Hunter-gatherers from different bands form fluid social networks that facilitate cultural innovation through recombination of cultural traditions.
Abstract
Although multilevel sociality is a universal feature of human social organization, its functional relevance remains unclear. Here, we investigated the effect of multilevel sociality on cumulative cultural evolution by using wireless sensing technology to map inter- and intraband social networks among Agta hunter-gatherers. By simulating the accumulation of cultural innovations over the real Agta multicamp networks, we demonstrate that multilevel sociality accelerates cultural differentiation and cumulative cultural evolution. Our results suggest that hunter-gatherer social structures [based on (i) clustering of families within camps and camps within regions, (ii) cultural transmission within kinship networks, and (iii) high intercamp mobility] may have allowed past and present hunter-gatherers to maintain cumulative cultural adaptation despite low population density, a feature that may have been critical in facilitating the global expansion of Homo sapiens.
INTRODUCTION
Multilevel sociality and a unique ability to accumulate culture are key human adaptations and evolved in ancestral humans who adopted a hunter-gatherer lifestyle. Hunter-gatherer multilevel sociality is defined by a uniquely fluid social structure, nuclear family units, high between-camp mobility, and multilocality (1–5). Genomic studies (6) have shown that fluid social structures already characterized expanding Upper Paleolithic human populations. Meanwhile, long-range cultural exchange in the Homo sapiens lineage date back to at least 320,000 years ago (7). The emergence of both multilevel sociality and advanced cumulative culture early in the human lineage suggests an evolutionary link between the two processes. To investigate the effect of multilevel sociality on the dynamics of cultural evolution in humans, we (i) mapped inter- and intraband social networks of Agta hunter-gatherers, (ii) designed agent-based simulations to model the virtual creation of a complex medicinal drug across the real Agta social network, and (iii) compared results of these simulations to similar simulations run across social structures lacking the unique features of hunter-gatherer multilevel sociality.
First, we mapped interactions among all adults in two multicamp Agta communities in the Philippines (seven forest camps over 36 km2, 53 adults, 28 females, and three coastal camps over 5 km of coast and 25 km2, 37 adults, 17 females; Fig. 1). We used wireless sensing technology (3) to record all dyadic interactions within 3 m, every hour, over a month. The weight of a dyadic link was defined as the number of times the dyad was recorded during the month. Data show that camps are connected by frequent migrations and visits, reflecting the reported high mobility of hunter-gatherers (8, 9). In the forest multicamp experiment, 35% (477 of 1378) of all possible inter- and intracamp dyads were recorded at least once (unweighted dyads), against 69% (458 of 666) in the coastal group. Intra- and intercamp networks varied in density. In the forest group, 59% (181 of 309) of possible intracamp dyads were recorded, against 28% of intercamp dyads (296 of 1069). In the coastal group, 85% (257 of 304) of the possible intracamp dyads were observed, against 56% of possible intercamp dyads (201 of 362). When weights are considered, we observe in both groups that intracamp dyads are more strongly connected. For example, only 38% of unweighted forest dyads were intracamp, while 53% (9321 of 17,555) of weighted dyads were intracamp. In the coastal group, 56% of unweighted dyads were intracamp, against 69% (10,439 of 15,224) of weighted dyads. In summary, coastal camps are denser and more interconnected than forest camps, intracamp dyads are more likely to be observed than intercamp dyads, and intracamp dyads are more strongly connected than intercamp dyads in both groups.
Fig. 1. Multicamp structures in Agta hunter-gatherers.
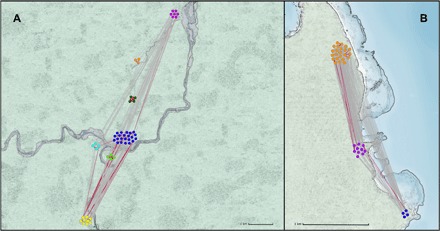
Figure displays individuals (dots) in camps (dot colors). Width of lines connecting individuals is proportional to dyadic weights (non-kin links: gray lines; close kin links: red lines). (A) Forest camps. (B) Coastal camps. Scale bars, 1 km. Locations of camps (seven forest and three coastal) and camp sizes are approximate.
Relatedness level is also a factor, with the proportion of close-kin dyads (both unweighted and weighted) being significantly higher among intra- than intercamp interactions (table S1), reflecting hunter-gatherer’s kin-based household structure and co-residence of mostly unrelated households in camps (1). Non-kin dyads, whose proportion is higher among inter- than intercamp interactions, play an important role in binding the multicamp structure together. Last, we found no sex biases in unweighted dyads in either coastal or forest groups (at both intra- and intercamp levels), reflecting the sex egalitarianism of hunter-gatherer societies (table S2) (1). When dyadic weights are considered, no consistent sex bias is found either, with an overrepresentation of male-female dyads at the expense of female-female dyads in both the forest and coastal camps, but no clear pattern regarding male-male dyads. Together, the results show a hierarchically structured multicamp social network, with households mostly consisting of close kin but dyads between households and between camps consisting mostly of non-kin, with few observed differences between men and women (3).
RESULTS
Next, we tested the potential effect of hierarchical multicamp network structure on cumulative cultural evolution. We adapted a computer-based experiment (10) to compare cultural evolution rates under distinct social network structures. We first ran the experiment as a simulation across the real Agta multicamp forest and coastal networks. In the simulations, agents had to find successive innovations by combining virtual medicinal plants, replicating actual processes observed in hunter-gatherer populations (11). Agents were originally given six medicinal plants, each deriving a drug with a medicinal value. In each round, two agents formed a dyad and combined three of their medicinal drugs, without repetition and selected in proportion to their medicinal value. The medicinal value of the resulting triad was calculated from the value of the three components (Fig. 2; see details in the Supplementary Materials). Of the possible 20 initial triads, we established that only one led to the creation of a superior phytomedicine A1 and another one to phytomedicine B1. Those two new, higher-order innovations became new ingredients added to the original set. A1 could be combined again in one unique drug triad to produce the superior phytomedicine A2, which, in its turn, was necessary in a triad producing A3. The same happened in the parallel trajectory of increasing medicinal value from B1 to B2 and B3. At the fourth and highest level of innovation in the simulation, a “crossover” or recombination of the two trajectories was necessary to produce the two phytomedicines with highest efficiency (crossover 1, which derived from triads A2, A3, and B3, or crossover 2, which required B2, B3, and A3). The virtual experiment was finished when either crossover 1 or 2 was found by two agents. This design aimed to reflect key components of cultural evolution [“ratchet effect,” incremental improvement, recombination, and innovation; (12)]. To implement the simulation across real hunter-gatherer networks, in each round t, an agent i was selected randomly, and its partner j was selected with a probability proportional to the weights of all dyadic interactions of i in the real hunter-gatherer network. When a new ingredient was found, it was automatically transmitted to all direct network neighbors of both i and j.
Fig. 2. Cumulative culture simulation.
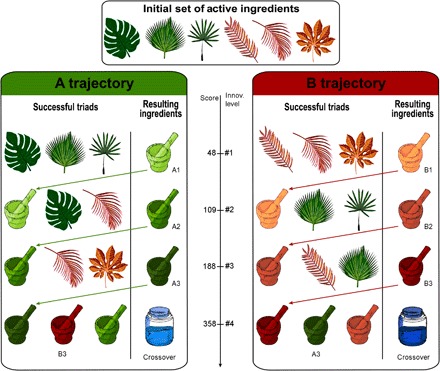
In our simulation, agents had to find successive innovations by combining virtual medicinal plants. They were given an initial set of six medicinal plants, which could be combined in triads to generate new drugs (A1, A2, A3, B1, B2, and B3) of increasing medicinal value. At the fourth level of innovation, a crossover of trajectories A and B produces the two medicines with highest efficiency (crossovers 1 and 2). The virtual experiment was finished when a crossover was found. Figure and simulation were adapted from (10).
We ran 1000 simulations across the real multicamp networks of forest and coastal groups. The forest group required, on average, 177.3 (SD ±250.5) trial rounds to find the crossover drug, and the coastal group required 516.7 (SD ±506.2) rounds (Fig. 3A and table S3). Next, we ran the same experiment over size-matching fully connected networks, where all individuals are network neighbors and hence any innovation is immediately transmitted to all network members. Crossovers took significantly more rounds in both the forest group (509.5, SD ±399.6; Wilcoxon rank test, W = 825,400, P < 10−15) and coastal group (698.7, SD ±569.2; Wilcoxon rank test, W = 620,870, P < 10−15). Therefore, the sparsely interconnected social structure of hunter-gatherer multicamps accelerates cultural evolution.
Fig. 3. Time to discovery of highest-level innovation (crossover).
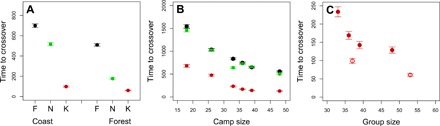
(A) Time to crossover in multicamp simulations (coast and forest) under three experimental treatments [black, fully connected (F); green, all neighbors copy (N); red, only close kin copy (K)]. (B) Time to crossover in six real individual camps. (C) Comparisons between real individual camps (red circles) and real multicamps of similar size (open circles), indicating independent effects of network size and structure. Vertical bars represent SEM.
Ethnographic studies have shown that hunter-gatherer medicinal plant knowledge is preferentially transmitted through kin networks rather than freely available to all network neighbors (11). Therefore, we repeated our experiment in real multicamp networks but limited transmission of new discoveries to close kin neighbors in the network (father, mother, offspring, siblings, and spouses). The result is further acceleration of innovation rates in comparison to transmission to all direct neighbors in size-matched fully connected networks, with crossovers now taking only 60.7 (SD ±103.7) rounds in the forest group (Wilcoxon rank test, W = 322,260, P < 10−15) and 99 (SD ±216.9) in the coastal group (Wilcoxon rank test, W = 125,560, P < 10−15) (Fig. 3A).
A recent study (3) has provided evidence that structural network properties of hunter-gatherer residential camps (global efficiency and clustering) maximize the efficiency of information transmission within camps. These properties result from households mostly consisting of close kin and households being interconnected through strong but more sparse non-kin dyads. We therefore replicated simulations as above, but here only considering intracamp interactions under three scenarios: fully connected networks, real hunter-gatherer networks with transmission to all neighbors, and real hunter-gatherer networks but limiting transmission to close kin only. We first used network data from six separate Agta camps, previously described in (3). In simulations across fully connected networks, time to produce the final medicine ranged from 557.9 to 1545.3 rounds, depending on the size of the camp (Fig. 3B). In real single-camp networks with transmission of innovations to all neighbors, time to crossover remains broadly similar (from 503.3 to 1460 rounds), with no significant differences between the two conditions in four of the six camps (table S3). In contrast, when transmission of discoveries is limited to close kin neighbors, we observe a significant acceleration in innovation rate, with average time to crossover reduced to between 128.7 and 680.8 rounds. For all six individual camps, transmission of innovations only to close kin halves time to crossover in comparison to transmission to all neighbors. There is also a positive effect of camp size (Fig. 3B), confirming the importance of demography in cultural evolution (13–17). However, the effects of social structure and size are independent (18, 19). This is demonstrated by our simulations across the two multicamp groups, which resulted in shorter times to crossover than in the case of single camps of approximately similar size (Fig. 3C). For example, in the coastal multicamp group (n = 37) with transmission to close kin only, mean time to crossover was 99 rounds, significantly shorter than for a single camp with equivalent population size, such as camp 4 (n = 36; 169 rounds; Wilcoxon rank test, W = 445,060, P = 0.00002), while in the forest multi-camp group (n = 53) with transmission to close kin only, mean time to crossover was 60.7 rounds, significantly shorter than for single camp 6 with equivalent size (n = 48; 128.7 rounds; Wilcoxon rank test, W = 545,960, P = 0.0004) (Fig. 3C and table S3). Thus, while intracamp social structure facilitates the evolution of cumulative culture due to kin clustering, sparsely connected multicamp social structures further accelerate cultural innovation rates.
We then asked how and why a multilevel social structure that restricts the flow of information can increase rates of cultural evolution. We thus analyzed the distribution of incremental innovations (A1, A2, A3, B1, B2, and B3) and recombination events (crossovers) across the social network at the end of simulations. Simulations based on transmission of innovations only to close kin revealed that the structuring of the two multicamp networks (with families within camps and camps within a multicamp) promotes cultural clustering, overall diversity, and faster times to crossover compared to transmission to all nodes in fully connected networks (Fig. 4). The reason is that fully connected networks promote faster transmission of innovations to all network members along one of the lineages (A or B). However, faster discoveries of incremental innovations along one trajectory happen at the expense of discoveries in the other lineage (Fig. 3B). Consequently, the populations become trapped in one of the lineages and unlikely to produce the crossover drug. For example, once the first innovation A1 is found and transferred to other individuals, drug triads including the new ingredient are, on average, superior to any of the other triads; therefore, B1 becomes less likely to be found; once A2 is found and transmitted to all other nodes, progress along the B trajectory becomes even less likely. In contrast, transmission across multilevel social structures of hunter-gatherers allows the coexistence of the two lineages among different kin or household clusters within camps and among distinct camps in a multicamp structure. The coexistence of cultural lineages promotes their faster recombination into higher-order cultural innovations (Fig. 3A). Thus, multilevel structuring favors the maintenance of cultural lineages and innovations in different parts of the network, due to a network memory of features that otherwise would be lost by single individuals.
Fig. 4. Timing and diversity of innovations across real multicamp social networks.
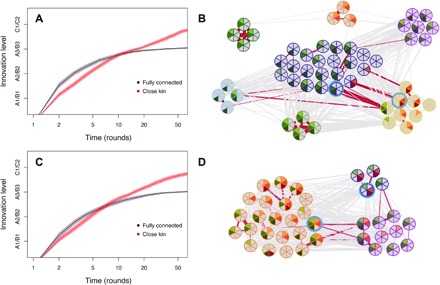
(A) Average innovation level (incremental steps A1, A2, A3, B1, B2, B3, and crossover event) by number of rounds, in fully connected (black) and real multicamp networks (forest group) with transmission only to close kin (red). Averages were drawn from 1000 simulations for each transmission scenario. Shaded areas around the curves are SEs estimated for each round and multiplied by 5 for better visualization. (B) Real multicamp network (forest group) showing innovation level achieved by each individual at the end of one typical simulation with transmission only to close kin (light green, A1; green, A2; dark green, A3; light red, B1; red, B2; dark red, B3; the two blue circled individuals are the ones finding the crossover). Innovations are clustered in different parts of the real network. (C and D) Same as above, but for the real multicamp coastal network.
Last, we asked how characteristics of hunter-gatherer sociality (high mobility, strong kinship ties, and non-kin dyads between households and camps) relate to the process of cultural accumulation. First, simulations show that as innovations become more complex from A1/B1 to crossovers, the contribution of non-kin dyads increases from 29 to 41% (forest group) and from 31 to 48% (coastal group; table S4). In addition, the contribution of intercamp dyads also increases from 31% (A1) to 48% (crossover) in the forest group and from 36 to 39% in the coastal group. Dyads formed by individuals from different camps (either kin or non-kin) often bridge together solutions from different parts of the network, which leads to the recombination of trajectories A and B into crossovers. This reveals the importance of high intercamp mobility of individuals and families to cumulative culture in hunter-gatherer societies.
DISCUSSION
Unlike nonhuman primates, extant hunter-gatherers exhibit a social structure containing clusters of nuclear families that co-reside with other unrelated families, a fluid social structure including both male and female migrations, and friendship dyads across camps (1–3). We have provided evidence that multiple levels of clustering in hunter-gatherer social networks accelerate cumulative cultural evolution. This occurs because multilevel social structuring restricts transmission of cultural innovations and allows the coexistence of multiple traditions or solutions to a similar problem in different parts of the network. The conclusion is consistent with differences in medicinal knowledge between BaYaka hunter-gatherers and African apes living in the same Central African forests (11). Of the 32 medicinal plants used by the BaYaka, 9 are used by gorillas and 6 are used by chimpanzees (20). However, no BaYaka individual in the sample had knowledge of all the 32 plants. This difference in knowledge breadth mirrors differences in social structure among the species and suggests a redefinition of the ratchet effect in humans: Cumulative culture involves not only the impossibility of recreation of cultural features by isolated individuals but also the emergence of knowledge specialization within populations. Accordingly, in our simulations across real networks, no individual ended up in possession of all innovations. This illustrates why cumulative culture is a product of human populations rather than individuals and suggests that the origin of knowledge specialization in humans took place in hunter-gatherer multilevel societies.
We propose that the multilevel structure observed in extant hunter-gatherers may explain the cultural dynamism of H. sapiens since its origins and its worldwide expansion. We believe that multilevel structuring already characterized Middle Stone Age populations emerging as early as 320,000 years ago, which were also known to have established trade dyads connecting sites up to 160 km apart (7, 21). As hunter-gatherers expanded within and then out of Africa in small and interconnected bands, potential consequences may have included cultural recombination preceding “local revolutions” such as the Upper Paleolithic (22) and genetic introgression among H. sapiens and other hominin species (23, 24).
MATERIALS AND METHODS
Collection of network data using motes
We have previously described the technology in detail (3), so here, we provided a shorter description. Motes are wireless sensing devices that store all between-device communications within a specified distance. The device we used was the UCMote Mini (with a TinyOS operating system). Individuals within a camp and in two areas including seven and three camps wore the motes from a period of 1 month. The motes created ad hoc networks and required no grounded infrastructure. Therefore, they had the advantage of collecting interactions even when a group of individuals was far from camp foraging.
Each device sent a message every hour that contained its unique ID, a time stamp, and the signal strength. These messages were stored by any other mote within a 3-m radius. Being within 3 m is a common threshold applied in behavioral studies of human and nonhuman primates to denote dyadic exchanges and close interactions, such as playing, hunting, foraging, and socializing.
Motes utilization
The motes were sealed into wristbands and belts (depending on size and preference), labeled with a unique number, and identified with colored string to avoid accidental swaps. We checked for armband swaps and made adjustments before data processing. Individuals wore motes uninterruptedly for 4 weeks and received a small compensation (thermal bottle and cooking utensils). If individuals arrived at a camp during data collection, they were promptly given a mote and entry time was recorded. Similarly, if an individual left a camp at any time before the end of data collection, the time they returned the mote was recorded. To ensure that swaps did not occur, individuals were asked twice daily to check if they were wearing the correct armband. All mote numbers were also checked when they were returned. Any swaps were recorded during data collection and adjusted in the final data processing by associating the individual with the correct mote at any given point during data collection.
Data recovery
Data were later downloaded via a PC side application written in Java. Data were only selected between 05:00 and 20:00 to avoid long hours of recording who slept in the same shelter. We ran raw data through a stringent data-processing system in Python to prevent data corruption. Data were matched to ID numbers and start-stop times of each mote. The result was a matrix with the number of recorded beacons for all possible dyads (or dyadic weights).
Ethical approval
Research project and fieldwork were approved by the University College London Ethics Committee (code 3086/003, Leverhulme Trust grant RP2011-R-045, 2011–2016).
Description of cultural evolution model
We performed agent-based simulations adapted from the model proposed by Derex and Boyd (10). The cultural repertoire of each individual is described by a binary vector (present/absent), where each entry represents a different ingredient or drug derived from a medicinal plant. Each ingredient has an intrinsic fitness or medicinal value. At time t = 0 (start of simulation), an agent is endowed with a set of six original ingredients (two drugs of value 10, two drugs of value 8, and two drugs of value 6). Their recombination into triads may lead, in some cases, to the discovery of new ingredients of higher fitness, which are then added to the original set.
Starting with the six original ingredients, one triad (combining three drugs of medicinal values 10, 8, and 6) produces a first cultural innovation of higher medicinal value A1. All other triads were considered as nonsuccessful and awarded no value. Next, another single triad including A1 plus the original ingredients produces a second drug A2 of even higher value; A2 is added to the drug set and is required for the creation of the higher-value drug A3. The same process generates a second trajectory B1, B2, and B3. The fitness of the new ingredients A1 and B1 is greater than the fitness of any other triad; the same is true for A2/A3 and B2/B3. At the final stage of the experiment, two possible crossover can be produced by the combination of ingredients from trajectories A and B (crossover 1 requiring A3, B3, and A2 and crossover 2 requiring A3, B3, and B2). Thus, cultural evolution from level 1 to 3 is vertical and defines two independent trajectories A and B, while cultural evolution from level 3 to 4 is horizontal and represents a rarer innovation leap. The values of the new ingredients were the same as in (10): A1 = B1 = 48; A2 = B2 = 109; A3 = B3 = 188; crossover 1 = crossover 2 = 358.
Simulations across fully connected networks
Simulations of the model were based on dyad interactions between agents i and j. First, we described the case of a fully connected network of N individuals, i.e., a well-mixed population of N agents with equal probability of interaction to each other. The process of cultural evolution was simulated by a Monte Carlo method with asynchronous update. The simulation proceeded in epochs (or rounds):
1. A focal agent i was uniformly and randomly selected with probability P = 1/N.
2. A second and neighboring agent j was also randomly and uniformly selected. Because the network was fully connected, agent j is selected with a fixed probability P = 1/(N − 1).
3. i and j respectively selected 2 and 1 objects from their set of ingredients (or vice versa, with probability P = 0.5), with probability proportional to their medicinal value, creating a drug triad.
4. If the combination led to a discovery, the new drug is added to the set of ingredients of both discoverers i and j; otherwise, nothing happens.
5. In case of discovery, all neighbors of i and j acquire the new discovery, too. Because the network was fully connected, all nodes or agents were connected, and the whole population acquired the new ingredient immediately following its discovery.
When all N individuals had been selected as focal agents in step 1, an epoch t ended. When a crossover was found at time t = T, the simulation ended.
Simulations across real hunter-gatherer networks
We simulated the model across the real weighted networks of Agta hunter-gatherers (derived both from two multicamp groups and from six individual camps). This was possible because of the modification of a few of the steps above. To simulate the process with transmission of discoveries to all neighbors, new steps 2 and 5 were defined as:
2. A second agent j, neighbor of i, was selected. For each neighbor j, selection probability was proportional to the weight of the dyadic link wij between i and j.
5. In case of discovery, only neighbors of i and neighbors of j acquired the new ingredient.
To simulate transmission of discoveries only to close kin across the real network, step 5 was further modified:
5. In case of discovery, only neighbors of i who were also close kin of i and neighbors of j who were also close kin of j acquire the new ingredient.
Supplementary Material
Acknowledgments
We would like to thank our assistants in the Philippines, as well as the Agta community. We also thank Institute of Anthropology students and staff, C. van Schaik and J. Bertranpetit, for comments on the manuscript. Funding: This project was funded by Leverhulme Trust grant RP2011-R-045, and Leverhulme Trust grant PLP-2017-323 (to A.B.M.). J.G.-G acknowledges support from MINECO and FEDER funds (Grant No. FIS2017-87519-P), and Gobierno de Aragon/Fondo Europeo de Desarrollo Regional (FEDER) (Grant No. E36_17R to FENOL group). Author contributions: Conceptualization: A.B.M. and L.V.; data curation: A.B.M., F.B., S.V., A.E.P., M.D., R.S., and D.S.; formal analysis: F.B., V.L., and L.V.; funding acquisition: A.B.M.; investigation: A.B.M., F.B. S.V., A.E.P., M.D., R.S., D.S., L.A., M.N., J.G.-G., V.L., and L.V.; methodology: A.B.M., F.B., S.V., J.G.-G., L.V., and V.L.; project administration: A.B.M.; supervision: A.B.M. and L.V.; software: S.V.; visualization: F.B., R.S., and L.V.; writing of original draft: A.B.M. and L.V.; writing review and editing: A.B.M., F.B., S.V., A.E.P., M.D., R.S., D.S., L.A., M.N., J.G.-G., V.L., and L.V. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/9/eaax5913/DC1
Table S1. Distribution of intracamp, intercamp, and total dyads by kinship level in forest and coastal multicamp groups.
Table S2. Distribution of intracamp, intercamp, and total dyads by sex composition in forest and coastal multicamp groups.
Table S3. Time to crossover as a function of camp size, network type, and transmission mode.
Table S4. Fraction of dyads classified by relatedness (close kin, extended kin, non-kin) or location (intra- and intercamp) and estimated at successive innovation level (A1/B1, A2/B2, A3/B3, crossover 1/2).
Data file S1. Simulation data: Time to crossover.
Data file S2. Simulation data: Innovation level.
Data file S3. Edge list, forest group.
Data file S4. Edge list, coastal group.
REFERENCES AND NOTES
- 1.Dyble M., Salali G. D., Chaudhary N., Page A., Smith D., Thompson J., Vinicius L., Mace R., Migliano A. B., Sex equality can explain the unique social structure of hunter-gatherer bands. Science 348, 796–798 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Dyble M., Thompson J., Smith D., Salali G. D., Chaudhary N., Page A. E., Vinicuis L., Mace R., Migliano A. B., Networks of food sharing reveal the functional significance of multilevel sociality in two hunter-gatherer groups. Curr. Biol. 26, 2017–2021 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Migliano A. B., Page A. E., Gómez-Gardeñes J., Salali G. D., Viguier S., Dyble M., Thompson J., Chaudhary N., Smith D., Strods J., Mace R., Thomas M. G., Latora V., Vinicius L., Characterization of hunter-gatherer networks and implications for cumulative culture. Nat. Hum. Behav. 1, 0043 (2017). [Google Scholar]
- 4.Hill K. R., Wood B. M., Baggio J., Hurtado A. M., Boyd R. T., Hunter-gatherer inter-band interaction rates: Implications for cumulative culture. PLOS ONE 9, e102806 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis H. M., Vinicius L., Strods J., Migliano A. B., High mobility explains demand sharing and enforced cooperation in egalitarian hunter-gatherers. Nat. Commun. 5, 5789 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sikora M., Seguin-Orlando A., Sousa V. C., Albrechtsen A., Korneliussen T., Ko A., Rasmussen S., Dupanloup I., Nigst P. R., Bosch M. D., Renaud G., Allentoft M. E., Margaryan A., Vasilyev S. V., Veselovskaya E. V., Borutskaya S. B., Deviese T., Comeskey D., Higham T., Manica A., Foley R., Meltzer D. J., Nielsen R., Excoffier L., Mirazon Lahr M., Orlando L., Willerslev E., Ancient genomes show social and reproductive behavior of early Upper Paleolithic foragers. Science 358, 659–662 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Brooks A. S., Yellen J. E., Potts R., Behrensmeyer A. K., Deino A. L., Leslie D. E., Ambrose S. H., Ferguson J. R., d’Errico F., Zipkin A. M., Whittaker S., Post J., Veatch E. G., Foecke K., Clark J. B., Long-distance stone transport and pigment use in the earliest Middle Stone Age. Science 360, 90–94 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Smith K. M., Larroucau T., Mabulla I. A., Apicella C. L., Hunter-gatherers maintain assortativity in cooperation despite high levels of residential change and mixing. Curr. Biol. 28, 3152–3157.e4 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Grove M., Hunter-gatherers adjust mobility to maintain contact under climatic variation. J. Archaeol. Sci. 19, 588–595 (2018). [Google Scholar]
- 10.Derex M., Boyd R., Partial connectivity increases cultural accumulation within groups. Proc. Natl. Acad. Sci. U.S.A. 113, 2982–2987 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salali G. D., Chaudhary N., Thompson J., Grace O. M., van der Burgt X. M., Dyble M., Page A. E., Smith D., Lewis J., Mace R., Vinicius L., Migliano A. B., Knowledge-sharing networks in hunter-gatherers and the evolution of cumulative culture. Curr. Biol. 26, 2516–2521 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Tennie C., Call J., Tomasello M., Ratcheting up the ratchet: On the evolution of cumulative culture. Philos. Trans. R. Soc. Lond. B 364, 2405–2415 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powell A., Shennan S., Thomas M. G., Late Pleistocene demography and the appearance of modern human behaviour. Science 324, 1298–1301 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Henrich J., Demography and cultural evolution: How adaptive cultural processes can produce maladaptive losses: The Tasmanian case. Am. Antiq. 69, 197–214 (2004). [Google Scholar]
- 15.Kline M. A., Boyd R., Population size predicts technological complexity in Oceania. Proc. R. Soc. Lond. B 277, 2559–2564 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.M. Smolla, E. Akçay, Cultural selection shapes network structure. Sci. Adv. 5, eaaw0609 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dyble M., The effect of dispersal on rates of cumulative cultural evolution. Biol. Lett. 14, 20180069 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derex M., Perreault C., Boyd R., Divide and conquer: Intermediate levels of population fragmentation maximize cultural accumulation. Philos. Trans. R. Soc. Lond. B 373, 20170062 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Creanza N., Kolodny O., Feldman M. W., Cultural evolutionary theory: How culture evolves and why it matters. Proc. Natl. Acad. Sci. U.S.A. 114, 7782–7789 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masi S., Gustafsson E., Saint Jalme M., Narat V., Todd A., Bomsel M.-C., Krief S., Unusual feeding behavior in wild great apes, a window to understand origins of self-medication in humans: Role of sociality and physiology on learning process. Physiol. Behav. 105, 337–349 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Blegen N., The earliest long-distance obsidian transport: Evidence from the ∼200 ka Middle Stone Age Sibilo School Road Site, Baringo, Kenya. J. Hum. Evol. 103, 1–19 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Greenbaum G., Friesem D. E., Hovers E., Feldman M. W., Kolodny O., Was inter-population connectivity of Neanderthals and modern humans the driver of the Upper Paleolithic transition rather than its product? Quat. Sci. Rev. 217, 316–329 (2019). [Google Scholar]
- 23.Pagani L., Lawson D. J., Jagoda E., Mörseburg A., Eriksson A., Mitt M., Clemente F., Hudjashov G., DeGiorgio M., Saag L., Wall J. D., Cardona A., Mägi R., Sayres M. A. W., Kaewert S., Inchley C., Scheib C. L., Järve M., Karmin M., Jacobs G. S., Antao T., Iliescu F. M., Kushniarevich A., Ayub Q., Tyler-Smith C., Xue Y., Yunusbayev B., Tambets K., Mallick C. B., Saag L., Pocheshkhova E., Andriadze G., Muller C., Westaway M. C., Lambert D. M., Zoraqi G., Turdikulova S., Dalimova D., Sabitov Z., Sultana G. N. N., Lachance J., Tishkoff S., Momynaliev K., Isakova J., Damba L. D., Gubina M., Nymadawa P., Evseeva I., Atramentova L., Utevska O., Ricaut F. X., Brucato N., Sudoyo H., Letellier T., Cox M. P., Barashkov N. A., Škaro V., Mulahasanovic´ L., Primorac D., Sahakyan H., Mormina M., Eichstaedt C. A., Lichman D. V., Abdullah S., Chaubey G., Wee J. T. S., Mihailov E., Karunas A., Litvinov S., Khusainova R., Ekomasova N., Akhmetova V., Khidiyatova I., Marjanović D., Yepiskoposyan L., Behar D. M., Balanovska E., Metspalu A., Derenko M., Malyarchuk B., Voevoda M., Fedorova S. A., Osipova L. P., Lahr M. M., Gerbault P., Leavesley M., Migliano A. B., Petraglia M., Balanovsky O., Khusnutdinova E. K., Metspalu E., Thomas M. G., Manica A., Nielsen R., Villems R., Willerslev E., Kivisild T., Metspalu M., Genomic analyses inform on migration events during the peopling of Eurasia. Nature 538, 238–242 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malaspinas A.-S., Westaway M. C., Muller C., Sousa V. C., Lao O., Alves I., Bergström A., Athanasiadis G., Cheng J. Y., Crawford J. E., Heupink T. H., Macholdt E., Peischl S., Rasmussen S., Schiffels S., Subramanian S., Wright J. L., Albrechtsen A., Barbieri C., Dupanloup I., Eriksson A., Margaryan A., Moltke I., Pugach I., Korneliussen T. S., Levkivskyi I. P., Moreno-Mayar J. V., Ni S., Racimo F., Sikora M., Xue Y., Aghakhanian F. A., Brucato N., Brunak S., Campos P. F., Clark W., Ellingvåg S., Fourmile G., Gerbault P., Injie D., Koki G., Leavesley M., Logan B., Lynch A., Matisoo-Smith E. A., McAllister P. J., Mentzer A. J., Metspalu M., Migliano A. B., Murgha L., Phipps M. E., Pomat W., Reynolds D., Ricaut F.-X., Siba P., Thomas M. G., Wales T., Wall C. M., Oppenheimer S. J., Tyler-Smith C., Durbin R., Dortch J., Manica A., Schierup M. H., Foley R. A., Lahr M. M., Bowern C., Wall J. D., Mailund T., Stoneking M., Nielsen R., Sandhu M. S., Excoffier L., Lambert D. M., Willerslev E., A genomic history of Aboriginal Australia. Nature 538, 207–214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/9/eaax5913/DC1
Table S1. Distribution of intracamp, intercamp, and total dyads by kinship level in forest and coastal multicamp groups.
Table S2. Distribution of intracamp, intercamp, and total dyads by sex composition in forest and coastal multicamp groups.
Table S3. Time to crossover as a function of camp size, network type, and transmission mode.
Table S4. Fraction of dyads classified by relatedness (close kin, extended kin, non-kin) or location (intra- and intercamp) and estimated at successive innovation level (A1/B1, A2/B2, A3/B3, crossover 1/2).
Data file S1. Simulation data: Time to crossover.
Data file S2. Simulation data: Innovation level.
Data file S3. Edge list, forest group.
Data file S4. Edge list, coastal group.


