Abstract
BACKGROUND AND PURPOSE:
Periventricular white matter injury is the common cause of spastic cerebral palsy. However, the early diagnosis of spastic cerebral palsy still remains a challenge. Our aim was to investigate whether infants with periventricular white matter injury with bilateral spastic cerebral palsy have unique lesions different from those in infants without cerebral palsy and to evaluate the efficiency of DTI in the early diagnosis of spastic cerebral palsy.
MATERIALS AND METHODS:
Infants with periventricular white matter injury and controls underwent MR imaging at 6–18 months of age. Fractional anisotropy was calculated from DTI. Cerebral palsy was diagnosed by 24–30 months of age. Subjects were divided into 3 groups: infants with periventricular white matter injury with bilateral spastic cerebral palsy, infants with periventricular white matter injury without cerebral palsy, and controls. Tract-Based Spatial Statistics and Automated Fiber Quantification were used to investigate intergroup differences. Receiver operating characteristic curves were used to assess the diagnostic accuracy of spastic cerebral palsy. Correlations between motor function scores and fractional anisotropy were evaluated along white matter tracts.
RESULTS:
There were 20, 19, and 33 subjects in periventricular white matter injury with spastic cerebral palsy, periventricular white matter injury without cerebral palsy, and control groups, respectively. Decreased fractional anisotropy in the corticospinal tract was only observed in infants with periventricular white matter injury with spastic cerebral palsy, whereas decreased fractional anisotropy in the posterior thalamic radiation and genu and splenium of the corpus callosum was seen in both periventricular white matter injury subgroups. Fractional anisotropy in the corticospinal tract at the internal capsule level was effective in differentiating infants with periventricular white matter injury with spastic cerebral palsy from those without cerebral palsy by a threshold of 0.53, and it had strong correlations with motor function scores.
CONCLUSIONS:
Corticospinal tract lesions play a crucial role in motor impairment related to spastic cerebral palsy in infants with periventricular white matter injury. Fractional anisotropy in the corticospinal tract at the internal capsule level could aid in the early diagnosis of spastic cerebral palsy with high diagnostic accuracy.
Periventricular white matter injury (PWMI) is a major form of white matter injury in both preterm and term infants and is the common cause of spastic cerebral palsy (SCP).1–3 MR imaging is effective in identifying PWMI and demonstrates periventricular white matter signal abnormality and/or volume loss, enlargement of the lateral ventricles, and thinning of the corpus callosum.3 On the basis of signs on MR imaging, PWMI can be classified into mild, moderate, and severe grades.4 Severe PWMI develops frequently into cerebral palsy (CP).4,5 However, the early diagnosis of SCP in infants with mild and moderate PWMI still remains a major challenge.
MR imaging and neurologic examinations are predictive tools for detecting the risk of CP.6–8 Compared with neurologic examinations, MR imaging is preferred for revealing the anatomic position and severity of brain lesions.6 However, conventional MR imaging has limitations in delineating white matter tracts precisely and has failed to differentiate individual tracts specifically.9 DTI is effective in overcoming the above limitations and improving the diagnostic accuracy.6 Patients with SCP typically present with motor deficits. DTI studies have revealed that the damage in the corticospinal tract (CST) is related to motor impairment.10–12 Meanwhile, the sensory pathways or commissural tract lesions or both are also involved in the motor deficits in children with SCP.13–15 However, the prerequisite white matter lesions accountable for SCP remain unclear.16 The exploration of the differences in white matter alterations between infants with PWMI with and without CP may provide clues for the identification of the responsible white matter tracts related to motor impairment.
In this study, the analyses of DTI data with Tract-Based Spatial Statistics (TBSS; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS) and Automated Fiber Quantification (AFQ; https://pypi.org/project/AFQ-Browser/) were used to compare the differences among groups of infants with PWMI with bilateral SCP, infants with PWMI without CP, and controls. The aim was to investigate whether infants with PWMI with bilateral SCP have unique lesions different from lesions in those without CP and to evaluate the early diagnostic efficiency of DTI for differentiating infants with SCP from those with PWMI without CP.
Materials and Methods
This was a retrospective cohort study approved by the institutional review board of the First Affiliated Hospital of Xi'an Jiaotong University.
Participants
Parents of the infants were informed of the potential risks of MR imaging. Written consent was obtained before the brain MR imaging examination.
Brain MR imaging was performed in infants 6–18 months of age, from July 2011 to January 2014. The reasons for MR imaging examination included suspected developmental delay, seizures, or other risks of cerebral disorders. The MR imaging scanner and sequences did not change throughout the study period. Next, these infants underwent follow-up neurologic examinations between 24 and 30 months of age, performed by a pediatric neurologist using several measurements, including motor milestones, primitive reflexes, postural reactions, muscle strength, and cognitive and psychomotor development assessment using the second edition of the Bayley Scales of Infant Development and the Gross Motor Function Classification System (GMFCS; https://research.cerebralpalsy.org.au/what-is-cerebral-palsy/severity-of-cerebral-palsy/gross-motor-function-classification-system/) assessment. Developmental delay was defined by a score of the Mental or Psychomotor Development Index of <85 according to the Bayley Scales of Infant Development assessment.
CP was diagnosed in infants on the basis of the follow-up examination results using the definition provided by the International Executive Committee in the United States.17 The infants with CP were classified into GMFCS levels I–V, representing mild-to-severe motor dysfunction.10 The GMFCS level zero represented infants without motor dysfunction in this study.
The inclusion criteria of infants with PWMI were as follows: 1) MR imaging performed between 6 and 18 months of age and 2) PWMI diagnosed by MR imaging. The severity of PWMI was graded as mild, moderate, or severe on the basis of the MR imaging signs as follows: 1) mild, abnormally high signal in the periventricular white matter on T2-weighted images with mild white matter reduction limited to the peritrigonal region; 2) moderate, abnormally high white matter signal on T2-weighted images, with moderate periventricular white matter decrease and irregular enlargement of the ventricles; 3) severe, large, or extensive cystic changes of periventricular white matter, with marked reduction of white matter and severe irregular enlargement of the ventricles (On-line Fig 1).4 The exclusion criteria included severe PWMI, intrauterine congenital infection, and unsuccessful follow-up neurologic examinations. According to the follow-up outcomes, infants with mild and moderate PWMI were divided into those with PWMI with bilateral SCP and those without CP after the exclusion of unilateral and nonspastic CP.
The infants with MR imaging performed between 6 and 18 months of age were selected as controls after excluding the following conditions: 1) abnormalities on MR imaging, including PWMI; dilated perivascular spaces; arachnoid cyst; hydrocephalus; congenital malformations; hematencephalon; subarachnoid space enlargement; encephalomalacia and so forth; 2) history of nervous system infection; preterm birth; hypoxic-ischemic encephalopathy and congenital heart disease; 3) seizures; developmental delay; and hypertonia; and 4) lack of follow-up neurologic examinations.
MR Imaging Protocols
MR imaging was performed on a 3T scanner (Signa HDxt; GE Healthcare, Milwaukee, Wisconsin). All infants were required to sleep soundly and wear sponge earplugs for hearing protection. The infants who could not remain still were sedated with 10% chloral hydrate (25–50 mg/kg) to reduce motion artifacts. The potential risks of chloral hydrate were fully explained to the parents. The patient selection, monitoring, and management were performed in strict compliance with the guidelines for monitoring and management of pediatric patients before, during, and after sedation for diagnostic and therapeutic procedures.18 Heart rate, transcutaneous oxygen saturation, and respiration rate were monitored throughout the procedure. All infants underwent the following MR imaging protocols: 3D T1-weighted imaging (TR = 10.2 ms, TE = 4.6 ms, slice thickness = 1 mm), T2-weighted imaging (TR = 6500 ms, TE = 124 ms, slice thickness = 4 mm), T2 FLAIR imaging (TR = 9600 ms, TE = 110 ms, TI = 2400 ms, slice thickness = 4 mm), and DTI. DTI was performed using a spin-echo echo-planar imaging sequence. The parameters for DTI were as follows: TR = 5500 ms, TE = 95 ms, flip angle = 90°, slice thickness = 4 mm without gap, FOV = 180 × 180 mm2, matrix = 128 × 128, b-values = 0, 1000 s/mm2 with 35 gradient directions.
Image Postprocessing and Data Analysis
Fractional anisotropy (FA) maps were obtained after brain extraction and eddy current correction using the FMRIB Software Library (FSL; http://www.fmrib.ox.ac.uk/fsl).19 Linear and nonlinear image registrations were used for alignment of the FA maps of all subjects to a selected FA map. An averaged image of the coregistered FA maps was created as the target map. Then, FA maps of all subjects were registered to the target map. A mean FA map and mean FA skeleton were created. The aligned FA map of each subject was projected onto the mean FA skeleton (threshold = 0.2). Voxelwise statistical analysis was performed to assess the intergroup differences in FA.
AFQ software was used to quantitatively analyze white matter tracts.20 Automatic fiber tract segmentation and cleaning were performed first. Then, FA values along the tracts were calculated by clipping each fiber to 100 equally spaced nodes. The CST, posterior thalamic radiation (PTR), genu of the corpus callosum (GCC), and splenium of corpus callosum (SCC) were evaluated. To characterize the properties of the CST in detail, we divided the CST into 3 parts according to the brain atlas21: CST at the cerebral peduncle level (CST-CP; nodes: 1–37), CST at the internal capsule level (CST-IC; nodes: 38–81), and CST at the corona radiata level (CST-CR; nodes: 82–100).
Statistical Analysis
Categoric variables were analyzed using the χ2 test in SPSS software (Version 17.0; IBM, Armonk, New York). Continuous variables were analyzed across groups using analysis of variance with the least significant difference test. Sex ratios were compared across tracts by using the χ2 test. Interrater reliability analyses for GMFCS were performed with the κ test. The variables of gestational age, birth weight, and age at MR imaging were adjusted during the intergroup comparisons and correlation analyses to remove their potential effects on the variations in DTI metrics. Intergroup comparisons in FA were performed by TBSS with the threshold-free cluster enhancement and family-wise error correction. In the AFQ analysis, a Mann-Whitney U test with a Bonferroni correction was used to assess the intergroup differences in FA. Receiver operating characteristic curves were used to assess the diagnostic performances of FA in different regions for differentiating infants with PWMI with bilateral SCP from those without CP. Correlations between FA values of the white matter tracts and motor function scores (GMFCS levels and Psychomotor Development Index) in infants with PWMI were analyzed by partial correlation analysis. P values < .05 were considered statistically significant for all tests.
Results
Demographic and Clinical Information
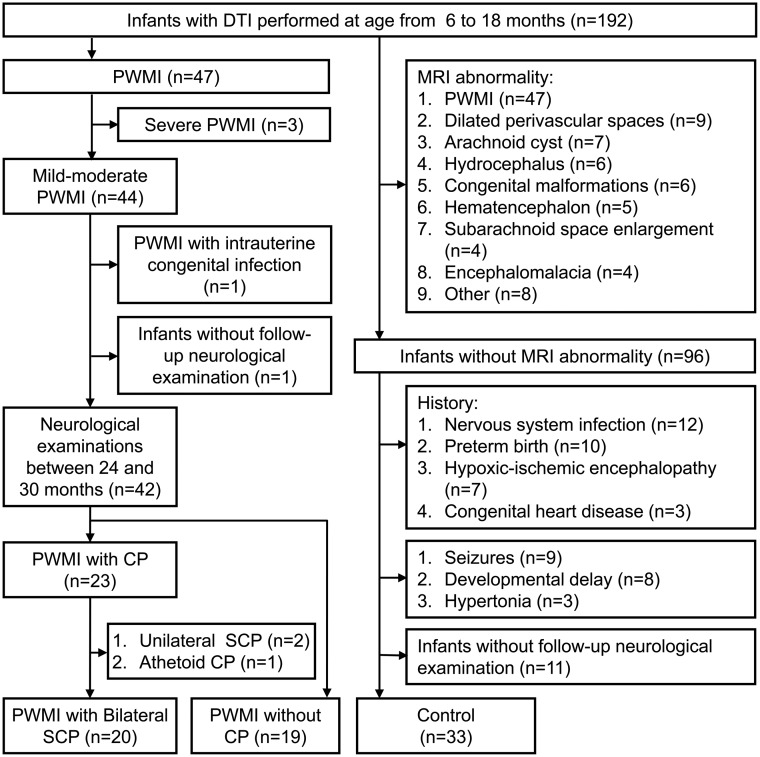
Thirty-nine infants with PWMI and 33 controls were finally enrolled on the basis of the inclusion and exclusion criteria (Fig 1). According to the follow-up diagnosis, the infants with PWMI were divided into infants with bilateral SCP (n = 20) and those without CP (n = 19) groups. The time between the MR imaging examination and diagnosis of SCP was 14.8 ± 3.2 months.
Fig 1.
Flow chart of study participants.
Among infants with PWMI with bilateral SCP, 7 (35%), 6 (30%), 4 (20%), and 3 (15%) infants were classified into GMFCS levels I, II, III, and IV, respectively. The κ value for the interrater reliability of the GMFCS assessment was 0.83. There was no significant difference in gestational age and birth weight between infants with PWMI with bilateral SCP and those without CP. The gestational age and birth weight in infants with PWMI with bilateral SCP and without CP were less than those in controls. Moreover, there was no significant difference across groups in the sex constituent ratio or age at MR imaging (Table 1). More details about the perinatal characteristics of the infants with PWMI are listed in On-line Table 1.
Table 1:
Demographics of infants with PWMI with bilateral spastic cerebral palsy, infants with PWMI without cerebral palsy, and controlsa
| Infants with PWMI with SCP (n = 20) | Infants with PWMI without CP (n = 19) | Controls (n = 33) |
P Values |
|||
|---|---|---|---|---|---|---|
| PWMI with SCP vs PWMI without CP | PWMI with SCP vs Control | PWMI without CP vs Control | ||||
| Sex (female/male) | 8:12 | 6:13 | 11:22 | .58 | .62 | .90 |
| Gestational age (wk) | 37.38 ± 1.90 (34.57–41.29) | 38.07 ± 2.45 (34.00–41.43) | 39.16 ± 1.14 (37.00–41.00) | .23 | <.01 | .04 |
| Age at MRI (mo) | 11.74 ± 2.13 (8.00–17.66) | 11.56 ± 2.99 (6.40–16.76) | 11.06 ± 2.38 (6.10–17.33) | .83 | .34 | .49 |
| Birth weight (kg) | 2.95 ± 0.47 (2.20–3.70) | 3.06 ± 0.60 (1.80–3.85) | 3.38 ± 0.50 (2.50–4.38) | .50 | .01 | .04 |
Data in columns 2–4 are mean and standard deviation (range).
TBSS Analysis of Intergroup Differences
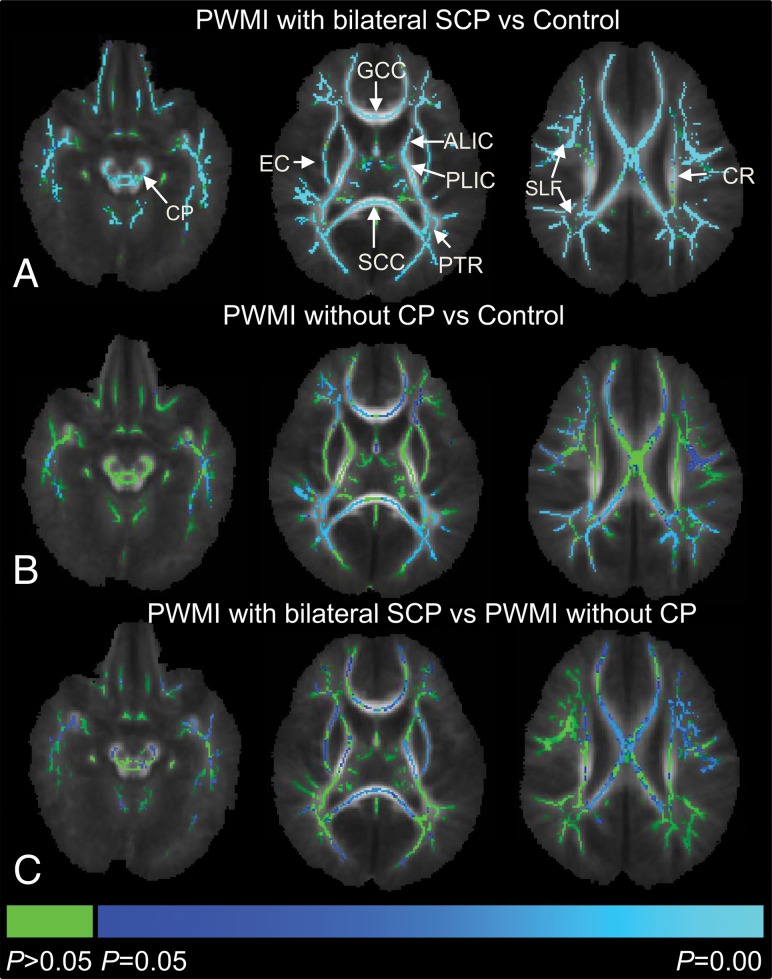
FA in widespread areas of white matter was lower in the infants with PWMI with bilateral SCP than that in the controls. In infants with PWMI without CP, decreased FA was found mainly in regions adjacent to the anterior horns, trigone, and posterior horns of the lateral ventricles. Infants with PWMI with bilateral SCP showed significantly lower FA values than infants with PWMI without CP in the bilateral CST-CP, CST-IC, CST-CR, external capsule, GCC, and SCC (Fig 2).
Fig 2.
Intergroup comparisons of fractional anisotropy values using TBSS. Blue and light blue regions show major white matter tracts with significantly decreased FA (P < .05) in infants with PWMI with bilateral SCP (A), infants with PWMI without CP (B) relative to controls, and infants with PWMI with bilateral SCP relative to those without CP (C). Green represents regions without significant differences (P > .05). ALIC indicates anterior limb of the internal capsule; CR, corona radiata; EC, external capsule; PLIC, posterior limb of the internal capsule; SLF, superior longitudinal fasciculus.
AFQ Analysis of Intergroup Differences along Tracts
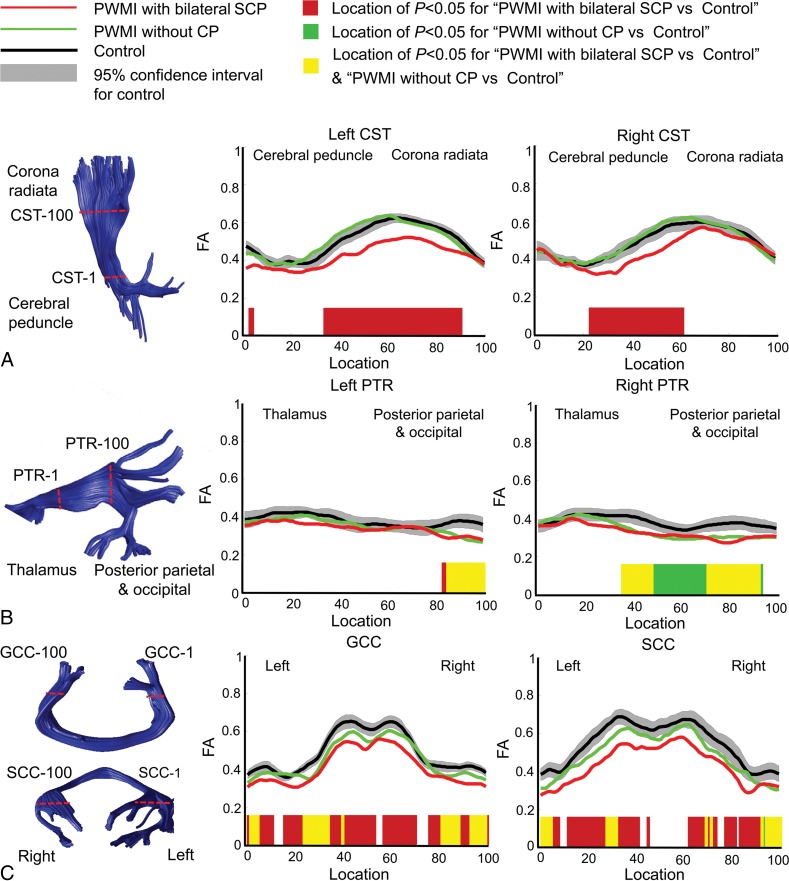
The tract profiles of infants with PWMI with bilateral SCP, infants with PWMI without CP, and controls are presented in Fig 3. Obvious deceases in FA values in infants with PWMI with bilateral SCP were found along the CST, mainly located at the internal capsule level, while these changes in FA were not found in infants with PWMI without CP. The PTR, GCC, and SCC showed significantly decreased FA values in both groups.
Fig 3.
Intergroup comparisons of fractional anisotropy among infants with PWMI with bilateral SCP, infants with PWMI without CP, and controls along tracts of the bilateral corticospinal tract (A), bilateral posterior thalamic radiation (B), and genu of the corpus callosum and splenium of the corpus callosum (C).
The receiver operating characteristic curves for the diagnostic performances of FA in different regions for differentiation of infants with PWMI with bilateral SCP from those without CP are depicted in On-line Fig 2. The area under the curve was larger in the CST than that in the PTR, GCC, or SCC (Table 2). Among the 3 parts of the CST, an FA threshold of 0.53 at the internal capsule level had the highest area under the curve value, which demonstrated high specificity and sensitivity.
Table 2:
Diagnostic performance of fractional anisotropy in different regions for differentiation of infants with PWMI with bilateral spastic cerebral palsy and those without cerebral palsy
| PWMI with SCP (Mean and standard deviation) | PWMI without CP (Mean and standard deviation) | Area under the Curve (95% CI) | Threshold | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| CST-L | 0.42 ± 0.06 | 0.50 ± 0.04 | 0.90 (0.75–0.98) | 0.48 | 94% | 72% |
| CST-R | 0.43 ± 0.06 | 0.50 ± 0.04 | 0.85 (0.68–0.95) | 0.49 | 93% | 67% |
| PTR-L | 0.34 ± 0.06 | 0.35 ± 0.05 | 0.55 (0.37–0.73) | 0.27 | 27% | 100% |
| PTR-R | 0.33 ± 0.07 | 0.34 ± 0.05 | 0.54 (0.35–0.72) | 0.36 | 67% | 50% |
| GCC | 0.41 ± 0.05 | 0.45 ± 0.05 | 0.72 (0.59–0.82) | 0.46 | 87% | 50% |
| SCC | 0.43 ± 0.11 | 0.49 ± 0.09 | 0.62 (0.49–0.74) | 0.40 | 40% | 88% |
| CST-CP-L | 0.36 ± 0.08 | 0.42 ± 0.04 | 0.74 (0.57–0.88) | 0.40 | 69% | 83% |
| CST-CP-R | 0.36 ± 0.06 | 0.42 ± 0.06 | 0.77 (0.60–0.90) | 0.40 | 73% | 72% |
| CST-IC-L | 0.48 ± 0.06 | 0.59 ± 0.05 | 0.95 (0.82–0.99) | 0.53 | 94% | 89% |
| CST-IC-R | 0.48 ± 0.07 | 0.58 ± 0.05 | 0.88 (0.72–0.97) | 0.53 | 93% | 78% |
| CST-CR-L | 0.43 ± 0.09 | 0.46 ± 0.09 | 0.60 (0.42–0.77) | 0.49 | 81% | 50% |
| CST-CR-R | 0.47 ± 0.10 | 0.48 ± 0.11 | 0.52 (0.34–0.70) | 0.59 | 100% | 17% |
Note:—L indicates left; R, right.
Correlation between Motor Function Scores and FA
The correlations between motor function scores and FA values along white matter tracts are presented in On-line Table 2. There were more significant correlations along the CST than along the PTR, GCC, and SCC (P < .05); and GMFCS levels were negatively correlated with FA values within the CST at the level of the internal capsule (left r = −0.80; right r = −0.79). The Psychomotor Development Index scores showed a positive correlation with FA values within the CST at the level of internal capsule (left r = 0.69; right r = 0.53).
Discussion
This study found that the spatial distribution of white matter alterations was different between the 2 PWMI groups. Structural integrity was more severely damaged in more widespread white matter areas in infants with PWMI with bilateral SCP than in those without CP. Furthermore, injured CST was found only in infants with PWMI with bilateral SCP. The FA threshold of 0.53 in the CST at the internal capsule level was useful for the differentiation of infants with PWMI with SCP from those without CP, and it had significant correlations with motor functions.
DTI with TBSS analysis is a powerful approach that effectively reveals alterations in the main white matter tracts of the whole brain. Several studies have reported the presence of FA reduction within widespread white matter areas, especially in the central and posterior white matter, in infants with PWMI with SCP.10,12,22 However, knowledge of the spatial distribution characteristics of white matter lesions in infants with PWMI with and without CP is limited. In this study, the infants with PWMI with SCP had lower FA and more widespread distribution than those without CP. Additionally, the age distribution of patients here was different from that in previous studies.10,12,15,22 We focused on 6- to 18-month-old infants with PWMI, which is the period before the definite diagnosis. Identification of the differences between infants with PWMI with SCP and those without CP during this time interval is beneficial for determining candidates at risk of SCP.
In this study, the white matter tracts potentially involved in SCP were further analyzed using the AFQ. Previously, Hoon et al13 had suggested that PTR injury altered the sensorimotor connections to the motor cortex and attenuated descending CST. However, our study showed that the PTR, SCC, and GCC were the injured regions in both PWMI subgroups, whereas decreased FA in CST was observed only in infants with PWMI with SCP. These results indicate that PTR injury may not be directly responsible for SCP. The CST, as the major projectional motor tract situated in close proximity to the periventricular white matter region, is vulnerable in patients with PWMI.23 CST lesions can interrupt the corticomotor circuit in executing movement and have also been shown to be involved in spasticity.24 These findings suggest that CST lesions are a prerequisite for bilateral SCP in infants with PWMI and provide clues for determining whether patients with PWMI will develop SCP.
Previous research has shown that an FA threshold of 0.5 within the CST was effective in differentiating CP and non-CP groups.25 Similarly, this study demonstrated that the FA thresholds in CST for the diagnosis of SCP were 0.48 (left) and 0.49 (right). Additionally, the current study revealed that the FA thresholds in both the left and right CST at the internal capsule level were 0.53, with high sensitivity and specificity. These were higher than results in previous MR imaging studies for detecting the risk of CP.8,26 The results obtained in this study suggest that CST at the internal capsule level is more suitable than the whole tract for the early diagnosis of SCP. Furthermore, the multiple regression results revealed that FA values of infants with PWMI were not correlated to age (On-line Table 3). This finding suggests that the FA threshold is applicable to the individual infants with PWMI between 6 and 18 months of age.
Apart from the determination of the risk of CP, the evaluation of the degree of motor impairment in CP can further facilitate the targeting of interventional strategies.27,28 Consistent with previous findings,23,24 FA in the CST was significantly correlated with motor function scores. A strong correlation was found along the CST at the internal capsule level, which may be associated with the vulnerability of white matter in the internal capsule, where the descending motor axons are densely concentrated.29,30 Moreover, there were more significant correlations along the CST than along the PTR, GCC, and SCC. It has been suggested that PTR lesions are responsible for the weakness in motor function in patients with CP because of the role of PTR in visuospatial performance.24 The corpus callosum may play an important role in dexterity and bimanual motor coordination.31 Overall, the results suggest that FA of the CST at the internal capsule level is suitable for assessing the motor function in infants with PWMI with SCP.
Nevertheless, this study has several limitations. First, it excluded severe PWMI because these cases accounted for a minority (3 subjects) of all the infants with PWMI. Moreover, it was relatively easy to determine the risk of CP in these infants. Second, unilateral SCP and nonspastic CP were not included due to the small sample sizes (2 with unilateral SCP and 1 with athetoid CP) and divergent MR imaging signs, such as asymmetric brain lesions in unilateral SCP and concurrent basal ganglia lesions in athetoid CP. Third, the FA threshold in the internal capsule of the CST was objectively extracted from an Automated Fiber Quantification. This threshold may be different from those obtained by other analysis methods, such as the ROI analysis. Fourth, this work tried to investigate the characteristics of DTI metric changes associated with SCP before the definite diagnosis. However, DTI was performed just several months ahead of the diagnosis of SCP. Whether SCP can be predicted by DTI in earlier periods needs further research. Finally, this was a retrospective study. Prospective research is required to test the accuracy of the early diagnosis of SCP.
Conclusions
CST lesions play a crucial role in motor impairment associated with SCP in infants with PWMI. FA in the CST at the internal capsule level could aid in the early diagnosis of SCP with high diagnostic accuracy.
Acknowledgments
The authors are grateful to Drs Liming Liu and Jie Yue in the Department of Child Health Care for the diagnosis of CP.
ABBREVIATIONS:
- CP
cerebral palsy
- CST
corticospinal tract
- CST-CP
CST at the cerebral peduncle level
- CST-CR
CST at the corona radiata level
- CST-IC
CST at the internal capsule level
- FA
fractional anisotropy
- GCC
genu of the corpus callosum
- GMFCS
Gross Motor Function Classification System
- PTR
posterior thalamic radiation
- PWMI
periventricular white matter injury
- SCC
splenium of the corpus callosum
- SCP
spastic cerebral palsy
Footnotes
This study was supported by the National Key Research and Development Program of China (2016YFC0100300); the National Natural Science Foundation of China (81471631, 81771810 and 81171317); the 2011 New Century Excellent Talent Support Plan of the Ministry of Education, China (NCET-11-0438); the Fundamental Research Funds for the Central Universities (xjj2018265); and the Fundamental Research Funds of the First Affiliated Hospital of Xi'an Jiaotong University (2017QN-09).
Preliminary data from this research were previously presented at: Annual Meeting of the International Society for Magnetic Resonance in Medicine, April 22–27, 2017; Honolulu, Hawaii.
References
- 1. Bax M, Tydeman C, Flodmark O. Clinical and MRI correlates of cerebral palsy: the European Cerebral Palsy Study. JAMA 2006;296:1602–08 10.1001/jama.296.13.1602 [DOI] [PubMed] [Google Scholar]
- 2. Back SA, Rivkees SA. Emerging concepts in periventricular white matter injury. Semin Perinatol 2004;28:405–14 10.1053/j.semperi.2004.10.010 [DOI] [PubMed] [Google Scholar]
- 3. Jauhari P, Singhi P, Sankhyan N, et al. . A comparison of spastic diplegia in term and preterm-born children. J Child Neurol 2018;33:333–39 10.1177/0883073817754175 [DOI] [PubMed] [Google Scholar]
- 4. Imamura T, Ariga H, Kaneko M, et al. . Neurodevelopmental outcomes of children with periventricular leukomalacia. Pediatr Neonatol 2013;54:367–72 10.1016/j.pedneo.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 5. Woodward LJ, Anderson PJ, Austin NC, et al. . Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 2006;355:685–94 10.1056/NEJMoa053792 [DOI] [PubMed] [Google Scholar]
- 6. Hadders-Algra M. Early diagnosis and early intervention in cerebral palsy. Front Neurol 2014;5:185 10.3389/fneur.2014.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morgan C, Crowle C, Goyen TA, et al. . Sensitivity and specificity of General Movements Assessment for diagnostic accuracy of detecting cerebral palsy early in an Australian context. J Paediatr Child Health 2016;52:54–59 10.1111/jpc.12995 [DOI] [PubMed] [Google Scholar]
- 8. Novak I, Morgan C, Adde L, et al. . Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr 2017;171:897–907 10.1001/jamapediatrics.2017.1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Son SM, Park SH, Moon HK, et al. . Diffusion tensor tractography can predict hemiparesis in infants with high risk factors. Neurosci Lett 2009;451:94–97 10.1016/j.neulet.2008.12.033 [DOI] [PubMed] [Google Scholar]
- 10. Lee JD, Park HJ, Park ES, et al. . Motor pathway injury in patients with periventricular leucomalacia and spastic diplegia. Brain 2011;134:1199–210 10.1093/brain/awr021 [DOI] [PubMed] [Google Scholar]
- 11. Skranes J, Vangberg T, Kulseng S, et al. . Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain 2007;130:654–66 10.1093/brain/awm001 [DOI] [PubMed] [Google Scholar]
- 12. Ceschin R, Lee VK, Schmithorst V, et al. . Regional vulnerability of longitudinal cortical association connectivity: associated with structural network topology alterations in preterm children with cerebral palsy. Neuroimage Clin 2015;9:322–37 10.1016/j.nicl.2015.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoon AH Jr, Stashinko EE, Nagae LM, et al. . Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol 2009;51:697–704 10.1111/j.1469-8749.2009.03306.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trivedi R, Agarwal S, Shah V, et al. . Correlation of quantitative sensorimotor tractography with clinical grade of cerebral palsy. Neuroradiology 2010;52:759–65 10.1007/s00234-010-0703-8 [DOI] [PubMed] [Google Scholar]
- 15. Nagae LM, Hoon AH Jr, Stashinko EE, et al. . Diffusion tensor imaging in children with periventricular leukomalacia: variability of injuries to white matter tracts. AJNR Am J Neuroradiol 2007;28:1213–22 10.3174/ajnr.A0534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Colver A, Fairhurst C, Pharoah PO. Cerebral palsy. Lancet 2014;383:1240–49 10.1016/S0140-6736(13)61835-8 [DOI] [PubMed] [Google Scholar]
- 17. Rosenbaum P, Paneth N, Leviton A, et al. . A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol 2007;109:8–14 [PubMed] [Google Scholar]
- 18. Coté CJ, Wilson S; American Academy of Pediatrics; American Academy of Pediatric Dentistry. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: update 2016. Pediatrics 2016;138. pii: e20161212 10.1542/peds.2016-1212 [DOI] [PubMed] [Google Scholar]
- 19. Smith SM, Jenkinson M, Woolrich MW, et al. . Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23:S208–19 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- 20. Yeatman JD, Dougherty RF, Myall NJ, et al. . Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One 2012;7:e49790 10.1371/journal.pone.0049790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oishi K, Faria AV, Yoshida S, et al. . Quantitative evaluation of brain development using anatomical MRI and diffusion tensor imaging. Int J Dev Neurosci 2013;31:512–24 10.1016/j.ijdevneu.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arrigoni F, Peruzzo D, Gagliardi C, et al. . Whole-brain DTI assessment of white matter damage in children with bilateral cerebral palsy: evidence of involvement beyond the primary target of the anoxic insult. AJNR Am J Neuroradiol 2016;37:1347–53 10.3174/ajnr.A4717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rešić B, Tomasović M, Kuzmanić-Šamija R, et al. . Neurodevelopmental outcome in children with periventricular leukomalacia. Coll Antropol 008;32(Suppl 1):143–47 [PubMed] [Google Scholar]
- 24. Scheck SM, Boyd RN, Rose SE. New insights into the pathology of white matter tracts in cerebral palsy from diffusion magnetic resonance imaging: a systematic review. Dev Med Child Neurol 2012;54:684–96 10.1111/j.1469-8749.2012.04332.x [DOI] [PubMed] [Google Scholar]
- 25. Murakami A, Morimoto M, Yamada K, et al. . Fiber-tracking techniques can predict the degree of neurologic impairment for periventricular leukomalacia. Pediatrics 2008;122:500–06 10.1542/peds.2007-2816 [DOI] [PubMed] [Google Scholar]
- 26. Thayyil S, Chandrasekaran M, Taylor A, et al. . Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics 2010;125:e382–95 10.1542/peds.2009-1046 [DOI] [PubMed] [Google Scholar]
- 27. Kendall GS, Melbourne A, Johnson S, et al. . White matter NAA/Cho and Cho/Cr ratios at MR spectroscopy are predictive of motor outcome in preterm infants. Radiology 2014;271:230–38 10.1148/radiol.13122679 [DOI] [PubMed] [Google Scholar]
- 28. Melhem ER, Hoon AH Jr, Ferrucci JT Jr, et al. . Periventricular leukomalacia: relationship between lateral ventricular volume on brain MR images and severity of cognitive and motor impairment. Radiology 2000;214:199–204 10.1148/radiology.214.1.r00dc35199 [DOI] [PubMed] [Google Scholar]
- 29. Jaspers E, Byblow WD, Feys H, et al. . The corticospinal tract: a biomarker to categorize upper limb functional potential in unilateral cerebral palsy. Front Pediatr 2016;3:112 10.3389/fped.2015.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rose J, Mirmiran M, Butler EE, et al. . Neonatal microstructural development of the internal capsule on diffusion tensor imaging correlates with severity of gait and motor deficits. Dev Med Child Neurol 2007;49:745–50 10.1111/j.1469-8749.2007.00745.x [DOI] [PubMed] [Google Scholar]
- 31. Weinstein M, Green D, Geva R, et al. . Interhemispheric and intrahemispheric connectivity and manual skills in children with unilateral cerebral palsy. Brain Struct Funct 2014;219:1025–40 10.1007/s00429-013-0551-5 [DOI] [PubMed] [Google Scholar]