Abstract
BACKGROUND AND PURPOSE:
Gadolinium enhanced MRI is routinely used for follow-up of patients with multiple sclerosis. Our aim was to evaluate whether enhancing multiple sclerosis lesions on follow-up MR imaging can be detected by visual assessment of unenhanced double inversion recovery and FLAIR sequences.
MATERIALS AND METHODS:
A total of 252 consecutive MRIs in 172 adult patients with a known diagnosis of multiple sclerosis were reviewed. The co-presence or absence of associated double inversion recovery and FLAIR signal abnormality within contrast-enhancing lesions was recorded by 3 neuroradiologists. In a subset of patients with prior comparisons, the number of progressive lesions on each of the 3 sequences was assessed.
RESULTS:
A total of 34 of 252 MRIs (13%) demonstrated 55 enhancing lesions, of which 52 (95%) had corresponding hyperintensity on double inversion recovery and FLAIR. All lesions were concordant between double inversion recovery and FLAIR, and the 3 enhancing lesions not visible on either sequence were small (<2 mm) and cortical/subcortical (n = 2) or periventricular (n = 1). A total of 17 (22%) of the 76 MRIs with a prior comparison had imaging evidence of disease progression: Ten (59%) of these showed new lesions on double inversion recovery or FLAIR only, 6 (35%) showed progression on all sequences, and 1 (6%) was detectable only on postcontrast T1, being located in a region of confluent double inversion recovery and FLAIR abnormality.
CONCLUSIONS:
There was a high concordance between enhancing lesions and hyperintensity on either double inversion recovery or FLAIR. Serial follow-up using double inversion recovery or FLAIR alone may capture most imaging progression, but isolated enhancing lesions in confluent areas of white matter abnormality could present a pitfall for this approach.
Multiple sclerosis affects approximately 727,324 individuals in the United States.1 Adherence to imaging follow-up is key to obtaining optimal clinical outcomes in patients with MS.2 Routine imaging follow-up, in particular, is important to guide therapy.3,4 MR imaging is currently the most sensitive available tool for monitoring inflammatory disease activity in patients with MS. Clinical assessments usually underestimate disease activity and burden compared with MRI.3 In addition, concordance between the clinical examination and MR imaging is essential for distinguishing frank relapses from pseudorelapses.
Brain MR imaging is recommended before the initiation or modification of disease-modifying therapies (DMTs) and approximately 6 months after a treatment change to allow sufficient time for new therapies to reach their therapeutic potential.3 Continued or worsening disease activity on MR imaging while the patient is on a DMT may prompt a change in therapy because it is indicative of a suboptimal therapeutic response.5-7 Additionally, many new lesions, especially those outside the more functionally eloquent regions of the brain, may be clinically silent.3 Therefore, for relapsing forms of MS, a follow-up brain MR imaging should be considered annually for at least the first 2–3 years after starting therapy or switching DMTs. More frequent surveillance may be indicated in clinically aggressive cases or unusual patterns of MR imaging lesions (eg, tumefactive MS).3 Finally, high-risk patients should have surveillance MR imaging performed every 3–6 months to assess progressive multifocal leukoencephalopathy.8
In the current routine clinical practice, the follow-up MR imaging for MS is performed by using intravenous gadolinium contrast. This is mainly due to prior studies reporting higher sensitivity of postcontrast MR imaging in detecting new MS lesions.9 However, with higher MR imaging magnet strength and new imaging sequences such as double inversion recovery (DIR), a sequence more sensitive to cortical and infratentorial lesions than FLAIR, this approach needs to be assessed and modified. Recent studies suggest that the use of contrast material at follow-up MR imaging does not change the diagnosis of interval disease progression in patients with MS.10-13 Most of these studies have used subtraction10 or computer-assisted detection (CAD)11 techniques to identify new MS lesions on 3D FLAIR10,11 or DIR sequences.10 This use is especially timely given growing concerns about deposition of free gadolinium in the brain and other organs after using gadolinium-based contrast agents.14-16 Furthermore, MS commonly affects populations 20–40 years of age17 with approximately 40 years of life expectancy,18 which translates into these patients undergoing >40 MR imaging examinations over the course of their disease.
However, the generalizability of the CAD approach is limited because such technologies are not yet widely available in most institutions and can be difficult to incorporate into a routine clinical workflow. In the current study, we sought to evaluate whether enhancing MS lesions on follow-up MR imaging can be detected by visual assessment of unenhanced DIR and FLAIR sequences. This study will provide information on identifying the subgroup of patients with MS who would benefit from gadolinium-enhanced MR follow-up imaging.
Materials and Methods
Institutional review board approval and a waiver of informed consent were obtained for this retrospective review. The study was Health Insurance Probability and Accountability Act–compliant.
Study Population
The Radiology Information System data base was searched for consecutive patients older than 18 years of age with a clinical diagnosis of MS who underwent brain MR imaging using the MS protocol at the outpatient radiology clinic of our institution between September 2016 and April 2018. Only patients with an established diagnosis of MS as determined by the medical record and documented by a neurologist were included. Patients with other coexisting conditions involving the central nervous system (stroke, vasculitis, tumor, migraine, epilepsy, and so forth) as determined from the medical record, history of prior neurosurgery, and incomplete or inadequate imaging were excluded. A total of 313 patients were screened, and 280 patients met the initial inclusion criteria, of whom 11 were excluded due to prior neurosurgery, 94 for coexisting conditions, and 3 for incomplete imaging, yielding 172 patients for recruitment.
MR Imaging
All included patients were scanned on the same 3T scanner (Magnetom Skyra; Siemens, Erlangen, Germany) using a dedicated MS MR imaging protocol, which included precontrast volumetric 3D-DIR, axial and sagittal 2D-FLAIR, DWI, gradient recalled-echo, postcontrast T2-weighted fast spin-echo, T1-weighted fast spin-echo, and 3D-MPRAGE sequences. During the study period, of the 172 patients, 111 had only 1 completed MR imaging examination with adequate image quality, while 48 patients had two, 8 patients had 3, 4 patients had 4, and one patient had 5 completed MR imaging examinations with adequate image quality, resulting in review of a total of 252 MR imaging examinations. Seventy-six examinations had at least 1 prior comparison included in the study.
For this study, we reviewed volumetric 3D-DIR (sagittal acquisition with 1-mm axial reformat; section thickness, 1.4 mm; FOV, 259 × 259 mm; matrix, 190 × 190; TE, 318 ms; TR, 7500 ms; variable flip angle; echo-train length, 234), axial 2D-T2 FLAIR (axial acquisition; section thickness, 3 mm; FOV, 178 × 220 mm; matrix, 320 × 182; TE, 81 ms; TR, 9000 ms; TI, 2500 ms; flip angle, 150°; echo-train length, 16), and axial volumetric 3D postcontrast T1-weighted MPRAGE (sagittal acquisition, 4-5 minutes after the intravenous administration of 0.1 mmol/kg of gadobenate dimeglumine [MultiHance; Bracco Diagnostics, Princeton, New Jersey] with 1-mm axial reformats; section thickness, 0.9 mm; FOV, 240 × 240 mm; matrix, 256 × 256; TE, 2.32 ms; TR, 1800 ms; flip angle, 8°; echo-train length, 1) sequences. The parameters for all sequences were the same in all patients.
Image Review
All images were anonymized and reviewed on a research DICOM viewer without knowledge of the patients’ demographics, clinical symptoms, or treatment. Three board-certified neuroradiologists with 10, 2, and 1 year of experience postfellowship training independently reviewed all 252 examinations. Total lesion burden on 2D-FLAIR and 3D-DIR was classified as mild (<10), moderate (11–20), and severe (>20 or confluent). This decision was made on the basis of the proposed contextual template for MS follow-up MR imaging by Mamlouk et al.19 The number and locations (cortical/juxtacortical, deep, periventricular, and infratentorial) of enhancing lesions was documented on the basis of 3D postcontrast T1-weighted MPRAGE, and whether these enhancing lesions were detectable on 3D-DIR or 2D-FLAIR by expert visual assessment was determined. For MR imaging examinations with a prior comparison (eg, if the patient had completed >1 examination during the study period), the reviewers also recorded the development of interval new lesions on each of the 3 sequences and whether this new lesion was seen on only 1 sequence or all of them. After the initial independent review, the raters reviewed all discrepant findings and reached a consensus if 2 of 3 raters agreed on a finding.
Statistical Analysis
Continuous variables were reported as median and interquartile range. Categoric variables were reported as numbers and frequencies. Association among categoric variables was computed using the Fisher exact test. Interobserver agreement was evaluated with the interclass correlation coefficient (κ). Statistical analysis was performed using STATA/SE Release 14.2, 2018 (StataCorp, College Station, Texas), with significance defined as P < .05.
Results
Patient Demographics
A total of 172 patients were included in the study, 131 (34%) women and 41 (24%) men, with a median age of 42 years (interquartile range, 32–50 years). Most patients, 160 (93%), had relapsing-remitting MS at the time of the first included MR imaging in our study, while 5 (3%) had primary-progressive MS, and 7 (4%) had secondary-progressive MS. The median time from initial symptoms to first MR imaging included in the study was 10 years (interquartile range, 4–12 years). The median times from initial symptoms to first MR imaging included in the study were 11, 12, and 12 years for patients with relapsing-remitting, primary-progressive, and secondary-progressive MS, respectively. Most patients (151, 88%) were on DMTs at the time of the first included MR imaging in our study. The 3 most common DMTs were tecfidera (n = 31), natalizumab (Tysabri) (n = 28), and glatiramer acetate injection (Copaxone) (n = 24).
Enhancing Lesions
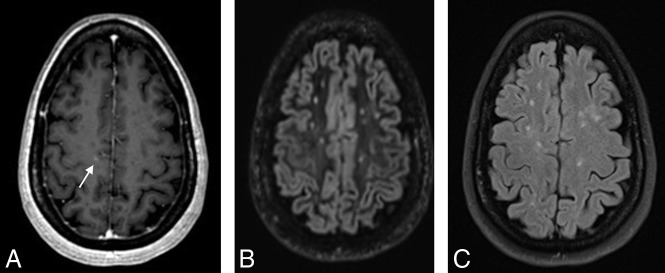
A total of 47 MRIs (19%) had mild T2 lesion burden, while 89 (35%) and 116 (46%) had moderate and severe T2 lesion burden, respectively (Table 1). A total of 55 enhancing lesions were present on 34 of 252 MRIs (13%). The presence of enhancing lesions was more common in examinations with moderate (12%) and severe (15%) T2 lesion burden than in those with mild (6%) T2 lesion burden (Table 1). Most enhancing lesions, 52 of 55 (95%), were also detectable on 3D-DIR and 2D-FLAIR. All enhancing lesions detectable on 3D-DIR were also detectable on 2D-FLAIR, and vice-versa. The 3 enhancing lesions not visible on both 3D-DIR and 2D-FLAIR sequences were all small (<2 mm), cortical/subcortical (n = 2) (Fig 1), or periventricular (n = 1) and occurred in examinations with moderate (n = 2) or severe lesion burden (n = 1).
Table 1:
The number of examinations categorized by T2 lesion burden and number of enhancing lesions
| T2 Lesion Burden | No. of MR Imaging Examinations | No. of MR Imaging Enhancing Lesions |
At Least 1 | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |||
| Mild (<10 lesions) | 47/252 (19%) | 44 | 3 | 3/252 (6%) | |||
| Moderate (11–20 lesions) | 89/252 (35%) | 78 | 7 | 3 | 1 | 11/252 (12%) | |
| Severe (>20 lesions) | 116/252 (46%) | 99 | 10 | 1 | 4 | 2 | 17/252 (15%) |
| Total | 252 | 221 | 20 | 4 | 4 | 3 | 34/252 (13%) |
Fig 1.
3D contrast-enhanced T1 MPRAGE (A) demonstrating a faint right frontal subcortical lesion (arrow) not visible on 3D DIR (B) and 2D FLAIR (C).
The interobserver agreement (κ) for enhancing lesions also detectable on 3D-DIR and 2D-FLAIR was 0.64, consistent with substantial agreement. However, the κ for enhancing lesions not visible on DIR or FLAIR was 0.10, consistent with only slight agreement.
Progression of Lesions across Time
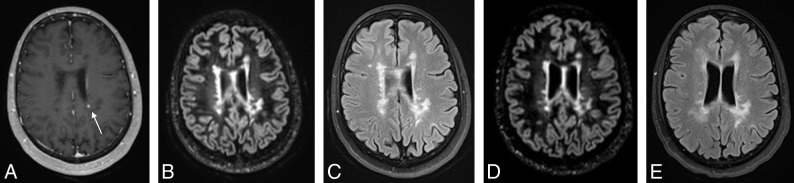
Seventy-six MRIs had a prior comparison in our study period, with a median interval of 236 days (interquartile range, 191–367 days) between examinations. Of these, a total of 17 (22%) had imaging evidence of progression, defined as new lesions (either on DIR/FLAIR or enhancing) compared with the most immediate prior MR imaging examination (Table 2). The presence of enhancing lesions was associated with new lesions on DIR and FLAIR (P < .001). Only one of these MR imaging examinations had a new lesion that was only detectable on postcontrast imaging as a single enhancing lesion in a region of confluent white matter abnormality that did not show interval change on DIR and FLAIR (Fig 2), because the lesion could not be differentiated from confluent periventricular white matter signal abnormality on DIR or FLAIR. Thus, 16 of 17 cases of progression could be detected on the basis of new DIR or FLAIR lesions alone (sensitivity, 94%).
Table 2:
Relationship between new signal abnormality and enhancement in patients with MS with prior comparison MR imaging examinations available
| No Enhancing Lesion | Enhancing Lesion Present | Total | |
|---|---|---|---|
| New DIR/FLAIR | 10 (13%) | 6 (8%) | 16 (21%) |
| Stable DIR/FLAIR | 59 (78%) | 1 (1%) | 60 (79%) |
| Total | 69 (91%) | 7 (9%) | 76 |
Fig 2.
3D contrast-enhanced T1 MPRAGE (A) demonstrating a left periventricular enhancing lesion (arrow) in a region of confluent white matter lesions that is not detectable as new between the more recent 3D-DIR (B) and 2D-FLAIR (C) and prior 3D-DIR (D) and 2D-FLAIR (E).
Discussion
In this retrospective review of patients with MS with brain MR imaging examination, our results demonstrate that most enhancing MS lesions (95%) can be identified on DIR and FLAIR sequences obtained using a 3T scanner and when assessed visually without any CAD or subtraction system. There were only 3 small (<2 mm) enhancing lesions without associated signal abnormality on DIR or FLAIR. This result is concordant with Karimian-Jazi et al,13 reporting that the enhancing lesions without correlates on FLAIR sequence are significantly smaller than those with correlates. These lesions may represent early breakdown of the blood-brain barrier before development of inflammation or edema.20 However, this likely represents a transient period early in the evolution of new MS lesions, with eventual development of concurrent T2 signal and resolution of enhancement generally within a month.21 With imaging follow-up frequency on the order of 6 months to a year, the probability of detecting such lesions is low, and their contribution to the overall evaluation of lesion progression is questionable. Indeed, when we evaluated serial imaging, only 1 of 76 examinations demonstrated lesion progression on the basis of lesion enhancement alone. In that case, the patient did not exhibit new or worsening clinical symptoms, and the patient was maintained on the existing treatment regimen, with clinical stability and resolution of the enhancing lesion on 2 subsequent follow-ups.
Our results are consistent with and augment recent works that used CAD11 and subtraction techiques10 by showing similar results with standard visual assessment by neuroradiologists. Given the high sensitivity of noncontrast imaging in detecting lesion progression and the lack of a definitive indication to alter clinical management even with a single small enhancing lesion, the cost and benefit of postcontrast MR imaging for routine follow-up of patients with MS should be re-evaluated. Our study is especially important because it evaluates the role of unenhanced DIR and FLAIR using standard visual assessment, which is likely the routine practice in most institutions. Furthermore, it will provide information to identify the subgroup of patients who would benefit from gadolinium-enhanced MR imaging for their follow-up MR imaging. Perhaps, a reasonable approach might be obtaining noncontrast follow-up in clinically stable patients with low-to-moderate lesion burden and reserving contrast-enhanced MR imaging for patients with worsening clinical symptoms or high lesion burden (eg, in which confluent lesions may limit the evaluation of T2 lesion progression). In these 2 scenarios, there is a higher chance that an enhancing lesion may make a difference in clinical management.
Our study is limited by retrospective methodology, small sample size, a short follow-up period, and a lower number of patients with available serial imaging. We intended to include only scans with the relatively recently adopted DIR sequence and to avoid potentially confounding technical factors. Therefore, we included only patients followed on a single 3T scanner at our outpatient clinic, where most of the patients with MS are being scanned. This approach limited the number of prior comparisons we could include and also limits the generalizability of our results to other magnet strengths. However, it is encouraging that other studies using different sequences and scanner types found similar findings. Furthermore, we did not evaluate the enhancing lesions on postcontrast TSE sequences and used only postcontrast MPRAGE sequences. Finally, although we evaluated the co-presence or absence of enhancing lesions (identified on 3D postcontrast sequences) on both 3D-DIR and 2D-FLAIR sequences and our results were concordant, comparison of a 2D with a 3D sequence is not optimal due to differences in section thickness. It is possible that very small lesions may not be apparent on thick-section 2D-FLAIR images due to volume averaging, though this was not observed in our dataset. With growing evidence that gadolinium is not necessary for the evaluation of lesion progression, the recently revised 2018 Consortium of MS Centers guidelines4 states that gadolinium-enhanced MR imaging is now optional for the follow-up of patients with MS to detect subclinical disease activity. We have already seen changes in the ordering pattern of our neurologists in favor of more noncontrast imaging, and a future prospective longitudinal study ideally including multiple institutions comparing the differences in clinical management and outcome of patients followed with the 2 imaging strategies would be helpful.
Conclusions
Our results add to growing evidence in the literature showing that the evaluation of noncontrast DIR and FLAIR sequences by neuroradiologists can detect most MS lesion progression on follow-up MR imaging examinations without intravenous contrast. Gadolinium-enhanced MR imaging should be considered only in select patients who may benefit from its minimally higher sensitivity and in whom detection of an enhancing lesion would alter management.
ABBREVIATIONS:
- CAD
computer-assisted detection
- DIR
double inversion recovery
- DMT
disease-modifying therapy
Footnotes
Disclosures: Gelareh Sadigh—UNRELATED: Grants/Grants Pending: Radiological Society of North America seed grant and Association of University Radiologists Radiology Research Academic Fellowship. Alex Waldman—UNRELATED: Grants/Grants Pending: Consortium of Multiple Sclerosis Centers Medical Student Research Scholarship (http://cmscfoundation.org/portfolio/2019-medical-student-research-scholarship/),* Radiological Society of North America Medical Student Grant (https://www.rsna.org/en/research/funding-opportunities/research-grants/medical-student-research-grant); Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: American Academy of Neurology, Consortium of Multiple Sclerosis Centers, and American Medical Student Association, Comments: American Academy of Neurology Annual Meeting Travel Scholarships in 2018 ($2000) and 2019 ($1000), Consortium of Multiple Sclerosis Centers Annual Meeting Travel Scholarship in 2019 ($2000), American Medical Student Association Leadership Retreat Travel Funding in 2018. Ranliang Hu—UNRELATED: Consulting Fee or Honorarium: Seimens Medical Solutions, Comments: speaker at Dual Energy Workshop; Payment for Lectures Including Service on Speakers Bureaus: paid to individual, Comments: Dual Energy Workshop; Payment for Development of Educational Presentations: Siemens, Comments: Dual Energy Workshop. *Money paid to institution.
Previously presented as an abstract at: Annual Meeting of the American Society of Neuroradiology, May 18–23, 2019; Boston, Massachusetts.
References
- 1.Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology 2019;92:e1029–40 10.1212/WNL.0000000000007035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lizan L, Comellas M, Paz S, et al. Treatment adherence and other patient-reported outcomes as cost determinants in multiple sclerosis: a review of the literature. Patient Prefer Adherence 2014;8:1653–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Traboulsee A, Simon JH, Stone L, et al. Revised recommendations of the Consortium of MS Centers Task Force for a Standardized MRI Protocol and Clinical Guidelines for the Diagnosis and Follow-Up of Multiple Sclerosis. AJNR Am J Neuroradiol 2016;37:394–401 10.3174/ajnr.A4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Consortium of. MS Centers MRI Protocol for the Diagnosis and Follow-up of MS and Clinical Guidelines for the Diagnosis and Follow-Up OF MS. 2018. revised guidelines. Februrary 2018. https://cdn.ymaws.com/mscare.site-ym.com/resource/collection/9C5F19B9-3489-48B0-A54B-623A1ECEE07B/2018MRIGuidelines_booklet_with_final_changes_0522.pdf. Accessed on May 9, 2019.
- 5.Rudick RA, Lee JC, Simon J, et al. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol 2006;60:236–42 10.1002/ana.20883 [DOI] [PubMed] [Google Scholar]
- 6.Sormani MP, Bonzano L, Roccatagliata L, et al. Magnetic resonance imaging as a potential surrogate for relapses in multiple sclerosis: a meta-analytic approach. Ann Neurol 2009;65:268–75 10.1002/ana.21606 [DOI] [PubMed] [Google Scholar]
- 7.Prosperini L, Gallo V, Petsas N, et al. One-year MRI scan predicts clinical response to interferon beta in multiple sclerosis. Eur J Neurol 2009;16:1202–09 10.1111/j.1468-1331.2009.02708.x [DOI] [PubMed] [Google Scholar]
- 8.Wattjes MP, Barkhof F.. Diagnosis of natalizumab-associated progressive multifocal leukoencephalopathy using MRI. Curr Opin Neurol 2014;27:260–70 10.1097/WCO.0000000000000099 [DOI] [PubMed] [Google Scholar]
- 9.Filippi M, Rovaris M, Bastianello S, et al. A comparison of the sensitivity of monthly unenhanced and enhanced MRI techniques in detecting new multiple sclerosis lesions. J Neurol 1999;246:97–106 10.1007/s004150050315 [DOI] [PubMed] [Google Scholar]
- 10.Eichinger P, Schon S, Pongratz V, et al. Accuracy of unenhanced MRI in the detection of new brain lesions in multiple sclerosis. Radiology 2019;291:429–35 10.1148/radiol.2019181568 [DOI] [PubMed] [Google Scholar]
- 11.Mattay RR, Davtyan K, Bilello M, et al. Do all patients with multiple sclerosis benefit from the use of contrast on serial follow-up MR imaging? A retrospective analysis. AJNR Am J Neuroradiol 2018;39:2001–06 10.3174/ajnr.A5828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saindane AM. Is Gadolinium-based contrast material needed for MRI follow-up of multiple sclerosis? Radiology 2019;291:436–37 10.1148/radiol.2019190319 [DOI] [PubMed] [Google Scholar]
- 13.Karimian-Jazi K, Wildemann B, Diem R, et al. Gd contrast administration is dispensable in patients with MS without new T2 lesions on follow-up MRI. Neurol Neuroimmunol Neuroinflamm 2018;5:e480 10.1212/NXI.0000000000000480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo BJ, Yang ZL, Zhang LJ.. Gadolinium deposition in brain: current scientific evidence and future perspectives. Front Mol Neurosci 2018;11:335 10.3389/fnmol.2018.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang H, Hii M, Le M, et al. Gadolinium deposition in deep brain structures: relationship with dose and ionization of linear gadolinium-based contrast agents. AJNR Am J Neuroradiol 2018;39:1597–1603 10.3174/ajnr.A5751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olchowy C, Cebulski K, Łasecki M, et al. The presence of the gadolinium-based contrast agent depositions in the brain and symptoms of gadolinium neurotoxicity: a systematic review. PLoS One 2017;12:e0171704 10.1371/journal.pone.0171704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause I, Kern S, Horntrich A, et al. Employment status in multiple sclerosis: impact of disease-specific and non-disease-specific factors. Mult Scler 2013;19:1792–99 10.1177/1352458513485655 [DOI] [PubMed] [Google Scholar]
- 18.Palmer AJ, van der Mei I, Taylor BV, et al. Modelling the impact of multiple sclerosis on life expectancy, quality-adjusted life years and total lifetime costs: evidence from Australia. Mult Scler 2019. Feb 26. [Epub ahead of print] 10.1177/1352458519831213 [DOI] [PubMed] [Google Scholar]
- 19.Mamlouk MD, Chang PC, Saket RR.. Contextual radiology reporting: a new approach to neuroradiology structured templates. AJNR Am J Neuroradiol 2018;39:1406–14 10.1177/1352458519831213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kermode AG, Thompson AJ, Tofts P, et al. Breakdown of the blood-brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis: pathogenetic and clinical implications. Brain 1990;113:1477–89 10.1093/brain/113.5.1477 [DOI] [PubMed] [Google Scholar]
- 21.Harris JO, Frank JA, Patronas N, et al. Serial gadolinium-enhanced magnetic resonance imaging scans in patients with early, relapsing-remitting multiple sclerosis: implications for clinical trials and natural history. Ann Neurol 1991;29:548–55 10.1002/ana.410290515 [DOI] [PubMed] [Google Scholar]