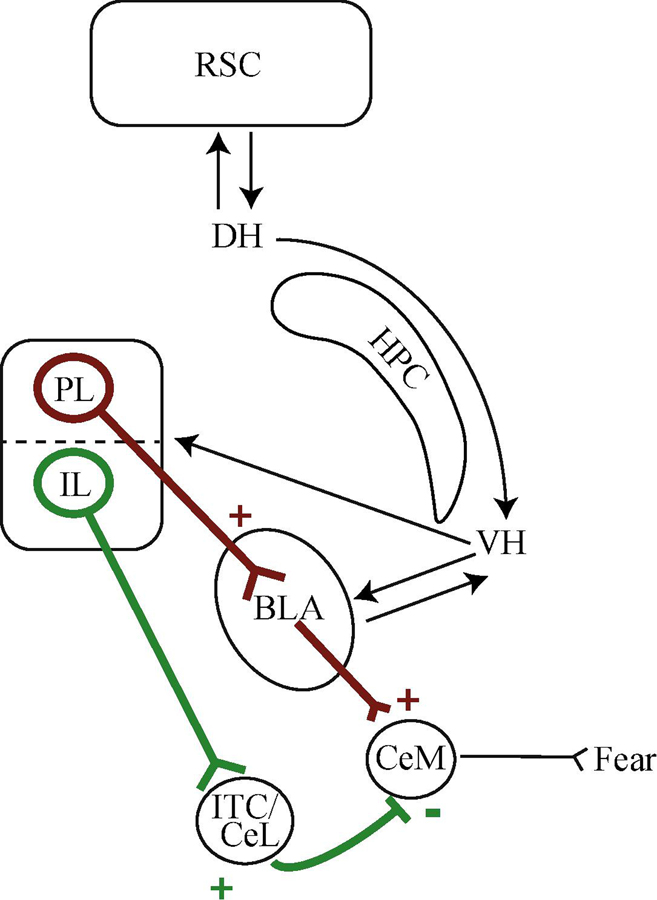
Figure 2. Schematic illustrating circuitry for fear acquisition and extinction.
The RSC forms reciprocal connections with DH and these projections are integral for contextual and trace fear acquisition and extinction. DH sends projections to VH, which forms connections with the mPFC. The PL and IL subregions differentially control fear-related memories. The PL projects to the BLA and to the CeM to support fear memories (red lines). The IL projects to the ITC or CeL to promote fear extinction (green lines). Abbreviations: retrosplenial cortex (RSC); hippocampus (HPC); dorsal hippocampus (DH); ventral hippocampus (VH); medial prefrontal cortex (mPFC); prelimbic region of the mPFC (PL); infralimbic region of the mPFC (IL); medial subdivision of the central nucleus of the amygdala (CeM); lateral subdivision of the central nucleus of the amygdala (CeL); intercalated cells (ITC).
2.2. Basic fear circuit (role of amygdala)
The amygdala consists of several nuclei and subnuclei, and is well-known for its role in auditory fear conditioning. Generally, auditory information about the CS converges on the lateral portion of the amygdala (LA), eventually leaving via the central nucleus (CE), resulting in defensive responding (i.e. fear expression; LeDoux, 2000). In contrast, background contextual information is routed from the hippocampus to basal amygdala (BA), then exits via CE (LeDoux et al., 2000). Both delay and trace fear conditioning are supported by amygdala function. Amygdala lesions disrupt delay fear learning (LeDoux, 1992), and delay fear conditioning leads to distinct changes in amygdala plasticity (Lee & Kim, 1998; Rumpel, LeDoux, Zador, & Malinow, 2005; Han et al., 2007, 2009; Reijmers, Perkins, Matsuo, & Mayford, 2007; Sehgal et al., 2014). LA neurons display increased expression of cAMP response element-binding protein (CREB) following delay fear conditioning (Han et al., 2007), and selective deletion of these CREB-expressing neurons impairs fear expression (Han et al., 2009). Trace fear memories are also dependent on intact amygdala function, as trace fear conditioning deficits are evident when the amygdala is lesioned (Selden, Everitt, Jarrard, & Robbins, 1991), inactivated (Guimarãis, Gregório, Cruz, Guyon, & Moita, 2011; Gilmartin et al., 2012; but see Raybuck & Lattal, 2011), when amygdala protein synthesis is disrupted (Kwapis et al., 2011), and following disruption of cholinergic signaling (Baysinger, Kent, & Brown, 2012). Together, these studies illustrate the importance of the amygdala in fear learning.
2.3. Fear circuit involving higher-order brain regions
2.3.1. Dorsal hippocampus
The hippocampus is pivotal for fear learning, with several studies suggesting a role for the dorsal subregion (DH) in trace fear conditioning. Trace fear learning is disrupted following electrolytic or cytotoxic DH lesions (McEchron et al., 1998; McEchron, Tseng, & Disterhoft, 2000; Quinn, Oommen, Morrison, & Fanselow, 2002; Chowdhury, Quinn, & Fanselow, 2005; Fendt, Fanselow, & Koch, 2005; Burman, Starr, & Gewirtz, 2006; Trivedi & Coover, 2006) as well as temporary DH inactivation using muscimol (Guimarãis et al., 2011; Raybuck & Lattal, 2011). Trace fear conditioning is also impaired when other forms of DH function are altered, including blockade of NMDARs (Misane et al., 2005; Quinn, Loya, Ma, & Fanselow, 2005; Wanisch, Tang, Mederer, & Wotjak, 2005; Seo, Pang, Shin, Kim, & Choi, 2008), impaired extracellular signal-regulated kinase (ERK) or CREB signaling (Peters, Kalivas, & Quirk, 2009; Huang, Chiang, Liang, Thompson, & Liu, 2010), disrupted protein synthesis (Runyan & Dash, 2005; Wanisch et al., 2005), or impaired function of the micro RNA mir-123 (Wang et al., 2013). Interestingly, while trace fear conditioning is adversely affected by disrupted DH functioning, delay fear conditioning remains intact (McEchron et al., 2000; Quinn et al., 2002; Burman et al., 2006; Chowdhury et al., 2005; Misane et al., 2005; Esclassan et al., 2009b; Raybuck & Lattal, 2011). This suggests DH function is selectively required for trace fear conditioning, rather than for fear learning in general.
2.3.2. Ventral hippocampus
In contrast to DH, ventral hippocampus (VH) also seems to support delay fear learning in addition to context and trace fear learning. For example, chemical lesions of VH disrupt delay fear (Richmond et al., 1999; Hunsaker & Kesner, 2008), while electrolytic VH lesions disrupt both context and delay fear memory (Maren & Holt, 2004). Furthermore, inhibition of VH protein synthesis disrupts context fear memory (Rudy & Matus-Amat, 2005), as does altered NMDAR function (Zhang, Bast, & Feldon, 2001), suggesting context fear memory depends on protein synthesis and intact NMDAR-mediated signaling in VH. Delay fear learning is also disrupted following muscimol inactivation of VH (Esclassan et al., 2009b; Sierra-Mercado, Padilla-Coreano, & Quirk, 2011), but it leaves context fear learning intact (Maren & Holt, 2004), suggesting context fear memory may be less sensitive to enhanced GABAA signaling in VH. Interestingly, delay fear conditioning enhances ERK activity in VH but not DH, and in males but not females (Gresack, Schafe, Orr, & Frick, 2009), suggesting sex may drive selective recruitment of VH to support delay fear memories. Thus, VH plays an important role in context and auditory delay fear memory.
VH function is also necessary for successful trace fear learning, as VH inactivation (Czerniawski, Yoon, & Otto, 2009; Gilmartin et al., 2012; Cox, Czerniawski, Ree, & Otto, 2013) or lesions (Yoon & Otto, 2007) disrupt trace fear conditioning. Some evidence supports a role for VH rather than DH in trace fear learning. Trace fear acquisition and expression are selectively disrupted by VH inactivation but not DH inactivation (Czerniawski et al., 2009), and while trace fear learning deficits are evident when VH is lesioned either before or after training, DH lesions only produce behavioral deficits when they occur after training (Yoon & Otto, 2007). Similarly, NMDAR blockade in DH only disrupts trace fear memory when it occurs before training, while NMDAR blockade in VH either before training or testing disrupts trace fear memory (Czerniawski, Ree, Chia, & Otto, 2012). Further, multiple measures of fear memory are sensitive to VH inactivation, including freezing to the intertrial interval, CS, and trace interval, whereas DH inactivation only impairs CS freezing (Cox et al., 2013). These data suggest that not only is VH important for trace fear memory, but that it may also be required for more aspects of trace fear encoding and retrieval than DH.
2.3.3. Retrosplenial cortex
The RSC is known to support contextual fear conditioning, and trace fear conditioning. For example, one recent study used c-fos genetic tagging to label RSC cells that were active during contextual fear conditioning. Optogenetic reactivation of the tagged RSC cells in a novel context (i.e. not the training context) induced high freezing responses in mice (Cowansage et al., 2014). Moreover, rodents with RSC lesions display impaired acquisition (Keene & Bucci, 2008a; Robinson, Poorman, Marder, & Bucci, 2012) and retrieval (Keene & Bucci, 2008a, 2008b) of contextual fear conditioning compared to control animals. Furthermore, infusions of a protein synthesis inhibitor, anisomycin (Kwapis, Jarome, Lee, Gilmartin, & Helmstetter, 2014; Kwapis et al., 2015) or NMDAR antagonists (Corcoran et al., 2011) in the RSC disrupted formation of context fear memories. Pharmacological blockade or lesions of the RSC attenuate retrieval of recent contextual fear conditioning (Keene & Bucci, 2008a, 2008b; Corcoran et al., 2011), however, RSC involvement is also evident in remotely acquired contextual fear memories (Corcoran et al., 2011; Tayler, Tanaka, Reijmers, & Wiltgen, 2013; Todd, Mehlman, Keene, DeAngeli, & Bucci, 2016). For example, NMDAR blockade or lesions of the rodent RSC impaired retrieval of remote memories approximately 8 weeks after contextual fear conditioning (Corcoran et al., 2011; Todd et al., 2016). Taken together, the RSC is necessary for retrieval of both recent and remote contextual fear memories.
Substantial evidence indicates that damage or inactivation of the RSC does not affect delay fear conditioning. Lesions to RSC made prior to or following delay fear conditioning did not impair fear expression in rats (Keene & Bucci 2008a, 2008b). Similarly, NMDAR blockade before or after delay fear conditioning did not affect acquisition or retrieval of fear in rodents (Corcoran et al., 2011; Kwapis et al., 2014, 2015). However, RSC is sensitive to the temporal relationship between the CS and US, and indeed, evidence suggests the RSC is necessary for trace fear conditioning. Infusions of a protein synthesis inhibitor in the RSC prior to training impaired acquisition of trace fear conditioning (Kwapis et al., 2015), and infusions of NMDAR antagonists following trace fear conditioning disrupted retrieval of trace fear memories (Kwapis et al., 2014, 2015). Through the use of selective chemogenetic approaches, inactivation of the RSC impaired retrieval of remote trace fear memories (Todd et al., 2016). Therefore, unlike delay fear conditioning, the RSC is necessary for both acquisition and retrieval of trace fear conditioning.
2.3.4. Medial prefrontal cortex
Trace fear encoding and retrieval critically depend on the prelimbic (PL) subregion of the mPFC. Trace fear memory is disrupted when PL is pharmacologically inactivated using the GABAA agonist muscimol (Gilmartin & Helmstetter, 2010), when PL neurons are optogenetically silenced (Gilmartin, Miyawaki, Helmstetter, & Diba, 2013), and when PL NMDARs are blocked (Gilmartin & Helmstetter, 2010; Gilmartin, Kwapis, & Helmstetter, 2013). Neurons in mPFC also display enhanced ERK phosphorylation (Runyan, Moore, & Dash, 2004) following trace fear conditioning, suggesting that fear learning alters kinase activity. The mPFC is also proposed to be a locus for long-term trace fear memory storage, as mPFC lesions made 1 month or more following trace fear conditioning lead to impaired responding to a conditioned cue (Quinn, Wied, Ma, Tinsley, & Fanselow, 2008; Beeman, Bauer, Pierson, & Quinn, 2013). These data fundamentally support a role for the mPFC in trace fear learning.
2.4. Extinction circuit
Many of the same major brain regions that have received attention for their importance in fear conditioning are also vital for extinction of conditioned fear. During extinction training, repeated presentations of the CS in the absence of an aversive stimulus gradually decreases conditioned responses (Bouton, 2002, 2004). Muscimol infusions into BLA, VH, or the infralimbic mPFC (IL) disrupt extinction of a delay fear memory, while muscimol infusion into PL has no effect on extinction learning (Sierra-Mercado et al., 2011). Context fear extinction likely involves activity in DH CA1, as expression of the immediate early gene (IEG) c-fos peaks 1 h following context fear conditioning and reverts to basal levels following five days of extinction (Tronson et al., 2009). Further, RSC is selectively recruited for extinction of trace fear rather than delay fear memories (Kwapis et al., 2014). Additional evidence suggests a role for mPFC-to-amygdala circuits in fear extinction. Delay fear extinction increased c-fos expression in IL-to-BA projection neurons relative to PL-to-BA projections or VH-to-BA projections (Orsini, Kim, Knapska, & Maren, 2011), suggesting specific fear circuits are preferentially recruited during extinction learning. Taken together, these studies highlight the importance of the amygdala, hippocampus, RSC, and mPFC in fear conditioning and extinction, and suggest that fear conditioning likely induces several forms of plasticity that underlie successful training. The next section will explore the evidence that suggests intrinsic plasticity is a critical substrate of fear conditioning and extinction.
3. Contributions of intrinsic plasticity to acquisition and extinction of fear learning
3.1. Early work
Alkon and colleagues provided early evidence for learning-related non-synaptic plasticity in invertebrates such as the mollusk, Hermissenda crassicornis (Alkon, 1974; Crow & Alkon, 1980). Furthermore, they demonstrated that increased excitability following learning was due to reduced A-type K+ currents, Ca2+-dependent K+ currents (Alkon et al., 1985) as well as increased intracellular Ca2+ concentration and protein phosphorylation (Alkon, 1984). Subsequent studies in vertebrates showed that classical conditioning of the cat eyeblink reflex was associated with enhanced excitability, and reduced rheobase (Brons & Woody, 1980). The first study to investigate learning-related changes using intracellular recordings in mammalian brain slices was carried out by Disterhoft and colleagues (Disterhoft et al., 1986). Using the hippocampal slice preparation, they demonstrated a learning-specific increase in the intrinsic excitability of rabbit CA1 neurons following acquisition of eyeblink conditioning. Moreover, Kapp and colleagues may have been the first to reveal that fear conditioning enhances neuronal excitability in fear-related structures such as the amygdala (Applegate, Frysinger, Kapp, & Gallagher, 1982; Pascoe & Kapp, 1985). Over the past two decades, there has been a steady increase in studies using in vitro recordings to demonstrate that both fear conditioning and extinction alter intrinsic excitability in multiple fear-related brain structures including the amygdala (Rosenkranz & Grace, 2002; Sehgal et al., 2014), hippocampus (McKay et al., 2009; Song et al., 2012; Zhang et al., 2017), and mPFC (Santini, Quirk, & Porter, 2008; Sepulveda-Orengo, Lopez, Soler-Cedeno, & Porter, 2013; Song, Ehlers, & Moyer, 2015). These studies will be discussed in greater detail in the next section.
3.2. How do fear learning and extinction modulate intrinsic excitability in fear-related brain structures?
3.2.1. Fear learning modulates excitability in the amygdala
Neurons in the amygdala undergo fear conditioning-induced intrinsic plasticity following olfactory and auditory delay fear conditioning (Cousens & Otto, 1998; Goosens & Maren, 2001). Olfactory fear conditioning enhances intrinsic excitability in LA neurons via reduced spike frequency adaptation (Rosenkranz & Grace, 2002). Interestingly, reward-based olfactory discrimination reduces spike frequency adaptation and the post-burst AHP, but olfactory fear conditioning increases spike frequency adaptation and has no effect on the AHP in basolateral amygdala (BLA) neurons (Motanis et al., 2012). This suggests the direction of intrinsic plasticity may depend on the subjective valuation of the stimuli used during training (i.e. appetitive vs. aversive).
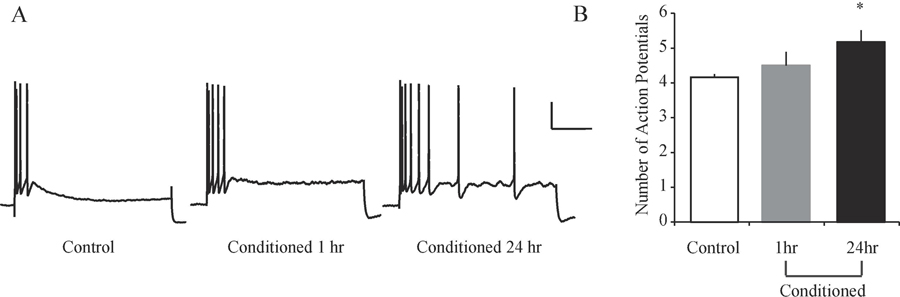
Amygdala neurons also undergo intrinsic plasticity following auditory delay fear conditioning, and more recent findings suggest a subset of neurons are changed and thus serve as engram-bearing neurons, or neurons that support the memory trace. Delay fear conditioning reduces spike frequency adaptation and the post-burst AHP in LA neurons, effectively increasing intrinsic excitability, and these changes occur in roughly one-third of the neuronal population studied (Sehgal et al., 2014). Further, as demonstrated in Figure 3 these changes in excitability are evident 24 h after fear conditioning, but not 1 h after conditioning, suggesting they are time-dependent (Sehgal et al., 2014). Delay fear conditioning also selectively increases spiking activity in Arc-positive LA neurons, which suggests those neurons activated by fear conditioning (i.e. those expressing Arc) selectively displayed increased excitability (Gouty-Colomer et al., 2016). Such specificity of intrinsic plasticity in LA neurons after delay fear conditioning suggests distinct neuronal populations support the memory trace.
Figure 3. Long-delay fear conditioning increases lateral amygdala neuronal excitability in a time-dependent manner.
(A) Representative traces illustrating spike frequency adaptation in response to a prolonged current injection in neurons from control and fear conditioned rats studied either 1-hr or 24-hr later. Note that LA pyramidal neurons from Control rats (n = 28) but not Conditioned-24hr rats (n = 28) display robust spike frequency adaptation. Scale bar, 20 mV, 200 ms. (B) Bar graphs illustrating the average number of APs elicited during prolonged current injection. LA pyramidal neurons from Conditioned-24hr rats fire significantly more APs than those from Control rats. Neurons from Conditioned-1hr rats (n = 13) are not significantly different from any other group. Asterisk (*) indicates p < 0.05 relative to LA neurons from Control rats. Abbreviations: lateral amygdala (LA); action potential (AP). Adapted from Sehgal et al., 2014.
3.2.2. Fear learning modulates excitability in the hippocampus
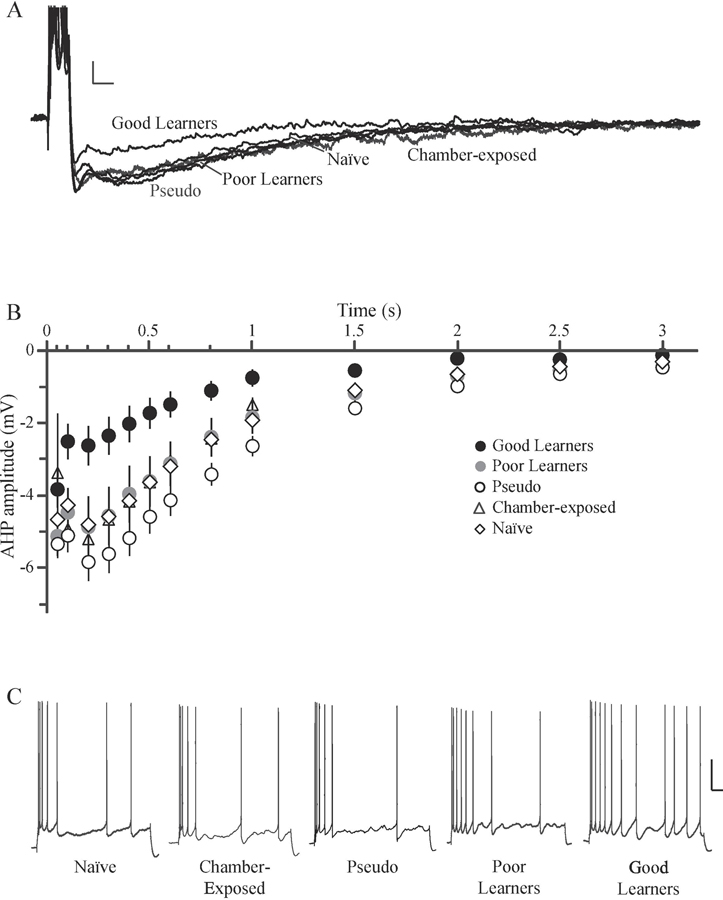
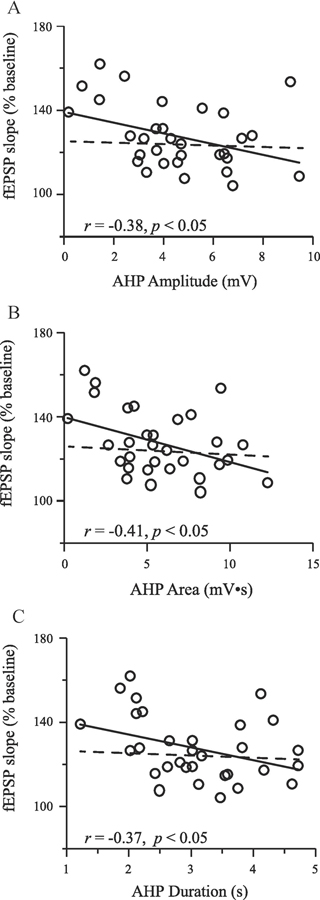
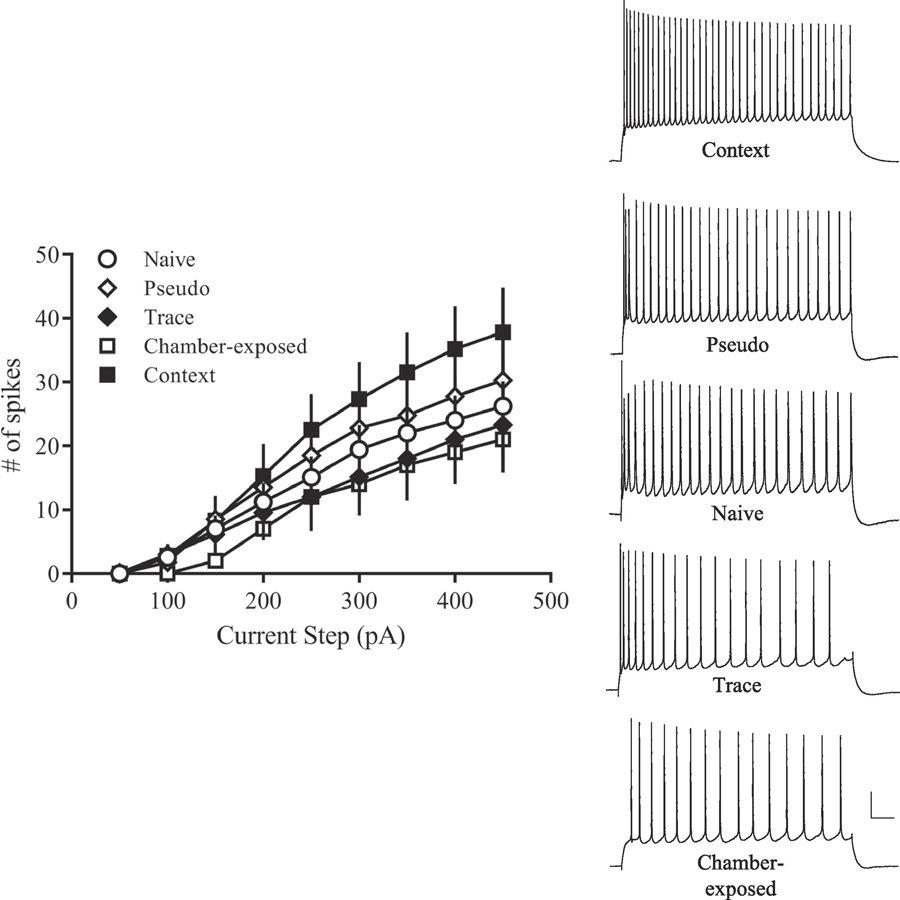
In the hippocampus, fear conditioning increases DH CA1 intrinsic excitability in the form of reduced AHP and reduced spike frequency adaptation (Kaczorowski & Disterhoft, 2009; McKay et al., 2009; Song et al., 2012). For example, McKay et al. (2009) demonstrate DH CA1 neurons display reduced spike frequency adaptation and reduced AHP following as little as three trace fear or context fear conditioning trials, and that this change in excitability is reversed by extinction learning. Work from our lab demonstrates that there is heterogeneity in trace fear conditioning, such that a subset of animals demonstrates good memory for the task (good learners), while others do not (poor learners). DH CA1 neurons from good learners display reduced post-bust AHPs and reduced spike frequency adaptation (Figure 4), suggesting that increased excitability is learning-specific (Song et al., 2012). Intrinsic excitability is also correlated with synaptic plasticity in DH neurons following fear learning. AHP amplitude, area, and duration are all negatively correlated with magnitude of LTP following trace fear conditioning (Figure 5), suggesting greater synaptic potentiation is correlated with a smaller AHP (Song et al., 2012).
Figure 4. Acquisition of trace fear conditioning increases intrinsic excitability of dorsal hippocampal CA1 pyramidal neurons.
(A) Representative traces of the post-burst AHP illustrating that DH CA1 neurons from good learners had smaller AHPs compared to those from poor learners, pseudoconditioned, chamber-exposed, and naïve rats. Scale bar, 2 mV, 100 ms. (B) Plot showing the time course of the post-burst AHP amplitude as a function of training condition. Neurons from good learners had a significantly smaller AHP compared to all other groups when measured at 0.1 – 0.8 s following current offset (p < 0.05). (C) AP output of DH CA1 neurons in response to a prolonged 1 s current injection. Notice that CA1 pyramidal neurons from good learners fired more APs than did CA1 neurons from poor learners, pseudoconditioned, chamber-exposed, or naïve rats. Scale bar, 20 mV, 100 ms. Abbreviations: dorsal hippocampus (DH); afterhyperpolarization (AHP); action potential (AP). Adapted from Song et al., 2012.
Figure 5. Synaptic plasticity is correlated with intrinsic excitability.
The magnitude of LTP was significantly correlated with the amplitude (A), area (B), and duration (C) of the post-burst AHP (solid lines). Data are mean values for each animal where both intrinsic excitability and synaptic plasticity were studied in the same slice. Interestingly, when good learners are removed from the plot, the correlation is no longer significant (dashed line indicates slope of the line in the absence of good learners). Abbreviations: long-term potentiation (LTP); afterhyperpolarization (AHP).
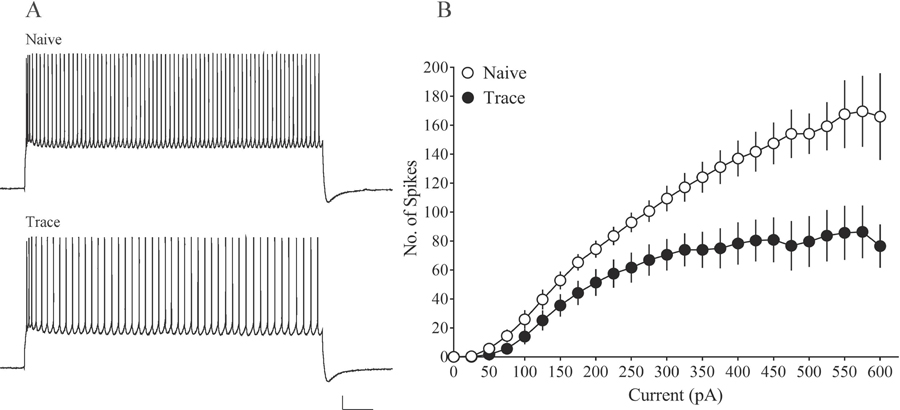
Since VH is also critical for fear learning, it is likely that fear conditioning modifies intrinsic plasticity in VH CA1 neurons as well. Indeed, preliminary findings from our lab indicate that context fear conditioning reduces spike frequency adaptation in VH CA1 neurons, reflecting increased intrinsic excitability (Figure 6). In subiculum neurons, which are a primary output of hippocampal CA1, contextual fear conditioning reduces the mAHP as well as the fAHP, and increases spiking activity in response to a 15 s current injection (Dunn et al., 2018). Notably, these changes are specific to regular-spiking neurons, and are not observed in burst-spiking neurons (Dunn et al., 2018), suggesting learning-related intrinsic plasticity in the subiculum is differentially regulated depending on firing type. Thus, several hippocampal subregions demonstrate learning-related intrinsic plasticity following fear conditioning, underlining the significance of the hippocampus in associative fear learning.
Figure 6. Context fear conditioning increases intrinsic excitability of VH CA1 pyramidal neurons.
Relative to controls and trace fear conditioned rats, VH neurons from context fear conditioned rats fire more action potentials in response to a 1 s depolarizing current injection, suggesting that intrinsic excitability is increased in Context neurons. Representative traces on the right show the number of action potentials elicited by a 450 pA current injection. Scale bar, 20 mV, 100 ms. Abbreviations: ventral hippocampus (VH).
3.2.3. Fear learning modulates excitability in the RSC
Although the RSC is necessary for both trace fear conditioning as well as trace extinction (Kwapis et al., 2014, 2015), no published studies have investigated whether intrinsic excitability of RSC neurons is altered as a function of trace fear learning. Preliminary findings from our lab demonstrate that following retrieval of trace fear memories, RSC neurons exhibited significantly decreased excitability compared to RSC neurons from naïve male rats (Figure 7). The reduced excitability of RSC neurons from trace fear conditioned rats may be due to a homeostatic mechanism, which may counterbalance increased excitatory synaptic inputs onto these neurons (Hayton, Lovett-Barron, Dumont, & Olmstead, 2010; Hayton, Olmstead, & Dumont, 2011). Synaptic transmission does not occur in isolation and compensatory mechanisms such as GABA transmission or decreased excitability are required to regulate neurons within their physiological firing rate, regardless of enhanced excitatory inputs (Zhang & Linden, 2003; Turrigiano, 2008). This regulatory feedback mechanism is prevalent in other cortical structures such as mPFC where elevated AMPA/NMDA receptor ratios (Hayton et al., 2010) and reduced intrinsic excitability (Hayton et al., 2011) were observed following learning of a response inhibition task. Similarly, intrinsic neuronal excitability in medium spiny neurons of the nucleus accumbens decreased over development (Kasanetz & Manzoni, 2009). Therefore, the reduction in intrinsic excitability in the RSC following trace fear learning may be due to a homeostatic mechanism, however, future work is required to elucidate synaptic mechanisms of learning-related plasticity in the RSC.
Figure 7. Trace fear conditioning increases spike frequency adaptation in retrosplenial cortical neurons.
(A) Representative traces demonstrating spike frequency adaptation in response to a 200 pA current injection. (B) In response to increasing current injections, retrosplenial neurons from trace fear conditioned rats have reduced intrinsic excitability compared to naïve rats. Scale bar, 10 mV, 500 ms.
3.2.4. Fear-related learning modulates intrinsic excitability in the mPFC
The mPFC has two subregions (PL and IL) that are morphologically and functionally distinct (Heidbreder & Groenewegen, 2003). Furthermore, intrinsic membrane properties differ between these two subregions in which IL neurons are more excitable compared to PL (Kaczorowski et al., 2012; Song & Moyer, 2017). Behaviorally, the subregions of the mPFC have dissociable roles, such that the PL is critical for fear expression whereas the IL inhibits fear behaviors after extinction (Vidal-Gonzalez, Vidal-Gonzalez, Rauch, & Quirk, 2006; Peters et al., 2009; Laurent & Westbrook, 2009; Sotres-Bayon & Quirk, 2010; Sierra-Mercado et al., 2011). Therefore, fear conditioning and extinction differentially modify intrinsic excitability in PL and IL neurons. For example, delay or context fear conditioning suppressed excitability and increased the sAHP of IL neurons (Santini et al., 2008; Soler-Cedeno, Cruz, Criado-Marrero, & Porter, 2016). Moreover, fear extinction induces burst firing (Santini et al., 2008; Santini & Porter, 2010) and enhances excitability (Sepulveda-Orengo et al., 2013) in IL neurons compared to controls. Extinction also reduced the fAHP in IL neurons (Santini et al., 2008; Santini & Porter, 2010; Sepulveda-Orengo et al., 2013). Since the fAHP is mediated by Ca2+-dependent K+ (SK) channels, blockade of these channels enhanced neuronal excitability in IL and promoted fear extinction (Criado-Marrero, Santini, & Porter, 2014). Thus, reduced IL excitability maintains fear learning whereas increased excitability and burst firing in IL regulates fear extinction.
3.3. Circuit-specific changes as a function of fear learning
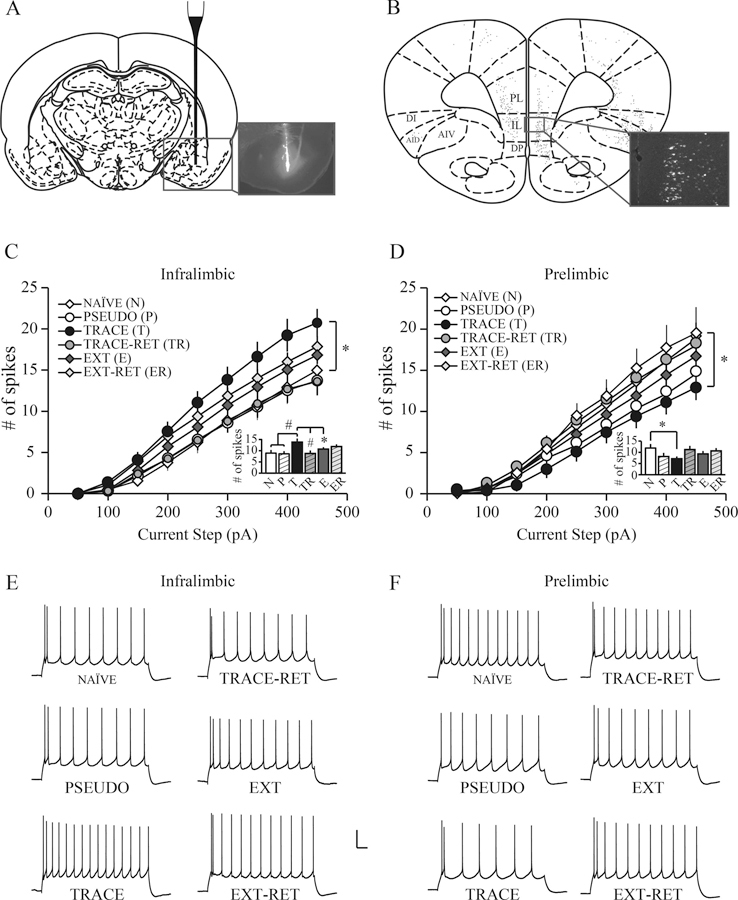
The above-mentioned section highlights valuable information regarding how fear learning modulates intrinsic excitability in multiple fear-related structures. However, it is important to consider that cortical structures such as the mPFC have a heterogeneous population of neurons that have distinct local circuit organization, interconnectivity, firing and morphological properties (Mason & Larkman, 1990; DeFelipe & Farinas, 1992; Morishima & Kawaguchi, 2006; Wang et al., 2006; Hattox & Nelson, 2007; Dembrow, Chitwood, & Johnston, 2010; Ferreira, Yousuf, Dalton, & Sheets, 2015). For example, mPFC forms reciprocal connections with subcortical brain structures such as the BLA (Hurley, Herbert, Moga, & Saper, 1991; Vertes, 2004; Gabbott, Warner, Jays, Salway, & Busby, 2005) and learning may alter mPFC neuronal activity in a circuit-specific manner. Therefore, our lab and others have used retrograde tracers to identify neurons in mPFC that project to the BLA (termed mPFC-BLA projection neurons) and tested whether these specific neurons undergo changes following fear learning (Song et al., 2015; Bloodgood, Sugam, Holmes, & Kash, 2018). Interestingly, trace fear conditioning reduced excitability in regular-spiking PL-BLA projection neurons (Figure 8) but enhanced excitability in burst-spiking PL-BLA projection neurons, which suggests that trace fear conditioning may modulate intrinsic excitability in mPFC-BLA projection neurons in a cell-type specific manner (Song et al., 2015).
Figure 8. Trace fear conditioning differentially modulates the intrinsic excitability of regular spiking mPFC neurons that project to the amygdala.
(A) Schematic diagram of a rat coronal section, showing that a glass pipette was used for the unilateral infusion of red fluorescent microspheres (Retrobeads) into the BLA (inset: fluorescence image showing infusion). (B) Coronal section showing the distribution of fluorescently labeled cortico-BLA projection neurons (inset: fluorescence image of IL-BLA projection neurons). (C) Trace fear conditioning significantly enhances the intrinsic excitability of IL-BLA projection neurons. Neurons from TRACE rats fired significantly more action potentials than those from NAÏVE rats (p < 0.05). (D) Trace fear conditioning significantly decreases the intrinsic excitability of PL-BLA projection neurons. Neurons from TRACE rats fired significantly fewer action potentials than those from NAÏVE rats (p < 0.05). In both IL and PL subregions, extinction reversed the conditioning-specific effect such that intrinsic excitability in EXT neurons was comparable with neurons from other groups and remained stable after EXT-RET. The conditioning-induced plasticity observed in TRACE rats was transient in both IL and PL subregions as the intrinsic excitability returned to naïve level after TRACE-RET. The insets in C and D show the average number of action potentials evoked by a 300 pA current injection in mPFC-BLA projection neurons (statistically different between TRACE and other groups: *p < 0.05, #p < 0.01). (E and F) Representative voltage sweeps showing the number of action potentials evoked by a 300 pA current injection in infralimbic and prelimbic neurons. Scale bar, 20 mV, 100 ms. Abbreviations: medial prefrontal cortex (mPFC); prelimbic region of the mPFC (PL); infralimbic region of the mPFC (IL); basolateral amygdala (BLA); trace fear conditioned (TRACE); extinction (EXT); trace retention (TRACE-RET); extinction retention (EXT-RET). Adapted from Song et al., 2015.
Although trace fear conditioning decreased excitability in regular-spiking PL-BLA neurons, it significantly enhanced excitability in regular-spiking IL-BLA projection neurons. Furthermore, as shown in Figure 8, extinction reversed the effects of trace fear conditioning by reducing excitability of IL-BLA neurons (Song et al., 2015). In contrast to these findings, another study reported enhanced excitability in IL-BLA neurons following extinction (Bloodgood et al., 2018). One possible reason for discrepancies in the results of these studies may be due to subregion specific effects as Song et al., 2015 exclusively recorded from layer 5 mPFC-BLA neurons, whereas Bloodgood et al., 2018 recorded from mPFC throughout layers 2/3 and 5. As mentioned earlier, neurophysiological properties and intrinsic neuronal excitability substantially differ between layers 2/3 and 5 in the rat mPFC (Song & Moyer, 2017) and may account for differences between studies.
Taken together, these studies strongly support a role for intrinsic plasticity as a neural substrate of associative fear conditioning. Modulation of intrinsic neuronal excitability likely reflects a learning-related consolidation mechanism, as these changes are transient (Sehgal et al., 2014), and are reversed by extinction learning in brain regions necessary for fear acquisition (Santini et al., 2008; McKay et al., 2009; Song et al., 2015). A common element among several of these studies is the observation that fear conditioning-induced intrinsic plasticity occurs within a subpopulation of neurons (Sehgal et al., 2014; Gouty-Colomer et al., 2016; Dunn et al., 2018) or within a circuit (Song et al., 2015; Bloodgood et al., 2018), which suggests these neurons are specific to the memory trace or engram. Although more research is needed to further explore this idea, a substantial amount of work has been done to establish intrinsic excitability as a critical component of learning-related plasticity following fear conditioning.
4. Future directions
Fear-related cortical brain structures including the perirhinal cortex, mPFC and RSC have heterogeneous neuronal populations that have distinct intrinsic and morphological properties (Moyer, McNay, & Brown, 2002; Chang & Luebke, 2007; Nye, Tuma, & Moyer, 2016), and they can be either glutamatergic or GABAergic (Nelson & Turrigiano, 2008). Moreover, we have shown that firing types are altered as a consequence of developmental age in RSC neurons. The ADP property, which has been shown to induce burst firing and synaptic plasticity, is absent in regular-spiking RSC neurons of juvenile rats (prior to postnatal day 30) and emerges during mid-adolescence (after postnatal day 30). These neurons are classified as regular-spiking ADP neurons (RSADP; Yousuf & Moyer, 2018). Interestingly, we note a subpopulation of adult RSC neurons that oscillate between single-spiking RSADP neurons and burst-firing neurons (Yousuf, Nye, & Moyer, 2019). Additionally, regular-spiking and burst-spiking neurons are differentially modified following fear learning. For example, trace fear conditioning increases excitability of burst-spiking neurons but decreases excitability of regular-spiking neurons within the mPFC (Song et al., 2015). In contrast, fear conditioning induced greater intrinsic excitability of regular-spiking but not burst-firing neurons of the hippocampus (Dunn et al., 2018). Diversity of firing patterns as well as alterations in cell types across development and learning raise multiple questions. The first question is whether intrinsic plasticity is capable of converting one cell type to another and if such changes can be induced by learning? Second, which cell types are preferentially recruited to stabilize activity within a neural circuit or to maintain homeostatic plasticity? A third question is whether the cell-specific nature of intrinsic excitability may promote flexible processes necessary for learning? For example, burst-spiking neurons that have distinct morphologies often project to thalamus, pons, and colliculus whereas regular-spiking neurons are more likely to project to cortex or to the striatum (Gao & Zheng, 2004; Le Be, Silberberg, Wang, & Markram, 2007). Thus, technologies that allow cell-specific tagging and manipulation with high temporal and spatial resolution can elucidate the precise functional roles of different firing types.
In addition to cell-type tagging, engram cell-specific tagging is required to investigate the intrinsic properties of neurons that are preferentially recruited to be part of a memory trace. Indeed, one study used a fluorescence-based Arc reporter to identify amygdala neurons activated during fear conditioning (Gouty-Colomer et al., 2016). Arc-expressing neurons exhibited increased excitability compared to non-activated neurons (Gouty-Colomer et al., 2016). Further, neurons that had had higher baseline excitability were selected into the fear memory trace, suggesting that intrinsic excitability determines neuronal selection. Although this study elucidates an important question regarding the pivotal role of intrinsic excitability in the fear memory engram, future work using multicellular recordings is required to reveal local microcircuit connectivity and precise cell-type specific mechanisms.
5. Conclusion
In conclusion, alterations in the intrinsic electrical properties of neurons can fundamentally modulate both the processing of information as well as the neuronal output, and these changes and their plasticity can have important implications for variations in fear learning. Understanding cell-specific and circuit-specific mechanisms associated with fear learning can shed light on emergence of behavioral phenotypes during development and aging, as well as in maladaptive fear responses that result from traumatic experiences or neurodegenerative disorders. Elucidating these mechanisms may provide more targeted neurobiologically-based approaches and facilitate treatment strategies for anxiety disorders and posttraumatic disorder.
Acknowledgements:
This work was supported by NIA grant R03-AG042814 (J.R. Moyer, Jr.) and a Research Growth Initiative from the University of Wisconsin–Milwaukee (J.R. Moyer, Jr.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WC (2008). Metaplasticity: tuning synapses and networks for plasticity. Nature Reviews Neuroscience, 9(5), 387. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, & Linden DJ (2000). Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nature neuroscience, 3(2), 109. [DOI] [PubMed] [Google Scholar]
- Alkon DL (1974). Associative training of Hermissenda. Journal of General Physiology, 64(1), 70–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon DL (1984). Calcium-mediated reduction of ionic currents: a biophysical memory trace. Science, 226(4678), 1037–1045. [DOI] [PubMed] [Google Scholar]
- Alkon DL, Sakakibara M, Forman R, Harrigan J, Lederhendler I, & Farley J (1985). Reduction of two voltage-dependent K+ currents mediates retention of a learned association. Behavioral and Neural Biology, 44(2), 278–300. [DOI] [PubMed] [Google Scholar]
- Applegate CD, Frysinger RC, Kapp BS, & Gallagher M (1982). Multiple unit activity recorded from amygdala central nucleus during Pavlovian heart rate conditioning in rabbit. Brain Research, 238(2), 457–462. [DOI] [PubMed] [Google Scholar]
- Azouz R, Jensen MS, & Yaari Y (1996). Ionic basis of spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. The Journal of Physiology, 492(1), 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baysinger AN, Kent BA, & Brown TH (2012). Muscarinic receptors in amygdala control trace fear conditioning. PLoS One, 7(9), e45720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeman CL, Bauer PS, Pierson JL, & Quinn JJ (2013). Hippocampus and medial prefrontal cortex contributions to trace and contextual fear memory expression over time. Learning & Memory, 20(6), 336–343. [DOI] [PubMed] [Google Scholar]
- Bevins RA, & Gould TJ (2012). Drug Conditioning. Encyclopedia of the Sciences of Learning, 1043–1046.
- Bloodgood DW, Sugam JA, Holmes A, & Kash TL (2018). Fear extinction requires infralimbic cortex projections to the basolateral amygdala. Translation Psychiatry, 8(1), 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME (2002). Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biology Psychiatry, 52(10), 976–986. [DOI] [PubMed] [Google Scholar]
- Bouton ME (2004). Context and behavioral processes in extinction. Learning & Memory, 11(5), 485–494. [DOI] [PubMed] [Google Scholar]
- Brons JF, & Woody CD (1980). Long-term changes in excitability of cortical neurons after Pavlovian conditioning and extinction. Journal of Neurophysiology, 44(3), 605–615. [DOI] [PubMed] [Google Scholar]
- Burman MA, Starr MJ, & Gewirtz JC (2006). Dissociable effects of hippocampus lesions on expression of fear and trace fear conditioning memories in rats. Hippocampus, 16(2), 103–113. [DOI] [PubMed] [Google Scholar]
- Chang YM, & Luebke JI (2007). Electrophysiological diversity of layer 5 pyramidal cells in the prefrontal cortex of the rhesus monkey: in vitro slice studies. Journal of Neurophysiology, 98(5), 2622–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury N, Quinn JJ, & Fanselow MS (2005). Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behavioral Neuroscience, 119(5), 1396–1402. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Coussens CM, Raymond CR, & Abraham WC (1999). Long-lasting increase in cellular excitability associated with the priming of LTP induction in rat hippocampus. Journal of Neurophysiology, 82(6), 3139–3148. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Donnan MD, Tronson NC, Guzmán YF, Gao C, Jovasevic V, … Radulovic J (2011). NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear memory. Journal of Neuroscience, 31(32), 11655–11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens G, & Otto T (1998). Both pre- and posttraining excitotoxic lesions of the basolateral amygdala abolish the expression of olfactory and contextual fear conditioning. Behavioral Neuroscience, 112(5), 1092–1103. [DOI] [PubMed] [Google Scholar]
- Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, & Mayford M (2014). Direct reactivation of a coherent neocortical memory of context. Neuron, 84(2), 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D, Czerniawski J, Ree F, & Otto T (2013). Time course of dorsal and ventral hippocampal involvement in the expression of trace fear conditioning. Neurobiology of Learning & Memory, 106, 316–323. [DOI] [PubMed] [Google Scholar]
- Criado-Marrero M, Santini E, & Porter JT (2014). Modulating fear extinction memory by manipulating SK potassium channels in the infralimbic cortex. Frontiers of Behavioral Neuroscience, 8, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow TJ, & Alkon DL (1980). Associative behavioral modification in hermissenda: cellular correlates. Science, 209(4454), 412–414. [DOI] [PubMed] [Google Scholar]
- Czerniawski J, Yoon T, & Otto T (2009). Dissociating space and trace in dorsal and ventral hippocampus. Hippocampus, 19(1), 20–32. [DOI] [PubMed] [Google Scholar]
- Czerniawski J, Ree F, Chia C, & Otto T (2012). Dorsal versus ventral hippocampal contributions to trace and contextual conditioning: differential effects of regionally selective NMDA receptor antagonism on acquisition and expression. Hippocampus, 22(7), 1528–1539. [DOI] [PubMed] [Google Scholar]
- Daoudal G, Hanada Y, & Debanne D (2002). Bidirectional plasticity of excitatory postsynaptic potential (EPSP)-spike coupling in CA1 hippocampal pyramidal neurons. Proceedings of the National Academy of Sciences, 99(22), 14512–14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis HP, & Squire LR (1984). Protein synthesis and memory: a review. Psychological bulletin, 96(3), 518. [PubMed] [Google Scholar]
- DeFelipe J, & Farinas I (1992). The pyramidal neuron of the cerebral cortex: morphological and chemical characteristics of the synaptic inputs. Progress in Neurobiology, 39(6), 563–607. [DOI] [PubMed] [Google Scholar]
- Dembrow NC, Chitwood RA, & Johnston D (2010). Projection-specific neuromodulation of medial prefrontal cortex neurons. Journal of Neuroscience, 30(50), 16922–16937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P-Y, Rotman Z, Blundon JA, Cho Y, Cui J, Cavalli V, … Klyachko VA (2013). FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron, 77(4), 696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detert JA, Kampa ND, & Moyer JR Jr. (2008). Differential effects of training intertrial interval on acquisition of trace and long-delay fear conditioning in rats. Behavioral neuroscience, 122(6), 1318. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Coulter DA, & Alkon DL (1986). Conditioning-specific membrane changes of rabbit hippocampal neurons measured in vitro. Proceedings of the National Academy of Sciences, 83(8), 2733–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disterhoft JF, Golden DT, Read HL, Coulter DA, & Alkon DL (1988). AHP reductions in rabbit hippocampal neurons during conditioning correlate with acquisition of the learned response. Brain research, 462(1), 118–125. [DOI] [PubMed] [Google Scholar]
- Dunn AR, Neuner SM, Ding S, Hope KA, O’Connell KMS, & Kaczorowski CC (2018). Cell-Type-Specific Changes in Intrinsic Excitability in the Subiculum following Learning and Exposure to Novel Environmental Contexts. eNeuro, 5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emptage NJ, & Carew TJ (1993). Long-term synaptic facilitation in the absence of short-term facilitation in Aplysia neurons. Science, 262(5131), 253–256. [DOI] [PubMed] [Google Scholar]
- Esclassan F, Coutureau E, Di Scala G, & Marchand AR (2009a). Differential contribution of dorsal and ventral hippocampus to trace and delay fear conditioning. Hippocampus, 19(1), 33–44. [DOI] [PubMed] [Google Scholar]
- Esclassan F, Coutureau E, Di Scala G, & Marchand AR (2009b). A cholinergic-dependent role for the entorhinal cortex in trace fear conditioning. Journal of Neuroscience, 29(25), 8087–8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber EL, & Sah P (2002). Physiological role of calcium-activated potassium currents in the rat lateral amygdala. Journal of Neuroscience, 22(5), 1618–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber EL, Delaney AJ, & Sah P (2005). SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nature neuroscience, 8(5), 635. [DOI] [PubMed] [Google Scholar]
- Fanselow MS (2000). Contextual fear, gestalt memories, and the hippocampus. Behavioural brain research, 110(1–2), 73–81. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, & Sterlace SR (2014). Pavlovian fear conditioning. The Wiley Blackwell handbook of operant and classical conditioning, 117–141.
- Fendt M, Fanselow MS, & Koch M (2005). Lesions of the dorsal hippocampus block trace fear conditioned potentiation of startle. Behavioral Neuroscience, 119(3), 834–838. [DOI] [PubMed] [Google Scholar]
- Ferreira AN, Yousuf H, Dalton S, & Sheets PL (2015). Highly differentiated cellular and circuit properties of infralimbic pyramidal neurons projecting to the periaqueductal gray and amygdala. Frontiers of Cellular Neuroscience, 9, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A, Magee J, & Johnston D (2004). LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nature neuroscience, 7(2), 126. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, & Busby SJ (2005). Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. Journal of Comparative Neurology, 492(2), 145–177. [DOI] [PubMed] [Google Scholar]
- Gao WJ, & Zheng ZH (2004). Target-specific differences in somatodendritic morphology of layer V pyramidal neurons in rat motor cortex. Journal of Comparative Neurolog, 476(2), 174–185. [DOI] [PubMed] [Google Scholar]
- Gasparini S, & DiFrancesco D (1999). Action of serotonin on the hyperpolarization-activated cation current (Ih) in rat CA1 hippocampal neurons. European Journal of Neuroscience, 11(9), 3093–3100. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, & Helmstetter FJ (2010). Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learning & Memory, 17(6), 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Kwapis JL, & Helmstetter FJ (2012). Trace and contextual fear conditioning are impaired following unilateral microinjection of muscimol in the ventral hippocampus or amygdala, but not the medial prefrontal cortex. Neurobiology of Learning & Memory, 97(4), 452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Kwapis JL, & Helmstetter FJ (2013). NR2A- and NR2B-containing NMDA receptors in the prelimbic medial prefrontal cortex differentially mediate trace, delay, and contextual fear conditioning. Learning & Memory, 20(6), 290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Miyawaki H, Helmstetter FJ, & Diba K (2013). Prefrontal activity links nonoverlapping events in memory. Journal of Neuroscience, 33(26), 10910–10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SS, & Rall W (1974). Changes of action potential shape and velocity for changing core conductor geometry. Biophysical journal, 14(10), 731–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, & Maren S (2001). Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learning & Memory, 8(3), 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouty-Colomer LA, Hosseini B, Marcelo IM, Schreiber J, Slump DE, Yamaguchi S, … Kushner SA (2016). Arc expression identifies the lateral amygdala fear memory trace. Molecular Psychiatry, 21(8), 1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene CC, Schwindt PC, & Crill WE (1994). Properties and ionic mechanisms of a metabotropic glutamate receptor-mediated slow afterdepolarization in neocortical neurons. Journal of Neurophysiology, 72(2), 693–704. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Schafe GE, Orr PT, & Frick KM (2009). Sex differences in contextual fear conditioning are associated with differential ventral hippocampal extracellular signal-regulated kinase activation. Neuroscience, 159(2), 451–467. [DOI] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Hu H, & Storm JF (2005). Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. The Journal of physiology, 566(3), 689–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarãis M, Gregório A, Cruz A, Guyon N, & Moita MA (2011). Time determines the neural circuit underlying associative fear learning. Frontiers of Behavioral Neuroscience, 5, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Dahmane S, & Andrade R (1998). Ionic mechanism of the slow afterdepolarization induced by muscarinic receptor activation in rat prefrontal cortex. Journal of Neurophysiology, 80(3), 1197–1210. [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, … Josselyn SA (2007). Neuronal competition and selection during memory formation. Science, 316(5823), 457–460. [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Hsiang HL, Buch T, Waisman A, … Josselyn SA (2009). Selective erasure of a fear memory. Science, 323(5920), 1492–1496. [DOI] [PubMed] [Google Scholar]
- Hattox AM, & Nelson SB (2007). Layer V neurons in mouse cortex projecting to different targets have distinct physiological properties. Journal of Neurophysiology, 98(6), 3330–3340. [DOI] [PubMed] [Google Scholar]
- Häusser M, Stuart G, Racca C, & Sakmann B (1995). Axonal initiation and active dendritic propagation of action potentials in substantia nigra neurons. Neuron, 15(3), 637–647. [DOI] [PubMed] [Google Scholar]
- Hayton SJ, Lovett-Barron M, Dumont EC, & Olmstead MC (2010). Target-specific encoding of response inhibition: increased contribution of AMPA to NMDA receptors at excitatory synapses in the prefrontal cortex. Journal of Neuroscience, 30(34), 11493–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayton SJ, Olmstead MC, & Dumont EC (2011). Shift in the intrinsic excitability of medial prefrontal cortex neurons following training in impulse control and cued-responding tasks. PLoS One, 6(8), e23885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, & Groenewegen HJ (2003). The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neuroscience Biobehavior Reviews, 27(6), 555–579. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, & Johnston D (1997). K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature, 387(6636), 869. [DOI] [PubMed] [Google Scholar]
- Hotson J, & Prince D (1980). A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. Journal of Neurophysiology, 43(2), 409–419. [DOI] [PubMed] [Google Scholar]
- Huang CH, Chiang YW, Liang KC, Thompson RF, & Liu IY (2010). Extra-cellular signal-regulated kinase 1/2 (ERK1/2) activated in the hippocampal CA1 neurons is critical for retrieval of auditory trace fear memory. Brain Research, 1326, 143–151. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, & Kesner RP (2008). Dissociations across the dorsal-ventral axis of CA3 and CA1 for encoding and retrieval of contextual and auditory-cued fear. Neurobiology of Learning & Memory, 89(1), 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, & Saper CB (1991). Efferent projections of the infralimbic cortex of the rat. Journal of Comparative Neurology, 308(2), 249–276. [DOI] [PubMed] [Google Scholar]
- Ingram E, & Fitzgerald HE (1974). Individual differences in infant orienting and autonomic conditioning. Developmental Psychobiology, 7(4), 359–367. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, & Medina JH (1998). On brain lesions, the milkman and Sigmunda. Trends in neurosciences, 21(10), 423–426. [DOI] [PubMed] [Google Scholar]
- Jensen MS, Azouz R, & Yaari Y (1996). Spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. The Journal of physiology, 492(1), 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Wolff SB, Luthi A, & LeDoux JE (2012). Controlling the elements: an optogenetic approach to understanding the neural circuits of fear. Biological Psychiatry, 71(12), 1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski CC, Disterhoft J, & Spruston N (2007). Stability and plasticity of intrinsic membrane properties in hippocampal CA1 pyramidal neurons: effects of internal anions. The Journal of physiology, 578(3), 799–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski CC, & Disterhoft JF (2009). Memory deficits are associated with impaired ability to modulate neuronal excitability in middle-aged mice. Learning & Memory, 16(6), 362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski CC, Davis SJ, & Moyer JR Jr. (2012). Aging redistributes medial prefrontal neuronal excitability and impedes extinction of trace fear conditioning. Neurobiology of Aging, 33(8), 1744–1757. [DOI] [PubMed] [Google Scholar]
- Kang H, Welcher AA, Shelton D, & Schuman EM (1997). Neurotrophins and time: different roles for TrkB signaling in hippocampal long-term potentiation. Neuron, 19(3), 653–664. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, & Manzoni OJ (2009). Maturation of excitatory synaptic transmission of the rat nucleus accumbens from juvenile to adult. Journal of Neurophysiology, 101(5), 2516–2527. [DOI] [PubMed] [Google Scholar]
- Keene CS, & Bucci DJ (2008a). Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behavioral neuroscience, 122(1), 89. [DOI] [PubMed] [Google Scholar]
- Keene CS, & Bucci DJ (2008b). Neurotoxic lesions of retrosplenial cortex disrupt signaled and unsignaled contextual fear conditioning. Behavioral neuroscience, 122(5), 1070. [DOI] [PubMed] [Google Scholar]
- Kholodar-Smith DB, Boguszewski P, & Brown TH (2008). Auditory trace fear conditioning requires perirhinal cortex. Neurobiology of Learning & Memory, 90(3), 537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, & Jung MW (2006). Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neuroscience & Biobehavioral Reviews, 30(2), 188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, & Bonhoeffer T (1995). Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proceedings of the National Academy of Sciences, 92(19), 8856–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Schiff JC, & Helmstetter FJ (2011). Memory consolidation in both trace and delay fear conditioning is disrupted by intra-amygdala infusion of the protein synthesis inhibitor anisomycin. Learning & Memory, 18(11), 728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lee JL, Gilmartin MR, & Helmstetter FJ (2014). Extinguishing trace fear engages the retrosplenial cortex rather than the amygdala. Neurobiology of Learning & Memory, 113, 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lee JL, & Helmstetter FJ (2015). The retrosplenial cortex is involved in the formation of memory for context and trace fear conditioning. Neurobiology of Learning & Memory, 123, 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, & Adams P (1986). Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. Journal of Neurophysiology, 55(6), 1268–1282. [DOI] [PubMed] [Google Scholar]
- Lancaster B, Hu H, Ramakers G, & Storm J (2001). Interaction between synaptic excitation and slow afterhyperpolarization current in rat hippocampal pyramidal cells. The Journal of Physiology, 536(3), 809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Kaiser K, & Sakmann B (1999). Calcium electrogenesis in distal apical dendrites of layer 5 pyramidal cells at a critical frequency of back-propagating action potentials. Proceedings of the National Academy of Sciences, 96(25), 14600–14604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, & Westbrook RF (2009). Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learning & Memory, 16(9), 520–529. [DOI] [PubMed] [Google Scholar]
- Le Be JV, Silberberg G, Wang Y, & Markram H (2007). Morphological, electrophysiological, and synaptic properties of corticocallosal pyramidal cells in the neonatal rat neocortex. Cerebral Cortex, 17(9), 2204–2213. [DOI] [PubMed] [Google Scholar]
- LeDoux JE (2000). Emotion circuits in the brain. Annual Reviews of Neuroscience, 23, 155–184. [DOI] [PubMed] [Google Scholar]
- Lee H, & Kim JJ (1998). Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. Journal of Neuroscience, 18(20), 8444–8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA (2004). Long-term potentiation and memory. Physiological Reviews, 84(1), 87–136. [DOI] [PubMed] [Google Scholar]
- Maren S, & Holt WG (2004). Hippocampus and Pavlovian fear conditioning in rats: muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behavioral Neuroscience, 118(1), 97–110. [DOI] [PubMed] [Google Scholar]
- Mayford M, Siegelbaum SA, & Kandel ER (2012). Synapses and memory storage. Cold Spring Harbor Perspectives in Biology, 4(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, & Disterhoft JF (1998). Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus, 8(6), 638–646. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Tseng W, & Disterhoft JF (2000). Neurotoxic lesions of the dorsal hippocampus disrupt auditory-cued trace heart rate (fear) conditioning in rabbits. Hippocampus, 10(6), 739–751. [DOI] [PubMed] [Google Scholar]
- McGaugh JL (2000). Memory--a century of consolidation. Science, 287(5451), 248–251. [DOI] [PubMed] [Google Scholar]
- McKay BM, Matthews EA, Oliveira FA, & Disterhoft JF (2009). Intrinsic neuronal excitability is reversibly altered by a single experience in fear conditioning. Journal of Neurophysiology, 102(5), 2763–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BM, Oh MM, Galvez R, Burgdorf J, Kroes RA, Weiss C, … Disterhoft JF (2012). Increasing SK2 channel activity impairs associative learning. Journal of Neurophysiology, 108(3), 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misane I, Tovote P, Meyer M, Spiess J, Ogren SO, & Stiedl O (2005). Time-dependent involvement of the dorsal hippocampus in trace fear conditioning in mice. Hippocampus, 15(4), 418–426. [DOI] [PubMed] [Google Scholar]
- Morishima M, & Kawaguchi Y (2006). Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex. Journal of Neuroscience, 26(16), 4394–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, & Baudry M (1986). Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature, 319(6056), 774–776. [DOI] [PubMed] [Google Scholar]
- Motanis H, Maroun M, & Barkai E (2012). Learning-induced bidirectional plasticity of intrinsic neuronal excitability reflects the valence of the outcome. Cerebral Cortex, 24(4), 1075–1087. [DOI] [PubMed] [Google Scholar]
- Moyer JR Jr., Thompson LT, & Disterhoft JF (1996). Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. Journal of Neuroscience, 16(17), 5536–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR Jr., Power JM, Thompson LT, & Disterhoft JF (2000). Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. Journal of Neuroscience, 20(14), 5476–5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR Jr., McNay EC, & Brown TH (2002). Three classes of pyramidal neurons in layer V of rat perirhinal cortex. Hippocampus, 12(2), 218–234. [DOI] [PubMed] [Google Scholar]
- Nelson SB, & Turrigiano GG (2008). Strength through diversity. Neuron, 60(3), 477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Saito H, & Abe K (1998). Medial amygdala stimulation produces a long-lasting excitatory postsynaptic potential/spike dissociation in the dentate gyrus in vivo. Brain Research, 794(1), 151–154. [DOI] [PubMed] [Google Scholar]
- Nye AN, Tuma J, & Moyer JR Jr. (2016). Neurons in the rat retrosplenial cortex are intrinsically heterogenous in firing type and morphology. In Abstract submitted for 46th annual conference of the Society for Neuroscience. San Diego, CA [Google Scholar]
- Oh MM, & Disterhoft JF (2015). Increased excitability of both principal neurons and interneurons during associative learning. The Neuroscientist, 21(4), 372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, & Maren S (2011). Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. Journal of Neuroscience, 31(47), 17269–17277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, … & Lu B (2004). Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science, 306(5695), 487–491. [DOI] [PubMed] [Google Scholar]
- Papoutsi A, Sidiropoulou K, & Poirazi P (2012). Memory beyond synaptic plasticity: The role of intrinsic neuronal excitability. Memory mechanisms in health and disease (ed. Giese KP), 53–80.
- Park J-Y, Remy S, Varela J, Cooper DC, Chung S, Kang H-W, … Spruston N (2010). A post-burst afterdepolarization is mediated by group I metabotropic glutamate receptor-dependent upregulation of Cav2. 3 R-type calcium channels in CA1 pyramidal neurons. PLoS biology, 8(11), e1000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe JP, & Kapp BS (1985). Electrophysiological characteristics of amygdaloid central nucleus neurons during Pavlovian fear conditioning in the rabbit. Behavioral Brain Research, 16(2–3), 117–133. [DOI] [PubMed] [Google Scholar]
- Pérez-Garci E, Gassmann M, Bettler B, & Larkum ME (2006). The GABAB1b isoform mediates long-lasting inhibition of dendritic Ca2+ spikes in layer 5 somatosensory pyramidal neurons. Neuron, 50(4), 603–616. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, & Quirk GJ (2009). Extinction circuits for fear and addiction overlap in prefrontal cortex. Learning & Memory, 16(5), 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, & LeDoux JE (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience, 106(2), 274–285. [DOI] [PubMed] [Google Scholar]
- Phillips RG, & LeDoux JE (1994). Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learning & Memory, 1(1), 34–44. [PubMed] [Google Scholar]
- Pike FG, Meredith RM, Olding AW, & Paulsen O (1999). Postsynaptic bursting is essential for ‘Hebbian’induction of associative long-term potentiation at excitatory synapses in rat hippocampus. The Journal of Physiology, 518(2), 571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo MM (2001). Neurotrophins as synaptic modulators. Nature reviews neuroscience, 2(1), 24. [DOI] [PubMed] [Google Scholar]
- Power JM, Bocklisch C, Curby P, & Sah P (2011). Location and function of the slow afterhyperpolarization channels in the basolateral amygdala. Journal of Neuroscience, 31(2), 526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese A, Ballerini L, Passani M, & Corradetti R (1994). EPSP-spike potentiation during primed burst-induced long-term potentiation in the CA1 region of rat hippocampal slices. Neuroscience, 62(4), 1021–1032. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Oommen SS, Morrison GE, & Fanselow MS (2002). Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus, 12(4), 495–504. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Loya F, Ma QD, & Fanselow MS (2005). Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus, 15(5), 665–674. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Wied HM, Ma QD, Tinsley MR, & Fanselow MS (2008). Dorsal hippocampus involvement in delay fear conditioning depends upon the strength of the tone-footshock association. Hippocampus, 18(7), 640–654. [DOI] [PubMed] [Google Scholar]
- Raybuck JD, & Lattal KM (2011). Double dissociation of amygdala and hippocampal contributions to trace and delay fear conditioning. PLoS One, 6(1), e15982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmers LG, Perkins BL, Matsuo N, & Mayford M (2007). Localization of a stable neural correlate of associative memory. Science, 317(5842), 1230–1233. [DOI] [PubMed] [Google Scholar]
- Richmond MA, Yee BK, Pouzet B, Veenman L, Rawlins JN, Feldon J, & Bannerman DM (1999). Dissociating context and space within the hippocampus: effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behavioral Neuroscience, 113(6), 1189–1203. [DOI] [PubMed] [Google Scholar]
- Robinson S, Poorman CE, Marder TJ, & Bucci DJ (2012). Identification of functional circuitry between retrosplenial and postrhinal cortices during fear conditioning. Journal of Neuroscience, 32(35), 12076–12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, & Grace AA (2002). Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature, 417(6886), 282–287. [DOI] [PubMed] [Google Scholar]
- Rudy JW, & Matus-Amat P (2005). The ventral hippocampus supports a memory representation of context and contextual fear conditioning: implications for a unitary function of the hippocampus. Behavioral Neuroscience, 119(1), 154–163. [DOI] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, & Malinow R (2005). Postsynaptic receptor trafficking underlying a form of associative learning. Science, 308(5718), 83–88. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Moore AN, & Dash PK (2004). A role for prefrontal cortex in memory storage for trace fear conditioning. Journal of Neuroscience, 24(6), 1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan JD, & Dash PK (2005). Inhibition of hippocampal protein synthesis following recall disrupts expression of episodic-like memory in trace conditioning. Hippocampus, 15(3), 333–339. [DOI] [PubMed] [Google Scholar]
- Saar D, Grossman Y, & Barkai E (1998). Reduced after-hyperpolarization in rat piriform cortex pyramidal neurons is associated with increased learning capability during operant conditioning. European Journal of Neuroscience, 10(4), 1518–1523. [DOI] [PubMed] [Google Scholar]
- Saar D, Grossman Y, & Barkai E (1999). Reduced synaptic facilitation between pyramidal neurons in the piriform cortex after odor learning. Journal of Neuroscience, 19(19), 8616–8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P (1996). Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends in neurosciences, 19(4), 150–154. [DOI] [PubMed] [Google Scholar]
- Sah P, & Bekkers JM (1996). Apical dendritic location of slow afterhyperpolarization current in hippocampal pyramidal neurons: implications for the integration of long-term potentiation. Journal of Neuroscience, 16(15), 4537–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria ER, Su H, & Yaari Y (2001). Initiation of network bursts by Ca2+-dependent intrinsic bursting in the rat pilocarpine model of temporal lobe epilepsy. The Journal of Physiology, 532(1), 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, & Porter JT (2008). Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. Journal of Neuroscience, 28(15), 4028–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, & Porter JT (2010). M-type potassium channels modulate the intrinsic excitability of infralimbic neurons and regulate fear expression and extinction. Journal of Neuroscience, 30(37), 12379–12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin PA (1975). Characteristics of CA1 neurons recorded intracellularly in the hippocampalin vitro slice preparation. Brain Research, 85(3), 423–436. [DOI] [PubMed] [Google Scholar]
- Sehgal M, Song C, Ehlers VL, & Moyer JR (2013). Learning to learn - intrinsic plasticity as a metaplasticity mechanism for memory formation. Neurobiology of Learning & Memory, 105, 186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal M, Ehlers VL, & Moyer JR (2014). Learning enhances intrinsic excitability in a subset of lateral amygdala neurons. Learning & Memory, 21(3), 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden NR, Everitt BJ, Jarrard LE, & Robbins TW (1991). Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience, 42(2), 335–350. [DOI] [PubMed] [Google Scholar]
- Seo DO, Pang MH, Shin MS, Kim HT, & Choi JS (2008). Hippocampal NMDA receptors are necessary for auditory trace fear conditioning measured with conditioned hypoalgesia in rats. Behavioral Brain Reseach, 192(2), 264–268. [DOI] [PubMed] [Google Scholar]
- Sepulveda-Orengo MT, Lopez AV, Soler-Cedeno O, & Porter JT (2013). Fear extinction induces mGluR5-mediated synaptic and intrinsic plasticity in infralimbic neurons. Journal of Neuroscience, 33(17), 7184–7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, & Quirk GJ (2011). Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology, 36(2), 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler-Cedeno O, Cruz E, Criado-Marrero M, & Porter JT (2016). Contextual fear conditioning depresses infralimbic excitability. Neurobiology of Learning & Memory, 130, 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Detert JA, Sehgal M, & Moyer JR Jr (2012). Trace fear conditioning enhances synaptic and intrinsic plasticity in rat hippocampus. Journal of Neurophysiology, 107(12), 3397–3408. [DOI] [PubMed] [Google Scholar]
- Song C, Ehlers VL, & Moyer JR (2015). Trace fear conditioning differentially modulates intrinsic excitability of medial prefrontal cortex–basolateral complex of amygdala projection neurons in infralimbic and prelimbic cortices. Journal of Neuroscience, 35(39), 13511–13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, & Moyer JR Jr (2017). Layer-and subregion-specific differences in the neurophysiological properties of rat medial prefrontal cortex pyramidal neurons. Journal of Neurophysiology, 119(1), 177–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, & Quirk GJ (2010). Prefrontal control of fear: more than just extinction. Current Opinions of Neurobiology, 20(2), 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N (2008). Pyramidal neurons: dendritic structure and synaptic integration. Nature Reviews Neuroscience, 9(3), 206. [DOI] [PubMed] [Google Scholar]
- Spruston N, Stuart G, & Häusser M (2016). Principles of dendritic integration. Dendrites, 351, 597. [Google Scholar]
- Stocker M, Krause M, & Pedarzani P (1999). An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proceedings of the National Academy of Sciences, 96(8), 4662–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm J (1987). Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. The Journal of Physiology, 385(1), 733–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF (1989). An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. The Journal of physiology, 409(1), 171–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GJ, & Häusser M (2001). Dendritic coincidence detection of EPSPs and action potentials. Nature Neuroscience, 4(1), 63. [DOI] [PubMed] [Google Scholar]
- Su H, Alroy G, Kirson ED, & Yaari Y (2001). Extracellular calcium modulates persistent sodium current-dependent burst-firing in hippocampal pyramidal neurons. Journal of Neuroscience, 21(12), 4173–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayler KK, Tanaka KZ, Reijmers LG, & Wiltgen BJ (2013). Reactivation of neural ensembles during the retrieval of recent and remote memory. Current Biology, 23(2), 99–106. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Watabe AM, Moody TD, Makhinson M, & O’dell TJ (1998). Postsynaptic complex spike bursting enables the induction of LTP by theta frequency synaptic stimulation. Journal of Neuroscience, 18(18), 7118–7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP, Mehlman ML, Keene CS, DeAngeli NE, & Bucci DJ (2016). Retrosplenial cortex is required for the retrieval of remote memory for auditory cues. Learning & Memory, 23(6), 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, & Luthi A (2015). Neuronal circuits for fear and anxiety. Nature Reviews of Neuroscience, 16(6), 317–331. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, & Coover GD (2006). Neurotoxic lesions of the dorsal and ventral hippocampus impair acquisition and expression of trace-conditioned fear-potentiated startle in rats. Behavioral Brain Research, 168(2), 289–298. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Schrick C, Guzman YF, Huh KH, Srivastava DP, Penzes P, … Radulovic J (2009). Segregated populations of hippocampal principal CA1 neurons mediating conditioning and extinction of contextual fear. Journal of Neuroscience, 29(11), 3387–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubokawa H, & Ross WN (1996). IPSPs modulate spike backpropagation and associated [Ca2+] i changes in the dendrites of hippocampal CA1 pyramidal neurons. Journal of Neurophysiology, 76(5), 2896–2906. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG (2008). The self-tuning neuron: synaptic scaling of excitatory synapses. Cell, 135(3), 422–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP (2004). Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse, 51(1), 32–58. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, & Quirk GJ (2006). Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learning & Memory, 13(6), 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Markram H, Goodman PH, Berger TK, Ma J, & Goldman-Rakic PS (2006). Heterogeneity in the pyramidal network of the medial prefrontal cortex. Nature Neuroscience, 9(4), 534–542. [DOI] [PubMed] [Google Scholar]
- Wanisch K, Tang J, Mederer A, & Wotjak CT (2005). Trace fear conditioning depends on NMDA receptor activation and protein synthesis within the dorsal hippocampus of mice. Behavioral Brain Research, 157(1), 63–69. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Hoffman DA, Migliore M, & Johnston D (2002). Dendritic K+ channels contribute to spike-timing dependent long-term potentiation in hippocampal pyramidal neurons. Proceedings of the National Academy of Sciences, 99(12), 8366–8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JB, & Rayner R (1920). Conditioned emotional reactions. Journal of Experimental Psychology, 3(1), 1. [Google Scholar]
- Yan H-D, Villalobos C, & Andrade R (2009). TRPC channels mediate a muscarinic receptor-induced afterdepolarization in cerebral cortex. Journal of Neuroscience, 29(32), 10038–10046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T, & Otto T (2007). Differential contributions of dorsal vs. ventral hippocampus to auditory trace fear conditioning. Neurobiology of Learning & Memory, 87(4), 464–475. [DOI] [PubMed] [Google Scholar]
- Young SR, Chuang SC, & Wong RK (2004). Modulation of afterpotentials and firing pattern in guinea pig CA3 neurones by group I metabotropic glutamate receptors. The Journal of Physiology, 554(2), 371–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf H, & Moyer JR (2018). Intrinsic excitability of retrosplenial cortical neurons varies as a function of age and sex. In Abstract submitted for 48th annual conference of the Society For Neuroscience. San Diego, CA [Google Scholar]
- Yousuf H, Nye AN, & Moyer JR Jr. (2019). Heterogeneity of Neuronal Firing Type and Morphology in Rat Retrosplenial Cortex. In review. [DOI] [PubMed]
- Zaitsev AV, & Anwyl R (2011). Inhibition of the slow afterhyperpolarization restores the classical spike timing-dependent plasticity rule obeyed in layer 2/3 pyramidal cells of the prefrontal cortex. Journal of Neurophysiology, 107(1), 205–215. [DOI] [PubMed] [Google Scholar]
- Zelcer I, Cohen H, Richter-Levin G, Lebiosn T, Grossberger T, & Barkai E (2005). A cellular correlate of learning-induced metaplasticity in the hippocampus. Cerebral Cortex, 16(4), 460–468. [DOI] [PubMed] [Google Scholar]
- Zhang WN, Bast T, & Feldon J (2001). The ventral hippocampus and fear conditioning in rats: different anterograde amnesias of fear after infusion of N-methyl-D-aspartate or its noncompetitive antagonist MK-801 into the ventral hippocampus. Behavioral Brain Research, 126(1–2), 159–174. [DOI] [PubMed] [Google Scholar]
- Zhang W, & Linden DJ (2003). The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nature Reviews Neuroscience, 4(11), 885. [DOI] [PubMed] [Google Scholar]
- Zhang D, Wang X, Wang B, Garza JC, Fang X, Wang J, … Lu XY (2017). Adiponectin regulates contextual fear extinction and intrinsic excitability of dentate gyrus granule neurons through AdipoR2 receptors. Molecular Psychiatry, 22(7), 1044–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]