Abstract
Background
Point-of-care testing (POCT) assays for chlamydia are being developed. Their potential impact on the burden of chlamydial infection in the United States, in light of suboptimal screening coverage, remains unclear.
Methods
Using a transmission model calibrated to data in the United States, we estimated the impact of POCT on chlamydia prevalence, incidence, and chlamydia-attributable pelvic inflammatory disease (PID) incidence, assuming status quo (Analysis 1) and improved (Analysis 2) screening frequencies. We tested the robustness of results to changes in POCT sensitivity, the proportion of patients getting treated immediately, the baseline proportion lost to follow-up (LTFU), and the average treatment delay.
Results
In Analysis 1, high POCT sensitivity was needed to reduce the chlamydia-associated burden. With a POCT sensitivity of 90%, reductions from the baseline burden only occurred in scenarios in which over 60% of the screened individuals would get immediate treatment and the baseline LTFU proportion was 20%. With a POCT sensitivity of 99% (baseline LTFU 10%, 2-week treatment delay), if everyone were treated immediately, the prevalence reduction was estimated at 5.7% (95% credible interval [CrI] 3.9–8.2%). If only 30% of tested persons would wait for results, the prevalence reduction was only 1.6% (95% CrI 1.1–2.3). POCT with 99% sensitivity could avert up to 12 700 (95% CrI 5000–22 200) PID cases per year, if 100% were treated immediately (baseline LTFU 20% and 3-week treatment delay). In Analysis 2, when POCT was coupled with increasing screening coverage, reductions in the chlamydia burden could be realized with a POCT sensitivity of 90%.
Conclusions
POCT could improve chlamydia prevention efforts if test performance characteristics are significantly improved over currently available options.
Keywords: chlamydia, point-of-care, diagnostics, screening, mathematical model
Point-of-care testing (POCT) could improve chlamydia prevention efforts if there is considerable improvement in currently available POCT performance.
(See the Editorial Commentary by Van Der Pol on pages 1824–5.)
Point-of-care testing (POCT) refers to testing done at or near the site of the patient’s care, where the result leads to immediate diagnosis and treatment and, consequently, improvements in the patient’s care. The World Health Organization (WHO) considers the lack of POCT for sexually transmitted infections (STI) to be an obstacle for global STI prevention [1]. Previous analyses, using static models, indicated that POCT for chlamydia infections could reduce the costs and incidence of pelvic inflammatory disease (PID), compared to standard testing, in the United States [2] and in the United Kingdom [3]. The lifetime risk of chlamydia and associated reproductive health outcomes remain at high levels in the United States [4], yet the population-level impact of POCT, accounting for transmission dynamics of chlamydia and current levels of chlamydia prevention, has not been evaluated.
A common feature in candidate POCT assays has been lower sensitivity, compared to nucleic acid amplification tests (NAATs) [5]. In high-burden settings with limited access to screening, POCT with moderate sensitivity may yield significant benefits [6, 7]. In the United States, NAATs with high sensitivity is the standard in diagnosing chlamydial infection [8], and chlamydia screening has been widely implemented [9]. Reduced test sensitivity may increase the risk of PID, as suggested in a retrospective cohort study in which women with negative antigen-based results had 17% higher odds of being diagnosed with PID over the next year, compared to women with negative NAAT results [10]. However, POCT could bring additional benefits in settings where delays between diagnosis and treatment, as well as the loss to follow-up (LTFU) of diagnosed individuals prior to treatment, are common [11–15]. Despite national guidelines recommending annual chlamydia screening in sexually active women <25 years old, annual chlamydia screening was estimated as 47–58% among sexually active women enrolled in different healthcare plans in the United States in 2017 [16]. POCT, coupled with other interventions, could facilitate wider access to chlamydia testing.
Several candidate POCT assays are in development; as they are introduced to the market, their potential impacts need to be evaluated in order to guide POCT adoption and to verify that they are able to improve chlamydia prevention. Implementing novel technologies requires forecasting factors that create uncertainty in their potential benefits, such as the willingness of patients to wait for their results. The aim of this study was to estimate the population-level health benefits that may be attained through the use of a future point-of-care test. We accounted for 2 possible avenues of improvement, and potential uncertainties in these: (1) reduced LTFUs and treatment delays; and (2) expanded screening coverage, facilitated by POCT together with other novel interventions. Using a mathematical transmission model calibrated to data in the United States, we evaluated the impact of a point-of-care test on the chlamydia prevalence and incidence, and chlamydia-attributable PID cases, under a range of different scenarios, reflecting uncertainty in the POCT characteristics and the status quo care conditions.
METHODS
Factors we considered to influence POCT are presented in Table 1. The potential effects of POCT were quantified by comparing scenarios in which POCT would be adopted to the status quo, using estimates of current prevention efforts that were generated by the calibrated chlamydia transmission model.
Table 1.
Key Point-of-Care Testing Parameters Considered
| Parameter | Importance for POCT | Empirical Estimates | Incorporated in the Analysis |
|---|---|---|---|
| Test sensitivity | Screening generally requires high specificity. To ensure acceptability by providers, the test needs to have high sensitivity [35, 36], and false negative test results also have a direct impact on chlamydia transmission dynamics. | A number of candidate POC tests are being rolled out. For example, the Atlas diagnostic platform is considered for use in the United States with sensitivity and specificity of >90% (Atlas Genetics io system, Bath, United Kingdom) [24]. | We include hypothetical test sensitivities of 90%, 95%, and 99% in the analysis. The sensitivity of the baseline model (status quo) is 99%. |
| Test turnaround time | A test with delayed results may be more feasible to develop than a test with instant results. The WHO suggests a 20-minute turnaround time as a goal [1]. | - There is a licensed test with a 90-minute turnaround time (Gene Transfer [37, 38]). - Among women screened for chlamydia, willingness to wait for a result and treatment is dependent on the wait required, with 61% willing to wait 20 minutes and 26% willing to wait 40 minutes [24]. |
We examined the impact on LTFU and treatment delays if 100%, 60%, or 30% wait for their test result. This represents immediate test results, a 20-minute test turnaround time, and a 40-minute test turnaround time, respectively. |
| Test setting | Test setting may affect the maximum screening coverage achievable (if testing remains only at clinics, maximum coverage is likely lower than if POCT were available over the counter). | Patients find home testing acceptable overall, but in focus groups, doubts were raised over the user-friendliness of the tests [35]. | We examined the impact of increased screening coverage resulting from a hypothetical point-of-care test being rolled out widely. We assumed screening coverage was raised by 50% from the current levels. |
| Loss to follow-up | POCT could decrease LTFU if test results and treatment are available at the test visit. LTFU is likely to vary depending on the healthcare setting and available resources. | - Philadelphia (1994): 3.8% of women screened for chlamydia, who were not presumptively treated, were lost to follow-up. Disease Intervention Specialists were involved in 55% of follow-ups to ensure treatment [11]. - Alabama STD clinic (1994): 26% of men and 20% of women with a positive screening test had not been treated in the 30 days after the initial visit [12]. - California HMO (2001): among 14–19 years, LTFU 3% [13]. - Washington DC, San Diego, and Los Angeles STD clinics (2002): LTFU 8–18% [14]. |
We analyzed 5%, 10%, and 20% LTFUs. |
| Treatment delay | POCT could decrease the delay between testing and treatment, which may reduce onward transmission and PID incidences, due to the shorter duration of infection. | - Philadelphia (1994): median time to treatment was 21 days [11]. - Alabama STD clinic (2002): median time to treatment was 13 days (range 4–59) [39]. - Washington DC, San Diego, and Los Angeles STD clinics (2002): median time to treatment between 7 and 18 days [14]. - California HMO (2001): 14–19 years, median time to treatment 6 days and 95% receiving treatment within 20 days [13]. - Massachusetts case report review (2016): median delay of 3 days (95% treated within 12 days) [15]. |
We analyzed 1, 2, and 3 weeks of delays. |
Abbreviations: HMO, Health Maintenance Organization; LTFU, loss to follow-up; PID, pelvic inflammatory disease; POC, point-of-care; POCT, point-of-care testing; STD, sexually transmitted disease; WHO, World Health Organization.
Chlamydia Transmission Model
We used a deterministic pair formation model capturing chlamydia transmission in a heterosexual population [17]. The model stratified the population by age, sex, partnership status, sexually risky behavior, and chlamydia infection status. Age was divided into 4 categories: 15–18, 19–24, 25–39, and 40–54 years old. Natural history was represented using a susceptible-infected-susceptible structure, differentiating asymptomatic and symptomatic infections and the first infection from subsequent infections. Testing symptomatic people was varied by sex, screening asymptomatic people was varied by sex and age, and natural recovery was varied by sex. Partner notification was modeled explicitly in long-term partnerships. The model was calibrated to United States age- and sex-specific estimates of chlamydia prevalences and case reports in a Bayesian framework. Prevalence estimates for ages 15–39 were obtained from the National Health and Nutrition and Examination Survey for the years 1999–2014 for each age group in the model, up to age 39 [18]. National case reports for the years 2000–2015 were obtained by sex and age groups [19]. We also calibrated the model to the proportion of 15- to 18-year-old individuals who reported ever having had sex, using data from the Youth Risk Behavioral Survey for 1999–2015 [20].
Development of Pelvic Inflammatory Disease due to Chlamydial Infection
The development of PID was included as a rate from the chlamydia-infected health states, assuming the risk of PID as constant over the course of the infection. The annual rate of PID due to chlamydia was estimated through evidence synthesis [21], and it is approximated well by a normally distributed variable with a mean of 0.154 and standard deviation of 0.049. We assumed the rate pertains to first-time infections, with a higher risk among those with repeat infections, at a fixed relative rate of 1.15, based on evidence from longitudinal studies [22, 23].
Outcomes and Analyses
We sampled 200 model simulations from the posterior distribution of the calibrated model and 40 values from the distribution of PID risk, resulting in 8000 parameter combinations used in this analysis. We assumed POCT would replace all chlamydia testing after the last year of model calibration, and the outcomes were estimated at equilibrium (40 years after POCT implementation) to quantify the intervention’s maximum impact. We compared each POCT scenario against the model-estimated status quo, estimating the existing chlamydia prevention efforts. In the status quo, we maintained screening at 2015 levels, as estimated in the calibrated model, and used a constant population size. We report the relative prevalence reduction, yearly infections averted, and yearly incident PID averted for women using means and 95% credible intervals [CrIs]. Women aged 15–24 years are most affected by chlamydia and were the focus of the analysis.
We completed 2 analyses: in Analysis 1, we implemented POCT without changing the screening rate; and in Analysis 2, we assumed that POCT was implemented with a modest increase in the screening rate, facilitated by other interventions (eg, testing outside the clinic setting). We varied some of the uncertainties that can influence the impact of POCT, the first 2 of which pertain to the point-of-care test characteristics and the last 2 of which pertain to population characteristics:
The sensitivity of the point-of-care test was varied between 90–99%.
The POCT turnaround time, and how it impacts patients’ willingness to wait for test results, was examined. In its most idealized form, POCT could remove 100% of LTFUs and treatment delays. This assumes the POCT results are generated quickly enough for everyone to receive their results and treatment during the same patient visit. A 20-minute wait time for POCT is the WHO goal [1], but only 60% of women screened for chlamydia indicated that they would be willing to wait for that amount of time, and 30% responded that they would be willing to wait 40 minutes [24]. In Analysis 1, we varied the proportion receiving immediate treatment between 30–100%. In Analysis 2, where increased screening was implemented, 30–60% received immediate treatment.
The baseline levels of LTFU in the population in the absence of POCT were varied between 5–20% (Table 1).
The baseline level of an average treatment delay in the absence of POCT was varied between 1–3 weeks (Table 1).
We assumed LTFUs and treatment delays pertained only to asymptomatic infections.
Accounting for Delayed Treatment and Loss to Follow-up
In the model, screening and treatment were operationalized simultaneously. The model-estimated rate of transition from infected to susceptible represents the effective treatment rate given a person was tested, retained in follow-up, and received treatment, and is equal to the inverse of the average duration from infection to treatment initiation (Obase) for asymptomatic women. For sexually active women 15–18 years old in 2015, the effective treatment rate was estimated as 0.69 per year (median, with interquartile range [IQR] 0.66–0.72). For women 19–24 years old, it was 0.52 (IQR 0.34–0.70), and for women 25–39 years old, it was 0.12 (IQR 0.10–0.15). A relative frequency was estimated for the same age groups of men and applied as a multiplier to the correspondent effective treatment rate for women in the same age group. For men 15–24 years old, the relative frequency was 0.14 (IQR 0.11–0.17) of the women’s rate, and for men 25–39 years old, the frequency was 0.02 (IQR 0.02–0.02) of the women’s rate. In the United States, no recommendations exist for screening heterosexual men, but men in high-burden areas are encouraged to be screened when resources allow [25]. Limited screening of men occurs in some institutional settings (such as the military [26], schools [27], and the US National Job Training Program [28]). For women and men aged 40–54 years, we assumed no routine screening.
Using the duration from infection to treatment initiation (Obase), we could back-calculate the duration between screening tests, depending on the assumptions about LTFU and treatment delay: in , T is the duration between screening tests, f is the proportion LTFU after screening, and D is the average duration between testing and treatment initiation among those not LTFU. POCT with a short test turnaround, which would remove LTFUs and treatment delays, could reduce the duration of infection by shortening time to treatment. Among those receiving testing and treatment on the same visit, the mean time to treatment initiation would be . If treatment were not immediate, the average duration to treatment initiation would be , where p is the proportion who wait for their test results. To increase the rate of screening by 20% from the levels estimated in the calibrated model, we used the duration between tests, T, and the assumed levels of LTFU and treatment delays: . This can be used in and , where .
RESULTS
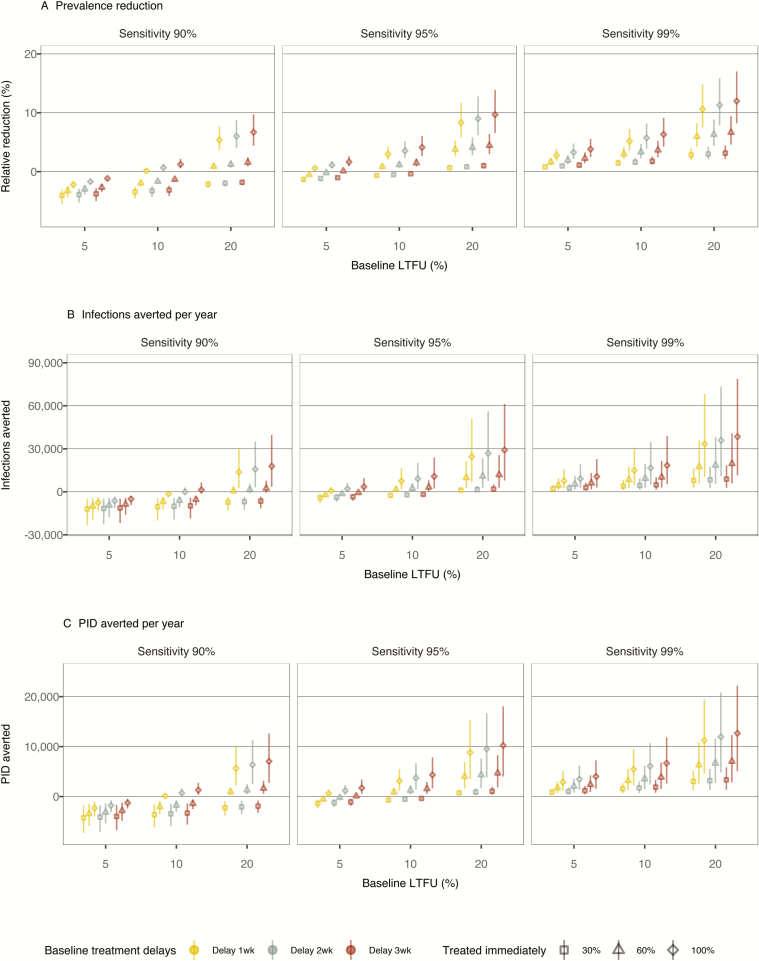
In Analysis 1, we considered scenarios in which all current testing would be replaced by POCT (Figure 1). POCT’s impact increases with increased test sensitivity, shorter turnaround times for POCT, and larger baseline LTFUs and treatment delays, which would be removed or reduced in the POCT scenarios. With a POCT sensitivity of 90%, reductions from the baseline burden only occurred in scenarios in which over 60% of the screened individuals would get immediate treatment and the baseline LTFU was 20%. When POCT sensitivity is on par with currently implemented testing (test sensitivity 99%), improvements were estimated across the analyzed scenarios.
Figure 1.
Impact of POCT in Analysis 1. Outcomes of the chlamydia burden among women aged 15–24, presented under different assumptions about POCT sensitivity (varied between 90–99%), the proportion of patients treated immediately (30–100%), the baseline proportion of LTFU (5–20%), and the average baseline delay between testing positive and being treated (1–3 weeks). (A) Prevalence reductions relative to baseline, (B) annual infections, and (C) annual PID cases averted are shown. Samples of 8000 simulations are plotted for each scenario. Abbreviations: LTFU, loss to follow-up; PID, pelvic inflammatory disease; POCT, point-of-care testing; wk, week.
In the calibrated model, the mean prevalence in women aged 15–24 years in equilibrium was 2.9% (95% CrI 2.4–3.4%). Using the scenario where the population baseline LTFU probability is 10% with a 2-week treatment delay as an example, implementing POCT with a sensitivity of 95% and with 100% of patients receiving immediate treatment was estimated to result in a prevalence reduction of 3.5% (95% CrI 2.3–5.1%), which corresponds to a chlamydia prevalence of 2.8% (95% CrI 2.3–3.3%). If only 30% waited for results and received immediate treatment, POCT would not be beneficial, but rather would result in a slight increase in prevalence, of 0.5% (95% CrI increase of 0.4–0.6%). If POCT sensitivity is 99%, more substantial benefits are possible; if 100% receives immediate treatment, the prevalence reduction was estimated at 5.7% (95% CrI 3.9–8.2%), corresponding to a chlamydia prevalence of 2.8% (95% CrI 2.2–3.2%). If only 30% waited for their results, the prevalence reduction would be 1.6% (95% CrI 1.1–2.3%).
Similar trends were observed for the other outcomes examined: POCT with 99% sensitivity could avert between 800 (95% CrI 300–1500) and 12 700 (95% CrI 5000–22 200) cases of PID per year, with the smaller number corresponding to 30% treated immediately, a 5% baseline LTFU rate, and a 1-week baseline delay, and the larger number corresponding to 100% treated immediately, a 20% baseline LTFU rate, and a 3-week baseline delay. The respective numbers for average yearly infections averted were estimated at 2100 (95% CrI 600–4500) and 38 400 (95% CrI 11 300–78 000).
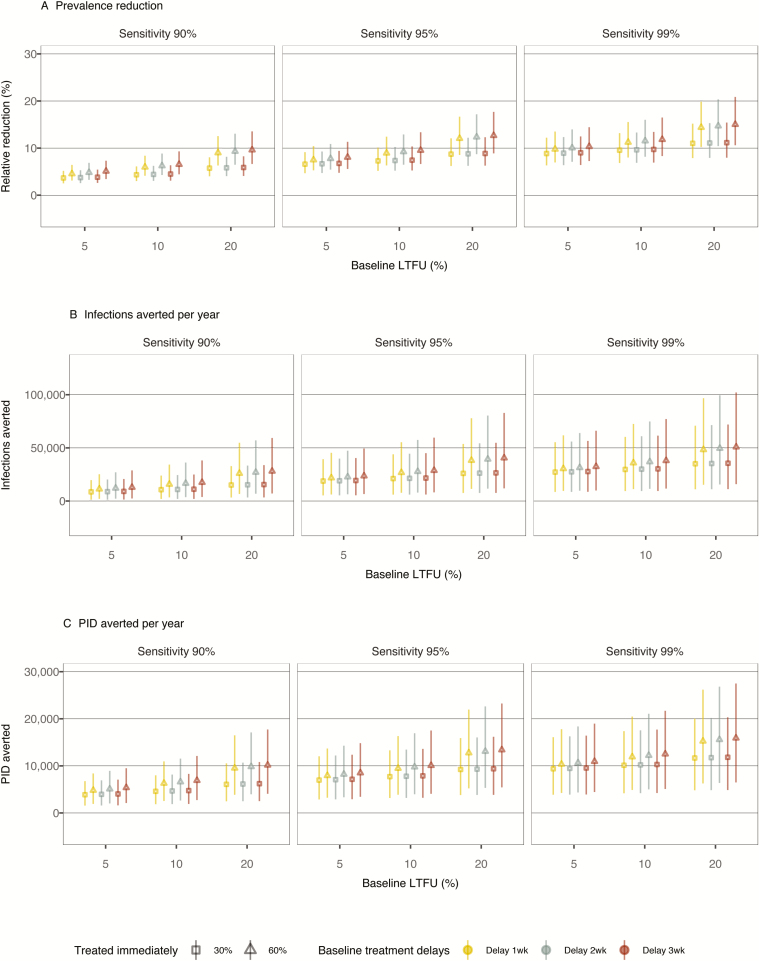
In Analysis 2, we considered additional scenarios in which POCT is accompanied by other improvements in chlamydia screening (Figure 2). With a 20% increase in screening frequency over baseline, we expect that reductions in the chlamydia burden compared to baseline would be seen with lower levels of POCT sensitivity than those required in Analysis 1. With sensitivity of 99%, 100% treated immediately, a 20% LTFU rate, and a 3-week treatment delay at baseline, POCT plus enhanced screening would reduce the prevalence by 15.0% (95% CrI 10.6–20.9%), corresponding to a new prevalence level of 2.5% (95% CrI 1.9–3.0%). The impact of immediate treatment was more pronounced when baseline LTFUs and treatment delays were larger.
Figure 2.
Impact of POCT if screening frequency is increased by 20% in Analysis 2. Outcomes of the chlamydia burden among women aged 15–24 are presented under different assumptions about POCT sensitivity (varied 90–99%), the proportion of patients treated immediately (30–60%), the baseline proportion of LTFU (5–20%), and the average baseline delay between testing positive and being treated (1–3 weeks). (A) Prevalence reductions relative to baseline, (B) annual infections, and (C) annual PID cases averted are shown. Samples of 8000 simulations are plotted for each scenario. Abbreviations. LTFU, loss to follow-up; PID, pelvic inflammatory disease; POCT, point-of-care testing; wk, week.
The findings for Analyses 1 and 2 were similar among women aged 25–39 and 40–54, with diminishing impact of POCT for the older age groups (Supplementary Figures 1–4).
DISCUSSION
In this study, we estimated the potential benefits achievable if chlamydia POCT were implemented in the United States under varied assumptions about the point-of-care test and population characteristics. We employed a transmission model of chlamydia, calibrated to national-level data on chlamydia epidemiology in the United States. Current prevention efforts for chlamydia are already maintaining a lower burden of infection compared to what would occur in the absence of screening [17]. A strength of the study is its ability to incorporate estimates of the impact of current prevention strategies, making assessments of the incremental benefits of novel strategies more robust. Improving chlamydia prevention over the status quo will likely require high standards from POCT. POCT with a sensitivity close to currently used NAATs could offer substantial additional benefits for chlamydia prevention. POCT with a lower sensitivity may increase the chlamydia burden, unless there are other factors coupled with POCT that can increase the level of screening at the population level.
Some of our assumptions may overestimate the impact of POCT, whilst others may underestimate its impact. The manner in which baseline LTFUs and treatment delays were incorporated may have overestimated the effect of POCT, as we adjusted the overall treatment rate instead of accounting for the heterogeneity in the population and improved outcomes that would occur for a subset of the population. Furthermore, if people were to reduce their risk behavior between receiving a positive diagnosis and being treated, this would overestimate the impact of POCT in our model, as we included no behavior changes. The estimate of the POCT impact would likely be lower using an individually based model, as an individual-based model is able to account for the individual-level variability in treatment times and LTFUs in the population. An individual based model would also account for the network-level factors whereby the main partners of an infected index case are likely to already be infected with chlamydia themselves, which would limit the onward transmission potential of the infection during the treatment delay and LTFU. The pair formation model framework utilized in this study captures some partnership dynamics, but not full network effects. However, the overall conclusion of the study remains robust to the underlying assumptions: knowing we are likely overestimating the impact of POCT strengthens the finding of needing a high-sensitivity point-of-care test to ensure beneficial outcomes at the population level. Our assumption that the impact of prolonged waiting times would cause patients who leave to experience the same level of LTFU and delay as in the baseline prior to POCT may underestimate the impact of POCT: even if patients leave before receiving test results, faster turnaround times for test results could help facilitate faster treatment times, as the Dean St. Express clinic has shown [29]. In the Dean St Express clinic, patients are contacted on the day of testing to inform them of the results, which has shortened treatment initiation times. If POCT were combined with other innovative models to expand chlamydia testing and to deliver test results to patients more quickly, this would diversify the ways in which POCT may be implemented. The study presents an evaluation of hypothetical point-of-care tests not yet available, and the results should be taken as exploratory in nature.
There are wider research needs regarding the practical aspects of POCT roll-out and the future of STI control in the presence of novel prevention strategies, which were outside the scope of this study but warrant future research. The inclusion of economic outcomes and cost effectiveness are further avenues for research. In the future, POCT for chlamydia may be part of a multiplex assay [30], which will require a more comprehensive analysis of benefits and harms. More research is also required if the direction of POCT is towards self-collection or even self-testing kits. Home testing can achieve a similar level of index case management as clinic-based testing, but it is not clear whether more infections are identified [31]. Testing outside the clinic setting may result in similar challenges as expedited partner therapy, with concerns about the prescription of antibiotics without seeing the patient [32, 33]. The surveillance of STIs is based on clinic and laboratory notifications of positive chlamydia test results, and the ability of surveillance to track changes in the disease burden may need to be realigned with novel testing methods if testing outside clinics became more common [34].
CONCLUSIONS
POCT can enhance chlamydia prevention efforts, but the magnitude of benefits depends on the state of care, including status quo levels of LTFUs and treatment delays, and the degrees to which POCT can improve these. These findings highlight the importance of understanding both the test characteristics and patient care–specific characteristics prior to the implementation of POCT. The evaluation of the technologies in development and in the implementation phase is a vital part of the improvement and planning of STI control. Aspects such as which outcomes to measure and what data to collect should be continually assessed. Candidate POCT assays for chlamydia are in development, and these have the potential to reduce the chlamydia burden if implemented in an effective manner.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Christian Testa for his advice on figure formatting.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the authors’ other affiliated institutions.
Financial support. This work was supported by the National Center for Human Immunodeficiency Virus, Viral Hepatitis, Sexually Transmitted Disease, and Tuberculosis Prevention Epidemiologic and Economic Modeling Agreement (grant number 5NU38PS004644) of the US Centers for Disease Control and Prevention.
Potential conflicts of interest. C. A. G. has been a paid educational speaker for Cepheid and has previously received research funds from Cepheid for Food and Drug Administration Clinical Trials, from which published data were used in estimating turnaround times of existing rapid tests in this study (references 37 and 38). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Sexually Transmitted Disease Prevention Conference, 27–30 August 2018, Washington, DC. Poster number POS 61.
References
- 1. World Health Organization. Point-of-care diagnostic tests (POCTs) for sexually transmitted infections (STIs). Geneva, Switzerland: World Health Organization, 2017. Available at: http://www.who.int/reproductivehealth/topics/rtis/pocts/en/. Accessed 31 March 2018. [Google Scholar]
- 2. Gift TL, Pate MS, Hook EW 3rd, Kassler WJ. The rapid test paradox: when fewer cases detected lead to more cases treated: a decision analysis of tests for Chlamydia trachomatis. Sex Transm Dis 1999; 26:232–40. [DOI] [PubMed] [Google Scholar]
- 3. Turner KM, Round J, Horner P, et al. An early evaluation of clinical and economic costs and benefits of implementing point of care NAAT tests for Chlamydia trachomatis and Neisseria gonorrhoea in genitourinary medicine clinics in England. Sex Transm Infect 2014; 90:104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chambers LC, Khosropour CM, Katz DA, Dombrowski JC, Manhart LE, Golden MR. Racial/ethnic disparities in the lifetime risk of Chlamydia trachomatis diagnosis and adverse reproductive health outcomes among women in King County, Washington. Clin Infect Dis 2018; 67:593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gaydos C, Hardick J. Point of care diagnostics for sexually transmitted infections: perspectives and advances. Expert Rev Anti Infect Ther 2014; 12:657–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hui BB, Wilson DP, Ward JS, et al. The potential impact of new generation molecular point-of-care tests on gonorrhoea and chlamydia in a setting of high endemic prevalence. Sex Health 2013; 10:348–56. [DOI] [PubMed] [Google Scholar]
- 7. Vickerman P, Watts C, Peeling RW, Mabey D, Alary M. Modelling the cost effectiveness of rapid point of care diagnostic tests for the control of HIV and other sexually transmitted infections among female sex workers. Sex Transm Infect 2006; 82:403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cook RL, Hutchison SL, Østergaard L, Braithwaite RS, Ness RB. Systematic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrhoeae. Ann Intern Med 2005; 142:914–25. [DOI] [PubMed] [Google Scholar]
- 9. The National Committee for Quality Assurance. The state of health care quality report. 2015. Available at: store.ncqa.org/index.php/catalog/product/view/id/2341/s/2015-state-of-health-care-quality-report/. Accessed 2 July 2019. [Google Scholar]
- 10. Davies B, Turner KME, Benfield T, et al. ; Danish Chlamydia Study Pelvic inflammatory disease risk following negative results from chlamydia nucleic acid amplification tests (NAATs) versus non-NAATs in Denmark: a retrospective cohort. PLOS Med 2018; 15:e1002483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Foglia G, Rhodes P, Goldberg M, St Louis ME. Completeness of and duration of time before treatment after screening women for Chlamydia trachomatis infections. Sex Transm Dis 1999; 26:421–5. [DOI] [PubMed] [Google Scholar]
- 12. Schwebke JR, Sadler R, Sutton JM, Hook EW 3rd. Positive screening tests for gonorrhea and chlamydial infection fail to lead consistently to treatment of patients attending a sexually transmitted disease clinic. Sex Transm Dis 1997; 24:181–4. [DOI] [PubMed] [Google Scholar]
- 13. Hwang LY, Tebb KP, Shafer MA, Pantell RH. Examination of the treatment and follow-up care for adolescents who test positive for Chlamydia trachomatis infection. Arch Pediatr Adolesc Med 2005; 159:1162–6. [DOI] [PubMed] [Google Scholar]
- 14. Wong D, Berman SM, Furness BW, Gunn RA, Taylor M, Peterman TA. Time to treatment for women with chlamydial or gonococcal infections: a comparative evaluation of sexually transmitted disease clinics in 3 US cities. Sex Transm Dis 2005; 32:194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoon J, Elder HR, Hawrusik R, Klevens RM, Roosevelt KA, Hsu KK. Does nonmetropolitan residence impact timely chlamydia treatment in Massachusetts? Sex Transm Dis 2018; 45:e52–6. [DOI] [PubMed] [Google Scholar]
- 16. The National Committee for Quality Assurance. Chlamydia screening in women Available at: https://www.ncqa.org/hedis/measures/chlamydia-screening-in-women/. Accessed 4 April 2019.
- 17. Rönn MM, Tuite AR, Menzies NA, et al. The impact of screening and partner notification on chlamydia prevalence and numbers of infections averted in the United States, 2000–2015: evaluation of epidemiologic trends using a pair-formation transmission model. Am J Epidemiol 2019; 188:545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dagnan D, Chadwick P, Proudlove J. Toward an assessment of suitability of people with mental retardation for cognitive therapy. Cognit Ther Res 2000; 24:627–36. [Google Scholar]
- 19. Centers for Disease Control and Prevention. NCHHSTP AtlasPlus Updated 2017. Available at: https://www.cdc.gov/nchhstp/atlas/index.htm. Accessed 16 November 2017.
- 20. Centers for Disease Control and Prevention. Youth risk behavior survey data & documentation Available at: https://www.cdc.gov/healthyyouth/data/yrbs/data.htm. Accessed 2 July 2019.
- 21. Trikalinos T. Pelvic inflammatory disease development due to chlamydia Available at: https://www.brown.edu/public-health/cesh/resources/technical-reports. Accessed 2 July 2019.
- 22. Davies B, Ward H, Leung S, et al. Heterogeneity in risk of pelvic inflammatory diseases after chlamydia infection: a population-based study in Manitoba, Canada. J Infect Dis 2014; 210(Suppl 2):S549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davies B, Turner KME, Frølund M, et al. ; Danish Chlamydia Study Group Risk of reproductive complications following chlamydia testing: a population-based retrospective cohort study in Denmark. Lancet Infect Dis 2016; 16:1057–64. [DOI] [PubMed] [Google Scholar]
- 24. Widdice LE, Hsieh YH, Silver B, Barnes M, Barnes P, Gaydos CA. Performance of the atlas genetics rapid test for Chlamydia trachomatis and women’s attitudes toward point-of-care testing. Sex Transm Dis 2018; 45:723–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. LeFevre ML; U.S. Preventive Services Task Force Screening for chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 161:902–10. [DOI] [PubMed] [Google Scholar]
- 26. Barnett SD, Brundage JF. Incidence of recurrent diagnoses of Chlamydia trachomatis genital infections among male and female soldiers of the US Army. Sex Transm Infect 2001; 77:33–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lewis FM, Newman DR, Anschuetz GL, Mettey A, Asbel L, Salmon ME. Partner meeting place is significantly associated with gonorrhea and chlamydia in adolescents participating in a large high school sexually transmitted disease screening program. Sex Transm Dis 2014; 41:605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Learner ER, Torrone EA, Fine JP, Pence BW, Powers KA, Miller WC. Chlamydia prevalence trends among women and men entering the National Job Training Program from 1990 through 2012. Sex Transm Dis 2018; 45:554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Whitlock GG, Gibbons DC, Longford N, Harvey MJ, McOwan A, Adams EJ. Rapid testing and treatment for sexually transmitted infections improve patient care and yield public health benefits. Int J STD AIDS 2018; 29:474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Birger R, Saunders J, Estcourt C, et al. Should we screen for the sexually-transmitted infection Mycoplasma genitalium? Evidence synthesis using a transmission-dynamic model. Sci Rep 2017; 7:16162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fajardo-Bernal L, Aponte-Gonzalez J, Vigil P, et al. Home-based versus clinic-based specimen collection in the management of Chlamydia trachomatis and Neisseria gonorrhoeae infections. Cochrane Database Syst Rev 2015; 9:CD011317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Niccolai LM, Winston DM. Physicians’ opinions on partner management for nonviral sexually transmitted infections. Am J Prev Med 2005; 28:229–33. [DOI] [PubMed] [Google Scholar]
- 33. Qin JZ, Diniz CP, Coleman JS. Pharmacy-level barriers to implementing expedited partner therapy in Baltimore, Maryland. Am J Obstet Gynecol 2018; 218:504.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peterman TA, Kreisel K, Habel MA, Pearson WS, Dittus PJ, Papp JR. Preparing for the chlamydia and gonorrhea self-test. Sex Transm Dis 2018; 45:e7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rompalo AM, Hsieh YH, Hogan T, et al. Point-of-care tests for sexually transmissible infections: what do “end users” want? Sex Health 2013; 10:541–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang W, Gaydos CA, Barnes MR, Jett-Goheen M, Blake DR. Comparative effectiveness of a rapid point-of-care test for detection of Chlamydia trachomatis among women in a clinical setting. Sex Transm Infect 2013; 89:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gaydos CA, Van Der Pol B, Jett-Goheen M, et al. ; Chlamydia trachomatis and Neisseria gonorrhoeae Study Group. Performance of the cepheid CT/NG Xpert rapid PCR test for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol 2013; 51:1666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gaydos CA. Review of use of a new rapid real-time PCR, the Cepheid GeneXpert® (Xpert) CT/NG assay, for Chlamydia trachomatis and Neisseria gonorrhoeae: results for patients while in a clinical setting. Expert Rev Mol Diagn 2014; 14:135–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Geisler WM, Wang C, Morrison SG, Black CM, Bandea CI, Hook EW 3rd. The natural history of untreated Chlamydia trachomatis infection in the interval between screening and returning for treatment. Sex Transm Dis 2008; 35:119–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.