Abstract
BACKGROUND AND PURPOSE:
Embryonal tumors with multilayered rosettes, C19MC-altered, are brain tumors occurring in young children, which were clearly defined in the 2016 World Health Organization classification of central nervous system neoplasms. Our objective was to describe the multimodal imaging characteristics of this new entity.
MATERIALS AND METHODS:
We performed a retrospective monocentric review of embryonal brain tumors and looked for embryonal tumors with multilayered rosettes with confirmed C19MC alteration. We gathered morphologic imaging data, as well as DWI and PWI data (using arterial spin-labeling and DSC).
RESULTS:
We included 16 patients with a median age of 2 years 8 months. Tumors were both supratentorial (56%, 9/16) and infratentorial (44%, 7/16). Tumors were large (median diameter, 59 mm; interquartile range, 48–71 mm), with absent (75%, 12/16) or minimal (25%, 4/16) peritumoral edema. Enhancement was absent (20%, 3/15) or weak (73%, 11/15), whereas intratumoral macrovessels were frequently seen (94%, 15/16) and calcifications were present in 67% (10/15). Diffusion was always restricted, with a minimal ADC of 520 mm2/s (interquartile range, 495–540 mm2/s). Cerebral blood flow using arterial spin-labeling was low, with a maximal CBF of 43 mL/min/100 g (interquartile range, 33–55 mL/min/100 g 5). When available (3 patients), relative cerebral blood volume using DSC was high (range, 3.5–5.8).
CONCLUSIONS:
Embryonal tumors with multilayered rosettes, C19MC-altered, have characteristic imaging features that could help in the diagnosis of this rare tumor in young children.
The classification of embryonal brain tumors has been redefined in the 2016 World Health Organization classification of central nervous system neoplasms,1 with the disappearance of the term “primitive neuroectodermal tumors.” Among the newly described entities, embryonal tumors with multilayered rosettes, C19MC-altered, were defined by amplification or gain of the C19MC region on chromosome 19 (19q13.42).2–4 These tumors include the previously known embryonal tumors with abundant neuropil and true rosettes (ETANTR, also referred to as embryonal tumors with multilayered rosettes [ETMR]), ependymoblastoma, and, in some cases, medulloepithelioma.2,4 They can be histopathologically suspected by LIN28A-positive immunostaining, but full confirmation warrants molecular confirmation of the C19MC alteration.
Because this type of tumor is rare and was only recently clearly isolated, reports of confirmed C19MC-altered tumors are scarce and most studies did not report detailed imaging data,2–14 except for the recent cohort of 7 patients reported by Wang et al.15 Nowak et al16–18 reported imaging data from 22 patients with ependymoblastoma and LIN28A immunostaining, but molecular confirmation was absent because these articles were published before the 2016 World Health Organization classification. These reports described ETMRs as large tumors with absent-to-moderate enhancement after contrast media injection and, when DWI images were available, a hypersignal on DWI. Nevertheless, because these tumors were rare and gathered during a long time, PWI data and detailed DWI data (such as ADC) were missing.
Our aim was to assess multimodal imaging characteristics of ETMR, C19MC-altered, through a retrospective review of molecularly confirmed cases, including DWI and PWI data.
Materials and Methods
Patients
We performed a retrospective review from 2005 to 2018 of the prospective data base of pediatric brain tumors from the Necker Enfants Malades Hospital, Paris, France, as well as a review of the pathology data base. We gathered all tumors previously considered as ependymoblastoma, medulloepithelioma, embryonal tumors with abundant neuropils and true rosettes, and ETMR. They were histopathologically confirmed by an experienced neuropathologist (A.T.-E.) and molecularly confirmed by the presence of a gain or an amplification of C19MC by the CC19MC/TPM4 FISH Probe kit, ref CT-PAC033 (CytoTest; https://www.cytotest.com/enn/index.asp). When MR images were available, patients were included in the study.
Ethics committee approval was obtained to study multimodal imaging of children's brain tumors. Agreement of patients was prospectively obtained to perform molecular testing of tumor samples and to use medical images for research purposes.
Imaging
MR imaging was performed at our institution using a Signa HDxt 1.5 T system (GE Healthcare, Milwaukee, Wisconsin) and a 12-channel head-neck-spine coil. Acquired sequences were the following: 3D-T1-weighted, 3D-T2-weighted, FLAIR, DTI, pseudocontinuous 3D-arterial spin-labeling (ASL), DSC if available, and 3D-T1-weighted imaging after contrast media injection. If available, postgadolinium T1-weighted spine imaging was also reviewed. Assessment of calcifications was performed using precontrast CT when available and T2*-weighted sequences (gradient-echo T2 or B0 of the DWI sequence) otherwise.
Acquisition parameters for the ASL sequence were as follows: TR/TE, 4428/10.5 ms; postlabeling delay, 1025 ms; 80 axial partitions; FOV, 240 × 240 mm; slice thickness, 4 mm; acquisition matrix, 8 spiral arms in each 3D partition with 512 points per arm; flip angle, 155°; acquisition time, 4 minutes 17 seconds. Acquisition parameters for the DTI sequence were the following: 24 directions, b-value = 1000 s/mm2, TR = 8000 ms, TE = minimum according to the specific absorption rate, slice thickness = 3 mm, FOV = 240 × 240 mm, matrix size = 512 × 512.
Two senior neuroradiologists (V.D.-R. and R.L.) analyzed MRIs in consensus. Images were qualitatively analyzed using a PACS.
DTI data were analyzed using an Advantage Workstation (Version 4.7; GE Healthcare) to obtain isotropic diffusion maps and average diffusion coefficient maps. ADC, CBF, relative CBF using ASL, and relative CBV using DSC were measured within the whole tumor and within the most diffusion-restricted area or the most perfused area (ROI size, 50 mm2). To obtain relative perfusion values, we used an ROI within the temporal cortex for ASL and a ROI in normal-appearing white matter for DSC.
Statistical Analysis
Data description was performed using proportions for categoric data and median and interquartile range (IQR) for quantitative data (ADC, CBF, and relative CBF using ASL, relative CBV and relative CBF using DSC).
Results
Patients
Among 2568 patients in the data base, 22 patients with suspected ETMR were histopathologically reviewed. Sixteen patients (12 girls, 4 boys) with ETMR, C19MC-altered, were included, with a median age of 2 years 8 months (range, 1 year 2 months to 11 years 10 months).
Imaging
Findings are reported in On-line Table 1 and On-line Table 2.
Both MR imaging and CT scans were available for 9/16 patients; 6/16 had only MR images, whereas 1/16 had only CT images (patient 14, who died within 1 day of her admission in the hospital). DWI with ADC was available for 13/16 patients; ASL, for 8/16 patients; and DSC, for 3/16 patients. Spinal imaging was available for 7/16 patients.
Tumors were always intra-axial, supratentorial in 9 cases (within hemispheres with variable involvement of the basal ganglia) (Figs 1 and 2) and infratentorial in 7 cases (2 lateralized within the tentorial incisure, equally supra- and infratentorial, posterior and lateral to the cerebral peduncle, with mass effect on the cerebral peduncle [On-line Fig 1]; 2 in the cerebellar vermis [Fig 3]; 1 in the fourth ventricle; and 2 within the brain stem [Fig 4 and On-line Fig 2]). Their median largest diameter was 59 mm (IQ, 48–71 mm), causing mass effect and frequent brain herniation. Peritumoral edema was rare (4/16) and not very extended when present.
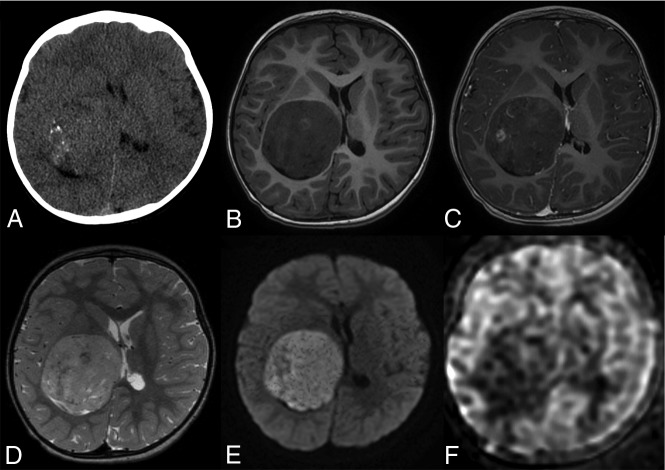
Fig 1.

Brain imaging of patient 6. Axial plane MR images show a large heterogeneous right temporal tumor, with mass effect and midline shift. The tumor displays hypointensity on the T1-weighted image (A), hyperintensity on the T2-weighted image (C), and weak enhancement after contrast media injection (B). An intratumoral vessel is seen (B). Diffusion signal is high (D) with low ADC. Diffusion is also restricted in the right occipital lobe (D) because of an ischemic injury caused by compression of the posterior cerebral artery.
Fig 2.
Brain imaging of patient 11. Axial plane image on CT (A) shows a right thalamic mass with microcalcifications. The tumor displays hypointensity on the T1-weighted image (B), hyperintensity on the T2-weighted image (D), and weak nodular enhancement after contrast media injection (C). An intratumoral vessel is seen (C). Diffusion signal is high (E) with low ADC. Cerebral blood flow (F) is low (maximum, 46 mL/min/100 g) within the tumor.
Fig 3.

Brain imaging of patient 3. Sagittal plane (A–C) MR images show a large midline vermian tumor, with mass effect on the fourth ventricle causing hydrocephalus. The tumor displays hypointensity on the T1-weighted image (A), hyperintensity on the T2-weighted image (C), and no enhancement after contrast media injection (B). Diffusion signal is high (D) with low ADC.
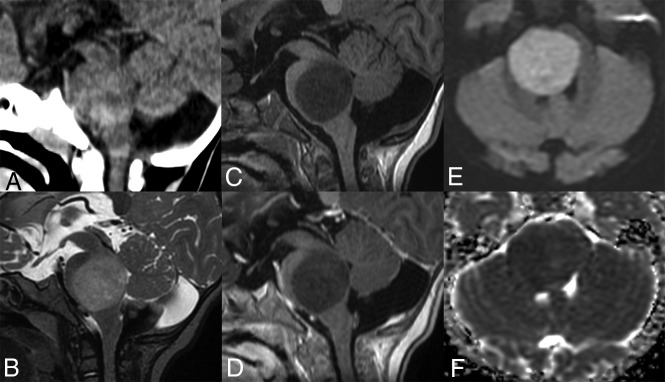
Fig 4.
Brain imaging of patient 4. Sagittal plane image on CT (A) shows a pontine isodense mass. The tumor displays hypointensity on the T1-weighted image (C), hyperintensity on the T2-weighted image (B), and no enhancement after contrast media injection (D). Diffusion signal is high (E) with low ADC (F).
No intracranial dissemination was seen at diagnosis, nor spinal metastasis when spine MR imaging was available.
Calcifications were present in 67% (10/15, Fig 2), and tumor density on CT was variable, though more frequently hyperdense (5/10). Tumors were always hypointense on T1-weighted images and hyperintense on T2-weighted and FLAIR images. Enhancement was weak and heterogeneous, sometimes nodular, except for the older patient (patient 16, 11 years of age) in whom the tumor had high and homogeneous enhancement. All patients except 1 (patient 15, On-line Fig 2) had curvilinear homogeneous enhancement connected to cortical veins, consistent with large intratumoral macroscopic veins (diameter, 1–2.5 mm).
Diffusion was always restricted, with a median minimal ADC measured at 520 mm2/s (IQR 495–540 mm2/s) and a median ADC within the whole tumor of 728 mm2/s (IQR, 611–802 mm2/s).
CBF using ASL was low, with a median value within the most perfused area of 43 mL/min/100 g (IQR, 33–55 mL/min/100 g) and a median value within the whole tumor of 30 mL/min/100 g (IQR, 24–40 mL/min/100 g). DSC-PWI was performed for only 3 patients (between 2 and 3 years of age) and showed high maximal and mean relative CBV (range, 3.5–5.8, and 1.7–2.7, respectively; On-line Table 2). DSC-derived relative CBF was also high (maximal range and mean range, 2.6–4.4 and 1.1–1.9, respectively).
Discussion
In this cohort, we report the characteristic imaging features of confirmed ETMR, C19MC-altered: large tumors with frequent calcifications, little-to-no edema, absent or weak contrast enhancement, intratumoral veins, restricted diffusion, and low CBF values using ASL.
Fifty-six percent of the tumors were hemispheric with variable basal ganglia involvement. This is slightly lower than previous larger clinical or pathologic cohorts, which reported 70%–76% supratentorial tumors.2–4,13,16 The distribution of posterior fossa tumors between the fourth ventricle/cerebellum and brain stem was also reported.3,15,16 Most interesting, we found 2 tumors lateralized within the tentorial incisure, equally supra- and infratentorial, posterior and lateral to the cerebral peduncle, with mass effect on the cerebral peduncle, which has rarely been reported.19–21 Nevertheless, because previous articles reported few imaging details, these tumors may have been classified as posterior fossa tumors or superior cerebellum vermis tumors. Their anatomic localization was difficult to define because of their large size.
Weak contrast enhancement is quite an original feature for this high-grade neoplasm and supports contrast enhancement not being a criterion for high-grade tumors in children.22 This absent or minimal enhancement was also reported in several cases of confirmed ETMR6,9,12,14,15 and in the LIN28A-stained ependymoblastoma cohort published by Nowak et al.16 The only outlier (patient 16) who had high contrast enhancement was clearly atypical because this patient was by far the oldest (11 years of age) and had high CBF. On the other hand, intratumoral large vessels were seen in all patients except 1 (patient 15, who had a mostly cystic tumor with peripherical tissular content). This feature was not detailed in previous reports, except in a few cases reports of histopathologically diagnosed ETANTR.23–26
Diffusion was restricted in all tumors, with a median ADC of 728 mm2/s for the whole tumor and 520 mm2/s for the area of maximum diffusion restriction. Hypersignal on DWI in confirmed ETMR was also previously reported,12,15 as well as in the ependymoblastoma cohort of Nowak et al,16 but no ADC values have been reported yet. This result is in line with the classic diffusion restriction in embryonal tumors in children, formerly known as primitive neuroectodermal tumors.27
CBF using ASL was low (ie, <50 mL/min/100 g) for most patients, with a median maximal CBF of 43 mL/min/100 g. To our knowledge, no previous PWI data were reported. This feature may be surprising for these high-grade neoplasms because high-grade pediatric tumors usually have high CBF using ASL.22,28–31 However, this is consistent with the study of Dangouloff-Ros et al22 on the posterior fossa, which reported that tumors with a moderate CBF (25–50 mL/min/100 g) but a weak contrast enhancement were high-grade neoplasms, including embryonal tumors. This CBF/enhancement ratio was not reported in hemispheric tumors, but supratentorial embryonal tumors (former primitive neuroectodermal tumors) were absent in this cohort.
Relative CBV using DSC was available for only 3 patients; it has become less frequently used in our institution after the introduction of the ASL sequence because it requires contrast media injection with high flow, which is difficult to obtain in young children. Relative CBV was high in all 3 cases (range, 3.5–5.8), even if CBF using ASL was as low as in other patients. This finding underlines the different pathophysiologic mechanisms involved in ASL and DSC PWI.32 The presence of calcifications in 2 of these patients (CT not available for the third patient) may have caused artifacts while using DSC and consequently less consistent results.
These homogeneous imaging characteristics may help to distinguish ETMR from other brain tumors in children between 1 and 4 years of age. Pilocytic astrocytoma is quite easily differentiated because it has no diffusion restriction and strong contrast enhancement.33 Ependymoma differs by its morphology, T2-weighted high signal, and variable DWI pattern.18,33 Most of all, some characteristics may be useful to distinguish ETMR from other embryonal brain tumors (ie, medulloblastoma and atypical teratoid/rhabdoid tumor, which may look like ETMR because they are aggressive tumors with high cellularity causing diffusion restriction).33,34 ETMR localization was different from that of classic medulloblastomas because it was not localized in the fourth ventricle (except in the one 11-year-old outlier patient). When localized in the cerebellar hemispheres (Sonic HedgeHog subgroup), medulloblastomas are different again because this subgroup has high contrast enhancement.34 Contrast uptake of ETMR was low, contrary to reported data concerning atypical teratoid/rhabdoid tumors.35 Furthermore, atypical teratoid/rhabdoid tumors usually have a much more necrotic pattern, with central cysts,35 than that in our ETMR cohort. Finally, medulloblastomas and atypical teratoid/rhabdoid tumors usually have high cerebral blood flow using ASL.22
Our study has several limitations. DSC was performed in only 3/16 patients, and one should use caution generalizing our relative CBV values. Also, we did not have spectroscopy data within the tumor because spectroscopy was not routinely performed in our institution.
Conclusions
We report the imaging data of 16 patients with confirmed ETMR, C19MC-altered. Imaging features were characteristic and should help to diagnose these rare tumors in young children between 1 and 4 years of age.
ABBREVIATIONS:
- ASL
arterial spin-labeling
- ETANTR
embryonal tumors with abundant neuropil and true rosettes
- ETMR
embryonal tumors with multilayered rosettes
- IQR
interquartile range
Footnotes
Disclosures: Jacques Grill—UNRELATED: Expert Testimony: Hoffmann-La Roche*; Grants/Grants Pending: Bristol-Myers Squibb, Novartis, Roche.* Pascale Varlet—UNRELATED: Consultancy: Hoffmann-La Roche Novartis*; Grants/ Grants Pending: Boehringer Ingelheim. *Money paid to the institution.
Paper previously presented at: Journées Francophones de Radiologie, October 12–15, 2018, Paris, France.
REFERENCES
- 1. Louis DN, Perry A, Reifenberger G, et al. . The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol (Berl) 2016;131:803–20 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 2. Korshunov A, Sturm D, Ryzhova M, et al. . Embryonal tumor with abundant neuropil and true rosettes (ETANTR), ependymoblastoma, and medulloepithelioma share molecular similarity and comprise a single clinicopathological entity. Acta Neuropathol (Berl) 2014;128:279–89 10.1007/s00401-013-1228-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Korshunov A, Remke M, Gessi M, et al. . Focal genomic amplification at 19q13.42 comprises a powerful diagnostic marker for embryonal tumors with ependymoblastic rosettes. Acta Neuropathol (Berl) 2010;120:253–60 10.1007/s00401-010-0688-8 [DOI] [PubMed] [Google Scholar]
- 4. Spence T, Sin-Chan P, Picard D, et al. . CNS-PNETs with C19MC amplification and/or LIN28 expression comprise a distinct histogenetic diagnostic and therapeutic entity. Acta Neuropathol (Berl) 2014;128:291–303 10.1007/s00401-014-1291-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ryzhova MV, Zheludkova OG, Ozerov SS, et al. . A new entity in WHO classification of tumors of the central nervous system–embryonic tumor with abundant neuropil and true rosettes: case report and review of literature. Zh Vopr Neirokhir Im N N Burdenko 2011;75:25–33 [PubMed] [Google Scholar]
- 6. Woehrer A, Slavc I, Peyrl A, et al. . Embryonal tumor with abundant neuropil and true rosettes (ETANTR) with loss of morphological but retained genetic key features during progression. Acta Neuropathol (Berl) 2011;122:787–90 10.1007/s00401-011-0903-2 [DOI] [PubMed] [Google Scholar]
- 7. Kleinschmidt-DeMasters BK, Boylan A, Capocelli K, et al. . Multinodular leptomeningeal metastases from ETANTR contain both small blue cell and maturing neuropil elements. Acta Neuropathol (Berl) 2011;122:783–85 10.1007/s00401-011-0894-z [DOI] [PubMed] [Google Scholar]
- 8. Nobusawa S, Orimo K, Horiguchi K, et al. . Embryonal tumor with abundant neuropil and true rosettes with only one structure suggestive of an ependymoblastic rosette. Pathol Int 2014;64:472–77 10.1111/pin.12196 [DOI] [PubMed] [Google Scholar]
- 9. Hervey-Jumper SL, Altshuler DB, Wang AC, et al. . The role of CD133+ cells in a recurrent embryonal tumor with abundant neuropil and true rosettes (ETANTR). Brain Pathol 2014;24:45–51 10.1111/bpa.12079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gessi M, Demir H, Goschzik T, et al. . MET T992I mutation in a case of ependymoblastoma/embryonal tumour with multilayered rosettes. J Clin Pathol 2014;67:1017–18 10.1136/jclinpath-2014-202563 [DOI] [PubMed] [Google Scholar]
- 11. Antonelli M, Korshunov A, Mastronuzzi A, et al. . Long-term survival in a case of ETANTR with histological features of neuronal maturation after therapy. Virchows Arch 2015;466:603–07 10.1007/s00428-015-1736-5 [DOI] [PubMed] [Google Scholar]
- 12. Sato H, Terakawa Y, Tsuyuguchi N, et al. . Embryonal tumor with abundant neuropil and true rosettes in the brainstem: case report. J Neurosurg Pediatr 2015;16:291–95 10.3171/2015.3.PEDS14727 [DOI] [PubMed] [Google Scholar]
- 13. Horwitz M, Dufour C, Leblond P, et al. . Embryonal tumors with multilayered rosettes in children: the SFCE experience. Childs Nerv Syst 2016;32:299–305 10.1007/s00381-015-2920-2 [DOI] [PubMed] [Google Scholar]
- 14. Tariq MU, Ahmad Z, Minhas MK, et al. . Embryonal tumor with multilayered rosettes, C19MC-altered: report of an extremely rare malignant pediatric central nervous system neoplasm. SAGE Open Med Case Rep 2017;5:2050313X17745208 10.1177/2050313X17745208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang B, Gogia B, Fuller GN, et al. . Embryonal tumor with multilayered rosettes, C19MC-altered: clinical, pathological, and neuroimaging findings. J Neuroimaging 2018;28:483–89 10.1111/jon.12524 [DOI] [PubMed] [Google Scholar]
- 16. Nowak J, Seidel C, Berg F, et al. . MRI characteristics of ependymoblastoma: results from 22 centrally reviewed cases. AJNR Am J Neuroradiol 2014;35:1996–2001 10.3174/ajnr.A4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nowak J, Seidel C, Pietsch T, et al. . Ependymoblastoma of the brainstem: MRI findings and differential diagnosis. Pediatr Blood Cancer 2014;61:1132–34 10.1002/pbc.24915 [DOI] [PubMed] [Google Scholar]
- 18. Nowak J, Seidel C, Pietsch T, et al. . Systematic comparison of MRI findings in pediatric ependymoblastoma with ependymoma and CNS primitive neuroectodermal tumor not otherwise specified. Neuro Oncol 2015;17:1157–65 10.1093/neuonc/nov063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maiti TK, Arimappamagan A, Mahadevan A, et al. . Rare pathologies in the posterior third ventricular region in children: case series and review. Pediatr Neurosurg 2015;50:42–47 10.1159/000369033 [DOI] [PubMed] [Google Scholar]
- 20. Al-Hussaini M, Abuirmeileh N, Swaidan M, et al. . Embryonal tumor with abundant neuropil and true rosettes: a report of three cases of a rare tumor, with an unusual case showing rhabdomyoblastic and melanocytic differentiation. Neuropathology 2011;31:620–25 10.1111/j.1440-1789.2011.01213.x [DOI] [PubMed] [Google Scholar]
- 21. Ferri Ñiguez B, Martínez-Lage JF, Almagro M-J, et al. . Embryonal tumor with abundant neuropil and true rosettes (ETANTR): a new distinctive variety of pediatric PNET—a case-based update. Childs Nerv Syst 2010;26:1003–08 10.1007/s00381-010-1179-x [DOI] [PubMed] [Google Scholar]
- 22. Dangouloff-Ros V, Deroulers C, Foissac F, et al. . Arterial spin labeling to predict brain tumor grading in children: correlations between histopathologic vascular density and perfusion MR imaging. Radiology 2016;281:553–66 10.1148/radiol.2016152228 [DOI] [PubMed] [Google Scholar]
- 23. Dunham C, Sugo E, Tobias V, et al. . Embryonal tumor with abundant neuropil and true rosettes (ETANTR): report of a case with prominent neurocytic differentiation. J Neurooncol 2007;84:91–98 10.1007/s11060-007-9346-y [DOI] [PubMed] [Google Scholar]
- 24. Manjila S, Ray A, Hu Y, et al. . Embryonal tumors with abundant neuropil and true rosettes: 2 illustrative cases and a review of the literature. Neurosurg Focus 2011;30:E2 10.3171/2010.10.FOCUS10226 [DOI] [PubMed] [Google Scholar]
- 25. Adamek D, Sofowora KD, Cwiklinska M, et al. . Embryonal tumor with abundant neuropil and true rosettes: an autopsy case-based update and review of the literature. Childs Nerv Syst 2013;29:849–54 10.1007/s00381-013-2037-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Govindan A, Vp M, Alapatt JP. Embryonal tumor with multilayered rosettes in a 3-year-old girl: case report. Turk Neurosurg 2017. January 8. [Epub ahead of print] 10.5137/1019-5149.JTN.19621-16.0 [DOI] [PubMed] [Google Scholar]
- 27. Shih RY, Koeller KK. Embryonal tumors of the central nervous system: from the radiologic pathology archives. Radiographics 2018;38:525–41 10.1148/rg.2018170182 [DOI] [PubMed] [Google Scholar]
- 28. Yeom KW, Mitchell LA, Lober RM, et al. . Arterial spin-labeled perfusion of pediatric brain tumors. AJNR Am J Neuroradiol 2014;35:395–401 10.3174/ajnr.A3670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kikuchi K, Hiwatashi A, Togao O, et al. . Correlation between arterial spin-labeling perfusion and histopathological vascular density of pediatric intracranial tumors. J Neurooncol 2017;135:561–69 10.1007/s11060-017-2604-8 [DOI] [PubMed] [Google Scholar]
- 30. Morana G, Piccardo A, Tortora D, et al. . Grading and outcome prediction of pediatric diffuse astrocytic tumors with diffusion and arterial spin labeling perfusion MRI in comparison with 18F-DOPA PET. Eur J Nucl Med Mol Imaging 2017;44:2084–93 10.1007/s00259-017-3777-2 [DOI] [PubMed] [Google Scholar]
- 31. Morana G, Tortora D, Staglianò S, et al. . Pediatric astrocytic tumor grading: comparison between arterial spin labeling and dynamic susceptibility contrast MRI perfusion. Neuroradiology 2018;60:437–46 10.1007/s00234-018-1992-6 [DOI] [PubMed] [Google Scholar]
- 32. Vidyasagar R, Abernethy L, Pizer B, et al. . Quantitative measurement of blood flow in paediatric brain tumours: a comparative study of dynamic susceptibility contrast and multi time-point arterial spin labelled MRI. Br J Radiol 2016;89:20150624 10.1259/bjr.20150624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Plaza MJ, Borja MJ, Altman N, et al. . Conventional and advanced MRI features of pediatric intracranial tumors: posterior fossa and suprasellar tumors. Am J Roentgenol 2013;200:1115–24 10.2214/AJR.12.9725 [DOI] [PubMed] [Google Scholar]
- 34. Dangouloff-Ros V, Varlet P, Levy R, et al. . Imaging features of medulloblastoma: conventional imaging, diffusion-weighted imaging, perfusion-weighted imaging, and spectroscopy—from general features to subtypes and characteristics. Neurochirurgie 2018. August 28. [Epub ahead of print] 10.1016/j.neuchi.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 35. Au Yong KJ, Jaremko JL, Jans L, et al. . How specific is the MRI appearance of supratentorial atypical teratoid rhabdoid tumors? Pediatr Radiol 2013;43:347–54 10.1007/s00247-012-2530-z [DOI] [PubMed] [Google Scholar]